Abstract
The parallel fibers (PFs) in the cerebellar cortex extend several millimeters along a folium in the mediolateral direction. The PFs are orthogonal to and cross several parasagittal zones defined by the olivocerebellar and corticonuclear pathways and the expression of molecular markers on Purkinje cells (PCs). The functions of these two organizations remain unclear, including whether the bands respond similarly or differentially to PF input. By using flavoprotein imaging in the anesthetized mouse in vivo, this study demonstrates that high-frequency PF stimulation, which activates a beamlike response at short latency, also evokes patches of activation at long latencies. These patches consist of increased fluorescence along the beam at latencies of 20–25 s with peak activation at 35 s. The long-latency patches are completely blocked by the type 1 metabotropic glutamate receptor (mGluR1) antagonist LY367385. Conversely, the AMPA and NMDA glutamate receptor antagonists DNQX and APV have little effect. Organized in parasagittal bands, the long-latency patches align with zebrin II-positive PC stripes. Additional Ca2+ imaging demonstrates that the patches reflect increases in intracellular Ca2+. Both the PLCβ inhibitor U73122 and the ryanodine receptor inhibitor ryanodine completely block the long-latency patches, indicating that the patches are due to Ca2+ release from intracellular stores. Robust, mGluR1-dependent long-term potentiation (LTP) of the patches is induced using a high-frequency PF stimulation conditioning paradigm that generates LTP of PF-PC synapses. Therefore, the parasagittal bands, as defined by the molecular compartmentalization of PCs, respond differentially to PF inputs via mGluR1-mediated release of internal Ca2+.
Keywords: Purkinje cell, metabotropic glutamate receptors, intracellular calcium release, flavoprotein imaging, parasagittal zones
the cerebellum has a prominent longitudinal architecture (Apps and Hawkes 2009; Hawkes and Herrup 1995). Both the climbing fiber projection from the inferior olive and the Purkinje cell (PC) corticonuclear projections are organized in parasagittal zones (Brodal and Kawamura 1980; Voogd 1967; Voogd and Bigare 1980). Mossy fiber projections also terminate in the granular layer to varying degrees in longitudinal zones (Ji and Hawkes 1994; Yaginuma and Matsushita 1989). Spinocerebellar and olivocerebellar afferent pathways activate parasagittally oriented responses in the cerebellar cortex (Ekerot and Larson 1973; Hanson et al. 2000; Llinas and Sasaki 1989; Oscarsson 1980; Welsh et al. 1995).
A parasagittal compartmentalization is present on PCs at the molecular level, exemplified by the parasagittal bands of zebrin II/aldolase C (Ahn et al. 1994; Brochu et al. 1990; Sillitoe and Hawkes 2002). Numerous other molecules are found on PCs in either a zebrin II-positive or the complimentary zebrin II-negative banding pattern (Apps and Hawkes 2009; Hawkes and Herrup 1995). Climbing and mossy fiber afferents show spatial correspondence with these markers (Apps and Garwicz 2000; Sugihara and Quy 2007; Voogd et al. 2003; Voogd and Ruigrok 2004), as do the responses evoked by peripheral inputs (Chen et al. 1996; Chockkan and Hawkes 1994; Hallem et al. 1999). The full functional significance of this parasagittal architecture remains unknown (Apps and Hawkes 2009).
Of interest for this study is the parasagittal organization of type 1 metabotropic glutamate receptors (mGluR1) and downstream signaling cascade. Implicated in regulating PC signaling and cerebellar function (Hartmann and Konnerth 2009), mGluR1 are expressed heavily on PCs (Grandes et al. 1994; Lein et al. 2007). Activation of mGluR1 leads to activation of phospholipase Cβ (PLCβ) with the production of inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). In turn, IP3 binds to IP3 receptors on the endoplasmic reticulum, releasing Ca2+ from intracellular stores (Finch and Augustine 1998; Linden et al. 1991; Llano et al. 1991a; Takechi et al. 1998). The PLCβ3 isoform is found in a subset of PCs in a zebrin II-positive banding pattern, whereas the PLCβ4 isoform and the splice variant, mGluR1b, are found in a complimentary pattern on zebrin II-negative PCs (Mateos et al. 2001; Sarna et al. 2006). Interestingly, the mGluR1b isoform has less potency in coupling to the PLC downstream signaling pathway than the mGluR1a isoform (Joly et al. 1995; Prezeau et al. 1996). The functional consequences of the parasagittal compartmentalization of the mGluR1 isoforms and intracellular signaling pathway are not known. However, it has been hypothesized that these differences in mGluR1 signaling pathways may result in differential synaptic plasticity among the parasagittal zones (Mateos et al. 2001; Paukert et al. 2010).
The parasagittal bands are crossed by the parallel fibers (PFs) in the molecular layer that extend for 3–5 mm in the mediolateral direction and make glutamatergic synapses with the dendrites of PCs and cerebellar interneurons. The PFs are hypothesized to play a central role in cerebellar functioning (Braitenberg et al. 1997; Ito 2006; Thach et al. 1992). Although it is generally assumed that PFs provide for relatively uniform, short-latency activation of their postsynaptic targets (Braitenberg 2002; Eccles et al. 1967), the effects of PF input may differ between bands, as shown recently by the parasagittal compartmentalization of molecular layer inhibition (Gao et al. 2006). The present study demonstrates that high-frequency PF stimulation activates patches at long latencies that align with the zebrin II and PLCβ3-positive zones. These patches are due to the release of Ca2+ from intracellular stores mediated through the mGluR1 signaling cascade.
MATERIALS AND METHODS
Animal preparation.
All animal procedures were approved by and conducted in conformity with the Institutional Animal Care and Use Committee of the University of Minnesota and in accordance with the American Physiological Society's “Guiding Principles for Research Involving Animals and Human Beings” (2002). Experimental details on the animal preparation and optical imaging techniques are described briefly, because the details have been provided in previous publications (Gao et al. 2006; Reinert et al. 2004; Wang et al. 2009).
Male FVB mice, ages 5–8 mo (Charles River Laboratories, Wilmington, MA), were anesthetized by induction with acepromazine (2.0 mg/kg im) followed by urethane (1.5 mg/kg ip). The electrocardiogram and response to pinch were monitored to assess the depth of anesthesia, with anesthetics supplemented as needed. The mice were mechanically ventilated, and body temperature was feedback-regulated. The animal was placed in a stereotaxic frame, a craniotomy exposed Crus I and II, and a watertight chamber of dental acrylic was created around the exposed folia. The chamber was filled and periodically rinsed with a gassed Ringer solution (Gao et al. 2006; Reinert et al. 2004). Various drugs in normal Ringer solution were applied to the exposed cerebellar surface, including 1) the dl-α-amino-3-hydroxy-5-methylisox azole-propionic acid (AMPA) receptor antagonist 6,7-dinitroquinoxaline-2,3-dione disodium salt (DNQX), 2) the mGluR1 receptor antagonist LY367385, and 3) the N-methyl-d-aspartate (NMDA) receptor antagonist d-(−)-2-amino-5-phosphonopentanoic acid (APV). The glutamate receptor antagonists were purchased from Tocris Bioscience (Ellisville, MO). To block downstream targets of the mGluR1 signaling pathway that release Ca2+ from intracellular stores, we used the following drugs: 1) the PLCβ inhibitor U73122 and 2) the ryanodine receptor (RyR) antagonist ryanodine (Tocris Bioscience). These latter two drugs were initially dissolved in ethanol and then diluted in Ringer solution to a final ethanol concentration of 1.0–1.5%.
To complement the flavoprotein autofluorescence imaging, we also used Ca2+ imaging. With the use of a glass micropipette, pressure microinjections (2 s with 100 kPa, PLI-100 microinjector; Medical Systems) are made into the molecular layer with a solution consisting of Oregon green 488 BAPTA-1/AM dissolved in DMSO plus 20% Pluronic F-127 solution and diluted in normal Ringer solution to a final concentration of 0.5 mM (Gao et al. 2006; Sarkisov and Wang 2008; Sullivan et al. 2005). The dye injections are visualized in the microscope, allowing for precise placement and control. Multiple injections at a depth of ∼200 μm were made to stain the exposed surface of Crus II with the Oregon green solution.
Optical imaging.
Images were acquired with a high-speed, cooled charge-coupled device (CCD) camera (Quantix 57 with a 535 × 512 CCD chip at 12-bit digitization or a Quantix 512SC with a 512 × 512 CCD chip at 16-bit digitization). Binning on the CCD chip is done to achieve a pixel resolution of ∼10 × 10 μm. The camera is focused ∼100 μm below the cerebellar surface. The light sources used a stabilized power supply.
Flavoprotein autofluorescence imaging used a band-pass excitation filter (455 ± 35 nm), an extended reflectance diachronic mirror (500 nm), and a >515-nm long-pass emission filter (Reinert et al. 2004). Ca2+ imaging used an excitation band-pass filter of 490–510 nm, a 515-nm diachronic mirror, and a band-pass filter of 520–530 nm (Gao et al. 2006; Sullivan et al. 2005).
Parallel fiber stimulation and LTP protocol.
Stimulation of the PFs was delivered by a parylene-coated microelectrode (2–5 MΩ) placed just below the surface of the cerebellar cortex (Gao et al. 2006; Reinert et al. 2004). Typical stimulation parameters were a train of 10 pulses (200 μA, 100 μs) at 100 Hz. In one series of experiments, the frequency of stimulation was varied from 10 to 500 Hz. At each frequency, 10 pulses were delivered and all other stimulation parameters kept constant. In another experiment, the amplitude of PF stimulation was varied from 50 to 400 μA and the other stimulation parameters were kept constant (10 pulses at 100 Hz, 100-μs duration). The effectiveness of 3 and 5 pulses at 100 Hz (50 μA and 100 μs) was also tested in 3 animals.
We also tested whether the long-latency patches undergo long-term potentiation (LTP) by using a high-frequency induction paradigm that generates LTP of the PF-PC synapses and PC-receptive fields in vivo (Jörntell and Ekerot 2002; Wang et al. 2009). The first 30 min were used to establish the baseline response to the PF test stimulation (5-min intervals). The “test” stimulation consisted of a train of 10 pulses at 100 Hz (175 μA, 150-μs duration). After this baseline period, the PF conditioning stimulation was applied (t = 0 min). The “conditioning” stimulation consisted of 15 pulses (175 μA, 150-μs duration) at 100 Hz every 3 s for 5 min (Jörntell and Ekerot 2002; Wang et al. 2009). To evaluate the effect of the conditioning stimulation, we applied the PF test stimulation at 5-min intervals for 120 min.
Analysis of the optical responses.
As detailed in previous publications (Chen et al. 2005; Dunbar et al. 2004), an image series consisting of 425 sequential frames was acquired (exposure time of 200 ms for each frame) in relation to PF stimulation. The first 20 frames collected before PF stimulation (control frames) provide a measure of the background fluorescence. The first step in the analysis is to generate a series of “difference” images by subtracting the average of the 20 control frames from each frame. These difference images are then divided by the average of the control frames on a pixel-by-pixel basis and converted into a percentage (ΔF/F), in which the intensity value of each pixel reflects the change in fluorescence intensity relative to the average of the control frames. Several methods are used to display the responses, including showing images of the ΔF/F, using either a grayscale or pseudocoloring. To display the optical responses in relation to the anatomy of the folia, the images were thresholded to highlight pixels above or below the mean ± 1.5 SD of the fluorescence in a region of the image of similar area without a response (i.e., typically Crus I). The thresholded pixels were then displayed on an image of the background fluorescence of the folia (Gao et al. 2003).
To quantify the responses to PF test stimulation, a region of interest (ROI) defined by the evoked beam or the long-latency patches was visually determined. The beamlike response to the PF test stimulation consists of an initial period of increase in fluorescence (light phase) followed by a longer duration decrease (Reinert et al. 2004, 2007). The former results from the oxidation of mitochondria flavoproteins in the postsynaptic neurons activated by glutamate and is tightly coupled to the strength of the stimulation (Brennan et al. 2006; Reinert et al. 2004, 2007; Shibuki et al. 2003). For a beam ROI, 5 frames (1 s) centered on the peak amplitude were averaged, and the average ΔF/F within the ROI was determined. For a patch ROI, 25 frames (5 s) were averaged around the peak. The same ROI was used throughout an experiment to quantify changes in the fluorescence. ANOVA was used to statistically assess the effect of a treatment on the response amplitude of the beam or patches (within-subject design with repeated measures, followed by Duncan's post hoc test, α = 0.05). The population response amplitudes are means ± SD, where n refers to the number of animals studied.
To analyze the effects of the LTP conditioning stimulation, we compared the responses in the baseline period with the responses following the conditioning stimulation (Wang et al. 2009). The latter was divided into early (0–60 min) and late phases (65–120 min). The flavoprotein responses within the ROI at each 5-min interval were normalized to the average response during the baseline. Using ANOVA (within-subject design with repeated measures), we tested for significant differences between the baseline period and the early and late phases (α = 0.05).
Field potential recordings.
Field potential recordings in the molecular layer of the responses to PF stimuli provided an electrophysiological assessment of the effect of several pharmacological agents. It is important to determine whether the PF volley and the short-latency postsynaptic response are affected by the blockers of the release of Ca2+ from internal stores (i.e., U73122 and ryanodine). Field potentials were recorded using glass microelectrodes (2 M NaCl, 2–5 MΩ), digitized at 25 KHz, and averaged (responses to 16 single PF stimuli at 1 Hz). The P1/N1 component was used as a measure of the presynaptic responses and the N2 component as a measure of the postsynaptic response (Eccles et al. 1967; Gao et al. 2003; Reinert et al. 2004). The field potentials are monitored before, during, and after washout of the drug and analyzed using an ANOVA (within-subject design with repeated measures).
Histology and immunohistochemistry.
A series of animals were used to determine the relation between the bands of decreased fluorescence and the parasagittal zonation revealed by anti-zebrin II and PLCβ3 immunostaining. As outlined above, optical imaging in Crus II was used to determine the locations of beam and long-latency patches evoked by PF stimulation. Next, lesions were generated on the surface of the molecular layer to serve as fiduciary makers by passing a direct current (50 μA for 30 s) using a PF stimulating electrode. The lesions were targeted to the center of the patches. After transcardiac perfusion with PBS containing 4% paraformaldehyde and coronal sectioning (40 μm), immunostaining of zebrin II was carried out (Eisenman and Hawkes 1993; Gao et al. 2006). Cerebellar sections were incubated at room temperature overnight with anti-zebrin II (1:100) and then with peroxidase-conjugated rabbit anti-mouse IgG for 1 h (Dako, Glostrup, Denmark). Immunoactivity was revealed by using diaminobenzidine as the peroxidase substrate. Sections with the lesions were recovered, and the zebrin II staining was compared with the locations of the long-latency patches evoked by PF stimulation. Also, immunostaining of PLCβ3 (anti-PLCβ3 at 1:200) was performed on alternating sections with the immunostaining for zebrin II to verify that these two molecules are in register as shown previously (Sarna et al. 2006).
RESULTS
High-frequency PF stimulation evokes long-latency patches.
With the use of flavoprotein imaging (Gao et al. 2006; Reinert et al. 2004), PF stimulation evokes a beamlike response that consists of an initial increase in fluorescence (on-beam light phase) followed by a long-latency decrease in fluorescence (dark phase). In addition, PF stimulation at 100 Hz evoked “patches” of increased fluorescence (Fig. 1A). The patches are located along the shorter latency, on-beam response region. In the example shown in Fig. 1A, right, four patches were evoked. Typically, two to five patches were evoked in Crus II with an average amplitude of 0.53 ± 0.17% ΔF/F. The patches developed after the fluorescence returned to the baseline following the dark phase (Fig. 1B). Although the number and amplitude of the patches varied in different mice (Fig. 1C), we attribute a large fraction of the variability to the limitations in imaging the entire folium. Crus II varies in orientation, shape, and contour among animals. Because the focus was optimized to the center of the folium, the more medial and lateral aspects were not always in the plane of focus or in view, and therefore the responses in these regions were more difficult to image. The latency of the patches was 20–25 s with peak activation at 35.0 ± 4.9 s (n = 5 mice, Fig. 1, B and C). Although there was also variability in the peak time, there was no obvious correlation between the peak time and the patch location on the folium (Fig. 1C). Also, there is no evidence that the location of the patches had any preferred spatial relationship to the surface vasculature (see Figs. 1 and 3–7). We will refer to these novel responses as “long-latency patches.”
Fig. 1.
Parallel fiber stimulation evokes long-latency patches. A: thresholded optical responses in Crus II overlaid on the background fluorescence are shown at 3 time frames relative to onset of PF stimulation as noted at top left. The typical biphasic, beamlike response was followed by long-latency patches of fluorescence increase (ΔF/F) located along the shorter latency beam. PF stimulation parameters consisted of 10 pulses of 200 μA, 100 μs at 100 Hz, and the same parameters were used throughout unless otherwise noted. The pseudocolor scale bar is shown at bottom. The gray stippled region reflects that only pixels either greater or less than the mean ± SD of the background fluorescence were displayed (i.e., thresholded). B: time courses of the evoked fluorescence response from the experiment shown in A, including 4 patches and an “off-patch” region between patches 3 and 4 as noted in right image in A. For numbering of the patches, we assigned the lateral-most patch as patch 1, the next most lateral as patch 2, and so on. C: peak time vs. peak amplitude of the evoked patches in 5 animals.
Fig. 3.
Frequency dependence of the long-latency patches. A: beam (top) and long-latency patches (bottom) evoked by different frequencies of PF stimulation, as indicated (only 5 of 8 tested frequencies are shown). All other stimulation parameters were kept constant (10 pulses of 200 μA, 100 μs). The patches were not evoked by 10-Hz stimulation, were present at 30 Hz, and peaked at 100 Hz. B: time courses of the evoked response in patch 3 (labeled at 50 Hz in A) at different PF stimulation frequencies. C: peak time of patches evoked by different PF stimulation frequencies (n = 5 mice). D: amplitude (mean ± SD) of the beam and patches as a function of stimulation frequency in 5 mice. Both the beam and the long-latency patches exhibited frequency dependence; however, the patches were not evoked at 10 Hz and showed a more pronounced dependence on the frequency of PF stimulation.
Fig. 4.
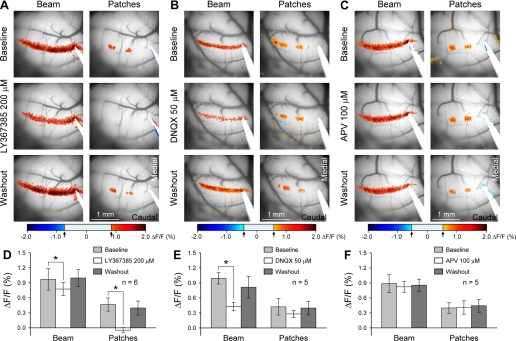
Long-latency patches are type 1 metabotropic glutamate receptor (mGluR1) dependent. A: mGluR1 receptor antagonist LY367385 (200 μM) abolished the long-latency patches completely and slightly reduced the beam. B: non-N-methyl-d-aspartate (NMDA) receptor antagonist 6,7-dinitroquinoxaline-2,3-dione disodium salt (DNQX; 50 μM) markedly reduced the beam but did not affect the long-latency patches. C: NMDA receptor antagonist d-(−)-2-amino-5-phosphonopentanoic acid (APV; 250 μM) did not affect the amplitude of the patch and beam responses. D–F: population data for the effects of the different glutamate receptor antagonists (LY367385, DNQX, and APV) on the beam and long-latency patches. *P < 0.05 indicates significant change (see results for statistical details for each drug). Stimulation parameters were 10 pulses of 200 μA, 100 μs at 100 Hz.
Fig. 5.
Long-latency patches evoked by PF stimulation are organized in parasagittal zones. A: experiment showing the position of the evoked beam and long-latency patches in response to PF stimulation at 4 anterior-posterior locations. B: composite image of the beams (red) and patches (blue) evoked by PF stimulation at 6 anterior-posterior positions on Crus II for the experiment in A. The beam and patches shift position with the site of stimulation and show that the long-latency patches are aligned in parasagittal bands. C–E: composite beam and patch results from similar experiments in 3 additional mice. Stimulation parameters were 10 pulses of 200 μA, 100 μs at 100 Hz.
Fig. 6.
Long-latency patches are aligned with zebrin II and PLCβ bands. A: thresholded image showing the beam and long-latency patches evoked by PF stimulation (10 pulses of 200 μA, 100 μs at 100 Hz). The 2 lateral patches (A and B) were lesioned with direct current. B: section through the folium in A stained with zebrin II antibodies. Three of the zebrin II bands (P6+, P5b+, and P4a+) are labeled (arrowheads). Inset shows that the locations of the lesions (a and b) correspond with the zebrin II-positive bands. C: another example showing the location of the lesions relative to zebrin II staining. The arrows show the lesions were in the granule cell layer below the P6+ and P5b+ zebrin II bands. D: 2 examples of the correspondence between zebrin II and PLCβ3 staining in Crus II. The P6+, P5b+, and P4a+ bands are labeled. Z II, zebrin II.
Fig. 7.
Inhibitory bands and long-latency patches are in spatial register. A, from left to right: at left is a pseudocolor image of the response to low-frequency PF stimulation (200 μA, 100 μs at 10 Hz for 10 s) taken at the end of stimulation (10 s). In the 2nd image, the inhibitory bands are outlined in blue. The 3rd image shows that high-frequency PF stimulation (10 pulses of 200 μA, 100 μs at 100 Hz) evokes long-latency patches at 40 s (outlined in red). Right image shows superimposition of the outlined patches and bands on a background image. B and C: 2 additional examples of the spatial relation between the inhibitory bands and the long-latency patches using the same conventions and PF stimulation parameters as described in A.
The long-latency patches are activated by a wide range of PF stimulation intensities. Long-latency patches were evoked at the lowest amplitude tested (50 μA, Fig. 2). For the example shown, two patches of increased fluorescence were evident in response to 10 pulses at 100 Hz (Fig. 2A, top images). At this stimulation amplitude, the beam and patch responses were reduced and the beam was quite narrow. In this and three additional mice, PF stimulation using five and three pulses (50 μA) at 100 Hz also evoked long-latency patches (Fig. 2A, middle and bottom images). In six mice, the amplitude of the PF stimulation was varied, with the other parameters kept constant. From 50 to 250 μA, the amplitude of the beam and long-latency patches increased in parallel (Fig. 2B). However, the beam and long-latency patches appear to have plateaued between 250 and 300 μA. The amplitudes of the beam and long-latency patches did not increase further at 350- and 400-μA stimulation and were not significantly different from the responses at 300 μA [beam and patch at 350 vs. 300 μA: F(1,3) = 2.0, P = 0.25 and F(1,3) = 1.3, P = 0.33; beam and patch at 400 vs. 300 μA: F(1,3) = 1.4, P = 0.33 and F(1,3) = 4.3, P = 0.13]. These results demonstrate that the long-latency patches are evoked at the lowest PF stimulation intensities that evoke the short-latency beam.
Fig. 2.
Long-latency patches as a function of the PF stimulation intensity. A: stimulation at 50 μA using 10, 5, and 3 pulses of 100 μs at 100 Hz evoked both the short-latency beam and the long-latency patches (see arrows in top image). The images of the long-latency patch responses (right) are from 32 s following the onset of PF stimulation. Responses are shown as pseudocolored images of ΔF/F as denoted by the scale bar. B: amplitude (mean ± SD) of the beam and long-latency patch responses as a function of the amplitude (50–400 μA) of PF stimulation with other stimulation parameters kept constant (10 pulses of 100 μs at 100 Hz). The population data in B are based on 6 mice studied at PF stimulations from 50 to 300 μA; 4 of these mice were also tested at 350- and 400-μA PF stimulation.
The long-latency patches depend on the frequency of PF stimulation. The patches were evoked by 20- but not 10-Hz PF stimulation, increased in amplitude as the frequency of PF stimulation increased [F(7,4) = 3.7, P = 0.0002], and were maximal with 100- to 200-Hz stimulation (Fig. 3). The amplitude of the patches was significantly different at each frequency (ANOVA followed by post hoc Bonferroni t-test, P < 0.05; Fig. 3, A and D, n = 5 mice). The peak time increased with stimulation frequency [F(6,24) = 10.31, P < 0.0001; Fig. 3, B and C]. The beam response was also dependent on the frequency of PF stimulation [F(7,4) = 12.8, P < 0.0001], as reported previously (Dunbar et al. 2004; Gao et al. 2006). However, the frequency dependence of the beam was less than that of the long-latency patches (Fig. 3D), since the beam amplitude increased by 35.3% between 20 and 200 Hz and the amplitude of the three patches increased on average 257%. The long-latency patches were not evident in our initial studies because typically low-frequency PF stimulation (10 Hz) and a briefer imaging time were used (Gao et al. 2006; Reinert et al. 2004, 2007).
Long-latency patches are mediated by mGluR1 receptors.
The frequency dependence of the long-latency patches prompted the hypothesis that mGluR1 receptors are involved, because activation of these receptors requires high-frequency PF input (Batchelor et al. 1997; Finch and Augustine 1998; Tempia et al. 1998). The mGluR1 receptor antagonist LY367385 (200 μM, n = 6 mice) abolished the long-latency patches (Fig. 4, A and D), decreasing the amplitude from 0.46 ± 0.13 to −0.05 ± 0.05 ΔF/F [F(1,5) = 60.93, P = 0.0006]. The amplitude of the beam also decreased significantly by 19.5% [F(1,5) = 22.81, P = 0.005], reflecting that the on-beam response evoked by PF stimulation also has an mGluR1 component, as reported previously (Wang et al. 2009). The percentage decrease for the beam was much less than that for the patches, consistent with the larger AMPA and smaller mGluR1 contributions to the evoked beam (Reinert et al. 2004; Wang et al. 2009). Blocking AMPA receptors with DNQX (50 μM) produced a small (0.42 ± 0.16 to 0.28 ± 0.08, n = 5) but nonsignificant reduction in the amplitude of the patches [F(1,4) = 5.04, P = 0.09; Fig. 4, B and E]. In contrast, DNQX resulted in a 55.6% decrease in the amplitude of the beam [F(1,4) = 108.43, P = 0.0005], similar to previous findings (Wang et al. 2009). Blocking NMDA receptors with APV (250 μM) had no effect on the amplitude of the patches [F(1,4) = 0.11, P = 0.75, n = 5] or the beam [F(1,4) = 0.75, P = 0.44; Fig. 4, C and F]. Therefore, the long-latency patches are highly mGluR1 dependent.
Long-latency patches are evoked in parasagittal zones.
Next, we investigated the topography of the long-latency patches. The experiment involved stimulating the PFs at several anterior-posterior positions on Crus II and determining the locations of the long-latency patches. As shown for the example in Fig. 5A, four patches were evoked at each PF stimulation position (only 4 of 6 PF stimulation positions are shown). The position of the beam and patches shifted with the different stimulation positions. The composite image of the beams (red) and long-latency patches (blue) at the different PF stimulation locations shows that the patches were aligned in parasagittal bands (Fig. 5B). The composite images in Fig. 5, C–E, illustrate the parasagittal zonation of the long-latency patches in three additional mice. Similar parasagittal banding for the long-latency patches was documented in seven mice.
To assess whether the parasagittal zones reflect the known parasagittal compartmentalization of PCs, we compared the position of the long-latency patches with the banding pattern obtained with anti-zebrin II staining in Crus II. As described in materials and methods, this involved 1) evoking the long-latency patches and determining their positions, 2) making a small DC lesion in the center of one or two patches, and 3) staining with anti-zebrin II antibodies after sectioning. An example showing the correspondence between the patches and zebrin II is shown in Fig. 6, A and B. In this example, the two lateral patches were lesioned (A and B in Fig. 6A) and after immunostaining were shown to be aligned with the two lateral zebrin II bands (a and b in Fig. 6B). These two lateral zebrin II bands correspond to the P6+ and P5+ in the terminology of Hawkes and colleagues (Eisenman and Hawkes 1993; Sillitoe and Hawkes 2002). Two more medial and smaller long-latency patches were also evoked by PF stimulation (C and D in Fig. 6A), and these patches were aligned with two smaller zebrin II bands (c and d in Fig. 6B). Another example of the correspondence between the location of the long-latency patches and zebrin II staining is shown in Fig. 6C. In this example, the lesions were slightly deeper in the granular layer (arrows) below the P6+ and P5b+ bands. These findings were replicated in six mice. Also, correspondence between the zebrin II and the PLCβ3 zones was found in Crus II in four mice (2 examples are shown in Fig. 6D), as shown previously (Sarna et al. 2006). Therefore, the long-latency patches are structural and in register with zebrin II and PLCβ3 bands.
We previously demonstrated that low-frequency PF stimulation activates molecular layer inhibition in parasagittal zones that are also aligned with zebrin II-positive bands (Gao et al. 2006; Moseley et al. 2006). Therefore, we examined the spatial relationship between the inhibitory bands and the long-latency patches. Three examples are shown in Fig. 7 in which the first column in each row shows the beam and bands evoked by 10-Hz PF stimulation. In the second column, the inhibitory bands are outlined in blue. In each animal, PF stimulation at 100 Hz evoked three or more long-latency patches (3rd column, outlined in red). The outlines of the inhibitory bands and patches are superimposed onto a background fluorescence image of the folium in the fourth column. The inhibitory bands and long-latency patches are aligned spatially with the long-latency patches in the center of the inhibitory bands. Similar results were obtained in six mice.
Long-latency patches are due to intracellular Ca2+ release.
Given that the long-latency patches are evoked by high-frequency PF stimulation and are mGluR1 dependent, we hypothesized that the long-latency patches are caused by mGluR1-mediated release of Ca2+ from intracellular stores. Therefore, we examined the long-latency patches using Oregon green 488 (see materials and methods). High-frequency PF stimulation evoked patches of increased fluorescence that occurred at long latencies (Fig. 8). As shown in the three examples, 100-Hz PF stimulation evoked both the beam and long-latency patches. Single-pulse or 10-Hz PF stimulation did not evoke the patch response (data not shown). Similar results were obtained in four mice using Ca2+ imaging.
Fig. 8.
Ca2+ imaging of the long-latency patches. A: Crus II was loaded with Oregon green, and the responses to PF stimulation were imaged. B: time course of a patch and off-patch response in Ca2+ imaging from the same experiment as in A. A beamlike response was evoked early, followed by long-latency patches. C–F: 2 additional examples of the long-latency patches evoked by PF stimulation using Ca2+ imaging. Stimulation parameters for each example were 15 pulses of 200 μA, 100 μs at 100 Hz.
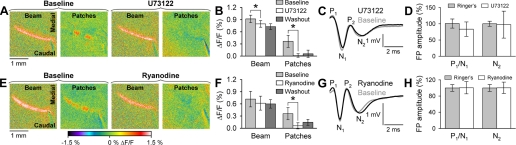
Next, the PLCβ inhibitor U73122 (50 μM) was tested and found to completely block the long-latency patches (Fig. 9, A and B). As evident in the example and population data, U73122 abolished the long-latency patches [F(1,4) = 33.33, P = 0.005, n = 5]. There was also a modest, but significant, 21% reduction in the short-latency, on-beam response [F(1,4) = 33.46, P = 0.004]. A reduction in the on-beam component is expected because PF stimulation results in a short-latency release of internal Ca2+ via activation of the mGluR1 receptors (Finch and Augustine 1998; Takechi et al. 1998). Washout of the U73122 resulted in some recovery of the long-latency patches, although no recovery of the beam (Fig. 9B), likely reflecting that U73122 acts intracellularly. Even in the slice preparation, U73122 is poorly reversible (Bell et al. 1998). The washout period was extensive, and for drugs that are reversible and do not act intracellularly, this typically results in substantial recovery of the responses (for example, see Fig. 4). Therefore, we are confident that the U73122 was removed from the extracellular space.
Fig. 9.
Long-latency patches are due to release of Ca2+ from internal stores. A: example of the beam and long-latency patches evoked by PF stimulation before (baseline) and after the application of U73122 (50 μM). Long-latency patches were completely blocked by U73122. B: population data for effects of U73122 on the evoked beam and long-latency patches (n = 5). C: example field potential (FP) recording of the response to PF stimulation before and during the application of U73122. The PF volley (P1/N1) and the postsynaptic response (N2) were not altered by U73122. D: population data for the effects of U73122 on the evoked FPs (n = 6). E: example of the effects of ryanodine (100 μM) on the beam and long-latency patches. Ryanodine completely suppressed the patches. F: population data for the effect of ryanodine on the beam and patches (n = 5). G: example of the effect of ryanodine on the PF volley (P1/N1) and the postsynaptic component (N2). H: population data for the effect of ryanodine on the evoked FPs (n = 4). Optical responses were evoked by 10 pulses of 200 μA, 100 μs at 100 Hz. FPs were evoked by PF stimulation using 100 μA, 100 μs at 1 Hz.
Field potential recordings were used to test the integrity of PF-PC synaptic transmission after the application of U73122. Neither the parallel fiber volley [P1/N1: F(1,5) = 5.9, P = 0.06, n = 6] nor the postsynaptic response [N2: F(1,5) = 0.02, P = 0.89] was significantly altered (Fig. 9, C and D). An additional control experiment was undertaken because dissolving the U73122 required ethanol. In three mice, adding 1.5% ethanol to the Ringer solution resulted in no significant change in the amplitude of the beam [F(1,2) = 0.50, P = 0.55, n = 3] or the long-latency patches [F(1,2) = 9.5, P = 0.10]. Also, 1.5% ethanol did not alter the PF volley [F(1,2) = 0.01, P = 0.92, n = 3] or postsynaptic response [F(1,2) = 0.02, P = 0.90]. Therefore, the block of the long-latency patches by U73122 is not due to the ethanol depressing the long-latency patches or to changes in the excitability of the PF-PC circuit.
A final test of the intracellular Ca2+ release hypothesis used the RyR antagonist ryanodine. Elevated intracellular Ca2+ triggers additional release via activation of ryanodine receptors (Kano et al. 1995; Llano et al. 1994). The type 1 RyR is the main ryanodine receptor type in PCs, and RyR1 and IP3 receptors share a common Ca2+ pool in PCs (Khodakhah and Armstrong 1997; Klein et al. 2007; Martin et al. 1998; Waniewski and Martin 1998). Ryanodine (100 μM) blocked the long-latency patches as shown for the example (Fig. 9E) and the population data [F(1,4) = 38.1, P = 0.004, n = 5; Fig. 9F]. Ryanodine did not produce a significant reduction in the on-beam response [F(1,4) = 6.9, P = 0.5]. Again, there was some recovery of the long-latency patches, but not of the beam, with washout (Fig. 9F). As discussed above for U73122, this likely reflects that ryanodine acts intracellularly, and although there is washout from the extracellular space, washout from the intracellular compartment is difficult to achieve in vivo. Field potential recordings (Fig. 9, G and H) show that ryanodine did not significantly alter the PF volley [P1/N1: F(1,3) = 0.08, P = 0.79, n = 4] or postsynaptic responses [N2: F(1,3) = 0.13, P = 0.74]; therefore, the loss of the long-latency patches is not due to a change in PF-PC synaptic transmission. The results of the U73122 and ryanodine experiments strongly support the hypothesis that the long-latency patches are due to release of Ca2+ from intracellular stores.
Long-latency patches exhibit LTP.
Recently, we demonstrated that high-frequency burst PF stimulation induces a postsynaptic, mGluR1-dependent LTP of the on-beam response (Wang et al. 2009). The long-latency patches exhibited LTP in response to the same high-frequency conditioning stimulation (Fig. 10). The example shows the LTP of the beam and the long-latency patches induced by this high-frequency conditioning stimulation (Fig. 10A). The patches were highly potentiated for at least 2 h with an increase in amplitude of 227 ± 86% above the baseline, in contrast to the 127 ± 7% increase of the beam (compare long-latency patch data in Fig. 10B and the beam data in Fig. 10C). The amplitude of the patches was potentiated for at least 2 h in both the early phase [F(1,4) = 9.8, P = 0.035] and the late phase 60 min following conditioning [F(1,4) = 35.4, P = 0.004]. In a control experiment, monitoring of the long-latency patches throughout the same period, but without the high-frequency conditioning (shaded curve), shows the amplitude of the patches remained constant and was not different from the baseline period [F(1,2) = 0.02, P = 0.91 early phase; F(1,2) = 0.61, P = 0.51 late phase; Fig. 10B].
Fig. 10.
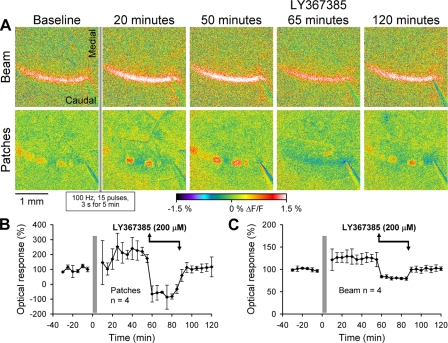
Induction of the long-latency patch long-term potentiation (LTP) is mGluR1 dependent. A: example images of the LTP evoked by the conditioning stimulus for both the evoked beam and long-latency patches. PF conditioning stimulation is denoted by the shaded vertical bar (15 pulses at 175 μA, 150 μs at 100 Hz every 3 s for 5 min). Each image shows the beam or patch response evoked by the “test” PF stimulation (10 pulses at 175 μA, 150 μs at 100 Hz). B: population data for the PF conditioning stimulation-evoked LTP of the patches. The potentiation was >200% above the baseline and persisted for 120 min (solid line). Average patch response is also shown in animals without conditioning stimulation (shaded line). C: population data for LTP of the beam response using the same conditioning paradigm. D: LTP of the long-latency patches was not blocked by the AMPA receptor antagonist (50 μM DNQX; solid line; n = 5); however, the LTP duration was shortened. Control responses with DNQX are shown (shaded line; n = 4). Error bars are ±SD. E: mGluR1 receptor antagonist LY367385 (200 μM) completely suppressed the induction of LTP of the long-latency patches (solid line; n = 5). Control data show responses with LY367385 but without the conditioning stimulation (shaded line; n = 4).
We tested whether the LTP of the patches is mGluR1 dependent (Wang et al. 2009). Application of LY367385 (200 μM) during the conditioning stimulation (Fig. 10E) completely suppressed the patches (compare Fig. 10E with Fig. 4D). On removal of LY367385, the long-latency patches recovered to baseline amplitude; however, there was no potentiation of the patches in either the early [F(1,4) = 2.6, P = 0.19] or late phase [F(1,4) = 0.28, P = 0.63]. The control experiment in which the LY367385 was applied without the conditioning stimulation shows the expected suppression of the long-latency patches, followed by return to baseline after washout of the mGluR1 antagonist (shaded curve). Application of the AMPA antagonist DNQX (50 μM) did not block the induction of LTP of the patches (Fig. 10D). During the early phase following induction stimulation, the amplitude of the patches increased 175 ± 50% above baseline [F(1,4) = 38.4, P = 0.004]. However, the duration of the LTP of the long-latency patches was shortened and returned to baseline at 80 min. The control experiment in which the DNQX was applied without the conditioning stimulation shows no changes in the long-latency patches during or following washout (shaded curve).
The final experiment evaluated whether the maintenance of long-latency patch LTP was dependent on mGluR1 receptors. Using the protocol described for Fig. 10, LTP of the beam and long-latency patches was induced by high-frequency PF stimulation. The amplitude of the patches increased 191 ± 72% above baseline [F(1,3) = 14.1, P = 0.03] for the 50-min period following LTP induction. The beam amplitude increased by 125 ± 12% [F(1,3) = 20.2, P = 0.02] for the same period. At 50 min following the conditioning stimulation, mGluR1 receptors were blocked with LY367385 (200 μM) for 30 min and then washed out (Fig. 11). As the example images (Fig. 11A) and population data (Fig. 11B) show, the LY367385 completely suppressed the long-latency patches [F(1,3) = 46.0, P = 0.007], demonstrating that the potentiated patches are mGluR1 dependent. During application of the LY367385, the amplitude of the long-latency patches is actually less than the baseline control because the patches are blocked and normally occur in the dark phase (Reinert et al. 2004). The dark phase is evident in the example image at 65 min during the LY367385 application (Fig. 11A). The potentiation of the beam (Fig. 11C) was also mGluR1 dependent, because the amplitude was suppressed during the LY367385 [F(1,3) = 77.3, P = 0.003]. The amplitude of the beam during the drug was also smaller than the baseline [F(1,3) = 718.6. P < 0.001], reflecting the mGluR1 component of the baseline response to PF stimulation (Wang et al. 2009). On washout, the amplitude of the long-latency patches returned to, and was not significantly different from, the baseline level [F(1,3) = 1.8, P = 0.27], implying that maintenance of the LTP requires continual mGluR1 activation. The LTP of the beam exhibited the same sensitivity to mGluR1 in that following washout of the LY367385, the amplitude returned to baseline control level and the responses during the baseline and washout periods were not significantly different [F(1,3) = 0.1, P = 0.76].
Fig. 11.
Maintenance of the long-latency patch LTP is mGluR1 dependent. A: example of beam and patch LTP evoked by conditioning PF stimulation (shaded vertical bar; 15 pulses at 175 μA, 150 μs at 100 Hz every 3 s for 5 min). Test PF stimulation was as described in Fig. 10. At 50 min following the conditioning stimulation, LY367385 (200 μM) was introduced into the chamber for 30 min and then washed out. Test PF stimulation was done during the application of the drug and for 60 min following washout. Example images show the LTP of the long-latency patches and beam and indicate that LY367385 suppressed the LTP of the patches and beam. On washout of LY367385, the long-latency patches and beam only recovered to baseline levels. B and C: population data (n = 4) for the effects of LY367385 on the maintenance of the LTP of the long-latency patches (B) and the beam (C).
DISCUSSION
Properties of the long-latency patches.
This study is the first report that PF stimulation evokes long-latency patches of activity in addition to the well-described beam of activity. The long-latency patches are dependent on high-frequency PF stimulation, suggesting the involvement of mGluR1 receptors on PCs (Batchelor et al. 1994; Finch and Augustine 1998; Klein et al. 2007; Shigemoto et al. 1992; Tempia et al. 1998). The application of glutamate receptor antagonists confirms that the long-latency patches are dependent on the activation of mGluR1 receptors and not on the activation of AMPA or NMDA receptors. Because mGluR1 receptors are not found on granule cell axons or PFs (Kinoshita et al. 1996; Mateos et al. 1999, 1999), the evidence demonstrates that the long-latency patches are generated postsynaptically.
Given that the long-latency patches are postsynaptic, we hypothesize that the patches originate in neurons, predominantly in PCs. The patches consist of increased fluorescence, consistent with a neuronal origin (Brennan et al. 2006; Reinert et al. 2004, 2007). Conversely, activation of glia generates a decrease in fluorescence and is responsible for the dark phase. The dark phase is thought to be the result of increased glycolysis in glia with the production of lactate, a reducing equivalent (Gao et al. 2009; Kasischke et al. 2004; Pellerin and Magistretti 1994; Reinert et al. 2004, 2007). Approximately 85–90% of PFs synapse on PCs (Harvey and Napper 1988; Palay and Chan-Palay 1974), and mGluR1 receptors are the major mGluR type on these neurons (Lein et al. 2007; Shigemoto et al. 1992). The location of the long-latency patches corresponds with zebrin II and PLCβ3-positive bands on PCs. To our knowledge, glia cells have not been reported to show such a molecular compartmentalization. Furthermore, both U73122 and ryanodine abolish the long-latency patches, and both IP3 and RyR receptors are heavily expressed in PCs (Kuwajima et al. 1992; Lein et al. 2007; Sharp et al. 1993).
Molecular layer interneurons are also activated by mGluR1 receptors (Karakossian and Otis 2004; Llano and Marty 1995), which results in intracellular Ca2+ release (Collin et al. 2009). However, activation of these increases in intracellular Ca2+ requires long-duration application of agonists as opposed to the short-duration PF stimulation used to evoke the long-latency patches. Furthermore, U73122 and ryanodine have very modest effects on the Ca2+ transients evoked in molecular layer interneurons (Collin et al. 2009), in sharp contrast to the complete blockade of the long-latency patches reported in the present study. Although we do not rule out involvement of cerebellar interneurons, the evidence suggests PCs are the major source of the long-latency patches.
We acknowledge that electrical stimulation generates a synchronous activation of PFs that is not physiological. However, it should be noted that PF stimulation has provided many insights into the organization and function of the cerebellar circuitry and continues to be widely used for both in vitro and in vivo studies. The experiments using different amplitudes of stimulation show that long-latency patches are evoked by the lowest amplitudes tested. Although an exhaustive search for the lowest levels of PF stimulation that can evoke the patches was not conducted, as few as three pulses at 50 μA evoked patches. Also, the amplitudes of the beam and patches plateau between 250 and 300 μA. These findings suggest that the long-latency patches are activated by approximately the same numbers of PFs as those needed to activate and detect a beam using flavoprotein imaging.
Long-latency patches are evoked in parasagittal bands.
Stimulation of the PFs at different anterior-posterior locations on Crus II showed that the long-latency patches are evoked in a parasagittal distribution. The location of the patches corresponds to the zebrin-II positive staining pattern and that of PLCβ3 (Mateos et al. 2001; Sarna et al. 2006). The observation that the patches align with zebrin II-positive and not zebrin II-negative bands may reflect the structural and physiological differences between mGluR1a and mGluR1b isoforms (for review, see Conn and Pin 1997). The mGluR1a isoform has a long carboxyl-terminal intracellular domain, and the mGluR1b isoform has a short one (Pin et al. 1992; Tanabe et al. 1992). Overall, the mGluR1b isoform exhibits less potency in coupling to PLC than does the mGluR1a isoform. Compared with mGluR1a, the mGluR1b isoform has slower activation of Cl− currents, markedly less constitutive activity, requires a higher concentration of agonists to activate, and results in lower levels of intracellular Ca2+ (Conn and Pin 1997; Joly et al. 1995; Pin et al. 1992; Prezeau et al. 1996). Therefore, selective expression of mGluR1b in zebrin II-negative bands is likely to contribute to the lack of long-latency patches in these regions.
Long-latency patches and intracellular Ca+2 release.
Activation of mGluR1 receptors on PCs activates an inward cation current (slow, excitatory postsynaptic current) at a much shorter latency (several hundred milliseconds) and faster time course (∼ 0.5–1 s) than that observed from the long-latency patches (Batchelor et al. 1994; Batchelor and Garthwaite 1997; Takechi et al. 1998). The slow excitatory current is due to a C-type transient receptor potential (TRPC) cation channel, specifically the TRPC3 cation channel (for review, see Hartmann and Konnerth 2009). Furthermore, Ca2+ entry through TRPC3 channels does not account for the Ca2+ release from internal stores (Canepari and Ogden 2006; Hartmann et al. 2008). Therefore, the long-latency patches are likely to involve a mechanism other than the slow postsynaptic current generated in PCs by TRPC3 channels. Blocking either PLCβ or RyR receptors completely suppresses the long-latency patches, demonstrating that the patches are generated by Ca2+ release from intracellular stores via the IP3 pathway and Ca2+-mediated Ca2+ release (Finch and Augustine 1998; Kano et al. 1995; Linden et al. 1994; Llano et al. 1994; Takechi et al. 1998).
The release of Ca2+ from intracellular stores has a host of effects on PC excitability as well as on short- and long-term plasticity. The effects include controlling PC excitability through the gating of small (SK)- (Netzeband and Gruol 2008) and large-conductance Ca2+-activated K+ (BK) channels (Canepari and Ogden 2006). The release of Ca2+ from intracellular stores following mGluR1 activation is also involved in the production of endocannabinoids that transiently decrease transmitter release from PFs (Duguid et al. 2007; Maejima et al. 2001, 2005). Furthermore, IP3-mediated release is required for long-term depression (LTD) at PF-PC synapses in vitro (Miyata et al. 2000). The long latency and protracted time course of the patches provides a mechanism by which a very brief PF input modulates PC intracellular activity and excitability for tens of seconds.
The mechanism for the long delay in activating the patches is not known. One possibility is a release-refill-release-type mechanism from intracellular Ca2+ stores found in both neuronal and nonneuronal systems (Berridge 1998; Tsien and Tsien 1990). In this scenario, PF stimulation results in an initial release of Ca2+ from intracellular stores (Finch and Augustine 1998; Takechi et al. 1998) and could account for the observed reduction in the beam response by U73122 (Fig. 9). This initial short-latency release is followed by refilling of the internal stores and a second release of Ca2+. In heterologous expression systems, activation of mGluRs can generate repetitive increases in Ca2+ from intracellular stores with a periodicity of tens of seconds (Kawabata et al. 1998). Another possible mechanism is that the long-latency patches are caused by oscillations in excitability of PCs driven by molecular layer interneurons. Recently, it was shown in the cerebellar slice that long-duration stimulation of molecular layer interneurons with receptor agonists evokes oscillations of internal Ca2+ release and corresponding oscillations in the excitability of these interneurons (Collin et al. 2009). The average oscillation period of the Ca2+ transients was reported to be 37.9 s, similar to the 35.0-s peak time observed for the patches. If brief PF stimulation results in oscillation in the interneurons in vivo, even for only one additional cycle, then the molecular layer interneurons would be expected to generate changes in excitability in PCs on these long time scales. However, it needs to be reiterated that the oscillations observed in the molecular layer interneurons are not blocked by U73122 or ryanodine (Collin et al. 2009), further evidence that the long-latency patches are generated in PCs and not in molecular layer interneurons.
Plasticity of the long-latency patches.
The long-latency patches exhibit marked LTP. The amplitude of the patches increases ∼200% above the baseline response compared with the ∼130% increase in the beam amplitude with the use of the same induction paradigm (Wang et al. 2009). The LTP of the long-latency patches greatly exceeds the amplitude of the LTP reported in cerebellar slices, either the presynaptic (Hirano 1991; Linden and Ahn 1999; Salin et al. 1996) or postsynaptic forms (Belmeguenai and Hansel 2005; Coesmans et al. 2004; Lev-Ram et al. 2002). The induction of the LTP is mGluR1 dependent, further implicating mGluR1 receptors and downstream signaling as the main pathway in the generation and control of the long-latency patches. The observation that LY367385 application following LTP induction completely suppressed the long-latency patches shows that the potentiated patches are mediated by mGluR1 receptors. The return of the amplitude of the patches to baseline following washout of the LY367385 suggests that continual mGluR1 activity is needed for the maintenance of the LTP. The LTP of the beam exhibits the same increase in mGluR1 responsiveness and mGluR1-dependent maintenance.
The mechanism underlying the marked potentiation of the long-latency patches is unresolved. The previously described LTP of the beam induced by the high-frequency induction paradigm may contribute (Wang et al. 2009), with the increase in the synaptic response augmenting internal Ca2+ release. Although the degree of the synaptic potentiation is markedly smaller than that of the long-latency patches, amplification could be achieved through the intracellular signaling cascade. It is also possible that changes in the mGluR1 signaling cascade, independent of the changes in synaptic transmission, lead to potentiation of the patches. Given the important role of protein kinase C (PKC) in PF-PC synaptic plasticity (Aiba et al. 1994; De Zeeuw et al. 1998; Kano et al. 1997; Linden and Connor 1991), we hypothesize that PKC activation is involved in LTP of the long-latency patches. The release of Ca2+ from intracellular stores is dependent on the phosphorylation state of mGluRs, which is under the control of PKC (Kawabata et al. 1996, 1998). Irrespective of the mechanism, the LTP of the long-latency patches provides a novel type of plasticity that can alter excitability, and therefore information processing, in PCs in spatially restricted bands. As noted above, many studies have implicated mGluR1 receptors in synaptic plasticity and motor learning in the cerebellum by knocking out or inhibiting mGluR1 receptors (Aiba et al. 1994; De Zeeuw et al. 1998; Kano et al. 1997). This raises the possibility that the effects observed in these previous studies may have involved interfering with the long-latency patches.
The spatial correspondence between the long-latency patches and the zebrin II-positive bands provides a further demonstration of differential synaptic plasticity among the parasagittal zones (Paukert et al. 2010; Sarna et al. 2006; Wadiche and Jahr 2005). The mGluR1 cascade may provide one possible mechanism for a parasagittally distributed plasticity, because some isoforms of mGluR1 and PLCβ have zebrin II staining patterns as well as different properties (Conn and Pin 1997; Mateos et al. 2001; Sarna et al. 2006). Another possible mechanism underlying the differential plasticity is the sequestration of glutamate by neuronal excitatory amino acid transporters (EAATs). The PC-specific EAAT4 transporter is expressed in a zebrin II band pattern (Dehnes et al. 1998; Gincel et al. 2007; Nagao et al. 1997). PF-PC LTD is dependent on activation of mGluR1 receptors (Aiba et al. 1994; Hartell 1994; Ichise et al. 2000); however, LTD of PF-PC synapses is greatly reduced in folia with high levels of EAAT4 expression (Wadiche and Jahr 2005). The loss of LTD in these folia is attributed to the EAAT4 removing the released glutamate, resulting in a limited activation of the mGluR1 receptors. Intriguingly, the long-latency patches and their plasticity described in this report are located within bands with high EAAT4 expression, providing an example in vivo of differential responsiveness and plasticity in relation to neuronal transporters. Whether there is a mechanistic connection between the long-latency patches and EAAT4 remains to be investigated.
The role of AMPA receptors is less clear, because DNQX did not block the induction of LTP but the duration of the LTP of the patches was shortened. The mechanism underlying this AMPA-mediated effect is not known but may involve the large decrease in PF-PC synaptic transmission caused by blocking AMPA receptors (Konnerth et al. 1990; Llano et al. 1991b). The overall reduction in PC excitability may alter the activation of PKC and/or other potential mediators of the LTP. Clearly, this needs further study.
Coupling between the transverse and parasagittal architectures.
These results provide for coupling between the transverse architecture of the PFs and the parasagittal organization of the cerebellar cortex, specifically to the molecular compartmentalization of PCs. Previously, we demonstrated that PF stimulation activates molecular layer inhibition in parasagittal bands and that the inhibitory bands can control the spatial aspects of the responses in the cerebellar cortex (Gao et al. 2006; Moseley et al. 2006). Climbing fiber inputs to zebrin II-positive bands release more glutamate and generate larger, longer duration AMPA-mediated excitatory currents in PCs than in zebrin II-negative zones (Paukert et al. 2010). The present study greatly expands these observations on the differential physiological properties of the parasagittal zones, showing that PFs also activate long-latency patches organized in parasagittal zones. Furthermore, the inhibitory bands and the long-latency patches are aligned, and both correspond spatially with the zebrin II- and PLCβ3-positive bands on PCs. The inhibitory bands and long-latency patches are optimally activated by different frequencies of PF stimulation, the inhibitory bands by low- and the long-latency patches by high-frequency stimulation. As discussed above, the LTP of the long-latency patches exemplifies that the parasagittal zones vary in their synaptic plasticity (Paukert et al. 2010; Sarna et al. 2006; Wadiche and Jahr 2005). Therefore, different parasagittal bands have highly differentiated responses and plasticity that are likely to underlie distinct functional roles.
GRANTS
This work was supported in part by National Institute of Neurological Disorders and Stroke Grants NS048944 and NS058901.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Lijuan Zhuo and Blake Ebner for animal preparation and histology, Michael McPhee for graphics, and Kris Bettin for preparation of the manuscript. We also thank Dr. Claudia Hendrix for help with the statistical analysis and Dr. Richard Hawkes for generously supplying the anti-zebrin II antibody.
REFERENCES
- Ahn AH, Dziennis S, Hawkes R, Herrup K. The cloning of zebrin II reveals its identity with aldolase C. Development 120: 2081–2090, 1994 [DOI] [PubMed] [Google Scholar]
- Aiba A, Kano M, Chen C, Stanton ME, Fox GD, Herrup K, Zwingman TA, Tonegawa S. Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell 79: 377–388, 1994 [PubMed] [Google Scholar]
- American Physiological Society Guiding principles for research involving animals and human beings. Am J Physiol Regul Integr Comp Physiol 283: R281–R283, 2002 [DOI] [PubMed] [Google Scholar]
- Apps R, Garwicz M. Precise matching of olivo-cortical divergence and cortico-nuclear convergence between somatotopically corresponding areas in the medial C1 and medial C3 zones of the paravermal cerebellum. Eur J Neurosci 12: 205–214, 2000 [DOI] [PubMed] [Google Scholar]
- Apps R, Hawkes R. Cerebellar cortical organization: a one-map hypothesis. Nat Rev Neurosci 10: 670–681, 2009 [DOI] [PubMed] [Google Scholar]
- Batchelor AM, Garthwaite J. Frequency detection and temporally dispersed synaptic signal association through a metabotropic glutamate receptor pathway. Nature 385: 74–77, 1997 [DOI] [PubMed] [Google Scholar]
- Batchelor AM, Knopfel T, Gasparini F, Garthwaite J. Pharmacological characterization of synaptic transmission through mGluRs in rat cerebellar slices. Neuropharmacology 36: 401–403, 1997 [DOI] [PubMed] [Google Scholar]
- Batchelor AM, Madge DJ, Garthwaite J. Synaptic activation of metabotropic glutamate receptors in the parallel fibre-Purkinje cell pathway in rat cerebellar slices. Neuroscience 63: 911–915, 1994 [DOI] [PubMed] [Google Scholar]
- Bell MI, Richardson PJ, Lee K. Characterization of the mechanism of action of tachykinins in rat striatal cholinergic interneurons. Neuroscience 87: 649–658, 1998 [DOI] [PubMed] [Google Scholar]
- Belmeguenai A, Hansel C. A role for protein phosphatases 1, 2A, and 2B in cerebellar long-term potentiation. J Neurosci 25: 10768–10772, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ. Neuronal calcium signaling. Neuron 21: 13–26, 1998 [DOI] [PubMed] [Google Scholar]
- Braitenberg V. In defense of the cerebellum. Ann NY Acad Sci 978: 175–183, 2002 [DOI] [PubMed] [Google Scholar]
- Braitenberg V, Heck D, Sultan F. The detection and generation of sequences as a key to cerebellar function: experiments and theory. Behav Brain Sci 20: 229–245, 1997 [PubMed] [Google Scholar]
- Brennan AM, Connor JA, Shuttleworth CW. NAD(P)H fluorescence transients after synaptic activity in brain slices: predominant role of mitochondrial function. J Cereb Blood Flow Metab 26: 1389–1406, 2006 [DOI] [PubMed] [Google Scholar]
- Brochu G, Maler L, Hawkes R. Zebrin II: a polypeptide antigen expressed selectively by Purkinje cells reveals compartments in rat and fish cerebellum. J Comp Neurol 291: 538–552, 1990 [DOI] [PubMed] [Google Scholar]
- Brodal A, Kawamura K. Olivocerebellar projection: a review. Adv Anat Embryol Cell Biol 64: 1–140, 1980 [PubMed] [Google Scholar]
- Canepari M, Ogden D. Kinetic, pharmacological and activity-dependent separation of two Ca2+ signalling pathways mediated by type 1 metabotropic glutamate receptors in rat Purkinje neurones. J Physiol 573: 65–82, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Gao W, Reinert KC, Popa LS, Hendrix CM, Ross ME, Ebner TJ. Involvement of Kv1 potassium channels in spreading acidification and depression in the cerebellar cortex. J Neurophysiol 94: 1287–1298, 2005 [DOI] [PubMed] [Google Scholar]
- Chen G, Hanson CL, Ebner TJ. Functional parasagittal compartments in the rat cerebellar cortex: an in vivo optical imaging study using neutral red. J Neurophysiol 76: 4169–4174, 1996 [DOI] [PubMed] [Google Scholar]
- Chockkan V, Hawkes R. Functional and antigenic maps in the rat cerebellum: zebrin compartmentation and vibrissal receptive fields in lobule IXa. J Comp Neurol 345: 33–45, 1994 [DOI] [PubMed] [Google Scholar]
- Coesmans M, Weber JT, De Zeeuw CI, Hansel C. Bidirectional parallel fiber plasticity in the cerebellum under climbing fiber control. Neuron 44: 691–700, 2004 [DOI] [PubMed] [Google Scholar]
- Collin T, Franconville R, Ehrlich BE, Llano I. Activation of metabotropic glutamate receptors induces periodic burst firing and concomitant cytosolic Ca2+ oscillations in cerebellar interneurons. J Neurosci 29: 9281–9291, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol 37: 205–237, 1997 [DOI] [PubMed] [Google Scholar]
- De Zeeuw CI, Hansel C, Bian F, Koekkoek SK, van Alphen AM, Linden DJ, Oberdick J. Expression of a protein kinase C inhibitor in Purkinje cells blocks cerebellar LTD and adaptation of the vestibulo-ocular reflex. Neuron 20: 495–508, 1998 [DOI] [PubMed] [Google Scholar]
- Dehnes Y, Chaudhry FA, Ullensvang K, Lehre KP, Storm-Mathisen J, Danbolt NC. The glutamate transporter EAAT4 in rat cerebellar Purkinje cells: a glutamate-gated chloride channel concentrated near the synapse in parts of the dendritic membrane facing astroglia. J Neurosci 18: 3606–3619, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid IC, Pankratov Y, Moss GW, Smart TG. Somatodendritic release of glutamate regulates synaptic inhibition in cerebellar Purkinje cells via autocrine mGluR1 activation. J Neurosci 27: 12464–12474, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar RL, Chen G, Gao W, Reinert KC, Feddersen R, Ebner TJ. Imaging parallel fiber and climbing fiber responses and their short-term interactions in the mouse cerebellar cortex in vivo. Neuroscience 126: 213–227, 2004 [DOI] [PubMed] [Google Scholar]
- Eccles JC, Ito M, Szentagothai J. The Cerebellum as a Neuronal Machine. Berlin: Springer, 1967 [Google Scholar]
- Eisenman LM, Hawkes R. Antigenic compartmentation in the mouse cerebellar cortex: zebrin and HNK-1 reveal a complex, overlapping molecular topography. J Comp Neurol 335: 586–605, 1993 [DOI] [PubMed] [Google Scholar]
- Ekerot CF, Larson B. Correlation between sagittal projection zones of climbing and mossy fibre paths in cat cerebellar anterior lobe. Brain Res 64: 446–450, 1973 [DOI] [PubMed] [Google Scholar]
- Finch EA, Augustine GJ. Local calcium signaling by inositol-1,4,5-trisphosphate in Purkinje cell dendrites. Nature 396: 753–756, 1998 [DOI] [PubMed] [Google Scholar]
- Gao W, Chen G, Ebner TJ. Flavoprotein imaging in the cerebellar cortex in vivo: cellular and metabolic basis and insights into cerebellar function. Proc SPIE 7180: 1–10, 2009 [Google Scholar]
- Gao W, Chen G, Reinert KC, Ebner TJ. Cerebellar cortical molecular layer inhibition is organized in parasagittal zones. J Neurosci 26: 8377–8387, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Dunbar RL, Chen G, Reinert KC, Oberdick J, Ebner TJ. Optical imaging of long-term depression in the mouse cerebellar cortex in vivo. J Neurosci 23: 1859–1866, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gincel D, Regan MR, Jin L, Watkins AM, Bergles DE, Rothstein JD. Analysis of cerebellar Purkinje cells using EAAT4 glutamate transporter promoter reporter in mice generated via bacterial artificial chromosome-mediated transgenesis. Exp Neurol 203: 205–212, 2007 [DOI] [PubMed] [Google Scholar]
- Grandes P, Mateos JM, Ruegg D, Kuhn R, Knopfel T. Differential cellular localization of three splice variants of the mGluR1 metabotropic glutamate receptor in rat cerebellum. Neuroreport 5: 2249–2252, 1994 [DOI] [PubMed] [Google Scholar]
- Hallem JS, Thompson JH, Gundappa-Sulur G, Hawkes R, Bjaalie JG, Bower JM. Spatial correspondence between tactile projection patterns and the distribution of the antigenic Purkinje cell markers anti-zebrin I and anti-zebrin II in the cerebellar folium crus IIa of the rat. Neuroscience 93: 1083–1094, 1999 [DOI] [PubMed] [Google Scholar]
- Hanson C, Chen G, Ebner TJ. Role of climbing fibers in determining the spatial patterns of activation in the cerebellar cortex to peripheral stimulation: an optical imaging study. Neuroscience 96: 317–331, 2000 [DOI] [PubMed] [Google Scholar]
- Hartell NA. Induction of cerebellar long-term depression requires activation of glutamate metabotropic receptors. Neuroreport 5: 913–916, 1994 [DOI] [PubMed] [Google Scholar]
- Hartmann J, Dragicevic E, Adelsberger H, Henning HA, Sumser M, Abramowitz J, Blum R, Dietrich A, Freichel M, Flockerzi V, Birnbaumer L, Konnerth A. TRPC3 channels are required for synaptic transmission and motor coordination. Neuron 59: 392–398, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann J, Konnerth A. Mechanisms of metabotropic glutamate receptor-mediated synaptic signaling in cerebellar Purkinje cells. Acta Physiol (Oxf) 195: 79–90, 2009 [Google Scholar]
- Harvey RJ, Napper RM. Quantitative study of granule and Purkinje cells in the cerebellar cortex of the rat. J Comp Neurol 274: 151–157, 1988 [DOI] [PubMed] [Google Scholar]
- Hawkes R, Herrup K. Aldolase C/zebrin II and the regionalization of the cerebellum. J Mol Neurosci 6: 147–158, 1995 [DOI] [PubMed] [Google Scholar]
- Hirano T. Differential pre- and postsynaptic mechanisms for synaptic potentiation and depression between a granule cell and a Purkinje cell in rat cerebellar culture. Synapse 7: 321–323, 1991 [DOI] [PubMed] [Google Scholar]
- Ichise T, Kano M, Hashimoto K, Yanagihara D, Nakao K, Shigemoto R, Katsuki M, Aiba A. mGluR1 in cerebellar Purkinje cells essential for long-term depression, synapse elimination, and motor coordination. Science 288: 1832–1835, 2000 [DOI] [PubMed] [Google Scholar]
- Ito M. Cerebellar circuitry as a neuronal machine. Prog Neurobiol 78: 272–303, 2006 [DOI] [PubMed] [Google Scholar]
- Ji Z, Hawkes R. Topography of Purkinje cell compartments and mossy fiber terminal fields in lobules II and III of the rat cerebellar cortex: spinocerebellar and cuneocerebellar projections. Neuroscience 61: 935–954, 1994 [DOI] [PubMed] [Google Scholar]
- Joly C, Gomeza J, Brabet I, Curry K, Bockaert J, Pin JP. Molecular, functional, and pharmacological characterization of the metabotropic glutamate receptor type 5 splice variants: comparison with mGluR1. J Neurosci 15: 3970–3981, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörntell H, Ekerot CF. Reciprocal bidirectional plasticity of parallel fiber receptive fields in cerebellar Purkinje cells and their afferent interneurons. Neuron 34: 797–806, 2002 [DOI] [PubMed] [Google Scholar]
- Kano M, Garaschuk O, Verkhratsky A, Konnerth A. Ryanodine receptor-mediated intracellular calcium release in rat cerebellar Purkinje neurones. J Physiol 487: 1–16, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Hashimoto K, Kurihara H, Watanabe M, Inoue Y, Aiba A, Tonegawa S. Persistent multiple climbing fiber innervation of cerebellar Purkinje cells in mice lacking mGluR1. Neuron 18: 71–79, 1997 [DOI] [PubMed] [Google Scholar]
- Karakossian MH, Otis TS. Excitation of cerebellar interneurons by group I metabotropic glutamate receptors. J Neurophysiol 92: 1558–1565, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasischke KA, Vishwasrao HD, Fisher PJ, Zipfel WR, Webb WW. Neural activity triggers neuronal oxidative metabolism followed by astrocytic glycolysis. Science 305: 99–103, 2004 [DOI] [PubMed] [Google Scholar]
- Kawabata S, Kohara A, Tsutsumi R, Itahana H, Hayashibe S, Yamaguchi T, Okada M. Diversity of calcium signaling by metabotropic glutamate receptors. J Biol Chem 273: 17381–17385, 1998 [DOI] [PubMed] [Google Scholar]
- Kawabata S, Tsutsumi R, Kohara A, Yamaguchi T, Nakanishi S, Okada M. Control of calcium oscillations by phosphorylation of metabotropic glutamate receptors. Nature 383: 89–92, 1996 [DOI] [PubMed] [Google Scholar]
- Khodakhah K, Armstrong CM. Inositol trisphosphate and ryanodine receptors share a common functional Ca2+ pool in cerebellar Purkinje neurons. Biophys J 73: 3349–3357, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita A, Ohishi H, Nomura S, Shigemoto R, Nakanishi S, Mizuno N. Presynaptic localization of a metabotropic glutamate receptor, mGluR4a, in the cerebellar cortex: a light and electron microscope study in the rat. Neurosci Lett 207: 199–202, 1996 [DOI] [PubMed] [Google Scholar]
- Klein B, Simura KJ, Flanders M. Timing of muscle activation in a hand movement sequence. Cereb Cortex 17: 803–815, 2007 [DOI] [PubMed] [Google Scholar]
- Konnerth A, Llano I, Armstrong CM. Synaptic currents in cerebellar Purkinje cells. Proc Natl Acad Sci USA 87: 2662–2665, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwajima G, Futatsugi A, Niinobe M, Nakanishi S, Mikoshiba K. Two types of ryanodine receptors in mouse brain: skeletal muscle type exclusively in Purkinje cells and cardiac muscle type in various neurons. Neuron 9: 1133–1142, 1992 [DOI] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Yuan XF, Zhang B, Zwingman TA, Jones AR. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445: 168–176, 2007 [DOI] [PubMed] [Google Scholar]
- Lev-Ram V, Wong ST, Storm DR, Tsien RY. A new form of cerebellar long-term potentiation is postsynaptic and depends on nitric oxide but not cAMP. Proc Natl Acad Sci USA 99: 8389–8393, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DJ, Ahn S. Activation of presynaptic cAMP-dependent protein kinase is required for induction of cerebellar long-term potentiation. J Neurosci 19: 10221–10227, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DJ, Connor JA. Participation of postsynaptic PKC in cerebellar long-term depression in culture. Science 254: 1656–1659, 1991 [DOI] [PubMed] [Google Scholar]
- Linden DJ, Dickinson MH, Smeyne M, Connor JA. A long-term depression of AMPA currents in cultured cerebellar Purkinje neurons. Neuron 7: 81–89, 1991 [DOI] [PubMed] [Google Scholar]
- Linden DJ, Smeyne M, Connor JA. Trans-ACPD, a metabotropic receptor agonist, produces calcium mobilization and an inward current in cultured cerebellar Purkinje neurons. J Neurophysiol 71: 1992–1998, 1994 [DOI] [PubMed] [Google Scholar]
- Llano I, DiPolo R, Marty A. Calcium-induced calcium release in cerebellar Purkinje cells. Neuron 12: 663–673, 1994 [DOI] [PubMed] [Google Scholar]
- Llano I, Dreessen J, Kano M, Konnerth A. Intradendritic release of calcium induced by glutamate in cerebellar Purkinje cells. Neuron 7: 577–583, 1991a [DOI] [PubMed] [Google Scholar]
- Llano I, Marty A. Presynaptic metabotropic glutamatergic regulation of inhibitory synapses in rat cerebellar slices. J Physiol 486: 163–176, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano I, Marty A, Armstrong CM, Konnerth A. Synaptic- and agonist-induced excitatory currents of Purkinje cells in rat cerebellar slices. J Physiol 434: 183–213, 1991b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Sasaki K. The functional organization of the olivo-cerebellar system as examined by multiple Purkinje cell recordings. Eur J Neurosci 1: 587–602, 1989 [DOI] [PubMed] [Google Scholar]
- Maejima T, Hashimoto K, Yoshida T, Aiba A, Kano M. Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron 31: 463–475, 2001 [DOI] [PubMed] [Google Scholar]
- Maejima T, Oka S, Hashimotodani Y, Ohno-Shosaku T, Aiba A, Wu D, Waku K, Sugiura T, Kano M. Synaptically driven endocannabinoid release requires Ca2+-assisted metabotropic glutamate receptor subtype 1 to phospholipase Cbeta4 signaling cascade in the cerebellum. J Neurosci 25: 6826–6835, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Chapman KE, Seckl JR, Ashley RH. Partial cloning and differential expression of ryanodine receptor/calcium-release channel genes in human tissues including the hippocampus and cerebellum. Neuroscience 85: 205–216, 1998 [DOI] [PubMed] [Google Scholar]
- Mateos JM, Azkue J, Sarria R, Kuhn R, Grandes P, Knopfel T. Localization of the mGlu4a metabotropic glutamate receptor in rat cerebellar cortex. Histochem Cell Biol 109: 135–139, 1998 [DOI] [PubMed] [Google Scholar]
- Mateos JM, Elezgarai I, Benitez R, Osorio A, Bilbao A, Azkue JJ, Kuhn R, Knopfel T, Grandes P. Clustering of the group III metabotropic glutamate receptor 4a at parallel fiber synaptic terminals in the rat cerebellar molecular layer. Neurosci Res 35: 71–74, 1999 [DOI] [PubMed] [Google Scholar]
- Mateos JM, Osorio A, Azkue J, Benitez R, Elezgaral I, Bilbao A, Diez J, Puente N, Kuhn R, Knopfel T, Hawkes R, Donate F, Grandes P. Parasagittal compartmentalization of the metabotropic glutamate receptor mGluR1b in the cerebellar cortex. Eur J Anat 5: 15–21, 2001 [Google Scholar]
- Miyata M, Finch EA, Khiroug L, Hashimoto K, Hayasaka S, Oda SI, Inouye M, Takagishi Y, Augustine GJ, Kano M. Local calcium release in dendritic spines required for long-term synaptic depression. Neuron 28: 233–244, 2000 [DOI] [PubMed] [Google Scholar]
- Moseley ML, Zu T, Ikeda Y, Gao W, Mosemiller AK, Daughters RS, Chen G, Weatherspoon MR, Clark HB, Ebner TJ, Day JW, Ranum LP. Bidirectional expression of CUG and CAG expansion transcripts and intranuclear polyglutamine inclusions in spinocerebellar ataxia type 8. Nat Genet 38: 758–769, 2006 [DOI] [PubMed] [Google Scholar]
- Nagao S, Kwak S, Kanazawa I. EAAT4, a glutamate transporter with properties of a chloride channel, is predominantly localized in Purkinje cell dendrites, and forms parasagittal compartments in rat cerebellum. Neuroscience 78: 929–933, 1997 [DOI] [PubMed] [Google Scholar]
- Netzeband JG, Gruol DL. mGluR1 agonists elicit a Ca2+ signal and membrane hyperpolarization mediated by apamin-sensitive potassium channels in immature rat Purkinje neurons. J Neurosci Res 86: 293–305, 2008 [DOI] [PubMed] [Google Scholar]
- Oscarsson O. Functional organization of olivary projection to the cerebellar anterior lobe. In: The Inferior Olivary Nucleus: Anatomy and Physiology, edited by Courville J. New York: Raven, 1980, p. 279– 293–290 [Google Scholar]
- Palay SL, Chan-Palay V. Cerebellar Cortex: Cytology and Organization. New York: Springer, 1974 [Google Scholar]
- Paukert M, Huang YH, Tanaka K, Rothstein JD, Bergles DE. Zones of enhanced glutamate release from climbing fibers in the mammalian cerebellum. J Neurosci 30: 7290–7299, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis - a mechanism coupling neuronal-activity to glucose-utilization. Proc Natl Acad Sci USA 91: 10625–10629, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pin JP, Waeber C, Prezeau L, Bockaert J, Heinemann SF. Alternative splicing generates metabotropic glutamate receptors inducing different patterns of calcium release in Xenopus oocytes. Proc Natl Acad Sci USA 89: 10331–10335, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prezeau L, Gomeza J, Ahern S, Mary S, Galvez T, Bockaert J, Pin JP. Changes in the carboxyl-terminal domain of metabotropic glutamate receptor 1 by alternative splicing generate receptors with differing agonist-independent activity. Mol Pharmacol 49: 422–429, 1996 [PubMed] [Google Scholar]
- Reinert KC, Dunbar RL, Gao W, Chen G, Ebner TJ. Flavoprotein autofluorescence imaging of neuronal activation in the cerebellar cortex in vivo. J Neurophysiol 92: 199–211, 2004 [DOI] [PubMed] [Google Scholar]
- Reinert KC, Gao W, Chen G, Ebner TJ. Flavoprotein autofluorescence imaging in the cerebellar cortex in vivo. J Neurosci Res 85: 3221–3232, 2007 [DOI] [PubMed] [Google Scholar]
- Salin PA, Malenka RC, Nicoll RA. Cyclic AMP mediates a presynaptic form of LTP at cerebellar parallel fiber synapses. Neuron 16: 797–803, 1996 [DOI] [PubMed] [Google Scholar]
- Sarkisov DV, Wang SS. Order-dependent coincidence detection in cerebellar Purkinje neurons at the inositol trisphosphate receptor. J Neurosci 28: 133–142, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarna JR, Marzban H, Watanabe M, Hawkes R. Complementary stripes of phospholipase Cbeta3 and Cbeta4 expression by Purkinje cell subsets in the mouse cerebellum. J Comp Neurol 496: 303–313, 2006 [DOI] [PubMed] [Google Scholar]
- Sharp AH, McPherson PS, Dawson TM, Aoki C, Campbell KP, Snyder SH. Differential immunohistochemical localization of inositol 1,4,5-trisphosphate- and ryanodine-sensitive Ca2+ release channels in rat brain. J Neurosci 13: 3051–3063, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuki K, Hishida R, Murakami H, Kudoh M, Kawaguchi T, Watanabe M, Watanabe S, Kouuchi T, Tanaka R. Dynamic imaging of somatosensory cortical activity in the rat visualized by flavoprotein autofluorescence. J Physiol 549: 919–927, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR1) in the central nervous system: an in situ hybridization study in adult and developing rat. J Comp Neurol 322: 121–135, 1992 [DOI] [PubMed] [Google Scholar]
- Sillitoe RV, Hawkes R. Whole-mount immunohistochemistry: a high-throughput screen for patterning defects in the mouse cerebellum. J Histochem Cytochem 50: 235–244, 2002 [DOI] [PubMed] [Google Scholar]
- Sugihara I, Quy PN. Identification of aldolase C compartments in the mouse cerebellar cortex by olivocerebellar labeling. J Comp Neurol 500: 1076–1092, 2007 [DOI] [PubMed] [Google Scholar]
- Sullivan MR, Nimmerjahn A, Sarkisov DV, Helmchen F, Wang SS. In vivo calcium imaging of circuit activity in cerebellar cortex. J Neurophysiol 94: 1636–1644, 2005 [DOI] [PubMed] [Google Scholar]
- Takechi H, Eilers J, Konnerth A. A new class of synaptic response involving calcium release in dendritic spines. Nature 396: 757–760, 1998 [DOI] [PubMed] [Google Scholar]
- Tanabe Y, Masu M, Ishii T, Shigemoto R, Nakanishi S. A family of metabotropic glutamate receptors. Neuron 8: 169–179, 1992 [DOI] [PubMed] [Google Scholar]
- Tempia F, Miniaci MC, Anchisi D, Strata P. Postsynaptic current mediated by metabotropic glutamate receptors in cerebellar Purkinje cells. J Neurophysiol 80: 520–528, 1998 [DOI] [PubMed] [Google Scholar]
- Thach WT, Goodkin HP, Keating JG. The cerebellum and the adaptive coordination of movement. Annu Rev Neurosci 15: 403–442, 1992 [DOI] [PubMed] [Google Scholar]
- Tsien RW, Tsien RY. Calcium channels, stores, oscillations. Annu Rev Cell Biol 6: 715–760, 1990 [DOI] [PubMed] [Google Scholar]
- Voogd J. Comparative aspects of the structure and fibre connexions of the mammalian cerebellum. Prog Brain Res 25: 94–134, 1967 [DOI] [PubMed] [Google Scholar]
- Voogd J, Bigare F. Topographical distribution of olivary and corticonuclear fibers in the cerebellum. A review. In: The Inferior Olivary Nucleus, edited by Courville J, deMontigny C, Lamarre Y. New York: Raven, 1980, p. 207–234 [Google Scholar]
- Voogd J, Pardoe J, Ruigrok TJH, Apps R. The distribution of climbing and mossy fiber collateral branches from the copula pyramidis and the paramedian lobule: congruence of climbing fiber cortical zones and the pattern of zebrin banding within the rat cerebellum. J Neurosci 23: 4645–4656, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voogd J, Ruigrok TJ. The organization of the corticonuclear and olivocerebellar climbing fiber projections to the rat cerebellar vermis: the congruence of projection zones and the zebrin pattern. J Neurocytol 33: 5–21, 2004 [DOI] [PubMed] [Google Scholar]
- Wadiche JI, Jahr CE. Patterned expression of Purkinje cell glutamate transporters controls synaptic plasticity. Nat Neurosci 8: 1329–1334, 2005 [DOI] [PubMed] [Google Scholar]
- Wang X, Chen G, Gao W, Ebner TJ. Long-term potentiation of the responses to parallel fiber stimulation in mouse cerebellar cortex in vivo. Neuroscience 162: 713–722, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waniewski RA, Martin DL. Preferential utilization of acetate by astrocytes is attributable to transport. J Neurosci 18: 5225–5233, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh JP, Lang EJ, Suglhara I, Llinas R. Dynamic organization of motor control within the olivocerebellar system. Nature 374: 453–457, 1995 [DOI] [PubMed] [Google Scholar]
- Yaginuma H, Matsushita M. Spinocerebellar projections from the upper lumbar segments in the cat, as studied by anterograde transport of wheat germ agglutinin-horseradish peroxidase. J Comp Neurol 281: 298–319, 1989 [DOI] [PubMed] [Google Scholar]