Abstract
Dysfunctions of neuronal and network excitability have emerged as common features in disorders associated with intellectual disabilities, autism, and seizure activity, all common clinical manifestations of Rett syndrome (RTT), a neurodevelopmental disorder caused by loss-of-function mutations in the transcriptional regulator methyl-CpG-binding protein 2 (MeCP2). Here, we evaluated the consequences of Mecp2 mutation on hippocampal network excitability, as well as synapse structure and function using a combination of imaging and electrophysiological approaches in acute slices. Imaging the amplitude and spatiotemporal spread of neuronal depolarizations with voltage-sensitive dyes (VSD) revealed that the CA1 and CA3 regions of hippocampal slices from symptomatic male Mecp2 mutant mice are highly hyperexcitable. However, only the density of docked synaptic vesicles and the rate of release from the readily releasable pool are impaired in Mecp2 mutant mice, while synapse density and morphology are unaffected. The differences in network excitability were not observed in surgically isolated CA1 minislices, and blockade of GABAergic inhibition enhanced VSD signals to the same extent in Mecp2 mutant and wild-type mice, suggesting that network excitability originates in area CA3. Indeed, extracellular multiunit recordings revealed a higher level of spontaneous firing of CA3 pyramidal neurons in slices from symptomatic Mecp2 mutant mice. The neuromodulator adenosine reduced the amplitude and spatiotemporal spread of VSD signals evoked in CA1 of Mecp2 mutant slices to wild-type levels, suggesting its potential use as an anticonvulsant in RTT individuals. The present results suggest that hyperactive CA3 pyramidal neurons contribute to hippocampal dysfunction and possibly to limbic seizures observed in Mecp2 mutant mice and RTT individuals.
Keywords: Rett syndrome, voltage-sensitive dye imaging, FM1–43, multiphoton excitation microscopy, electron microscopy
altered synapse formation during brain development has long been suggested to contribute to deficits in cognitive function and adaptive behavior such as those observed in neurological disorders associated with intellectual disabilities. The X-linked neurodevelopmental disorder Rett syndrome (RTT; MIM 312750) is the leading cause of severe intellectual disabilities in females, affecting 1:10,000–15,000 births worldwide (Neul and Zoghbi 2004). RTT individuals are born healthy and achieve standard developmental milestones until 6–18 mo, when they begin a regression period associated with loss of acquired cognitive, social, and motor skills (Armstrong 1997). Later, RTT individuals develop breathing irregularities, impaired motor abilities, stereotypical behaviors including a characteristic hand wringing, dysfunctions in different forms of communication and social withdrawal, cognitive deficits, and recurrent partial and generalized seizures (Armstrong 1997; Hagberg et al. 1983). Convulsive and silent (i.e., absence) seizures are more severe and frequent in RTT individuals between 7 and 12 yr of age (Steffenburg et al. 2001), but despite a small reduction in their frequency, they significantly impair their quality of life (Glaze et al. 1998; Huppke et al. 2007; Jian et al. 2006).
More than 90% of RTT individuals carry loss-of-function mutations in a single gene located in the Xq28 chromosome (Chahrour and Zoghbi 2007; Percy and Lane 2005), which encodes the DNA-binding protein methyl-CpG-binding protein 2 (MeCP2) (Amir et al. 1999). Originally, MeCP2 was described to bind methylated CpG sites in DNA and repress gene transcription by recruiting corepressor and histone deacetylase complexes (HDACs), thus altering the structure of genomic DNA (Nan et al. 1998). However, recent work has demonstrated that out of all the genes dysregulated in the hypothalamus of both MECP2 overexpressing and Mecp2 knockout mice, the majority (∼85%) was activated in overexpressing mice and downregulated in deficient mice, suggesting that MeCP2 has a broader gene transcription role than originally thought (Chahrour et al. 2008).
Different mouse models of RTT, either lacking Mecp2 (Mecp2tm1.1Bird and Mecp2tm1Tam) or expressing a truncated nonfunctional protein (Mecp2tm1.1Jae and Mecp2308), recapitulate several behavioral features of RTT (Chen et al. 2001; Guy et al. 2001; Pelka et al. 2006; Shahbazian et al. 2002). For clarity, the mouse strains that express a truncated protein will be called “mutant” (i.e., Mecp2tm1.1Jae and Mecp2308) (Chen et al. 2001; Shahbazian et al. 2002), and the ones showing no signs of MeCP2 protein expression will be called “knockouts” (Mecp2tm1.1Bird and Mecp2tm1Tam) (Guy et al. 2001; Pelka et al. 2006). Consistent with the seizures observed in RTT individuals (Steffenburg et al. 2001), Mecp2308 mutant mice displayed repetitive generalized myoclonic jerks associated with high-amplitude bilateral cortical spikes and wave discharges in the electroencephalogram (EEG) (Shahbazian et al. 2002). Mecp2 knockout mice also have seizure episodes with recurrent abnormal EEG discharges in somatosensory cortex (D'Cruz et al. 2010; Pelka et al. 2006). However, electrophysiological recordings from hippocampal and cortical pyramidal neurons in acute slices or primary cultures from Mecp2 knockout or mutant mice showed reduced neuronal activity, which was attributed to either impaired glutamatergic synaptic transmission (Dani et al. 2005; Nelson et al. 2006) or excitatory synapse number (Chao et al. 2007). On the other hand, increased input/output relationships, reduced paired-pulse facilitation (PPF), and enhanced synaptic depression during high-frequency stimulation all suggest enhanced transmitter release in Mecp2-deficient neurons (Asaka et al. 2006; Moretti et al. 2006; Nelson et al. 2006). In addition, the frequency of spontaneous rhythmic field potentials in area CA3 was reduced in slices from Mecp2 knockout mice, rendering them prone to hyperexcitability (Zhang et al. 2008). Together, these alterations in glutamatergic synaptic transmission likely underlie the impairment of long-term potentiation (LTP) in area CA1, as well as in hippocampal-dependent learning and memory in Mecp2 knockout and mutant (Asaka et al. 2006) and Mecp2308 mutant mice (Moretti et al. 2006).
Here, we evaluated the consequences of a Mecp2 loss-of-function mutation on hippocampal synapse structure and function, as well as network excitability using a combination of imaging and electrophysiological approaches in acute slices from male Mecp2tm1.1Jae mutant mice (Chen et al. 2001). Quantitative electron microscopy and estimations of synaptic vesicle release with FM1–43 revealed a selective impairment in docked synaptic vesicles and the readily releasable pool (RRP) at excitatory and inhibitory synapses of Mecp2tm1.1Jae mutant mice. However, imaging the amplitude and spatiotemporal spread of neuronal depolarizations with voltage-sensitive dyes (VSD) revealed that the CA1 and CA3 regions of hippocampal slices from Mecp2tm1.1Jae mutant mice were highly hyperexcitable. These differences in network excitability were not observed in surgically isolated CA1 minislices, and blockade of GABAergic inhibition enhanced VSD signals to the same extent in Mecp2tm1.1Jae mutant mice and wild-type mice, suggesting that network excitability originated in area CA3. Indeed, extracellular multiunit recordings revealed a higher level of spontaneous firing of CA3 pyramidal neurons in slices from symptomatic Mecp2tm1.1Jae mutant mice. The neuromodulator adenosine reduced the amplitude and spatiotemporal spread of VSD signals evoked in CA1 of Mecp2 mutant slices to wild-type levels, suggesting its potential use as anticonvulsant in RTT individuals. The present results suggest that hyperactive CA3 pyramidal neurons contribute to hippocampal dysfunction and possibly the limbic seizures observed in Mecp2 mutant and knockout mice, as well as RTT syndrome individuals.
MATERIAL AND METHODS
Animals.
Breeding pairs of mice lacking exon 3 of the X-chromosome-linked Mecp2 gene (B6.Cg-Mecp2tm1.1Jae, “Jaenisch” strain; C57BL/6 background) (Chen et al. 2001) were purchased from the Mutant Mouse Regional Resource Center at the University of California, Davis. A colony was established at The University of Alabama at Birmingham (UAB) by breeding wild-type males with heterozygous Mecp2tm1.1Jae mutant females, following the original breeding scheme (Chen et al. 2001), which is recommended by the supplier. Genotyping was performed by PCR of sample DNA from tail clips. Hemizygous male mice of the “Jaenisch” Mecp2tm1.1Jae mutant strain are healthy until 5–6 wk of age, when they begin acquiring RTT-like motor symptoms, such as hypoactivity, hind limb elevation, and reflex impairments (Asaka et al. 2006; Chen et al. 2001). For the present studies, the experimental subjects were hemizygous Mecp2tm1.1Jae mutant males (called Mecp2 mutants) and wild-type male mice that were littermates of the same age. Mecp2 mutant females were used as breeders to maintain the colony. Mice between postnatal days 16–23 (P16–23) were considered presymptomatic, while those between P40–62 began to display one or more RTT-like phenotypes and were considered as symptomatic. Animals were handled and housed according to the Committee on Laboratory Animal Resources of the National Institutes of Health. All experimental protocols were annually reviewed and approved by the Institutional Animal Care and Use Committee of UAB.
Acute slices.
Mice were anesthetized with ketamine (100 mg/kg ip) and euthanized by rapid decapitation. For imaging and extracellular recordings, the brain of P16–55 mice was rapidly dissected and submerged in ice-cold low Ca2+ artificial cerebrospinal fluid (aCSF) for dissection and cutting that contained the following (in mM): 125 NaCl, 3.5 KCl, 3.0 MgCl2, 1.0 CaCl2, 26 NaHCO3, and 10 glucose, and was saturated with 95% O2-5% CO2 (pH 7.4). Three-hundred-micrometer-thick slices from the dorsal or ventral hippocampus were prepared using a vibratome (HM650V; Micron, Walldorf, Germany). Slices were allowed to recover for at least 1 h at room temperature in an interface chamber filled with a recording aCSF that contained the following (in mM): 125 NaCl, 3.5 KCl, 1.25 MgCl2, 2.5 CaCl2, 26 NaHCO3, and 10 glucose, and was saturated with 95% O2-5% CO2 (pH 7.4).
VSD imaging.
After recovery, slices were stained with the voltage-sensitive fluorescent dye N-(3-triethylammoniumpropyl)-4-{4-[4-(diethylamino)phenyl]butadienyl}pyridinium dibromide (RH-414; 30 μM) for 45 min at room temperature and with continuous bubbling with 95% O2-5% CO2. Excess dye was washed for at least 20 min in an immersion chamber perfused (1.5 ml/min) with aCSF at room temperature that contained the following (in mM): 125 NaCl, 3.5 KCl, 1.25 MgCl2, 2.5 CaCl2, 26 NaHCO3, and 10 glucose, and was saturated with 95% O2-5% CO2 (pH 7.4). RH-414 (Abs/Em = 532/716 nm) was excited with 535 ± 40 nm light from a shutter-controlled 100-W halogen lamp driven by a regulated power supply (Hewlett Packard, Palo Alto, CA). RH-414 fluorescence emission (>590 nm) was collected by a ×10 0.5 NA Plan-Neofluar objective (Carl Zeiss, Thornwood, NY) and detected by a NeuroPDA 464-element photodiode array (Red Shirt Imaging, Fairfield, CT) mounted under an inverted microscope (Zeiss Axiovert 135TV). Individual photodiode current was sampled at 1.6 kHz, amplified, digitized, and stored on a computer running Neuroplex software (Red Shirt Imaging), as described previously (Bandyopadhyay et al. 2005).
To image membrane depolarization in area CA1 with RH-414, Schaffer collaterals in stratum radiatum were stimulated with single current pulses (200 μs) of at least four different current intensities (10–90 μA) delivered through a bipolar electrode connected to a constant-current stimulus isolator (S8800 Stimulator, Grass Instruments). Mossy fibers in s. lucidum were stimulated with single pulses or with short trains of 10 pulses at 100 Hz to image responses in area CA3. Field excitatory postsynaptic potentials (fEPSPs) were simultaneously recorded with an extracellular electrode connected to an Axoclamp-2B amplifier (Molecular Devices, Sunnyvale, CA) and filled with aCSF positioned either in CA1 s. radiatum or in CA3 s. lucidum, which allowed setting the stimulus intensity in the middle of the input/output curve for every slice. The spatiotemporal pattern of RH-414 signals with respect to each individual slice and the stimulating and recording electrodes was established by overlapping the image of the photodiode array obtained with a video camera and a dissecting microscope.
Fluorescence measurements during image sequences were normalized to the resting light intensity measured for each photodiode in the array. RH-414 bleaching was corrected by imaging sequences in the absence of stimulation. RH-414 optical signals are represented as percentage changes in fluorescence intensity during illumination without stimulation and during neuronal activity (ΔF/F). Membrane depolarization causes a decrease in RH-414 fluorescence intensity but for illustrative purposes following established conventions, we displayed ΔF/F signals with a “rainbow” style pseudocolor scale with increasing color “warmth” corresponding to higher depolarizations (i.e., low ΔF/F). Regions of interest (ROI) including active and surrounding inactive areas were chosen for analysis of the amplitude of ΔF/F signals. The spatial spread of RH-414 signals was obtained from the distance between the two extreme photodiodes in the array that showed ΔF/F levels twice the baseline noise. Similarly, the duration of RH-414 signals was obtained from the interval between the first and the last frames of a sequence that showed ΔF/F levels twice the baseline noise.
Multiphoton imaging of vesicular release from presynaptic terminals.
After recovery, slices were transferred to an immersion chamber mounted on the stage of a custom-modified multiphoton excitation microscope, and continuously perfused (1.5 ml/min) with aCSF at room temperature containing the following (in mM): 121 NaCl, 5.0 KCl, 1.25 KH2PO4, 1.3 MgSO4, 2.5 CaCl2, 17.6 NaHCO3, and 10 glucose, and was saturated with 95% O2-5% CO2 (pH 7.4). Slices were stained with the fluorescent dye N-(3-triethylammoniumpropyl)-4-[4-(dibutylamino)styryl]pyridinium dibromide (FM1–43; 5 μM, in 0.1% DMSO) in the presence of 10 μM 6-cyano-7-nitroquinoxalene-2,3-dione (CNQX) and 50 μM dl-2-amino-5-phosphonovaleric acid (APV) to block dl-α-amino-3-hydroxy-5-methylisox azole-propionic acid (AMPA) and N-methyl-d-aspartate receptors (NMDA), respectively. After 5 min in this staining solution, two different pools of recycling synaptic vesicles were labeled by the following protocols: 1) the total recycling pool (TRP) was loaded by a 15-min exposure to hyperkalemic aCSF (40 mM KCl, equimolar substitution with NaCl); and 2) the RRP was loaded by a brief (25 s) exposure to hypertonic aCSF (adjusted to 800 mOsm with sucrose). In both cases, dye uptake into synaptic terminals was allowed to proceed in normal aCSF for 2 additional min by the ensuing endocytosis of synaptic vesicles. To prevent spontaneous vesicle fusion that would have caused FM1–43 destaining, slices were perfused with Ca2+-free aCSF (equimolar substitution with Mg2+) containing glutamate receptor antagonists for at least 30 min before the start of the imaging experiments (Mathew et al. 2008; Stanton et al. 2003; Tyler et al. 2006; Winterer et al. 2006).
FM1–43 was excited with 840 nm of light from a Ti-sapphire laser (Chameleon; Coherent, Santa Clara, CA). Infrared laser intensity was controlled by an external Pockels cell (Conoptics, Danbury, CT) before entering a modified FV300 scanhead (Olympus, Center Valley, PA) attached to an upright BX50WI microscope (Olympus) and never exceeded 50 mW at the back focal plane of the objective lens. FM1–43 fluorescence was collected with a ×60 0.9 NA water immersion objective (LUMPlanFI/IR2; Olympus) and detected in nondescanned mode by a GaAsP avalanche PMT (R3896; Hamamatsu, Bridgewater, NJ) located at the nosepiece of the microscope (Mathew et al. 2008). Laser scanning, image formation and focus control was performed using FluoView Tiempo software (Olympus).
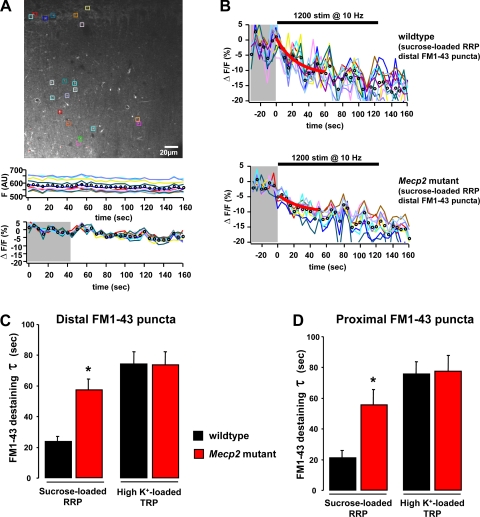
Five-minute-long image sequences consisted of 512 × 512 pixel frames (72 × 72 μm) and were acquired every 5 s from the center of the slice (25–60 μm below the surface). After 9 frames of baseline, afferent Schaffer collaterals were stimulated with a 10-Hz train of 1,200 pulses (100 μs, 40 μA) using an aCSF-filled patch electrode connected to a constant-current stimulus isolator (Iso-FLEX; AMPI, Jerusalem, Israel). The stimulating electrode was located 40–60 μm away from the imaging area. During offline analyses, ROIs (∼2 × 4 μm2) were defined surrounding individual FM1–43 puncta either in CA1 s. radiatum (presumptive excitatory synapses on distal apical dendrites) or in CA1 s. pyramidale (presumptive inhibitory synapses on cell bodies), as described previously (Axmacher et al. 2004; Brager et al. 2003). Any FM1–43 puncta that showed lateral displacement from the ROI within the 5-min time-lapse sequence were discarded from the analyses. The average pixel intensity within each ROI was first background subtracted and then normalized by the average intensity of the first nine frames before afferent stimulation (ΔF/F). FM1–43 bleaching during 5-min-long image sequences in the absence of afferent stimulation was negligible (1st frame 595.12 ± 35.56 in arbitrary units of fluorescence intensity vs. 60th frame 558.89 ± 32.04; 6.16 ± 0.4% bleaching; n = 8 slices; see Fig. 4A) and therefore not subtracted from traces during stimulation. Destaining time courses were generated by normalizing each ROI time course to its starting intensity. The rate of activity-dependent FM1–43 destaining was calculated from the time constant (τ) of single exponentials fitted to the first 11 frames (55 s) after the start of afferent stimulation (Mathew et al. 2008; Stanton et al. 2005; Tyler et al. 2006). With the use of IgorPro software (WaveMetrics, Lake Oswego, OR), all exponential curve fittings yielded values of χ2-test ≤ 18.54, which is statistically significant at the P < 0.05 level for 10 degrees of freedom.
Fig. 4.
Imaging of synaptic vesicle recycling with FM1–43 in acute slices by multiphoton excitation microscopy. A: representative example of the CA1 region of a wild-type slice stained with FM1–43. Note colored regions of interest over distal (s. radiatum) and proximal (s. pyramidale) FM1–43 puncta. Plots are a representative example of FM1–43 bleaching during a 5-min-long image sequence in the absence of afferent stimulation both as raw fluorescence intensity (F) in arbitrary units (AU; top) and as ΔF/F ratio (bottom) for comparison with ΔF/F traces of afferent stimulation-evoked FM1–43 destaining shown in B. B: representative examples of the time course of FM1–43 destaining from the sucrose-loaded readily releasable pool (RRP) evoked by afferent Shaffer collateral fiber stimulation in a wild-type slice (top) and a Mecp2 mutant slice (bottom). Horizontal bar denotes the onset and duration of afferent stimulation, and gray area denotes the 9-frame baseline period for calculation of ΔF/F. C: time course of activity-dependent FM1–43 destaining from distal puncta within CA1 s. radiatum. D: time course of activity-dependent FM1–43 destaining from proximal puncta within CA1 s. pyramidale. The inverse of the time constant of single exponential fittings to the first 11 frames of ΔF/F destaining traces was used for statistical comparisons. Note that the sucrose-loaded RRP of vesicles destained with a faster rate than the total recycling pool (TRP) loaded by hyperkalemic solutions. Data are means ± SD. *P < 0.05, symptomatic vs. wild-type.
Extracellular recordings of fEPSPs in CA1 and of multiunit activity in CA3.
After recovery, slices were transferred to an interface chamber (Fine Science Tools, Foster City, CA) and perfused (1 ml/min) with oxygenated aCSF (95% O2-5% CO2) at 30°C in a humidified atmosphere. Electrical signals from aCSF-filled glass microelectrodes (1–3 MΩ) were acquired with an Axoclamp-2B amplifier, digitized (Digidata 1320A), and stored on a computer using Clampex (Molecular Devices). Extracellular fEPSPs were evoked by stimulation of Schaffer collaterals with a bipolar electrode placed in CA1 s. radiatum (A365 stimulus isolator; WPI, Sarasota, FL) and acquired from the same dendritic region at 4 kHz. To obtain an input/output response curve, afferent Schaffer collaterals were stimulated at increasing stimulus amplitudes (0, 5, 10, 15, 20, 30, 40, 50, 60, 70, 80, 90, and 100 μA). Pairs of pulses at different interstimulus intervals (ISIs; 10, 20, 50, 100, 150, 200, 250, and 300 ms) were delivered to Schaffer collaterals to measure PPF. The initial slope of the fEPSP was measured using Clampfit 10.0 (Molecular Devices). Spontaneous multiunit activity was recorded during 5-min epochs from the cell body layer (s. pyramidale) of area CA3 and acquired at 10 kHz. Detecting an antidromic spike concomitant with a dendritic fEPSP in CA1 s. radiatum evoked by Schaffer collateral stimulation confirmed that the spontaneous multiunit activity originated from CA3 pyramidal neurons (Cohen and Miles 2000).
Quantitative electron microscopy.
Mice were deeply anesthetized with ketamine (100 mg/kg) and euthanized by transcardiac perfusion with ∼200 ml of half strength Karnovsky's fixative (2.5% glutaraldehyde, 2% formaldehyde, and 2 mM CaCl2 in 100 mM cacodylate buffer, pH 7.4). After 1 h, the brain was removed and placed in the same fixative for an additional 2 h. Four-hundred-micrometer-thick frontal sections were cut with a McIlwain tissue chopper (Mickle Laboratory Engineering, Gomshall, Surrey, England). After being rinsed with cacodylate buffer, sections including the dorsal hippocampus were stained with ice-cold reduced osmium solution (K-ferrocyanide and 1% OsO4 in 100 mM cacodylate buffer) and warmed up to at 37°C for 2.5 min in a laboratory microwave (Pelco 3450 Laboratory Microwave Processor; Ted Pella, Redding, CA). After being rinsed with cacodylate buffer, sections were stained in ice-cold 1% aqueous uranyl acetate followed by microwave irradiation at 37°C for 2.5 min. After being rinsed, sections were dehydrated in a graded acetone series (50, 70, 90, and 100%; 2 times each) at 37°C for 40 s in the microwave. Finally, sections were embedded flat between two plastic coverslips (Thermanox; Nunc, Naperville, IL) with Poly/Bed 812 (Electron Microscopy Science, Fort Washington, PA) for 24 h at 60°C. CA1 s. radiatum and s. pyramidale were located in semithin sections (0.5 μm) stained with toluidine blue. After being trimmed, ultrathin sections (60–80 nm) were prepared with a diamond knife in an Ultracut-S ultramicrotome (Reichert) and stained with uranyl acetate and lead citrate. Ultrathin sections were imaged at 60–80 keV in a FEI-Tecnai transmission electron microscope equipped with a digital CCD camera (2976 by 2976 pixels; Gatan). Low magnification (×2,100) images were used for analyses of synapse density, while high magnification (×16,000) images were used for analyses of synaptic vesicle distributions within individual synapses.
Presumptive excitatory synapses were identified by the higher electron density of their postsynaptic membrane specializations compared with the presynaptic membrane thickening (i.e., asymmetric), as well as by their location on the head of dendritic spines, which in turn were identified by a continuous connection with the parent dendrite and the lack of microtubules and mitochondria in the spine head (Peters et al. 1991). Sampling areas for presumptive excitatory asymmetric spine synapses were restricted to CA1 s. radiatum at least 70 μm away from the pyramidal cell layer (i.e., s. pyramidale). Presumptive inhibitory synapses were identified by the comparable electron density of their pre- and postsynaptic membrane specializations (i.e., symmetric) and their location on dendritic shafts and somata (Peters et al. 1991). Sampling areas for presumptive inhibitory symmetric shaft synapses were restricted to proximal regions of CA1 s. radiatum no more than 50 μm away from pyramidal somata in s. pyramidale. To reduce the bias of selecting the larger synapses in individual thin sections, only synapses with presynaptic terminals and active zones of similar magnitude were included in the analyses; this approach yields comparable results to random sampling of synapses (Pozzo-Miller et al. 1999; Tartaglia et al. 2001; Tyler and Pozzo-Miller 2001) and serially sectioned synapses (Harris and Sultan 1995; Kushner et al. 2005; Schikorski and Stevens 1997). The total area of neuropil sampled in Mecp2 mutant mice was 13,059 μm2 (for asymmetric spine synapses) and 10,643 μm2 (for symmetric shaft synapses). The total area of neuropil sampled in wild-type mice was 15,196 μm2 (for spine synapses) and 13,987 μm2 (for shaft synapses); these neuropil areas were used to normalize the number of synapses. Small clear synaptic vesicles within presynaptic terminals were counted in two distinct and mutually exclusive domains: 1) vesicles were considered docked if they were in contact or in close apposition with the presynaptic active zone (up to ∼50 nm, i.e., 1 vesicle diameter); this number was normalized to the length of the active zone [identified by the apposing postsynaptic density (PSD)]; and 2) vesicles in the reserve pool were defined as those within the perimeter of the presynaptic terminal, excluding docked vesicles; this number was normalized to the area of the presynaptic terminal (Dickinson-Nelson and Reese 1983; Pozzo-Miller et al. 1999; Tyler and Pozzo-Miller 2001). All quantitative analyses of digital electron microscopy images were performed using ImageJ software (NIH).
Drugs.
Some compounds were dissolved in DMSO (0.01%) and others directly into the aCSF. Drugs were kept frozen in stock solutions and dissolved in aCSF before each experiment. RH-414 was purchased from Anaspec (San Jose, CA); FM1–43 was purchased from InVitrogene (Eugene, OR); CNQX and APV were purchased from Tocris (Ellisville, MO); 4-aminopyridine (4-AP) and bicuculline methiodide were purchased from Sigma (St. Louis, MO).
Statistical analyses.
Averages of multiple measurements are presented as means ± SE, while averages of single measurements are means ± SD. Data were statistically analyzed using unpaired Student's t-test or ANOVA test using the Prism software package (GraphPad Software,). Probability values <0.05 were considered statistically significant (i.e., P < 0.05, <5% probability that the observations are due to chance). When lower than this cut-off value, the actual P values up to four decimal points are given in the results (rather than just the statement “greater than” or “less than”) to provide readers with more detailed information regarding the outcome of the statistical analyses. Compromise power analyses were performed to determine the statistical power given the number of observations, sample means, and SD, using G*Power (Erdfelder et al. 1996). These power analyses yielded values of statistical power (1-β; where β is the Type-II error) >0.95 (i.e., 95% confidence of accepting the null hypothesis when is true).
RESULTS
Area CA1 of hippocampal slices from symptomatic Mecp2 mutant mice is hyperexcitable and shows enhanced 4-AP-induced epileptiform activity.
To evaluate the consequences of a loss-of-function Mecp2 mutation on hippocampal network activity, we performed VSD imaging in acute slices from Mecp2 mutant male mice and their wild-type littermates. Dorsal hippocampal slices were stained with RH-414 (30 μM; Fig. 1A), and its fluorescence signals evoked by afferent stimulation were detected with a 464-element array of fast-responding photodiodes (Bandyopadhyay et al. 2005; Carlson and Coulter 2008; Chang and Jackson 2003; Grinvald et al. 1988). Figure 1B shows two representative examples of the spatiotemporal pattern of VSD signals evoked in area CA1 by a single pulse stimulation of afferent Schaffer collaterals in slices from a symptomatic (P55) Mecp2 mutant mouse (right) and a wild-type littermate (left); the arrow points to the location of the stimulating electrode in CA1 s. radiatum (Fig. 1A, top left), which in this particular example delivered a single 30-μA current pulse (200-μs duration). Note that the hexagonal array covers all area CA1, as shown by the red LED reference points visible in Fig. 1A. Although the VSD stains the plasma membrane of pyramidal cells, interneurons, and glial cells, optical signals were considered to reflect depolarization of postsynaptic pyramidal neurons because 1) as in previous studies of hippocampal slices (Grinvald et al. 1982), they were consistent and correlated temporally with extracellular recordings of fEPSPs from the same site, which are widely interpreted in terms of voltage changes in pyramidal neurons (Andersen et al. 1980); 2) interneurons are not expected to contribute significantly because of their lower density (estimated ratio 10:1 pyramidal neurons to interneurons; Spruston and McBain 2007); 3) glial cells are numerous, but evoked responses in hippocampal glial cells have much smaller amplitudes and slower time courses as demonstrated by patch clamp recording (Bergles and Jahr 1997), field potential recording (Diamond et al. 1998), and voltage imaging (Kojima et al. 1999); and 4) lastly, evoked VSD signals in CA1 are enhanced after induction of LTP (Aihara et al. 2005; Chang and Jackson 2006), while glutamate transporter currents recorded in glial cells (and thus glial VSD signals) are not affected by LTP induction (Diamond et al. 1998; Hosokawa et al. 2003; Luscher et al. 1998; Momose-Sato et al. 1999; Saggau et al. 1986).
Fig. 1.
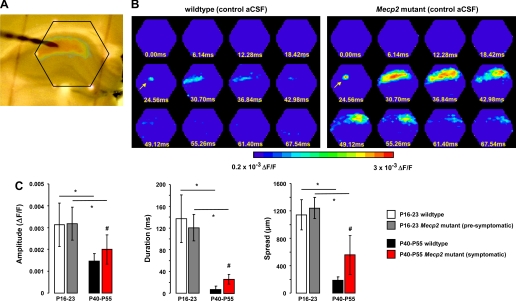
Voltage-sensitive dye (VSD) imaging revealed pronounced hyperexcitability in area CA1 of acute hippocampal slices from symptomatic Mecp2 mutant mice. A: dorsal hippocampal slice with a stimulation electrode in CA1 stratum radiatum to stimulate afferent Schaffer collaterals. Note the red LEDs marking the hexagonal photodiode array. B: representative examples of the spatiotemporal pattern of VSD signals evoked in area CA1 by stimulation of afferent Schaffer collaterals in slices from a symptomatic Mecp2 mutant mouse (right) and a wild-type littermate (left). Arrow points to the location of the stimulation electrode. In this and Figs. 2–8, normalized changes in RH-414 fluorescence (ΔF/F) are represented with a pseudocolor scale (warmer colors indicate larger depolarizations). C: amplitude and spatiotemporal pattern of VSD signals evoked in area CA1 by stimulation of afferent Schaffer collaterals in slices from symptomatic Mecp2 mutant, presymptomatic Mecp2 mutant, and age-matched wild-type littermate controls. Data are means ± SD. aCSF, artificial cerebrospinal fluid; P, postnatal day. #P < 0.05, symptomatic vs. wild-type; *P < 0.05, presymptomatic vs. symptomatic Mecp2 mutant, young wild-type vs. older wild-type.
With the use of this approach, it is evident that a single afferent stimulation of similar intensity (30 μA) evoked larger, longer lasting, and more widespread VSD signals in slices from symptomatic Mecp2 mutant mice than in ones from wild-type littermates. The peak amplitude of VSD signals in slices from P35-P42 symptomatic Mecp2 mutant mice (2 × 10−3 ± 0.67 × 10−3 ΔF/F; means ± SD; n = 13 slices from 7 mice) was significantly larger than those evoked in wild-type slices (1.47 × 10−3 ± 0.34 × 10−3 ΔF/F; n = 15 slices/5 mice; P = 0.0116; Fig. 1C). In addition, the duration of VSD signals were significantly longer in Mecp2 mutant slices (25.65 ± 8.84 ms) compared with those in wild-type slices (7.58 ± 6.23ms; P < 0.0001; Fig. 1C). Similarly, the VSD signals spread over a significantly larger region of area CA1 in Mecp2 mutant slices (555.8 ± 285.3 μm) than in wild-type slices (190 ± 48 μm; P < 0.0001; Fig. 1C).
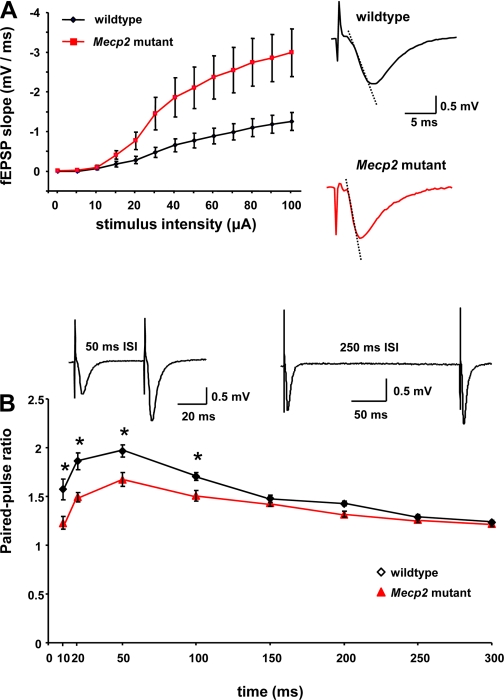
Such large differences in the amplitude and spatiotemporal pattern of VSD signals evoked by similar afferent stimulation intensities suggest an alteration in basal synaptic transmission at Schaffer collateral-CA1 synapses in Mecp2 mutant slices. Indeed, the fitted slope (m) of the linear portion of the input-output relationship of fEPSP slopes vs. afferent stimulation intensities was significantly larger in Mecp2 mutant slices (23 ± 4.6; means ± SE; n = 10 slices/4 mice) than in wild-type slices (11 ± 2.3; n = 10 slices/4 mice; P = 0.0310; Fig. 2A). In addition, short-term plasticity was impaired in slices from Mecp2 mutant mice: the PPF ratio was significantly lower at the shortest inter-stimulus intervals (10, 20, 50, and 100 ms) in Mecp2 mutant slices (n = 10 slices/4 mice) than in slices from their wild-type littermates [n = 9 slices/4 mice; F(15,8) = 35.86, P < 0.0001, repeated measures one-way ANOVA; P < 0.05, post hoc Newman-Keuls; Fig. 2B].
Fig. 2.
Basal excitatory synaptic transmission and short-term plasticity at CA3-CA1 synapses. A: input-output relationship between stimulus intensity and slope of field excitatory postsynaptic potentials (fEPSPs) evoked in area CA1 by Schaffer collateral stimulation. Fitted slopes (m) of the linear portion of these input-output curves were used for statistical comparisons. Insets show representative traces of field EPSPs in Mecp2 mutant and wild-type slices (dotted line represents the slope of the fEPSP used for measurements). B: paired-pulse stimulation at different interstimulus intervals (ISIs; 10, 20, 50, and 100 ms). *P < 0.0001, repeated-measures one-way ANOVA; P < 0.05, post hoc Newman-Keuls test. Data means ± SE. Inset: representative traces of field EPSPs at 50- and 250-ms interpulse intervals in a slice from a wild-type mouse.
To further confirm the hyperexcitable state of hippocampal slices from symptomatic Mecp2 mutant mice, we tested their susceptibility to 4-AP, a K+ channel blocker that induces epileptiform activity in hippocampal slices, likely due to enhanced glutamate release by lengthening presynaptic action potentials (Buckle and Haas 1982; Perreault and Avoli 1991; Rutecki et al. 1987; Storm 1990; Voskuyl and Albus 1985). Supplemental Fig. S1A shows representative examples of VSD responses evoked in CA1 by single Schaffer collateral stimulation before and after the application of 125 μM 4-AP in slices from a wild-type control mouse (top) and a Mecp2 mutant littermate (bottom), which confirmed that bath application of 4-AP induced epileptiform activity (Supplemental Material for this article is available online at the J Neurophysiol website). This epileptiform activity was reflected by a significant enhancement of the amplitude, duration, and spatial spread of VSD signals evoked in CA1 by single-pulse Schaffer collateral stimulation in slices from either Mecp2 mutant mice or their wild-type littermates (P < 0.05; Supplemental Fig. S1B). Consistent with a hyperexcitable network, the effect of 4-AP was significantly larger in slices from symptomatic Mecp2 mutant mice compared with those from wild-type littermates. The peak amplitude of VSD signals in Mecp2 mutant slices in the presence of 4-AP (5 × 10−3 ± 1.35 × 10−3 ΔF/F; n = 10 slices/6 mice) was significantly larger than in 4-AP-treated wild-type slices (3.1 × 10−3 ± 1.2 × 10−3 ΔF/F; n = 15 slices/5 animals; P = 0.0010; Supplemental Fig. S1B). Similarly, the duration (72.82 ± 32.29 vs. 33.16 ± 11.77 ms; P = 0.0004) and spatial spread (1,448 ± 252.6 vs. 700 ± 312.7 μm; P < 0.0001) were significantly larger in 4-AP-treated Mecp2 mutant slices than in wild-type slices exposed to this K+ channel blocker (Supplemental Fig. S1B).
In addition to increasing the amplitude and spatiotemporal spread of VSD signals associated with the fEPSP, 4-AP also evoked delayed depolarizations in CA1 s. radiatum but only in a small subset of wild-type slices (2 of 15; Supplemental Fig. S2A). These delayed VSD signals observed in the presence of 4-AP started after 93–223 ms from afferent stimulation, and their amplitudes varied between 1.5 × 10−3 and 1.6 × 10−3 ΔF/F, with durations of 233–426 ms and spatial spreads of 675–1,050 μm (Supplemental Fig. S2B). Consistent with a hyperexcitable network, delayed depolarizations evoked in the presence of 4-AP were more common in slices from Mecp2 mutant mice (4/10), although they had a longer delay from afferent stimulation (214.1 ± 138.3 ms; Supplemental Fig. S2A). The amplitude of these delayed VSD signals in the presence of 4-AP averaged 1.5 × 10−3 ± 4.1 × 10−4 ΔF/F, with a duration of 268.8 ± 196.9 ms and a spatial spread of 937.5 ± 417.6 μm, which were not significantly different than those observed in wild-type slices (P > 0.05 in all cases; Supplemental Fig. S2B).
Another effect of 4-AP was the appearance of even more delayed secondary VSD signals in the dentate gyrus, starting between 211–224 ms after a single pulse stimulation to Schaffer collaterals, which were observed only in 2 out of 15 wild-type slices (Supplemental Fig. S2A). The amplitude of these secondary VSD signals in the dentate gyrus varied between 2.4 × 10−3-2.2 × 10−3 ΔF/F, with durations between 233–395 ms and spatial spreads between 1,050–975 μm (Supplemental Fig. S2C). Again consistent with a hyperexcitable network, these secondary responses evoked in the presence of 4-AP were more common in slices from Mecp2 mutant mice (6/10), being detectable in the dentate gyrus 233.6 ± 95.6 ms after a single pulse stimulation to Schaffer collaterals (Supplemental Fig. S2A). Secondary VSD signals in the dentate gyrus averaged 2.26 × 10−3 ± 6 × 10−4 ΔF/F, with a duration of 301.1 ± 105.1 ms and a spatial spread of 1,100 ± 271.1 μm, which were not significantly different than those observed in wild-type slices (P > 0.05 in all cases; Supplemental Fig. S2C).
Representative examples of delayed Schaffer collateral evoked VSD signals in CA1 and of secondary synaptic current-evoked VSD signals in DG in the presence of 4-AP are shown in Supplemental Fig. S2A. These observations demonstrate that hippocampal slices from symptomatic Mecp2 mutant mice are more prone to 4-AP-induced epileptiform activity than wild-type slices. The fact that a higher proportion of Mecp2 mutant slices exhibited 4-AP-induced delayed depolarizations in area CA1 and secondary responses in dentate gyrus after a single pulse stimulation of Schafer collaterals also demonstrate that these slices are more excitable than those from wild-type mice and prone to epileptiform activity.
Area CA1 of presymptomatic Mecp2 mutant mice is as excitable as that of wild-type mice, but it shows an impaired developmental reduction of network excitability.
Consistent with a neurodevelopmental disorder, the amplitude and spatiotemporal pattern of VSD signals evoked in CA1 by single pulse Schaffer collateral stimulation were not significantly different in slices from younger presymptomatic Mecp2 mutant mice compared with slices from their wild-type littermates (P16–23). The peak VSD signal amplitude in Mecp2 mutant slices (3.2 × 10−3 ± 0.07 × 10−3 ΔF/F; n = 22 slices/6 mice) was not significantly different from that in wild-type slices (3.2 × 10−3 ± 0.09 × 10−3 ΔF/F; n = 26 slices/8 mice; P = 0.8358; Fig. 1C). In addition, the duration of VSD signals in presymptomatic Mecp2 mutant slices (120.6 ± 24.55 ms) was not significantly different than in wild-type slices (137 ± 43.6 ms; P = 0.124; Fig. 1C). Also, the spatial spread of VSD signals was not significantly different between presymptomatic Mecp2 mutant slices (1,241 ± 154.8) and wild-type slices (1,142 ± 218.7; P = 0.0832; Fig. 1C).
Also consistent with a “critical period” of network hyperexcitability described by intracellular recordings in hippocampal and neocortical slices during the second and third postnatal week (Hablitz 1987; Swann and Brady 1984; Swann and Hablitz 2000), the amplitude and spatiotemporal pattern of VSD signals evoked in CA1 by single pulse Schaffer collateral stimulation were significantly higher in slices from younger mice (P16–23) than in those from older mice (P40–55), irrespective of their genotypes (P < 0.05 in all cases; Fig. 1C). However, this developmental reduction in VSD signals was impaired in slices from Mecp2 mutant mice: there was only a 36.9% reduction in peak VSD signal amplitude between P16–23 and P40–55, compared with a significantly larger 53.1% decrease in wild-type slices during a comparable period. Similarly, the duration of VSD signals decreased 78.7% in Mecp2 mutant during this developmental period, compared with a significantly larger 94.5% reduction in wild-type slices. Similarly, the spatial spread of VSD signals decreased only 55.2% in Mecp2 mutant slices, compared with a significantly larger 83.4% decrease in wild-type slices during a comparable period. Taken together, these observations demonstrate that area CA1 of hippocampal slices from symptomatic Mecp2 mutant mice persists in a hyperexcitable state due to an impaired developmental reduction of network excitability.
Excitatory and inhibitory synapses in CA1 s. radiatum of Mecp2 mutant mice have a selective deficit in synaptic vesicles docked at their active zone.
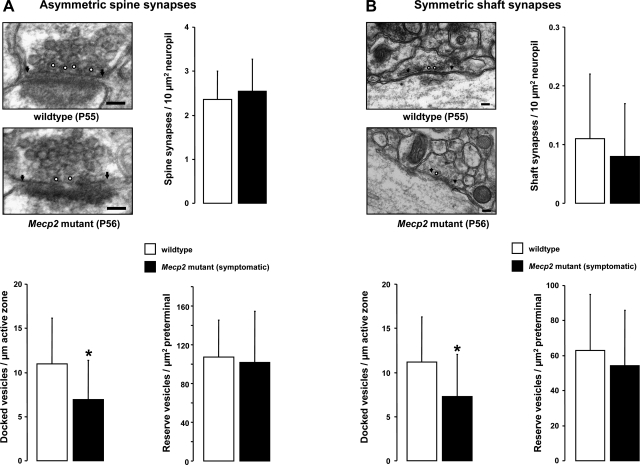
We next performed quantitative electron microscopy of synapses in s. radiatum of area CA1 of symptomatic Mecp2 mutant mice and their wild-type littermates (P55–56). Presumptive excitatory synapses were identified by their location on dendritic spines and thickest postsynaptic densities (i.e., asymmetric spine synapses), while presumptive inhibitory synapses were identified by their location on dendritic shafts and thinner postsynaptic densities (i.e., symmetric shaft synapses) (Peters et al. 1991). Asymmetric synapses on dendritic spines and symmetric synapses on dendritic shafts had similar structural organization in Mecp2 mutant mice and their wild-type littermates, as well as the normal complement of intracellular organelles and plasma membrane specializations (Figs. 3, A and B, top left). In addition, the density of asymmetric spine synapses in Mecp2 mutant mice was not significantly different than in their wild-type littermates (Mecp2 mutant: 2.54 ± 0.73 synapses per 10 μm2 of neuropil, n = 4 mice, vs. wild-type: 2.36 ± 0.64 synapses/10 μm2, n = 4; P > 0.05; Fig. 3A, top right). Similarly, the density of symmetric shaft synapses was comparable between the genotypes (Mecp2 mutant: 0.08 ± 0.09 synapses/10 μm2, n = 4, vs. wild-type: 0.11 ± 0.11 synapses/10 μm2, n = 4; P > 0.05; Fig. 3B, top right).
Fig. 3.
Quantitative analysis of synapse ultrastructure in hippocampal area CA1. A: representative electron micrographs of asymmetric synapses on dendritic spines in a symptomatic Mecp2 mutant and a wild-type littermate. B: representative electron micrographs of symmetric synapses on dendritic shafts in a symptomatic Mecp2 mutant and a wild-type littermate. Arrowheads indicate the edges of the postsynaptic density, and docked vesicles are marked with white circles. Bar graphs show the number of synapses normalized per 10 μm2 of neuropil, the number of docked synaptic vesicles normalized per active zone length, and the number of synaptic vesicles in the reserve pool normalized per terminal area. Data are means ± SD. *P < 0.05, symptomatic vs. wild-type.
Quantitative analyses of the distribution of synaptic vesicles within presynaptic terminals revealed a specific impairment in the vesicle pool docked at the active zones of both asymmetric spine synapses and symmetric shaft synapses of Mecp2 mutant mice. The density of docked vesicles in spine synapses of Mecp2 mutant mice was significantly lower than in wild-type littermates [Mecp2 mutant: 6.92 ± 4.49 docked vesicles per μm of active zone length (DV/μm), n = 200 synapses, 4 mice, vs. wild-type 11 ± 5.16 DV/μm, n = 193 synapses, 4 mice; P < 0.05; Fig. 3A, bottom left]. Similarly, the density of docked vesicles in shaft synapses was significantly lower in Mecp2 mutant mice (7.29 ± 4.74 DV/μm, n = 24 synapses, 4 mice) compared with wild-type littermates (11.2 ± 5.152 DV/μm, n = 48 synapses, 4 mice; P < 0.05; Fig. 3, B, bottom left).
These impairments were selective for docked vesicles, because the density of all the other synaptic vesicles within presynaptic terminals of spine synapses from Mecp2 mutant mice [101.9 ± 52.74 reserve vesicles per μm2 of terminal area (RV/μm2), n = 200 synapses, 4 mice] was similar to that in wild-type mice (107.1 ± 38.06 RV/μm2, n = 193 synapses, 4 mice; P > 0.05; Fig. 3A, bottom right). Similarly, the density of reserve vesicles in shaft synapses was not significantly different between genotypes (Mecp2 mutant: 54.2 ± 31.86 RV/μm2, n = 24 synapses, 4 mice, vs. wild type: 63.11 ± 31.97 RV/μm2, n = 48 synapses, 4 mice; P > 0.05; Fig. 3B, bottom right). Finally, neither the area of presynaptic terminals nor the length of the active zones was significantly different between Mecp2 mutant mice and their wild-type littermates (P > 0.05; Supplemental Fig. S3).
Excitatory and inhibitory synapses in CA1 s. radiatum of Mecp2 mutant mice have a selective deficit in activity-dependent release from the RRP of synaptic vesicles.
Multiphoton excitation microscopy of activity-dependent destaining of FM1–43 (5 μM) was used to assess vesicular release from two distinct pools of synaptic vesicles in acute hippocampal slices: the TRP labeled with hyperkalemic solutions (40 mM K+, 15 min), and the RRP stained with hyperosmotic solutions (800 mOsm, 25 s) (Mathew et al. 2008; Stanton et al. 2003; Tyler et al. 2006; Winterer et al. 2006). In addition, the selective distribution of excitatory and inhibitory synapses on apical dendrites or around cell bodies in hippocampal slices was exploited to classify FM1–43 fluorescence puncta as presumptive glutamatergic or GABAergic presynaptic terminals, respectively (Axmacher et al. 2004; Brager et al. 2003). Figure 4A shows a multiphoton image of area CA1 of a representative slice after FM1–43 uptake. Note the cell body layer at the top, and colored ROIs over individual FM1–43 fluorescence puncta (∼1 × 1 μm), either around cell bodies in s. pyramidale (proximal puncta, presumptive GABAergic) or apical dendrites in s. radiatum (distal puncta, presumptive glutamatergic). After washout of extracellular FM1–43, stimulation of Schaffer collaterals with 1,200 pulses (100 μs, 40 μA) at 10 Hz was used to evoke synaptic vesicle release during multiphoton excitation microscopy of FM1–43 fluorescence.
Figure 4B illustrates the time courses of evoked destaining from the RRP of distal FM1–43 puncta labeled with hyperosmotic solutions in CA1 s. radiatum of a wild-type slice (top) and a Mecp2 mutant slice (bottom). Activity-dependent vesicular release from the sucrose-loaded RRP of these presumptive excitatory terminals in CA1 s. radiatum was significantly slower in hippocampal slices from symptomatic Mecp2 mutant mice than in their wild-type littermates (P48–68): the time constant of FM1–43 destaining was 57.4 ± 7.1 s in Mecp2 mutant slices (103 puncta from n = 9 slices/3 mice), while the RRP destained with a shorter time constant of 23.8 ± 3.32 s in wild-type slices (78 puncta from n = 7 slices/3 mice; P < 0.05; Fig. 4C). This impairment was selective for the RRP, because the time constant of FM1–43 destaining from the K+-loaded TRP of presumptive excitatory distal puncta was not affected by Mecp2 deletion (Mecp2 mutant: 73.65 ± 8.42 s, 95 puncta from n = 13 slices/8 mice, vs. wild-type: 74.06 ± 8.07 s, 74 puncta from n = 11 slices/7 mice; P > 0.05; Fig. 4C). Note that, consistent with a readily available vesicular pool (Stanton et al. 2003), the destaining kinetics of sucrose-loaded FM1–43 puncta was ∼34% faster than that of K+-loaded ones.
Presumptive inhibitory, proximal FM1–43 puncta around cell bodies in s. pyramidale also showed a selective impairment in vesicular release from the RRP: the time constant of FM1–43 destaining from the sucrose-loaded pool was larger in Mecp2 mutant slices than in wild-type ones (Mecp2 mutant: 55.6 ± 9.9 s, 84 puncta from n = 8 slices/4 mice, vs. wild-type: 20.9 ± 4.9 s, 47 puncta from n = 5 slices/3 mice; P < 0.05; Fig. 4D). On the other hand, the time constant of FM1–43 destaining from the K+-loaded TRP was not significantly different between the genotypes (Mecp2 mutant: 77.52 ± 10.42 s, 44 puncta from n = 4 slices/3 mice, vs. wild-type: 75.53 ± 8.14 s, 72 puncta from n = 8 slices/4 mice; P > 0.05; Fig. 4D). Also for proximal FM1–43 puncta, the destaining kinetics was ∼34% faster in sucrose-loaded terminals than in K+-loaded ones.
Together with the quantitative electron microscopy observations, these results demonstrate that a loss-of-function Mecp2 mutation causes selective impairments in the size and release kinetics of the RRP of synaptic vesicles within presynaptic terminals of both excitatory and inhibitory synapses in area CA1. These results also suggest that such subtle differences in synapse structure and function may not be a major contributor to the large differences in VSD signals evoked in area CA1 of slices from Mecp2 mutant and wild-type mice.
GABAergic disinhibition enhanced VSD signals in CA1 to a comparable extent in Mecp2 mutant and wild-type minislices, and isolation from CA3 inputs eliminated the differences in CA1 excitability.
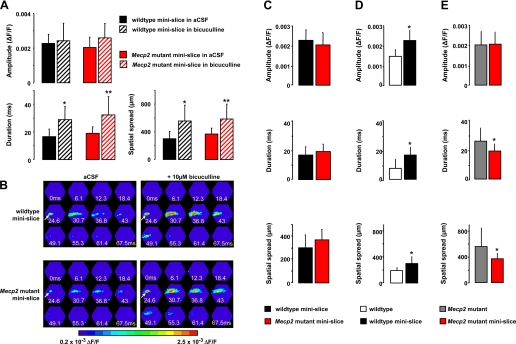
To assess the contribution of GABAergic synaptic inhibition to VSD signals evoked by Schaffer collateral stimulation in area CA1, we used the GABAA receptor antagonist bicuculline. As commonly done in electrophysiological studies, we performed these experiments in so-called CA1 minislices, where CA1 is surgically isolated from CA3 to prevent epileptiform activity generated by the loss of GABAergic inhibition in the highly interconnected CA3 pyramidal neurons.
We first tested whether blocking GABAA receptors affected VSD responses evoked by stimulating Schaffer collaterals downstream of the surgical cut, i.e., proximal to the imaging area. Consistent with blockade of GABAergic feed-forward inhibition, bicuculline (10 μM) significantly increased the duration of VSD signals in CA1 minislices from wild-type mice (16.64 ± 5.39 vs. 28.8 ± 9.6 ms, n = 10 minislices/4 mice; P < 0.05; Fig. 5A). In addition, VSD signals spread more in the presence of bicuculline (300 ± 106.1 vs. 555 ± 229.7 μm; P < 0.05), but their peak amplitude was not affected (aCSF: 2.2 × 10−3 ± 5.2 × 10−4 ΔF/F vs. 2.4 × 10−3 ± 1 × 10−3; P > 0.05; Fig. 5A). Similar effects of blocking GABAergic feed-forward inhibition were observed in CA1 minislices from Mecp2 mutant mice: the duration (18.92 ± 4.75 vs. to 32.2 ± 13.23 ms) and spatial spread (368.2 ± 85.21 vs. 586.4 ± 214.3 μm) of VSD signals were significantly increased by bicuculline (n = 11 minislices/3 mice; P < 0.05), while their amplitude was not affected (aCSF: 2 × 10−3 ± 5.9 × 10−4 ΔF/F vs. bicuculline: 2.6 × 10−3 ± 8.3 × 10−4; P > 0.05; Fig. 5A). Furthermore, the effect of bicuculline on the duration and spatial spread of VSD signals was comparable in both Mecp2 mutant and wild-type mice (P > 0.05 for all cases). Figure 5B shows representative examples of VSD responses evoked in CA1 by single Schaffer collateral stimulation before and after the application of bicuculline in minislices from a wild-type control mouse (top) and a Mecp2 mutant littermate (bottom).
Fig. 5.
Surgically isolating CA3 from CA1 in Mecp2 mutant slices prevented hyperexcitability in CA1, where GABAergic inhibition is intact. A: amplitude and spatiotemporal pattern of VSD signals evoked in area CA1 by stimulation of afferent Schaffer collaterals in the presence of bicuculline in minislices from symptomatic Mecp2 mutant and wild-type littermate controls. Data are means ± SD. *P < 0.0005, aCSF vs. bicuculline in wild-type minislices by ANOVA; **P < 0.0005, aCSF vs. bicuculline in Mecp2 mutant minislices by ANOVA. B: representative examples of the spatiotemporal pattern of VSD signals evoked in area CA1 by stimulation of afferent Schaffer collaterals in minislices from a wild-type mouse (top) and a symptomatic Mecp2 mutant littermate (bottom), before and after the application of the GABAA inhibitor bicuculline (10 μM). Arrow points to the location of the stimulating electrode. C: amplitude and spatiotemporal pattern of VSD signals evoked in area CA1 by stimulation of afferent Schaffer collaterals in minislices from symptomatic Mecp2 mutant and wild-type littermate controls. Data are means ± SD. *P < 0.05, Student's t-test of Mecp2 mutant minislices vs. wild-type minislices. D: amplitude and spatiotemporal pattern of VSD signals evoked in area CA1 by stimulation of afferent Schaffer collaterals in wild-type slices. Data are expressed as means ± SD. *P < 0.05, Student's t-test of wild-type intact slices vs. wild-type minislices. E: amplitude and spatiotemporal pattern of VSD signals evoked in area CA1 by stimulation of afferent Schaffer collaterals in Mecp2 mutant slices. Data are means ± SD. *P < 0.05, Student's t-test of Mecp2 mutant intact slices vs. Mecp2 mutant minislices.
We next assessed the consequences of the surgical isolation itself on VSD signals evoked in CA1 by stimulating Schaffer collaterals downstream of the cut. Intriguingly, the differences in VSD signals evoked in CA1 of intact slices from Mecp2 mutant and wild-type mice were absent in surgically isolated CA1 minislices (Fig. 5C): the peak amplitude of VSD signals in Mecp2 mutant minislices (2 × 10−3 ± 5.9 × 10−4 ΔF/F; n = 11 minislices/3 mice) was not significantly different than in those from wild-type mice (2.2 × 10−3 ± 5.2 × 10−4 ΔF/F; n = 10 minislices/4 mice; P > 0.05). Similarly, the duration (18.92 ± 4.75 vs. 16.64 ± 5.39 ms) and spatial spread (368.2 ± 85.21 vs. 300 ± 106.1 μm) of VSD signals in Mecp2 mutant minislices were no longer different than in those from wild-type mice (P > 0.05 in all cases).
Did surgical isolation from area CA3 decrease VSD signals in CA1 minislices from Mecp2 mutant mice to wild-type levels or increase VSD signals in wild-type slices? Surprisingly, the amplitude, duration and spatial spread of VSD signals in CA1 minislices were significantly larger than in intact slices from wild-type mice (Fig. 5D): the peak amplitude was 2.2 × 10−3 ± 5.2 × 10−4 ΔF/F in minislices (n = 10 minislices/4 mice) compared with 1.47 × 10−3 ± 0.34 × 10−3 ΔF/F in intact slices (n = 15 slices/5 mice; P < 0.05). Similarly, VSD signals were significantly longer in minislices (16.64 ± 5.39 ms) than in intact slices (7.58 ± 6.23 ms), while they spread over a larger region of CA1 (300 ± 106.1 vs. 190 ± 48 μm; P < 0.05).
In contrast, the duration and spread of VSD signals in CA1 minislices from Mecp2 mutant mice were significantly smaller compared with those evoked in intact slices (Fig. 5E): VSD signals lasted 18.92 ± 4.75 ms in Mecp2 mutant minislices (n = 11 minislices/3 mice), compared with 25.65 ± 8.84 ms in intact slices (n = 13 slices/7 mice; P < 0.05), while they spread over 368.2 ± 85.21 μm of CA1 in minislices, compared with 555.8 ± 285.3 μm in intact slices (P < 0.05). The peak amplitude of VSD signals was not significantly different in minislices (2 × 10−3 ± 5.9 × 10−4 ΔF/F) compared with intact slices from Mecp2 mutant mice (2 × 10−3 ± 0.67 × 10−3 ΔF/F; P > 0.05).
These results demonstrate that GABAergic feed-forward inhibition within area CA1 is not affected by Mecp2 deletion, and together with the observations on synaptic ultrastructure and synaptic vesicle release, strongly suggest that the hyperexcitability in CA1 originates from the upstream CA3 region.
Frequency of spontaneous multiunit activity in CA3 is higher in slices from Mecp2 mutant mice than in those from wild-type littermates.
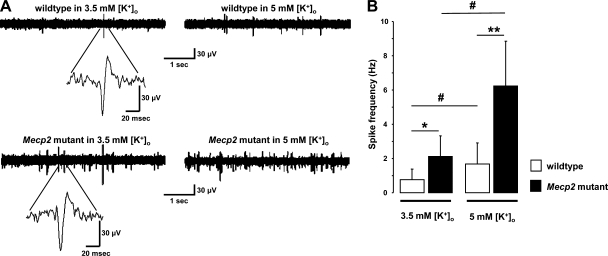
The fact that surgical isolation from area CA3 abolished the differences in VSD signals evoked in CA1 by Schaffer collateral stimulation suggested that CA3 pyramidal neuron activity contributes to the hyperexcitability observed downstream in area CA1. As revealed by extracellular multiunit recordings from CA3 s. pyramidale (Fig. 6A), the frequency of spontaneous spikes is higher in slices from (P54–62) symptomatic Mecp2 mutant mice (2.11 ± 1.22 Hz; n = 11 slices/4 mice) compared with that in slices from wild-type littermates (0.75 ± 0.63 Hz; n = 12 slices/5 mice; P < 0.05; Fig. 6B). These recordings of multiunit spikes were sensitive to differences in neuronal excitability because raising extracellular K+ from 3.5 to 5 mM significantly increased the frequency of spontaneous spikes in slices from both genotypes (P < 0.05, compared with recordings in 3.5 mM K+; Fig. 6B). Consistent with a hyperexcitable network, the effect of raising extracellular K+ on multiunit spike frequency was significantly larger in Mecp2 mutant slices (2.9-fold increase, to 6.25 ± 2.61 Hz; n = 6 slices/4 mice) than in wild-type slices (2.2-fold, to 1.68 ± 1.23 Hz; n = 8 slices/4 mice; P < 0.05; Fig. 6B).
Fig. 6.
Spontaneous neuronal firing is higher in CA3 of Mecp2 mutant mice than in wild-type littermates. A: representative traces of extracellular recordings from CA3 s. pyramidale of wild-type (top) and Mecp2 mutant slices (bottom). Insets: spontaneous multiunit spikes at higher temporal resolution. B: frequency of spontaneous multiunit spikes. Extracellular K+ concentration was raised to confirm the neuronal origin of spikes and to show the heightened susceptibility of Mecp2 mutant slices to membrane depolarization. Data are means ± SD. #P < 0.0001, wild type or Mecp2 symptomatic in 3.5 m K+ vs. 5 mM K+ by ANOVA; *P < 0.0001, symptomatic vs. wild-type in 3.5 m K+ by ANOVA; **P < 0.0001, symptomatic vs. wild-type in 5 mM K+ by ANOVA.
These results demonstrate that the level of spontaneous firing of CA3 pyramidal neurons is higher in slices from symptomatic Mecp2 mutant mice and suggest that such activity drives the hyperexcitability observed in area CA1 when stimulating Schaffer collaterals, the axons of CA3 pyramidal neurons. In the next series of experiments, we followed the spatiotemporal spread of neuronal depolarization across areas CA3 and CA1 by stimulating mossy fibers, the axons of dentate granule cells that synapse onto pyramidal neurons, and interneurons in the hilus and CA3 s. lucidum.
Spatiotemporal spread of neuronal depolarizations evoked in area CA3 by mossy fiber stimulation is enhanced in slices from Mecp2 mutant mice.
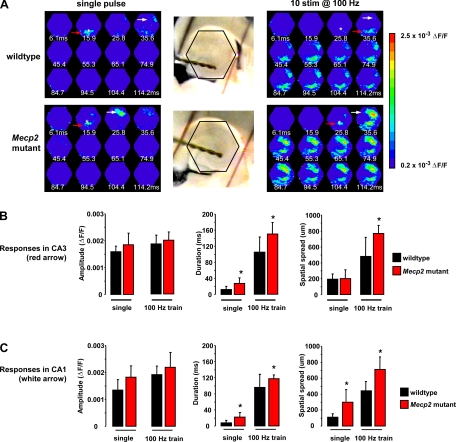
To preserve the integrity of the mossy fiber projection from the dentate gyrus to area CA3, acute slices from the ventral hippocampus were used for the next series of experiments. Figure 7A, left, shows representative examples of the spatiotemporal pattern of VSD signals evoked in area CA3 by a single stimulation pulse given to afferent mossy fibers in slices from a wild-type mouse (top) and a symptomatic (P50–61) Mecp2 mutant littermate (bottom); the asterisks point to the location of the stimulating electrode in CA3 s. lucidum, which delivered a single 20-μA current pulse (200-μs duration). VSD signals evoked by a single pulse stimulation of mossy fibers lasted significantly longer in slices from symptomatic Mecp2 mutant mice (27.2 ± 14 ms; n = 12 slices/4 mice) than in slices from wild-type littermates (12.8 ± 7.6 ms; n = 5 slices/4 mice; P = 0.048). On the other hand, neither the amplitude (Mecp2 mutant: 1.84 ± 0.43 × 10−3 ΔF/F vs. wild-type: 1.59 ± 0.21 × 10−3 ΔF/F) nor the spatial spread (Mecp2 mutant: 200 ± 112.3 μm vs. wild-type: 195 ± 30 μm) of voltage-dye signals was statistically different between slices from Mecp2 mutants and their wild-type littermates (P > 0.05; Fig. 7B).
Fig. 7.
VSD signals evoked in CA3 and CA1 by mossy fiber stimulation show frequency-dependent facilitation and were enhanced in Mecp2 mutant slices. A: representative examples of the spatiotemporal pattern of VSD signals evoked in area CA3 (red arrow) and CA1 (white arrow) by stimulation of afferent mossy fibers in slices from a symptomatic Mecp2 mutant mouse and a wild-type littermate. Asterisk shows the location of the stimulating electrode. Ventral hippocampal slices with stimulation electrodes in CA3 s. lucidum to stimulate afferent mossy fibers. Note the red LEDs marking the hexagonal photodiode array. B: amplitude and spatiotemporal pattern of VSD signals evoked in area CA3 by mossy fiber stimulation. C: amplitude and spatiotemporal pattern of VSD signals evoked in area CA1 by mossy fiber stimulation. Data are means ± SD. *P < 0.05, Student's t-test of symptomatic vs. wild-type.
To overcome the low initial release probability of mossy fiber-CA3 pyramidal neuron synapses, we next evoked VSD signals with short trains of pulses at frequencies known to induce frequency-dependent facilitation (Henze et al. 2000; McBain 2008). Indeed, VSD signals evoked in CA3 with a train of 10 stimulus pulses (200 μs, 20 μA) at 100 Hz were significantly longer lasting and spread more than those evoked by a single pulse in slices from both genotypes (Fig. 7A, right; P < 0.05 for all cases), demonstrating the consequence of frequency-dependent facilitation on neuronal depolarizations. Consistent with a hyperexcitable network, VSD signals evoked by a brief 100-Hz train lasted significantly longer in slices from Mecp2 mutant mice (149.7 ± 29.4 ms; n = 12 slices/4 mice) than those in wild-type slices (105 ± 37.97 ms; n = 10 slices/4 mice; P = 0.0055). Likewise, the spatial spread of VSD signals was wider in Mecp2 mutant slices (768.8 ± 101.8 μm) compared with that in wild-type slices (480 ± 237.7 μm; P = 0.0011). However, the amplitude of VSD signals was not significantly different between the genotypes (Mecp2 mutant: 1.99 × 10−3 ± 0.31 × 10−3 ΔF/F vs. wild-type: 1.86 × 10−3 ± 0.3 × 10−3 ΔF/F; P = 3285; Fig. 7B).
Stimulation of afferent mossy fibers also evokes larger neuronal depolarizations in area CA1 of slices from Mecp2 mutant mice.
Consistent with the spread of neuronal depolarizations through the hippocampal network as revealed by VSD imaging (Chang and Jackson 2006; Nakagami et al. 1997), a single-pulse stimulation to afferent mossy fibers also evoked VSD signals at the CA2/CA1 border, which sometimes spread over area CA1 (white arrow in Fig. 7A); these signals were also enhanced in slices from symptomatic Mecp2 mutant mice compared with those in wild-type slices. VSD signals evoked in CA2/CA1 were significantly longer in Mecp2 mutant slices (21.8 ± 11.6 ms; n = 11 slices/4 mice) than in wild-type slices (7.5 ± 6.3 ms; n = 4 slices/4 mice; P = 0.035). Likewise, VSD signals spread significantly more towards CA1 s. radiatum in Mecp2 mutant slices (300 ± 157.3 μm) than in wild-type slices (112.5 ± 43.3 μm; P = 0.0386). However, VSD signal amplitudes at CA2/CA1 were not significantly different between the genotypes (Mecp2 mutant: 1.8 ± 0.42 × 10−3 ΔF/F vs. wild-type: 1.34 ± 0.38 × 10−3 ΔF/F; P = 0.0697; Fig. 7C).
This enhanced spatiotemporal spread of neuronal depolarizations in CA1 of Mecp2 mutant slices was also evident for VSD signals evoked by brief trains of high frequency stimulation to mossy fibers (Fig. 7A). VSD signals evoked by a 100-Hz train were significantly longer in slices from Mecp2 mutant mice (118 ± 8.99 ms; n = 11 slices/4 mice) than in wild-type slices (95.78 ± 32.64 ms; n = 4 slices/4 mice; P = 0.0348). The spatial spread of VSD signals was also larger in Mecp2 mutant slices (712.5 ± 158.3 μm) than in wild-type slices (442.5 ± 119.6 μm; P = 0.0003). However, the amplitudes of VSD signals were not significantly different between the genotypes (Mecp2 mutant: 2.19 × 10−3 ± 0.55 × 10−3 ΔF/F vs. wild-type: 1.92 × 10−3 ± 0.32 × 10−3 ΔF/F; P = 1,784; Fig. 7C).
Thus the entire hippocampal network is hyperexcitable and more prone to display epileptiform activity in slices from symptomatic Mecp2 mutant mice, a condition that seems to originate from a high level of spontaneous activity in the pyramidal cell body layer of area CA3.
Adenosine reduces hippocampal excitability in slices of Mecp2 mutant mice to levels comparable to those observed in wild-type slices.
Dysfunction of endogenous neuromodulators such as adenosine has been proposed to underlie epileptiform activity (Boison 2008; DeLorenzo et al. 2007). G-protein-coupled adenosine receptors are mainly localized in presynaptic terminals, where they modulate neurotransmitter release (Wu and Saggau 1997). Adenosine inhibits glutamatergic synaptic transmission release, while GABAergic transmission is mostly unaffected in hippocampal slices (Lambert and Teyler 1991; Thompson et al. 1992; Wu and Saggau 1994), as well as in cortical slices (Mathew et al. 2008). Indeed, A1-receptor agonists are effective in seizure suppression (Boison 2008), and adenosine prevented epileptiform activity in hippocampal slices exposed to Mg2+-free aCSF (Kovac et al. 2008). Considering that it has been proposed as an anticonvulsant (Boison 2005), we tested whether adenosine could normalize hyperexcitability of hippocampal slices from Mecp2 mutant mice.
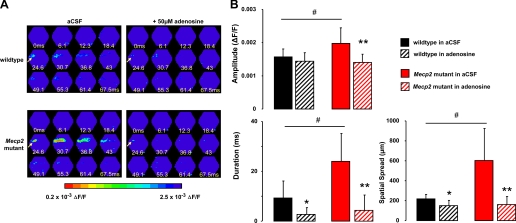
Figure 8A shows representative examples of VSD responses evoked in CA1 by single Schaffer collateral stimulation before and after the application of 50 μM adenosine in slices from a wild-type control mouse (top) and a Mecp2 mutant littermate (bottom). Consistent with a neuromodulatory action, the effect of adenosine in wild-type slices was subtle, reducing only their duration (9.35 ± 6.8 ms vs. adenosine: 2.73 ± 2.66 ms; n = 9 slices/3 mice; P = 0.0384) and spatial spread (216.7 ± 45.1 μm vs. adenosine: 150 ± 53.03 μm; P = 0.011), without affecting their amplitude (1.6 × 10−3 ± 0.2 × 10−3 ΔF/F vs. adenosine: 1.4 × 10−3 ± 0.2 × 10−3 ΔF/F; P = 0.2758; Fig. 8B). On the other hand, adenosine reduced the amplitude and spatiotemporal spread of Schaffer collateral-evoked VSD signals in CA1 of slices from symptomatic Mecp2 mutant mice. The amplitude was reduced from 2 × 10−3 ± 0.5 × 10−3 ΔF/F to 1.4 × 10−3 ± 0.2 × 10−3 ΔF/F (n = 7 slices/4 mice; P = 0.0189). Likewise, adenosine shortened the duration (23.96 ± 11.2 ms vs. adenosine: 4.39 ± 6.1 ms; P = 00161) and reduced the spatial spread (603.6 ± 321.3 μm vs. adenosine: 160.7 ± 80.2 μm; P = 0.0041; Fig. 8B). Most importantly, adenosine eliminated the differences in the amplitude and spatiotemporal spread of Schaffer collateral-evoked VSD signals in CA1 between Mecp2 mutant and wild-type slices (P > 0.05; Fig. 8B). These results are consistent with a selective neuromodulatory action by adenosine and provide support for its potential therapeutic use as an anticonvulsant in RTT individuals.
Fig. 8.
Adenosine prevents hyperexcitability in area CA1 of Mecp2 mutant slices. A: representative examples of the spatiotemporal pattern of VSD signals evoked in area CA1 by stimulation of afferent Schaffer collaterals in slices from a symptomatic Mecp2 mutant mouse and a wild-type littermate before and after the application of adenosine (50 μM). Arrow points to the location of the stimulation electrode. B: amplitude and spatiotemporal pattern of VSD signals evoked in area CA1 by stimulation of afferent Schaffer collaterals in the presence of adenosine in slices from symptomatic Mecp2 mutant and wild-type littermate controls. Data are means ± SD. *P < 0.005, aCSF vs. adenosine in wild-type slices by ANOVA; **P < 0.005, aCSF vs. adenosine in Mecp2 mutant slices by ANOVA; #P < 0.05 Mecp2 mutant vs. wild-type slices in aCSF by ANOVA.
DISCUSSION
Here, we have presented several novel observations on the excitability state of the hippocampal network in Mecp2 mutant mice. VSD imaging revealed that area CA1 is hyperexcitable and more sensitive to the epileptogenic agent 4-AP, and this feature is absent in CA1 minislices lacking excitatory input from area CA3. Together with subtle impairments in synaptic ultrastructure and activity-dependent vesicle recycling in area CA1, these results indicate that the hyperexcitability observed in CA1 originates upstream in the CA3 region. Indeed, spontaneous neuronal firing is higher in the CA3 pyramidal cell body layer of Mecp2 mutant slices than in wild-type controls. Altogether, these results provide a cellular and network mechanism underlying the characteristic hippocampal dysfunction and seizure disorders in RTT individuals and Mecp2-based mouse models of RTT.
We set out to understand the synaptic bases of network excitability in the hippocampus of Mecp2 mutant mice because of its well-known seizure susceptibility. The prevalence of reported seizures in RTT individuals is close to 80% (Glaze et al. 2010; Jian et al. 2007; Percy and Lane 2005), where the EEG presents with focal, multifocal, and generalized epileptiform abnormalities, as well as rhythmic slow (theta) activity primarily in the frontal-central regions (Glaze 2005, 2002; Glaze et al. 1987). Similar cortical EEG discharges and seizures episode were observed in Mecp2308 mice expressing a nonfunctional truncated protein (Shahbazian et al. 2002) and in Mecp2 knockout mice (D'Cruz et al. 2010; Pelka et al. 2006). It should be noted that most studies in these Mecp2-based mouse lines have used male hemizygous mice (i.e., Mecp2−/y) because they consistently develop a severe and characteristic behavioral phenotype much earlier than female heterozygous mice (i.e., Mecp2−/+), which express a mosaic pattern of wild-type and mutant cells due to X-chromosome inactivation (XCI). However, XCI is not uniform in female heterozygous mice from the Mecp2tm1.1Jae and Mecp2308/X mutant lines or from the Mecp2tm1.1Bird knockout strains, being skewed towards the wild-type Mecp2 allele (Braunschweig et al. 2004; Young and Zoghbi 2004). Intriguingly, wild-type cells in Mecp2tm1.1Jae mutant and Mecp2tm1.1Bird knockout mice express lower levels of Mecp2 protein than in wild-type mice (Braunschweig et al. 2004). Therefore, the delayed appearance and variability of the behavioral phenotypes observed in Mecp2−/+ heterozygous female mice could be caused by the combination of mosaic expression of mutant Mecp2, the degree of XCI unbalance, as well as reduced Mecp2 levels in wild-type cells. To simplify the analyses by reducing the contribution of these contributing factors, Mecp2−/y male hemizygous mice are used as a more homogenous population, which seem more amenable for experimental work in the laboratory. There are, however, a number of studies that compare RTT-like phenotypes between Mecp2−/+ heterozygous female and Mecp2−/y hemizygous male mice and their wild-type littermates, and all agree that female heterozygous mice display a delayed onset, milder phenotype than male hemizygous mice [e.g., Belichenko et al. (2009); Bissonnette and Knopp (2008); D'Cruz et al. (2010); Isoda et al. (2010); Jugloff et al. (2008); Kondo et al. (2008); Lonetti et al. (2010); Metcalf et al. (2006); Roux et al. (2010); Stearns et al. (2007); Ward et al. (2008)].
Optical imaging of VSDs directly demonstrated the widespread depolarization of granule cell dendrites by sprouting mossy fibers in kainate-treated rats (Otsu et al. 2000). The only voltage dye imaging study of the entire hippocampal circuit disruption in a chronic model of temporal lobe epilepsy revealed that the temporoammonic input is transformed from a primarily inhibitory to a powerfully excitatory input to area CA1 of slices from pilocarpine-treated rats, without changes in the gate-keeping function of the dentate gyrus and reduced coupling between CA3 to CA1 via Schaffer collaterals (Ang et al. 2006). Since there is pronounced neuronal cell loss and disruption of pyramidal cell layers in this chronic model (Dinocourt et al. 2003; Mello et al. 1993), features that are absent in Mecp2 mutant mice (Chen et al. 2001), the parsimonious interpretation of our results is that enhanced neuronal activity in a region with high levels of recurrent excitatory connections as area CA3 leads to increased excitatory drive to downstream CA1.
Whether hippocampal dysfunction in Mecp2-deficient mice results from altered intrinsic and/or synaptic functional properties or from altered neuronal and/or synaptic morphology is still unclear. For example, the lower frequency of spontaneous action potential firing in pyramidal neurons of acute cortical slices from Mecp2 mutant mice was attributed to impaired glutamatergic synaptic transmission (Dani et al. 2005). Consistently, the frequency of spontaneous action potential-independent miniature excitatory postsynaptic currents (mEPSCs) is lower in cultured hippocampal neurons from Mecp2 knockout mice than in wild-type cells, while PPF is smaller and synaptic depression during high frequency stimulation is enhanced, which suggest an increased release probability of evoked responses (Nelson et al. 2006). On the other hand, mEPSCs frequency was lower and evoked autaptic EPSCs smaller in Mecp2-deficient cultured neurons, which resulted from fewer (but with similar properties) glutamatergic autapses per neuron (Chao et al. 2007). Whether these differences are due to the widely different cell densities of micro-islands vs. mass cultures [e.g., Liu et al. (2009)] is unclear at this time.
In contrast with impaired spontaneous synaptic transmission, the smaller PPF and enhanced input/output relationship and synaptic depression during high-frequency stimulation all suggest enhanced transmitter release in Mecp2-deficient neurons (Asaka et al. 2006; Moretti et al. 2006; Nelson et al. 2006). The apparent discrepancy between spontaneous and evoked synaptic transmission is somewhat surprising but not without precedent, since it has been proposed that spontaneous tetrodotoxin-resistant mEPSCs and action potential-evoked EPSCs originate from different synaptic vesicle pools (Fredj and Burrone 2009; Mathew et al. 2008; Sara et al. 2005) [but see Groemer and Klingauf (2007), reviewed by Hablitz et al. (2009)]. In addition, the frequency of spontaneous rhythmic field potentials in area CA3 was lower in slices from Mecp2 knockout mice, rendering them prone to hyperexcitability (Zhang et al. 2008). Altogether, these alterations in glutamatergic synaptic transmission likely underlie the impairment of LTP in area CA1 of Mecp2 knockout (Asaka et al. 2006) and Mecp2308 mutant mice (Moretti et al. 2006). We interpret that impaired LTP in hippocampal slices from Mecp2-deficient mice is a consequence of a hyperexcited network with already potentiated glutamatergic synapses (i.e., occlusion by saturated LTP). Indeed, epileptiform activity in disinhibited slice cultures prevented LTP at CA3-CA3 associational connections (Debanne et al. 2006), as well as at CA3-CA1 synapses (Abegg et al. 2004). Thus impaired plasticity at hippocampal synapses could be responsible for the learning and memory impairments in animal models (Ricceri et al. 2008) and intellectual disabilities in RTT individuals (Baptista et al. 2006; von Tetzchner et al. 1996).
Our observations with VSDs are consistent with enhanced evoked synaptic transmission in area CA1 of Mecp2-deficient mice and allowed us to discover that it results from a hyperactive CA3 region and not from altered synapses within CA1. Indeed, the number of asymmetric spine synapses (presumptive excitatory) and symmetric shaft synapses (presumptive inhibitory) within CA1 s. radiatum was comparable between symptomatic Mecp2 mutant and wild-type mice. Consistently, Mecp2308 mice have comparable spine synapse densities (Moretti et al. 2006), while the levels of the presynaptic proteins synaptotagmin, synaptophysin, and synaptobrevin were not affected in Mecp2 knockout mice (Asaka et al. 2006). Intriguingly, spine synapses in Mecp2308 mice had comparable docked vesicle numbers but smaller PSDs than in wild-type controls (Moretti et al. 2006). Considering the tight correlation between the sizes of presynaptic active zones and PSDs (Harris and Sultan 1995; Schikorski and Stevens 1997), the observations in Mecp2308 mice would suggest a higher density of docked vesicles at the active zone than in wild-type controls. On the other hand, our measurements revealed fewer docked vesicles per active zone (normalized to PSD length) in both asymmetric spine and symmetric shaft synapses, without differences in any other synaptic parameters (presynaptic terminal area, PSD length, number of all vesicles per terminal, normalized to terminal area).
Would those subtle differences in synaptic vesicle distribution affect presynaptic terminal function? Indeed, multiphoton excitation imaging of FM1–43 revealed that activity-dependent release from the RRP (but not from the TRP) of presynaptic terminals in both CA1 s. radiatum (presumptive excitatory) and s. pyramidale (presumptive inhibitory) was slower in slices from Mecp2 mutant mice than in those from wild-type controls, consistent with the selective differences in docked vesicle density at both types of synapses. Alternatively, slower activity-dependent FM1–43 destaining from presumptive excitatory terminals in s. radiatum could result from reduced synaptic depression during afferent stimulation or lower initial release probability (which is inconsistent with reduced PPF and increased input-output relationship). Further experiments are needed to differentiate between these possibilities, as well as to define the relationship between initial release probability and the rate of FM1–43 destaining from sucrose-loaded RRP vesicles.
Together with the quantitative electron microscopy observations, these results indicate that CA1 hyperexcitability does not result from synaptic changes within the CA1 network, consistent with the VSD imaging results in CA1 minislices isolated from CA3 in the absence of GABAergic inhibition. Instead, hyperactivity within CA3 seems to contribute to the hyperexcitability of intact slices, as suggested by the increased frequency of spontaneous firing recorded in CA3 s. pyramidale. Furthermore, voltage-dye signals in CA3 evoked by a single stimulation pulse to mossy fibers were longer lasting in Mecp2 mutant slices, spreading to CA1 more than in wild-type slices, suggesting impaired GABAergic recruitment (McBain 2008). Consistent with frequency-dependent facilitation of mossy fiber inputs to CA3 (Lawrence et al. 2004; McBain 2008; Salin et al. 1996), a brief high-frequency train enhanced voltage dye signals in CA3, which then spread to area CA1. The fact that voltage-dye responses evoked in CA3 and CA1 by brief high-frequency stimulation lasted longer and spread more in Mecp2 mutant slices than in wild-type controls is also consistent with impaired GABAergic recruitment in CA3 by mossy fiber activity. Experiments currently under way suggest that the hyperactivity of CA3 pyramidal neurons in Mecp2 mutant slices originates from impaired GABAergic input (G. Calfa, M. Amaral, and L. Pozzo-Miller, unpublished observations).
From the developmental point of view, and consistent with a “critical period” of network hyperexcitability revealed by intracellular recordings in hippocampal and neocortical slices during the second and third postnatal weeks (Hablitz 1987; Swann and Brady 1984; Swann and Hablitz 2000), CA1 excitability was enhanced in slices from younger mice (P16 - 23) than in those from older mice (P40–55), irrespective of their genotypes. The fact that CA1 excitability was comparable in slices from presymptomatic Mecp2 mutants and young wild-type controls supports the developmental nature of the mouse disorder and RTT. In addition, these results suggest an impaired developmental reduction of network excitability likely mediated by GABAergic inhibition in the highly interconnected CA3 region.
Together with the fact that it inhibits transmitter release from glutamatergic terminals without affecting GABAergic transmission (Lambert and Teyler 1991; Mathew et al. 2008; Thompson et al. 1992; Wu and Saggau 1994), the in vitro reversal of hippocampal hyperexcitability in Mecp2 mutant slices by adenosine reported here supports its use as an anticonvulsant (Boison 2005). Adenosine also prevented epileptiform activity in hippocampal slices exposed to Mg2+ free aCSF (Kovac et al. 2008). Additional deficits in neuromodulatory signaling may contribute to our observations. Since norepinephrine modulates seizure threshold (Bengzon et al. 1999), and the locus coeruleus is the exclusive source of noradrenergic input to the hippocampus (Berridge and Waterhouse 2003), the reduced tyrosine hydroxylase content in locus coeruleus neurons and lower cortical norepinephrine levels reported in Mecp2 mutant mice (Taneja et al. 2009) are highly relevant to our observations of an hyperexcitable hippocampal network.
In summary, the hippocampal network of Mecp2 mutant mice is hyperexcitable due to a hyperactive CA3 region. Such network alterations likely underlie the characteristic hippocampal dysfunction and seizure disorders in RTT individuals and Mecp2-based mouse models of RTT.
GRANTS
This work was supported by International Rett Syndrome Foundation Postdoctoral Fellowship (to G. Calfa; IRSF0804) and by National Institute of Neurological Disorders and Stroke Grants NS40593, NS057780, and NS-065027 (to L. Pozzo-Miller).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Edward Phillips and Alison Margolies for technical assistance; Drs. Rachel Penton and Robin Lester (UAB) for sharing the extracellular field potential rig; Dr. Takafumi Inoue (Waseda University, Tokyo, Japan) for acquisition and analysis software; the Molecular Engineering Core of the Alabama Neuroscience Blueprint Center for mouse genotyping; and the UAB High Resolution Imaging Facility, UAB Intellectual and Developmental Disabilities Research Center (P30-HD38985), and UAB Neuroscience and Blueprint Cores (P30-NS47466, P30-NS57098) for use of the electron microscope and laser scanning multiphoton excitation microscope.
REFERENCES
- Abegg MH, Savic N, Ehrengruber MU, McKinney RA, Gahwiler BH. Epileptiform activity in rat hippocampus strengthens excitatory synapses. J Physiol 554: 439–448, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aihara T, Kobayashi Y, Tsukada M. Spatiotemporal visualization of long-term potentiation and depression in the hippocampal CA1 area. Hippocampus 15: 68–78, 2005 [DOI] [PubMed] [Google Scholar]
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet 23: 185–188, 1999 [DOI] [PubMed] [Google Scholar]
- Andersen P, Silfvenius H, Sundberg SH, Sveen O. A comparison of distal and proximal dendritic synapses on CA1 pyramids in guinea-pig hippocampal slices in vitro. J Physiol 307: 273–299, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang CW, Carlson GC, Coulter DA. Massive and specific dysregulation of direct cortical input to the hippocampus in temporal lobe epilepsy. J Neurosci 26: 11850–11856, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong DD. Review of Rett syndrome. J Neuropathol Exp Neurol 56: 843–849, 1997 [DOI] [PubMed] [Google Scholar]
- Asaka Y, Jugloff DG, Zhang L, Eubanks JH, Fitzsimonds RM. Hippocampal synaptic plasticity is impaired in the Mecp2-null mouse model of Rett syndrome. Neurobiol Dis 21: 217–227, 2006 [DOI] [PubMed] [Google Scholar]
- Axmacher N, Winterer J, Stanton PK, Draguhn A, Muller W. Two-photon imaging of spontaneous vesicular release in acute brain slices and its modulation by presynaptic GABAA receptors. Neuroimage 22: 1014–1021, 2004 [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay S, Gonzalez-Islas C, Hablitz JJ. Dopamine enhances spatiotemporal spread of activity in rat prefrontal cortex. J Neurophysiol 93: 864–872, 2005 [DOI] [PubMed] [Google Scholar]
- Baptista PM, Mercadante MT, Macedo EC, Schwartzman JS. Cognitive performance in Rett syndrome girls: a pilot study using eyetracking technology. J Intellect Disabil Res 50: 662–666, 2006 [DOI] [PubMed] [Google Scholar]
- Belichenko NP, Belichenko PV, Mobley WC. Evidence for both neuronal cell autonomous and nonautonomous effects of methyl-CpG-binding protein 2 in the cerebral cortex of female mice with Mecp2 mutation. Neurobiol Dis 34: 71–77, 2009 [DOI] [PubMed] [Google Scholar]
- Bengzon J, Hansson SR, Hoffman BJ, Lindvall O. Regulation of norepinephrine transporter and tyrosine hydroxylase mRNAs after kainic acid-induced seizures. Brain Res 842: 239–242, 1999 [DOI] [PubMed] [Google Scholar]
- Bergles DE, Jahr CE. Synaptic activation of glutamate transporters in hippocampal astrocytes. Neuron 19: 1297–1308, 1997 [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev 42: 33–84, 2003 [DOI] [PubMed] [Google Scholar]
- Bissonnette JM, Knopp SJ. Effect of inspired oxygen on periodic breathing in methy-CpG-binding protein 2 (Mecp2) deficient mice. J Appl Physiol 104: 198–204, 2008 [DOI] [PubMed] [Google Scholar]
- Boison D. Adenosine and epilepsy: from therapeutic rationale to new therapeutic strategies. Neuroscientist 11: 25–36, 2005 [DOI] [PubMed] [Google Scholar]
- Boison D. The adenosine kinase hypothesis of epileptogenesis. Prog Neurobiol 84: 249–262, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brager DH, Luther PW, Erdelyi F, Szabo G, Alger BE. Regulation of exocytosis from single visualized GABAergic boutons in hippocampal slices. J Neurosci 23: 10475–10486, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunschweig D, Simcox T, Samaco RC, LaSalle JM. X-Chromosome inactivation ratios affect wild-type MeCP2 expression within mosaic Rett syndrome and Mecp2−/+ mouse brain. Hum Mol Genet 13: 1275–1286, 2004 [DOI] [PubMed] [Google Scholar]
- Buckle PJ, Haas HL. Enhancement of synaptic transmission by 4-aminopyridine in hippocampal slices of the rat. J Physiol 326: 109–122, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson GC, Coulter DA. In vitro functional imaging in brain slices using fast voltage-sensitive dye imaging combined with whole-cell patch recording. Nat Protoc 3: 249–255, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, Zoghbi HY. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science 320: 1224–1229, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahrour M, Zoghbi HY. The story of Rett syndrome: from clinic to neurobiology. Neuron 56: 422–437, 2007 [DOI] [PubMed] [Google Scholar]
- Chang PY, Jackson MB. Heterogeneous spatial patterns of long-term potentiation in rat hippocampal slices. J Physiol 576: 427–443, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang PY, Jackson MB. Interpretation and optimization of absorbance and fluorescence signals from voltage-sensitive dyes. J Membr Biol 196: 105–116, 2003 [DOI] [PubMed] [Google Scholar]
- Chao HT, Zoghbi HY, Rosenmund C. MeCP2 controls excitatory synaptic strength by regulating glutamatergic synapse number. Neuron 56: 58–65, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RZ, Akbarian S, Tudor M, Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat Genet 27: 327–331, 2001 [DOI] [PubMed] [Google Scholar]
- Cohen I, Miles R. Contributions of intrinsic and synaptic activities to the generation of neuronal discharges in in vitro hippocampus. J Physiol 524: 485–502, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Cruz JA, Wu C, Zahid T, El-Hayek Y, Zhang L, Eubanks JH. Alterations of cortical and hippocampal EEG activity in MeCP2-deficient mice. Neurobiol Dis 38: 8–16, 2010 [DOI] [PubMed] [Google Scholar]
- Dani VS, Chang Q, Maffei A, Turrigiano GG, Jaenisch R, Nelson SB. Reduced cortical activity due to a shift in the balance between excitation and inhibition in a mouse model of Rett syndrome. Proc Natl Acad Sci USA 102: 12560–12565, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debanne D, Thompson SM, Gahwiler BH. A brief period of epileptiform activity strengthens excitatory synapses in the rat hippocampus in vitro. Epilepsia 47: 247–256, 2006 [DOI] [PubMed] [Google Scholar]
- DeLorenzo RJ, Sun DA, Blair RE, Sombati S. An in vitro model of stroke-induced epilepsy: elucidation of the roles of glutamate and calcium in the induction and maintenance of stroke-induced epileptogenesis. Int Rev Neurobiol 81: 59–84, 2007 [DOI] [PubMed] [Google Scholar]
- Diamond JS, Bergles DE, Jahr CE. Glutamate release monitored with astrocyte transporter currents during LTP. Neuron 21: 425–433, 1998 [DOI] [PubMed] [Google Scholar]
- Dickinson-Nelson A, Reese TS. Structural changes during transmitter release at synapses in the frog sympathetic ganglion. J Neurosci 3: 42–52, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinocourt C, Petanjek Z, Freund TF, Ben-Ari Y, Esclapez M. Loss of interneurons innervating pyramidal cell dendrites and axon initial segments in the CA1 region of the hippocampus following pilocarpine-induced seizures. J Comp Neurol 459: 407–425, 2003 [DOI] [PubMed] [Google Scholar]
- Erdfelder E, Faul F, Buchner A. GPOWER: A general power analysis program. Behav Res Methods 28: 1–11, 1996 [DOI] [PubMed] [Google Scholar]
- Fredj NB, Burrone J. A resting pool of vesicles is responsible for spontaneous vesicle fusion at the synapse. Nat Neurosci 12: 751–758, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaze DG. Neurophysiology of Rett syndrome. J Child Neurol 20: 740–746, 2005 [DOI] [PubMed] [Google Scholar]
- Glaze DG. Neurophysiology of Rett syndrome. Ment Retard Dev Disabil Res Rev 8: 66–71, 2002 [DOI] [PubMed] [Google Scholar]
- Glaze DG, Frost JD, Jr, Zoghbi HY, Percy AK. Rett's syndrome: correlation of electroencephalographic characteristics with clinical staging. Arch Neurol 44: 1053–1056, 1987 [DOI] [PubMed] [Google Scholar]
- Glaze DG, Percy AK, Skinner S, Motil KJ, Neul JL, Barrish JO, Lane JB, Geerts SP, Annese F, Graham J, McNair L, Lee HS. Epilepsy and the natural history of Rett syndrome. Neurology 74: 909–912, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaze DG, Schultz RJ, Frost JD. Rett syndrome: characterization of seizures versus non-seizures. Electroencephalogr Clin Neurophysiol 106: 79–83, 1998 [DOI] [PubMed] [Google Scholar]
- Grinvald A, Frostig RD, Lieke E, Hildesheim R. Optical imaging of neuronal activity. Physiol Rev 68: 1285–1366, 1988 [DOI] [PubMed] [Google Scholar]
- Grinvald A, Manker A, Segal M. Visualization of the spread of electrical activity in rat hippocampal slices by voltage-sensitive optical probes. J Physiol 333: 269–291, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groemer TW, Klingauf J. Synaptic vesicles recycling spontaneously and during activity belong to the same vesicle pool. Nat Neurosci 10: 145–147, 2007 [DOI] [PubMed] [Google Scholar]
- Guy J, Hendrich B, Holmes M, Martin JE, Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat Genet 27: 322–326, 2001 [DOI] [PubMed] [Google Scholar]
- Hablitz JJ. Spontaneous ictal-like discharges and sustained potential shifts in the developing rat neocortex. J Neurophysiol 58: 1052–1065, 1987 [DOI] [PubMed] [Google Scholar]
- Hablitz JJ, Mathew SS, Pozzo-Miller L. GABA vesicles at synapses: are there 2 distinct pools? Neuroscientist 15: 218–224, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg B, Aicardi J, Dias K, Ramos O. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett's syndrome: report of 35 cases. Ann Neurol 14: 471–479, 1983 [DOI] [PubMed] [Google Scholar]
- Harris KM, Sultan P. Variation in the number, location and size of synaptic vesicles provides an anatomical basis for the nonuniform probability of release at hippocampal CA1 synapses. Neuropharmacology 34: 1387–1395, 1995 [DOI] [PubMed] [Google Scholar]
- Henze DA, Urban NN, Barrionuevo G. The multifarious hippocampal mossy fiber pathway: a review. Neuroscience 98: 407–427, 2000 [DOI] [PubMed] [Google Scholar]
- Hosokawa T, Ohta M, Saito T, Fine A. Imaging spatio-temporal patterns of long-term potentiation in mouse hippocampus. Philos Trans R Soc Lond B Biol Sci 358: 689–693, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppke P, Kohler K, Brockmann K, Stettner GM, Gartner J. Treatment of epilepsy in Rett syndrome. Eur J Paediatr Neurol 11: 10–16, 2007 [DOI] [PubMed] [Google Scholar]
- Isoda K, Morimoto M, Matsui F, Hasegawa T, Tozawa T, Morioka S, Chiyonobu T, Nishimura A, Yoshimoto K, Hosoi H. Postnatal changes in serotonergic innervation to the hippocampus of methyl-CpG-binding protein 2-null mice. Neuroscience 165: 1254–1260, 2010 [DOI] [PubMed] [Google Scholar]
- Jian L, Nagarajan L, de Klerk N, Ravine D, Bower C, Anderson A, Williamson S, Christodoulou J, Leonard H. Predictors of seizure onset in Rett syndrome. J Pediatr 149: 542–547, 2006 [DOI] [PubMed] [Google Scholar]
- Jian L, Nagarajan L, de Klerk N, Ravine D, Christodoulou J, Leonard H. Seizures in Rett syndrome: an overview from a one-year calendar study. Eur J Paediatr Neurol 11: 310–317, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jugloff DG, Vandamme K, Logan R, Visanji NP, Brotchie JM, Eubanks JH. Targeted delivery of an Mecp2 transgene to forebrain neurons improves the behavior of female Mecp2-deficient mice. Hum Mol Genet 17: 1386–1396, 2008 [DOI] [PubMed] [Google Scholar]
- Kojima S, Nakamura T, Nidaira T, Nakamura K, Ooashi N, Ito E, Watase K, Tanaka K, Wada K, Kudo Y, Miyakawa H. Optical detection of synaptically induced glutamate transport in hippocampal slices. J Neurosci 19: 2580–2588, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo M, Gray LJ, Pelka GJ, Christodoulou J, Tam PP, Hannan AJ. Environmental enrichment ameliorates a motor coordination deficit in a mouse model of Rett syndrome–Mecp2 gene dosage effects and BDNF expression. Eur J Neurosci 27: 3342–3350, 2008 [DOI] [PubMed] [Google Scholar]
- Kovac S, Sirin Y, Speckmann EJ, Gorji A. Different regional neuroinhibitory effects of adenosine on stimulus-induced patterns of bioelectric activity of rat hippocampal and neocortical tissues. Neuroscience 152: 547–557, 2008 [DOI] [PubMed] [Google Scholar]
- Kushner SA, Elgersma Y, Murphy GG, Jaarsma D, van Woerden GM, Hojjati MR, Cui Y, LeBoutillier JC, Marrone DF, Choi ES, De Zeeuw CI, Petit TL, Pozzo-Miller L, Silva AJ. Modulation of presynaptic plasticity and learning by the H-ras/extracellular signal-regulated kinase/synapsin I signaling pathway. J Neurosci 25: 9721–9734, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert NA, Teyler TJ. Adenosine depresses excitatory but not fast inhibitory synaptic transmission in area CA1 of the rat hippocampus. Neurosci Lett 122: 50–52, 1991 [DOI] [PubMed] [Google Scholar]
- Lawrence JJ, Grinspan ZM, McBain CJ. Quantal transmission at mossy fibre targets in the CA3 region of the rat hippocampus. J Physiol 554: 175–193, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Dean C, Arthur CP, Dong M, Chapman ER. Autapses and networks of hippocampal neurons exhibit distinct synaptic transmission phenotypes in the absence of synaptotagmin I. J Neurosci 29: 7395–7403, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonetti G, Angelucci A, Morando L, Boggio EM, Giustetto M, Pizzorusso T. Early environmental enrichment moderates the behavioral and synaptic phenotype of MeCP2 null mice. Biol Psychiatry 67: 657–665, 2010 [DOI] [PubMed] [Google Scholar]
- Luscher C, Malenka RC, Nicoll RA. Monitoring glutamate release during LTP with glial transporter currents. Neuron 21: 435–441, 1998 [DOI] [PubMed] [Google Scholar]
- Mathew SS, Pozzo-Miller L, Hablitz JJ. Kainate modulates presynaptic GABA release from two vesicle pools. J Neurosci 28: 725–731, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain CJ. Differential mechanisms of transmission and plasticity at mossy fiber synapses. Prog Brain Res 169: 225–240, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello LE, Cavalheiro EA, Tan AM, Kupfer WR, Pretorius JK, Babb TL, Finch DM. Circuit mechanisms of seizures in the pilocarpine model of chronic epilepsy: cell loss and mossy fiber sprouting. Epilepsia 34: 985–995, 1993 [DOI] [PubMed] [Google Scholar]
- Metcalf BM, Mullaney BC, Johnston MV, Blue ME. Temporal shift in methyl-CpG binding protein 2 expression in a mouse model of Rett syndrome. Neuroscience 139: 1449–1460, 2006 [DOI] [PubMed] [Google Scholar]
- Momose-Sato Y, Sato K, Arai Y, Yazawa I, Mochida H, Kamino K. Evaluation of voltage-sensitive dyes for long-term recording of neural activity in the hippocampus. J Membr Biol 172: 145–157, 1999 [DOI] [PubMed] [Google Scholar]
- Moretti P, Levenson JM, Battaglia F, Atkinson R, Teague R, Antalffy B, Armstrong D, Arancio O, Sweatt JD, Zoghbi HY. Learning and memory and synaptic plasticity are impaired in a mouse model of Rett syndrome. J Neurosci 26: 319–327, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagami Y, Saito H, Matsuki N. Optical recording of trisynaptic pathway in rat hippocampal slices with a voltage-sensitive dye. Neuroscience 81: 1–8, 1997 [DOI] [PubMed] [Google Scholar]
- Nan X, Ng HH, Johnson CA, Laherty CD, Turner BM, Eisenman RN, Bird A. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature 393: 386–389, 1998 [DOI] [PubMed] [Google Scholar]
- Nelson ED, Kavalali ET, Monteggia LM. MeCP2-dependent transcriptional repression regulates excitatory neurotransmission. Curr Biol 16: 710–716, 2006 [DOI] [PubMed] [Google Scholar]
- Neul JL, Zoghbi HY. Rett syndrome: a prototypical neurodevelopmental disorder. Neuroscientist 10: 118–128, 2004 [DOI] [PubMed] [Google Scholar]
- Otsu Y, Maru E, Ohata H, Takashima I, Kajiwara R, Iijima T. Optical recording study of granule cell activities in the hippocampal dentate gyrus of kainate-treated rats. J Neurophysiol 83: 2421–2430, 2000 [DOI] [PubMed] [Google Scholar]
- Pelka GJ, Watson CM, Radziewic T, Hayward M, Lahooti H, Christodoulou J, Tam PP. Mecp2 deficiency is associated with learning and cognitive deficits and altered gene activity in the hippocampal region of mice. Brain 129: 887–898, 2006 [DOI] [PubMed] [Google Scholar]
- Percy AK, Lane JB. Rett syndrome: model of neurodevelopmental disorders. J Child Neurol 20: 718–721, 2005 [DOI] [PubMed] [Google Scholar]
- Perreault P, Avoli M. Physiology and pharmacology of epileptiform activity induced by 4-aminopyridine in rat hippocampal slices. J Neurophysiol 65: 771–785, 1991 [DOI] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster H. The Fine Structure of the Nervous System. Neurons and Their Supporting Cells. New York: Oxford University Press, 1991 [Google Scholar]
- Pozzo-Miller LD, Gottschalk W, Zhang L, McDermott K, Du J, Gopalakrishnan R, Oho C, Sheng ZH, Lu B. Impairments in high-frequency transmission, synaptic vesicle docking, and synaptic protein distribution in the hippocampus of BDNF knockout mice. J Neurosci 19: 4972–4983, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricceri L, De Filippis B, Laviola G. Mouse models of Rett syndrome: from behavioural phenotyping to preclinical evaluation of new therapeutic approaches. Behav Pharmacol 19: 501–517, 2008 [DOI] [PubMed] [Google Scholar]
- Roux JC, Panayotis N, Dura E, Villard L. Progressive noradrenergic deficits in the locus coeruleus of Mecp2 deficient mice. J Neurosci Res 88: 1500–1509, 2010 [DOI] [PubMed] [Google Scholar]
- Rutecki PA, Lebeda FJ, Johnston D. 4-Aminopyridine produces epileptiform activity in hippocampus and enhances synaptic excitation and inhibition. J Neurophysiol 57: 1911–1924, 1987 [DOI] [PubMed] [Google Scholar]
- Saggau P, Galvan M, ten Bruggencate G. Long-term potentiation in guinea pig hippocampal slices monitored by optical recording of neuronal activity. Neurosci Lett 69: 53–58, 1986 [DOI] [PubMed] [Google Scholar]
- Salin PA, Scanziani M, Malenka RC, Nicoll RA. Distinct short-term plasticity at two excitatory synapses in the hippocampus. Proc Natl Acad Sci USA 93: 13304–13309, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara Y, Virmani T, Deak F, Liu X, Kavalali ET. An isolated pool of vesicles recycles at rest and drives spontaneous neurotransmission. Neuron 45: 563–573, 2005 [DOI] [PubMed] [Google Scholar]
- Schikorski T, Stevens CF. Quantitative ultrastructural analysis of hippocampal excitatory synapses. J Neurosci 17: 5858–5867, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazian M, Young J, Yuva-Paylor L, Spencer C, Antalffy B, Noebels J, Armstrong D, Paylor R, Zoghbi H. Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron 35: 243–254, 2002 [DOI] [PubMed] [Google Scholar]
- Spruston N, McBain C. Structural and functional properties of hippocampal neurons. In: The Hippocampus Book, edited by Andersen P, Morris R, Amaral D, Bliss T, O'Keefe J. New York: Oxford University Press, 2007, p. 133–201 [Google Scholar]
- Stanton PK, Winterer J, Bailey CP, Kyrozis A, Raginov I, Laube G, Veh RW, Nguyen CQ, Muller W. Long-term depression of presynaptic release from the readily releasable vesicle pool induced by NMDA receptor-dependent retrograde nitric oxide. J Neurosci 23: 5936–5944, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton PK, Winterer J, Zhang XL, Muller W. Imaging LTP of presynaptic release of FM1–43 from the rapidly recycling vesicle pool of Schaffer collateral-CA1 synapses in rat hippocampal slices. Eur J Neurosci 22: 2451–2461, 2005 [DOI] [PubMed] [Google Scholar]
- Stearns NA, Schaevitz LR, Bowling H, Nag N, Berger UV, Berger-Sweeney J. Behavioral and anatomical abnormalities in Mecp2 mutant mice: a model for Rett syndrome. Neuroscience 146: 907–921, 2007 [DOI] [PubMed] [Google Scholar]
- Steffenburg U, Hagberg G, Hagberg B. Epilepsy in a representative series of Rett syndrome. Acta Paediatr 90: 34–39, 2001 [DOI] [PubMed] [Google Scholar]
- Storm JF. Potassium currents in hippocampal pyramidal cells. Prog Brain Res 83: 161–187, 1990 [DOI] [PubMed] [Google Scholar]
- Swann JW, Brady RJ. Penicillin-induced epileptogenesis in immature rat CA3 hippocampal pyramidal cells. Brain Res 314: 243–254, 1984 [DOI] [PubMed] [Google Scholar]
- Swann JW, Hablitz JJ. Cellular abnormalities and synaptic plasticity in seizure disorders of the immature nervous system. Ment Retard Dev Disabil Res Rev 6: 258–267, 2000 [DOI] [PubMed] [Google Scholar]
- Taneja P, Ogier M, Brooks-Harris G, Schmid DA, Katz DM, Nelson SB. Pathophysiology of locus ceruleus neurons in a mouse model of Rett syndrome. J Neurosci 29: 12187–12195, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia N, Du J, Tyler WJ, Neale E, Pozzo-Miller L, Lu B. Protein synthesis-dependent and -independent regulation of hippocampal synapses by brain-derived neurotrophic factor. J Biol Chem 276: 37585–37593, 2001 [DOI] [PubMed] [Google Scholar]
- Thompson SM, Haas HL, Gahwiler BH. Comparison of the actions of adenosine at pre and postsynaptic receptors in the rat hippocampus in vitro. J Physiol 451: 347–363, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WJ, Pozzo-Miller LD. BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses. J Neurosci 21: 4249–4258, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WJ, Zhang XL, Hartman K, Winterer J, Muller W, Stanton PK, Pozzo-Miller L. BDNF increases release probability and the size of a rapidly recycling vesicle pool within rat hippocampal excitatory synapses. J Physiol 574: 787–803, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Tetzchner S, Jacobsen KH, Smith L, Skjeldal OH, Heiberg A, Fagan JF. Vision, cognition and developmental characteristics of girls and women with Rett syndrome. Dev Med Child Neurol 38: 212–225, 1996 [DOI] [PubMed] [Google Scholar]
- Voskuyl RA, Albus H. Spontaneous epileptiform discharges in hippocampal slices induced by 4-aminopyridine. Brain Res 342: 54–66, 1985 [DOI] [PubMed] [Google Scholar]
- Ward BC, Agarwal S, Wang K, Berger-Sweeney J, Kolodny NH. Longitudinal brain MRI study in a mouse model of Rett Syndrome and the effects of choline. Neurobiol Dis 31: 110–119, 2008 [DOI] [PubMed] [Google Scholar]
- Winterer J, Stanton PK, Müller W. Direct monitoring of vesicular release and uptake in brain slices by multiphoton excitation of the styryl dye FM1–43. Biotechniques 40: 343–351, 2006 [DOI] [PubMed] [Google Scholar]
- Wu LG, Saggau P. Adenosine inhibits evoked synaptic transmission primarily by reducing presynaptic calcium influx in area CA1 of hippocampus. Neuron 12: 1139–1148, 1994 [DOI] [PubMed] [Google Scholar]
- Wu LG, Saggau P. Presynaptic inhibition of elicited neurotransmitter release. Trends Neurosci 20: 204–212, 1997 [DOI] [PubMed] [Google Scholar]
- Young JI, Zoghbi HY. X-chromosome inactivation patterns are unbalanced and affect the phenotypic outcome in a mouse model of rett syndrome. Am J Hum Genet 74: 511–520, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, He J, Jugloff DG, Eubanks JH. The MeCP2-null mouse hippocampus displays altered basal inhibitory rhythms and is prone to hyperexcitability. Hippocampus 18: 294–309, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.