Abstract
Neocortex network activity changes from a deactivated state during quiescence to an activated state during arousal and vigilance. In urethane-anesthetized rats, cortical activation is readily produced by either stimulating the brainstem reticular formation or by application of cholinergic agonists into the thalamus. We studied the effects of cortical activation on spontaneous activity and sensory responses in the barrel cortex. Cortical activation leads to a suppression of low-frequency sensory responses and to a reduction in their variability due to the abolishment of up and down membrane potential fluctuations in cortical cells. Overall, sensory responses become sharper and more reliable during cortical activation.
Keywords: somatosensory, cholinergic and noradrenergic thalamic stimulation, desynchronized, arousal, whisker
the spontaneous network activity of the neocortex undergoes significant changes during different behavioral states (Steriade et al. 1993; Vanderwolf 1988). Cortical deactivation consists of large-amplitude slow rhythms that are highly synchronized among neuronal populations and occur during states of drowsiness, slow-wave sleep, and surgical anesthesia. Cortical activation consists of low amplitude fast rhythms that appear to be asynchronous between neuronal populations and occur during arousal, vigilance, and paradoxical sleep.
Widespread forebrain activation is produced in anesthetized animals by electrically stimulating the brainstem reticular formation (BRF) (Castro-Alamancos and Oldford 2002; Moruzzi and Magoun 1949). A more selective somatosensory (barrel) cortex activation is produced by application of cholinergic agonists into the somatosensory thalamus, while cortical deactivation results from the application of noradrenergic agonists into the somatosensory thalamus (Hirata and Castro-Alamancos 2010). Both BRF stimulation and cholinergic thalamic stimulation enhance the firing rate of ventroposterior medial (VPM) thalamocortical cells with little or no effect on principal trigeminal complex (Pr5) cells (Aguilar and Castro-Alamancos 2005; Castro-Alamancos and Oldford 2002; Hirata et al. 2006), and this increased firing directly leads to cortical activation (Hirata and Castro-Alamancos 2010). The less selectivity of BRF stimulation implies that it may also produce cortical activation through other means in addition to increased thalamocortical firing, such as by activating the basal forebrain (Metherate et al. 1992).
An important question is how cortical deactivation and activation states affect sensory responses in the barrel cortex (Castro-Alamancos 2009; 2004b). Cortical neurons respond maximally to deflection of a principal whisker (PW) and may respond more weakly to deflection of several adjacent whiskers (AWs) (Armstrong-James and Fox 1987; Simons 1978). Simultaneous (multiwhisker) stimulation of the PW and the AWs produces the strongest responses in the barrel cortex (Hirata and Castro-Alamancos 2008). In the present study, we investigated the effects of cortical activation caused by BRF stimulation or cholinergic thalamic stimulation on field potential (FP), single-unit, and subthreshold (intracellular) sensory responses in the barrel cortex. We found that a main effect of cortical activation is to decrease the variability of sensory responses.
METHODS
Surgery.
Spague-Dawley rats (male, 300–350 g) were used in this study and cared for in accordance with National Institutes of Health Guidelines for Laboratory Animal Welfare. All experiments were approved by the Drexel University Institutional Animal Care and Use Committee. Rats were anesthetized with urethane (1.5 g/kg ip) and placed in a stereotaxic frame. All skin incisions and frame contacts with the skin were injected with lidocaine (2%). A unilateral craniotomy extended over the parietal cortex. Small incisions were made in the dura as necessary. Body temperature was automatically maintained constant with a heating pad at 37°C. The level of anesthesia was monitored with FP recordings and limb-withdrawal reflexes and kept constant at a control state using supplemental doses of urethane. The control state consists of large-amplitude slow oscillatory cortical FP activity (with predominance of 0.1–4 Hz), absence of whisker movements, and pinch withdrawal reflex.
Electrophysiology.
In every experiment, a tungsten electrode was lowered into the depth of the barrel cortex (0.6–1 mm) contralateral to the stimulated whiskers to record FP and multiunit activity. In addition, a single-unit (extracellular) or an intracellular electrode was lowered adjacent to the FP electrode (<300 μm horizontally) to record from cells located in layers 2/3 (200–700 μm in depth) and 4 (700–950 μm). To conduct single-unit recordings in the barrel cortex, a high impedance (5–30 MΩ) glass electrode filled with artificial cerebrospinal fluid (aCSF) was used. These electrodes yield highly isolated single units; usually the spike from only one cell is present with a high signal-to-noise ratio. The single units included in this study were all regular spiking (RS) based on spike width, as previously described (Hirata and Castro-Alamancos 2008).
To conduct intracellular recordings in the barrel cortex, a high-impedance (80–120 MΩ) sharp electrode filled with K-acetate (2 M) was lowered into the vicinity of the FP electrode. All intracellular recordings included in the study had overshooting action potentials and a stable membrane potential (Vm) for >30 min. Intracellular recordings were usually done without any constant current (DC = 0 nA) or with a small amount of constant negative current (DC = −0.2 nA). Current pulses used to determine intrinsic firing and input resistance were 500 ms in duration delivered every 5–10 s. Input resistance was measured using negative current pulses (−0.3 nA). Continuous periods of Vm recordings, excluding brief periods of current pulses or afferent stimulation, lasting 5–10 min in each state, were used to derive distributions of counts at each Vm (1-mV bin), which indicate the amount of time that the neuron spends at each Vm. These distributions were further analyzed by measuring the kurtosis or peakedness of the distribution and obtaining the peak Vm value by fitting each distribution with a nonlinear Gaussain, as described previously (Hirata and Castro-Alamancos 2010).
BRF stimulation.
The method used to produce forebrain activation in the anesthetized rat was to stimulate the BRF in the area of the laterodorsal tegmentum, as described previously (Castro-Alamancos and Oldford 2002). BRF stimulation was delivered using a bipolar stimulating electrode (200-μm diameter concentric) placed contralateral to the stimulated whiskers. The coordinates for BRF stimulation were as follows: posterior, 9; lateral, 0.7; depth, 5–6. These coordinates were slightly adjusted so that high-frequency stimulation (100 Hz for 1 s) produced robust activation consisting of a transformation of the cortical FP activity from a large-amplitude slow activity to a small-amplitude fast activity, without whisker motion. In each experiment, the BRF electrode was placed in the indicated coordinates and then adjusted vertically within the indicated range (5–6 mm) while BRF stimulation was applied (range of 140–200 μA), until a location was found that produced a minimum suppression (>50%) of the low-frequency (0.1–10 Hz) fast Fourier transform power spectrum measured in the FP recording during the first 2 s after BRF stimulation.
Microdialysis.
To apply drugs into the somatosensory thalamus, a microdialysis cannula (250-μm diameter, 2-mm long membrane) was placed around the following coordinates: posterior = 3, lateral = 2–3, depth = 4–6, as previously described (Hirata et al. 2006). The cannula entered into the brain at an angle (∼30°) from the midline. aCSF was continuously infused through the probe at 2–4 μl/min. aCSF contained the following (in mM): 126 NaCl, 3 KCl, 1.25 NaH2PO4, 26 NaHCO3, 1.3 MgSO4 7H2O, 10 dextrose, and 1 CaCl2 2H2O. Norepinephrine (NE) and carbachol (CA) were used at 0.5–1 mM and 0.2–0.5 mM, respectively. Under the same experimental conditions used here, these doses were previously shown to significantly affect the spontaneous firing and whisker-evoked responses of thalamic cells (Castro-Alamancos 2002; Hirata et al. 2006) and to concomitantly affect the spontaneous network activity of the barrel cortex (Hirata and Castro-Alamancos 2010). CA is not easily metabolized by cholinesterase and remains active for quite some time after application. Thus we made no effort to washout the effect of CA. Instead, we directly substituted CA with NE in the medium as a way to immediately reverse the effects of CA. This rapidly changes the tonic firing of VPM cells from a high rate during CA to nil during NE (Hirata et al. 2006). Thus, as previously shown, application of CA in somatosensory thalamus increases the firing of VPM neurons and suppresses the firing of thalamic reticular nucleus (nRt) neurons. In contrast, application of NE in somatosensory thalamus increases the firing of nRt neurons and abolishes the firing of VPM neurons.
Based on experience, and a typical exchange of ∼10% (reverse dialysis recovery), the effective doses used during microdialysis are ∼10 times those used during direct application in slices. Based on diffusion experiments using arrays of recording electrodes at different distances from the probe, we have estimated the spread of these drugs to be <1 mm in the horizontal plane away from the membrane (Hirata et al. 2006). This affects most of the somatosensory thalamus (Paxinos and Watson 1982), since it is centered in VPM and spreads anterior into nRt [as shown previously by Hirata et al. (2006)] and posterior into the medial sector of the posterior nucleus of the thalamus. However, other thalamic nuclei, including intralaminar or midline nuclei, are too far to be affected by the diffusion [for discussion, see Hirata and Castro-Alamancos (2010)].
Whisker stimulation.
Sensory stimulation consisted of independently deflecting six individual whiskers using six different whisker stimulators. Once the tungsten electrode was in the barrel cortex, the whiskers were trimmed to a length of ∼15 mm and a hand-held probe was used to identify the PW, the whisker evoking the strongest audible multiunit response. The hand-held mapping was then confirmed by placing a whisker stimulator on the PW and five other stimulators on AWs surrounding the PW. The PW always produced the most robust response, i.e., shortest latency and largest amplitude FP response. The five AWs were selected as those producing the most robust responses following the PW. Each of the selected whiskers was inserted into a glass micropipette (1/0.5 mm outer/inner diameter) that was glued to the membrane of a miniature speaker. Each whisker was inserted into the micropipette for ∼5 mm, leaving ∼10 mm from the end of the micropipette to the skin. Application of a 1-ms square current pulse to the speaker deflected the micropipette and the whiskers inside. The resulting whisker deflection is very low amplitude (∼2°) and very high velocity (∼2,000°/s) stimulus. The whisker stimulators were oriented in the preferred direction to produce the largest response as determined with the hand probe. Each of the six whisker stimulators were driven by counter/timer boards controlled with Labview software (National Instruments). Multiwhisker stimulation consisted in stimulating the PW and the five AWs simultaneously.
During BRF stimulation experiments, we applied in succession multiwhisker and PW trains (10 stimuli at 10 Hz) separated by 10 s (0.1 Hz) for a total of 30 trials each. Thus each control trial lasted 20 s and contained multiwhisker and PW stimulation. Next, we applied the same 30 trials but we included a train of BRF stimulation (1 s, 100 Hz) every 10 s ending 0.5–1 s before the onset of multiwhisker and PW stimulation (0.5 s in most cases). This was followed by another 30 trials of control multiwhisker and PW stimulation. For intracellular recording experiments, we mainly focused on multiwhisker stimulation. The BRF stimulation was set at a relatively low intensity (<200 μA) that produced a smaller activating effect on FP activity than possible with stronger currents. This was important so that many trials could be obtained (n = 30) and so that the effects were readily reversible. Indeed, stronger BRF stimulation was found to significantly augment the effects reported here, but this was impractical because it required longer periods of time to reverse the effects. The effects of BRF stimulation on spontaneous activity were determined by measuring the 500-ms period between the offset of the BRF stimulation and the onset of the whisker stimulation.
During thalamic CA and NE microdialysis experiments, whisker stimulation (10 stimuli at 10 Hz) was delivered according to the following protocols. A single-whisker trial consisted of an initial 2 s without whisker stimulation, during which spontaneous activity was measured, followed by stimulation delivered to each of six whiskers at 2-s intervals (the order of whisker stimulation was randomly selected). The first whisker was stimulated 2 s after the trial began, the second whisker was stimulated 4 s after the trial started, and so on, so that the sixth (last) whisker stimulus was delivered 12 s after the start of the trial. Thus a single trial contained stimuli for all six whiskers and lasted a total of 14 s. A multiwhisker trial lasted 5 s and consisted of an initial 2 s without whisker stimulation, during which spontaneous activity was measured, followed by stimulation delivered simultaneously to the six whiskers. Every trial was repeated at least 30 times to derive poststimulus time histograms (PSTHs) and to average FP and intracellular responses. For intracellular recording experiments, we mainly focused on multiwhisker stimulation.
Throughout the study, low-frequency whisker stimulation refers to the 1st stimulus in the 10-Hz train (delivered at 0.1 Hz), while high-frequency whisker stimulation refers to the 10th stimulus in the 10-Hz train.
Data analysis and population.
If the data were considered normally distributed, according to the Shapiro-Wilk normality test, we used parametric statistics. Otherwise, we used nonparametric tests. In general, normally distributed data was first tested for a significant main effect using the repeated-measures ANOVA followed by multiple comparisons with Tukey's test. Nonparametric comparisons consisted of the Wilcoxon signed ranks test.
There are four types of experiments in this study that involved the following number of animals and intracellular/single-unit/FP recordings: 1) extracellular recordings during BRF stimulation (n = 15 experiments from 7 rats); 2) intracellular recordings during BRF stimulation (n = 13 cells from 8 rats); 3) extracellular recordings during CA and NE thalamic stimulation (n = 13 experiments from 13 rats); and 4) intracellular recordings during CA thalamic stimulation (n = 8 cells from 8 rats). In BRF stimulation experiments, more than one cell was recorded from the same rat.
Histology.
At the end of the experiments, the animals were given an overdose of sodium pentobarbital and either perfused through the heart with saline followed by paraformaldehyde (4%) or the brain was directly extracted and placed in the fixative. The brains were then sectioned in the coronal plane using a vibratome (80–100 μm) and processed for Nissl's staining to confirm the location of the microdialysis probe in the somatosensory thalamus.
RESULTS
Effects of BRF stimulation on spontaneous activity in the barrel cortex: intracellular correlates.
Cholinergic stimulation of the somatosensory thalamus induces activation in the barrel cortex characterized by a shift in FP activity from a large-amplitude slow oscillation pattern to a small amplitude fast activity pattern, which is corresponded at the intracellular level by the abolishment of up and down Vm fluctuations and a concomitant reduction of spontaneous firing rate for most RS cortical cells (Hirata and Castro-Alamancos 2010). In addition, BRF stimulation produces similar effects on FP activity in the barrel cortex and a similar reduction of spontaneous single-unit firing rate for most RS cortical cells (Castro-Alamancos and Oldford 2002), but the intracellular correlates of these effects are not known. Thus we investigated the intracellular correlates of the effects of BRF stimulation on the spontaneous activity of barrel cortex cells.
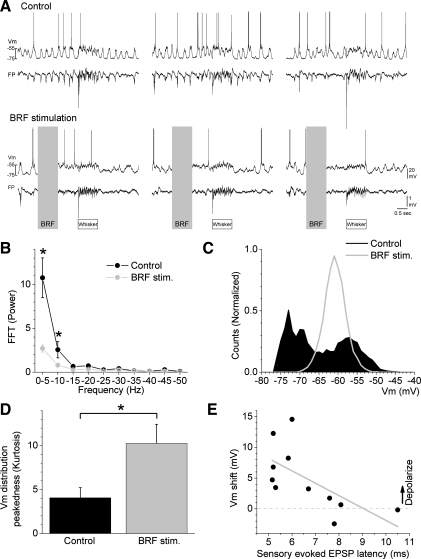
Intracellular recordings were obtained from cells in layers 2–4 that based on intrinsic firing properties fell in the category of RS cells with different degrees of adaptation. Figure 1A shows an example of a layer 4 cell (750 μm in depth) during control conditions and during BRF stimulation. During control conditions, the cell displays large-amplitude fluctuations in Vm and up and down states that are tightly synchronized with the simultaneously recorded FP activity (Fig. 1A); the Vm of this cell fluctuated between −75 and −55 mV, and the up state corresponded to a sharp negativity in the FP. During BRF stimulation, the low-frequency range (0.1–10 Hz) of the FP power spectrum was suppressed (Fig. 1B), the up and down states (large Vm fluctuations) of the cell were abolished, and the Vm settled around −62 mV (Fig. 1C). Thus the Vm distribution became much sharper resulting in increased peakedness (kurtosis).
Fig. 1.
Effect of brainstem reticular formation (BRF) stimulation on spontaneous intracellular activity in barrel cortex. A: intracellular membrane potential (Vm) and field potential (FP) traces recorded during control and during BRF stimulation. Top traces: multiwhisker evoked responses (10 stimuli at 10 Hz; whisker) during control. Bottom traces: multiwhisker evoked responses starting 1 s after BRF stimulation (1 s at 100 Hz). All measurements of spontaneous activity shown in B–E were taken during the first 0.5 s after BRF stimulation. B: group data showing fast Fourier transform (FFT) power spectrum of FP activity during control and BRF stimulation (*P < 0.05 control vs. BRF stimulation). C: plot of Vm distribution for the cell in A during control and during BRF stimulation. D: peakedness (kurtosis) of the Vm distribution (means ± SE) during control and during BRF stimulation. *P < 0.01. E: Vm peak shift during BRF stimulation as a function of multiwhisker excitatory postsynaptic potential (EPSP) onset latency.
The effect of BRF stimulation was tested in several cells (n = 13) with similar effects. Figure 1, D and E, shows population data of different measures obtained from these cells. First, to estimate the peakedness of the Vm distribution, we measured the kurtosis of the distribution during control and during BRF stimulation. We found a significant increase in kurtosis during BRF stimulation (P < 0.01; Fig. 1D). Second, to measure the shift in the peak Vm, we fitted each Vm distribution with a nonlinear Gaussian function to derive the peak of the distribution and then subtracted the control and BRF stimulation peak values (Fig. 1E). A positive value indicates that the cell tended to depolarize during BRF stimulation, whereas a negative value indicates that the cell tended to hyperpolarize. BRF stimulation significantly depolarized most of the cells tested. Taken together, the mean depolarization was 3.6 ± 1 mV (n = 9; P < 0.01). However, two of the cells showed a small hyperpolarization caused by BRF (3.5 ± 2 mV). If those cells are excluded, then the average depolarization is 5.7 ± 0.5 mV (n = 7; P < 0.01). Thus, although depolarization is not a consistent effect of BRF stimulation among cells, a suppression of Vm fluctuations occurs in all cells activated by BRF stimulation.
We noticed that the depolarizing effect of BRF stimulation depended on the onset latency of the cell to sensory stimulation. The mean onset latency of multiwhisker (sensory)-evoked excitatory postsynaptic potentials (EPSPs) was 6.2 ± 0.3 ms (5.1- to 10.5-ms range; n = 13); cells with the shorter multiwhisker EPSP latencies are likely to be directly contacted by thalamocortical synapses while those with the longer latencies are more likely to receive sensory inputs mostly through other cortical (thalamocortical recipient) cells. We found a significant negative correlation between the multiwhisker EPSP onset latency and the effect of BRF stimulation on Vm (n = 11; r = −0.7; P < 0.01; Fig. 1E), so that the shorter the onset latency the larger the depolarization. This result leads us to suggest that cortical cells that receive direct thalamocortical synapses may be the ones that are more robustly depolarized by BRF stimulation, which makes functional sense because BRF stimulation strongly drives the firing rate of thalamocortical cells in VPM (Castro-Alamancos 2002; Castro-Alamancos and Oldford 2002).
Taken together, these results indicate that BRF stimulation affects the spontaneous activity in the barrel cortex in a way similar to the activation produced by cholinergic thalamic stimulation (Hirata and Castro-Alamancos 2010); both consist of a suppression of up and down Vm fluctuations, and a tendency of the Vm to depolarize from the down state, which appears to be stronger in putative thalamocortical-recipient cells.
Effect of BRF stimulation on sensory responses in the barrel cortex: extracellular correlates.
Previous work (Castro-Alamancos and Oldford 2002) has shown that barrel cortex FP and single-unit responses evoked by whisker deflections are mostly suppressed by BRF stimulation. Here, we compared the effect of BRF stimulation on multiwhisker (6 whisker stimulated simultaneously) vs. single-whisker responses (evoked by the PW alone). In the barrel cortex, multiwhisker responses are significantly stronger than PW responses (Hirata and Castro-Alamancos 2008), but the possible differential effects of BRF stimulation on these distinct responses is unknown.
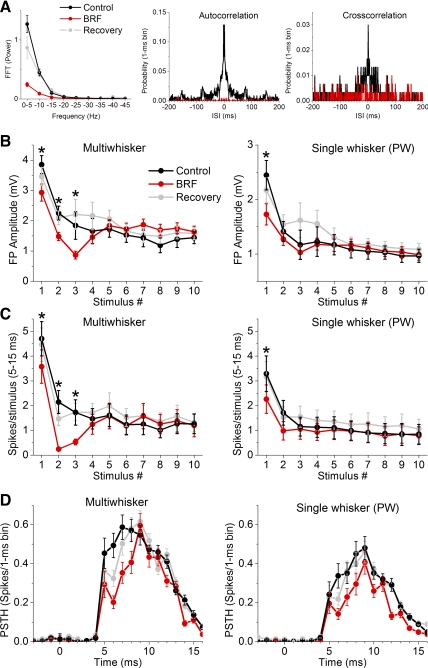
FP and single-unit recordings were obtained from two electrodes close-by in the barrel cortex (see methods). The effect of BRF stimulation was tested on spontaneous activity and on multiwhisker and PW responses. In these experiments, BRF stimulation produced a robust activating effect on spontaneous FP activity that consisted in a significant suppression of low-frequency activity (0.1–10 Hz; P < 0.01; n = 15) in the fast Fourier transform power spectrum (Fig. 2A), with no significant effect on higher frequencies (10–50 Hz; not significant). In addition, the mean spontaneous firing of most cells was significantly suppressed by BRF stimulation (0.92 ± 0.2 vs. 0.26 ± 0.08 Hz, control vs. BRF; n = 15), although some cells increased firing. This was accompanied by a sharp suppression of high-frequency firing in the autocorrelation function (Fig. 2A), and a suppression of synchronous firing between the two cortical electrodes in the cross-correlation function (Fig. 2A) in a sample experiment where this was tested. Thus, as previously described (Moruzzi and Magoun 1949; Steriade et al. 1993), BRF activation transforms spontaneous barrel cortex activity from a synchronous oscillatory state to an asynchronous nonoscillatory state.
Fig. 2.
Effect of BRF stimulation on FP and single-unit sensory responses. A: group data showing FFT power spectrum of FP activity during control and BRF stimulation for the experiments in B. Also shown are autocorrelation and cross-correlation functions from sample experiments during control and BRF stimulation. B: negative peak FP amplitude for barrel cortex responses, measured within a 5- to 30-ms window poststimulus during control, BRF stimulation, and recovery after BRF stimulation. The x-axis shows the responses evoked by each of the 10 stimuli in the 10-Hz train for multiwhisker and principal whisker (PW) stimulation. *P < 0.05, significant differences between control and BRF stimulation for each stimulus in the train. C: single-unit barrel cortex responses, measured during a 5- to 15-ms window poststimulus, evoked during control, BRF stimulation, and recovery after BRF stimulation. The x-axis shows the responses evoked by each of the 10 stimuli in the 10-Hz train for multiwhisker and PW stimulation. *P < 0.05, significant differences between control and BRF stimulation for each stimulus in the train. D: overlaid population poststimulus time histograms (PSTHs; 1-ms bins ± SE) of low-frequency multiwhisker and PW responses evoked during control and BRF stimulation.
During control (cortical deactivation) conditions, the FP peak amplitude and the spikes per stimulus (spike probability; 5–15 ms poststimulus) evoked by multiwhisker (Fig. 2, B–D, left) or PW stimulation (Fig. 2, B–D, right) at 10 Hz are significantly depressed compared with the low-frequency (first) stimulus in the train. Thus the first response in the train is unadapted while the last responses in the train are adapted. In between unadapted and adapted responses are a few (∼3) transition responses before a steady state is reached [see Hirata et al. (2009)]. The mean effects of BRF stimulation on sensory responses measured using FP and unit activity were similar. The FP peak amplitude (Fig. 2B) and the spike probability (Fig. 2C) of responses evoked by multiwhisker and PW stimulation were significantly suppressed by BRF activation, but this occurred only for the unadapted (first) and transition responses in the 10-Hz train and not for the subsequent adapted (high-frequency) responses (Fig. 2C). Thus BRF activation significantly suppressed both the unadapted and transition responses, with little effect on adapted responses. Interestingly, the suppression caused by BRF activation was particularly strong for the transition stimuli during multiwhisker stimulation (i.e., second or third response in the 10-Hz train).
Figure 2D shows the effect of BRF stimulation on each 1-ms bin of the PSTHs evoked by low-frequency stimulation (first stimulus in a 10-Hz train). During multiwhisker stimulation, the first bin (shortest latency) with a significant response (5 ms) and the following three bins (i.e., 5–8 ms) were significantly suppressed by BRF activation (P < 0.05; n = 15), while during PW responses the first bin with a significant response (5 ms) was not significantly suppressed by BRF activation. These results indicate that BRF stimulation more strongly suppresses the shortest latency responses during multiwhisker stimulation than during PW stimulation. The average effect of BRF stimulation on unadapted sensory responses is usually suppression (Castro-Alamancos 2004a; Castro-Alamancos and Oldford 2002), but there was some variability among the recorded cells. Here we found that the unadapted responses of about one-third of the cells (4 out of 15) displayed little change (<15%) in spike probability during BRF stimulation.
Effect of BRF stimulation on sensory responses in the barrel cortex: intracellular correlates.
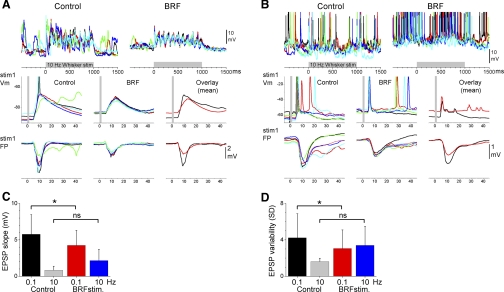
We next studied the effects of BRF stimulation on barrel cortex intracellular responses evoked by 10-Hz trains (10 stimuli) of multiwhisker stimuli (6 whiskers stimulated simultaneously including the PW). Figure 3 shows examples of the effect of BRF stimulation on two different cells recorded from the barrel cortex. Figure 3, left, middle, right, shows responses evoked by five stimulus trials (different colors) during control (left) and BRF stimulation (right or middle). The bottom displays close-ups of the response to the first stimulus and simultaneously recorded FP responses in the barrel cortex, and the average responses during control (black trace) and BRF stimulation (red trace) are overlaid at the right. Figure 3A shows a typical cell in which BRF stimulation suppressed the spontaneous up and down Vm fluctuations, slightly depolarized the Vm, and suppressed the evoked EPSP. Figure 3B shows a cell that was strongly driven by the whisker stimulation during control conditions so that usually several action potentials were triggered within the first 20 ms poststimulus. During BRF stimulation, the cells increased spontaneous firing rate but the responses to the whisker stimulation were reduced to a single short-latency (>20 ms) spike followed later by spontaneous firing, which made the response much sharper. Thus, despite the robust depolarization and increase in spontaneous firing, the evoked response became sharper (limited to short-latency evoked spikes) and was suppressed in the number of evoked spikes.
Fig. 3.
Effect of BRF stimulation on intracellular multiwhisker responses. A and B: examples from 2 different cells showing the effect of BRF stimulation on intracellular potentials evoked by a 10-Hz multiwhisker stimulus train (10 stimuli at 10 Hz). Five trials are overlaid per panel, except in the panel that overlays mean traces. Before averaging the traces were filtered using a median filter to attenuate action potentials amplitudes. Bottom: simultaneously recorded FP responses. C: population data showing the effect of BRF stimulation on the slope of short-latency EPSPs evoked by low-frequency (1st stimulus in the 10-Hz train delivered at 0.1 Hz) and high-frequency (10th stimulus in the 10-Hz train) multiwhisker stimulation. D: population data showing the effect of BRF stimulation on short-latency EPSP trial-by-trial variability calculated by comparing the SD of the slope measurements from each cell in C [*P < 0.05; not significant (ns)].
There are two main effects of BRF stimulation on the rising slope of EPSPs evoked by low-frequency (first stimulus in the 10-Hz train) multiwhisker stimulation. Most cells show a reduction of EPSP slope while a few cells are unaffected, but none of the cells showed an increase in slope. Thus BRF stimulation produced a significant reduction of the short-latency (5–10 ms; Fig. 3C) EPSP rising slope for low-frequency (P < 0.05; n = 13) multiwhisker responses, while the EPSP slope of high-frequency (10th stimulus in the 10-Hz train) multiwhisker responses was not significantly affected (P = 0.5; n = 13). The reduction in slope did not appear to be a simple consequence of a reduction in driving force caused by Vm depolarization, because cells that were not significantly depolarized by BRF stimulation could also show a reduction in EPSP rising slope. We did not measure the amplitude of inhibitory postsynaptic potentials that followed the sensory-evoked short-latency EPSPs because this longer latency region of the response could be contaminated by evoked up states that were highly variable between cells and experiments (note that we did not use ketamine anesthesia, which makes up states more constant). As in slices (Rigas and Castro-Alamancos 2007), the long-latency up states are clearly separable from the short-latency EPSPs we measured here but not from the inhibitory postsynaptic potentials.
We also found that trial-by-trial EPSP variability was much larger during control than during activation produced by BRF stimulation. This may be due to the presence of slow oscillations (up and down Vm fluctuations) during control and their abolishment during BRF activation. Thus BRF stimulation significantly reduced the trial-by-trial variability (SD) of the EPSP slope for low-frequency (P < 0.05; n = 13; Fig. 3D) but not for high-frequency (P = 0.5; n = 13) responses. Similarly, the simultaneously recorded FP peak amplitude for low-frequency (P < 0.01; n = 13) and high-frequency (P < 0.01; n = 13) responses were both significantly reduced by BRF stimulation. Thus sensory-evoked responses are more variable during control than during activation produced by BRF stimulation.
In conclusion, BRF stimulation has the following main effects on spontaneous and sensory responses in the barrel cortex. First, BRF stimulation suppresses the spontaneous low-frequency (0.1–10 Hz) FP activity and the corresponding up and down Vm fluctuations in all cortical cells. Second, BRF stimulation suppresses the peak amplitude of sensory FP responses and this is related to a reduction of the rising slope of sensory-evoked EPSPs for some cells in the barrel cortex. Finally, BRF stimulation makes sensory responses more reliable (less variable).
Effects of cholinergic and noradrenergic thalamic stimulation on sensory responses in the barrel cortex: extracellular correlates.
Based on previous work (Hirata and Castro-Alamancos 2010) and the current study, spontaneous barrel cortex activity is driven to activation by either BRF stimulation or cholinergic thalamic stimulation and to deactivation by noradrenergic thalamic stimulation. Here we tested if the cortical activation and deactivation produced by cholinergic and noradrenergic thalamic stimulation affect single-whisker and multiwhisker sensory responses in the barrel cortex. Single-whisker stimulation was delivered to the center receptive field (PW) or the surround receptive field (5 different AWs; Aw1-Aw5). Multiwhisker stimulation involved simultaneous stimulation of the center and surround receptive field (6 whiskers together, ALL). Whisker stimulation consisted of a train of 10 stimuli delivered at 10 Hz. We obtained FP and unit recordings from two electrodes close-by in the barrel cortex (see methods). All experiments produced data of the effects of cholinergic and noradrenergic thalamic stimulation on FP responses, but we show only the effects of cholinergic thalamic stimulation on single-unit responses because all cells were held stably during this period.
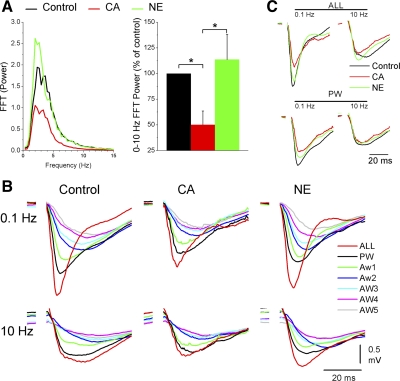
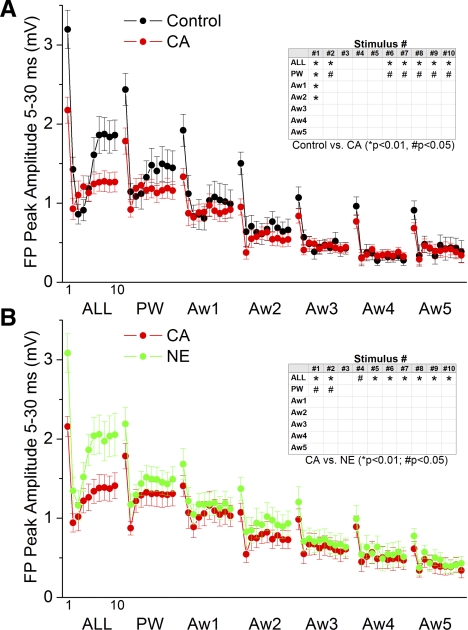
Regarding FP activity, Fig. 4 shows the effects of control, cholinergic thalamic stimulation (CA) and noradrenergic thalamic stimulation (NE) on spontaneous FP activity (Fig. 4A) and whisker-evoked FP responses (Fig. 4, B and C, and Fig. 5, A and B) in the barrel cortex. As previously described (Hirata and Castro-Alamancos 2010), cholinergic thalamic stimulation leads to a robust activating effect in the barrel cortex consisting in a suppression of slow oscillations, while noradrenergic thalamic stimulation leads to a deactivating effect consisting in the restoration of slow oscillations (Fig. 4A). Figure 4B overlays typical FP responses evoked by the different stimuli (ALL, PW, and Aw1-5) during each of the network states, while Fig. 4C overlays responses evoked by low-frequency (first stimulus in the 10-Hz train) and high-frequency (10th stimulus in the 10-Hz train) multiwhisker (ALL) and PW stimuli during the different states. Figure 5, A and B, plots the peak amplitude of whisker-evoked cortical FP responses measured during a 5- to 30-ms time window poststimulus. Cholinergic thalamic stimulation (Fig. 5A) significantly suppressed (P < 0.01; n = 13) low-frequency responses (stimulus #1) evoked by multiwhisker, PW and two AWs (Aw1-2) stimulation, and transition responses (stimulus #2) evoked by multiwhisker and PW stimulation. In addition, cholinergic thalamic stimulation suppressed high-frequency responses (stimulus #7–10) evoked by multiwhisker (P < 0.01) and PW (P < 0.05) stimulation. The effects on sensory responses produced by cholinergic thalamic stimulation were mostly reversed by Noradrenergic thalamic stimulation. Thus, compared with cholinergic thalamic stimulation, noradrenergic thalamic stimulation (Fig. 5B) significantly enhanced (P < 0.01; n = 13) low-frequency responses (stimulus #1) and transition responses (stimulus #2) evoked by multiwhisker and PW stimulation. Noradrenergic thalamic stimulation also enhanced high-frequency responses (stimulus #4–10) evoked by multiwhisker stimulation (P < 0.01).
Fig. 4.
Effect of cholinergic and noradrenergic thalamic stimulation on FP sensory responses. A: FFT power spectrum of FP activity during control and cholinergic (CA) and noradrenergic (NE) thalamic stimulation plotted as a FFT function (left) or integrated between 0–10 Hz (right). B: average FP traces evoked by low-frequency (1st stimulus in the 10-Hz train delivered at 0.1 Hz) or high-frequency (10th stimulus in the 10-Hz train) multiwhisker (ALL) or PW stimulation during control and cholinergic and noradrenergic thalamic stimulation. C: average FP traces as in B, including also single-whisker responses evoked by AWs (Aw1-5).
Fig. 5.
Population data showing the effect of cholinergic and noradrenergic thalamic stimulation on FP sensory responses. A: FP responses, measured during a 5- to 30-ms window poststimulus, evoked during control and during cholinergic thalamic stimulation. The x-axis shows the responses evoked by each of the 10 stimuli in the 10-Hz train. Each block of 10 stimuli correspond (left to right) to multiwhisker stimulation delivered simultaneously to all 6 whiskers (ALL) or single-whisker stimulation of the PW and of each of the 5 AWs (Aw1-Aw2). Inset marks with an asterisk the responses that showed a significant effect as determined with a pair-wise comparison. B: similar to A, but responses during cholinergic thalamic stimulation are compared with those during noradrenergic thalamic stimulation.
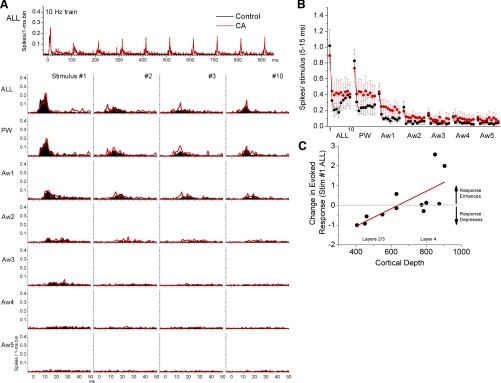
Regarding single-unit activity, Fig. 6 shows the effects of control and cholinergic thalamic stimulation on single-unit responses in the barrel cortex. Despite the robust and consistent effects on FP responses, the effects on single-unit responses were much more variable. Figure 6A overlays PSTHs for multiwhisker and single-whisker stimulation (ALL, PW, and Aw1-5) evoked by the first, transition (stimulus #2–3), and last stimulus in the 10-Hz train. Figure 6B shows the spike per stimulus (5–15 ms) of whisker-evoked responses in the barrel cortex during control and cholinergic thalamic stimulation. There were no significant differences in evoked responses between control and cholinergic thalamic stimulation. The lack of significant differences may have been due to the large variability in cholinergic thalamic stimulation effects between different cortical cells. Figure 6C shows this variability by plotting the effect of cholinergic thalamic stimulation on multiwhisker responses evoked by low-frequency stimulation (stimulus #1). The responses of cells located around layer 4 (600–900 μm in depth) were either unaffected or enhanced by cholinergic thalamic stimulation, while the responses of cells located in the upper layers (200–600 μm) tended to be suppressed by cholinergic thalamic stimulation. Thus there was a significant correlation between cortical depth and the effect of cholinergic thalamic stimulation on low-frequency multiwhisker responses (r = 0.47; P < 0.01; Fig. 6C). Even if the two cells located in layer 4 that show the largest response enhancement (Fig. 6C) are removed, the correlation is still significant (r = 0.46; P < 0.01). We next considered separately upper layer cells (n = 6) and found a significant suppression (P < 0.05) caused by cholinergic thalamic stimulation when these cells are considered as a group, while the remaining layer 4 cells show no significant change when considered as a group (P = 0.3).
Fig. 6.
Effect of cholinergic thalamic stimulation on single-unit sensory responses. A: population PSTHs show responses evoked by simultaneous multiwhisker (ALL) or single whisker stimulation of the PW and 5 AWs (Aw1-Aw5) during control (black traces) and during cholinergic thalamic stimulation (CA; red traces). Whisker stimulation consisted of 10 stimuli (stimulus #1 through #10) delivered at 10 Hz. At the bottom, only the responses to stimulus #1, #2, #3, and #10 of the 10-Hz train are shown. B: single-unit barrel cortex responses, measured during a 5- to 15-ms window poststimulus, evoked during control (black symbols) and cholinergic thalamic stimulation (CA; red symbols). The x-axis shows the responses evoked by each of the 10 stimuli in the 10-Hz train. Each block of 10 stimuli correspond (left to right) to multiwhisker stimulation delivered simultaneously to all 6 whiskers (ALL), single-whisker stimulation of the PW and of each of the 5 AWs (Aw1-Aw2). Pair-wise comparisons (between the same responses) revealed that there were no significant differences between control and CA (hence the inset shown in Fig. 5 is omitted). C: plot of cortical depth of regular spiking cells (x-axis) as a function of the effect that cholinergic thalamic stimulation had on low-frequency multiwhisker responses (y-axis). The y-axis shows the ratio between CA/control responses (spikes/stimulus for the 5- to 15-ms response window) taken from the first stimulus in the 10-Hz train of multiwhisker (ALL) stimulation. So that zero means that the response was not changed, a value of +1 means that the CA response increased by 100%, while a value of −1 means that the CA response decreased by 100% compared with control (i.e., was nil). A linear regression fitting the data is plotted (red). Low-frequency multiwhisker responses of cells located around layer 4 (600–900 μm) tend to not change or enhance during cholinergic thalamic stimulation, while the responses of cells in upper layers (600–200 μm) tend to depress.
In conclusion, cortical activation driven by cholinergic thalamic stimulation produces a consistent suppression of FP sensory responses and a highly variable effect on individual cortical cells, which depends greatly on the location of the cell in the cortical network. This suggests that the main effect of cortical activation is on subthreshold sensory responses, and this is tested ahead with intracellular recordings.
Effects of cholinergic and noradrenergic thalamic stimulation on sensory responses in the barrel cortex: intracellular correlates.
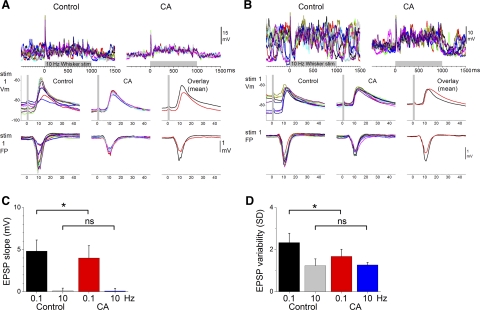
Next we determined the effects of cholinergic thalamic stimulation on subthreshold (intracellular) sensory responses triggered by multiwhisker stimulation at 10 Hz. Figure 7 shows examples from two different cells recorded in the barrel cortex. Figure 7, left, middle, right, shows responses evoked by 10 stimulus trials (different colors) during control (left) and cholinergic thalamic stimulation (right or middle). The bottom displays close-ups of the response to the first stimulus and simultaneously recorded FP responses in the barrel cortex, and the average responses during control (black trace) and cholinergic thalamic stimulation (red trace) are overlaid at the right. Figure 7A shows a typical cell in which cholinergic thalamic stimulation suppressed the spontaneous up and down Vm fluctuations and reduced the evoked EPSP. Figure 7B shows another cell in which cholinergic thalamic stimulation produced similar effects on up and down Vm fluctuations, but the EPSP was only slightly affected. As per BRF stimulation, there are two main effects of cholinergic thalamic stimulation on the rising slope of EPSPs evoked by low-frequency multiwhisker stimulation. Some cells show a reduction of EPSP slope while other cells are unaffected, but none of the cells showed an increase in slope. Taking all cells together, cholinergic thalamic stimulation produced a significant reduction of the short-latency (5–10 ms; Fig. 7C) EPSP rising slope of low-frequency (P < 0.05; n = 8) multiwhisker responses, while the EPSP slope of high-frequency responses was not significantly affected (P = 0.5).
Fig. 7.
Effect of cholinergic thalamic stimulation on intracellular multiwhisker responses. A and B: examples from 2 different cells showing the effect of cholinergic thalamic stimulation on intracellular potentials evoked by a 10-Hz multiwhisker stimulus train (10 stimuli at 10 Hz). Ten trials are overlaid per panel, except in the panel that overlays mean traces. Before averaging, the traces were filtered using a median filter to attenuate action potentials amplitudes. Bottom: simultaneously recorded FP responses. C: population data showing the effect of cholinergic thalamic stimulation on the slope of short-latency EPSPs evoked by low-frequency (1st stimulus in the 10-Hz train delivered at 0.1 Hz) and high-frequency (10th stimulus in the 10-Hz train) multiwhisker stimulation. D: population data showing the effect of cholinergic thalamic stimulation on short-latency EPSP trial-by-trial variability calculated by comparing the SD of the slope measurements from each cell in C (*P < 0.05).
Similar to the effect of BRF, we found that trial-by-trial EPSP variability was larger during control than during activation produced by cholinergic thalamic stimulation. As already mentioned, this appears to be related to the presence of slow oscillations (up and down states; Vm fluctuations) during control and their abolishment during cholinergic thalamic stimulation (Hirata and Castro-Alamancos 2010). Thus cholinergic thalamic stimulation significantly reduced the trial-by-trial variability (SD) of the EPSP slope for low-frequency (P < 0.05; n = 8; Fig. 7D) but not for high-frequency (P = 0.3) responses. Moreover, the simultaneously recorded FP peak amplitude for low-frequency (P < 0.01) and high-frequency (P < 0.05) responses were both significantly reduced by BRF stimulation. In the barrel cortex, sensory responses are more variable during control than during cortical activation produced by either BRF stimulation or cholinergic thalamic stimulation. Thus a main effect of cortical activation is a reduction in the variability of the evoked EPSPs.
DISCUSSION
Effects of cortical activation on spontaneous barrel cortex activity.
Cortical activation caused by either cholinergic thalamic stimulation (Hirata and Castro-Alamancos 2010) or BRF stimulation (this study) is associated with a reduction of spontaneous firing rate for most RS cortical cells, which is related to the abolishment of up and down Vm fluctuations. However, during activation, the Vm of cortical cells does not simply drift to the Vm of the up state, or become even more depolarized towards the reversal potential of excitatory inputs (∼0 mV), as would be expected if only thalamocortical (excitatory) synapses were being stimulated in neocortex. Instead, we previously found that cortical activation caused by cholinergic thalamic stimulation is associated with increased firing of fast spiking (FS) inhibitory neurons in layer 4 (Hirata and Castro-Alamancos 2010), likely because FS cells are tightly coupled to thalamocortical cells (Swadlow 1995). This would ensure that a balance between excitation and inhibition is reached to avoid the runaway excitation that would occur otherwise in the barrel cortex (Okun and Lampl 2008; Shu et al. 2003). Thus, during activation, the excitatory drive from thalamocortical firing appears to be effectively countered by the inhibitory drive from FS cells.
Both BRF stimulation and cholinergic thalamic stimulation enhance the spontaneous firing and sensory responsiveness of thalamocortical cells, while noradrenergic thalamic stimulation suppresses the spontaneous firing of thalamocortical cells but still enhances the sensory responsiveness of thalamocortical cells (Castro-Alamancos 2002; Hirata et al. 2006). Thus neuromodulation within the thalamus establishes two distinct thalamocortical modes, both of which are highly effective at relaying sensory inputs to the cortex but that differ in spontaneous activity (noise) levels. The cholinergic thalamocortical mode, which is also produced by BRF stimulation, has abundant presynaptic noise (thalamocortical cell activity) but little postsynaptic noise (Vm fluctuations are abolished during cortical activation). The noradrenergic thalamocortical mode, which is in part similar to surgical anesthesia, has nil presynaptic noise but plenty of postsynaptic noise (Vm fluctuations are present during cortical deactivation).
Effects of cortical activation on short-latency sensory responses in the barrel cortex.
We found three main effects of cortical activation on sensory responses that depended on the method used. First, BRF stimulation suppressed low-frequency FP and unit responses, and the suppression was stronger for the stronger responses caused by multiwhisker stimulation (compared to PW responses). Second, like BRF stimulation, cholinergic thalamic stimulation suppressed low-frequency FP responses, but there was less effect on cortical single units, which tended to be suppressed only if located in upper layers (i.e., not in layer 4). Finally, at the intracellular level, both BRF stimulation and cholinergic thalamic stimulation tend to reduce the rising slope of the short-latency EPSP but also reduce the trial-by-trial variability of the EPSPs so that during activation responses are more reliable.
Regarding the suppression of low-frequency sensory responses, this has been explained by the increased firing of thalamocortical cells during activation, which leads to the depression of thalamocortical synapses (Boudreau and Ferster 2005; Castro-Alamancos 2004a; Castro-Alamancos and Oldford 2002; Stoelzel et al. 2009; Swadlow and Gusev 2001). In addition, depolarization in cortical cells caused by up states has been shown to have complex effects on sensory responses. Up states increase the responsiveness of barrel cortex cells in vivo to artificial EPSPs, but EPSPs driven by sensory stimuli recruit less excitatory conductance in cortical cells during up states (Hasenstaub et al. 2007). Likewise, the cortical activation produced by cholinergic thalamic stimulation increases the excitability of cortical cells to current pulses (Hirata and Castro-Alamancos 2010), but as shown here sensory responses tend to be suppressed. Using thalamocortical slices, we recently found that depolarization during up states makes EPSPs reach threshold faster and more successfully (Rigas and Castro-Alamancos 2009). Even though cortical up states may not be completely equal to cortical activation (e.g., thalamocortical relay may not be as efficacious during up states as during activation), the previous findings may explain the variable effects of cortical activation caused by cholinergic thalamic stimulation on single-unit sensory responses. Since cells tend to depolarize in layer 4 during this state, these cells may tend to increase their responses as a consequence of depolarization, while cells in upper layers may be less prone to this enhancing effect because they do not tend to depolarize during cholinergic thalamic stimulation. Moreover, there tends to be a significant difference between activation caused by BRF stimulation and by cholinergic thalamic stimulation, so that the former usually produces stronger sensory suppression. This may be due to the fact that BRF stimulation (Castro-Alamancos and Oldford 2002) leads to higher firing rates in thalamocortical cells (10–40 vs. 2–10 Hz) than cholinergic thalamic stimulation (Hirata et al. 2006). Future work must also explore putative differences between the effects that cortical activation produced by cholinergic thalamic stimulation and BRF stimulation may have on intrinsic cortical cell properties.
The effects of cortical activation on sensory responses are more prominent for low-frequency responses while high-frequency (adapted) responses tend to be unaffected likely because adaptation per se causes the same sharpening and suppressing effects as cortical activation (Castro-Alamancos 2004a; Moore 2004; Moore et al. 1999). Cortical activation produced by cholinergic thalamic stimulation appears to be highly functional because primary thalamic nuclei, unlike nonspecific activating systems, have rather restricted cortical projections and therefore are capable of producing area-restricted activation that may be useful for modality-specific selective sensory processing related to selective attention demands (Hirata and Castro-Alamancos 2010). Here we found that cortical activation leads to sharper and more reliable sensory responses as opposed to wider and variable sensory responses during deactivated states.
GRANTS
This work was supported by the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- Aguilar JR, Castro-Alamancos MA. Spatiotemporal gating of sensory inputs in thalamus during quiescent and activated states. J Neurosci 25: 10990–11002, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong-James M, Fox K. Spatiotemporal convergence and divergence in the rat S1 “barrel” cortex. J Comp Neurol 263: 265–281, 1987 [DOI] [PubMed] [Google Scholar]
- Boudreau CE, Ferster D. Short-term depression in thalamocortical synapses of cat primary visual cortex. J Neurosci 25: 7179–7190, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Different temporal processing of sensory inputs in the rat thalamus during quiescent and information processing states in vivo. J Physiol 539: 567–578, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Absence of rapid sensory adaptation in neocortex during information processing states. Neuron 41: 455–464, 2004a [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Dynamics of sensory thalamocortical synaptic networks during information processing states. Progr Neurobiol 74: 213–247, 2004b [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Cortical up and activated states: implications for sensory information processing. Neuroscientist 15: 625–634, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Oldford E. Cortical sensory suppression during arousal is due to the activity-dependent depression of thalamocortical synapses. J Physiol 541: 319–331, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenstaub A, Sachdev RN, McCormick DA. State changes rapidly modulate cortical neuronal responsiveness. J Neurosci 27: 9607–9622, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata A, Aguilar J, Castro-Alamancos MA. Noradrenergic activation amplifies bottom-up and top-down signal-to-noise ratios in sensory thalamus. J Neurosci 26: 4426–4436, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata A, Aguilar J, Castro-Alamancos MA. Influence of subcortical inhibition on barrel cortex receptive fields. J Neurophysiol 102: 437–450, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata A, Castro-Alamancos MA. Cortical transformation of wide-field (multiwhisker) sensory responses. J Neurophysiol 100: 358–370, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata A, Castro-Alamancos MA. Neocortex network activation and deactivation states controlled by the thalamus. J Neurophysiol 103: 1147–1157, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metherate R, Cox CL, Ashe JH. Cellular bases of neocortical activation: modulation of neural oscillations by the nucleus basalis and endogenous acetylcholine. J Neurosci 12: 4701–4711, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CI. Frequency-dependent processing in the vibrissa sensory system. J Neurophysiol 91: 2390–2399, 2004 [DOI] [PubMed] [Google Scholar]
- Moore CI, Nelson SB, Sur M. Dynamics of neuronal processing in rat somatosensory cortex. Trends Neurosci 22: 513–520, 1999 [DOI] [PubMed] [Google Scholar]
- Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol 1: 455–473, 1949 [PubMed] [Google Scholar]
- Okun M, Lampl I. Instantaneous correlation of excitation and inhibition during ongoing and sensory-evoked activities. Nat Neurosci 11: 535–537, 2008 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic, 1982 [DOI] [PubMed] [Google Scholar]
- Rigas P, Castro-Alamancos MA. Thalamocortical up states: differential effects of intrinsic and extrinsic cortical inputs on persistent activity. J Neurosci 27: 4261–4272, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigas P, Castro-Alamancos MA. Impact of persistent cortical activity (up states) on intracortical and thalamocortical synaptic inputs. J Neurophysiol 102: 119–131, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, McCormick DA. Turning on and off recurrent balanced cortical activity. Nature 423: 288–293, 2003 [DOI] [PubMed] [Google Scholar]
- Simons DJ. Response properties of vibrissa units in rat SI somatosensory neocortex. J Neurophysiol 41: 798–820, 1978 [DOI] [PubMed] [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science 262: 679–685, 1993 [DOI] [PubMed] [Google Scholar]
- Stoelzel CR, Bereshpolova Y, Swadlow HA. Stability of thalamocortical synaptic transmission across awake brain states. J Neurosci 29: 6851–6859, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swadlow HA. Influence of VPM afferents on putative inhibitory interneurons in S1 of the awake rabbit: evidence from cross-correlation, microstimulation, and latencies to peripheral sensory stimulation. J Neurophysiol 73: 1584–1599, 1995 [DOI] [PubMed] [Google Scholar]
- Swadlow HA, Gusev AG. The impact of “bursting” thalamic impulses at a neocortical synapse. Nat Neurosci 4: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- Vanderwolf CH. Cerebral activity and behavior: control by central cholinergic and serotonergic systems. Int Rev Neurobiol 30: 225–340, 1988 [DOI] [PubMed] [Google Scholar]