Abstract
We analyzed the receptive field information conveyed by interspike intervals (ISIs) in the auditory cortex. In the visual system, different ISIs may both code for different visual features and convey differing amounts of stimulus information. To determine their potential role in auditory signal processing, we obtained extracellular recordings in the primary auditory cortex (AI) of the cat while presenting a dynamic moving ripple stimulus and then used the responses to construct spectrotemporal receptive fields (STRFs). For each neuron, we constructed three STRFs, one for short-ISI events (ISI < 15 ms); one for isolated, long-ISI events (ISI > 15 ms); and one including all events. To characterize stimulus encoding, we calculated the feature selectivity and event information for each of the STRFs. Short-ISI spikes were more feature selective and conveyed information more efficiently. The different ISI regimens of AI neurons did not represent different stimulus features, but short-ISI spike events did contribute over-proportionately to the full spike train STRF information. Thus short-ISIs constitute a robust representation of auditory features, and they are particularly effective at driving postsynaptic activity. This suggests that short-ISI events are especially suited to provide noise immunity and high-fidelity information transmission in AI.
Keywords: information theory, receptive field, synergy, dynamic moving ripple
the relationships between spikes, as described by interspike intervals (ISI), have been implicated in many aspects of neuronal information processing. For example, pairs of spikes that are separated by short time intervals have a greater chance of synaptic transmission than isolated action potentials (Usrey et al. 2000), and burst spikes have been shown to have special roles in Hebbian learning (Pike et al. 1999) and in selective communication between neurons (Izhikevich et al. 2003). ISI coding of temporal information in auditory cortex also appears to be more informative than firing rate or spike-time precision alone (Imaizumi et al. 2010).
Sensory neurons can be characterized by their receptive fields, which are commonly estimated via spike-triggered average or covariance techniques (Schwartz et al. 2006). All spike-triggered techniques, however, make the implicit assumption that single spikes are independent from one another. Whether the model is a tuning curve or a spike-triggered average receptive field, it is assumed that each spike makes an independent and equal contribution to a neuron's stimulus-response relationship. The independence assumption, however, is not valid for all events, in particular with regard to spike bursts, which usually exhibit short ISIs.
In this study, we address the role of the first-order ISI in primary auditory cortex in stimulus encoding. The first-order ISI is the time between adjacent spikes in a spike train. The duration of a spike's preceding ISI affects the probability of eliciting a postsynaptic spiking response (Usrey et al. 2000), and relatively simple model neurons are capable of displaying selectivity for ISIs (Wilson et al. 2001). Short-ISI bursting activity can also constitute viable, even ideal, stimulus encoding schemes (Krahe and Gabbiani 2004; Oswald et al. 2007). Additionally, studies in the visual system have shown that different ISIs may convey distinct features of visual stimuli, as well as convey different amounts of stimulus information to subsequent sensory processing stages (Rathbun et al. 2007; Reich et al. 2000). These ideas suggest that it is possible that auditory cortical neurons use ISIs to distinguish different stimulus representations.
To address this possibility, we separated spikes into two categories: those with short preceding ISIs (1–15 ms) and those with long preceding ISIs (>15 ms). While spikes in these ISI categories did not encode different stimulus features, we did find that short-ISI spikes have the potential to play particularly significant roles in the overall stimulus encoding of neurons in auditory cortex. Our results, when paired with the result that downstream neurons seem to be able to respond preferentially to particular ISIs, indicate that ISI discrimination could be a prime candidate for implementing a temporal code in auditory cortical circuits. Neurons that respond preferentially to short-ISI input events may provide either greater coding efficiency or, potentially, robustness to neural noise.
METHODS
Electrophysiological methods and stimulus design have been described in previous reports (Atencio and Schreiner 2008; Miller et al. 2001; Miller and Schreiner 2000). A brief description follows.
Electrophysiology.
All procedures were carried out in compliance with the University of California, San Francisco, Institutional Animal Care and Use Committee as well as the guidelines of the National Institutes of Health. Adult cats (n = 3) were initially sedated with ketamine (30 mg/kg) and acepromazine (0.15 mg/kg) and then anesthetized with pentobarbital sodium (15–30 mg/kg) for the surgical procedure. During recording, an areflexic state was maintained via constant infusion of ketamine (2–11 mg/kg/h) and diazepam (0.05–0.2 mg·kg·−1h−1).
Recordings were made in a sound-shielded anechoic chamber (IAC, Bronx, NY), and stimuli were delivered monaurally, via a closed speaker system (diaphragms from Stax, Saitama, Japan), to the contralateral ear. Extracellular recordings were made using 16-channel microelectrode arrays (NeuroNexus Technologies, Ann Arbor, MI). Electrodes were linearly arranged, with a spacing of 150 μm between contacts on the array. Each electrode contact had an area of 177 μm2.
Neural traces were bandpass filtered between 600 and 6,000 Hz and recorded with a Neuralynx (Bozeman, MT) Cheetah recording system at sampling rates between 18 and 27 kHz. Traces were sorted offline using a Bayesian spike-sorting algorithm to obtain isolated single units (Lewicki 1994).
Stimuli.
Neurons were presented with a 15-min dynamic moving ripple (Escabí and Schreiner 2002). The dynamic moving ripple is a temporally varying broadband stimulus spanning frequencies between 500 and 40,000 Hz with 50 sinusoidal carriers per octave. At any given moment, the stimulus envelope is determined by a combination of temporal and spectral modulation parameters. In our ripple, we spanned a temporal modulation range between −40 Hz (upward sweep) and 40 Hz (downward sweep) and a spectral modulation range between 0 cycles/octave and 4 cycles/octave. Maximum modulation depth of the spectrotemporal envelope was 40 dB.
For 88 neurons, we also presented 50 trials of a 30-s dynamic moving ripple segment. All parameters of the 30-s segment were identical to the 15-min dynamic moving ripple.
Spectrotemporal receptive fields.
Reverse correlation was used to construct spectrotemporal receptive fields (STRFs) from each of our spike trains (Aertsen et al. 1981; deCharms et al. 1998; Escabí and Schreiner 2002; Klein et al. 2000; Theunissen et al. 2000). The STRF is the average spectrotemporal stimulus envelope preceding each spike. Positive (red) regions in the STRF indicate that stimulus energy at that frequency and time will drive the neural activity above the average firing rate, while negative (blue) regions have the opposite effect.
Inhomogeneous Poisson model.
To evaluate how well a spike train can be modeled by an inhomogeneous Poisson process, we estimated the time-varying instantaneous rate function of the neuron and generated simulated spike trains to compare to our real spike trains. We estimated the rate function r(t) via a linear-nonlinear-Poisson (LNP) model (Schwartz et al. 2006) as follows:
where
The linear component of our LNP model consists of x(t), the convolution between the STRF and the entire stimulus, while the nonlinear component consists of the empirical spiking nonlinearity f(x) for each neuron (Aguera y Arcas et al. 2003; Atencio and Schreiner 2008). This spiking nonlinearity is static and may be arbitrarily nonlinear.
As a final step, we also normalized r(t) so that the average firing rate would match the average firing rate in the real spike trains. By doing this, we avoided effects in the model caused by overestimating or underestimating the overall firing rate of the neuron.
Similarity index and feature selectivity.
The similarity between a stimulus segment and the STRF was evaluated using a similarity index (SI) (DeAngelis et al. 1999; Reich et al. 2000). The SI is equivalent to the Pearson correlation coefficient, and may be calculated according to:
where STRF·Stim is the dot product and ‖STRF‖ and ‖Stim‖ are the norms of the vectorized versions of the STRF and stimulus arrays. We note that SIs are related to projection values, but differ in that SIs are scaled to a range from 1 to −1, where a value near 1 indicates that the stimulus is well matched to the STRF, while a value near −1 indicates the stimulus has features opposite in sign to the STRF. If the SI is 0, then the STRF and the stimulus feature are uncorrelated.
The SI can also be used to compare two STRFs. In this case, the STRF-STRF SI is calculated as
where STRFA and STRFB are two STRFs.
The feature selectivity index (FSI) (Atencio and Schreiner 2008; Escabí and Schreiner 2002; Miller et al. 2001) quantifies the match between the STRF and the individual stimulus segments that were used to calculate the STRF. We calculated the FSI as follows:
where
Aprior and Aspike represent the area under the cumulative distribution functions (CDFs) of the prior and spike-conditioned probability distributions (d) of SI values, respectively. In calculating Aprior, we used the complete set of all SIs between the stimulus and the STRF, while for Aspike, we used only SIs associated with the occurrence of spiking events. FSIs can have values ranging from 0 to 1. Neurons that only spike when the stimulus and STRF are well-matched will have Aspike values that are relatively small, driving Aprior − Aspike toward Aprior and thus the FSI value toward 1. Randomly firing neurons will have Aspike very close to Aprior, driving Aprior − Aspike toward 0 and thus the FSI value toward 0. An FSI of 0 indicates that a neuron is no more feature-selective than a randomly spiking unit while a neuron with an FSI of 1 only responds to stimuli that are perfectly matched to its receptive field. Note that the FSI is closely related to the shape of a neuron's spiking nonlinearity and indicates the degree to which a stimulus must match the neuron's STRF in order for a spike to be fired.
Information.
We followed previously reported methodologies (Brenner et al. 2000; Sharpee et al. 2004) to find the event information (I) for each STRF. The event information is defined as the mutual information between the spiking response and the stimulus, calculated by:
P(x) was calculated by computing x for each stimulus segment in the ripple stimulus, without regard to whether the segment corresponded to a spike. P(x | spk) was calculated by computing x for each stimulus segment associated with a spike.
Bias corrections.
We extrapolated our feature selectivity and information calculations to correct for the finite size of our data set (Brenner et al. 2000; Strong et al. 1998). To do this, we took 25 random sections each of 50, 53, 56, 59, 62, 66, 71, 76, 83, and 90% of the data and calculated the FSI and information values. By plotting these values against the inverse of the data portion (1/0.50, 1/0.53, 1/0.56, etc.), fitting a line to the plot, and finding the ordinate intersect, we obtained FSI and information values extrapolated for infinite data set size.
Since the number of spikes in the short- or long-ISI categories is less than the total, we also corrected for spike number differences in these distributions. As each ISI category has a different number of spikes for each neuron, we calculated separate correction values for every ISI category for every cell. For each ISI-specific spike train, we generated 50 randomly resampled spike trains and recalculated the FSI and information values. Each of the resampled spike trains consisted of spikes selected at random (without replacement) from the full spike train. The number of spikes in each resampled subset was matched to the number of events in each ISI-specific spike train. We corrected according to:
where FSIISI and IISI are the ISI-specific FSI and information values,
and
are the mean values for the 50 resampled spike trains, and FSIfull and Ifull are the values obtained from the full (non-ISI-specific) spike train. In a case where there is no bias due to STRF estimation with subsets of spikes,
and
will be equal to FSIfull and Ifull, respectively, and thus FSIISI,corrected = FSIISI and IISI,corrected = IISI.
Spike pattern synergy.
We followed previously reported methodologies (Brenner et al. 2000) to calculate the synergy displayed by spikes within a spike pattern. The synergy in an ISI event is given by:
where I(ISI) is the information conveyed by a particular ISI event type and I(spk) is the information conveyed by single spikes. We subtract two times the single-spike information because each ISI event consists of two spikes. Positive synergy indicates that an ISI event type conveys more information than the sum of the information conveyed by its independent spike components.
We calculated our synergy values using multitrial data from repeated presentations of a short segment of dynamic moving ripple, which allowed us to generate peristimulus time histograms (PSTHs) and calculate event information as follows (Brenner et al. 2000):
where rISI(t) and rspk(t) are, respectively, the time-varying rates of ISI events and spikes obtained from the PSTHs and
and
are their averages over time. This method of calculating information is model independent, so our synergy results are unbiased by model assumptions.
We corrected for biases in our multitrial information calculations in two ways (Brenner et al. 2000). First, we corrected for the finite number of trials. To do this, we took 25 random selections each of 80, 83, 87, 91, and 95% of the trials and calculated the information values. By fitting a line to the plot of the information values vs. the inverse of the data portions and finding the ordinate intersect, we extrapolated an information value for an infinite number of trials. Second, we varied the bin size in each PSTH from 5 to 19 ms at 1-ms intervals and calculated the extrapolated infinite-trial information values. We then fitted a line to the plot of these information values vs. bin size, found the ordinate intersect, and obtained an information value for an infinitely small bin size.
RESULTS
ISI histograms.
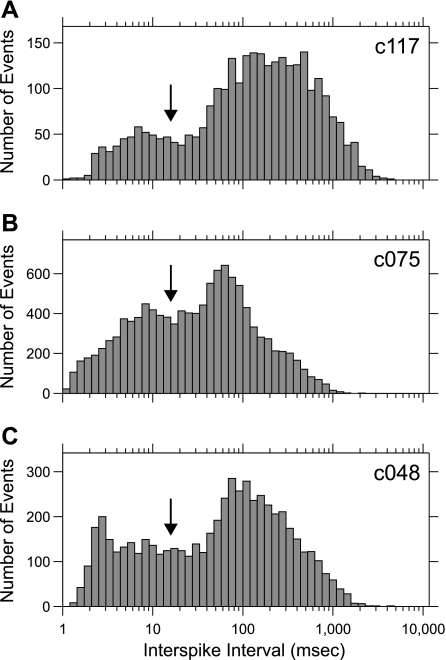
Log-ISI histograms (Reich et al. 2000) from the full spike trains were constructed to view the distribution of ISIs for each studied neuron (Fig. 1). Log-ISI histograms (log-ISIHs) display features of the ISI distributions that are not apparent in linearly scaled ISI histograms. The ISI histograms had a main peak associated approximately with the driven rate of the neuron and often had a noticeable separate peak at shorter ISIs. When we identified the border between each of the peaks by eye (borders for the three example histograms in Fig. 1 are indicated by downward-pointing arrows), we found that these borders had a mean location at 16.2 ms (median: 15.6 ms) and a SD of 8.5 ms.
Fig. 1.
Example interspike interval (ISI) histograms. Each histogram has 50 bins logarithmically spaced from ISIs of 1 ms up to 10,000 ms. Arrows indicate manually selected borders between the 2 peaks of each distribution. Cell IDs are shown at top right each histogram.
We hypothesized that the large number of short-ISI events reflected a distinct mode of spiking behavior that may have its own stimulus-encoding scheme. We thus separated each spike train into two subsets, one containing spikes with short preceding ISIs and the other containing spikes with long preceding ISIs. We erred on the side of including fewer events in the short-ISI group, selecting 15 ms to serve as the dividing point between short- and long-ISI categories.
We used a static division rather than individual cutoff points for each neuron because a static division allowed us to makes comparisons across neurons without observer biases. The 15-ms boundary also has significance in that it is similar to estimated cortical membrane time constants (Koch et al. 1996), as well as the average width of cross-correlation peaks between auditory cortical neurons (Eggermont 2000). It is also approximately the upper ISI for which the second spike in a pair has an elevated likeliness of eliciting a postsynaptic spike response (Usrey et al. 2000). We note that with a 15-ms dividing point, there were on average 3.7 times more long-ISI than short-ISI events.
For our analysis, due to computational considerations, we included 105 out of 133 neurons on the basis that they had at least 200 spiking events in both their short- and long-ISI subsets. These neurons, on average, had 1,793 (median: 1,275) short-ISI events with a SD of 1,550 events. On average, they had 4,525 (median: 4,087) long-ISI events with a SD of 2,470 events.
ISI coding.
Modulations in firing rate have long been shown to account for significant amounts of information in sensory neurons, but temporal coding strategies may also be important in the neural representation of sensory information (Engel et al. 1992; Imaizumi et al. 2010; Middlebrooks et al. 1994; Richmond et al. 1987). In rate-coding models, it is assumed that spikes are generated independently of each other, whereas in temporal coding models, the emphasis is on the generation of spike patterns.
By focusing on a very simple spike pattern, spike doublets, this study seeks to determine whether stimulus representations change across different spike pattern responses. In separating short-ISI events from long-ISI events, however, there is the possibility that we have not identified two separate spiking patterns but have rather separated spikes that occur during periods of stimulus-driven high firing rates from those that occur during periods of low firing rates. If this is the case, then any observations we make distinguishing short-ISI spikes from long-ISI spikes may be a simple consequence of time-varying rate fluctuations during the response of a neuron to the ripple stimulus.
Therefore, as a preliminary analysis, we tested whether an independent-spiking rate model would predict the observed ISI distributions in our data. To do this, we estimated the firing rate function of each neuron in response to our stimulus and used it to generate simulated spike trains. Because our subsequent SI, feature selectivity, and information measures were all based on the STRF, we used an STRF-based linear-nonlinear-Poisson model (see methods) to estimate firing rates. For each time bin, we calculated the projection value between the STRF and the stimulus, and we transformed this projection value into a firing rate by applying the empirically derived spiking nonlinearity function (see methods). Once we calculated firing rates for the entire stimulus trial, we generated simulated spikes for a hypothetical Poisson process with the given firing rate function. We then compared the ISI distributions of these simulated spike trains to those of the real spike trains.
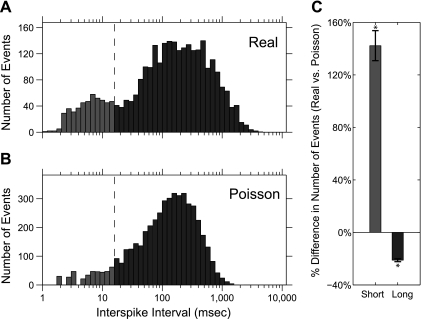
Figure 2A shows the ISI histogram of a neuron while Fig. 2B shows the ISI histogram of its Poisson model simulated spike train. For this neuron, there was a decrease in the salience of the short-ISI peak in the histogram of the simulated spike train. Figure 2C shows the relative difference in the number of short-ISI and long-ISI events for the simulated and real spike trains across all neurons. We found that real spike trains had 142.2% more (median: 125.8%) short-ISI events than rate-coded simulated spike trains. Conversely, there were on average 21.2% fewer (median: 20.1%) long-ISI events in the real responses. We found that both of these differences were significant (P < 0.01, t-test). Note that these differences were obtained without incorporating refractory periods in the LNP models, which would serve to decrease the chances of short-ISI events in the simulated spike trains. The inclusion of the spiking nonlinearity, however, enabled our model to capture effects such as thresholding and high feature selectivity that could potentially influence the shape of ISI distributions by generating more short-ISI events. The LNP model results indicate that a simple rate-coding model does not capture the real distribution of ISI events in the data.
Fig. 2.
Real and Poisson (simulated) ISI histograms. A: ISI histogram of a real spike train (cell c117 from Fig. 1A). Dotted line indicates the static division between the short- and long-ISI categories at 15 ms. B: ISI histogram of the simulated spike train obtained from the rate-coded Poisson model of the same neuron. Total number of simulated spikes matches that of the real spike train. C: relative numbers of short and long ISI events in real vs. Poisson-simulated spike trains across the population of neurons. Positive values indicate that there are more of those events in the real spike train. Standard error bars are shown.
There remains the possibility that more general spiking models (e.g., gamma processes) or more sophisticated receptive field models (e.g., multi-order Wiener kernels) may be better able to simulate the ISI distributions seen in the real data. Ideally, however, we would test for the presence of interval coding in auditory cortical neurons using a model-independent method. For 88 neurons, we presented repeated trials of 30-s dynamic moving ripple segments; this allowed us to calculate spike pattern synergy (Brenner et al. 2000). Spike pattern synergy is a model-independent method of quantifying whether a pattern of spikes conveys more information than the sum of the information conveyed by each of the spike elements independently (see methods). In our case, the spike patterns under consideration are ISI events constructed from two spikes, so we calculate synergy as the difference between ISI-event information and two times the single-spike information.
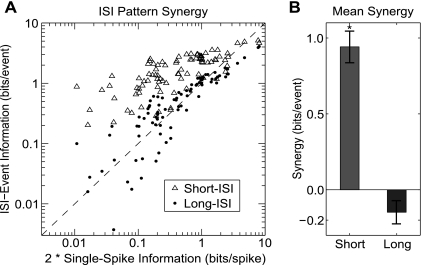
Figure 3A compares the information conveyed by ISI events to twice the single-spike information for each neuron. Points along the dotted line indicate that the two spikes constituting the ISI event convey information independently; therefore, the ISI event conveys no additional information beyond the sum of its parts. Points below the dotted line indicate the spikes convey redundant information. Points above the dotted line indicate that the spikes in an ISI event act synergistically to convey more information than both spikes could independently. Figure 3B shows the mean synergy in short- and long-ISI events. On average, short-ISI events displayed 0.94 bits of synergy per event, meaning that short-ISI events conveyed nearly one bit of information more than would be expected from any independent-spiking model. This positive synergy was significant (P < 0.01, t-test). In contrast, long-ISI events displayed −0.15 bits of synergy per event. This negative synergy was not significantly different from 0 (P > 0.1, t-test), indicating that the spikes constituting long-ISI events are effectively acting independently.
Fig. 3.
Synergy in ISI patterns. A: comparison of information conveyed by short- and long-ISI events to 2 times the information conveyed by single spikes. Points above the dotted line indicate that the 2 spikes that constitute the ISI event are synergistic; i.e., the ISI event conveys more information than the sum of the information conveyed by the 2 spikes independently. Points below the dotted line indicate the 2 spikes in the ISI event contribute redundant information. Points directly on the dotted line indicate the 2 spikes in the ISI event convey independent information. B: mean synergy values for short- and long-ISI events. Spikes in short-ISI events are significantly synergistic (P < 0.01, t-test) while spikes in long-ISI events are not (P > 0.1, t-test). SE bars are shown.
We defined the timing of each ISI event as the timing of the second spike of each pair. We wished to test whether the observed synergy results were dependent on this definition, so as an additional analysis, we recalculated synergy values for short- and long-ISI events. In this case, however, we took the timing of the first spike of each pair (instead of the second spike) as the timing of each ISI event. We found that short-ISI events still displayed positive synergy, on average 0.85 bits per event and that this synergy was significant (P < 0.01, t-test). Long-ISI events displayed −0.14 bits of synergy per event on average, although this negative synergy was not significantly different from 0 (P > 0.1, t-test).
Comparing the synergy results for the first- and second-spike definitions of ISI event timing, we found that while there was no significant difference in synergy for long-ISI events (P > 0.1, paired t-test), the second-spike definition of ISI event timing yielded significantly greater synergy for short-ISI events than the first-spike definition (P < 0.01, paired t-test). This suggests that while short-ISI spike pairs constitute special encoding elements, the timing of the second spike in each pair may be more reliable and thus convey greater information.
These results show that auditory cortical neurons display a significant degree of interval coding that cannot be explained by independent-spiking models. First, the LNP model simulations suggest that the preponderance of short-ISI events cannot be explained by a simple rate-coding model. Second, the synergy results show that the individual spikes in a short-ISI event combine in a highly nonindependent way.
STRFs.
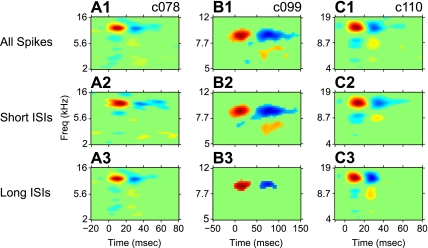
To determine the stimulus features encoded by short and long ISIs, we constructed STRFs for each ISI category. Figure 4 shows the full-response STRFs of three example neurons along with their ISI-specific STRFs. In some cases, we observed that the long-ISI STRF contained components of the full-response STRF that were absent from the short-ISI STRF (Fig. 4, A1–A3). In other cases, we observed the converse, where the short-ISI STRF contained components absent in the long-ISI STRF (Fig. 4, B1–B3). Last, there were cases where both the short-ISI and long-ISI STRFs were largely similar to the STRF of the full spike train (Fig. 4, C1–C3).
Fig. 4.
Example spectrotemporal receptive fields (STRFs) based on different ISIs. Each column contains the STRFs for one neuron; cell IDs are shown in the top right of the first row. First row: STRFs constructed from all the spikes in the response; second row: STRFs for short-ISI (<15 ms) spikes; third row: STRFs for long-ISI (>15 ms) spikes. A1–A3: example neuron where the long-ISI STRF shares minor inhibitory sideband structures with the full-response STRF that are absent in the short-ISI STRF. B1–B3: example neuron where the short-ISI STRF has an excitatory sideband structure absent in the long-ISI STRF. C1–C3: example neuron where the full-response, short-ISI, and long-ISI STRFs are largely similar.
In the vast majority of cases, we were unable to find clear qualitative differences between the ISI-specific STRFs and the full-response STRFs. Short- and long-ISIs had STRFs that were generally well correlated with the full-response STRFs. On average, short-ISI STRFs had an SI of 0.68 with the full-response STRFs (median: 0.70; SD: 0.12), while long-ISI STRFs on average had an SI of 0.86 (median: 0.87; SD: 0.07). Thus different categories of ISI events generally have similar stimulus features in primary auditory cortex.
STRF-STRF and stimulus-STRF similarity.
Initially, it seems that because long-ISI spikes generate STRFs that are more similar to the full-response STRFs, they may contribute more to the overall stimulus representations of auditory cortex neurons than short-ISI spikes. Of note, however, is that short-ISI STRFs achieve relatively high similarity to the full-response STRFs despite being constructed from generally less than half as many spikes as the long-ISI STRFs.
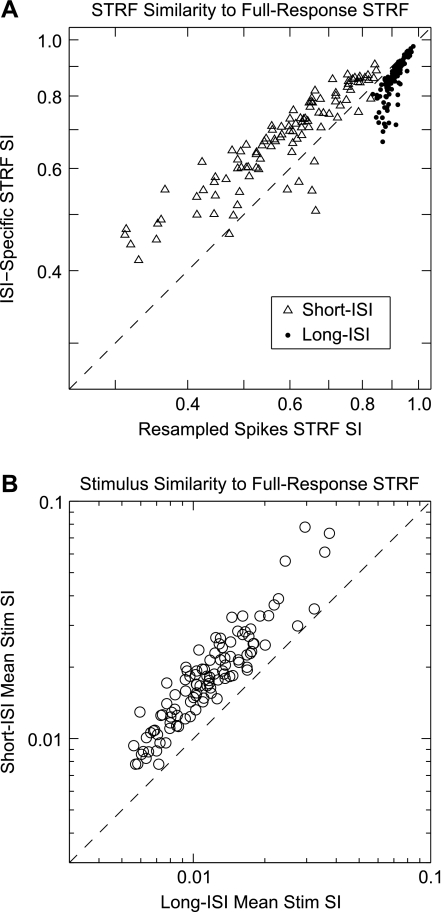
A fairer comparison of ISI-specific STRFs and their similarity to the full-response STRF needs to control for the number of spikes used in STRF construction. We wished to see whether each ISI-specific STRF was more or less similar than expected to the full-response STRF given the number of spikes used to construct the ISI-specific STRF. To establish the expected similarity for a given number of spikes, we generated resampled response trains that consisted of spikes randomly drawn (without replacement) from the total spiking response. We then used these resampled trains to construct STRFs that were then compared with the full-response STRFs. This resampling procedure preserves the relative rate modulations of the original full spike train, since the likelihood of drawing spikes from a particular time period is proportional to the firing rate during that period. By matching the number of spikes in the resampled trains to that of an ISI-specific train, we could then determine whether an ISI-specific STRF similarity was above or below the value expected given the number of spikes. For each ISI-specific STRF, Fig. 5A shows the similarity of each ISI-specific STRF to the full-response STRF. This similarity is then compared with the mean similarity achieved by 50 spike-number-matched resampled spike trains. Our results show that short-ISI STRFs are more similar than expected, while long-ISI STRFs are less similar to the full-response STRF than expected. On average, short-ISI STRFs were 15.8% more similar to the full-response STRFs than the resampled spike trains (median: 15.4%), while long-ISI STRFs were on average 5.7% less similar (median: 4.0%). Both of these differences were significant (P < 0.01, signed-rank test).
Fig. 5.
Comparison of STRF and spike-triggered stimulus similarity to full-response STRFs. A: comparison of ISI-specific STRF similarity to mean similarity of 50 resampled spike trains. Resampled spike trains consist of spikes randomly selected (without replacement) from the full spike train matching the number of events in the ISI-specific spike trains. Points above the dotted diagonal line indicate that the ISI-specific STRF was more similar than expected to the full-response STRF while points below the dotted line indicate the ISI-specific STRF was less similar than expected. B: comparison of mean similarity of stimuli triggered to either short or long-ISI spikes to the full-response STRF. Stimuli associated with short-ISI events are on average more similar to the full-response STRF than those associated with long-ISI events. SI, similarity index.
We also addressed how short or long-ISI spikes contributed to receptive field properties by considering stimulus encoding on a per-spike basis. To that end, we calculated the similarity between the full-response STRF and the individual stimuli that triggered a spike (Fig. 5B). This measure describes how well matched a stimulus had to be to the full-response receptive field of a neuron for that neuron to respond with a particular ISI event. On average, short-ISI-triggered stimuli were 58.4% more similar to the full-response STRFs than long-ISI-triggered stimuli (median: 54.9%). We found this difference to be statistically significant (P < 0.01, signed-rank test). When considered on a per-event basis, short-ISI spikes are responses to stimuli that are more similar to the full-response STRFs, indicating that they encode the feature of that STRF more reliably. This means that spike-for-spike, short-ISIs are represented over-proportionately in the overall receptive field properties of auditory cortical neurons.
Feature selectivity.
For each STRF, we calculated a feature selectivity index (see Methods). The feature selectivity index (FSI) is a measure of how closely the stimuli that elicited a spike match the neuron's STRF. In the case of our ISI-specific STRFs, the FSI is a measure of the stimulus similarity for spikes in either the short- or long-ISI categories.
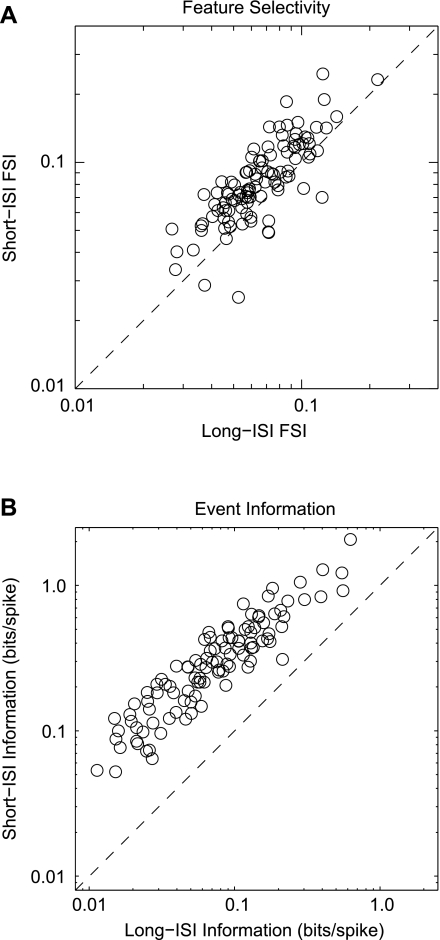
Figure 6A compares the feature selectivity of short- and long-ISI spikes for each neuron. On average, short-ISI spikes had FSI values 28.9% greater (median: 27.5%) than long-ISI spikes. We found that 88 of 105 neurons had higher FSI values associated with short-ISIs compared with long-ISIs. These results may explain the lack of noticeable estimation noise in the short-ISI STRFs. Greater feature selectivity means that the range of stimuli that comprise the STRF are less variable and thus better matched to the STRF. In turn, this leads to stimuli that evoked short-ISIs being less variable than stimuli associated with long-ISIs. This may explain why, despite the relative scarcity of short-ISI events, we were able to obtain well-defined STRFs with the short-ISI spike trains.
Fig. 6.
Comparison of STRF feature selectivity and event information for short- and long-ISI events. A: comparison of short- and long-ISI feature selectivity index values. Short-ISI events are significantly more feature selective than long-ISI events (P < 0.01, signed-rank test). B: comparison of short- and long-ISI event information. Short-ISI events convey more bits per spike than long-ISI events (P < 0.01, signed-rank test). FSI, feature selectivity index.
Information.
We can quantify the information conveyed by the spikes associated with short- and long-ISIs by calculating the event information for each of the ISI-specific spike trains (see methods). Event information is a more general measure of neural encoding than feature selectivity since it does not make any assumptions about the manner of information encoding or decoding. The event information simply quantifies how much information a spike can convey about the sound stimulus. Event information and feature selectivity are related in that a highly feature-selective neuron will also convey a high amount of event information, since spike-triggered stimuli will be more similar to the resulting receptive field of a neuron. The converse, however, is not necessarily true; a neuron can convey significant amounts of event information without being particularly feature-selective. For example, although visual complex cells are not very selective for spatial details, they do transmit significant amounts of information about image statistics such as texture and motion energy (Hammond and MacKay 1977; Van Essen et al. 1992). If an auditory neuron conveys a great amount of event information but has a low FSI, then the neuron does not convey information about specific spectrotemporal features, but it still conveys information about other aspects of the stimulus.
We found that short- and long-ISIs conveyed different amounts of information (Fig. 6B). There is a clearer distinction between short- and long-ISI spikes in event information than in feature selectivity: for every auditory cortex neuron, short-ISI spikes conveyed more event information than long-ISI spikes and on average conveyed 320.3% more bits per spike (median: 288.6%).
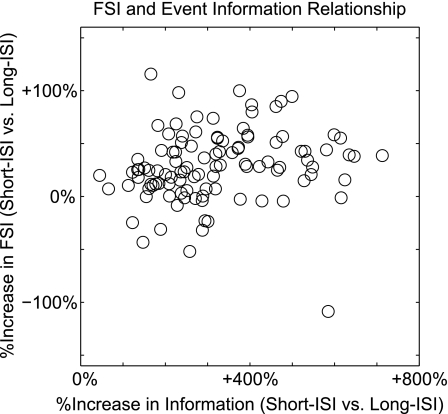
In our feature selectivity analysis, we found 17 neurons where long-ISIs were more feature-selective than short ISIs. Even for these neurons, however, the long-ISI events conveyed less information per event than the short-ISI spikes. Figure 7 shows the relationship between the percent increase in event information and the percent increase in feature selectivity for short-ISI spikes vs. long-ISI spikes. While feature selectivity and event information tend to be loosely correlated, we found that the correlation between the two measures was not statistically significant in our data set (P > 0.1, t-test). This indicates that the large differences observed in event information cannot be entirely attributed to differences in feature selectivity. That is, short-ISI spikes do not necessarily convey greater stimulus information only because they are more feature-selective. Similarly, long-ISI spikes do not necessarily convey less stimulus information only because they are less feature-selective. The population medians for short- and long-ISI spikes, however, differed significantly in both feature selectivity and information measures (P < 0.01, signed-rank test).
Fig. 7.
Relationship between increases in feature selectivity and increases in event information. These 2 measures were not significantly correlated (P > 0.1, t-test).
DISCUSSION
A goal of this study was to determine if spike doublets, with their enhanced probability of postsynaptic propagation, also have privileged stimulus-encoding properties. We addressed this question by separating spikes into different categories based on their preceding ISIs and determining how each of those ISI categories encoded stimulus information.
We found that short-ISI spikes constituted particularly reliable representations of the overall stimulus encoding of auditory cortical neurons. Compared with other spikes, they conveyed more stimulus information, were more feature selective, and contributed over-proportionately to each neuron's overall receptive field properties. Conversely, we found that long-ISI spikes were relatively poor encoders of stimulus information, were less feature selective, and spike-for-spike contributed less to the overall STRF of each neuron. We found that pairs of short-ISI spikes convey over three times as much information as long-ISI spikes, well over what we would expect from summing two independent information sources.
Our analysis focused on single neurons, but if we consider these neurons as part of a cortical network, our results gain an increased significance. Biophysically, spikes with short preceding ISIs are more likely than spikes with long preceding ISIs to elicit postsynaptic spiking responses (Usrey et al. 2000). As there is also a significant difference between the effectiveness of stimulus information encoding by short- and long-ISI spikes, this asymmetry effectively acts to amplify the signal-to-noise ratio of the transmitted neural code. Thus the most reliable sources of sound information, short-ISI spikes, are also the most likely to transmit their information to postsynaptic targets while the less reliable long-ISI spikes are not as likely to drive postsynaptic responses.
Short-ISI spikes may also play a role in maintaining synchrony between neurons when important sound stimuli are present. It has been suggested that spontaneous activity does not affect perception in the auditory system largely because spontaneous activity is generally uncorrelated across auditory neurons (Eggermont 1992; Johnson and Kiang 1976). Correlated activity, however, may be important for maintaining temporal precision as information is propagated through neural pathways (Kimpo et al. 2003; Reyes 2003). By driving postsynaptic spikes more reliably than isolated spikes, short-ISI events from a neuron may be well suited to drive multiple target neurons synchronously, thus providing a mechanism of eliciting correlated activity downstream despite the presence of significant levels of spontaneous spiking.
Maintaining temporal precision via synchronous firing may be particularly relevant to the encoding requirements of the auditory system. Audition is generally thought of as a “fast” modality: spike timing differences as small as 3 ms in the auditory cortex have been shown to guide behavior, whereas the visual cortex has been shown to have temporal discrimination limits closer to 15 ms (Yang et al. 2008; Yang and Zador 2010). Because small differences in temporal structure can be behaviorally relevant in audition, the auditory system in particular may require an encoding scheme that can preserve short timescales. Short-ISI events may be the kind of quick neural responses required to implement such a fast temporal code.
While it is conceivable that a rapidly modulating rate code might be able to capture the short-timescale fluctuations of sound stimuli, our LNP model simulations suggested that the auditory cortex encodes sound information by utilizing events with specific ISIs. The independent spiking model was unable to account for the disproportionately large number of short-ISI events in the real spike trains. This underestimation of short-ISI events may be caused by the fact that our rate-coding model does not take into account factors such as backpropagation of spikes from dendrites (Doiron et al. 2007) or membrane current dynamics (Canavier et al. 1991) that predispose neurons to fire off doublets or bursts. These spiking dynamics, however, deal with the mechanisms of generating spikes and do not explain the improved stimulus encoding properties observed for short-ISI events nor do they explain the synergistic fashion in which the two spikes in a short-ISI event combine to convey greater information than both of the spikes would individually. Regardless of how ISI events are generated, our results suggest that auditory cortex neurons may fire off pairs of spikes as temporal patterns to signal the occurrence of certain stimulus features in a fashion not captured by rate-coding models.
The question now arises: what is the mechanism that leads short-ISI spikes to be so significantly more informative? One simple explanation may be that doublets occur when a stimulus transitions from a state that is sufficient to elicit a spike to a state that is so near optimal that it provides enough drive to elicit a spike within the relative refractory period. The need for the second spike to overcome the relative refractory period would mean that they could only very rarely be noise driven, explaining the increased event information and feature selectivity in our results. Our synergy results appear to support this hypothesis. Using the timing of the first spike to define the timing of short-ISI events yielded significant synergy but not as much as when using the second spike to define short-ISI event times. This suggests that while the timing of the first spike in a doublet conveys a reasonably high amount of information, it is the timing of the second spike where even greater information is conveyed. These results correspond to what one would expect if the timing of the second spike has to coincide with the presentation of a near-optimal stimulus state to overcome the relative refractory period caused by the first spike.
Another possible explanation for the improved encoding properties of doublets is that the timing of short-ISI spikes coincides with the general excitability of a neuron; that is, whether the neuron is in a depolarized “up” or a hyperpolarized “down” state. In fact, models of auditory cortex neurons that incorporate representations of these excitability states have been shown to generate more short-ISI events, resulting in bimodal ISI distributions highly similar to those observed in our data (Britvina and Eggermont 2007). As cortical neurons tend to be desensitized to thalamic inputs during up states (Watson et al. 2008), it is possible that during up states, neurons are only able to fire quick pairs of spikes when there is a preponderance of coincident thalamic inputs, corresponding to great certainty in the presence of a stimulus. This would explain the heightened feature selectivity we observed for short-ISI events. In the rat barrel cortex, however, it has been shown that spikes during down states tend to display greater stimulus selectivity than spikes during up states (Hasenstaub et al. 2007). The results in barrel cortex, though, were averaged across all spikes and thus did not differentiate spikes based on preceding ISIs. This leaves the possibility that within up states, spikes with short preceding ISIs may have the improved stimulus encoding properties that we observed.
Another possible mechanism of how short-ISI spikes come to be more informative than other spikes is that our results may be a reflection of a combined effect between Hebbian synaptic plasticity and the increased postsynaptic spiking efficacy of short-ISI spikes. According to Hebbian theory, a synapse becomes strengthened when the presynaptic neuron “repeatedly and persistently takes part in firing” the postsynaptic neuron (Hebb 1949). Double spikes (i.e., short-ISI spikes) are more likely than single spikes (i.e., long-ISI spikes) to elicit postsynaptic responses and would thus be more likely to strengthen synapses between neurons. It is feasible then that Hebbian learning may create neural circuits that are predisposed toward neurons that fire off double spikes because those are the cells that are most likely to maintain strong connections with their postsynaptic targets. Bursting activity has been shown to be necessary for inducting long-term potentiation in synapses in the hippocampus (Pike et al. 1999), and in the visual cortex it has been shown that burst activity firing above 50 Hz also drives long-term potentiation (Froemke et al. 2006). Of note, the 15-ms cutoff puts our short-ISI events in a burst rate category of ∼67 Hz and above. In the auditory cortex, mechanisms for synaptic strengthening may predispose circuits to select for neurons that fire off double spikes or bursts when there is great certainty of the presence of a sound feature.
While our data may not be suited to directly address the question of how short-ISI spikes come to be more informative than other spikes, we see that not all spikes play an equal role in stimulus encoding. By looking at a simple timing relationship, the ISI, we are able to discern two significantly distinct populations of spike events. We find that short-ISI spikes are particularly important in primary auditory cortex stimulus encoding and have the potential to provide low-noise, robust, and efficient representations of sound features.
GRANTS
This work was supported by National Institutes of Health Grants DC-02260 and MH-077970, the Coleman Memorial Fund, and Hearing Research Inc.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Brian Malone, Patrick Hullett, and Loren Frank for suggestions on the analysis and the manuscript.
REFERENCES
- Aertsen AM, Olders JH, Johannesma PI. Spectro-temporal receptive fields of auditory neurons in the grassfrog. III. Analysis of the stimulus-event relation for natural stimuli. Biol Cybern 39: 195–209, 1981 [DOI] [PubMed] [Google Scholar]
- Aguera y Arcas B, Fairhall AL, Bialek W. Computation in a single neuron: Hodgkin and Huxley revisited. Neural Comput 15: 1715–1749, 2003 [DOI] [PubMed] [Google Scholar]
- Atencio CA, Schreiner CE. Spectrotemporal processing differences between auditory cortical fast-spiking and regular-spiking neurons. J Neurosci 28: 3897–3910, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner N, Strong SP, Koberle R, Bialek W, de Ruyter van Steveninck RR. Synergy in a neural code. Neural Comput 12: 1531–1552, 2000 [DOI] [PubMed] [Google Scholar]
- Britvina T, Eggermont JJ. A Markov model for interspike interval distributions of auditory cortical neurons that do not show periodic firings. Biol Cybern 96: 245–264, 2007 [DOI] [PubMed] [Google Scholar]
- Canavier CC, Clark JW, Byrne JH. Simulation of the bursting activity of neuron R15 in aplysia: role of ionic currents, calcium balance, and modulatory transmitters. J Neurophysiol 66: 2107–2124, 1991 [DOI] [PubMed] [Google Scholar]
- DeAngelis GC, Ghose GM, Ohzawa I, Freeman RD. Functional micro-organization of primary visual cortex: receptive field analysis of nearby neurons. J Neurosci 19: 4046–4064, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- deCharms RC, Blake DT, Merzenich MM. Optimizing sound features for cortical neurons. Science 280: 1439–1443, 1998 [DOI] [PubMed] [Google Scholar]
- Doiron B, Oswald AM, Maler L. Interval coding. II. Dendrite-dependent mechanisms. J Neurophysiol 97: 2744–2757, 2007 [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. Neural interaction in cat primary auditory cortex. Dependence on recording depth, electrode separation, and age. J Neurophysiol 68: 1216–1228, 1992 [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. Sound-induced synchronization of neural activity between and within three auditory cortical areas. J Neurophysiol 83: 2708–2722, 2000 [DOI] [PubMed] [Google Scholar]
- Engel AK, Konig P, Kreiter AK, Schillen TB, Singer W. Temporal coding in the visual cortex: new vistas on integration in the nervous system. Trends Neurosci 15: 218–226, 1992 [DOI] [PubMed] [Google Scholar]
- Escabí MA, Schreiner CE. Nonlinear spectrotemporal sound analysis by neurons in the auditory midbrain. J Neurosci 22: 4114–4131, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC, Tsay IA, Raad M, Long JD, Dan Y. Contribution of individual spikes in burst-induced long-term synaptic modification. J Neurophysiol 95: 1620–1629, 2006 [DOI] [PubMed] [Google Scholar]
- Hammond P, MacKay DM. Differential responsiveness of simple and complex cells in cat striate cortex to visual texture. Exp Brain Res 30: 275–296, 1977 [DOI] [PubMed] [Google Scholar]
- Hasenstaub A, Sachdev RN, McCormick DA. State changes rapidly modulate cortical neuronal responsiveness. J Neurosci 27: 9607–9622, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO. The Organization of Behavior: A Neuropsychological Theory. New York: Wiley, 1949 [Google Scholar]
- Imaizumi K, Priebe NJ, Sharpee TO, Cheung SW, Schreiner CE. Encoding of temporal information by timing, rate, and place in cat auditory cortex. PLos One 5: e11531, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izhikevich EM, Desai NS, Walcott EC, Hoppensteadt FC. Bursts as a unit of neural information: selective communication via resonance. Trends Neurosci 26: 161–167, 2003 [DOI] [PubMed] [Google Scholar]
- Johnson DH, Kiang NY. Analysis of discharges recorded simultaneously from pairs of auditory nerve fibers. Biophys J 16: 719–734, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimpo RR, Theunissen FE, Doupe AJ. Propagation of correlated activity through multiple stages of a neural circuit. J Neurosci 23: 5750–5761, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DJ, Depireux DA, Simon JZ, Shamma SA. Robust spectrotemporal reverse correlation for the auditory system: optimizing stimulus design. J Comput Neurosci 9: 85–111, 2000 [DOI] [PubMed] [Google Scholar]
- Koch C, Rapp M, Segev I. A brief history of time (constants). Cereb Cortex 6: 93–101, 1996 [DOI] [PubMed] [Google Scholar]
- Krahe R, Gabbiani F. Burst firing in sensory systems. Nat Rev Neurosci 5: 13–23, 2004 [DOI] [PubMed] [Google Scholar]
- Lewicki MS. Bayesian modeling and classification of neural signals. Neural Computation 6: 1005–1030, 1994 [Google Scholar]
- Middlebrooks JC, Clock AE, Xu L, Green DM. A panoramic code for sound location by cortical neurons. Science 264: 842–844, 1994 [DOI] [PubMed] [Google Scholar]
- Miller LM, Escabí MA, Schreiner CE. Feature selectivity and interneuronal cooperation in the thalamocortical system. J Neurosci 21: 8136–8144, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LM, Schreiner CE. Stimulus-based state control in the thalamocortical system. J Neurosci 20: 7011–7016, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald AM, Doiron B. Maler Interval coding L. I. Burst interspike intervals as indicators of stimulus intensity. J Neurophysiol 97: 2731–2743, 2007 [DOI] [PubMed] [Google Scholar]
- Pike FG, Meredith RM, Olding AW, Paulsen O. Rapid report: postsynaptic bursting is essential for “Hebbian” induction of associative long-term potentiation at excitatory synapses in rat hippocampus. J Physiol 518: 571–576, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathbun DL, Alitto HJ, Weyand TG, Usrey WM. Interspike interval analysis of retinal ganglion cell receptive fields. J Neurophysiol 98: 911–919, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich DS, Mechler F, Purpura KP, Victor JD. Interspike intervals, receptive fields, and information encoding in primary visual cortex. J Neurosci 20: 1964–1974, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes AD. Synchrony-dependent propagation of firing rate in iteratively constructed networks in vitro. Nat Neurosci 6: 593–599, 2003 [DOI] [PubMed] [Google Scholar]
- Richmond BJ, Optican LM, Podell M, Spitzer H. Temporal encoding of two-dimensional patterns by single units in primate inferior temporal cortex. I. Response characteristics. J Neurophysiol 57: 132–146, 1987 [DOI] [PubMed] [Google Scholar]
- Schwartz O, Pillow JW, Rust NC, Simoncelli EP. Spike-triggered neural characterization. J Vis 6: 484–507, 2006 [DOI] [PubMed] [Google Scholar]
- Sharpee T, Rust NC, Bialek W. Analyzing neural responses to natural signals: maximally informative dimensions. Neural Comput 16: 223–250, 2004 [DOI] [PubMed] [Google Scholar]
- Strong SP, Koberle R, van Steveninck RR, Bialek W. Entropy and information in neural spike trains. Phys Rev Lett 80: 197–200, 1998 [Google Scholar]
- Theunissen FE, Sen K, Doupe AJ. Spectral-temporal receptive fields of nonlinear auditory neurons obtained using natural sounds. J Neurosci 20: 2315–2331, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usrey WM, Alonso JM, Reid RC. Synaptic interactions between thalamic inputs to simple cells in cat visual cortex. J Neurosci 20: 5461–5467, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Anderson CH, Felleman DJ. Information processing in the primate visual system: an integrated systems perspective. Science 255: 419–423, 1992 [DOI] [PubMed] [Google Scholar]
- Watson BO, MacLean JN, Yuste R. UP states protect ongoing cortical activity from thalamic inputs. PLos One 3: e3971, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson NR, Bodnar DA, Skovira JF, Land BR. Processing of auditory midbrain interspike intervals by model neurons. J Comput Neurosci 10: 151–172, 2001 [DOI] [PubMed] [Google Scholar]
- Yang Y, DeWeese MR, Otazu GH, Zador AM. Millisecond-scale differences in neural activity in auditory cortex can drive decisions. Nat Neurosci 11: 1262–1263, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Zador AM. Differential sensitivity of different sensory cortices to behaviorally relevant timing differences (Abstract). Front Neurosci Conference Abstract: Computational and Systems Neuroscience 2010, 2010 [Google Scholar]