Abstract
A series of isatin analogs containing a hydrophilic group, including a pyridine ring, ethylene glycol group, and a triazole ring, have been synthesized, and their inhibition potency for caspase-3 was measured both in vitro (i.e. recombinant enzyme) and in whole cells (HeLa cells). The analogs having a hydrophilic group, including 12, 13, 16, 38, and 40, have dramatically increased activity in vitro and in HeLa cells compared to the corresponding unsubstituted N-phenyl isatin analogs.
Keywords: caspase-3, apoptosis, cell death
The caspase family of cysteine proteases consists of two different classes of enzymes involved in apoptosis, the initiator caspases and the executioner caspases.1 The initiator caspases, which include caspase-2, -8, -9, and -10, are located at the top of the signaling cascade; their primary function is to activate the executioner caspases, caspase-3, -6, and -7. The executioner caspases are responsible for the physiological changes (e.g., cleavage of the DNA repair enzyme poly(ADP-ribose) polymerase-1, nuclear laminins, and cytoskeleton proteins) and morphological changes (DNA strand breaks, nuclear membrane damage, and membrane blebbing) that occur in apoptosis. Apoptosis plays an important role in a wide variety of normal cellular processes including fetal development, tissue homeostasis, and maintenance of the immune system. However, abnormal apoptosis has been observed in a large number of pathological conditions, including ischemia-reperfusion injury (stroke and myocardial infarction), cardiomyopathy, neurodegeneration (Alzheimer's Disease, Parkinson's Disease, Huntington's Disease, ALS), sepsis, Type I diabetes, and allograft rejection.2-3 The development of drugs that can halt the process of apoptosis has been an active area of research in the pharmaceutical industry.2 In addition, the benefits of many drugs, especially antitumor drugs, can be attributed to their activation of the apoptotic process.4-9 Therefore, the development of a noninvasive imaging procedure that can study the process of apoptosis in a variety of disease states and monitor the ability of a drug to either induce or halt apoptosis would be of tremendous value to the research and clinical community.
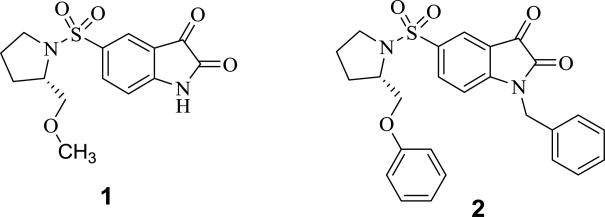
Recently, a number of isatin-based inhibitors of caspase-3/7 have been reported.10-22 Structure-activity relationship studies have revealed that the following structural changes to 1 (Figure 1) resulted in a dramatic improvement in potency for inhibiting caspase-3 activity in enzymatic activity assays: a) replacement of the 2-methoxymethyl group with a phenoxymethyl or 2-pyridin-3-yl-oxymethyl moiety; 2) replacement of the pyrrolidine ring with an azetidine ring; and, 3) the introduction of an alkyl or arylalkyl on the isatin nitrogen group.10,12 For example, isatin analog in 2 (Figure 1) had a significantly enhanced potency for inhibiting caspase-3 activity in enzymatic assays relative to 1; however, the potency for inhibiting caspase-3 activity using a whole cell assay was not as high as that observed in enzymatic assays using the recombinant enzyme. The introduction of a benzyl group on the isatin nitrogen and replacement of the methoxymethyl group with a phenoxymethyl group dramatically increased the lipophilicity of the compound; this increased lipophilicity is expected to have a negative effect on the ability of the inhibitor to cross the cell membrane and inhibit caspase-3 in the whole cell assay versus in an in vitro enzymatic assay using recombinant protein.10 The goal of the current study is to extend the structure-activity relationship study of this class of compounds by introducing a hydrophilic group in the isatin nitrogen, including an oligo-ethylene glycol group or a triazole ring, in order to reduce the lipophilicity of the isatin analogs and improve the potency for inhibiting caspase-3 activity in the whole cell assay. Here we report a new series of isatin analogs as caspase-3 inhibitors with improved potency for inhibiting caspase-3 activity in both the in vitro enzyme assay and in cyclosporine-induced caspase activation in HeLa cells.
Figure 1.
Structures of the lead compounds for the current study.
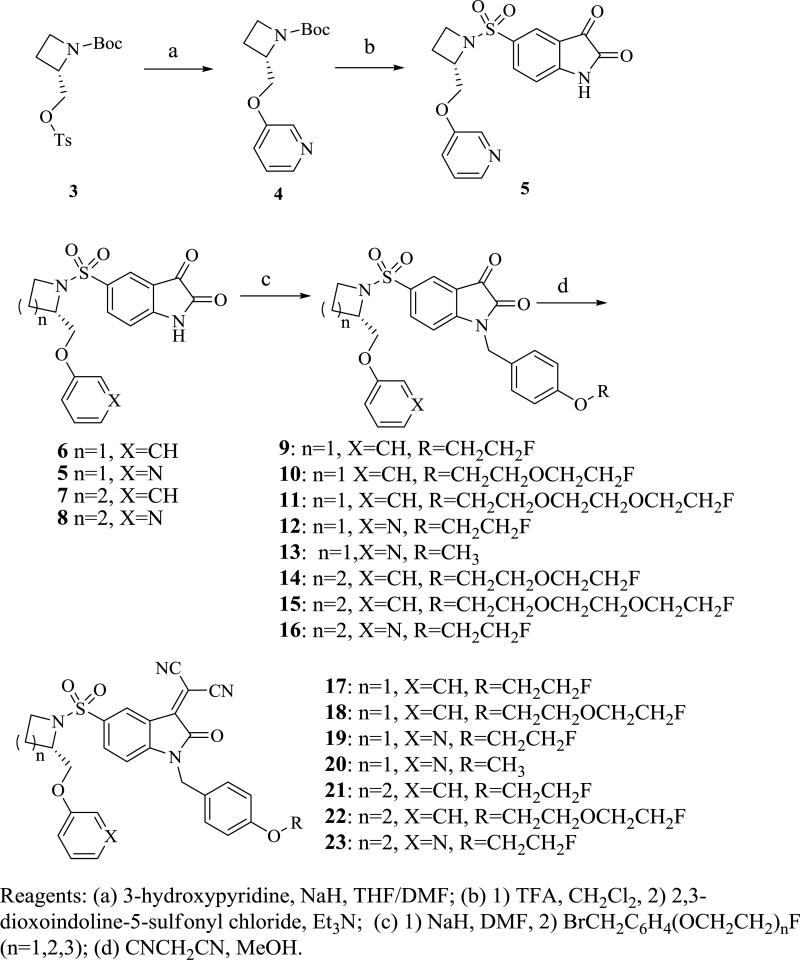
The synthesis of 5-(2-phenoxymethyl-pyrrolidine-1-sulfonyl)isatin and 5-(2-phenoxymethyl-azetidine-1-sulfonyl)isatin analogs is shown in Scheme 1. The pyridin-3-yloxy group was introduced via displacement of the tosylate group of 3 with sodium pyridin-3-olate in DMF to afford N-Boc-2-(phenoxymethyl)azetidine 4. The N-Boc group of 4 was removed with TFA and the secondary amine was coupled with 2,3-dioxoindoline-5-sulfonyl chloride in THF using triethylamine as an acid scavenger to afford the (S)-5-(2-((pyridin-3-yloxy)methyl)azetidin-1-ylsulfonyl)indoline-2,3-dione 5. The isatin nitrogen was alkylated by treatment of 5 with sodium hydride in DMF at 0 °C followed by addition of 1-(bromomethyl)-4-(2-fluoroethoxy)benzene or 1-(chloromethyl)-4-methoxybenzene to give compounds 12 and 13. The isatin compound 12 or 13 were treated with malononitrile in methanol to give the corresponding isatin Michael addition acceptor (IMA) analogs 19 and 20 as a red solid. Similarly, the isatin nitrogen of 6-8, was alkylated with alkyl halide intermediates to give 9-11, and 14-16, which were converted to their IMA analogs 17, 18, 21-23, respectively. The preparation of alkyl halide intermediates is shown in Scheme 2.
Scheme 1.
Synthesis of the pegylated analogs.
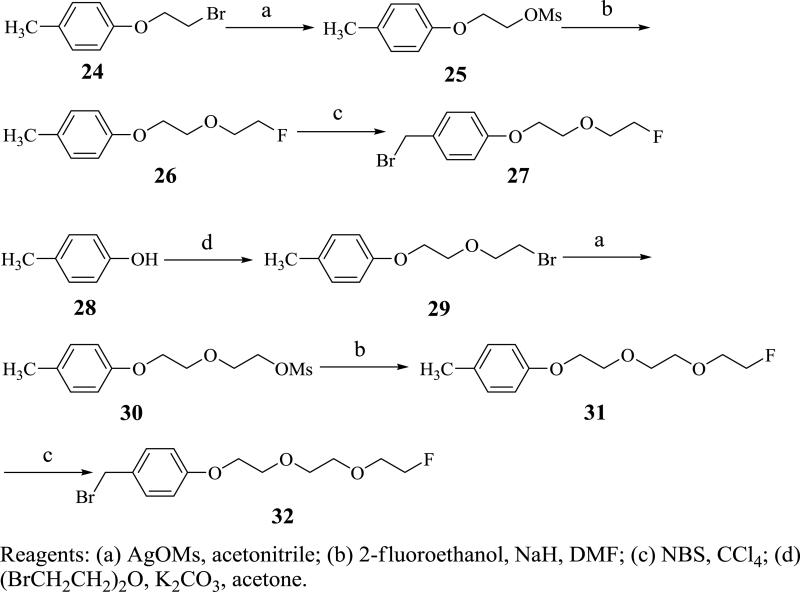
Scheme 2.
Synthesis of intermediates 27 and 32.
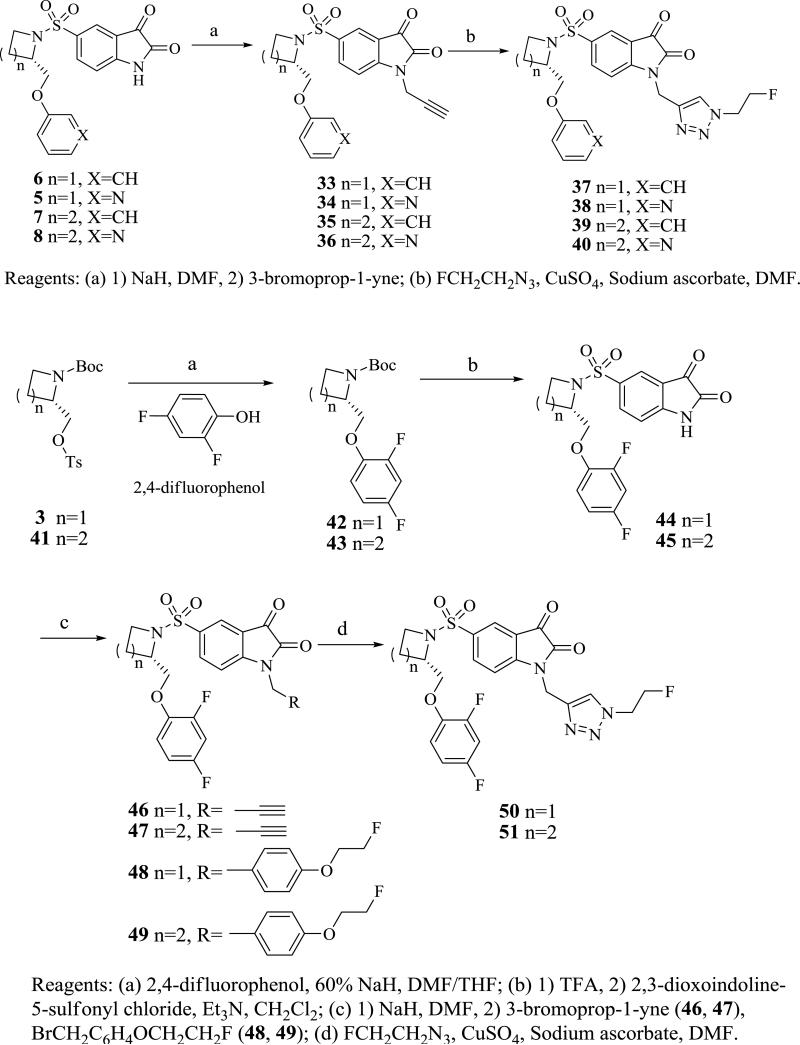
The synthesis of the triazole-containing isatin analogs is shown in Scheme 3. The isatin nitrogen of 5-8 was first alkylated by treatment of 5-8 with sodium hydride in DMF at 0 °C followed by addition of propargyl bromide to give alkyne analogs 33-36, respectively. The triazole analogs 37-40 were prepared by copper(I) catalyzed 1,3-dipolar cycloaddition (i.e., “Click reaction”) of 2-fluoroethylazide with 33-36, respectively. While this research was ongoing, Aboagye and colleagues reported a series of triazole-containing isatin analogs containing a fluorine-substituted benzene ring in the phenoxymethyl moiety. 20,21 These analogs showed high caspase-3 inhibition potency and improved in vivo stability relative to the corresponding unsubstituted phenoxymethyl analogs.20,21 In fact, [18F]51 has shown potential for imaging chemotherapy-induced caspase-3 activation in microPET imaging studies of an animal model of lymphoma.21 Therefore 2,4-difluorophenyloxy isatin analogs 48-50 were also prepared using the same procedure as that of 12 and 37, respectively (Scheme 3) and compared with 51 in the enzymatic and whole cell assays for inhibiting caspase-3 activity.
Scheme 3.
Synthesis of the triazole analogs.
The inhibition potency of the isatin and representative IMA analogs for recombinant human caspase-3 and other caspases in the enzyme assays was assessed using a standard fluorometric assay described previously;12 the IC50 values for each compound are summarized in Table 1. All of the tested compounds have no inhibition of caspase-8, and little or very weak inhibition of caspase-1. The isatin analogs 9-16 and their IMA analogs 17-23 show high potency and selectivity for the inhibition of caspase-3. Compounds 9-16 show comparable inhibition potency for caspase-7, whereas the IMA analogs 17-23 show weaker inhibition for caspase-7 than for caspase-3. The pyridine-containing analogs, 5-(2-pyridin-3-yl-oxymethylpyrrolidine-1-sulfonyl)isatin 16 (IC50: 3.1 nM) and its IMA analog 23 (IC50: 7.5 nM) have higher potency for inhibition of caspase-3 in the enzyme assays than the corresponding phenoxymethyl-containing analog, WC-II-89 (IC50: 9.7 nM) and its IMA analog 21 (IC50: 18.3 nM). The same trend holds for 5-(2-pyridin-3-yl-oxymethylazetidine-1-sulfonyl)isatin and its IMA analog (12 IC50: 3.6 nM, 19 IC50: 7.1 nM) versus the corresponding phenoxymethyl-containing analogs (9 IC50: 8.6 nM, and 17 IC50: 22.4 nM). The IMA analogs have higher inhibition potencies for caspase-6 than their corresponding isatin analogs, which is consistent with our previous observations.15 The 2,4-difluorophenoxymethyl-containing analogs, 5-(2-(2,4-difluorophenoxymethyl)pyrrolidine-1-sulfonyl)isatin 49 and 5-(2-(2,4-difluorophenoxymethyl)azetidine-1-sulfonyl)isatin 48, have high selectivity and enzymatic activity for inhibition of caspase-3 and caspase-7. The triazole analogs, compounds 37-40, 50, and 51, also showed high selectivity and activity for inhibition of caspase-3 and caspase-7 in the enzyme assays. It is interesting to note that the triazole-containing isatin analogs have a high potency for inhibiting caspase-7 activity, with IC50 values for inhibiting caspase-7 similar to that for caspase-3 (e.g. 38 IC50: 5.6 nM and 40 IC50: 4.5 nM for caspase-3 vs 38 IC50: 6.1 nM and 40 IC50: 3.8 nM for caspase-7). Otherwise, for most of the isatin compounds reported, their inhibition potency for caspase-3 is usually 3-4 times higher than that for caspase-7. To our knowledge, this is the first report of caspase inhibitors displaying inhibition potencies for caspase-7 similar to or better than for caspase-3.
Table 1.
Inhibition activities for caspase-1, -3, -6, -7, and -8.
| Compoundsa | IC50 (nM)b |
Log Pc | ||||
|---|---|---|---|---|---|---|
| Caspase-1 | Caspase-3 | Caspase-6 | Caspase-7 | Caspase-8 | ||
| 9 | >20000 | 8.6±1.0 | 4930±1240 | 26.1±1.5 | >25000 | 3.63 |
| 10 | >50000 | 27.9±3.6 | >10000 | 28.9±4.1 | >50000 | 3.23 |
| 11 | >25000 | 26.9±4.4 | 2987±118 | 19.2±1.2 | >50000 | 2.87 |
| 12 | >10000 | 3.6±0.5 | 8700±140 | 17.6±0.4 | >50000 | 2.57 |
| 13 | >15000 | 3.3±0.1 | 9200±600 | 10.2±1.1 | >50000 | 2.34 |
| 14 | >25000 | 10.9±1.2 | >10000 | 11.9±2.0 | >50000 | 3.79 |
| 15 | >50000 | 13.7±1.0 | 7666±1361 | 17.6±0.4 | >50000 | 3.44 |
| 16 | >20000 | 3.1±0.4 | 6900±850 | 11.3±0.6 | >50000 | 3.13 |
| 17 | 4775±178 | 22.4±2.1 | 747±91 | 292±60 | >25000 | 3.08 |
| 18 | 5733±1102 | 24.4±2.0 | 1012±55 | 68.3±6.9 | >50000 | 2.68 |
| 19 | 2900±90 | 7.1±0.6 | 509±55 | 45.0±4.3 | >50000 | 2.02 |
| 20 | >36000 | 6.0±0.8 | 450±43 | 50.0±11.6 | >50000 | 1.80 |
| 21 | >10000 | 18.3±0.4 | 927±35 | 96.3±20.7 | >50000 | 3.65 |
| 22 | >10000 | 23.3±0.4 | 1165±256 | 70.0±4.5 | >50000 | 3.25 |
| 23 | 3500±960 | 7.5±0.2 | 770±119 | 26.5±1.3 | >50000 | 2.59 |
| 37 | >20000 | 11.0±0.2 | 6950±915 | 18.0±2.8 | >50000 | 1.22 |
| 38 | >20000 | 5.6±0.5 | 7750±676 | 6.1±1.8 | >50000 | 0.16 |
| 39 | >20000 | 12.2±1.5 | 9967±1079 | 12.4±1.4 | >50000 | 1.78 |
| 40 | >20000 | 4.5±0.7 | 13450±636 | 3.8±0.2 | >50000 | 0.73 |
| 48 | >50000 | 12.0±2.1 | 3475±727 | 15.4±1.3 | >50000 | 3.79 |
| 49 | >50000 | 15.2±1.5 | 9125±2647 | 12.3±2.2 | >50000 | 4.36 |
| 50 | >20000 | 13.4±2.0 | 4700±712 | 15.3±2.5 | >50000 | 1.39 |
| 51 | >20000 | 10.8±0.4 | 4425±532 | 11.6±1.1 | >50000 | 1.95 |
| Ac-IETD-CHO | 4.2±0.2 | |||||
| Ac-YVAD-CHO | 8.4±0.6 | |||||
| Ac-VEID-CHO | 8.0±2.0 | |||||
| Ac-DEVD-CHO | 2.2±0.4 | 2.9±0.4 | ||||
All tested compounds in the manuscript possess a purity of at least 95% as determined by elemental analysis.
IC50 values are the mean ± SD of at least three independent assays.
Calculated value using the ClogP
The Apo-One® Homogeneous Caspase-3/7 Assay (Promega Corporation, Madison, WI) was used to screen potential apoptosis inhibitors against caspase-3 activity in cell culture following the manufacturer's protocol with slight modifications. HeLa cells, grown under appropriate cell culture conditions, were plated at a density of 1-2×104 cells per well in 96-well format and allowed to adhere overnight at 37°C in a 5% CO2 atmosphere. Cells were then induced into apoptosis following treatment with 0.5 μg/ml staurosporine in the presence of potential caspase-3 inhibitors. After induction, a cell permeable caspase-3/7 substrate Z-DEVD-R110 was added and the hydrolysis of the substrate was measured using a Victor3V microplate fluorometer (Perkin Elmer Inc., Waltham, MA) at excitation wavelength 485nm and emission wavelength 535nm. Dose-response curves were plotted and EC50 values were determined for each compound. EC50 values were calculated from no less than three independent experiments. Data from the whole cell assays are shown in Table 2 and 3. The phenoxymethyl analog 5-(2-phenoxymethylazetidine-1-sulfonyl)isatin 6 (EC50: 9.57 μM) has a slightly higher inhibition potency for caspase-3 in whole cell assays than the 5-(2-phenoxymethylpyrrolidine-1-sulfonyl)isatin 7 (EC50: 11.50 μM), but the more hydrophilic pyridine analog, 5-(2-pyridin-3-yl-oxymethylpyrrolidine-1-sulfonyl)isatin 8 (EC50: 1.83 μM) has the highest potency for inhibiting caspase-3 in the whole cell assay. A good correlation exists between the relative whole cell assays with their in vitro enzyme assays for caspase-3 or -7 inhibition activities.11,12,15 As reported previously, alkylation of the isatin nitrogen with methyl, benzyl or a substituted benzyl group also resulted in an increase in potency for inhibition of caspase-3 in the whole cell assay. The azetidine analogs, 5-(2-phenoxymethylazetidine-1-sulfonyl)isatin 52 and 55, have higher potency for inhibition of caspase-3 than their corresponding pyrrolidine analogs, 5-(2-phenoxymethylpyrrolidine-1-sulfonyl)isatin 53 and 2, and the pyridine-containing 5-(2-pyridin-3-yl-oxymethylpyrrolidine-1-sulfonyl)isatin analogs have increased potency for inhibiting caspase-3 in whole cell assays compared with their corresponding phenoxymethyl-isatin analogs. As shown in Table 3, the results from the whole cell assay are mirrored in the enzyme assay results shown in Table 1. For the 5-(2-phenoxymethylpyrrolidine-1-sulfonyl)isatin analogs, starting from the fluoroethoxy benzyl analog (WC-II-89), increasing the chain with an ethylene glycol unit resulted in higher potency for inhibiting caspase-3 in the whole cell assay as well as in the enzyme assays (WC-II-89 EC50: 4.50 μM, 14 EC50: 1.69 μM, and 15 EC50: 1.30 μM, respectively). For the phenoxymethyl-isatin analogs, the introduction of a triazole group also resulted in improved inhibition potency for caspase-3 in the whole cell assay compared with their corresponding fluoroethoxybenzyl analogs (e.g. 37 vs 9; 39 vs WC-II-89). Similarly, the pyridine-containing isatin analogs always give rise to the most potent inhibition for caspase-3 in the whole cell assay, and even the introduction of triazole group did not increase the potency further as shown above. The 2,4-difluorophenoxymethyl-containing isatin analogs afforded comparable results with the phenoxymethyl isatin analogs in the whole cell assay. It is worthwhile to mention that compound 51, which was reported by the Aboagye and colleagues20 as a potential PET imaging tracer, also has very good inhibition potency for caspase-3 in the whole cell assay.
Table 2.
Inhibition activities for caspase-3 in HeLa cell.
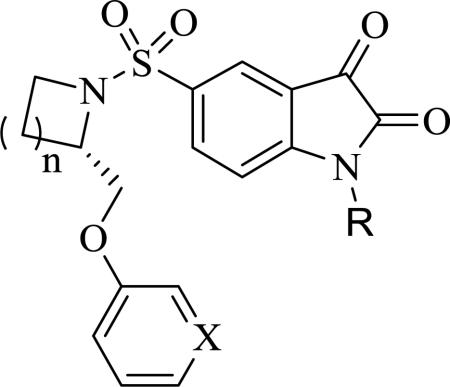 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compd. | n | X | R | EC50 (μM)a | Compd. | n | X | R | EC50 (μM)a |
| 6 | 1 | CH | H | 9.57±2.27 | 55 b | 1 | CH | Phenyl | 2.80±0.72 |
| 7 | 2 | CH | H | 11.50±1.50 | 2 | 2 | CH | Phenyl | 3.67±0.76 |
| 8 | 2 | N | H | 1.83±0.29 | 56 b | 2 | N | Phenyl | 0.65±0.18 |
| 52 b | 1 | CH | CH3 | 3.25±0.25 | 5 b | 1 | CH | 4-MeO-Phenyl | 3.07±1.01 |
| 53 b | 2 | CH | CH3 | 7.67±2.02 | 13 | 1 | N | 4-MeO-Phenyl | 0.45±0.12 |
| 54 b | 2 | N | CH3 | 1.80±0.26 | 58 b | 2 | N | 4-MeO-Phenyl | 0.45±0.15 |
EC50 values are the mean ± SD of at least three independent assays
reference 12.
Table 3.
Inhibition activities for caspase-3 in HeLa cells.
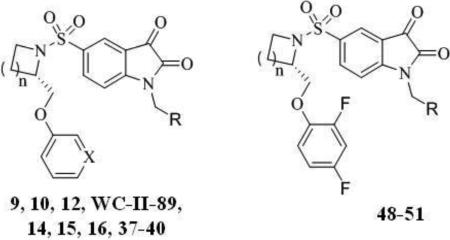 | ||||
|---|---|---|---|---|
| Compd. | n | X | R | EC50 (μM)a |
| 9 | 1 | CH |
|
2.00±0.44 |
| 10 | 1 | CH |
|
2.15±0.45 |
| WC-II-89 b | 2 | CH |
|
4.50±1.10 |
| 14 | 2 | CH |
|
1.69±0.18 |
| 15 | 2 | CH |
|
1.30±0.25 |
| 12 | 1 | N |
|
0.38±0.07 |
| 16 | 2 | N |
|
0.39±0.09 |
| 37 | 1 | CH |
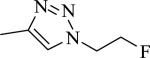
|
0.80±0.06 |
| 38 | 1 | N |
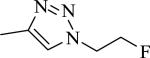
|
0.42±0.10 |
| 39 | 2 | CH |
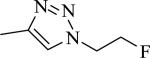
|
0.93±0.21 |
| 40 | 2 | N |
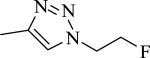
|
0.35±0.04 |
| Compd. | n | R | EC50 (μM)a | |
| 48 | 1 |
|
1.39±0.40 | |
| 49 | 2 |
|
2.50±0.80 | |
| 50 | 1 |
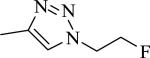
|
1.06±0.20 | |
| 51 | 2 |
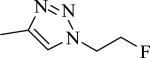
|
0.68±0.39 | |
IC50 values are the mean ± SD of at least three independent assays
reference 14.
The current study was focused on the development of new caspase-3 inhibitors as radiotracers that are specific for imaging apoptosis using PET, as well as drugs for the treatment of diseases associated with unregulated apoptosis. As an efficient drug or radiotracer for PET imaging, the inhibitor has to penetrate the cell easily in vivo to arrive at the target. For the neurodegenerative diseases, the inhibitors need to cross the blood-brain barrier by a passive diffusion process. The 5-(2-phenoxymethylpyrrolidine-1-sulfonyl)isatin analogs having a pegylated moiety in the N-benzyl region displayed an increase in potency for inhibiting caspase-3 in the whole cell assay and a lower Log P value relative to WC-II-89. Molecular modeling studies have also provided evidence of a possible hydrophilic interaction between pyridyloxymethyl moiety and the S3 binding domain of caspase-3.12,23 In addition to reducing the log P of the compound, this pyridine-for-benzene substitution results in a favorable π- π interaction as well as a hydrogen bond formation between the pyridine nitrogen and Phe381 in the active site of caspase-3. Consequently, the pyridyloxymethyl isatin analogs have a higher potency for inhibiting caspase-3 in both the in vitro enzyme assay and the whole cell assay than the corresponding benzoxymethyl isatin analogs. Furthermore, alkylation of the isatin nitrogen with a substituted triazole ring resulted in a further decrease in Log P value and an increase in potency for inhibiting caspase-3 in whole cell assay.
In conclusion, we synthesized a series of isatin analog compounds as caspase-3 inhibitors and evaluated their activities for inhibiting caspase-3 in enzyme and whole cell assays. The isatin analogs containing a hydrophilic group, such as a pyridine ring, polyethyleneglycol group, and a triazole ring, resulted in a dramatic increase in inhibiting caspase-3 and -7 in both the in vitro enzyme assays using recombinant enzyme and in HeLa cells treated with cyclosporine.
Supplementary Material
Acknowledgment
This research was funded by CA121952 and HL13851 awarded by the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data. Synthetic procedures, NMR and Elemental analysis data can be found in the online version of this article.
References
- 1.Denault J–B, Salvesen GS. Chem. Rev. 2002;102:4489. doi: 10.1021/cr010183n. [DOI] [PubMed] [Google Scholar]
- 2.Reed JC. Nature Rev. Drug Discovery. 2002;1:111. doi: 10.1038/nrd726. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez I, Matsuura K, Ody C, Nagata S, Vassalli P. J. Exp. Med. 1996;184:2067. doi: 10.1084/jem.184.5.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White E. Gene Dev. 1996;10:1. doi: 10.1101/gad.10.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Ashkenazi A, Dixit VM. Science. 1998;281:1305. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 6.Evan G, Littlewood T. Science. 1998;281:1317. doi: 10.1126/science.281.5381.1317. [DOI] [PubMed] [Google Scholar]
- 7.Green DR, Reed JC. Science. 1998;281:1309. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 8.Thornberry NA, Lazebnik Y. Science. 1998;281:1312. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 9.Dive C, Hickman JA. Br. J. Cancer. 1991;64:192. doi: 10.1038/bjc.1991.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee D, Long SA, Adams JL, Chan G, Vaidya KS, Francis TA, Kikly K, Winkler JD, Sung CM, Debouck C, et al. J. Biol. Chem. 2000;275:16007. doi: 10.1074/jbc.275.21.16007. [DOI] [PubMed] [Google Scholar]
- 11.Lee D, Long SA, Murray JH, Adams JL, Nuttall ME, Nadeau DP, Kikly K, Winkler JD, Sung CM, Ryan MD, et al. J. Med. Chem. 2001;44:2015. doi: 10.1021/jm0100537. [DOI] [PubMed] [Google Scholar]
- 12.Chu W, Zhang J, Zeng C, Rothfuss J, Tu Z, Chu Y, Reichert DE, Welch MJ, Mach RH. J. Med. Chem. 2005;48:7637. doi: 10.1021/jm0506625. [DOI] [PubMed] [Google Scholar]
- 13.Kopka K, Faust A, Keul P, Wagner S, Breyholz HJ, Höltke C, Schober O, Schäfers M, Levkau B. J. Med. Chem. 2006;49:6704. doi: 10.1021/jm051217c. [DOI] [PubMed] [Google Scholar]
- 14.Zhou D, Chu W, Rothfuss J, Zeng C, Xu J, Jones L, Welch MJ, Mach RH. Bioorg. Med. Chem. Lett. 2006;16(19):5041. doi: 10.1016/j.bmcl.2006.07.045. [DOI] [PubMed] [Google Scholar]
- 15.Chu W, Rothfuss J, D'Avignon A, Zeng C, Zhou D, Hotchkiss RS, Mach RH. J. Med. Chem. 2007;50:3751. doi: 10.1021/jm070506t. [DOI] [PubMed] [Google Scholar]
- 16.Faust A, Wagner S, Law MP, Hermann S, Schnöckel U, Keul P, Schober O, Schäfers M, Levkau B, Kopka K. Q. J. Nucl. Med. Mol. Imaging. 2007;51(1):67. [PubMed] [Google Scholar]
- 17.Podichetty AK, Wagner S, Schröer S, Faust A, Schäfers M, Schober O, Kopka K, Haufe G. J. Med. Chem. 2009;52(11):3484. doi: 10.1021/jm8015014. [DOI] [PubMed] [Google Scholar]
- 18.Podichetty AK, Faust A, Kopka K, Wagner S, Schober O, Schäfers M, Haufe G. Bioorg. Med. Chem. 2009;17(7):2680. doi: 10.1016/j.bmc.2009.02.048. [DOI] [PubMed] [Google Scholar]
- 19.Kopka K, Faust A, Keul P, Wagner S, Breyholz HJ, Höltke C, Schober O, Schäfers M, Levkau B. J. Med. Chem. 2006;49(23):6704. doi: 10.1021/jm051217c. [DOI] [PubMed] [Google Scholar]
- 20.Smith G, Glaser M, Perumal M, Nguyen QD, Shan B, Arstad E, Aboagye EO. J. Med. Chem. 2008;51(24):8057. doi: 10.1021/jm801107u. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen QD, Smith G, Glaser M, Perumal M, Arstad E, Aboagye EO. Proc. Natl. Acad. Sci. U S A. 2009;106(38):16375. doi: 10.1073/pnas.0901310106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Havran LM, Chong DC, Childers WE, Dollings PJ, Dietrich A, Harrison BL, Marathias V, Tawa G, Aulabaugh A, Cowling R, Kapoor B, Xu W, Mosyak L, Moy F, Hum WT, Wood A, Robichaud AJ. Bioorg. Med. Chem. 2009;17(22):7755–68. doi: 10.1016/j.bmc.2009.09.036. [DOI] [PubMed] [Google Scholar]
- 23.Wang Q, Mach RH, Reichert DE. J. Chem. Inf .Model. 2009;49(8):1963. doi: 10.1021/ci900144x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.