Abstract
Recent studies have provided evidence that temporal coding contributes significantly to encoding taste stimuli at the first central relay for taste, the nucleus of the solitary tract (NTS). However, it is not known whether this coding mechanism is also used at the next synapse in the central taste pathway, the parabrachial nucleus of the pons (PbN). In the present study, electrophysiological responses to taste stimuli (sucrose, NaCl, HCl, and quinine) were recorded from 44 cells in the PbN of anesthetized rats. In 29 cells, the contribution of the temporal characteristics of the response to the discrimination of various taste qualities was assessed. A family of metrics that quantifies the similarity of two spike trains in terms of spike count and spike timing was used. Results showed that spike timing in 14 PbN cells (48%) conveyed a significant amount of information about taste quality, beyond what could be conveyed by spike count alone. In another 14 cells (48%), the rate envelope (time course) of the response contributed significantly more information than spike count alone. Across cells there was a significant correlation (r = 0.51; P < 0.01) between breadth of tuning and the proportion of information conveyed by temporal dynamics. Comparison with previous data from the NTS (Di Lorenzo PM and Victor JD. J Neurophysiol 90: 1418–31, 2003 and J Neurophysiol 97: 1857–1861, 2007) showed that temporal coding in the NTS occurred in a similar proportion of cells and contributed a similar fraction of the total information at the same average level of temporal precision, even though trial-to-trial variability was higher in the PbN than in the NTS. These data suggest that information about taste quality conveyed by the temporal characteristics of evoked responses is transmitted with high fidelity from the NTS to the PbN.
Keywords: gustatory system, brainstem, neural coding
in studies of neural coding of sensory stimuli, it is not uncommon for electrophysiological studies to focus on a single structure in the central pathway. In such cases, the characterization of the sensory representation in one structure can provide a context with which to interpret neural coding in structures further upstream. That is, if the same form of neural coding is identified in multiple structures in a neural pathway, the argument that these mechanisms are in fact an essential system-wide method for communicating information is substantially strengthened. Here we present a study of information processing of taste stimuli in the parabrachial nucleus of the pons (PbN), the second relay in the central gustatory pathway and compare it to what is known about neural processing of taste in the nucleus of the solitary tract (NTS), the primary source of taste-related information to the PbN. The focus of our investigation is on the analysis of the temporal characteristics of taste responses, defined as temporal coding.
Information about stimuli of particular taste qualities (sweet, sour, salt, bitter, and perhaps umami) conveyed by peripheral nerves converges onto multisensitive cells in the NTS. The broad sensitivity of the majority of NTS cells often makes spike count an ambiguous signal for identification of taste quality. Under those conditions, a method of encoding that utilizes the temporal features of the response for stimulus discrimination may be better suited to the task. Previous studies (Di Lorenzo and Victor 2003) have shown that approximately half of NTS cells utilize temporal coding in the representation of taste. Further, temporal coding can disambiguate taste stimuli of similar quality but different chemical composition (Roussin et al. 2008) as well as individual taste qualities when presented at different concentrations (Chen et al. 2011). Moreover, the temporal characteristics of taste responses contribute information about the components of binary mixtures of tastants of different qualities, especially in cells that are broadly tuned across taste qualities (Di Lorenzo et al. 2009a).
In the rodent gustatory system, the main target of the NTS is the PbN. The neural circuitry that interconnects the NTS and PbN is complex and involves subnuclei with connections to areas controlling orofacial and ingestive behaviors as well as reward. In both rat and hamster, the rostral central NTS, the subnucleus that receives most of the afferent input from peripheral nerves innervating taste buds (Whitehead 1988; Lundy and Norgren 2004) sends the majority of its output to the waist area of the PbN. This area includes the central medial and ventral lateral nuclei and the cells that are scattered within the portion of the brachium between them (Norgren 1978; Travers 1988). The waist area then sends a heavy projection back to the ventral subnucleus of the NTS, which in turn sends projections to the underlying medullary reticular formation, an area containing premotor circuits for taste-evoked orofacial behaviors (Travers and Norgren 1983; Halsell et al. 1996; Karimnamazi and Travers 1998). There are also direct projections from the waist area to the medial reticular formation as well as ascending projections to the thalamus, amygdala, hypothalamus, and insular cortex [reviewed in Lundy and Norgren (2004)]. Most of these forebrain connections are reciprocal, suggesting a widely distributed and highly interactive circuit [see Katz et al. (2002) and Simon et al. (2006)]. It is therefore an open question as to whether information about taste stimuli conveyed by spike timing in the NTS would also be evident the PbN.
The purpose of the present study was to evaluate temporal coding of taste stimuli in the PbN in the context of what is known about temporal coding in the NTS. Results show that temporal coding contributes a significant proportion of the total information conveyed by taste-evoked spike trains in the PbN. Further, temporal coding in the PbN occurs with the same prevalence and with the same level of temporal precision as that found in the NTS even though trial-to-trial variability in spike count increases. Collectively, these data support the idea that information conveyed by the temporal characteristics of taste responses is preserved at the second synapse in the central gustatory system.
MATERIALS AND METHODS
Subjects.
Thirty-four male Sprague-Dawley rats (350–450 g) were used in this study. Rats were given unrestricted access to food and water and were pair housed with a 12-h light-dark schedule. A plastic tube was placed in each cage to provide environmental stimulation. Animal care was in accord with the requirements of the Institutional Animal Care and Use Committee of Binghamton University.
Surgery.
Before surgery, rats were anesthetized with urethane (1.5 g/kg ip, in 2 doses given 20 min apart). Supplemental injections of urethane (0.1 ml) were delivered as needed to maintain anesthesia. Robinul (glycopyrrolate), a peripheral anticholinergic agent (0.0004 g/kg, 10% in isotonic saline), was administered subcutaneously to facilitate breathing when necessary. Body temperature was maintained at 35–37°C during surgery with a rectal thermistor probe connected to a heating pad (FHC, Bowdoinham, ME).
Animals were tracheotomized to facilitate breathing during stimulus delivery. Their head was mounted in a stereotaxic instrument with upper incisor bar positioned 5 mm below the interaural line. Skin and fascia were removed, and a nontraumatic head holder was secured to the skull with stainless steel screws and dental cement. This allowed better access to the mouth without the obstruction of the ear and tooth bars. The occipital bone and underlying meninges were removed, and a small area of the posterior cerebellum was gently aspirated to provide access to the obex.
Taste stimuli and stimulus delivery.
Taste stimuli consisted of 0.1 M NaCl, 0.01 M HCl, 0.01 M quinine, and 0.5 M sucrose. These concentrations have been shown to elicit half-maximal potentials in the CT nerve of the rat (Ganchrow and Erickson 1970; Ogawa et al. 1974) and matched those used in our previous studies of the NTS (Chen et al. 2011; Di Lorenzo and Victor 2003, 2007; Di Lorenzo et al. 2009a; Roussin et al. 2008). Taste stimuli were made from reagent-grade chemicals dissolved in distilled water and were delivered at room temperature. The stimulus delivery system consisted of stimulus-filled reservoirs pressurized with compressed air and connected via polyethylene tubing to perforated stainless steel tubes placed in the mouth. Tastant delivery was controlled by computer activation of a solenoid valve interposed between the reservoir and the tongue. Tastants were delivered at a flow rate of 5 ml/s. The taste solution bathed the whole mouth; this was verified by application of methylene blue through the system. Each stimulus trial consisted of 10 s of spontaneous activity, 10 s of prestimulus distilled water, 5 s of tastant, 5 s of pause, and 20 s of a distilled water rinse. The inter-trial interval was 2 min. Stimuli were presented in repeated trials for as long as the cell remained well isolated. For any given stimulus, all other stimuli were presented before it was repeated.
Electrophysiological recording and testing.
Electrophysiological recordings were performed with etched tungsten microelectrodes (18–20 MΩ, 1 V at 1 kHz; FHC). The electrode was lowered through the cerebellum above the pons located 5.4 mm anterior and 1.8 mm lateral to the obex and 5–6 mm below the cerebellar surface. Signals were amplified (model P511; Grass Technologies, West Warwick, RI) and fed to a computer. The activity was digitized with an analog-to-digital interface (model 1401; Cambridge Electronic Designs, Cambridge, UK) and was processed with Spike2 software (Cambridge Electronic Designs). Single cells were identified by periodically delivering a 0.1 M NaCl solution followed by a water rinse as the electrode was slowly lowered through the brain. Once a background response to NaCl was detected, every well-isolated cell thereafter was tested with all four taste stimuli. Cell isolation was based on the consistency of the waveform shape using template matching and principal component analysis. A signal-to-noise ratio of 3:1 was required for cell isolation. Isolated cells were tested with the exemplars of the four basic taste qualities yielding the “response profile” of the cell, defined as the relative response rates across tastants. The cell was tested for as long as it remained isolated allowing for multiple presentations of the same stimulus. Spike timing (1-ms precision) was calculated with respect to the onset of each stimulus delivery.
Data analysis.
The magnitude of response to a given tastant was defined as the mean firing rate (spikes per second) during the first 2 s of tastant delivery minus the average firing rate (spikes per second) during the 5 s of water delivery immediately preceding taste stimulus onset. A taste response was considered to be significant if it was 2.5 SDs greater than the average spontaneous firing rate. The breadth of tuning of taste-responsive cells was calculated with the uncertainty measure (Smith and Travers 1979). The formula for uncertainty was
where k (scaling factor) = 1.66 for four stimuli and Pi is the proportion of response to stimulus i relative to the summed responses to all four stimuli. Values ranged from 0 to 1.0 with 0 corresponding to a cell responsive to only one stimulus and 1.0 corresponding to a cell equally responsive to all four stimuli. The absolute values of inhibitory taste responses were used for the analysis of breadth of tuning with the uncertainty measure [see Smith and Travers (1979) for a discussion]. We labeled this measure “U” for uncertainty rather that “H” as in Smith and Travers' article (1979) to avoid confusion with the “H” value that indicated information calculated in the analyses of temporal coding, described below.
Metric space analyses of temporal patterns of response.
The analytical methods described in Victor and Purpura (1996, 1997) provide a rigorous way to determine whether stimulus evoked spike trains have the potential to carry information about the taste stimuli. A detailed description of this analysis as it has been applied to electrophysiological recordings in the taste system has been published previously (Di Lorenzo and Victor 2003). Briefly, the analysis derives a family of metrics that measure “distance” (i.e., dissimilarity) between spike trains. Each of these metrics represents the “cost” of transforming one spike train into another by changing a different aspect of the spike trains that are being compared. These include the number of spikes and the precise timing of spikes. The simplest of this family of metrics represents the difference in the number of spikes contained in two spike trains associated with two responses. To calculate cost in this case, each spike that is either deleted or added incurs a cost of “1,” so that this metric, Dcount, is simply the arithmetic difference between the number of spikes in each response.
To measure the difference between two spike trains in terms of the arrangement of spikes in time requires a definition of how close in time two spikes need to occur to be considered equivalent. In the family of metrics described by Victor and Purpura (1996, 1997), the similarity of the timing of spikes in two responses is calculated at a variety of levels of precision, measured by a parameter called “q.” The cost of adding or deleting a spike is set at “1” as in Dcount and, in addition, the cost of moving a spike by an amount of time t is set at qt where q is in units of 1/s. The resulting metric for spike timing is called Dspike[q]. For each metric, the information conveyed at various levels of precision (values of q) was calculated, and the value of q at which information is maximized was obtained (see Di Lorenzo and Victor 2003; Victor and Purpura 1996, 1997). Thus the relative contribution of spike count and spike timing to the information conveyed by taste responses can be quantified.
Importantly, there are several additional analyses that serve as controls for the possibility of spurious results. These are detailed in Victor and Purpura (1996, 1997). First, the values of H calculated from observed responses were compared with the values of H calculated from a data set in which the observed responses were randomly assigned to the various clusters of tastant. This served as a control for the statistical effects of a finite data set and was called Hshuffle. Second, to distinguish between the firing rate envelope (time course of response) and the precise firing pattern, we applied metric space analysis to surrogate data sets created by randomly exchanging spikes between individual responses belonging to the same tastant. These surrogate data sets, called Hexchange, had poststimulus time histograms that were identical to those of the actual responses, with the identical number of spikes for each trial. If the value of H for the actual response data was greater than the value of Hexchange (means ± 2 SD), we concluded that the information contributed by spike timing in individual trials was contributing to taste coding, above and beyond that contributed by the rate envelope and spike count alone.
Histology.
At the end of each experiment, a lesion was produced through the recording electrode. (0.1 mA DC for 5 s) at the final recording site. The rat was then overdosed with urethane and perfused transcardially with isotonic saline (0.15 M NaCl) and formol saline (10% formaldehyde in isotonic saline). The brain was removed and processed for histological reconstruction of the recording site(s). Frozen sections (80 μm) were mounted on gelatinized slides and stained with cresyl violet.
RESULTS
General response characteristics.
Responses to exemplars of the four prototypical taste qualities were recorded from 44 PbN cells. Thirty-nine of 44 cells were tested with multiple trials of each stimulus (range = 2 to 26 trials; mean = 11.8 ± 1.13; median = 10). The average spontaneous rate across all cells was 3.9 ± 0.6 spikes per second. When cells were categorized by the stimulus that evoked the highest magnitude of response, 28 cells were NaCl best, 7 cells were HCl best, 5 cells were quinine best, and 4 cells were sucrose best. The average response magnitudes to the four taste stimuli (±SE) were as follows: sucrose, 5.65 ± 1.15; NaCl, 20.13 ± 2.47; HCl, 11.10 ± 1.70, and quinine, 10.74 ± 1.64. The mean breadth of tuning across taste stimuli as quantified by the Uncertainty measure was U = 0.78 ± 0.02 with a range of U = 0.32 to U = 0.99.
Variability in response magnitude with repeated presentations of a given stimulus was assessed by calculating the coefficient of variation (CV; SD/mean). The mean CV across tastants in all cells was 0.45 ± 0.04. Levels of variability across trials were similar for all tastants tested: the CV for NaCl = 0.41 ± 0.07, for HCl = 0.49 ± 0.09, for quinine = 0.43 ± 0.06, and for sucrose = 0.49 ± 0.06. A one-way ANOVA applied to these data showed no significant effects of stimulus [F(3,123) = 0.31; P > 0.05]. Across cells and stimuli, the average CV for the best stimulus of a cell (0.31 ± 0.04) was significantly smaller (P < 0.01, Student's t-test) than the average CV for non-best stimuli (CV = 0.54 ± 0.05). This reflected the fact that taste stimuli that evoked higher response magnitudes showed relatively less variability across trials than those that evoked smaller responses as evidenced by a significant negative correlation between response magnitude and CV (r = −0.49; P < 0.001).
Temporal coding of taste stimuli.
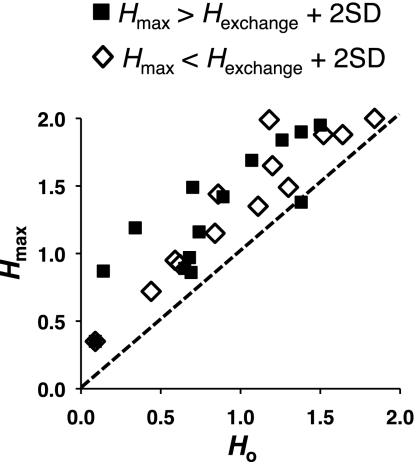
Twenty-nine cells tested with seven or more trials of each taste stimulus were analyzed for temporal coding. This number of trials per stimulus was the minimum number that would provide meaningful results using metric space analyses. The amount of information (in bits) conveyed by spike timing was compared with that provided by spike count alone (Hcount). The maximum information possible was two bits, corresponding to perfect discrimination among four distinct stimuli. Information conveyed by spike timing conveyed more information than spike count alone in 28 of 29 cells as shown in Fig. 1. When the maximum information conveyed by spike timing (Hmax) was no greater than the information conveyed by the exchange-resampled control +2 SD, then the rate envelope (time course) of the response, rather than precise spike timing is the informative characteristic of the cell's responses. This was observed in 14 (of 29, 48%) cells. When Hmax is greater than the value of the exchange-resampled control +2 SD, then there is a significant contribution of spike timing to the information conveyed by the responses. This occurred in the remaining 14 cells (of 29, 48%). There was one cell where spike count alone conveyed more information than spike timing or the rate envelope of the response.
Fig. 1.
Plot of the amount of information (in bits) contributed by spike count alone (Ho) vs. the maximum amount of information contributed by spike count plus the temporal features of the response (Hmax). ■: Cells with responses that showed a significant contribution of spike timing, i.e., Hmax > Hexchange + 2 SD.
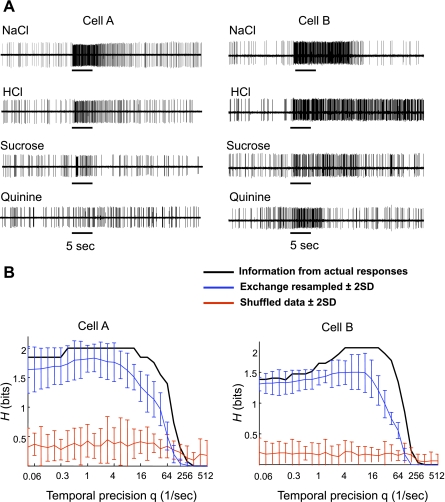
Figure 2 shows the raw data and temporal coding analyses from two cells. In Fig. 2A, left, responses from a cell that is relatively narrowly tuned to NaCl (U = 0.78) are shown. The information plot associated with this cell, shown in Fig. 2B, left, shows that the information conveyed by spike count alone (Hcount = 1.84; the value of the information plot for actual responses at q = 0) may support nearly perfect discrimination among the four tastants (which corresponds to H=2). The contribution of spike timing (information from spike timing is greater than that shown by the exchange-resampled control analyses) adds the remaining 0.16 bits for a total of 2.0 bits at q values between 8 and 16. In contrast, Fig. 2, A and B, right, shows the responses and information plot from a broadly tuned cell (U = 0.90) with a significant contribution of spike timing at q values between 4 and 32. This cell responded to all four taste stimuli. In this case, spike timing contributed 35% more information than spike count alone.
Fig. 2.
Taste responses and results of temporal coding analyses in a relatively narrowly tuned and a broadly tuned parabrachial nucleus of the pons (PbN) cell. A: raw data showing responses to the basic taste qualities in a relatively narrowly tuned cell (cell A) and a broadly tuned cell (cell B). B: information plots associated with cells A and B shown in A. In both cells, spike timing contributes significantly more information about taste quality than either spike count alone, as indicated by the value of the plot of the information from actual responses at q = 0, or the rate envelope of response, as indicated by the plot of the exchange resampled data. However, in cell A, spike count alone contributes nearly all of the information necessary to discriminate among four tastants (2.0 bits).
The proportional contribution of temporal coding (including the contribution of the temporal envelope) was calculated with the following formula:
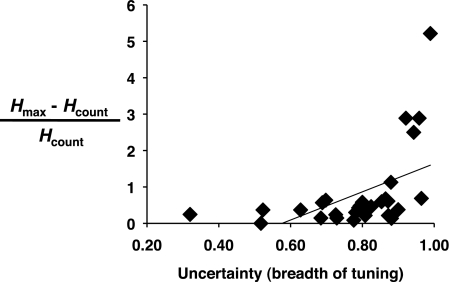
There was a significant positive correlation between the breadth of tuning of PbN cells and the proportional contribution of temporal coding such that cells that were broadly tuned showed greater information conveyed by temporal coding (r = 0.51; P < 0.001; see Fig. 3). Although it might appear that this significant correlation was driven by the contribution of four cells that are plotted above the rest, the relationship between breadth of tuning and temporal coding remains significant when the analysis is recalculated without those four cells (r = 0.40; P < 0.05). In contrast, there was no relationship between the best stimulus of a cell and the proportion of total information conveyed by temporal coding. Most cells were either N best (n = 20) or H best (n = 6), and a comparison between the proportion of information contributed by temporal coding for these two groups was not statistically significant (P > 0.27).
Fig. 3.
Plot of the uncertainty measure (U, breadth of tuning) vs. the proportion of information that was conveyed by the temporal features of the response (Hmax − Hcount/Hcount). Line on plot shows result of linear regression.
Comparison of temporal coding in the PbN and the NTS.
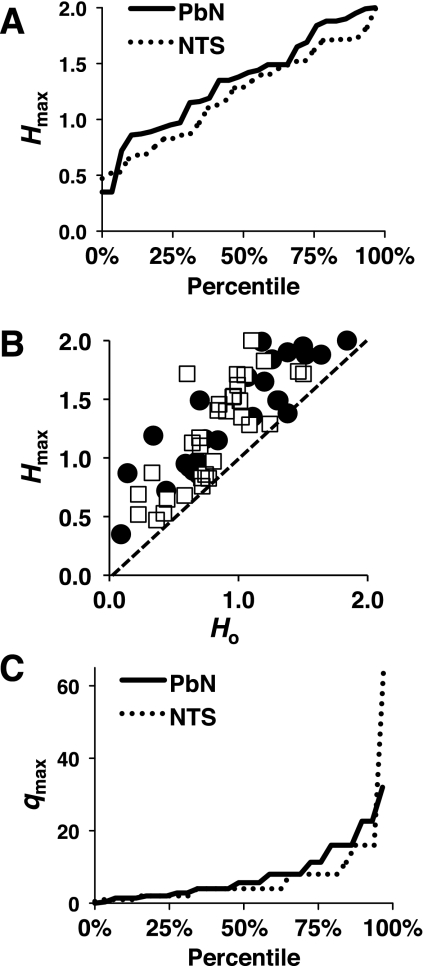
Data from the present study were compared with data collapsed from two previous studies of temporal coding in the NTS (n = 32 cells total; Di Lorenzo and Victor 2003, 2007). In general, results showed that taste responses in the PbN were more variable across trials than those in the NTS but that measures of temporal coding in the PbN were not significantly different than those in the NTS. Specifically, the average CV of taste responses in the PbN (0.45 ± 0.04) was significantly larger than the average CV of taste responses in the NTS (CV = 0.32 ± 0.02; P < 0.01, Student's t-test). However, the average total amount of information conveyed by spike count and spike timing (Hmax) was similar across structures: 1.33 ± 0.09 among PbN cells and 1.21 ± 0.08 in the NTS (P > 0.3, Student's t-test). Figure 4A shows the values of Hmax in all PbN and NTS cells, plotted as percentiles. Information conveyed by spike count alone (Hcount) was also similar in the two structures: 0.94 ± 0.09 in PbN and 0.82 ± 0.06. In Fig. 4B, Hcount is plotted against Hmax to illustrate the relative contribution of spike timing to the total amount of information. The dotted line in the diagonal shows the condition where spike timing does not contribute any information to the total. In this plot, it can be seen that PbN and NTS cells are intermingled, suggesting that there is no difference between these two nuclei in the relative contribution of spike timing to the total amount of information. In fact, the average proportion of the total information contributed by spike timing was 0.81 ± 0.21 across PbN cells and 0.58 ± 0.09 across NTS cells. Although these values are different, the difference is not statistically reliable (P = 0.3, Student's t-test). Much of the difference can be explained by a few PbN cells that show a very small Hcount, so that even a relatively small Hmax will produce a very large proportionate contribution of spike timing. The median values for this proportion were more similar across structures (0.43 for the PbN and 0.53 for the NTS). Finally, the average level of temporal precision at which information is at a maximum (qmax) was 7.90 ± 1.45 in the PbN and 7.12 ± 0.99 in the NTS. Not surprisingly, the distribution of qmax values across PbN and NTS cells, plotted as percentiles, is nearly identical (Fig. 4C). Collectively, these data show that information conveyed by both spike count and spike timing is preserved as it is conveyed from the NTS to the PbN and, further, that spike timing is significant at the same level of temporal precision in both structures.
Fig. 4.
Comparison of temporal coding in the PbN and nucleus of the solitary tract (NTS). A: graph of Hmax in all PbN and NTS cells, plotted as percentiles. B: plot of the amount of information (in bits) contributed by spike count alone (Ho) vs. the maximum amount of information contributed by spike count plus the temporal features of the response (Hmax) for PbN cells (■) and NTS cells (□). C: distribution of values of qmax (a measure of temporal precision) at which information is at a maximum value (Hmax), plotted as percentiles.
Histology.
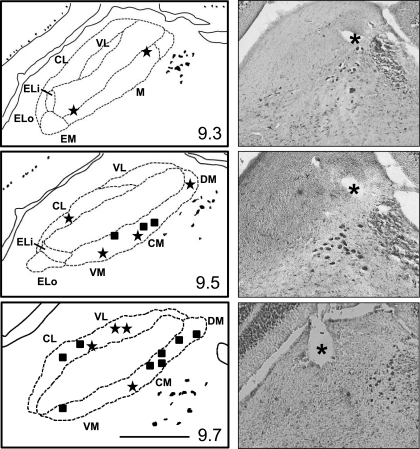
Lesions corresponding to the locations of 21 of 29 cells analyzed for temporal coding were confined within the PbN (see Fig. 5). The lesions were largely concentrated in the caudal PbN, located 9.8 mm caudal to bregma. The number of lesions decreased precipitously in more rostral planes. Lesions were most often located on the dorsal and ventral borders of the PbN, encompassing the ventrolateral, ventromedial, dorsal medial, and central medial subnuclei.
Fig. 5.
Histological results showing recording site for 21 cells. Left: line drawings of coronal sections at various AP levels through the PbN. Numbers in bottom right of each drawing indicate distance in millimeters caudal to bregma. Line in bottom right of bottom drawing indicates 0.5 mm. DM, dorsomedial n.; CM, central medial n.; VM, ventromedial n.; VL, ventral lateral n.; CL, central lateral n.; ELo, external lateral outer n.; external lateral inner n. Right: photomicrographs of coronal sections showing lesions (asterisks) marking recording sites at AP levels corresponding to the line drawings to the left.
DISCUSSION
Analyses of taste responses in single cells in the PbN showed that temporal coding provided a significant advantage over rate coding in nearly every cell in spite of significant trial-to-trial variability in response magnitude. In particular, spike timing contributed a significant amount of information about taste quality above and beyond that contributed by spike count alone in 48% of the cells. In another 48% of the cells, the rate envelope of the response was more informative than spike count. Comparison with previously published data recorded from taste-responsive NTS cells (Di Lorenzo and Victor 2003, 2007) provided evidence that PbN cells show significantly more trial-to-trial variability than NTS cells. Even so, the distribution, amount, and proportion of information contributed by the temporal features of taste responses were similar to those observed in the PbN in the present study. The temporal precision with which spike timing conveyed information was also similar in NTS and PbN. Further, results showed that broadly tuned PbN cells, like those in the NTS (Di Lorenzo and Victor 2003; Di Lorenzo et al. 2009a), generally encode more information using spike timing than cells that are narrowly tuned. Altogether, these data imply that PbN cells use the temporal features of taste-evoked spike trains to convey information about taste quality and that this information is transmitted from the NTS to the PbN with high fidelity, even in the face of an increase in trial-to-trial variability in response magnitude.
The widespread incidence of temporal coding among PbN cells reported here points to the temporal features of taste responses in this region as an informative, but relatively neglected, aspect of taste responses. While the presence of temporal coding has been well documented in the NTS (Di Lorenzo and Victor 2003; Roussin et al. 2008), few reports have touched on this issue in the PbN, and none have quantified the information contributed by temporal coding. Early on, Perrotto and Scott (1976) and Scott and Perrotto (1980) described taste quality-specific time courses (rate envelopes) of the average peristimulus-time histogram (PSTH) of PbN responses. Perrotto and Scott (1976) also noted that the ratio of the magnitude of the phasic component of the response (usually the number of spikes in the second 100-ms time bin of the PSTH) to the later tonic component (number of spikes in 0.3–1.3 s of the PSTH) of the response varied systematically according to taste quality. More detailed analyses of taste responses in the rabbit PbN using principal components analyses of the normalized responses, however, suggested that only the hedonic valence (pleasant or unpleasant) of a tastant could be signaled by the time course of response (Di Lorenzo and Schwartzbaum 1982). By examining the fine temporal characteristics of spike trains, the present data further suggest that the time course of response can also convey information about taste quality in about half of the population of PbN cells. Later, Erickson et al. (1994) used a fuzzy set approach to derive prototypical time courses from PSTHs across cells. Each taste response was then assigned a “loading” that measured the association of that response with each of the prototypical time courses. With the use of this method, the time courses of each cell's response could be accurately reconstructed. From these data, Erickson et al. (1994) speculated that the prototypical time courses originated in the receptor and that the actual time course of any given response was the result of the convergence of inputs originating from different receptor processes. In effect, the argument was that the time course of response was not a function of the cell but of the interaction of various peripherally derived processes. While this idea is not at all inconsistent with the present data, we show that spike timing, as well as the time course of response, can be used to distinguish among tastants of different qualities.
Although the present report highlights the similarities in the quantitative aspects of temporal coding in NTS and PbN, there is ample reason to suspect that this information may be used in different ways. That is, the PbN is thought to be involved in conditioned taste-visceral associations while the NTS may be more concerned with unconditioned taste-evoked ingestion and behavioral taste reactivity [reviewed in Lundy (2008)]. In the NTS, lick-contingent electrical stimulation with temporal patterns of pulses that mimic actual electrophysiological responses to particular taste qualities can evoke specific and predictable taste sensations in rats (Di Lorenzo and Hecht 1993; Di Lorenzo et al. 2003a, 2009b), observations that underscore the functionality of temporal coding in the NTS. Given the different function of the PbN, it is an open question as to whether the same type of stimulation in the PbN would produce similar effects. On the other hand, PbN/NTS projections may allow information conveyed by temporal coding in the PbN to amplify the signal conveyed by spike timing in the NTS. In this context, it is worth noting that there are projections from the PbN to ventral subnucleus of the NTS (Karimnamazi and Travers 1998), an area that then projects to oromotor nuclei in the reticular formation (Halsell et al. 1996) where spike timing may be critical to the selection of appropriate oromotor behaviors (Venugopal et al. 2010).
Trial-to-trial variability.
Considered in the context of data from the chorda tympani nerve (CT: a branch of the facial nerve that innervates taste buds on the rostral 2/3 of the tongue; Ogawa et al. 1973) and the NTS (Di Lorenzo and Victor 2003, 2007), present data from the PbN extend a trend of increasing trial-to-trial variability from peripheral to central structures along the gustatory neuraxis. In a study of trial-to-trial variability of taste responses recorded from CT fibers, for example, Ogawa et al. (1973) reported that the average CV ranged between 0.1 and 0.25. The mean CV of CT fibers as calculated from Table 1 of Ogawa et al. (1973) was 0.19 ± 0.02. This value was significantly lower than the average CV across NTS cells (0.32 ± 0.02; P < 0.01, Student's t-test). In turn, the average CV across PbN cells (0.44 ± 0.03) was significantly greater than that in the NTS (P < 0.01, Student's t-test).
Escalating trial-to-trial variability in the central gustatory pathway may be due to increasing complexity in the network of interconnections as the sensory signal ascends through the brain. That is, as the signal is transmitted from structure to structure, there are more and more loops of information that can influence responding of single cells and/or ensembles (see Jones et al. 2007). Related to this point, Fontanini and Katz (2008) cited evidence from a number of sensory systems and neural structures to argue that trial-to-trial variability may be an essential feature of normal sensory processing. That is, they maintained that, rather than reflecting noise in the system, this type of variability may be an expression a naturally fluctuating state of the neural network. These fluctuations can reflect variables such as attention (Fontanini and Katz 2006) or context (Di Lorenzo et al. 2003b) for example. In their work on the gustatory cortex, Katz and colleagues (Jones et al. 2007; Fontanini and Katz 2006, 2008) have shown that ensembles of cortical cells traverse through stimulus-specific stereotypic sequences of states (defined as coordinated firing rates across cells) when taste stimuli are presented. From trial-to-trial, however, the length of time that the network remains in each state may expand or contract, but the sequence remains the same. Such network dynamics may also be present in the PbN cells considering the rich network of intra- and extranuclear connections (Cho et al. 2003; Li et al. 2005; Di Lorenzo and Monroe 1992, 1995).
Conservation of information conveyed by temporal coding in the PbN.
In spite of a significant increase in trial-to-trial variability in response magnitude, the present data show that the information conveyed by spike timing is conserved as it is passed from NTS to PbN. Of course, the overall similarity in the prevalence and precision of temporal coding between these two structures does not directly imply that the PbN merely mirrors the spike patterns relayed from the NTS. However, in a study of simultaneously recorded pairs of taste-responsive cells, one from the NTS and the other from the PbN, we showed that PbN cells that were functionally connected to NTS cells did indeed follow the activity of NTS cells spike by spike in a roughly damped oscillatory pattern for the first 3 s of the response (Di Lorenzo and Monroe 1997; Di Lorenzo et al. 2009c). As the drive from the NTS cells diminishes, the responses from the PbN, though still robust, become increasingly independent from those in the NTS. Since our analyses of taste responses focused on the initial 2 s of response, it is possible that information conveyed by spike timing in PbN cells was transmitted directly from relay cells in the NTS. That would be consistent with the observation that the amount of information conveyed by spike timing and the temporal precision with which the information was conveyed were identical in NTS and PbN cells. In addition, taste-responsive PbN cells that used temporal coding were located in the central medial and ventral lateral regions of the PbN, an area that receives dense synaptic input from the NTS (Herbert et al. 1990; Halsell and Travers 1997).
It can also be hypothesized that the conservation of information through temporal coding as it is transferred from the NTS to the PbN may be the result of a common descending drive from forebrain structures such as the lateral hypothalamus (Li et al. 2005; Cho et al. 2003), bed nucleus of the stria terminalis (Li and Cho 2006), central nucleus of the amygdala (Cho et al. 2003; Li et al. 2005), and gustatory cortex (Di Lorenzo and Monroe 1992, 1995). However, although both NTS and PbN receive input from the same structures, the character (excitatory or inhibitory) and selectivity of the influences can differ [reviewed in Lundy (2008)]. Moreover, the proportion of cells in each of these structures that projects to both the NTS and PbN is <20% (Kang and Lundy 2009), supporting the idea that the NTS and PbN receive differential modulatory influences. It is therefore unlikely that centrifugal feedback is responsible for similarities with respect to temporal coding of tastants in NTS and PbN cells.
Conclusions.
In the present study, spike timing in taste-responsive PbN cells was found to significantly contribute information about taste quality in about half of the sample, with broadly tuned PbN cells conveying proportionately more information than narrowly tuned cells. The fraction of the total amount of information conveyed by temporal coding in the PbN and the temporal precision at which information from temporal coding was maximized was identical to that in the NTS, even though trial-to-trial variability was higher in the PbN than in the NTS. In all, these data show that the neural representation of taste stimuli through the temporal characteristics of the taste-evoked spike trains is strikingly similar in both PbN and NTS, suggesting a high fidelity of synaptic transmission from one structure to the other. Although there is evidence that the temporal characteristics of taste responses can be “read” by cells in the NTS (Di Lorenzo and Hecht 1993; Di Lorenzo et al. 2003, 2009b), a corresponding demonstration that the same applies to the PbN awaits further experimentation.
GRANTS
P. M. Di Lorenzo was supported by National Institute on Deafness and Other Communication Disorders Grant RO1-DC-006914. J. Victor was supported in part by MH68012, “Algorithms and Informatics for Analysis of Neural Coding” (Principal Invesitgator: D. Gardner).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We acknowledge the substantial technical contribution of Daniel Platt. Histological processing of tissue was done by Obeda Lavin for which we are also grateful.
REFERENCES
- Chen JY, Victor JD, Di Lorenzo PM. Temporal coding of intensity of NaCl and HCl in the nucleus of the solitary tract of the rat. J Neurophysiol 105: 697–711, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YK, Li CS, Smith DV. Descending influences from the lateral hypothalamus and amygdala converge onto medullary taste neurons. Chem Senses 28: 155–171, 2003 [DOI] [PubMed] [Google Scholar]
- Di Lorenzo PM, Monroe S. Corticofugal input to taste-responsive units in the parabrachial pons. Brain Res Bull 29: 925–930, 1992 [DOI] [PubMed] [Google Scholar]
- Di Lorenzo PM, Monroe S. Corticofugal influence on taste responses in the nucleus of the solitary tract in the rat. J Neurophysiol 74: 258–272, 1995 [DOI] [PubMed] [Google Scholar]
- Di Lorenzo PM, Monroe S. Transfer of information about taste from the nucleus of the solitary tract to the parabrachial nucleus of the pons. Brain Res 763; 167–181, 1997 [DOI] [PubMed] [Google Scholar]
- Di Lorenzo PM, Hecht GS. Perceptual consequences of electrical stimulation in the gustatory system. Behav Neurosci 107: 130–138, 1993 [DOI] [PubMed] [Google Scholar]
- Di Lorenzo PM, Hallock RM, Kennedy DP. Temporal coding of sensation: mimicking taste quality with electrical stimulation of the brain. Behav Neurosci 117: 1423–1433, 2003a [DOI] [PubMed] [Google Scholar]
- Di Lorenzo PM, Lemon CH, Reich CG. Dynamic coding of taste stimuli in the brainstem: effects of brief pulse of taste stimuli on subsequent taste responses. J Neurosci 23: 8893–8902, 2003b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lorenzo PM, Schwartzbaum JS. Coding of gustatory information in the pontine parabrachial nuclei of the rabbit: Temporal patterns of neural response. Brain Res 251: 245–257, 1982 [DOI] [PubMed] [Google Scholar]
- Di Lorenzo PM, Victor JD. Taste response variability and temporal coding in the nucleus of the solitary tract of the rat. J Neurophysiol 90: 1418–1431, 2003 [DOI] [PubMed] [Google Scholar]
- Di Lorenzo PM, Victor JD. Neural coding mechanisms for flow rate in taste-responsive cells in the nucleus of the solitary tract of the rat. J Neurophysiol 97: 1857–1861, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lorenzo PM, Chen JY, Victor JD. Quality time: representation of a multimodal sensory domain through temporal coding. J Neurosci 29: 9227–9238, 2009a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lorenzo PM, Leshchinskiy S, Moroney DN, Ozdoba JM. Making time count: functional evidence for temporal coding of taste sensation. Behav Neurosci 123: 14–25, 2009b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lorenzo PM, Platt D, Victor JD. Information processing in the parabrachial nucleus of the pons. Ann NY Acad Sci 1170: 365–371, 2009c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson RP, Di Lorenzo PM, Woodbury MA. Classification of taste responses in brain stem: membership in fuzzy sets. J Neurophysiol 71: 2139–2150, 1994 [DOI] [PubMed] [Google Scholar]
- Fontanini A, Katz DB. State-dependent modulation of time-varying gustatory responses. J Neurophysiol 96: 3183–3193, 2006 [DOI] [PubMed] [Google Scholar]
- Fontanini A, Katz DB. Behavioral states, network states, and sensory response variability. J Neurophysiol 100: 1160–1168, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganchrow JR, Erickson RP. Neural correlates of gustatory intensity and quality. J Neurophysiol 33: 768–783, 1970 [DOI] [PubMed] [Google Scholar]
- Herbert H, Moga HM, Saper CB. Connections of the parabrachial nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol 293: 540–580, 1990 [DOI] [PubMed] [Google Scholar]
- Halsell CB, Travers SP, Travers JB. Ascending and descending projections from the rostral nucleus of the solitary tract originate from separate neuronal populations. Neuroscience 72: 185–97, 1996 [DOI] [PubMed] [Google Scholar]
- Halsell CB, Travers SP. Anterior and posterior oral cavity responsive neurons are differentially distributed among parabrachial subnuclei in rat. J Neurophysiol 78: 920–938, 1997 [DOI] [PubMed] [Google Scholar]
- Jones LM, Fontanini A, Sadacca BF, Miller P, Katz DB. Natural stimuli evoke dynamic sequences of states in sensory cortical ensembles. Proc Natl Acad Sci USA 104: 18772–18777, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Lundy RF. Terminal field specificity of forebrain efferent axons to brainstem gustatory nuclei. Brain Res 1248: 76–85, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimnamazi H, Travers JB. Differential projections from the gustatory responsive parabrachial regions to the medulla and forebrain. Brain Res 813: 283–302, 1998 [DOI] [PubMed] [Google Scholar]
- Katz DB, Nicolelis MA, Simon SA. Gustatory processing is dynamic and distributed. Curr Opin Neurobiol 12: 448–454, 2002 [DOI] [PubMed] [Google Scholar]
- Li CS, Cho YK, Smith DV. Modulation of parabrachial taste neurons by electrical and chemical stimulation of the lateral hypothalamus and amygdala. J Neurophysiol 93: 1183–1196, 2005 [DOI] [PubMed] [Google Scholar]
- Li CS, Cho YK. Efferent projection from the bed nucleus of the stria terminalis suppresses activity of taste-responsive neurons in the hamster parabrachial nuclei. Am J Physiol Regul Integr Comp Physiol 291: R914–R926, 2006 [DOI] [PubMed] [Google Scholar]
- Lundy RF, Jr, Norgren R. Activity in the hypothalamus, amygdala and cortex generates bilateral and convergent modulation of pontine gustatory neurons. J Neurophysiol 91: 1143–1157, 2004 [DOI] [PubMed] [Google Scholar]
- Lundy RF., Jr Gustatory hedonic value: potential function for forebrain control of brainstem taste processing. Neurosci Biobehav Rev 32: 1601–1606, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgren R. Projections from the nucleus of the solitary tract in the rat. Neuroscience 3: 207–218, 1978 [DOI] [PubMed] [Google Scholar]
- Ogawa H, Sato M, Yamashita S. Variability in impulse discharges in rat chorda tympani fibers in response to repeated gustatory stimulations. Physiol Behav 11: 469–479, 1973 [DOI] [PubMed] [Google Scholar]
- Perrotto RS, Scott TR. Gustatory neural coding in the pons. Brain Res 110: 283–300, 1976 [DOI] [PubMed] [Google Scholar]
- Roussin AT, Victor JD, Chen JY, Di Lorenzo PM. Variability in responses and temporal coding of tastants of similar quality in the nucleus of the solitary tract of the rat. J Neurophysiol 99: 644–655, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SA, de Araujo IE, Gutierrez R, Nicolelis MA. The neural mechanisms of gustation: a distributes processing code. Nat Rev Neurosci 7: 890–901, 2006 [DOI] [PubMed] [Google Scholar]
- Scott TR, Perrotto RS. Intensity coding in pontine taste area: gustatory information is processed similarly throughout rat's brain stem. J Neurophysiol 44: 739–750, 1980 [DOI] [PubMed] [Google Scholar]
- Smith DV, Travers JB. A metric for the breadth of tuning of gustatory neurons. Chem Senses 4: 215–229, 1979 [Google Scholar]
- Travers JB. Efferent projections from the anterior nucleus of the solitary tract of the hamster. Brain Res 457: 1–11, 1988 [DOI] [PubMed] [Google Scholar]
- Travers JB, Norgren R. Afferent projections to the oral motor nuclei in the rat. J Comp Neurol 22: 280–298, 1983 [DOI] [PubMed] [Google Scholar]
- Venugopal S, Boulant JA, Chen Z, Travers JB. Intrinsic membrane properties of pre-oromotor neurons in the intermediate zone of the medullary reticular formation. Neuroscience 168: 21–47, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor JD, Purpura KP. Nature and precision of temporal coding in visual cortex: a metric space analysis. J Neurophysiol 76: 1310–1326, 1996 [DOI] [PubMed] [Google Scholar]
- Victor JD, Purpura KP. Sensory coding in cortical neurons. Recent results and speculations. Ann NY Acad Sci 835: 330–352, 1997 [DOI] [PubMed] [Google Scholar]
- Whitehead MC. Neuronal architecture of the nucleus of the solitary tract in the hamster. J Comp Neurol 276: 547–572, 1988 [DOI] [PubMed] [Google Scholar]