Abstract
In the stationary hand, static joint-position sense originates from multimodal somatosensory input (e.g., joint, skin, and muscle). In the moving hand, however, it is uncertain how movement sense arises from these different submodalities of proprioceptors. In contrast to static-position sense, movement sense includes multiple parameters such as motion detection, direction, joint angle, and velocity. Because movement sense is both multimodal and multiparametric, it is not known how different movement parameters are represented by different afferent submodalities. In theory, each submodality could redundantly represent all movement parameters, or, alternatively, different afferent submodalities could be tuned to distinctly different movement parameters. The study described in this paper investigated how skin input and muscle input each contributes to movement sense of the hand, in particular, to the movement parameters dynamic position and velocity. Healthy adult subjects were instructed to indicate with the left hand when they sensed the unseen fingers of the right hand being passively flexed at the metacarpophalangeal (MCP) joint through a previously learned target angle. The experimental approach was to suppress input from skin and/or muscle: skin input by anesthetizing the hand, and muscle input by unexpectedly extending the wrist to prevent MCP flexion from stretching the finger extensor muscle. Input from joint afferents was assumed not to play a significant role because the task was carried out with the MCP joints near their neutral positions. We found that, during passive finger movement near the neutral position in healthy adult humans, both skin and muscle receptors contribute to movement sense but qualitatively differently. Whereas skin input contributes to both dynamic position and velocity sense, muscle input may contribute only to velocity sense.
Keywords: anesthesia, cutaneous, muscle spindle, proprioception
the proprioceptive system is relatively unique because its input arises from several different receptor submodalities located in different tissues (i.e., skin, joint capsule, tendon, muscle, ligamentous, and connective tissue; e.g., Windhorst 2007). Since the late 1970s, until recent times, muscle spindles were viewed by many as the most important of these receptors (Burgess et al. 1982; Gandevia 1996; Matthews 1988; McCloskey 1978), presumably exerting a dominant influence on the senses of body posture and movement (Proske and Gandevia 2009). However, during nerve recordings from stretch and pressure receptors in the skin of the hand (Burke et al. 1988; Edin and Abbs 1991; Edin 1992; 2004; Grill and Hallett 1995; Hulliger et al. 1979) and the knee (Edin 2001), skin receptors have been shown to fire reliably in relation to the position and movement of nearby joints (Aimonetti et al. 2007). Psychophysical analyses of healthy human subjects have shown that motion detection and sensations of direction and total joint displacement occur when skin receptors are stimulated electrically or by skin stretch (Collins and Prochazka 1996; Collins et al. 2000; Collins et al. 2005; Edin and Johansson 1995). When skin stretch was combined with muscle vibration, the latter being a stimulus for muscle spindle Ia afferents and skin receptors in the vicinity of the vibrator, the psychophysical effects of the input from cutaneous and muscle afferents appeared to add to each other, albeit not proportionally (Collins et al. 2000; Collins et al. 2005). Parallel investigations have also implicated articular afferents as contributing information about position and movement in the hand, again in an additive manner with skin and muscle afferents (Ferrell et al. 1987; Ferrell and Craske 1992; Gandevia and McCloskey 1976). The additive nature of each of these signals, proportionally or not, suggests that each type of afferent responds redundantly to the same motion parameter(s), the sum of which determines the magnitude of sensation. However, it remains unclear how each afferent submodality differentially contributes to the quality of sensation.
Although these previous studies provide convincing evidence of nonmuscular afferent contributions to position and movement sense, the quality of these various contributions remains unclear. In many previous studies, psychophysical assessment was made with the limb held stationary (e.g., Collins and Prochazka 1996; Collins et al. 2000; Collins et al. 2005; Edin and Johansson 1995) and with artificial sensory stimulation of only one or two types of afferents without stimulation of the remaining proprioceptive submodalities normally activated by movement. Moreover, these studies quantified only a limited component of movement sense, including simple motion detection, direction, and the final extent of motion (e.g., Gandevia and McCloskey 1976). These studies did not quantify movement sense during movement because they measured the sense of displacement at the end of a movement (i.e., at zero velocity), or they evaluated movement illusions in the absence of real movement.
Movement sense is actually more complex and multiparametric than many previous studies imply. Movement sense, as a whole, consists of the senses of motion, direction, dynamic (instantaneous) position, and velocity, each of these parameters being represented by one or more types of sensory receptors. One cannot assume that stimulation of any particular type of afferent influences the senses of all movement parameters quantitatively or qualitatively in the same way because it is not presently understood how each submodality contributes to an integrated sense of movement. Moreover, to assess the specific contributions of different proprioceptive afferents to the senses of dynamic position and velocity, neither of which has been investigated in these previous studies, dynamic position and velocity need to be quantified during an actual (i.e., not illusory) movement.
The study reported here investigated how skin input and muscle input contribute to movement sense of the hand, focusing on the senses of dynamic position and velocity during movement. The experiment was designed to investigate whether the movement information contributed by skin and muscle receptors is parametrically redundant or distinct. Healthy human subjects performed a movement task requiring them to indicate with one hand when the unseen fingers of the other hand were rotated through a target (see also Cordo 1990; Cordo et al. 1994; Bevan et al. 1994; Cordo et al. 1995). The overall bias and variability of positional errors were used to quantify dynamic position sense, and the dependence of bias errors on movement velocity was used as an indicator of velocity sense (Cordo et al. 1994).
Four experimental conditions were used: 1) no intervention, 2) skin input suppressed, 3) muscle input suppressed, and 4) both skin and muscle input suppressed. Skin input was suppressed by regional anesthesia of the entire hand and distal forearm, and muscle input was suppressed by passively extending the wrist joint during metacarpophalangeal (MCP) flexion, to minimize stretch of the extensor digitorum by MCP flexion. Because the task was performed near the neutral positions of the MCP joints, joint afferents were assumed not to contribute significantly to movement sense in this task (Burgess and Clark 1969; Burke et al. 1988; Ferrell 1980; Skoglund 1956).
METHODS
Subjects.
Seventeen healthy adult human subjects (ages 20–57 yr) were recruited to participate in this study after providing written informed consent according to procedures approved by the Oregon Health and Science University Institutional Review Board. Nine of the 17 participated in the main experiment reported here. Subjects qualified for the study if they had no history of neuromuscular or somatosensory deficits or allergic reactions to local anesthetics. The subjects received monetary compensation for their participation.
Experimental apparatus.
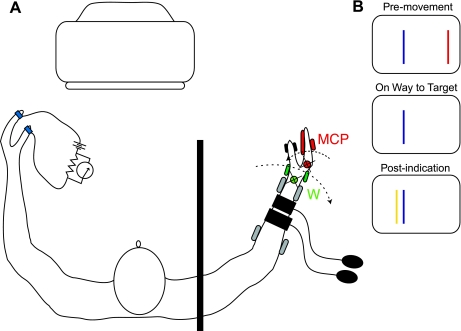
Each subject was seated at a table, with the right forearm resting in a limb-motion device that passively flexed all four fingers of the right hand at the MCP joints and with the left forearm resting freely on an armrest. During MCP motion, a load cell measured the combined resistive torque of the four fingers (Fig. 1A). The limb-motion device was also used to extend passively the subject's wrist. Two different motors actuated finger motion and wrist motion, each of which was controlled independently by microprocessors. In the occasional trial when the wrist was extended, this wrist movement usually coincided with finger flexion although the converse was not true; most trials with finger motion did not include wrist motion. The subject's right upper arm was abducted about 30 degrees at the shoulder, and the right elbow was flexed to an angle chosen by each participant on the basis of comfort (typically ∼120 degrees). Two U-shaped padded cuffs supported and constrained the right forearm just proximal to the wrist and just distal to the elbow (2 pairs of gray ellipsoids in Fig. 1A). The right hand was inserted into a fixture so that the device held the hand in a thumb-up position. The right thumb, held in an extended posture, rested on a stationary, curved platform (pair of black ellipsoids) that did not move with the fingers; this thumb platform held the thumb out of the way of the finger motion. The axes of rotation for MCP and wrist motion were adjusted to correspond to those of the subject's actual finger MCP and wrist joints (red and green circles and Xs). A metal plate and a rigid pad (pair of red ellipsoids) held the interphalangeal joints of the four fingers at 13 degrees, 0 degrees representing the fingers extending in a straight line from the dorsum of the hand. Once the arm was resting in the device, imposed hand movements were limited to flexion and extension at the wrist and/or flexion and extension at the four MCP joints of the fingers. Each motor controlled the velocity, direction, and angle of either finger or wrist motion. The thumb and index finger of the left hand were fit with a light-weight, low-voltage (5 V) contact device that signaled the loss of contact between the tips of these two digits.
Fig. 1.
Experimental setup. A: top-down view with the subject sitting at the limb-motion device. Two motors apply movement independently to the hand, one at the wrist (green) and the other at the fingers (red). Two sphygmomometer cuffs are applied to the right forearm and a contact device to the left thumb and index finger. An opaque shield (vertical black bar) prevented the subject from seeing the right arm. A computer monitor provided visual feedback. B: graphical presentation of constant error (CE) at 3 points during a trial; red line denotes the MCP starting position, blue line denotes the target angle, and yellow line denotes the metacarpophalangeal (MCP) angle at which the subject indicated target arrival, in this example resulting in a small overshoot. The distance from the red line to the blue line is equivalent to 12 degrees of MCP flexion.
Target-detection task.
One second after a computer-generated tone, the device passively flexed all four fingers of the right hand at the MCP joints, beginning from 13 degrees and ending at 33 degrees, a total of 20-degree flexion. To ensure that the subjects detected this finger motion on the basis of the sensory input associated with the actual movement, rather than taking a cue from a preparatory tone, catch trials were incorporated pseudorandomly into the trial sequence (see below). The task required of the subject in this experiment was to indicate when the fingers of the right hand passed through a target angle located at 25 degrees, 12 degrees flexed relative to the starting position, by suddenly breaking contact between the left thumb and index finger. Earplugs allowed the subject to hear the preparatory tone but prevented the subject from picking up any sounds from the limb-motion device related to the speed of joint rotation. An opaque screen (vertical black bar in Fig. 1A) blocked the subject's view of the arm to ensure that the subject used proprioceptive, but not visual, cues to detect target arrival. To quantify velocity sense and to minimize the subject's use of internally sensed time lapse to predict target arrival, the device rotated the fingers at one of seven velocities (8, 10, 12, 14, 16, 18, and 20 degrees/s) in a pseudorandom order. The velocity range of 8–20 degrees/s was used to provide a behaviorally realistic combination of movement times (0.6–1.5 s) and distance (12 degrees) to the target, affording the subjects sufficient time and distance to use sensory information to perform the task.
A computer screen provided the subject with information about the size of any undershoot or overshoot, at the moment that the subject broke contact between the left thumb and index finger (see Fig. 1B). Software scaled the horizontal extent of the computer screen to correspond to a 20-degree range of the MCP joint, with stationary vertical lines marking the starting (red) and target (blue) positions. A third, dynamically positioned line (yellow) appeared when the subject opened the left hand to feed back to the subject the angle of the MCP joints when the subject opened the left thumb and index finger. This feedback, at the end of each trial, kept the subject's perception of target angle location calibrated throughout the experiment, rather than allowing it to drift (e.g., Brown et al. 2003). In the example illustrated in Fig. 1B, the target angle was overshot by 2 degrees. In some trials, the limb-motion device passively extended the wrist at the same time that it flexed the MCP joints to prevent MCP flexion from stretching the long finger extensor. In those trials involving wrist extension, feedback of undershoot and overshoot errors was not provided (i.e., the yellow line did not appear) to prevent errors in these wrist extension trials from influencing the subject's perception of the target location.
Selection and training of subjects.
All subjects participated in a preliminary series of trials in which only the fingers were rotated, the purpose of which was to identify subjects who were clearly using velocity sense to perform the target-detection task employed in this experiment. This preliminary testing was necessary because previous studies revealed that subjects sometimes use time rather than, or in addition to, velocity sense to perform this task (e.g., Verschueren et al. 1998). In one to three preliminary sessions conducted a few days before the actual experiment, each subject practiced the basic task. If, after three (or fewer) practice sessions, the slope of the relationship between MCP velocity and constant error was <0.25 s, the individual was included in the study. The rationale for this cut-off was that slopes >0.25 s indicate that the individual was using time estimation instead of, or in addition to, sensory feedback to perform the task (see Cordo et al. 1994), and we wished to determine the effects of our interventions on velocity (and dynamic position) sense. If included in this study, each subject performed, on the day of the experiment, five to ten training trials with MCP velocity set to 16 degrees/s. These training trials were carried out just before each of two runs of the protocol began. In the first run, the subject performed the target detection task with hand sensation intact, and, in the second, the subject performed the task with the hand anesthetized.
Suppression of muscle receptor input.
To suppress muscle receptor input at each of the seven MCP flexion velocities, a procedure was performed with each subject to identify the corresponding seven wrist extension velocities that minimized extensor digitorum stretch by the MCP flexion. During this procedure, which was carried out just before each experiment, MCP torque was monitored and wrist extension velocity was systematically varied until resistance of the subject's MCP joints to passive finger flexion was minimized. We expected that each of these empirically determined pairs of MCP and wrist velocities would strongly attenuate the signal from finger extensor muscle spindles, despite the ongoing MCP flexion (see Nerve recording procedure, below). When, during an experiment, the limb-motion device extended the wrist at the same time as it flexed the MCP joints, the velocity of wrist rotation was set to that previously determined to minimize MCP stretch for that subject. The amplitude of wrist rotation was always 22 degrees. Thus each subject's set of wrist velocities was unique. In several subjects, we marked a pair of dots in ink on the skin 0.5 cm apart over the MCP and wrist joints (ventrally and dorsally). We then measured the effect of a 22-degree wrist extension on the skin overlying the ventral MCP joint, as well as the effect of 22-degree MCP flexion on the skin overlying the dorsal wrist. We found that no measurable skin stretch was transmitted from the wrist to the MCP joint or vice versa.
Suppression of skin receptor input.
During the first run of the protocol in each experimental session, the subject's hand sensation was intact, but, in the second run, the subject's hand was anesthetized. To induce hand anesthesia, two sphygmomometer cuffs were applied to the right forearm, one 2–3 cm proximal to the wrist joint, and the other just proximal to the first (Fig. 1A). The purpose of using two cuffs was to minimize ischemic pain during the experiment by, ultimately, inflating the more distal of the two around an anesthetized area of the forearm. Before inflating either cuff, a 22-gauge IV catheter was inserted under sterile conditions into a prominent vein on back of the subject's hand and flushed with saline to prevent clotting of the catheter. The clenched hand was then exsanguinated by a thin, 5-cm wide rubber strap wrapped tightly around it (ESMark Band). To extend the exsanguination to the middle of the forearm and to maintain it throughout the experiment, the more distal of the two pressure cuffs was inflated to 150 mmHg, followed by inflation of the proximal cuff to 250 mmHg; the rubber strap was removed from the hand, and the distal cuff was deflated. Finally, 20 ml 0.3% Ropivacaine was injected into the hand and distal forearm through the IV catheter, and then the catheter was removed. Ropivacaine, an amino amide local anesthetic, produces reversible but prolonged conduction block with a very low incidence of adverse or allergic reaction. Over time, the Ropivacaine infiltrated all regions of the hand and the distal part of the forearm, up to the more proximal of the two pressure cuffs, completely blocking all nerve conduction to and from the hand.
After the injection, the progression of hand anesthesia was monitored by mechanically probing the hand with Semmes-Weinstein monofilaments (TouchTest, North Coast Medical) and by spraying the skin of the hand with a coolant (Gebauer's Spray and Stretch). In response to probing and spraying, which the subjects could neither see or hear, they verbally reported if they detected the stimulus. A total of 13 monofilament-probing sites were mapped out on the dorsal and ventral surfaces of the hand that are innervated by the radial, medial, and ulnar nerves. Coolant spraying was less systematically applied, usually to areas just reported to be insensitive to probing. Anesthesia was deemed complete when the subject could no longer detect the coolant spray or probing from a 300-gauge monofilament at any site. The distal cuff was then inflated to 150 mmHg around the anesthetized part of the wrist, the proximal cuff was deflated, and the second run of the protocol began. The distal pressure cuff remained inflated for the duration of the protocol (∼30 min).
Experimental protocol.
The target-detection protocol, consisting of 80 pseudorandomly ordered trials, was carried out twice on the day of the experiment, first with hand sensation intact, and second, with the hand anesthetized. In 49 trials, only the fingers were rotated, seven trials at each of the seven MCP velocities. In 21 trials, the wrist was passively extended as the fingers were flexed to minimize stretch of the finger extensor muscle (see Suppression of muscle receptor input, above). Three of these 21 trials were carried out at each MCP velocity, but all 21 were randomly intermixed with the other trials. The 10 remaining trials were catch trials, seven of which involved only wrist extension (1 at each of the 7 empirically determined wrist velocities), and three of which involved no movement at either joint. Before the first run of the protocol, the subjects were informed about the catch trials and instructed not to respond if the fingers did not rotate at the MCP joint.
During the first run of the protocol, with hand sensation intact, a single pressure cuff was applied just proximal to the wrist to control for any mechanical effects of the inflated pressure cuff when the hand was later anesthetized. The cuff was inflated to 150 mmHg for 10 trials (2–3 min), then deflated for 1 min, during which no trials were run, to reestablish perfusion in the hand. This procedure was repeated every 10 trials for a total of eight times during the protocol. The second run of the protocol was identical except the hand was anesthetized, and the distal pressure cuff remained inflated for the duration of the protocol.
Nerve-recording procedure.
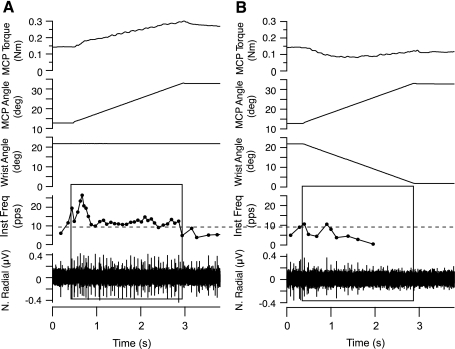
In a control procedure designed to verify that wrist extension attenuated muscle spindle input during MCP rotation, microneurographic recordings (Gandevia and Hales 1997) were performed on three of the nine subjects participating in the main experiment but on a separate day as the main experiment. As described above, each of these three subjects was assayed for a unique set of seven wrist-extension velocities that minimized finger resistance to seven corresponding velocities of MCP flexion. Once the optimal wrist velocities were determined, the experimenter inserted a tungsten microelectrode (UNA55FOM, FHC) percutaneously into the radial nerve to record from a single muscle spindle Ia axon. Muscle spindle Ia afferents were identified on the basis of stretch response to pressure applied to the tendon and the muscle belly, absence of a response to light skin stimulation over the same locations, poststretch silent period, and strong response to joint rotation and tendon vibration (see also Cordo et al. 2002; Edin and Vallbo 1990; Gandevia and Hales 1997). On the basis of the dynamic responses of muscle afferents to joint rotation, the afferents were tentatively classified as Ia, but we cannot exclude the possibility that one or more was a group II afferent. When a suitable recording was obtained, the MCP joints were flexed at one of the seven velocities and the discharge from the afferent was recorded. The next trial involved the identical MCP flexion, but the wrist was also extended at its corresponding velocity. As shown in Fig. 2A for a representative extensor digitorum muscle spindle Ia afferent with a resting discharge of 5–10 pulses/s (pps) at the initial MCP position of 13 degrees, 8 degrees/s passive flexion at the MCP joint alone produced a typical stretch response from the afferent. However, when the wrist was simultaneously extended (Fig. 2B), the identical MCP rotation generated much less torque resistance, and the afferent produced only a few action potentials. We recorded, thusly, from four muscle spindle afferents, all with similar responses.
Fig. 2.
Wrist extension minimizes stretch of the finger extensor muscle during MCP flexion. MCP torque, MCP angle, wrist angle, instantaneous frequency (Inst Freq) of muscle spindle firing, and the raw neurogram (N. Radial) are shown in A and B, top to bottom. A: wrist movement did not occur, and the muscle spindle Ia afferent fired briskly in response to MCP flexion (black box). B: wrist extension reduced the afferent response to MCP flexion to just a few impulses (black box).
Analysis of data.
Data recorded during the main experiment included wrist angle, MCP angle, MCP torque, and the voltage from the indicator device attached to the subject's left thumb and index finger. These four signals were digitized at 500 samples/s. The nerve recording in the microneurography procedure was digitized at 20K samples/s.
The data for each subject were analyzed as follows. For each trial, the velocity of finger rotation was quantified as the slope of the linear regression of angular position vs. time over the time course of each movement, not including the initial acceleration or subsequent deceleration in the calculation. In addition, the constant error (CE) was calculated for each trial involving MCP rotation as the difference between the target angle and MCP angle at the moment the subject indicated the fingers passed through the target. For each subject, CE was averaged across all like-movement trials (i.e., MCP only or MCP plus wrist) at the same MCP velocity to allow us to calculate the average CE and the variable error (VE) at each velocity (see below). CE was also averaged over all like-movement trials, irrespective of the MCP velocity, to assess the accuracy of each subject's perception of the target location. The CE feedback provided at the end of each trial served to maintain a consistent perception of target location throughout the protocol. Associated with each average CE calculation at each MCP velocity was a corresponding VE calculation, which is the SD of the average CE. The seven VE values were averaged across velocities to obtain an overall average VE. Finally, the individual CE values for all like-movement trials were plotted against the MCP velocity, and a linear regression was calculated (see Fig. 4). The slope of the CE vs. MCP velocity relationship was independent of the overall average CE and VE. The slope of the relationship between CE and velocity estimated how well subjects compensated for the varying MCP velocity when performing the task, with zero slope representing full compensation. Thus the slope provides an indirect measurement of the subjects' velocity perception. Independent CE, VE, and slope measurements were obtained for trials with MCP rotation only and for trials with MCP plus wrist rotation.
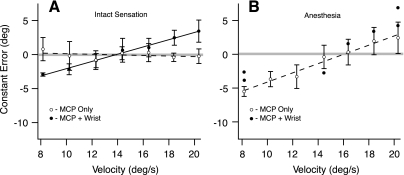
Fig. 4.
Data from a single subject, exemplifying the different effects from the 2 interventions. A: in the unanesthetized hand, wrist extension increased the slope of the CE vs. MCP velocity relationship without affecting average CE or variable error (VE). Open and filled circles represent average CE. B: hand anesthesia increased the slope of the CE vs. MCP velocity relationship while also increasing the average CE or VE. ○, average CE; ●, single trial CE.
To determine whether wrist extension or hand anesthesia influenced CE, VE, or the slope of CE vs. velocity, two paired t-tests were carried out on each of these three dependent variables. In one set of t-tests, the anesthesia condition was compared with the no-anesthesia condition, but this analysis was limited to trials with MCP flexion only. In the other set of t-tests, trials with simultaneous MCP flexion and wrist extension were compared with those with MCP flexion only, but this analysis was limited to trials with intact hand sensation. Subjects were often unaware of MCP motion when both interventions (i.e., wrist extension and hand anesthesia) were combined, so the data obtained during this combination of experimental conditions were incomplete and were not analyzed beyond calculating the percentage of no-response trials.
RESULTS
Effects of wrist extension and anesthesia on dynamic position and velocity sense.
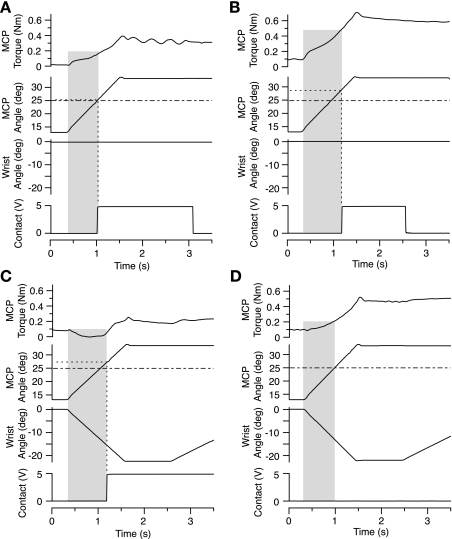
Both interventions, hand anesthesia and wrist extension, influenced the subjects' accuracy with the target detection task, as illustrated by the representative data in Fig. 3. Performance was accurate without intervention (Fig. 3A), with the subject opening the left hand precisely as the MCP joints passed through the target angle (i.e., intersection of 2 dashed lines). When the hand was anesthetized (Fig. 3B), the subject produced a 4-degree overshoot. When the wrist was extended (Fig. 3C), MCP torque decreased to zero, and the subject overshot the target by 2 degrees. When the hand was anesthetized and the wrist was extended (Fig. 3D), the subject did not respond, even though the fingers rotated through a 20-degree arc at 20 degrees/s.
Fig. 3.
Hand anesthesia and/or wrist extension affected performance of the movement task. Representative single-trial data are shown from a representative subject. Interventional conditions were no intervention (A), hand anesthesia only (B), wrist extension only (C), and wrist extension and hand anesthesia (i.e., both interventions) (D). In B and C, the indicator movement (Contact) was delayed; in D, the subject was unaware of the MCP movement and so did not respond. Note the decrease in MCP torque resulting from wrist extension (shaded regions in C and D).
Task performance was quantified by measures of error (average CE and VE) and by the dependence of CE on MCP velocity. Figure 4A illustrates the effect of wrist extension when hand sensation was intact. When the MCP joint was flexed without intervention (dashed line and open circles), task performance was accurate (average CE = −0.06 degrees), precise (average VE = 1.31 degrees), and independent of MCP velocity (slope of CE vs. MCP velocity = −0.04 s).
In contrast, when the wrist was extended during MCP flexion (Fig. 4A, solid line and filled circles), the slope of CE vs. velocity increased to 0.53, but with negligible changes in average CE (+0.29 degrees) and VE (−0.18 degrees). Thus this subject's ability to compensate for different MCP velocities diminished when muscle afferent input was suppressed by wrist extension, but his ability to distinguish dynamically the MCP angle was relatively unaffected.
Figure 4B illustrates the effect of hand anesthesia on this subject's task performance. During hand anesthesia, when only the fingers moved (open circles and dashed line), this subject's average CE was 1.17 degrees, VE was 1.65 degrees, and the slope was 0.68 s. Thus, the subject's ability to compensate for finger velocity and to distinguish MCP angle dynamically were both compromised by hand anesthesia (i.e., compare open circles and dashed lines in Fig. 4A with those in Fig. 4B). When the wrist was extended during MCP rotation in the anesthetized hand (i.e., with both interventions), this subject responded in only 7 of 21 trials, as he did not consistently detect the movement (filled circles in Fig. 4B).
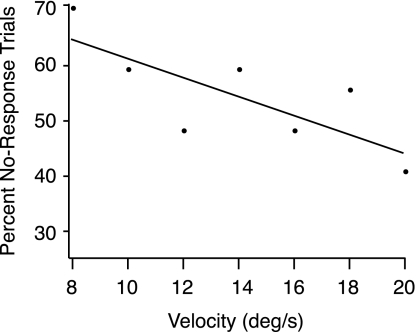
The combination of both interventions, wrist extension and hand anesthesia, left most subjects unable to detect MCP rotation in most trials, but the percent of no-response trials increased as the speed of MCP rotation decreased (Fig. 5). When the MCP joint rotated at 20 degrees/s, the subjects failed to detect the movement 40% of the time, whereas when velocity was 8 degrees/s, the subjects failed to detect the movement 70% of the time. Therefore, the combination of wrist extension and hand anesthesia was sufficient, in many cases, to eliminate completely the sensation of motion. The lack of response in most trials with both interventions prevented the acquisition of sufficient data to analyze more fully this combination of experimental conditions. In contrast, the subjects never failed to detect MCP rotation with only one (or no) intervention.
Fig. 5.
Awareness of MCP motion depended on velocity when the 2 interventions were combined. Averaged data from the 9 enrolled subjects showed that, for 8 degree/s MCP rotations, subjects were unaware of the movement 70% of the time, whereas for 20 degrees/s, they were unaware of the movement 40% of the time.
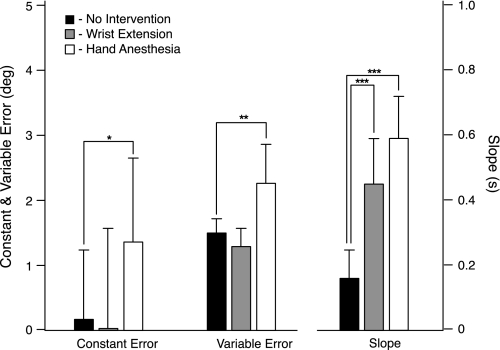
Statistical analysis of all nine subjects' data confirmed that wrist extension and hand anesthesia affected task performance qualitatively differently (Fig. 6 and Table 1). A t-test (n = 9) comparing CE with and without wrist extension showed that, with hand sensation intact, wrist extension had no effect on CE (P = 0.83), as illustrated in Fig. 6 (Fig. 6, left, solid and shaded bars). In contrast, a t-test comparing CE with and without hand anesthesia showed that, in the absence of wrist extension, hand anesthesia caused the subjects to overshoot the target more (P < 0.05) than when sensation was intact (Fig. 6, left, solid and open bars). Similarly, VE was unaffected by wrist extension (P = 0.06; Fig. 6, middle, solid and shaded bars), but VE increased when the hand was anesthetized (P ≤ 0.001; Fig. 6, middle, solid and open bars). Thus anesthesia diminished the perception of dynamic position, but wrist extension did not. In contrast, the slope of the relationship between CE and MCP velocity increased significantly with both wrist extension and hand anesthesia. With wrist extension, the slope increased from 0.16 to 0.45 s (P ≤ 0.0001; Fig. 6, right, solid and shaded bars), and when the hand was anesthetized, from 0.16 to 0.59 s (P ≤ 0.0001; Fig. 6, right, solid and open bars). Thus anesthesia and wrist extension both diminished the subject's ability to compensate for different MCP velocities.
Fig. 6.
Summary results for all subjects. Wrist extension had no effect on CE, whereas hand anesthesia increased CE relative to the no-intervention condition (leftmost 3 bars). Wrist extension had no effect on VE, whereas hand anesthesia increased VE relative to the no-intervention condition (middle 3 bars). Both wrist extension and hand anesthesia interventions increased the slope of the CE vs. MCP velocity relationship relative to the no-intervention condition (rightmost 3 bars). Significance, *P ≤ 0.05, **P ≤ 0.001, ***P < 0.0001.
Table 1.
Dynamic position and velocity analysis
| Constant Error, degrees |
Variable Error, degrees |
Slope, s |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Subject | FO/IS | FW/IS | FO/A | FO/IS | FW/IS | FO/A | FO/IS | FW/IS | FO/A |
| 1 | −0.06 | 0.23 | −1.17 | 1.31 | 1.13 | 1.65 | −0.04 | 0.53 | 0.68 |
| 2 | 2.39 | 2.20 | 3.76 | 1.61 | 1.54 | 2.97 | 0.20 | 0.58 | 0.56 |
| 3 | −0.96 | −1.73 | 1.84 | 1.84 | 1.81 | 1.69 | 0.17 | 0.46 | 0.78 |
| 4 | −0.03 | 0.02 | 0.80 | 1.66 | 0.97 | 1.96 | 0.24 | 0.26 | 0.43 |
| 5 | 0.77 | −2.68 | 0.89 | 1.33 | 1.27 | 2.14 | 0.18 | 0.57 | 0.69 |
| 6 | 0.65 | 1.59 | 2.15 | 1.56 | 1.26 | 2.71 | 0.23 | 0.38 | 0.40 |
| 7 | −1.32 | −0.50 | 1.23 | 1.63 | 0.90 | 1.88 | 0.20 | 0.58 | 0.56 |
| 8 | −0.03 | 0.09 | 1.57 | 1.55 | 1.24 | 2.20 | 0.22 | 0.45 | 0.68 |
| 9 | 0.12 | 1.09 | 1.21 | 1.14 | 1.39 | 2.41 | 0.06 | 0.21 | 0.54 |
| Avg | 0.17 | 0.03 | 1.36 | 1.51 | 1.28 | 2.18 | 0.16 | 0.45 | 0.59 |
| SD | 1.07 | 1.54 | 1.31 | 0.21 | 0.28 | 0.45 | 0.09 | 0.14 | 0.13 |
FO/IS, finger only/intact sensation; FW/IS, finger-wrist/intact sensation; FO/A, finger only/anesthesia.
Catch trials.
Subjects' responses to catch trials demonstrated that they were detecting actual MCP movement, rather than responding to wrist rotation or to the tone 1 s before the onset of movement. Two types of catch trials were used in this study, one without any movement and one with wrist extension only. In the protocol run with intact hand sensation, subjects never responded in a catch trial. In the protocol run using the anesthetized hand, some subjects occasionally responded even though the MCP joint did not move (Table 2). For catch trials involving no movement of either the MCP joint or wrist (n = 3), the subjects never responded. However, for catch trials involving wrist rotation only, the subjects responded, on average, in 1.2 out of 7 (17%) of these trials.
Table 2.
Catch trials (hand anesthesia protocol)
| Subject | Wrist-Only Catch Trials (n = 7) | Percent of All trials | No-Movement Catch Trials (n = 3) |
|---|---|---|---|
| 1 | 1 | 14.3 | 0 |
| 2 | 1 | 14.3 | 0 |
| 3 | 1 | 14.3 | 0 |
| 4 | 0 | 0.0 | 0 |
| 5 | 0 | 0.0 | 0 |
| 6 | 2 | 28.6 | 0 |
| 7 | 3 | 42.9 | 0 |
| 8 | 1 | 14.3 | 0 |
| 9 | 2 | 28.6 | 0 |
| Avg | 1.2 | 17.5 | 0 |
DISCUSSION
The principal findings of this study were that 1) both wrist extension and hand anesthesia interventions adversely affected the slope of the CE vs. MCP velocity relationship and 2) only the hand anesthesia intervention adversely affected the average CE and VE. These two findings suggest that, for the MCP joint near the neutral position, the sense of dynamic position (represented by CE and VE) can be derived principally from skin afferent input, whereas the sense of velocity (indirectly represented by slope) is derived from both muscle and skin afferent input. These results suggest that the proprioceptive information provided by skin and muscle spindle Ia afferents are not entirely redundant in a parametric sense and that each submodality contributes differently to movement sense of the hand (see also Gandevia 1996, p. 163).
Sensory effects of MCP flexion and the two interventions.
Passive flexion of the subjects' MCP joints by our limb-motion device likely evoked sensory responses from skin-pressure receptors in the fingertips, skin-stretch receptors on the dorsum of the hand, and muscle receptors in the long finger extensor. On the other hand, MCP flexion probably did not evoke a response from MCP joint afferents or muscle receptors in the intrinsic muscles of the hand, at least not sufficiently to influence task performance. Joint receptors are sensitive to motion primarily at the extremes of the joint space, and, even there, some articular afferents respond to both flexion and extension of the joint (Burke et al. 1988; Burgess and Clark 1969; Skoglund 1956). In the MCP flexion-extension plane, active contraction of the lumbrical and interossei muscles contributes to flexion torque (Buford et al. 2005; Revol and Servant 2008), and stimulation of muscle afferents in the intrinsic muscles evokes a sensation of MCP extension (Gandevia 1985), suggesting that intrinsic muscle receptors did not contribute to the perception of MCP flexion in this experiment.
Passive extension of the wrist was used to suppress muscle input from the long finger extensor, as demonstrated by microneurographic recordings (e.g., Fig. 2). Wrist extension likely also activated skin-stretch receptors on the ventral surface of the wrist as well as muscle afferents in the wrist flexors muscles. However, because the perceptual motor task in this study did not involve the wrist, this sensory input presumably evoked by wrist rotation is unlikely to have influenced the subjects' performance, as supported by our results of wrist-extension catch trials, which rarely evoked a response from the subjects. Neither 22-degree wrist extension nor 20-degree MCP flexion measurably stretched the skin at the other joint (see methods).
Although the wrist extension intervention suppressed sensory input from extensor digitorum, it is unlikely to have completely eliminated this input (e.g., Fig. 2). However, the muscle activity that remained was insufficient, in most trials, to preserve the subjects' ability to detect MCP motion when the hand was anesthetized. In contrast, regional anesthesia of the hand with the Bier block is likely to have completely eliminated sensory activity, not only from skin receptors of the hand and distal forearm, but also from articular receptors, muscle receptors in the intrinsic muscles of the hand, thermoreceptors, and nociceptors. The same anesthetic procedure (i.e., Bier block) is often used during hand surgery (e.g., Brill et al. 2004).
It might be argued that, to evoke a detectable dynamic position signal from extensor digitorum muscle receptors, the MCP velocity must be >20 degrees/s, which is rather slow for a natural finger movement. This hypothesis, if correct, could explain the differences in the effects of the two interventions on dynamic position and velocity sensitivity. Perhaps at higher velocities, wrist extension would have adversely affected average CE and VE. However, previous studies of motion detection during very slow finger movements (e.g., 2 degrees/min) have shown that the velocity threshold for motion detection is roughly two orders of magnitude lower than the slowest MCP velocity used in the present study (Clark et al. 1986; Taylor and McCloskey 1990). Moreover, Cordo and colleagues (2002) showed that muscle spindle Ia firing patterns are strongly correlated with dynamic position at velocities as low as 2 degrees/s, below the slowest velocity used in the present study. Therefore, the slow MCP velocities used in this study would appear not to explain why suppressing extensor digitorum sensory input had no effect on average CE and VE.
In addition to sensory input, corollary discharge is produced during active movement and contributes to movement sense (e.g., Gandevia et al. 2006). As all movements in this experiment were passive, there would have been no corollary discharge associated with MCP flexion movements imposed by our arm-motion device. Several previous studies have reported that movement sense is more accurate during active, compared with passive movement (Fuentes and Bastian 2010; Paillard and Brouchon 1968), presumably a result of the presence of corollary discharge or fusimotor drive in active movements. However, not all movements in our daily activities are completely active because, in some movements, gravitational and inertial forces act on the joints in a functional manner. Nevertheless, it would be important to replicate the present study with active movement before generalizing the conclusions of the present study to all types of volitional movement.
How proprioceptive input is used in this perceptual motor task.
As with any proprioceptively controlled movement, a key feature of our perceptual motor task is that it required prediction; that is, any action to be taken (e.g., indicator movement) lagged the relevant sensory input because of nerve conduction and computational delays (Cordo et al. 1994). The authors of the 1994 study hypothesized that the central nervous system could perform this perceptual motor task with one of two distinct strategies. The first requires a real-time computation of the angular distance moved during the sensorimotor delay (∼220 ms), which would necessarily require the central nervous system to “know” the velocity and the sensorimotor delay (computational strategy). Alternatively, such a computation would be unnecessary if, at the primary afferent level, dynamic position and velocity information were interdependently represented (e.g., Grill and Hallett 1995), thereby exaggerating the perception of dynamic position as a function of increasing velocity (noncomputational strategy). The computational strategy requires that dynamic position and velocity information be dissociable, whereas the noncomputational strategy does not. For the noncomputation strategy to be accurate, the proportionality between dynamic position and velocity input would have to be calibrated to the sensorimotor delay, which would differ for movements of the arm or leg, for example. How such a calibration could be accomplished remains an open question.
Although the computational and noncomputational strategies could conceivably result in perfect performance of our perceptual motor task, several other imperfect strategies are also possible that would result in systematic errors. In one (velocity timing), the central nervous system would first detect the movement velocity and then initiate the indicator movement after an elapsed time. The accuracy of the velocity-timing strategy depends on the subject's ability to discriminate and remember the correct timing for each speed. It is unlikely that subjects used this strategy, at least by itself, because we used seven different velocities separated by only 2 degrees/s in our protocols. Remembering seven different velocities, 2 degrees apart, pushes the limit of information capacity (Clark et al. 1995) and velocity discrimination (Grill and Hallett 1995; Kerr and Worringham 2002) in healthy humans. However, some subjects might have used the velocity-timing strategy for some velocities, perhaps reacting as soon as possible upon detecting the fastest one or two velocities.
Another imperfect alternative (dynamic-position strategy) requires the central nervous system to detect dynamic position, but not velocity, whereby the subject initiates all indicator movements at the same (or random) joint angle. One outcome of the dynamic position strategy would be a CE vs. velocity slope equal to the loop delay (∼0.22 s). It is possible that the subjects whose data were excluded from the present analysis employed such a velocity-independent strategy.
Our results do not distinguish unambiguously which of these different strategies was used by our subjects in this target detection task, but we are confident that almost all of the subjects chosen for this study were using velocity information because of the slope criterion for exclusion. Because of the wide variation in performance observed among all 17 subjects initially enrolled for this study, it seems likely that different individuals employ different strategies, perhaps on the basis of different abilities to detect and use dynamic position and velocity information.
Multimodal function in proprioception.
Clark and colleagues (1985, p. 1530) noted that, “a major difficulty with demonstrating the importance of intramuscular receptors in proprioception and with clarifying the role of skin and joint receptors stems from a failure to distinguish sufficiently between a static-position sense and a movement sense… . ” This statement suggests that some receptors might contribute to static-position sense and others, to movement sense. However, there may be additional reasons why proprioception is multimodal.
First, proprioceptive afferents appear to serve several distinct functions (e.g., Gandevia 1996, p. 163). Proprioceptors, even within a single submodality, are known to be differentially sensitive to movement because of their location. Muscle spindles in different compartments of a muscle (e.g., Chanaud et al. 1991; English et al. 1993) or in different muscles respond differently to movements in different directions at the same joint (Bergenheim et al. 2000; Jones et al. 2001; Ribot-Ciscar et al. 2003).
Second, different submodalities of proprioceptors have different sensitivities to movement parameters in different parts of the joint space. Joint afferents, if they contribute at all (Proske and Gandevia 2009), are primarily sensitive to movement at the extremes of the joint space (Burke et al. 1988; Burgess and Clark 1969; Ferrell 1980; Skoglund 1956), whereas muscle spindle Ia afferents appear to be tuned primarily to the central region of the joint space (Cordo et al. 2002). Thus the movement parameters signaled by articular and muscle afferents may be qualitatively the same, but both articular and muscle afferents may be required to signal these same movement parameters over the full range of motion. The differential sensitivity of the population of skin receptors through the range of motion of a joint is presently unknown.
Third, at least in the hand, skin receptors may be more optimally located than muscle receptors to transduce finger flexion-extension movements. One reason is that the long finger muscles are multiarticular, and, therefore, muscle receptors from an individual muscle, at least, cannot by themselves differentiate which joint(s) is moving (e.g., Burgess et al. 1982). Another reason is that all four tendons of the long finger extensor or flexor muscles are attached to a single muscle, which makes the execution of individuated finger movements more complex (e.g., Lang and Scheiber 2004), creating potential ambiguity about which finger is moving, on the basis of muscle receptor input alone. Finally, the muscle bellies of the long finger muscles, where the muscle receptors are located, are remotely connected to the finger joints via long tendons. Such transduction at a distance might introduce errors (e.g., noise) into the signaling of finger flexion/extension.
Fourth, and most relevant to the study here, different types of proprioceptors may be anatomically and physiologically adapted to transduce different movement parameters. The results of this study support this hypothesis for proprioceptors because, in the central region of the MCP joint space, dynamic position sense appears to be influenced by skin receptors but not muscle receptors, whereas velocity sense is influenced by both. This hypothesized “distribution of labor” for signaling different proprioceptive parameters seems a more plausible explanation for the multimodal nature of the proprioceptive system than the more parsimonious alternative of receptor redundancy.
GRANTS
This work was supported by the National Institutes of Health (R01 NS061304).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors are indebted to our experimental subjects, who contributed to preliminary investigations and the experiment reported in this paper, and to Dr. George Knafl (School of Nursing, Univ. of N. Carolina) for reviewing the manuscript.
REFERENCES
- Aimonetti JM, Hospod V, Roll JP, Robot-Cisar E. Cutaneous afferents provide a neuronal population vector that encodes the orientation of human ankle movement. J Physiol 580: 649–658, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergenheim M, Ribot-Cisar E, Roll JP. Proprioceptive population coding of two-dimensional limb movements in humans: I. Muscle spindle feedback during spatially oriented movements. Exp Brain Res 134: 301–310, 2000 [DOI] [PubMed] [Google Scholar]
- Bevan L, Cordo P, Carlton L, Carlton M. Proprioceptive coordination of movement sequences: discrimination of joint angle versus angular distance. J Neurophysiol 71: 1862–1872, 1994 [DOI] [PubMed] [Google Scholar]
- Brill S, Middleton W, Fisher A. Bier's block: 100 years old and still going strong. Acta Anesthesiol 48: 117–1122, 2004 [DOI] [PubMed] [Google Scholar]
- Brown LE, Rosenbaum DA, Sainburg RL. Limb position drift: implications for control of posture and movement. J Neurophysiol 90: 3105–3118, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buford WL, Koh S, Andersen CR, Viegas SF. Analysis of intrinsic-extrinsic muscle function through interactive 3-dimensional kinematic simulation and cadaver studies. J Hand Surg Am 30: 1267–1275, 2005 [DOI] [PubMed] [Google Scholar]
- Burgess PR, Wei JY, Clark FJ, Simon J. Signaling of kinesthetic information by peripheral sensory receptors. Annu Rev Neurosci 5: 171–187, 1982 [DOI] [PubMed] [Google Scholar]
- Burgess PR, Clark FJ. Characteristics of knee-joint receptors in the cat. J Physiol 203: 317–335, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Gandevia SC, Macefield G. Responses to passive movement of receptors in joint, skin and muscle of the human hand. J Physiol 402: 347–361, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanaud CM, Pratt CA, Loeb GE. Functionally complex muscles of the cat hindlimb. II. Mechanical and architectural heterogeneity within the biceps femoris. Exp Brain Res 85: 257–270, 1991 [DOI] [PubMed] [Google Scholar]
- Clark FJ, Burgess RC, Chapin JW, Lipscomb WT. Role of intramuscular receptors in the awareness of limb position. J Neurophysiol 54: 1529–1540, 1985 [DOI] [PubMed] [Google Scholar]
- Clark FJ, Burgess RC, Chapin JW. Proprioception with the proximal interphalangeal joint of the index finger. Evidence for a movement sense without a static position sense. Brain 109: 1195–1208, 1986 [DOI] [PubMed] [Google Scholar]
- Clark FJ, Larwood KJ, Davis ME, Deffenbacher KA. A metric for assessing acuity in positioning joints and limbs. Exp Brain Res 107: 73–79, 1995 [DOI] [PubMed] [Google Scholar]
- Collins DF, Prochazka A. Movement illusions evoked by ensemble cutaneous input from the dorsum of the hand. J Physiol 496: 857–871, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DF, Refschauge KM, Gandevia SC. Sensory integration in the perception of movements at the human metacarpophalangeal joint. J Physiol 529: 505–515, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DF, Refshauge KM, Todd G, Gandevia SC. Cutaneous receptors contribute to kinesthesia at the index finger, elbow, and knee. J Neurophysiol 94: 1699–1706, 2005 [DOI] [PubMed] [Google Scholar]
- Cordo PJ. Kinesthetic control of a multijoint movement sequence. J Neurophysiol 63: 161–172, 1990 [DOI] [PubMed] [Google Scholar]
- Cordo P, Carlton L, Bevan L, Carlton M, Kerr GK. Proprioceptive coordination of movement sequences: role of velocity and position information. J Neurophysiol 71: 1848–1861, 1994 [DOI] [PubMed] [Google Scholar]
- Cordo P, Gurfinkel VS, Bevan L, Kerr GK. Proprioceptive consequences of tendon vibration during movement. J Neurophysiol 74: 1675–1688, 1995 [DOI] [PubMed] [Google Scholar]
- Cordo PJ, Flores-Vieira C, Verschueren SM, Inglis JT, Gurfinkel V. Position sensitivity of human muscle spindles: single afferent and population representations. J Neurophysiol 87: 1186–1195, 2002 [DOI] [PubMed] [Google Scholar]
- Edin BB, Vallbo AB. Classification of human muscle stretch receptor afferents: a Bayesian approach. J Neurophysiol 63: 1314–1322, 1990 [DOI] [PubMed] [Google Scholar]
- Edin BB, Abbs JH. Finger movement responses of cutaneous mechanoreceptors in the dorsal skin of the human hand. J Neurophysiol 65: 657–670, 1991 [DOI] [PubMed] [Google Scholar]
- Edin BB. Quantitative analysis of static strain sensitivity in human mechanoreceptors from hairy skin. J Neurophysiol 67: 1105–1113, 1992 [DOI] [PubMed] [Google Scholar]
- Edin BB, Johansson N. Skin strain patterns provide kinaesthetic information to the human central nervous system. J Physiol 487: 243–251, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edin BB. Cutaneous afferents provide information about knee joint movements in humans. J Physiol 531: 289–297, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edin BB. Quantitative analyses of dynamic strain sensitivity in human skin mechanoreceptors. J Neurophysiol 92: 2322–2343, 2004 [DOI] [PubMed] [Google Scholar]
- English AW, Wolf SL, Segal RL. Compartmentalization of muscles, and their motor nuclei: the partitioning hypothesis. Phys Ther 73: 857–867, 1993 [DOI] [PubMed] [Google Scholar]
- Ferrell WR. The adequacy of stretch receptors in the cat knee joint for signaling joint angle throughout a full range of movement. J Physiol 299: 85–99, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell WR, Gandevia SC, McCoskey DI. The role of joint receptors in human kinaesthesia when intramuscular receptors cannot contribute. J Physiol 386: 63–71, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell WR, Craske B. Contribution of joint and muscle afferents to position sense at the human proximal interphalangeal joint. Exp Physiol 77: 331–342, 1992 [DOI] [PubMed] [Google Scholar]
- Fuentes CT, Bastian AJ. Where is your arm? Variations in proprioception across space and tasks. J Neurophysiol 103: 164–171, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC, McCloskey I. Joint sense, muscle sense, and their combination as position sense, measured at the distal interphalangeal joint of the middle finger. J Physiol 260: 387–407, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC. Illusory movements produced by electrical stimulation of low-threshold muscle afferents from the hand. Brain 108: 965–982, 1985 [DOI] [PubMed] [Google Scholar]
- Gandevia SC. Kinesthesia: roles for afferent signals and motor commands. In: Handbook of Physiology, edited by Rowell LB, Shepherd JT. Oxford, UK: Oxford University Press, 1996, p. 128–172 [Google Scholar]
- Gandevia SC, Hales JP. The methodology and scope of human microneurography. J Neurosci Methods 27: 123–136, 1997 [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Smith J, Crawford M, Proske U, Taylor JL. Motor commands contribute to human position sense. J Physiol 571: 703–710, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill SE, Hallett M. Velocity sensitivity of human muscle spindle afferents and slowly adapting type II cutaneous mechanoreceptors. J Physiol 489: 593–602, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulliger M, Nordh E, Thelin AE, Vallbö A. The responses of afferent fibres from the glabrous skin of the hand during voluntary finger movements in man. J Physiol 291: 370–392, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KE, Wessberg J, Vallbo AB. Directional tuning of human forearm muscle afferents during voluntary wrist movements. J Physiol 536: 635–647, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr GK, Worringham CJ. Velocity perception and proprioception. In: Sensorimotor Control of Movement and Posture, edited by Gandevia SG, Proske U, Stuart DG. New York: Kluwer Academic/Plenum Publishing, 2002, p. 79–86 [DOI] [PubMed] [Google Scholar]
- Lang CE, Scheiber MH. Human finger independence: limitations due to passive mechanical coupling versus active neuromuscular control. J Neurophysiol 92: 2802–2810, 2004 [DOI] [PubMed] [Google Scholar]
- Matthews PBC. Proprioceptors and their contribution to somatosensory mapping: complex messages require complex processing. Can J Physiol Pharmacol 66: 430–438, 1988 [DOI] [PubMed] [Google Scholar]
- McCloskey DI. Kinesthetic sensibility. Physiol Rev 58: 762–820, 1978 [DOI] [PubMed] [Google Scholar]
- Paillard J, Brouchon M. Active and passive movements in the calibration of position sense. In: The Neuropsychology of Spatially Oriented Behavior, edited by SJ Freedman. Homewood IL: Dorsey, 1968, p. 37– 762–55 [Google Scholar]
- Proske U, Gandevia SC. The kinaesthetic senses. J Physiol 587: 4139–4146, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribot-Ciscar E, Bergenheim M, Albert F, Roll JP. Proprioceptive population coding of limb position in humans. Exp Brain Res 149: 512–519, 2003 [DOI] [PubMed] [Google Scholar]
- Revol M, Servant JM. Paralysis of the intrinsic muscles of the hand. Chir Main 27: 1–11, 2008 [DOI] [PubMed] [Google Scholar]
- Skoglund D. Anatomical and physiological studies of knee joint innervation in the cat. Acta Physiol Scand Suppl 124: 1–101, 1956 [PubMed] [Google Scholar]
- Taylor JL, McCloskey DI. Ability to detect angular displacements of the fingers made at an imperceptibly slow speed. Brain 143: 157–166, 1990 [DOI] [PubMed] [Google Scholar]
- Verscheuren SM, Cordo PJ, Swinnen SP. Representation of wrist joint kinematics by the ensemble of muscle spindles from synergistic muscles. J Neurophysiol 79: 2265–2276, 1998 [DOI] [PubMed] [Google Scholar]
- Windhorst U. Muscle proprioceptive feedback and spinal networks. Brain Res Bull 73: 155–202, 2007 [DOI] [PubMed] [Google Scholar]