Abstract
The small GTPase Ran is essential for spindle assembly. Ran is proposed to act through its nuclear import receptors importin α and/or importin β to control the sequestration of proteins necessary for spindle assembly. To date, the molecular mechanisms by which the Ran pathway functions remain unclear. Using purified proteins, we have reconstituted Ran-regulated microtubule binding of the C-terminal kinesin XCTK2, a kinesin important for spindle assembly. We show that the tail of XCTK2 binds to microtubules and that this binding is inhibited in the presence of importin α and β (α/β) and restored by addition of Ran-GTP. The bipartite nuclear localization signal (NLS) in the tail of XCTK2 is essential to this process, because mutation of the NLS abolishes importin α/β-mediated regulation of XCTK2 microtubule binding. Our data show that importin α/β directly regulates the activity of XCTK2 and that one of the molecular mechanisms of Ran-regulated spindle assembly is identical to that used in classical NLS-driven nuclear transport.
INTRODUCTION
The process of chromosome congression and segregation is mediated by the mitotic spindle, which is comprised of microtubules (MTs) and their associated proteins. Proper spindle assembly requires the activities of both plus-end– and minus-end–directed MT motors, nonmotor MT-associated proteins, and other essential non-MT–associated proteins (Compton, 2000). Minus-end–directed cytoplasmic dynein and plus-end–directed Eg5 play key roles in the organization of spindle MTs in Xenopus egg extracts and in cells (Gaglio et al., 1996; Heald et al., 1996; Merdes et al., 1996; Walczak et al., 1998). In addition, the mitotic minus-end–directed C-terminal kinesins function as MT cross-linkers to promote proper spindle assembly in many organisms (Endow and Komma, 1996; Walczak et al., 1997, 1998; Matuliene et al., 1999; Mountain et al., 1999; Ovechkina and Wordeman, 2003) and KIFC1 and KIFC5 C-terminal kinesins may serve a similar cross-linking function during spermatogenesis (Navolanic and Sperry, 2000; Yang and Sperry, 2003). The mechanism that regulates this MT cross-linking is currently unknown.
Recently, the GTPase Ran was shown to be sufficient to induce MT aster formation as well as bipolar spindle assembly in egg extracts (Carazo-Salas et al., 1999; Kalab et al., 1999; Ohba et al., 1999; Wilde and Zheng, 1999; Nachury et al., 2001). Subsequently, it was reported to be involved in regulating numerous mitotic spindle assembly processes, including MT nucleation and dynamics (Wilde and Zheng, 1999; Carazo-Salas et al., 2001; Wilde et al., 2001), MT motor activity (Wilde et al., 2001), and nuclear envelope assembly (Bamba et al., 2002; Hetzer et al., 2002; Zhang et al., 2002). It is believed that Ran provides positional cues by the generation of a steep gradient of Ran-GTP between the condensed chromosomes and the surrounding cytoplasm (Kalab et al., 2002; Trieselmann and Wilde, 2002). After nuclear envelope breakdown, the localized concentration of Ran-GTP around chromatin is thought to contribute to proper spindle assembly and to be maintained by the chromatin-associated nucleotide exchange factor RCC1 (Bilbao-Cortes et al., 2002; Moore et al., 2002; Li et al., 2003). The molecular mechanisms by which Ran affects these mitotic processes are largely unknown, but they seem to involve the nuclear import proteins importin α and importin β (Nachury et al., 2001; Wiese et al., 2001; Askjaer et al., 2002; Dasso, 2002; Kalab et al., 2002; Zhang et al., 2002; Quimby and Dasso, 2003; Tsai et al., 2003).
Thus far, the MT binding proteins TPX2 and NuMA were identified as proteins that bind to importin α and induce spontaneous MT aster formation when added in excess to Xenopus extracts (Gruss et al., 2001; Nachury et al., 2001; Wiese et al., 2001; Schatz et al., 2003). This suggests that these proteins may be early mediators in the Ran pathway that lead to the formation of bipolar spindles. This has led to a working model in which importin β or a complex of importin α/β sequesters proteins necessary for spindle assembly, such as TPX2 and NuMA, so that upon nuclear envelope breakdown, high levels of Ran-GTP promote the release of spindle-promoting factors, allowing them to function (Kuersten et al., 2001; Walczak, 2001; Dasso, 2002; Hetzer et al., 2002). Consistent with this idea, importin α/β binding to TPX2 inhibits Aurora A MT-dependent activation in vivo and in vitro (Eyers et al., 2003; Tsai et al., 2003) and mutation of the importin α binding site in TPX2 allows mutant TPX2 to induce aster formation in extracts at concentrations below those required for wild-type TPX2 (Schatz et al., 2003). This provides evidence that the pathway used for nuclear protein import is conserved in spindle assembly. In addition, Ran increases the plus-end–directed MT motor activity in egg extract asters, in part due to Eg5 activity (Wilde et al., 2001), but it has not been demonstrated to directly regulate the MT binding or organizational activities of any microtubule motors.
We hypothesized that Ran and importin α/β do likely influence the activity of mitotic motor proteins. From our previous work, we know that the Xenopus C-terminal kinesin XCTK2 (Xenopus C-terminal kinesin 2) is required for spindle formation in egg extracts (Walczak et al., 1997). Specifically, loss of XCTK2 cross-linking activity by antibody addition to extracts results in a modest decrease in the efficiency of bipolar spindle assembly and results in spindle structures with splayed poles, whereas addition of excess XCTK2 stimulates the formation of bipolar spindles (Walczak et al., 1997; Walczak et al., 1998). XCTK2 function seems to be redundant with the activity of the minus-end–directed motor dynein, because coaddition of XCTK2 and dynein antibodies results in a large increase in the proportion of spindles with unfocused poles (Walczak et al., 1998). XCTK2 was also found to be the limiting component of a large protein complex and coimmunoprecipitates with 95- and 105-kDa proteins. We envisioned that XCTK2 might be a protein regulated by Ran because it is important for spindle assembly and is nuclear during interphase (Walczak et al., 1997). Because the molecular weight of the 95-kDa protein present in the XCTK2 complex is similar to the molecular weight of importin β, we speculated that XCTK2 might bind to importin α/β and be regulated by Ran. We provide biochemical data to support the model that Ran regulates the cross-linking activity of XCTK2 to promote spindle bipolarity by removing the inhibitory effect of importin α/β.
MATERIALS AND METHODS
Protein Expression and Purification
For expression of the bacterially purified recombinant proteins His6-S-importin β (human importin β1), His6-S-importin βΔ, importin α-His6 (Xenopus importin α1a), GST-XCTK2-NM (G-NM; amino acids 2–289), His6-XCTK2-NM (H-NM; amino acids 2–289), GST-XCTK2-Motor (G-M; amino acids 290–643), GST-RanL43E, His6-RanL43E, and GST-RanT24N DNA plasmids were induced in BL21(DE3) bacteria, and the protein A-importin α-ED-His6 construct was induced in M15[pREP4]. Induction was carried out in the presence of 0.1 mM isopropyl β-d-thiogalactoside for 3–5 h. GST-XCTK2-NM, GST-RanL43E, GST-RanT24N, and His6-XCTK2-NM were induced at 37°C, and the other constructs were induced at 20°C. Cells were pelleted, resuspended in phosphate-buffered saline (50 mM phosphate buffer pH 7.4), pelleted, frozen in liquid nitrogen, and stored as cell pellets until needed.
His6-S-importin β, His6-S-importin βΔ, importin α-His6, protein A-importin α-ED-His6, His6-RanL43E, and His6-XCTK2-M were purified on Ni-nitrilotriacetic acid (NTA) agarose and GST-RanL43E and GST-RanT24N were purified on glutathione agarose by using published procedures (Chi et al., 1997; Wilde and Zheng, 1999). GST-XCTK2-NM and His6-XCTK2-NM were purified on glutathione agarose and Ni-NTA, respectively, and XCTK2 was purified from baculovirus infected Sf-9 cells as described previously (Walczak et al., 1997). All bacterially purified proteins were dialyzed into XB (10 mM HEPES, pH 7.2, 100 mM KCl, 25 mM NaCl, 50 mM sucrose, 0.1 mM EDTA, 0.1 mM EGTA) before being aliquoted, flash frozen in liquid nitrogen and stored at –80°C.
Site-directed Mutagenesis
PSORT, a Web-based protein motif prediction program (http://psort.nibb.ac.jp), was used to predict putative NLS sequences within the amino acid sequence of XCTK2. The three predicted NLS sequences were mutated by sequential mutagenesis on the His6-XCTK2-NM and GST-XCTK2-NM DNA plasmids using the QuikChange site-directed mutagenesis system (Stratagene, La Jolla, CA) to generate the NLS mutant plasmids. Based on the published nucleotide sequence of XCTK2 (accession number U82809), four primers were designed to modify the lysine and/or arginine codons to codons that encode for alanine. Primer NLS1a (5′G GAC TCC ACA GAC GCA GCG GTC CAA GTG GCT TCC CG) mutates K6 and K7; primer NLS1b2a (5′GCT TCC CGT TTG CCA GTG CCT CCG GCG GCA GCA TAT GTC TCT AAT GAT G) mutates K19, R20, and K21; primer NLS2b (5′GAA AAT CAA GAG CAG ATG CAG GCG GCG GCT CTC AGA TCC TCC CTA GAG TC) modifies R34, K35, and R36; and NLS3 modifies (5′GCA GCC ATT GGC GCT GAA GCG GCG GCG GCT GCT GCT TGG GAT CTT AAG G) K116, K117, K118, and R119. Modified nucleotides are in italics. The coding region of each mutant construct was sequenced to verify that no extraneous mutations were introduced. Protein was expressed from the induction of the resulting plasmids and purified as described above.
S-Protein Agarose and Ni-NTA Pull-Down Assays
Per reaction, 0.7 pmol of purified importin β and/or importin α and 0.35 pmol of XCTK2, His-/GST-NM domain, or motor domain protein were incubated with 30 μl of S-protein agarose (Novagen, Madison, WI) or Ni-NTA agarose (QIAGEN, Valencia, CA) in 10% FPLC buffer (20 mM PIPES, pH 7.2, 1 mM MgCl2, 1 mM EGTA, 0.1 mM EDTA, 100 mM KCl) with 2 mM MgATP for 45 min at 4°C with rotation. The protein/bead complexes were pelleted and then washed. For the pull-downs, the beads were washed two times with 10% FPLC buffer, 2 mM MgATP and two times with Tris-buffered saline-Triton X (20 mM Tris, pH 7.5, 150 mM NaCl, 0.1% Triton X-100), and then the protein was eluted with 1× SDS-PAGE sample buffer. For the bind and release experiments, the protein/bead complexes were washed three times with 10% FPLC buffer, 2 mM MgATP, resuspended in the same buffer, and then aliquoted to separate tubes for mock buffer or 25 μM Ran addition. The Ran/bead solution was incubated at room temperature for 15 min, pelleted, and washed as for the pull-down experiment. Equivalent volumes of supernatant and pellet fractions were electrophoresed on 10% SDS-PAGE gels and either stained with Coomassie Brilliant Blue R250 or transferred to Protran (Schleicher & Schuell, Keene, NH) and probed with anti-CTP1 (2 μg ml–1) (Walczak et al., 1997), which recognizes the motor domain, followed by donkey anti-rabbit immunoglobulin (Ig) horseradish peroxidase (HRP)-linked whole antibody (1:20,000; Amersham Biosciences, Piscataway, NJ) and developed using SuperSignal West Pico Chemiluminescent Substrate (Pierce Chemical, Rockford, IL).
Cytostatic Factor-arrested Extracts, Immunofluorescence, and Immunoprecipitations
Cytostatic factor (mitotic) and cycled cytostatic factor arrested extracts were prepared as described previously (Desai et al., 1999a) from Xenopus laevis laid eggs. Spindles were induced with Xenopus sperm and exogenous nonmotor (NM) domain was added to 0.13 μM. Spindle and import reactions were allowed to procede for 30 min before 20 μl were fixed, spun onto coverslips, and processed for immunofluorescence as described previously (Desai et al., 1999a). Coverslips were probed with either anti-XCTK2 (1 μg ml–1) or anti-GST (2.6 μg ml–1) followed by donkey anti-rabbit fluorescein isothiocyanate. Images were taken on a Nikon E600 epifluorescence microscope with a 60× 1.4 numerical aperture objective. The microscope is equipped with a Roper Micromax 1300Y camera and is under control of MetaMorph Software (Universal Imaging, Downingtown, PA). All images were taken at equal exposures and processed equivalently in Adobe Photoshop before assembling figures in Adobe Illustrator.
Immunoprecipitations were performed as described previously (Walczak et al., 1997) except anti-XCTK2, nonimmune rabbit IgG, and anti-glutathione S-transferase (GST) were covalently coupled to the Affi-prep protein A beads (Bio-Rad, Hercules, CA) (Harlow and Lane, 1999). Where indicated, 25 μM purified GST-RanL43E, His-RanL43E, His-RanT24N, or GST-RanT24N was added to the immunoprecipitation reactions. Equivalent volumes of eluted protein were electrophoresed on 10% SDS-PAGE gels and stained with Coomassie or transferred to Protran. Western blots were probed with anti-importin β (1 μg μl–1; Sigma-Aldrich, St. Louis, MO) or anti-importin α (1:1000; from Mary Dasso, National Institutes of Health, Bethesda, MD) followed by sheep anti-mouse Ig HRP-linked whole antibody (1:20,000; Amersham Biosciences, Piscataway, NJ) or donkey anti-rabbit Ig HRP-linked whole antibody (1:20,000) and developed by chemiluminescence as described above.
Microtubule Pelleting and Microtubule Affinity
MTs were polymerized from purified tubulin with 0.5 mM guanosine-5′-[(α,β)-methyleno]triphosphate (Jena Bioscience) and 10 μM paclitaxel at a 5 μM final tubulin concentration for 30 min at 37°C. Polymerized MTs were pelleted at 90,000 rpm in a Beckman TLA100 rotor and resuspended in BRB80 (80 mM PIPES, pH 6.8, 1 mM MgCl2, 1 mM EGTA), 1 mM dithiothreitol (DTT), 10 μM paclitaxel. MTs were then diluted to a working concentration in BRB80, 1 mM DTT. MT pelleting reactions were set up in 10% FPLC buffer with either 2 mM MgATP, 2 mM MgADP + 20 mM inorganic phosphate (Pi), 10 mM MgATP, or 5 mM MgAMP-PNP. The reactions were started by the addition of MTs to the reaction in a 1:1 ratio. The XCTK2 to MT ratio was 1:3 unless otherwise indicated. For the initial pelleting assays, XCTK2 or NM domain was incubated with 1:32 M ratio of XCTK2 to importin α/β (0.0625 μM XCTK2, monomer, to 2 μM importin α/β). Casein was added to 1 mg ml–1 to stabilize proteins at these low protein concentrations. For all assays, protein and MTs were incubated in a 40- or 50-μl final volume for 15 min at room temperature before pelleting. After centrifugation, the supernatants were removed as the soluble fraction, and the pellets were resuspended in an equal volume of 2× sample buffer and then diluted to a final volume of 80 or 100 μl with 1/2× FPLC buffer, 1/2× BRB80, 0.5 mM DTT. Sample buffer was added to the supernatants for a final volume of 80 or 100 μl. For microtubule-pelleting assays in the presence of Ran, either 25 μM GST-RanL43E, GST-RanT24N, His6-RanL43E, or His6-RanT24N was added to the MT–protein complexes 15 min after MT addition and then incubated for an additional 15 min at room temperature before pelleting. Equal volumes of supernatant and pellet fractions were electrophoresed on 10% SDS-PAGE gels and stained with Coomassie Blue or transferred to Protran. Western blots were probed with either anti-XCTK2 (0.5 μg ml–1) or anti-CTP1 (2 μg ml–1) as described above. For MT pelleting onto coverslips, rhodamine-labeled MTs (6:1 unlabeled to labeled) were polymerized in 0.5 mM guanosine-5′-[(α,β)-methyleno]triphosphate, BRB80, 1 mM DTT, and reactions were fixed and sedimented through a glycerol cushion as described previously (Desai et al., 1999b; Desai and Walczak, 2001).
The apparent Kd,MTs of XCTK2 or GST-XCTK2-NM domain constructs at 0.35 μM (monomer concentration) was determined with increasing concentrations of MTs (0.0875–2.8 μM) in 1/2× BRB80, 0.5 mM DTT, 5% FPLC buffer without added nucleotide for 15 min and pelleted as described above. Additional affinities for XCTK2 were determined identically except 2 mM MgADP, 20 mM Pi was added to the reaction. Equal volumes of supernatants and pellets were electrophoresed and the gels stained with Coomassie as described above. Gels were scanned and the apparent Kd of the constructs determined similarly to that described for Ncd (Foster et al., 1998). Briefly, the amount of pelleted protein was determined from the densitometry of scanned gels by using NIH Image. Nonspecific pelleting of the XCTK2 proteins was determined with 0 μM MTs for each experiment. Per experiment, the amount of protein that pelleted at each MT concentration was corrected for the nonspecific pelleting by subtraction. The amount bound in terms of monomeric protein (micromolar) was then plotted against the total MT concentration defined as the concentration of tubulin dimer (micromolar). Using the GraFit 5 software package, the data were fit to the equation below:
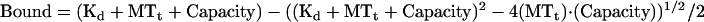 |
where bound is the monomeric micromolar amount of XCTK2, GST-XCTK2-NM, or NLS-2 that pelleted, Kd is the dissociation constant, MTt is the total MT concentration in terms of tubulin dimer, and capacity is the maximum micromolar amount of XCTK2 or NM domain that will pellet under these conditions. The apparent Kd in the presence of importin α and/or importin β was determined similarly. The values shown for each construct are from at least three separate experiments. The binding curves displayed are the fit from the averaged data points, but the enumerated Kd and capacity values are the average and SE of the mean from the individual experiments. F tests for variance equivalence and Student's t tests based on the variance equivalence were performed using EXCEL.
RESULTS
Ran-GTP Regulates the Association of Importin α and Importin β with XCTK2 in Xenopus Egg Extracts
We sequenced the 95-kDa protein that coimmunoprecipitates with XCTK2 by using two separate antibodies (Walczak et al., 1997) and found that 12 of the 14 peptides sequenced were homologous to mouse importin β. In additional anti-XCTK2 immunoprecipitations from cytostatic factor-arrested (mitotic) egg extracts, antibodies to importin β recognized the 95-kDa band, confirming the identity of this protein (Figure 1A, lane 2, middle). Because importin β associates with importin α in the import of proteins into the nucleus, the immunoprecipitations were also probed for importin α. The importin α antibodies recognized a 55-kDa band in the anti-XCTK2 immunoprecipitates, indicating that importin α also associates with XCTK2 (Figure 1A, lane 2, bottom).
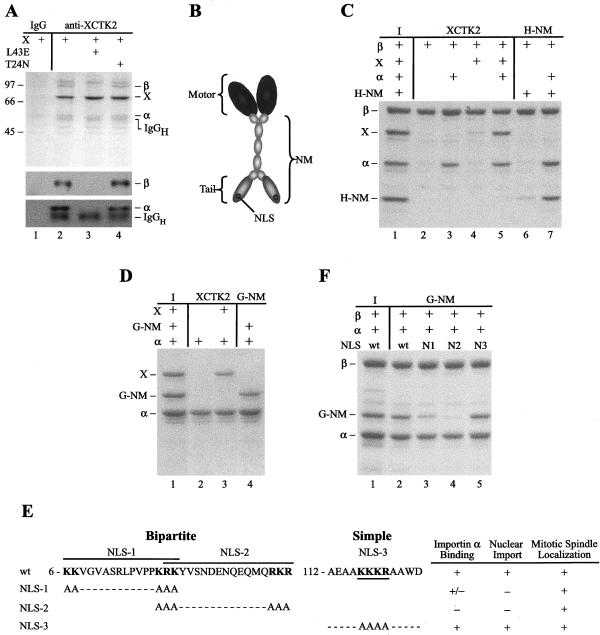
Figure 1.
Importin α and importin β associate with XCTK2 in a Ran-dependent manner via a bipartite NLS in the tail of XCTK2. (A) Coomassie-stained gel (top) and importin β (middle) or importin α (bottom) Western blots of XCTK2 immunoprecipitations from Xenopus egg extracts. Lane 1, immunoprecipitation control with nonimmune rabbit immunoglobulin (IgG). Lane 2–4, immunoprecipitations with anti-XCTK2. The 95-kDa band was used for protein sequencing and identified as importin β (β). The immunoprecipitated XCTK2 protein (X) and coimmunoprecipitated importin α (α) bands are also indicated. Lane 3, XCTK2 immunoprecipitation in the presence of purified RanL43E (L43E). Lane 4, immunoprecipitation in the presence of purified RanT24N (T24N). Importin α is slightly larger than the heavy chain of the anti-XCTK2 antibody (IgGH) and migrates slower by SDS-PAGE as seen on the Coomassie-stained gel and the importin α Western blot. The positions of molecular weight standards (kilodaltons) are indicated to the left of the figure. (B) Diagram of the domains of XCTK2. XCTK2 is composed of an N-terminal NM and a C-terminal motor domain (Motor). The NM domain consists of the globular tail (Tail), which contains the NLS-(NLS) and MT-binding sites, and the central α-helical stalk that is important for dimerization. (C and D) Coomassie stained gels of S-importin β pull-downs (C) and importin α-His pull-downs (D). Lane 1 of parts C and D are input (I) amounts of purified protein. The remaining lanes contain the indicated combinations of proteins. The NM domain of XCTK2 associates with importin β through importin α and can bind directly to importin α. (E) Amino acid sequences of the three putative NLS sequences contained within the tail of XCTK2 and the three NLS mutants generated. The corresponding amino acid numbers are indicated to the left of the NLS and the individual putative NLS sequences are underlined or overlined. The consensus sequence for a bipartite NLS is 2 K/R 10–12 amino acids 3 K/R and for a simple NLS is KKxK. Data from importin α-His pull-downs, nuclear import, and spindle localization are indicated to the right of the mutant sequences and indicate binding, import or localization (+), partial binding (+/–), or no binding or no import (–). (F) S-Importin β pull-downs of the three putative NLS mutants. Lane 1 contains the input (I) amounts of purified protein. The remaining lanes contain the indicated combinations of importin α, importin β, and either wild-type (wt) or NLS-mutated versions of G-NM. The NLS-1 mutant (N1) partially binds to importin α, whereas the NLS-2 mutant (N2) binds importin α the least compared with wt G-NM. Mutation of the simple NLS does not affect importin α binding (N3). The input amount of only wt G-NM is shown, but the NLS mutant versions had similar amounts of input protein.
To test whether Ran regulated the association of importin α and importin β with XCTK2, we performed additional immunoprecipitations from extracts with the anti-XCTK2 antibody in the presence of RanL43E (L43E), an effector domain mutant that binds tightly to GTP (Lounsbury et al., 1996), or in the presence of RanT24N (T24N), a dominant negative allele that strongly associates with its nucleotide exchange factor RCC1 (Dasso et al., 1994; Klebe et al., 1995). Addition of RanL43E to immunoprecipitation reactions abolished the ability of importin α and importin β to coimmunoprecipitate with XCTK2 (Figure 1A, lane 3). In contrast, addition of RanT24N did not affect the association of importin α and β with XCTK2 (Figure 1A, lane 4). These results show that Ran regulates the association of XCTK2 with importin α and importin β in mitotic egg extracts.
Importin α/β Bind Directly to XCTK2 through a Bipartite NLS in the Nonmotor Domain of XCTK2
Because egg extracts are a complex mixture of proteins, it is possible that the Ran-regulated association of importin α and β with XCTK2 is mediated by other factors. To investigate whether XCTK2 could bind directly to importin α and/or importin β, we performed pull-down experiments by using purified proteins and a fragment of XCTK2 that contained the N-terminal globular tail and the central α-helical stalk that we refer to as the NM domain (Figure 1B). Pull-down experiments with importin β showed that full-length XCTK2 and a His-tagged version of the NM domain of XCTK2 (H-NM) did not bind well to importin β alone (Figure 1C, lanes 4 and 6). However, in the presence of importin α, both associated with importin β (Figure 1C, lanes 5 and 7). Because importin α and β are the nuclear import receptors for proteins that contain classical NLS sequences and because nuclear import by importin β is often mediated by importin α (Chook and Blobel, 2001), we performed additional pull-down experiments with importin α alone. Both XCTK2 and a GST-tagged version of the NM domain of XCTK2 (G-NM) bound directly to importin α (Figure 1D, lanes 3–4), whereas a fragment of XCTK2 containing only the motor domain was not able to bind to importin α (our unpublished data). As an additional control, we performed pull-down experiments with the ED mutant of importin α that does not bind to bipartite NLS sequences due to a mutation in each NLS binding pocket (E389R and D189K) (Conti et al., 1998; Gruss et al., 2001) and found that the NM domain of XCTK2 did not associate with mutant importin α (our unpublished data). These results suggest that the binding of XCTK2 to importin α is mediated by a bipartite NLS in the NM domain of XCTK2 and that the association of XCTK2 with importin β in extracts is through importin α.
We examined the sequence of XCTK2 and identified three putative classical NLS sequences in the N-terminal globular tail of XCTK2 (Figure 1E). Two were bipartite and one represented a simple NLS (Conti et al., 1998). To define the NLS responsible for the binding of XCTK2 to importin α, sequential site-directed mutagenesis was performed to mutate the lysine and/or arginine residues to alanine residues in the three putative NLS sequences (Figure 1E). We then assayed the mutant G-NM proteins for binding to importin α by using in vitro pull-down assays. Mutation of NLS-1 (amino acids 6–21) resulted in partial inhibition of importin α/β binding (Figure 1F, lane 3). Mutation of the second putative bipartite NLS (NLS-2, amino acids 19–36) resulted in essentially full inhibition of importin α binding (Figure 1F, lane 4), whereas mutation of NLS-3, the putative simple NLS (amino acids 116–119), had no detectable effect on the NM domain binding to importin α (Figure 1F, lane 5). These results suggest that NLS-2, residing in the tail of XCTK2, is the major NLS.
We next tested the competency of the wild-type and mutant G-NM proteins to undergo nuclear import in egg extracts. Mutation of either NLS-1 or NLS-2 caused a defect in nuclear import, whereas mutation of NLS-3 had no effect (Figure 1E). We suspect that the nuclear import defect of the NLS-1 mutant is a consequence of the amino acid sequence overlap with NLS-2. It has been shown that modification of lysine and/or arginine residues in the head or tail of a bipartite NLS can confer an import defect (Ishii et al., 1996; Efthymiadis et al., 1997; Taniguchi et al., 2002). None of the putative NLS mutations conferred a disruptive effect on MT localization in extracts, because all G-NM proteins localized to bipolar spindles (Figure 1E), identical to the C-terminal kinesin CHO2 stalk-tail construct transfected into Chinese hamster ovary cells (Matuliene et al., 1999). From these results, we conclude that NLS-2 is the bona fide NLS sequence in the XCTK2 tail and that this sequence is responsible for importin α binding to XCTK2.
Ran Can Directly Regulate the Binding of Importin α/β to XCTK2
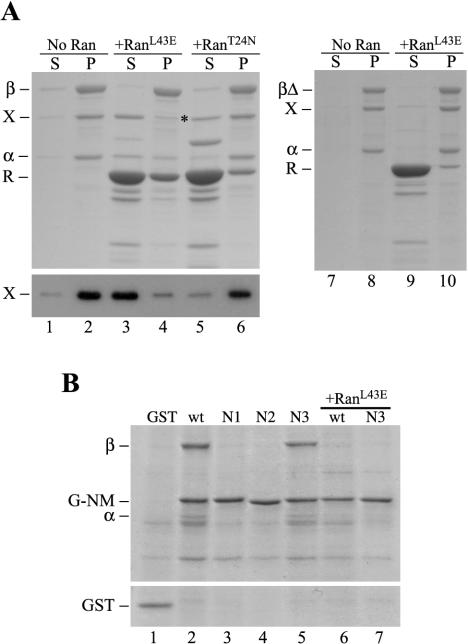
The above-mentioned experiments established that XCTK2 associates with importin β through the direct binding of importin α to a bipartite NLS in the tail of XCTK2. We wanted to know whether Ran regulated this association in a manner similar to the nuclear import pathway. We devised a bind and release experiment where we first formed an importin β/importin α/XCTK2 ternary complex on agarose beads and then incubated the complex with buffer, RanL43E, or RanT24N to look for release of protein. We expected that if Ran regulated the binding of the importins to XCTK2, then incubation of the ternary complex with RanL43E would result in the release of XCTK2 and importin α from importin β bound to the agarose beads. Incubation of this complex with concentrations of RanL43E that are sufficient to induce asters in spindle assembly reactions (Wilde and Zheng, 1999; Wilde et al., 2001) resulted in the near complete release of XCTK2 from importin β (Figure 2A, lanes 3 and 4), whereas incubation with RanT24N did not release XCTK2 or importin α from the beads (lanes 5 and 6). Similar results were obtained using either the H-NM or G-NM proteins. We determined that this Ran effect is directly dependent on the Ran binding domain of importin β by repeating the experiment with a deletion construct of importin β in which the Ran-binding domain is deleted (Nachury et al., 2001), called importin βΔ. In this experiment, RanL43E did not dissociate XCTK2 from importin βΔ (Figure 2A, lanes 9–10). These results establish that Ran regulates the interaction between importin α/β and XCTK2 in a reconstituted system by using purified proteins.
Figure 2.
XCTK2 binding to importin α/β is regulated by the nucleotide state of Ran and is dependent on the Ran-binding domain of importin β. Coomassie-stained gels and anti-CTP1 immunoblot of the supernatants and pellets of S-importin β and S-importin βΔ (βΔ) bind and release assays in the presence and absence of Ran (R). (A) S-importin β bind and release assay with importin α-His and XCTK2 in the absence (No Ran, lanes 1 and 2) and presence of GST-RanL43E-GTP (RanL43E, lanes 3 and 4) or GST-RanT24N-GDP (RanT24N, lanes 5 and 6). The asterisk indicates a contaminating, comigrating band from the RanT24N purification. Bottom, an immunoblot of the same fractions probed with the anti-CTP1 antibody. Right, S-importin βΔ bind and release assay with importin α and XCTK2 in the absence (lanes 7 and 8) and presence of GST-RanL43E-GTP (lanes 9 and 10). (B) Anti-GST immunoprecipitation of GST (lane 1), G-NM (wt, lane 2), NLS-1 (N1, lane 3), NLS-2 (N2, lane 4), and NLS-3 (N3, lane 5). G-NM (lane 6) and NLS-3 mutant (lane 7) anti-GST immunoprecipitations in the presence of His-RanL43E (+RanL43E).
To demonstrate that the NLS in the tail of XCTK2 is responsible for importin α/β binding in a more physiological context, we performed anti-GST immunoprecipitations from mitotic egg extracts to which we added either wild-type or NLS-mutant versions of the GST-tagged NM proteins. Immunoprecipitation of exogenously added wild-type G-NM or the NLS-3 mutant resulted in the coimmunoprecipitation of importin α and importin β (Figure 2B, lanes 2 and 5). In contrast, importin α and importin β failed to coimmunoprecipitate with the NLS-1 or NLS-2 mutant (lanes 3 and 4), consistent with their nuclear import defect. Similarly to full-length XCTK2, addition of RanL43E abolished the ability of importin α and β to coimmunoprecipitate with wild-type G-NM and NLS-3 (lanes 6 and 7). Together, our results demonstrate that Ran regulates the direct binding of importin α/β to the tail of XCTK2 via the same mechanism used for the nuclear import of proteins containing classical NLS sequences (Chook and Blobel, 2001; Kuersten et al., 2001).
Importin α/β Sequester the Microtubule-binding Activity of the XCTK2 Tail
XCTK2 and other members of the C-terminal kinesin family are proposed to function by cross-linking and sliding MTs in the spindle via their motor domains and globular tails (Chandra et al., 1993; Walczak et al., 1997; Karabay and Walker, 1999; Matuliene et al., 1999; Mountain et al., 1999). The motor domain binds to MTs in an ATP-dependent manner, whereas the tail binds MTs in an ATP-independent manner through undefined and nonconserved MT binding sites (Chandra et al., 1993; Karabay and Walker, 1999; Matuliene et al., 1999; Karabay and Walker, 2003). A significant amount of research has been done on the motor domains of C-terminal kinesins, but little work has been done with regards to the tails, and the mechanism for regulating the cross-linking activity has not been elucidated. Because importin α/β bind to XCTK2 through the tail, we envisioned that importin α/β might modulate the MT cross-linking activity of XCTK2 by sequestering the MT binding of the tail and that this activity would be regulated by Ran.
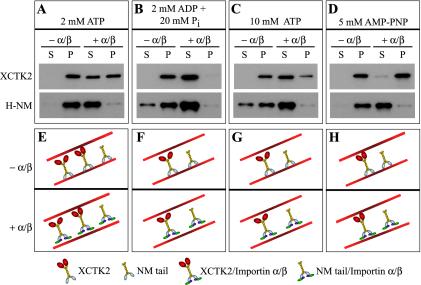
We first wanted to test the effects of importin α/β on the ability of XCTK2 to bind to MTs. If importin α/β affects the MT binding of the tail, then the ability of full-length XCTK2 to bind to MTs would vary with nucleotide condition, whereas the H-NM and G-NM proteins would bind to MTs independent of nucleotide condition. In the presence of 2 mM MgATP, XCTK2 and the H-NM protein cosedimented with MTs in the absence of importin α/β. In contrast, in the presence of importin α/β, ∼50% of full-length XCTK2 and all of the H-NM protein no longer cosedimented with MTs (Figure 3A). The motor domain of XCTK2 probably still bound MTs under this physiological ATP condition and could account for the partial amount of XCTK2 that bound to MTs in the presence of importin α/β. These results imply that importin α/β sequestered only the NM domain away from MTs (Figure 3E). As a test for the independence between the tail binding to MTs and the motor domain binding to MTs, we next assayed the ability of XCTK2 to bind MTs in the presence of high concentrations of MgATP or MgADP plus Pi. These conditions inhibit only the MT binding activity of the motor domain of C-terminal kinesins (Chandra et al., 1993; Foster et al., 1998). In the presence of either 2 mM MgADP + 20 mM Pi or 10 mM MgATP, importin α/β efficiently inhibited the MT binding activity of full-length XCTK2 (Figure 3, B and C, F and G). In contrast, in the presence of the nonhydrolyzable ATP analog AMP-PNP, which prevents the motor head from releasing MTs (Chandra et al., 1993; Foster et al., 1998; Wendt et al., 2002), importin α/β no longer inhibited XCTK2 from binding to MTs (Figure 3, D and H). Under all conditions tested, the H-NM protein bound to MTs and its binding was inhibited by importin α/β. These results suggest that importin α/β likely functions to inhibit or moderate the MT cross-linking activity of XCTK2 by binding to the tail.
Figure 3.
Importin α/β inhibits the nonmotor microtubule binding domain in full-length XCTK2 from binding microtubules. Immunoblots of the supernatants and pellets of XCTK2 and His-tagged NM domain microtubule pelleting assays under different nucleotide conditions, without (– α/β) and with (+ α/β) a 32:1 ratio of importin α/β to XCTK2. XCTK2 blots were probed with anti-CTP1 and H-NM blots were probed with the anti-XCTK2. (A) 2 mM MgATP. (B) 2 mM MgADP + 20 mM Pi. (C) 10 mM MgATP. (D) 5 mM MgAMP-PNP. (E–H) Schematic representations of the results in a–d. (E) In 2 mM MgATP and the absence of importin α/β (green and purple), XCTK2 can bind to and pellet with MTs either through the tail (gray domain) only, by cross-linking MTs through the motor domain (red domain) and the tail or through the motor domain only (our unpublished data). The NM domain (gray and yellow domains) binds tightly to MTs. In the presence of importin α/β (E, bottom), the population of XCTK2 that only binds to MTs through the tail is sequestered away from MTs, whereas XCTK2 that cross-links MTs can still bind to and pellet with MTs through its motor domain. (F and G) In the presence of 2 mM MgADP + 20 mM Pi or 10 mM MgATP, the motor domain does not bind tightly to MTs. Thus, in the absence of importin α/β, XCTK2 and H-NM bind to and pellet with MTs, whereas in the presence of importin α/β, both XCTK2 and H-NM are sequestered away from MTs. (H) In the presence of 5 mM MgAMP-PNP, the motor domain binds tightly to MTs with no effect by importin α/β.
Importin α/β Compete with Microtubules for G-NM
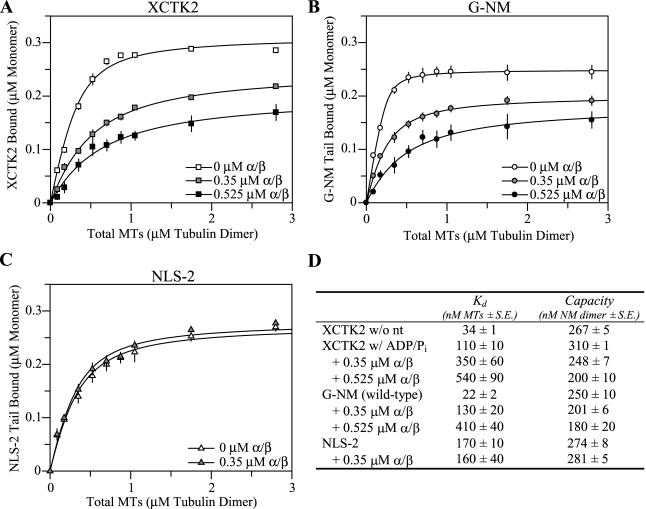
The working model of importin α/β sequestering spindle assembly factors in the absence of Ran-GTP dictates that importin α/β must compete effectively with the substrates of the proteins important in spindle assembly. To begin to understand the molecular mechanism by which importin α/β compete with MTs for binding to the XCTK2 tail, we determined the affinity of XCTK2 for MTs and the affinity of the NM domain for MTs in the absence or presence of various concentrations of importin α/β. We first measured the apparent affinity of XCTK2 for MTs in the absence of nucleotide and in the presence of 2 mM MgADP + 20 mM Pi. XCTK2 had a threefold stronger apparent affinity for MTs in the absence of nucleotide (Kd,MT = 34 ± 1 nM) compared with its affinity in the presence of ADP + Pi (Kd,MT = 110 ± 10 nM; p < 0.001), suggesting that the MT pelleting of the NM domain can be uncoupled from the ATP-dependent MT binding of the motor domain. Because we were interested in how importin α/β regulated the tail of XCTK2, we performed additional measurements for full-length XCTK2 in the presence of ADP + Pi. Addition of importin α/β to 0.35 μM caused a threefold decrease in affinity (p < 0.05) and addition of 0.525 μM importin α/β caused a fivefold reduction in the affinity of XCTK2 for MTs (p < 0.05) (Figure 4, A and D). Under these same conditions, the capacity of XCTK2 for MTs was reduced by 20 and 35% (p < 0.05) (Figure 4, A and D).
Figure 4.
Importin α/β compete with microtubules for the XCTK2 tail and alter the affinity and capacity of G-NM for microtubules in a dose-dependent manner that is dependent on the bipartite NLS. Averaged ligand binding curves for full-length XCTK2 (A), wild-type G-NM (B), and NLS-2 mutant (C) in the absence (open symbols) and presence of importin α/β at 0.35 μM (shaded symbols) or 0.525 μM (black symbols). Concentrations of XCTK2 and G-NM are in terms of protein monomer and MTs in terms of tubulin dimer. (D) Affinity and capacity values determined from the ligand binding curves in A–C. The resulting MT affinities (Kd) and maximum binding (capacity) are presented in terms of nanomolar concentrations ± the SE of the mean of at least three separate experiments.
To characterize the ability of the NM tail to bind to MTs in the presence of importin α/β in more detail and without constraints potentially imposed by the motor domain, similar affinity assays were performed with the G-NM protein. The Kd of G-NM for MTs was 22 ± 2 nM with complete MT binding (Figure 4, B and D). The affinity of the G-NM protein was fivefold higher than for full-length XCTK2 in the presence of ADP + Pi (p < 0.01), suggesting the motor domain likely imposed additional constraints on the conformation of the stalk in full-length XCTK2 but not in our stalk-tail construct (Wendt et al., 2002). Although these are apparent Kd values, the values in this range and resulting curves are highly suggestive of very tight binding affinity and suggest a binding stoichiometry of one tubulin dimer per one NM domain.
Similar to full-length XCTK2, the G-NM protein also had reduced affinity and capacity for MTs in the presence of importin α/β (Figure 4B). Addition of a twofold molar amount of importin α/β resulted in a sixfold decrease in affinity (p < 0.05) and a 20% decrease in capacity (p < 0.05). Threefold molar excess of importin α/β resulted in a 19-fold decrease in affinity (p < 0.01) and 28% decrease in capacity (p < 0.05) (Figure 4D). Note that this effect was strikingly similar to full-length XCTK2. Addition of an eightfold molar excess (1.4 μM) of importin α/β resulted in the inability of the NM domain to bind to MTs (our unpublished data). These results indicate that the XCTK2 tail has a greater affinity for importin α/β than for MTs and that importin α/β not only reduce the affinity of the tail for MTs but also prevent the tail from binding MTs.
To test the importance of the NLS in importin α/β-regulated inhibition of MT binding, similar experiments were performed with the NLS-2 mutant. Because importin α/β are unable to bind to NLS-2 (Figure 1E) we expected that importin α/β would not affect the affinity of NLS-2 for MTs. Indeed, importin α/β did not have a statistically significant effect on the affinity (p = 0.70) for MTs or the capacity (p = 0.50) (Figure 4, C and D). These results demonstrate that importin α/β directly influence the MT binding activity of XCTK2 through the NLS-2 sequence that interacts with importin α. Unexpectedly, the NLS-2 mutant protein displayed an eightfold reduced affinity for MTs compared with the wild-type G-NM (p < 0.001) but not a reduced capacity (p = 0.12). Although the MT binding sites of other C-terminal kinesin tails have not been defined, it is generally believed that the positively charged residues are important for MT binding (Woehlke et al., 1997; Karabay and Walker, 1999; Kikkawa et al., 2000). The reduced affinity for MTs suggests that mutation of the six lysine/arginine residues in the tail of our constructs either alters the MT binding site or results in conformational changes that are allosterically transferred to the MT binding site.
Ran-GTP Promotes the Binding of the Tail to Microtubules by Releasing the Sequestering Activity of Importin α/β
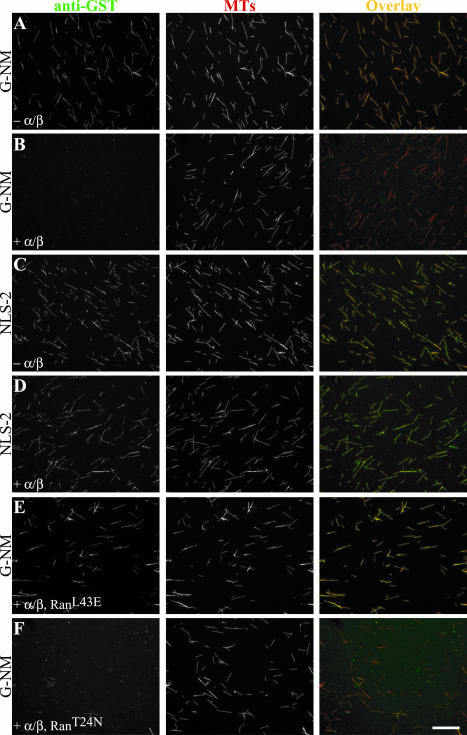
Because Ran can regulate the association of XCTK2 with importin α/β, we predicted that Ran could also regulate the ability of XCTK2 to bind to MTs. We developed a visual assay to examine the G-NM protein binding to MTs. The G-NM protein colocalized with MTs in the absence of importin α/β but had drastically reduced binding in the presence of a fourfold molar excess of importin α/β (Figure 5, A and B). In contrast, the MT binding of the NLS-2 mutant was not affected by the presence of importin α/β (Figure 5, C and D). Addition of RanL43E to the G-NM/importin α/β ternary complex restored the ability of the G-NM protein to bind MTs (Figure 5E), whereas addition of RanT24N had little effect (Figure 5F). Classical MT pelleting assays using either full-length XCTK2 or H-NM gave similar results. These data show that Ran-GTP regulates the binding of the tail of XCTK2 to MTs in the presence of importin α/β and is dependent upon the NLS.
Figure 5.
Ran-GTP promotes XCTK2 microtubule binding in the presence of importin α/β. Immunofluorescence analysis of G-NM and NLS-2 MT binding in the absence (– α/β) and presence of importin α/β (+ α/β) and Ran (+ RanL43E or + RanT24N). (A and B, E and F) Micrographs of reconstituted Ran regulation of the MT binding of the G-NM domain to rhodamine-labeled MTs. (C and D) Micrographs of NLS-2 MT binding in the absence and presence of importin α/β. Bar, 20 μm for all panels.
DISCUSSION
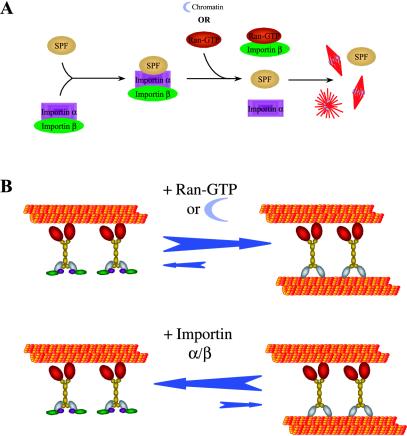
The small GTPase Ran is essential for spindle assembly and regulates diverse spindle assembly processes (Kahana and Cleveland, 1999; Kuersten et al., 2001; Walczak, 2001; Dasso, 2002; Hetzer et al., 2002; Quimby and Dasso, 2003). Although several spindle processes regulated by Ran have been identified, it is unclear how Ran functions at the molecular level to organize the MTs within the spindle because most of these experiments were performed in egg extracts. One proposed model to account for Ran action in extracts is that importin α and importin β function as a complex to inhibit spindle formation by sequestering a set of proteins required for the process of spindle assembly (Gruss et al., 2001; Nachury et al., 2001; Walczak, 2001; Wiese et al., 2001). The model proposes that importin α/β binds to proteins with spindle-promoting activities in mitotic extracts, such that upon chromatin or exogenous Ran-GTP addition, the importins dissociate, which then activates the spindle-promoting factors (Figure 6A). Given the diversity of the spindle assembly processes regulated by Ran, the mechanism by which Ran acts must be robust and yet specific. We propose that for spindle assembly it is essential for importin α and β to be present in vast molar excess in the extract relative to proteins important in spindle formation and that importin α/β and/or importin β be able to effectively compete with the substrates of the spindle-promoting factors. The former seems to be true because importin α and β are estimated to be present at 10–20 μM in extracts (Jans et al., 2000) relative to an extract concentration of only ∼100 nM for TPX2 (Gruss et al., 2001) and ∼40 nM for NuMA (Merdes and Cleveland, 1997). We have estimated an extract concentration of XCTK2 to be only ∼10 nM, well below the endogenous concentration of importin α and importin β (Walczak et al., 1997). Furthermore, our previous work shows that all of the XCTK2 in egg extracts is present in a large complex and that upon addition of recombinant XCTK2 to 100 nM, the added XCTK2 is completely incorporated into the large complex, suggesting it is a limiting component of that complex (Walczak et al., 1997). The work presented here demonstrates that importin α/β binds to XCTK2 and is likely a component of the XCTK2 complex. Together, these results are supportive of the idea that importin α/β are in sufficient quantities to sequester the known spindle-promoting factors, as well as many others.
Figure 6.
Models for Ran-regulated spindle assembly. (A) One proposed model of Ran-induced spindle assembly. The importin α/β complex sequesters factors that possess spindle-promoting activity (SPF) whereupon exogenous Ran-GTP or chromatin addition, Ran-GTP binds to importin β and causes the dissociation of importin α/β from the spindle promoting factor. The “activated” spindle-promoting activities then promote spindle assembly. (B) Our proposed model of Ran-induced XCTK2 MT cross-linking. The importin α/β complex sequesters the cross-linking activity of XCTK2 by binding tightly to the tail of XCTK2 and thus prevents it from binding to MTs. With the addition of Ran-GTP, the sequestering activity of importin α/β is inhibited, thereby promoting XCTK2 MT cross-linking.
More importantly, to effectively inhibit spindle assembly, importin α/β must be able to successfully compete with the substrates of the spindle-promoting factors. Using purified proteins, we show here that importin α/β and Ran oppose one another to regulate the ability of XCTK2, a protein with spindle promoting activity, to bind to MTs. Specifically, by tightly binding to the tail of XCTK2 through a bipartite NLS, importin α/β inhibit the activity of the tail. They do so by competing with MTs and efficiently sequestering the MT binding domain of XCTK2 that results in the inability of XCTK2 to cross-link MTs. This result is consistent with previous observations that the importin α/β complex has a very high affinity for NLS sequences (2–180 nM) (Fanara et al., 2000; Jans et al., 2000; Catimel et al., 2001; Harreman et al., 2003) and that high salt concentrations are required to cause the release XCTK2 from MTs by the addition of ATP (Walczak et al., 1997). In addition, neither importin α nor importin β alone has a significant effect on the affinity of XCTK2 for MTs even though XCTK2 can bind to importin α alone in pull-down experiments (our unpublished data). These results are consistent with previous studies that showed that the affinity of importin α for NLS sequences increases up to 300-fold in the presence of importin β (Efthymiadis et al., 1997; Hu and Jans, 1999; Fanara et al., 2000), which is likely due to importin β binding to and sequestering the autoinhibitory sequence in importin α (Kobe, 1999; Fanara et al., 2000; Hodel et al., 2001; Harreman et al., 2003). This suggests that importin α cannot compete with MTs in the absence of importin β because of a reduced affinity for the XCTK2 tail. Thus, the Ran-regulated mechanism used for the nuclear import of proteins is extremely well suited for regulating spindle assembly because it evolved for the efficient import of diverse proteins into the nucleus.
These results provide the first report of importin α/β directly affecting the MT-binding activity of a MT motor in an NLS-dependent manner, which could provide a very effective mechanism to regulate spindle formation. Our findings are distinct from the recent report showing that importin α mediates TPX2-induced MT nucleation because in that study importin α does not inhibit TPX2 MT association (Schatz et al., 2003). For XCTK2, we envision two models of how importin α/β binding might influence activity. If the importin α/β binding site on the XCTK2 tail were distinct from the MT binding site, then it might be possible for XCTK2 to simultaneously bind to both MTs and to importin α/β. This situation would result in incomplete sequestering by importin α/β. On the other hand, if the importin α/β binding site on the XCTK2 tail coincides with the MT binding site or if importin α/β binding sterically obstructs the MT binding site, complete sequestering would occur. We favor the latter scenario in which importin α/β sterically obstruct the MT binding site of the tail such that there is maximal sequestering of the MT binding activity. This is supported by several experimental findings. First, the addition of anti-XCTK2 NM antibodies to egg extracts causes mislocalization of XCTK2 toward the poles (Walczak et al., 1997). These antibodies must be sterically blocking the MT binding site in the tail of XCTK2. Second, importin β in addition to importin α is required to prevent the NM domain from binding to MTs, suggesting that importin β physically occludes the MT binding site. This steric exclusion mechanism is similar to the way in which the mammalian Partner of inscuteable, LGN, inhibits the MT binding and stabilization activity of NuMA (Du et al., 2002). We predict that where Ran-GTP levels are highest, Ran-GTP would have the greatest consequence on the activities of factors that are completely sequestered by importin α/β or importin β. Together, these data provide a mechanism by which Ran can discretely regulate many activities through importin α/β.
How can this molecular mechanism be incorporated into what we know about the physiological function of XCTK2 in spindle assembly? We know that cross-linking MTs by XCTK2 or its homologs is important for bipolar spindle assembly and is needed to help focus spindle poles. In agreement with this function, C-terminal kinesins are localized to both the MTs of the mitotic spindle and spindle poles (Walczak et al., 1997, 1998; Matuliene et al., 1999; Mountain et al., 1999). Specifically, we previously demonstrated that in addition to a moderate decrease in spindle formation, addition of an antibody raised against the NM domain of XCTK2 to spindle assembly reactions results in the absence of XCTK2 localization on spindle MTs and enrichment at spindle poles, suggesting that the antibody inhibits XCTK2 cross-linking activity. Consistent with this idea, addition of the same antibody to MT-binding experiments results in the prevention of XCTK2-induced MT bundling (Walczak et al., 1997). We have recently found that when the wild-type and NLS-2 mutant NM domains are added to aster assembly reactions in extracts, asters with differing morphology assemble. In addition, the localization of the NM domain proteins to MTs in these extracts is influenced by the presence of Ran (our unpublished data). Thus, the localization of Ran-GTP specifically around chromatin (Bamba et al., 2002; Kalab et al., 2002; Moore et al., 2002) and spindle poles (Keryer et al., 2003) puts Ran at precisely the correct place for the activation of C-terminal kinesins as well as additional essential players in spindle assembly. Here, we provide experimental data that supports a model for Ran regulation of XCTK2 MT cross-linking whereby importin α/β effectively compete with MTs to bind to and inhibit XCTK2 cross-linking activity where Ran-GTP levels are low (Figure 6B), e.g., outside the boundaries of the spindle.
Driven by the positional cues of the Ran-GTP gradient within the spindle, XCTK2 will only cross-link MTs in the vicinity of the chromosomes and the spindle where MT levels are high. The cross-linking activity at the chromosomes would help organize the MTs, and then as XCTK2 moved toward the minus-ends, the cross-linking activity would help focus the MTs into discrete poles. We expect XCTK2 homologs to be regulated similarly in cells. Our modeling shows that a steep Ran-GTP gradient can be established in cells (Li et al., 2003) and that perturbation of RCC1 localization or ectopic expression of Ran-GTP in the cytoplasm of cells causes spindle defects (Moore et al., 2002). The existence of a Ran-GTP gradient is well established in egg extracts; however, it is not clear whether a similar gradient is established in cells as mathematical modeling has led to different conclusions (Görlich et al., 2003; Li et al., 2003). To resolve this issue, experimental measurements of the Ran-GTP gradient is necessary. We speculate that in light of importin α/β and Ran precisely regulating the ability of the XCTK2 tail to bind to MTs that even a slight Ran-GTP gradient may be sufficient to provide positional cues. Additionally, due to the complexity of bipolar spindle assembly it will be essential in the future to elucidate the physiological contribution of the NLS sequence in XCTK2 and its homologs both in extracts and in cells. Finally, upon completion of mitosis XCTK2 and its homologs are likely transported into the nucleus at telophase to prevent unwanted MT cross-linking during interphase.
In summary, we show here that XCTK2 MT cross-linking activity is regulated by the importin α/β and Ran pathway through a classical bipartite NLS and that importin α/β can uniquely regulate XCTK2 by directly interfering with the MT binding site of the XCTK2 tail. This provides a biochemical mechanism for the functional observation that MT cross-linking induced by XCTK2 is important for bipolar spindle assembly. In the future, it will be of great interest to identify all of the spindle components that interact with the effectors of Ran and to determine how Ran temporally and spatially regulates their diverse functions.
Acknowledgments
We thank Kathleen Hertzer, Sarah Johnstone, Susan Kline-Smith, Jane Stout, and Chris Wiese for critical review of the manuscript; Steve Adam for the His-S-importin β/βΔ and importin α-His constructs; Iain Mattaj for the protein A-importin α ED-His construct; Mary Dasso for the importin α antibody; and Ona Martin for purification of His6-RanL43E and His6-RanT24N. We also thank Susan Gilbert and Martin Stone for their assistance with fitting the MT binding data. This work was supported by grants from the National Institutes of Health (to C.E.W. and Y.Z.) and by a fellowship from the Walther Cancer Institute (to S.E.M.). C.E.W. is a Scholar of the Leukemia and Lymphoma Society.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–07–0454. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-07-0454.
References
- Askjaer, P., Galy, V., Hannak, E., and Mattaj, I.W. (2002). Ran GTPase cycle and importins α and β are essential for spindle formation and nuclear envelope assembly in living Caenorhabditis elegans embryos. Mol. Biol. Cell 13, 4355–4370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamba, C., Bobinnec, Y., Fukuda, M., and Nishida, E. (2002). The GTPase Ran regulates chromosome positioning and nuclear envelope assembly in vivo. Curr. Biol. 12, 503–507 [DOI] [PubMed] [Google Scholar]
- Bilbao-Cortes, D., Hetzer, M., Langst, G., Becker, P.B., and Mattaj, I.W. (2002). Ran binds to chromatin by two distinct mechanisms. Curr. Biol. 12, 1151–1156 [DOI] [PubMed] [Google Scholar]
- Carazo-Salas, R.E., Gruss, O.J., Mattaj, I.W., and Karsenti, E. (2001). Ran-GTP coordinates regulation of microtubule nucleation and dynamics during mitotic-spindle assembly. Nat. Cell Biol. 3, 228–234 [DOI] [PubMed] [Google Scholar]
- Carazo-Salas, R.E., Guarguaglini, G., Gruss, O.J., Segref, A., Karsenti, E., and Mattaj, I.W. (1999). Generation of GTP-bound Ran by RCC1 is required for chromatin-induced mitotic spindle formation. Nature 400, 178–181 [DOI] [PubMed] [Google Scholar]
- Catimel, B., Teh, T., Fontes, M.R., Jennings, I.G., Jans, D.A., Howlett, G.J., Nice, E.C., and Kobe, B. (2001). Biophysical characterization of interactions involving importin-α during nuclear import. J. Biol. Chem. 276, 34189–34198 [DOI] [PubMed] [Google Scholar]
- Chandra, R., Salmon, E.D., Erickson, H.P., Lockhart, A., and Endow, S.A. (1993). Structural and functional domains of the Drosophila ncd microtubule motor protein. J. Biol. Chem. 268, 9005–9013 [PubMed] [Google Scholar]
- Chi, N.C., Adam, E.H., and Adam, S.A. (1997). Different binding domains for Ran-GTP and Ran-GDP/RanBP1 on nuclear import factor p97. J. Biol. Chem. 272, 6818–6822 [DOI] [PubMed] [Google Scholar]
- Chook, Y.M., and Blobel, G. (2001). Karyopherins and nuclear import. Curr. Opin. Struct. Biol. 11, 703–715 [DOI] [PubMed] [Google Scholar]
- Compton, D.A. (2000). Spindle assembly in animal cells. Annu. Rev. Biochem. 69, 95–114 [DOI] [PubMed] [Google Scholar]
- Conti, E., Uy, M., Leighton, L., Blobel, G., and Kuriyan, J. (1998). Crystallographic analysis of the recognition of a nuclear localization signal by the nuclear import factor karyopherin α. Cell 94, 193–204 [DOI] [PubMed] [Google Scholar]
- Dasso, M. (2002). The Ran GTPase: theme and variations. Curr. Biol. 12, R502–R508. [DOI] [PubMed] [Google Scholar]
- Dasso, M., Seki, T., Azuma, Y., Ohba, T., and Nishimoto, T. (1994). A mutant form of the Ran/TC4 protein disrupts nuclear function in Xenopus laevis egg extracts by inhibiting the RCC1 protein, a regulator of chromosome condensation. EMBO J. 13, 5732–5744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai, A., Murray, A., Mitchison, T.J., and Walczak, C.E. (1999a). The use of Xenopus egg extracts to study mitotic spindle assembly and function in vitro. Methods Cell Biol. 61, 385–412 [DOI] [PubMed] [Google Scholar]
- Desai, A., Verma, S., Mitchison, T.J., and Walczak, C.E. (1999b). Kin I kinesins are microtubule-destabilizing enzymes. Cell 96, 69–78 [DOI] [PubMed] [Google Scholar]
- Desai, A., and Walczak, C.E. (2001). Assays for microtubule destabilizing kinesins. In: Kinesin Protocols, ed. I. Vernos, Totowa, NJ: Humana Press, 109–121 [DOI] [PubMed]
- Du, Q., Taylor, L., Compton, D.A., and Macara, I.G. (2002). LGN blocks the ability of NuMA to bind and stabilize microtubules: a mechanism for mitotic spindle assembly regulation. Curr. Biol. 12, 1928–1933 [DOI] [PubMed] [Google Scholar]
- Efthymiadis, A., Shao, H., Hubner, S., and Jans, D.A. (1997). Kinetic characterization of the human retinoblastoma protein bipartite nuclear localization sequence (NLS) in vivo and in vitro. A comparison with the SV40 large T-antigen NLS. J. Biol. Chem. 272, 22134–22139 [DOI] [PubMed] [Google Scholar]
- Endow, S.A., and Komma, D.J. (1996). Centrosome and spindle function of the Drosophila Ncd microtubule motor visualized in live embryos using Ncd-GFP fusion proteins. J. Cell Sci. 109, 2429–2442 [DOI] [PubMed] [Google Scholar]
- Eyers, P.A., Erikson, E., Chen, L.G., and Maller, J.L. (2003). A novel mechanism for activation of the protein kinase Aurora A. Curr. Biol. 13, 691–697 [DOI] [PubMed] [Google Scholar]
- Fanara, P., Hodel, M.R., Corbett, A.H., and Hodel, A.E. (2000). Quantitative analysis of nuclear localization signal (NLS)-importin α interaction through fluorescence depolarization. Evidence for auto-inhibitory regulation of NLS binding. J. Biol. Chem. 275, 21218–21223 [DOI] [PubMed] [Google Scholar]
- Foster, K.A., Correia, J.J., and Gilbert, S.P. (1998). Equilibrium binding studies of non-claret disjunctional protein (Ncd) reveal cooperative interactions between the motor domains. J. Biol. Chem. 273, 35307–35318 [DOI] [PubMed] [Google Scholar]
- Gaglio, T., Saredi, A., Bingham, J., Hasbani, J., Gill, S.R., Schroer, T.A., and Compton, D.A. (1996). Opposing motor activities are required for the organization of the mammalian mitotic spindle pole. J. Cell Biol. 135, 399–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görlich, D., Seewald, M.J., and Ribbeck, K. (2003). Characterization of Randriven cargo transport and the RanGTPase system by kinetic measurements and computer simulation. EMBO J. 22, 1088–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss, O.J., Carazo-Salas, R.E., Schatz, C.A., Guarguaglini, G., Kast, J., Wilm, M., Le Bot, N., Vernos, I., Karsenti, E., and Mattaj, I.W. (2001). Ran induces spindle assembly by reversing the inhibitory effect of importin α on TPX2 activity. Cell 104, 83–93 [DOI] [PubMed] [Google Scholar]
- Harlow, E., and Lane, D. (1999). Immunoaffinity purification. In: Using Antibodies: A Laboratory Manual, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 321–325
- Harreman, M.T., Hodel, M.R., Fanara, P., Hodel, A.E., and Corbett, A.H. (2003). The auto-inhibitory function of importin α is essential in vivo. J. Biol. Chem. 278, 5854–5863 [DOI] [PubMed] [Google Scholar]
- Heald, R., Tournebize, R., Blank, T., Sandaltzopoulos, R., Becker, P., Hyman, A., and Karsenti, W. (1996). Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature 382, 420–425 [DOI] [PubMed] [Google Scholar]
- Hetzer, M., Gruss, O.J., and Mattaj, I.W. (2002). The Ran GTPase as a marker of chromosome position in spindle formation and nuclear envelope assembly. Nat. Cell Biol. 4, E177–E184. [DOI] [PubMed] [Google Scholar]
- Hodel, M.R., Corbett, A.H., and Hodel, A.E. (2001). Dissection of a nuclear localization signal. J. Biol. Chem. 276, 1317–1325 [DOI] [PubMed] [Google Scholar]
- Hu, W., and Jans, D.A. (1999). Efficiency of importin α/β-mediated nuclear localization sequence recognition and nuclear import. Differential role of NTF2. J. Biol. Chem. 274, 15820–15827 [DOI] [PubMed] [Google Scholar]
- Ishii, N., Minami, N., Chen, E.Y., Medina, A.L., Chico, M.M., and Kasamatsu, H. (1996). Analysis of a nuclear localization signal of Simian virus 40 major capsid protein Vp1. J. Virol. 70, 1317–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jans, D.A., Xiao, C.-Y., and Lam, M.H.C. (2000). Nuclear targeting signal recognition: a key control point in nuclear transport. Bioessays 22, 532–544 [DOI] [PubMed] [Google Scholar]
- Kahana, J.A., and Cleveland, D.W. (1999). Beyond nuclear transport: Ran-GTP as a determinant of spindle assembly. J. Cell Biol. 146, 1205–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalab, P., Pu, R.T., and Dasso, M. (1999). The Ran GTPase regulates mitotic spindle assembly. Curr. Biol. 9, 481–484 [DOI] [PubMed] [Google Scholar]
- Kalab, P., Weis, K., and Heald, R. (2002). Visualization of a Ran-GTP gradient in interphase and mitotic Xenopus egg extracts. Science 295, 2452–2456 [DOI] [PubMed] [Google Scholar]
- Karabay, A., and Walker, R.A. (1999). Identification of microtubule binding sites in the Ncd tail domain. Biochemistry 38, 1838–1849 [DOI] [PubMed] [Google Scholar]
- Karabay, A., and Walker, R.A. (2003). Identification of Ncd tail domain-binding sites on the tubulin dimer. Biochem. Biophys. Res. Commun. 305, 523–528 [DOI] [PubMed] [Google Scholar]
- Keryer, G., Fiore, B.D., Celati, C., Lechtreck, K.F., Mogensen, M., Delouvée, A., Lavia, P., Bornens, M., and Tassin, A.-M. (2003). Part of Ran is associated with AKAP450 at the centrosome: involvement in microtubule organizing activity. Mol. Biol. Cell 14, 4260–4271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa, M., Okada, Y., and Hirokawa, N. (2000). 15 Å resolution model of the monomeric kinesin motor, KIF1A. Cell 100, 241–252 [DOI] [PubMed] [Google Scholar]
- Klebe, C., Bischoff, F.R., Ponstingl, H., and Wittinghofer, A. (1995). Interaction of the nuclear GTP-binding protein Ran with its regulatory proteins RCC1 and RanGAP1. Biochemistry 34, 639–647 [DOI] [PubMed] [Google Scholar]
- Kobe, B. (1999). Autoinhibition by an internal nuclear localization signal revealed by the crystal structure of mammalian importin α. Nat. Struct. Biol. 6, 388–397 [DOI] [PubMed] [Google Scholar]
- Kuersten, S., Ohno, M., and Mattaj, I.W. (2001). Nucleocytoplasmic transport: Ran, beta and beyond. Trends Cell Biol. 11, 497–503 [DOI] [PubMed] [Google Scholar]
- Li, H.Y., Wirtz, D., and Zheng, Y. (2003). A mechanism of coupling RCC1 mobility to RanGTP production on the chromatin in vivo. J. Cell Biol. 160, 635–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounsbury, K.M., Richards, S.A., Carey, K.L., and Macara, I.G. (1996). Mutations within the Ran/TC4 GTPase. J. Biol. Chem. 271, 32834–32841 [DOI] [PubMed] [Google Scholar]
- Matuliene, J., Essner, R., Ryu, J.-H., Hamaguchi, Y., Baas, P.W., Haraguchi, T., Hiraoka, Y., and Kuriyama, R. (1999). Function of a minus-end-directed kinesin-like motor protein in mammalian cells. J. Cell Sci. 112, 4041–4050 [DOI] [PubMed] [Google Scholar]
- Merdes, A., and Cleveland, D.W. (1997). Pathways of spindle pole formation: different mechanisms; conserved components. J. Cell Biol. 138, 953–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merdes, A., Ramyar, K., Vechio, J.D., and Cleveland, D.W. (1996). A complex of NuMA and cytoplasmic dynein is essential for mitotic spindle assembly. Cell 87, 447–458 [DOI] [PubMed] [Google Scholar]
- Moore, W.J., Zhang, C., and Clarke, P.R. (2002). Targeting of RCC1 to chromosomes is required for proper mitotic spindle assembly in human cells. Curr. Biol. 12, 1442–1447 [DOI] [PubMed] [Google Scholar]
- Mountain, V., Simerly, C., Howard, L., Ando, A., Schatten, G., and Compton, D.A. (1999). The kinesin-related protein, HSET, opposes the activity of Eg5 and cross-links microtubules in the mammalian mitotic spindle. J. Cell Biol. 147, 351–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachury, M.V., Maresca, T.J., Salmon, W.C., Waterman-Storer, C.M., Heald, R., and Weis, K. (2001). Importin β is a mitotic target of the small GTPase Ran in spindle assembly. Cell 104, 95–106 [DOI] [PubMed] [Google Scholar]
- Navolanic, P.M., and Sperry, A.O. (2000). Identification of isoforms of a mitotic motor in mammalian spermatogenesis. Biol. Reprod. 62, 1360–1369 [DOI] [PubMed] [Google Scholar]
- Ohba, T., Nakamura, M., Nishitani, H., and Nishimoto, T. (1999). Self-organization of microtubule asters induced in Xenopus egg extracts by GTP-bound Ran. Science 284, 1356–1358 [DOI] [PubMed] [Google Scholar]
- Ovechkina, Y., and Wordeman, L. (2003). Unconventional motoring: an overview of the Kin C and Kin I kinesins. Traffic 4, 367–375 [DOI] [PubMed] [Google Scholar]
- Quimby, B.B., and Dasso, M. (2003). The small GTPase Ran: interpreting the signs. Curr. Opin. Cell Biol. 15, 338–344 [DOI] [PubMed] [Google Scholar]
- Schatz, C.A., Santarella, R., Hoenger, A., Karsenti, E., Mattaj, I.W., Gruss, O.J., and Carazo-Salas, R.E. (2003). Importin α-regulated nucleation of microtubules by TPX2. EMBO J. 22, 2060–2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi, E., Toyoshima-Morimoto, F., and Nishida, E. (2002). Nuclear translocation of plk1 mediated by its bipartite nuclear localization signal. J. Biol. Chem. 277, 48884–48888 [DOI] [PubMed] [Google Scholar]
- Trieselmann, N., and Wilde, A. (2002). Ran localizes around the microtubule spindle in vivo during mitosis in Drosophila embryos. Curr. Biol. 12, 1124–1129 [DOI] [PubMed] [Google Scholar]
- Tsai, M.-Y., Wiese, C., Cao, K., Martin, O., Donovan, P., Ruderman, J., Prigent, C., and Zheng, Y. (2003). A Ran signalling pathway mediated by the mitotic kinase Aurora A in spindle assembly. Nat. Cell Biol. 5, 242–248 [DOI] [PubMed] [Google Scholar]
- Walczak, C.E. (2001). Ran hits the ground running. Nat. Cell Biol. 3, E69–E70. [DOI] [PubMed] [Google Scholar]
- Walczak, C.E., Verma, S., and Mitchison, T.J. (1997). XCTK 2, A Kinesin-related protein that promotes mitotic spindle assembly in Xenopus laevis egg extracts. J. Cell Biol. 136, 859–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak, C.E., Vernos, I., Mitchison, T.J., Karsenti, E., and Heald, R. (1998). A model for the proposed roles of different microtubule based motor proteins in establishing spindle bipolarity. Curr. Biol. 8, 903–913 [DOI] [PubMed] [Google Scholar]
- Wendt, T.G., Volkmann, N., Skiniotis, G., Goldie, K.N., Muller, J., Mandelkow, E., and Hoenger, A. (2002). Microscopic evidence for a minus-end-directed power stroke in the kinesin motor ncd. EMBO J. 21, 5969–5978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese, C., Wilde, A., Moore, M.S., Adam, S.A., Merdes, A., and Zheng, Y. (2001). Role of importin-β in coupling Ran to downstream targets in microtubule assembly. Science 291, 653–656 [DOI] [PubMed] [Google Scholar]
- Wilde, A., Lizarraga, S.B., Zhang, L., Wiese, C., Gliksman, N.R., Walczak, C.E., and Zheng, Y. (2001). Ran stimulates spindle assembly by altering microtubule dynamics and the balance of motor activities. Nat. Cell Biol. 3, 221–227 [DOI] [PubMed] [Google Scholar]
- Wilde, A., and Zheng, Y. (1999). Stimulation of microtubule aster formation and spindle assembly by the small GTPase Ran. Science 284, 1359–1362 [DOI] [PubMed] [Google Scholar]
- Woehlke, G., Ruby, A.K., Hart, C.L., Ly, B., Hom-Booher, N., and Vale, R.D. (1997). Microtubule interaction site of the kinesin motor. Cell 90, 207–216 [DOI] [PubMed] [Google Scholar]
- Yang, W., and Sperry, A.O. (2003). C-terminal kinesin motor KIFC1 participates in acrosome biogenesis and vesicle transport. Biol. Reprod. 69, 1719–1729 [DOI] [PubMed] [Google Scholar]
- Zhang, C., Hutchins, J.R.A., Mühlhäusser, P., Kutay, U., and Clarke, P.R. (2002). Role of importin-β in the control of nuclear envelope assembly by Ran. Curr. Biol. 12, 498–502. [DOI] [PubMed] [Google Scholar]