Abstract
The mammalian main olfactory bulb receives a significant noradrenergic input from the locus coeruleus. Norepinephrine (NE) is involved in acquisition of conditioned odor preferences in neonatal animals, in some species-specific odor-dependent behaviors, and in adult odor perception. We provide a detailed review of the functional role of NE in adult rodent main olfactory bulb function. We include cellular, synaptic, network, and behavioral data and use computational simulations to tie these different types of data together.
Keywords: computation, neuromodulation
the locus coeruleus (LC), which releases norepinephrine (NE), is one of several neuromodulatory nuclei with widely distributed ascending projections to cortical brain regions (Foote et al. 1983). LC neurons are activated by novel or target stimuli (Foote et al. 1980; Vankov et al. 1995) and are important in the regulation of vigilance (reviewed in Aston-Jones et al. 2000; Berridge and Waterhouse 2003). During waking, LC neurons simultaneously fire a brief burst of action potentials in response to nonnoxious sensory stimuli of all modalities, but especially those that are novel or salient [e.g., signal reward (Aston-Jones and Bloom 1981; Aston-Jones et al. 2000)].
At the neural level, work in somatosensory, auditory, olfactory, and visual circuits has revealed complex, often subtle effects of NE on cellular excitability that dramatically alter neuronal responses to sensory input. Some of the better characterized effects include 1) reduction of spontaneous but not stimulus-evoked firing, thereby increasing signal-to-noise ratios, 2) specific augmentation of stimulus-evoked excitation, and 3) conversion of subthreshold excitatory inputs to suprathreshold spiking responses (Devilbiss and Waterhouse 2000, 2004; Hirata et al. 2006; McLean and Waterhouse 1994; Mouradian et al. 1991; Waterhouse et al. 1990). NE also increases the temporal precision between excitatory afferent input and postsynaptic responses, enhancing synchrony between sensory input and spike output (Kossl and Vater 1989; Lecas 2004; Moxon et al. 2007). In the main olfactory bulb (MOB) and olfactory (piriform) cortex, NE has a potentiating effect on weak sensory inputs similar to its effect in other sensory systems (Bouret and Sara 2002; Ciombor et al. 1999; Hayar et al. 2001; Jiang et al. 1996). NE also decreases response latencies for evoked responses in the MOB and piriform cortex, improving the temporal precision of sensory responses.
NE is deeply integrated into the olfactory system. Olfactory cues increase the discharge of LC neurons in behaving animals (Aston-Jones and Bloom 1981) and trigger rapid increases in NE levels in MOB as well as the accessory olfactory bulb (AOB) (Brennan et al. 1990; Kaba and Keverne 1988; Kaba et al. 1989). LC projections to the AOB are vital for the formation of memories involved in the regulation of pregnancy (Bruce effect) and maternal behavior (Brennan et al. 1990; Kaba and Keverne 1988; Kaba et al. 1989). In neonatal animals, NE-β receptor activation in the MOB plays a critical role in the rapid learning of conditioned odor preferences (reviewed in Moriceau and Sullivan 2004a). This LC-dependent olfactory imprinting, however, is developmentally transient; although the behavioral effects of bulbar NE modulation in adult rodents have been less studied, they are likely to be more measured and conditional than those in neonates (Moriceau and Sullivan 2004b). More recently, increased attention has been paid by several laboratories to noradrenergic modulation of MOB processing in adult animals; in this review we describe these data, including cellular, network, and behavioral results. We present a comprehensive model of how NE effects on bulbar processing results in the perceptual effects described in the literature.
Olfactory Bulb Noradrenergic Innervation
The MOB receives a dense noradrenergic projection from the LC that terminates in all but the most superficial layers [(Shipley et al. 1985); Fig. 1A]. Approximately 40% of LC neurons (400–600 of 1,600 cells) project to the rat MOB (Shipley et al. 1985). NE fibers preferentially target the internal plexiform layer and the granule cell layer, and to a lesser extent the mitral cell layer and external plexiform layer (McLean et al. 1989). Generally paralleling this NE fiber distribution, each of the three major NE receptor subtypes (α1, α2, β) are expressed in multiple layers of the MOB, and individual MOB neurons appear to express multiple NE receptor subtypes (for review, see Ennis and Hayar 2008). For example, mitral cells express all three NE receptor subtypes, and granule cells express α1 and α2 receptors (Ennis and Hayar 2008).
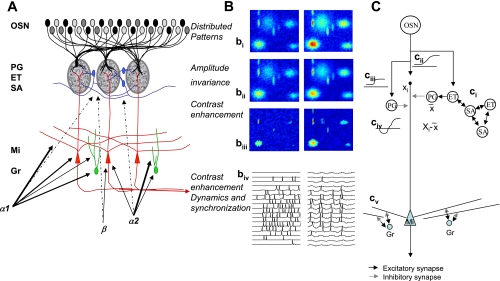
Fig. 1.
Olfactory bulb processing. A: schematic of olfactory bulb organization. Olfactory sensory neurons (OSN) expressing a common receptor, and therefore exhibiting similar odorant receptive fields, project to 2 common glomeruli in the main olfactory bulb (MOB). Within this glomerulus, OSNs make excitatory synapses onto mitral and tufted cells and the primary output neurons of the MOB, as well as glomerular layer interneurons comprising periglomerular (PG) and external tufted (ET) cells. Most PG cells (∼70%) and a third type of interneuron, short axon cells (SA), are not directly activated by OSNs. PG, ET, and SA cells form intricate networks within the glomerular layer that have been proposed to perform operations such as normalization, contrast enhancement, and synchronization. In deeper processing layers, mitral cells (Mi) interact with at least 1 other class of interneurons, granule cells (Gr). These provide extensive feedback and lateral interactions between Mi cells by interacting with their elongated secondary dendrites. This layer of processing is thought to be involved in creating olfactory bulb gamma rhythms and generating synchronized spike patterns. Noradrenergic inputs from the locus coeruleus activate 3 classes of noradrenergic receptors distributed across the MOB. α1-Receptors are thought to be predominantly located on Mi and Gr cell bodies, as well as secondary dendrites, with a sparser distribution in the glomerular layers; α2-receptors are mainly located on granule cells with a sparse distribution on Mi cell bodies and the glomerular layer; and β-receptors have been reported on Mi cell bodies and in the glomerular layer. B: schematic of glomerular input layer and Mi cell activity patterns in response to odor stimulation. bi-–biii show simulated distributed odor responses at 2 concentrations, with the lower concentration at left and high concentration at right. The 2-dimensional simulations are color coded, with red indicating high, and dark blue low, levels of activity. bii shows the same patterns after amplitude invariance processing has been performed by the network of local interneurons, and biii shows the same pattern after contrast enhancement. The patterns in biii represent the end result of the glomerular computations transmitted to deeper layers by Mi cells. The details of these computations are given by Cleland et al. (2007) and Cleland and Sethupathy (2006). biv shows how spikes generated in Mi cells in response to activation patterns (left) are transformed into sparser, oscillatory and highly synchronized spike patterns (right) by the interactions with deeper interneuron networks (Escanilla et al. 2010; Mandairon et al. 2006b). C: neural networks that could perform bulbar computations. ci indicates amplitude invariance computation as proposed by Cleland et al. (2007). ET cells sample OSN activity across the glomerular layer. A localized network of SA and ET cells, densely interconnected, calculates the sum or average activity amplitude (X̄) across the glomerular layer and projects this average activity onto inhibitory PG cells. PG cells inhibit Mi cells, hence Mi cells, which sample a single data point Xi from OSN, inputs process Xi − X̄, corresponding to a z-score normalization. cii–civ: local contrast enhancement as proposed by Cleland and Sethupathy (2006). OSN odor responses (cii), schematically drawn as response amplitude as a function of receptor-odorant affinity, activate Mi cells and PG cells. Because of the higher input resistance of PG cells, the corresponding response curves are steeper and more easily saturated (ciii). Because PG cells locally inhibit Mi cells, the resulting net effect on Mi cells is a Mexican hat function (civ). Contrast enhancement and amplitude invariance calculations are performed simultaneously and partially by the same neurons; the result is an activity pattern with higher contrast and relative amplitude invariance, as depicted in bi–biii. This activity is transformed again in deeper layers by dynamic interactions with Gr cells. Current thinking assumes that in this layer, activity patterns conveyed as Poisson-distributed spike trains (biv, left) are modulated and a fast oscillation in the gamma range is superposed on these spike trains, modulating them in time and creating periodically synchronized patterns as depicted schematically in biv (right). cv: interactions between Mi cell secondary dendrites and Gr cells provide feedback (within the same column) and lateral (between columns) inhibitory interactions. These have been proposed to create oscillatory dynamics, synchronization, and/or contrast enhancement.
Physiological considerations suggest that the broad NE fiber innervation of the MOB is paralleled by diffuse NE release in response to LC activation. LC neuronal activity exhibits tonic increases and decreases at the population level in a behavioral state-dependent manner, and LC neurons fire synchronously in response to excitatory input (Aston-Jones and Bloom 1981; Berridge and Waterhouse 2003). Therefore, even if individual LC axons terminate with sublaminar specificity in the MOB, functionally the parallel activity of the ensemble of LC neurons that project to MOB should lead to diffuse synaptic release of NE. However, there may be laminar differences in concentrations of synaptically released NE, and perhaps the magnitude of subsequent postsynaptic effects, that are proportional to the density of NE axon terminals. The degree of postsynaptic receptor activation will ultimately depend on the proximity of the receptors to NE terminal release sites. Unfortunately, critical data to assess these issues (subcellular localization of noradrenergic receptors in the MOB and their relationship to NE terminal release sites) are unavailable. Nevertheless, given these considerations, it stands to reason that NE modulation of the MOB network and its impact on olfactory processing cannot be extracted by an understanding of NE's action at a specific receptor or MOB neuronal subtype. Equally importantly, a premise of this review based on emerging and parallel behavioral and electrophysiological findings is that the impact of NE varies dynamically as a function of the extracellular concentration and the constellation of receptors engaged by the prevailing NE level.
Olfactory Bulb Network and Processing
The MOB in rodents has been extensively described in a number of review articles. We briefly review the main neuronal types and their interactions as well as hypothesized function in odor processing before describing the details of noradrenergic action onto these neurons (Fig. 1A).
Distributed patterns of activity in response to volatile chemical stimuli (odorants) are transmitted to the olfactory bulb via olfactory sensory neuron (OSN) axons that terminate in the glomeruli of its input layer; each set of OSNs transmits a particular odor-receptive field to the glomerulus it projects to (Fig. 1A). The olfactory bulb is believed to filter and transform these incoming sensory data, performing normalization, contrast enhancement, signal-to-noise regulations, and other types of operations before conveying the processed olfactory information to several different secondary olfactory structures via mitral/tufted cell axon collaterals (Cleland and Linster 2003). Neuromodulatory inputs such as NE, acetylcholine, and serotonin modulate and change these functions of the olfactory bulb, presumably adapting the performed computations to maximize processing dependent on the specific behavioral demands on the animal (reviewed in Mandairon and Linster 2009).
Concentration invariance, or normalization of odor representations, is a function that has been proposed for bulbar processing (Cleland et al. 2007); this function may rely on the dense glomerular networks of external tufted, short axon, and periglomerular cells described by Aungst et al. (2003) (Fig. 1, B and C). Cholinergic and serotonergic inputs acting on subpopulations of these neurons could modulate the processing, leading to concentration invariant odor representations.
Contrast enhancement is a common property of sensory systems that narrows (sharpens) sensory representations by specifically inhibiting neurons on the periphery of the representation, thus enhancing the contrast between signal and background (Fig. 1B). Contrast enhancement of odor representations in the olfactory bulb is thought to be mediated by inhibitory neurons in both the glomerular layer [periglomerular cells (Cleland and Sethupathy 2006; Linster and Gervais 1996; Linster and Hasselmo 1997)] and granule cell layer [granule cells (Urban 2002; Urban and Arevian 2009; Yokoi et al. 1995)]. A number of computational models have proposed solutions for this important function, including lateral interactions between glomeruli (Linster and Gervais 1996; Linster and Hasselmo 1997), computations local to each glomerulus (Cleland and Sethupathy 2006) (Fig. 1, B and C), and local and lateral interactions between mitral and granule cells (Urban and Arevian 2009) (Fig. 1, B and C). Modulatory inputs acting on the excitability and synaptic interactions in these bulbar layers could therefore change these computations and modulate the degree of contrast enhancement performed in a given behavioral situation. Behavioral data in which modulatory functions in the MOB have been disturbed clearly point to contrast enhancement being an important function of the MOB (reviewed in Mandairon and Linster 2009). For example, enhancing cholinergic activity in the MOB increased rats' ability to discriminate between chemically very similar odorants, whereas blockade of cholinergic receptors decreased this discrimination; these results can be predicted by known effects of cholinergic inputs onto glomerular circuits mediating contrast (Chaudhury et al. 2009; Linster and Cleland 2002; Mandairon et al. 2006a).
At the level of glomerular processing, changes in activation patterns, i.e., which mitral cells are responsive to any given odorant, are thought to be dominant, and the functions described above modulate these activation patterns. In deeper layers, modulation of spike timing and synchronization may affect contrast of odor representations (Fig. 1, B and C), since the dynamics of bulbar processing seem to be mainly regulated by the interactions between mitral and granule cells. Modulatory inputs on mitral and granule cells can potentially affect the dynamics of neural activity as well as spike timing and degrees of synchronization. Levels of synchronization among bulbar outputs have been proposed to contribute to odor processing and learning experimentally (Beshel et al. 2007; Brea et al. 2009; Kay et al. 2009; Nusser et al. 2001). Modulatory inputs to granule and mitral cells can potentially regulate oscillatory dynamics of olfactory bulb processing and hence change the processing of odors, as proposed early on by Freeman and colleagues (Di Prisco and Freeman 1985; Gray et al. 1986) and shown recently in slices of young rats by Schoppa and colleagues (Gire and Schoppa 2008; Pandipati et al. 2010).
In summary, modulatory inputs have the capacity to regulate bulbar processing at many levels and have been shown to do so in a variety of paradigms and levels of investigation: glomerular modulation regulates contrast and normalization processing, whereas deeper layer modulation regulates contrast, dynamics, and synchronization properties. We next review data pertaining to the noradrenergic modulation of these different functionalities and summarize these data with a comprehensive model of bulbar processing and noradrenergic modulation of this processing.
Noradrenergic Modulation of Bulbar Processing: Electrophysiological Evidence
Because this review is meant to focus on noradrenergic modulation of adult rodent olfaction, we have focused the review of cellular and synaptic effects on those data obtained in young to adult rodents. Data from neonatal rodents and data from other species are discussed when necessary to make a specific point only but are not extensively reviewed in this article. As extensively reviewed by Sullivan and colleagues (Moriceau and Sullivan 2004b; Sullivan and Wilson 2003), noradrenergic modulation and the expression of noradrenergic receptors in the olfactory bulb of rodents changes dramatically after postnatal days 9 and 10 (P9 and P10) in rats. The role of noradrenergic modulation in odor learning, as well as the receptors involved in learning, change after the critical period; this behavioral observations has also been confirmed in brain slice recordings: there is a marked increase in the density of β-receptor binding in the rat olfactory bulb from P12 to P30 (Woo and Leon 1995). The expression of mRNA for the α2A-receptor subtype increases until P21, but levels are lower in the adult olfactory bulb (Winzer-Serhan et al. 1997), and the effects of NE as well as the specific receptors involved in these effects differed in slices obtained in younger (P9–P13) or older rats (P19–P21) (Pandipati et al. 2010). For the sake of consistency and focus on adult perception and learning, we therefore only discuss results obtained in animals after the critical learning period and only briefly mention results in younger animals.
Modulation of mitral cell responsiveness to low-amplitude sensory stimuli.
Activation of LC in anesthetized rats enhanced mitral cell spiking responses to weak (i.e., perithreshold) but not strong (i.e., suprathreshold) stimulation of the olfactory epithelium (Jiang et al. 1996). The LC-evoked enhancement occurred without consistent changes in mitral cell spontaneous firing rate, although a subset of cells showed a small decrease in activity. Consistent with these in vivo findings, application of low concentrations of NE (1 μM) selectively increased mitral cell spike discharge to perithreshold intensity olfactory nerve (ON) stimulation in rat MOB slices (Ciombor et al. 1999). NE also decreased the inhibitory period following ON stimulation-evoked spikes. The NE response enhancement was due to a reduction of spike response failures to ON stimulation, rather than an increase of the incidence of evoked-spikes for threshold ON stimuli. Thus NE converts a previously subthreshold excitatory synaptic response into a suprathreshold, spike-triggering response, similar to the effect of NE at α1-receptors in the cortex (Mouradian et al. 1991; Waterhouse et al. 1990). The perithreshold ON response enhancement, but not the decrease in inhibition, was mimicked by α1-receptor agonists, although the magnitude of the enhancement was less than that for NE (Ciombor et al. 1999; Hayar et al. 2001). This indicates that the effect of NE may involve multiple noradrenergic receptors. Together, these findings suggest that NE release alters mitral cell excitability in a manner that could increase their sensitivity to weak or subthreshold ON input, perhaps to improve the detection of weak odorants. The onset latency for ON-evoked spikes was significantly reduced by NE, thereby more closely synchronizing mitral cell output to sensory input, similar to NE actions in a number of cortical circuits including olfactory cortex (Bouret and Sara 2002; Devilbiss and Waterhouse 2000; Waterhouse et al. 2000).
Modulation of mitral cell responsiveness to odors.
The impact of NE on cellular responses to odors has been examined in only few studies. Repetitive pairing of LC stimulation and odors producing excitation of a mitral cell was found to produce a long-lasting (4 h) suppression of subsequent responses of the cell to the paired odor in anesthetized mice (Shea et al. 2008). This suppression was prevented by combined blockade of α- and β-receptors near the recorded mitral cell. Behavioral testing 24 h after such pairing indicated that mice investigated the paired odor less than an unpaired odor, suggesting that LC stimulation-odor pairing produces a memory expressed by decreased interest specific for the paired odor. LC-induced suppression of mitral cell excitatory odor responses, when put into context with the preceding studies showing that LC stimulation or NE application enhances mitral cell responses to perithreshold intensity ON stimulation, show that NE modulation is not a simple effect independent of time course or experimental protocol. Differences in species, the duration or magnitude of LC activation, and the mitral cell sensory response may be important factors; however, behavioral studies in mice suggest that NE may play an important role in behavioral habituation to odorants (Guerin et al. 2008), and these data are discussed in more detail below.
Modulation of mitral and granule cell properties.
Historically, findings obtained in turtle and cell cultures of neonatal rats led to the conclusion that a major action of NE is mitral cell disinhibition subsequent to inhibition of, or decreased GABA release from, granule cells (Jahr and Nicoll 1982; Trombley 1992; Trombley and Shepherd 1992). Subsequent studies in juvenile and adult rat MOB slices demonstrated noradrenergic agonists did not effect ON stimulation-evoked field potentials (FPs) recorded in the glomerular layer or ON stimulation-evoked postsynaptic currents (EPSCs) in mitral cells (Hayar et al. 2001). This suggests that NE-evoked enhancement of ON stimulation-evoked mitral cell spiking is mediated by actions on MOB neurons. Interestingly, a relatively high concentration of NE (30 μM) suppressed ON stimulation-evoked FPs and EPSCs by activating inhibitory presynaptic D2 dopamine receptors on ON terminals. Thus high concentrations of NE may have nonspecific inhibitory effects that dampen ON input. Since low concentrations of NE or α1-receptor agonist did not modulate the strength of ON input, how does NE and α1-receptor activation enhance mitral cell responses to weak ON input observed in extracellular studies (Ciombor et al. 1999; Jiang et al. 1996)? One possibility is that NE (1–30 μM), acting at α1-receptors, directly depolarizes and induces an inward current in mitral cells (Hayar et al. 2001). This effect is mediated by closure of a leak potassium current as determined by the reversal potential and increased membrane resistance associated with the inward current. These results indicate that NE-induced activation of the α1-receptor produces a small depolarization that moves the mitral cell membrane potential toward spike threshold, whereas the resistance increase boosts somatic depolarization to synaptic inputs. Together, these effects cooperate to increase the probability of spike initiation in response to weak ON synaptic input (Hayar et al. 2001).
Individual MOB neurons appear to express multiple NE receptor subtypes that have differing affinities for NE (Ramos and Arnsten 2007). Therefore, the effect of NE on MOB neurons and the MOB network is likely to vary as a function of the extracellular concentration of NE. To investigate this question, the effects of varying NE concentrations on GABAergic inhibition of mitral cells as assessed by spontaneous inhibitory postsynaptic current (IPSC) activity were examined in rat MOB slices (Nai et al. 2009). The lowest concentrations of NE used reduced IPSC frequency, with further increases in concentration producing an inverted-U profile characterized by an intermediate-dose increase in IPSCs and a high-dose decline in IPSCs toward baseline level. Specifically, NE concentrations ≤1 μM suppressed IPSCs, whereas 1–30 μM increased IPSCs; concentrations >30 μM reversed the facilitation. These effects were presynaptically mediated by NE actions on GABAergic inputs to mitral cells. As determined by subtype-specific agonists and antagonists, the low concentration suppression was mediated by the α2-receptor, whereas the intermediate concentration facilitation was mediated by α1-receptors (Nai et al. 2009). At the intermediate NE levels, the α1-receptor stimulatory effect appears to overrides the α2-receptor inhibitory effect on GABA release (Fig. 2, A and B).
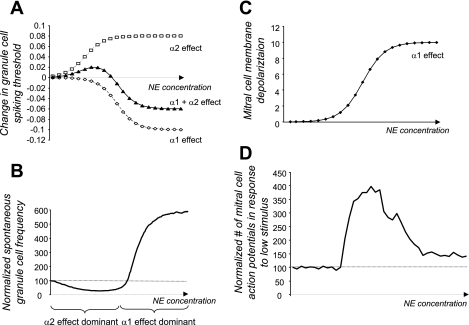
Fig. 2.
Effects of norepinephrine (NE) modulation on cellular parameters. A: NE modulates Gr cell spontaneous firing (Nai et al. 2010). The measured effects of NE activation of α1- and α2-receptors on Gr cell-mediated spontaneous inhibitory postsynaptic currents (IPSCs) was simulated by changes in Gr cell firing thresholds [in abstract parameters; equations described in detail by Escanilla et al. (2010)]. α1- and α2-receptor activation modulate spontaneous IPSCs in opposite directions, each with their own concentration dependency. The summated effect is a nonmonotonic modulation of firing threshold. B: effect of threshold modulation on Gr cell spontaneous firing. The size of the effect was adjusted to data reported by Ennis and colleagues (Nai et al. 2009, 2010). C: modulation of membrane potential by NE in model Mi cells. The size of the effect was modeled after data reported by Hayar et al. (2001). In the model, spontaneous activity was not effected by this effect, but responses to low-threshold stimuli were modulated. D: modulation of response probability to low-threshold stimuli in Mi cells. Both modulation of inhibition by α2 and α2 effects on Gr cell firing and direct modulation of Mi cell membrane potential were taken into account. Mi cell responses to low-amplitude stimuli were modulated in a nonmonotonic manner by simulated NE due to the additive effects of NE receptor activation.
The concentration profile for the effects of NE on the level of GABAergic inhibition of mitral cells was well matched by direct effects of NE on granule cell excitability in rat MOB slices. Specifically, low concentrations of NE (0.1–1.0 μM) or α2-receptor agonists hyperpolarized and inhibited granule cell spontaneous or evoked spike discharge (Nai et al. 2010). By contrast, 10 μM NE or α1-receptor agonists depolarized and increased granule cell discharge. These effects appear to be mediated by opposing actions on granule cell potassium currents, with α2-receptor activation increasing, and α1-receptor activation inhibiting, potassium currents (Nai et al. 2010). These studies indicate that the differential affinities of NE receptor subtypes allow for differential modulation of GABA release and olfactory processing as a function of the level of NE release, which in turn is regulated by behavioral state (Berridge and Waterhouse 2003).
Relevant to the preceding studies is the consideration of how the concentration range of exogenously applied NE correlates with levels of synaptically released NE. In anesthetized rats, where LC spontaneous discharge rate averages 1–2 Hz, basal extracellular concentrations of NE in the rat olfactory bulb as measured by in vivo microdialysis are 0.3–0.4 nM (El-Etri et al. 1999); tonic increases in LC discharge to 14 Hz elicited by intra-LC microinjection of acetylcholine elevated NE levels to ∼1.0 nM. However, NE levels at synaptic release sites may be substantial higher than values obtained via in vivo microdialysis. Also, microdialysis samples are collected over many minutes and may not accurately reflect phasic or peak changes in NE levels. More sensitive electrochemical measurements of phasic NE release in the cerebellum of anesthetized rats indicate that 10-Hz LC electrical stimulation increases NE levels to 1–2 μM (Bickford-Wimer et al. 1991). The later value is compatible with the effects of submicromolar to low micromolar concentrations of bath-applied NE on mitral cell IPSCs and ON stimulation-evoked spiking summarized above. Unfortunately, detailed measurements of basal and phasic, LC-evoked NE levels in the olfactory bulb in unanesthetized rats are unavailable.
Resulting modulation of network properties.
The combined effects of NE receptor activation on bulbar cell types results in complex and concentration-dependent effects on network processing. NE infusion (100 μM) into the MOB of awake rabbits increased the amplitude of gamma (40–80 Hz) components of the MOB EEG and also potentiated the spatial EEG pattern elicited by novel odors (Gray et al. 1986). In the same preparation, β-receptor antagonist infusion prevented transient alterations of the MOB EEG elicited by repetitive presentation of a reinforced, but not unreinforced, odor (Gray et al. 1986). These observations can be ascribed to the increased excitation of both mitral and granule cells (see Modulation of mitral and granule cell properties for details) leading to a stronger feedback coupling between these cell types, which naturally results in higher oscillatory power (see Fig. 3A).
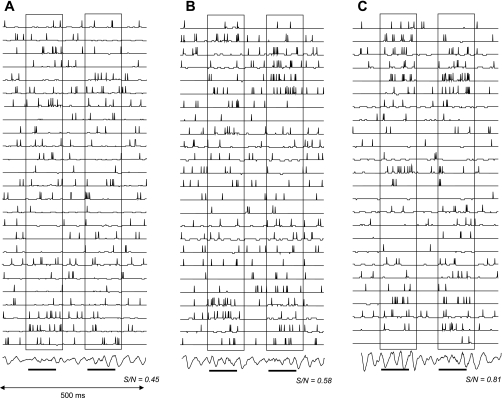
Fig. 3.
Modulation of model odor responses. Membrane potential and action potentials in a subset of olfactory bulb model Mi cells are shown in response to very low concentration odor stimuli; odor presentation is indicated by lower horizontal bars and is highlighted by outlined boxes. Odor stimulation activated OSNs to 10% of their maximal activation level. The model is modulated with no (A), low (B; mainly α2 effects), and high levels of NE (C; mainly α1 effects). The NE modulation corresponds to the negative and positive peaks of the modulation function in Fig. 2A. Signal-to-noise ratio (S/N) for each simulation is indicated below the traces and is calculated as the number of spikes evoked during odor stimulation divided by the total number of spikes evoked during the 500-ms simulation. Note that Mi cell odor responses are enhanced with respect to background activity when low NE levels are simulated and S/N is increased. At high NE levels, Mi cell responses to odorants are further increased on a background of lower spontaneous activity, and S/N further increases. Note that although the total number of spikes over the course of a 500-ms simulation does not substantially change, the distribution of spikes during spontaneous and odor activity does change. The traces at bottom represent model field potentials recorded in the Gr cell layer.
Several studies have investigated the effects of NE on the well-characterized FP evoked by stimulation of the lateral olfactory tract (LOT), which antidromically activates mitral cells and engages the mitral cell-to-granule cell synapse. LC stimulation was reported to have no effect on LOT-evoked FPs recorded in the rat granule cell layer (Perez et al. 1987). A subsequent study in rats using a paired-pulse LOT stimulation protocol reported that LC activation had no effect on the conditioning pulse-evoked FP in the granule cell layer. However, LC stimulation elicited an initial phasic decrease followed by a long-lasting increase in paired-pulse depression of the evoked FP (Okutani et al. 1998). This biphasic effect, attributed to β-receptor activation, was interpreted as an NE-elicited reduction and then facilitation of granule cell-mediated inhibition of mitral cells. Another study reported that NE infusion (0.1–1.0 mM) into the rat MOB, acting at α1- but not α2- or β-receptors, increased the LOT conditioning pulse-evoked granule cell layer FP; mitral cell antidromic responses were unaffected, suggesting that NE increases synaptic activation of granule cells (Mouly et al. 1995), a finding compatible with increased excitation of granule cells. In summary, the studies above indicate that LC stimulation or NE infusion 1) has no effect on mitral cells or granule cells (Perez et al. 1987), 2) biphasically inhibits and then facilitates granule cell-mediated inhibition via β-receptors (Okutani et al. 1998), and 3) facilitates activation of granule cells and subsequent granule cell-mediated inhibition via α1-receptors (Mouly et al. 1995). It is important to note that interpretation of paired-pulse tests at mitral cell-granule cell reciprocal dendrodendritic synapses are especially problematic if NE simultaneously acts on pre- and postsynaptic sites under study.
Noradrenergic Modulation of Olfactory Bulb Processing
As described in the previous sections, NE has multiple effects on bulbar cellular and synaptic parameters in the olfactory bulb, and these effects are strongly concentration dependent. Together these individual actions lead to changes in network processing that can be difficult to predict due to the nonlinearity of cellular processes and the differential binding affinities of the receptors involved. Interestingly, despite the fact that all NE receptor types have been localized across the glomerular layer, no data as to modulation of glomerular processing by NE inputs are available. Hence, the following functional discussions focus on deeper layer processing. One must keep in mind that inconsistencies between electrophysiological findings and behavioral data can potentially be explained by the missing information pertaining to glomerular modulation by NE. As detailed above, it is believed that contrast enhancement functions, for example, are performed in the glomerular layer; hence, any effect NE would have on the networks involved would modulate odor perception and learning.
Figure 2 shows the effects of NE activation of α1- and α2-receptors on simulated granule and mitral cells. Because NE activation of α1- and α2-receptors creates opposite effects on granule cell activation, low concentrations of NE decrease granule cell firing (Fig. 2A; simulated by increased spiking threshold) and with it mitral cell inhibition, whereas at higher concentrations, α1 effects are stronger and granule cell activation is increased (Fig. 2A; simulated by decreased firing threshold), resulting in stronger inhibition of mitral cells. The net effect, shown as changes in granule cell spontaneous firing in Fig. 2B, with magnitudes comparable to those recorded in slices (Nai et al. 2009), is that of a decrease followed by an increase in firing. In addition to the modulation of granule cell firing, activation of α1-receptors depolarizes mitral cells (simulated by a change in membrane depolarization; Fig. 2C) and hence counterbalances the effects of increased inhibition on this particular response. As a consequence, mitral cell responsiveness to very low concentration odor stimuli increases with increasing NE concentrations despite increasing tonic inhibition on mitral cells (Fig. 2D). At higher NE concentrations, responses to weak stimuli are decreased with respect to the peak response due to the additive effects of α1- and α2-receptor activation (Fig. 2D). This particular combination of effects could explain why low odor detection is improved by bulbar NE without affecting odor discrimination capabilities to the extent predicted by increased responsiveness of mitral cells alone. Figure 3, A–C, shows examples of simulated network responses during spontaneous and odor-elicited activity in a simulation of olfactory bulb. These three examples illustrate the modulation of network properties based on changes in cellular properties in a computational model of olfactory bulb processing. Changes in signal-to-noise ratio are highlighted by comparing the magnitude of stimulus responses to spontaneous activities, and changes in dynamics are highlighted by comparing stimulus-evoked FP oscillations in the three cases. The network was stimulated with very low odor concentrations (best OSN response was 10% of maximally possible response), and no-NE, low-NE (α2 only), and high-NE (α1 + α2) modulation was simulated using the cellular parameters shown in Fig. 2. At low NE concentrations, granule cell spontaneous activity decreases and mitral cell spontaneous activity is slightly higher than under control conditions. However, mitral cell responsiveness to odor stimulation is increased; as a consequence, the overall signal-to-noise ratio increases. At higher NE concentrations, increased granule activity decreases mitral cell spontaneous activity; during odor stimulation, this effect is counteracted by the α1-mediated increase of mitral cell responsiveness. In combination, these two effects lead to a net increase in signal-to-noise ratio compared with control. Overall, the relative response to odor compared with spontaneous activity is enhanced, as described previously in detail (Hasselmo et al. 1997, Escanilla et al. 2010).
Simulation of NE modulation in a computational model of olfactory bulb processing showed that 1) activation of granule and mitral cells by α1-receptor activation increases oscillations and synchrony among olfactory bulb mitral cells, 2) the combination of granule and mitral cell activation leads to enhanced responses to low threshold stimuli, and 3) signal-to-noise ratio in the model is improved in mitral cells in response to odor stimulation (Escanilla et al. 2010). In the model these effects were prominent when NE concentrations were modeled to be in the range of α1-receptor activation (Fig. 3C). In the low concentration range, α2-receptor activation dominates and leads mainly to a small increase in mitral cell activation due to a decrease in granule cell inhibition (Fig. 2, C and D). Overall, odor detection, calculated as the distance between spontaneous and odor-activated activity, increases and then decreases as NE levels in the model increase (Fig. 4A). The nonmonotonic curves qualitatively follow those of the combination of α1 and α2 effects at the cellular level (Fig. 2, B and D). Discrimination between two highly overlapping odorants, calculated as the distance between odor-evoked network activities, increases with increasing NE concentration. Figure 3 shows that although stimulus-evoked responses are more easily detected at low NE concentration, the responses to two different but overlapping odorants are more apparent at higher NE concentration. The increase in discrimination is due to increased inhibition paired with depolarization in mitral cells in response to α1 activation mainly (Fig. 4B). In addition to modulation of rate-based activation patterns used to calculate these distances, oscillatory power and synchronization between mitral cells is also modulated by levels of NE inputs in the model, as shown previously in Escanilla et al. (2010) (Figs. 3 and 4D).
Fig. 4.
Detection and discrimination of very low concentration stimuli in model. A–C: NE modulation was simulated by varying the spiking threshold of Gr cells and Mi cell depolarization according to the sigmoid functions shown in Fig. 2. Odors were modeled as very low concentration stimuli that activated ∼30% of the available OSN population to varying degrees but to maximally 10% of their possible response magnitude. Details of OSN and odor simulations can be found in Escanilla et al. (2010) and Mandairon et al. (2006b). A: magnitude of odor detection in model neurons as a function of NE modulation. Population responses of model neurons to a simulated complex odor were compared with spontaneous activity by calculating the normalized Euclidean distance between the firing rate activation patterns [see for modeling details Escanilla et al. (2010) and Mandairon et al. (2006b)]. Odor detection in the model varied in a nonmonotonic fashion, roughly replicating that of Mi cell responsiveness depicted in Fig. 2C. B: magnitude of odor discrimination in model neurons as a function of NE modulation in the model. The magnitude of discrimination was computed as the normalized Euclidean distance between the population responses to 2 highly similar odorants. Odor discrimination increased as a function of NE modulation at medium to high NE concentrations. C: effect of NE modulation on only Mi cells in the model. If only Mi cells are affected by NE in the model, odor detection increases at the expense of discrimination. D: synchronization index (number of synchronous spikes/total number of spikes during odor response) as a function of NE modulation.
The computational model also puts into evidence that activation of α1-receptors in mitral cells alone, although enhancing odor detection, would not increase discrimination (Fig. 4C). If noradrenergic depolarization of mitral cells is accompanied by increased inhibition from granule cells through NE, the net result is enhanced detection (by lowering mitral cell response threshold, as well as enhanced discrimination) by increasing inhibition and making weakly activated mitral cells silent. Although NE depolarization of mitral cells alone entrains granule cells and leads to increased inhibition of mitral cells, in the present model this is not sufficient to offset the NE depolarization of mitral cells to obtain good odor discrimination. When NE activates only one cell type, the system becomes unbalanced and the result is an enhancement of detection accompanied by low discrimination. The simultaneous activation of mitral and granule cells also leads to enhanced oscillations that increase signal-to noise ratio as well as spike synchronization among mitral cells (Fig. 3).
Behavioral Results
Olfactory perception and learning can be measured with a number of different paradigms in rodents, such as habituation, perceptual learning, discrimination learning, and social interactions. Because of the choice of paradigms, odors, concentration, and other variables, it is relatively difficult to directly compare results from different studies. It is therefore useful to consider these studies in light of the previously discussed cellular, synaptic, and network effects of noradrenergic modulation to interpret them within a common framework.
A study by Royet et al. (1983) showed that food-deprived rats with lesions of noradrenergic inputs to the olfactory system were more likely to eat novel foods than control rats. The authors concluded that NE modulates the bulbar response to food odors. A follow-up study showed that in rats with similar lesions, mitral cell responses to food odorants were enhanced (Gervais and Pager 1983). In light of the previously discussed cellular effects, mitral cell excitability could be increased because of missing noradrenergic excitation of granule cells, leading to higher excitability in mitral cells.
With respect to general odor detection, a recent study by Escanilla et al. (2010) showed that rats infused with additional NE into their olfactory bulb detected odorants at substantially lower concentrations than saline-infused control rats. Given that NE increases mitral cell excitability in vitro as well as in vivo and increases responses to weak olfactory nerve stimuli, these behavioral data support the idea that bulbar NE increases the sensitivity of the MOB to low-intensity stimuli. The behavioral NE effect was mainly mediated by α1-receptors in these studies: blockade of α1-receptors abolished the effect of NE infusions; these data are in agreement with the brain slice results discussed above (Nai et al. 2009, 2010).
Habituation, a commonly used paradigm to test olfactory perception and memory in rodents, has been reported to be affected by manipulations of the noradrenergic system. In a habituation paradigm, animals form a memory to an odorant to which they are repeatedly exposed. The duration and specificity of this memory can be assessed by presenting the same or novel odorants after memory formation. Guan et al. (1993b) reported that in mice in which noradrenergic inputs to the MOB were lesioned via 6-hydroxydopamine infusions, the discrimination of general odorants after habituation, i.e., the specificity of the habituation memory, was impaired. In contrast, discrimination between social cues was not affected: general lesions of LC neurons decreased investigatory responses to urinary odorants in mice but did not change the discrimination of different urinary cues (Guan et al. 1993a,b). This result was contradictory to a later study showing a general inability to form the habituation memory after DSP-4 lesions in mice, albeit in this study general odorants instead of urinary stimuli were used and NE depletion was not specific to the olfactory bulb (Guerin et al. 2008). In the latter study, habituation to an olfactory stimulus was restored in DSP-4-lesioned mice when NE was reintroduced bilaterally into the MOBs, strongly suggesting that bulbar NE is important for the formation of a habituation memory (Guerin et al. 2008). These data are supported by later electrophysiological data showing strong adaptation of mitral odor responses when odor stimulation was paired with stimulation of LC neurons (Shea et al. 2008).
In agreement with Guan et al., the role of bulbar NE in discrimination of odorants has also been shown in experiments by Mandairon et al. (2006a) in which bulbar NE receptors were specifically blocked; in these experiments the specificity of the formed habituation memory was decreased when α1- but not α2- or β-receptors were blocked in the MOB (Mandairon et al. 2008). Escanilla et al. (2010) support these data by showing an increase in odor discrimination at relatively low odor concentrations when NE is added to the MOB (Escanilla et al. 2010). In summary, all experiments using a habituation-dishabituation paradigm to test odor discrimination show that bulbar NE modulates discrimination after memory formation. Modulation of discrimination between perceptually similar odorants can be predicted from the combination of increased excitability and increased inhibition evidenced in brain slice experiments and put into context by computational modeling of these results. The results pertaining to the formation of the habituation memory are contradictory and may be due to differences in the behavioral paradigms used, specifically the time course of odor presentations, which has been shown to be modulated by NE (Veyrac et al. 2007).
In contrast to the spontaneous discrimination between odorants tested when a habituation task is used, discrimination in a rewarded forced-choice task was only affected when all NE receptors were blocked: similar results were obtained in two very different behavioral paradigms in mice (Doucette et al. 2007) and rats (Mandairon et al. 2008). The former study showed that in an automated successive go-no-go task, mice with all bulbar NE receptors blocked were significantly impaired in learning a discrimination between highly similar binary mixtures only. Discrimination of “easy” odor pairs was not affected in this experiment (Doucette et al. 2007). Similarly, in a naturalistic simultaneous forced-choice discrimination task, rats' ability to discriminate perceptually very similar odorants was impaired when all bulbar NE receptors were blocked but not when individual receptors were blocked (Mandairon et al. 2008).
In summary, current odor discrimination experiments in adult rodents support the idea that NE changes odor processing in the MOB in such a manner as to support specific odor representations and to facilitate the separation of representations with highly similar input patterns. At low odor concentrations and in a spontaneous discrimination task, α1-receptors seem to be mainly involved in this process, whereas in a reward-motivated task, all three receptor types interact. Figure 5 summarizes behavioral results that are in agreement with the computational results shown in Fig. 4. Odor detection and discrimination at very low stimulus concentrations (10–4 Pa) are graphed as a function of NE concentration infused into the OB during the behavioral task. Note that the magnitude of detection and discrimination is not a monotonic function of NE concentration, as would be predicted from the nonmonotonic effects on cellular parameters discussed above. An increase in discrimination of odorants with similar bulbar representations could be achieved by 1) increased granule cell excitability leading to inhibition of weakly activated mitral cells and 2) increased mitral and granule cell excitability leading to stronger feedback and, as a consequence, higher oscillatory power and synchronization between responsive mitral cells. This effect would be mainly through α1-receptors as described above. In a reward-driven discrimination task, the animals are highly motivated, and one can speculate that higher order structures are involved in processing of odor-reward associations. Blockade of all three receptor types is necessary to impair rats or mice in a reward-motivated task, suggesting that bulbar processing differs between spontaneous and reward-motivated tasks or that the downstream processing of bulbar information is different (Doucette et al. 2007; Mandairon et al. 2008). This apparent difference in the bulbar role across tasks has been observed in a number of experiments. For example, habituation memory was not affected by broad lesions of feedback to the MOB from central structures, whereas learning of a reward-motivated discrimination was (Kiselycznyk et al. 2006). In contrast, blockade of cholinergic receptors in the MOB, individual or global, only affected habituation memory but not discrimination learning in a reward-motivated task (Chaudhury et al. 2009; Linster et al. 2001; Mandairon et al. 2006a). The effects of different modulators may be a means to distinguish between the role the MOB plays in different types of behaviors relying on olfaction.
Fig. 5.
Summary of behavioral results. Behavioral results are reanalyzed from data previously published by Escanilla et al. (2010) and Mandairon et al. (2006b). A: rats with cannulas for drug infusions in both MOBs were tested on odor detection and discrimination using a habituation ask. Odors were presented in the home cage using a weighing dish with odor impregnated filter paper. B: rats were first habituated to the behavioral context by 3 successive presentations of mineral only (MO). Odor detection was then tested by presenting a novel odorant (O1; detection). After 3 habituation trials to this odorant, discrimination was tested by a single presentation of a second, chemically and perceptually similar odorant (O2). C and D: behavioral results. Rats were originally tested at a range of odor concentrations with vapor partial pressures varying from 10−2 to 10−6 Pa. Data presented pertain to the lowest concentration used only. Rats were infused with a range of NE concentrations as shown. C: odor detection magnitude at the lowest odor concentration tested (10−6 Pa) as a function of NE concentration. Detection magnitude is calculated as (O1 − H4)/(O1 + H4), where O1 is the first response to the novel odor and H4 is the response during the last habituation trial to mineral oil. This index varies between 0 (O1 = H4, no detection) and 1 (O1 >> H4, high detection) and is normalized with respect to individual differences in sniff times. Note that detection magnitude follows a nonmonotonic function of NE concentration, as would be predicted by the nonmonotonic dependency of cellular modulation by NE. D: odor discrimination magnitude at the lowest odor concentration tested (10−6 Pa) as a function of NE concentration. Discrimination magnitude is calculated as (O2 − O1)/(O2 + O1), where O2 is the response to the second odor and O1 is the response during the last trial with O1. The index varies between 0 (O2 = O1, no discrimination) and 1 (O2 >> O1, high discrimination) and is normalized with respect to individual sniff times.
Discussion
The olfactory system is an ideal model to study sensory modulation by NE inputs. First, the MOB has a dense NE innervation; second, it is accessible to experimentation at many levels; third, it is relatively segregated from other brain structures; and fourth, its inputs and outputs are well characterized. Brain slice experiments in combination with behavioral pharmacology and computational modeling show that it is the combination of NE effects on mitral and granule cells that creates the behaviorally observed modulation of odor detection and discrimination (Fig. 6). Activation of α1- and α2-receptors creates nonlinearities due to the relative affinities of these receptors. How these nonlinearities are behaviorally relevant needs to be further investigated. Effects of β-receptors have not yet been determined in behavioral studies, other than the fact that effects on rewarded odor discrimination tasks can only be observed when all receptor types are blocked. Further brain slice experiments elucidating NE's effects at β-receptors are needed to design specific behavioral experiments to test the role of this receptor in adult rodent odor processing. Thus far, results on the role of NE in early olfactory processing are in good agreement with results from other systems: a modulation of signal-to-noise ratio, detection of low-amplitude stimuli, and discrimination between similar stimuli.
Fig. 6.
Summary of NE effects in the OB. Activation of α1-receptors depolarizes Mi cells and increases spontaneous activity in Gr cell. The net result of these actions is an increase in excitability accompanied by an increase in oscillatory power. Behaviorally, this results in lower detection and discrimination thresholds.
In general, converging data from electrophysiological studies of cellular-membrane properties of MOB neurons and behavioral assays of perception reviewed presently indicate that NE biases the MOB network to improve odor detection and discrimination. Despite these advances, many questions about NE function in the MOB remain. The cellular effects of NE on other MOB neuronal subtypes, such as tufted cells and non-granule cell interneurons, as well as whether and how NE modulates centrifugal input from olfactory cortical structures, are unknown. Such studies will be critical to better understand how NE modifies the MOB network. In other systems, NE is known to alter network properties in a manner to sharpen stimulus representation. In visual cortex, NE enhances selectivity for stimulus speed and direction (McLean and Waterhouse 1994). In the cochlear nucleus and auditory cortex, NE refines receptive fields, increasing selectively for pure tones (Manunta and Edeline 1999, 2004). In the thalamus, NE refines whisker-receptive fields to the most strongly tuned or principal whisker (Hirata et al. 2006). Although cellular and behavioral data suggest that NE may impact similarly on mitral cell odor-receptive fields, recordings of odor responses during LC activation or NE infusion are necessary to evaluate this possibility.
GRANTS
This work was supported by National Institutes of Health Grant CRCNS DC008702 (to C. Linster and M. Ennis).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- Aston-Jones G, Bloom FE. Norepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci 1: 887–900, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Cohen J. Locus coeruleus and regulation of behavioral flexibility and attention. Prog Brain Res 126: 165–182, 2000 [DOI] [PubMed] [Google Scholar]
- Aungst JL, Heyward PM, Puche AC, Karnup SV, Hayar A, Szabo G, Shipley MT. Centre-surround inhibition among olfactory bulb glomeruli. Nature 426: 623–629, 2003 [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev 42: 33–84, 2003 [DOI] [PubMed] [Google Scholar]
- Beshel J, Kopell N, Kay LM. Olfactory bulb gamma oscillations are enhanced with task demands. J Neurosci 27: 8358–8365, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickford-Wimer P, Pang K, Rose GM, Gerhardt GA. Electrically-evoked release of norepinephrine in the rat cerebellum: an in vivo electrochemical and electrophysiological study. Brain Res 558: 305–311, 1991 [DOI] [PubMed] [Google Scholar]
- Bouret S, Sara SJ. Locus coeruleus activation modulates firing rate and temporal organization of odour-induced single-cell responses in rat piriform cortex. Eur J Neurosci 16: 2371–2382, 2002 [DOI] [PubMed] [Google Scholar]
- Brea JN, Kay LM, Kopell NJ. Biophysical model for gamma rhythms in the olfactory bulb via subthreshold oscillations. Proc Natl Acad Sci USA 106: 21954–21959, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan P, Kaba H, Keverne EB. Olfactory recognition: a simple memory system. Science 250: 1223–1226, 1990 [DOI] [PubMed] [Google Scholar]
- Chaudhury D, Escanilla O, Linster C. Bulbar acetylcholine enhances neural and perceptual odor discrimination. J Neurosci 29: 52–60, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciombor KJ, Ennis M, Shipley MT. Norepinephrine increases rat mitral cell excitatory responses to weak olfactory nerve input via alpha-1 receptors in vitro. Neuroscience 90: 595–606, 1999 [DOI] [PubMed] [Google Scholar]
- Cleland TA, Johnson BA, Leon M, Linster C. Relational representation in the olfactory system. Proc Natl Acad Sci USA 104: 1953–1958, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland TA, Linster C. Central olfactory processing. In: Handbook of Olfaction and Gustation (2nd ed.), edited by Doty RL. New York: Marcel Dekker, 2003, p. 165–180 [Google Scholar]
- Cleland TA, Sethupathy P. Non-topographical contrast enhancement in the olfactory bulb. BMC Neurosci 7: 7, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devilbiss DM, Waterhouse BD. The effects of tonic locus ceruleus output on sensory-evoked responses of ventral posterior medial thalamic and barrel field cortical neurons in the awake rat. J Neurosci 24: 10773–10785, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devilbiss DM, Waterhouse BD. Norepinephrine exhibits two distinct profiles of action on sensory cortical neuron responses to excitatory synaptic stimuli. Synapse 37: 273–282, 2000 [DOI] [PubMed] [Google Scholar]
- Di Prisco GV, Freeman WJ. Odor-related bulbar EEG spatial pattern analysis during appetitive conditioning in rabbits. Behav Neurosci 99: 964–978, 1985 [DOI] [PubMed] [Google Scholar]
- Doucette W, Milder J, Restrepo D. Adrenergic modulation of olfactory bulb circuitry affects odor discrimination. Learn Mem 14: 539–547, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis M, Hayar A. Physiology of the olfactory bulb. In: The Senses: a Comprehensive Reference, edited by Basbaum A, Kaneko A, Shepherd G. San Diego, CA: Academic Press, 2008, vol. 4, p. 641–686 [Google Scholar]
- Escanilla O, Arrellanos A, Karnow A, Ennis M, Linster C. Noradrenergic modulation of behavioral odor detection and discrimination thresholds in the olfactory bulb. Eur J Neurosci 32: 458–468, 2010 [DOI] [PubMed] [Google Scholar]
- El-Etri MM, Griff ER, Ennis M, Shipley MT. Evidence for cholinergic regulation of basal norepinephrine release in the rat olfactory bulb. Neuroscience 93: 611–617, 1999 [DOI] [PubMed] [Google Scholar]
- Foote SL, Aston-Jones G, Bloom FE. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc Natl Acad Sci USA 77: 3033–3037, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote SL, Bloom FE, Aston-Jones G. Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol Rev 63: 844–914, 1983 [DOI] [PubMed] [Google Scholar]
- Gervais R, Pager J. Olfactory bulb excitability selectively modified in behaving rats after local 6-hydroxydopamine treatment. Behav Brain Res 9: 165–179, 1983 [DOI] [PubMed] [Google Scholar]
- Gire DH, Schoppa NE. Long-term enhancement of synchronized oscillations by adrenergic receptor activation in the olfactory bulb. J Neurophysiol 99: 2021–2025, 2008 [DOI] [PubMed] [Google Scholar]
- Gray CM, Freeman WJ, Skinner JE. Chemical dependencies of learning in the rabbit olfactory bulb: acquisition of the transient spatial pattern change depends on norepinephrine. Behav Neurosci 100: 585–596, 1986 [DOI] [PubMed] [Google Scholar]
- Guan X, Blank J, Dluzen D. Depletion of olfactory bulb norepinephrine by 6-OHDA disrupts chemical cue but not social recognition responses in male rats. Brain Res 622: 51–57, 1993a [DOI] [PubMed] [Google Scholar]
- Guan X, Blank JL, Dluzen DE. Role of olfactory bulb norepinephrine in the identification and recognition of chemical cues. Physiol Behav 53: 437–441, 1993b [DOI] [PubMed] [Google Scholar]
- Guerin D, Peace ST, Didier A, Linster C, Cleland TA. Noradrenergic neuromodulation in the olfactory bulb modulates odor habituation and spontaneous discrimination. Behav Neurosci 122: 816–826, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Linster C, Patil M, Ma D, Cekic M. Noradrenergic suppression of synaptic transmission may influence cortical signal-to-noise ratio. J Neurophysiol 77: 3326–3339, 1997 [DOI] [PubMed] [Google Scholar]
- Hayar A, Heyward PM, Heinbockel T, Shipley MT, Ennis M. Direct excitation of mitral cells via activation of alpha1-noradrenergic receptors in rat olfactory bulb slices. J Neurophysiol 86: 2173–2182, 2001 [DOI] [PubMed] [Google Scholar]
- Hirata A, Aguilar J, Castro-Alamancos MA. Noradrenergic activation amplifies bottom-up and top-down signal-to-noise ratios in sensory thalamus. J Neurosci 26: 4426–4436, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr CE, Nicoll RA. Noradrenergic modulation of dendrodendritic inhibition in the olfactory bulb. Nature 297: 227–229, 1982 [DOI] [PubMed] [Google Scholar]
- Jiang M, Griff ER, Ennis M, Zimmer LA, Shipley MT. Activation of locus coeruleus enhances the responses of olfactory bulb mitral cells to weak olfactory nerve input. J Neurosci 16: 6319–6329, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaba H, Keverne EB. The effect of microinfusions of drugs into the accessory olfactory bulb on the olfactory block to pregnancy. Neuroscience 25: 1007–1011, 1988 [DOI] [PubMed] [Google Scholar]
- Kaba H, Rosser A, Keverne B. Neural basis of olfactory memory in the context of pregnancy block. Neuroscience 32: 657–662, 1989 [DOI] [PubMed] [Google Scholar]
- Kay LM, Beshel J, Brea J, Martin C, Rojas-Libano D, Kopell N. Olfactory oscillations: the what, how and what for. Trends Neurosci 32: 207–214, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselycznyk CL, Zhang S, Linster C. Role of centrifugal projections to the olfactory bulb in olfactory processing. Learn Mem 13: 575–579, 2006 [DOI] [PubMed] [Google Scholar]
- Kossl M, Vater M. Noradrenaline enhances temporal auditory contrast and neuronal timing precision in the cochlear nucleus of the mustached bat. J Neurosci 9: 4169–4178, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecas JC. Locus coeruleus activation shortens synaptic drive while decreasing spike latency and jitter in sensorimotor cortex. Implications for neuronal integration. Eur J Neurosci 19: 2519–2530, 2004 [DOI] [PubMed] [Google Scholar]
- Linster C, Cleland TA. Cholinergic modulation of sensory representations in the olfactory bulb. Neural Netw 15: 709–717, 2002 [DOI] [PubMed] [Google Scholar]
- Linster C, Garcia PA, Hasselmo ME, Baxter MG. Selective loss of cholinergic neurons projecting to the olfactory system increases perceptual generalization between similar, but not dissimilar, odorants. Behav Neurosci 115: 826–833, 2001 [DOI] [PubMed] [Google Scholar]
- Linster C, Gervais R. Investigation of the role of interneurons and their modulation by centrifugal fibers in a neural model of the olfactory bulb. J Comput Neurosci 3: 225–246, 1996 [DOI] [PubMed] [Google Scholar]
- Linster C, Hasselmo M. Modulation of inhibition in a model of olfactory bulb reduces overlap in the neural representation of olfactory stimuli. Behav Brain Res 84: 117–127, 1997 [DOI] [PubMed] [Google Scholar]
- Mandairon N, Ferretti CJ, Stack CM, Rubin DB, Cleland TA, Linster C. Cholinergic modulation in the olfactory bulb influences spontaneous olfactory discrimination in adult rats. Eur J Neurosci 24: 3234–3244, 2006a [DOI] [PubMed] [Google Scholar]
- Mandairon N, Linster C. Odor perception and olfactory bulb plasticity in adult mammals. J Neurophysiol 101: 2204–2209, 2009 [DOI] [PubMed] [Google Scholar]
- Mandairon N, Peace S, Karnow A, Kim J, Ennis M, Linster C. Noradrenergic modulation in the olfactory bulb influences spontaneous and reward-motivated discrimination, but not the formation of habituation memory. Eur J Neurosci 27: 1210–1219, 2008 [DOI] [PubMed] [Google Scholar]
- Mandairon N, Stack C, Kiselycznyk C, Linster C. Broad activation of the olfactory bulb produces long-lasting changes in odor perception. Proc Natl Acad Sci USA 103: 13543–13548, 2006b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manunta Y, Edeline JM. Effects of noradrenaline on frequency tuning of auditory cortex neurons during wakefulness and slow-wave sleep. Eur J Neurosci 11: 2134–2150, 1999 [DOI] [PubMed] [Google Scholar]
- Manunta Y, Edeline JM. Noradrenergic induction of selective plasticity in the frequency tuning of auditory cortex neurons. J Neurophysiol 92: 1445–1463, 2004 [DOI] [PubMed] [Google Scholar]
- McLean J, Waterhouse BD. Noradrenergic modulation of cat area 17 neuronal responses to moving visual stimuli. Brain Res 667: 83–97, 1994 [DOI] [PubMed] [Google Scholar]
- McLean JH, Shipley MT, Nickell WT, Aston-Jones G, Reyher CK. Chemoanatomical organization of the noradrenergic input from locus coeruleus to the olfactory bulb of the adult rat. J Comp Neurol 285: 339–349, 1989 [DOI] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Corticosterone influences on Mammalian neonatal sensitive-period learning. Behav Neurosci 118: 274–281, 2004a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Unique neural circuitry for neonatal olfactory learning. J Neurosci 24: 1182–1189, 2004b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouly AM, Elaagouby A, Ravel N. A study of the effects of noradrenaline in the rat olfactory bulb using evoked field potential response. Brain Res 681: 47–57, 1995 [DOI] [PubMed] [Google Scholar]
- Mouradian RD, Sessler FM, Waterhouse BD. Noradrenergic potentiation of excitatory transmitter action in cerebrocortical slices: evidence for mediation by an alpha 1 receptor-linked second messenger pathway. Brain Res 546: 83–95, 1991 [DOI] [PubMed] [Google Scholar]
- Moxon KA, Devilbiss DM, Chapin JK, Waterhouse BD. Influence of norepinephrine on somatosensory neuronal responses in the rat thalamus: a combined modeling and in vivo multi-channel, multi-neuron recording study. Brain Res 1147: 105–123, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nai Q, Dong HW, Hayar A, Linster C, Ennis M. Noradrenergic regulation of GABAergic inhibition of main olfactory bulb mitral cells varies as a function of concentration and receptor subtype. J Neurophysiol 101: 2472–2484, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nai Q, Dong HW, Linster C, Ennis M. Activation of alpha1 and alpha2 noradrenergic receptors exert opposing effects on excitability of main olfactory bulb granule cells. Neuroscience 169: 882–892, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Kay LM, Laurent G, Homanics GE, Mody I. Disruption of GABAA receptors on GABAergic interneurons leads to increased oscillatory power in the olfactory bulb network. J Neurophysiol 86: 2823–2833, 2001 [DOI] [PubMed] [Google Scholar]
- Okutani F, Kaba H, Takahashi S, Seto K. The biphasic effect of locus coeruleus noradrenergic activation on dendrodendritic inhibition in the rat olfactory bulb. Brain Res 783: 272–279, 1998 [DOI] [PubMed] [Google Scholar]
- Pandipati S, Gire DH, Schoppa NE. Adrenergic receptor-mediated disinhibition of mitral cells triggers long-term enhancement of synchronized oscillations in the olfactory bulb. J Neurophysiol 104: 665–674, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez H, Hernandez A, Almli CR. Locus coeruleus stimulation modulates olfactory bulb evoked potentials. Brain Res Bull 18: 767–770, 1987 [DOI] [PubMed] [Google Scholar]
- Ramos BP, Arnsten AF. Adrenergic pharmacology and cognition: focus on the prefrontal cortex. Pharmacol Ther 113: 523–536, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royet JP, Gervais R, Araneda S. Effect of local 6-OHDA and 5,6-DHT injections into the rat olfactory bulb on neophobia and learned aversion to a novel food. Behav Brain Res 10: 297–309, 1983 [DOI] [PubMed] [Google Scholar]
- Shea SD, Katz LC, Mooney R. Noradrenergic induction of odor-specific neural habituation and olfactory memories. J Neurosci 28: 10711–10719, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley MT, Halloran FJ, de la Torre J. Surprisingly rich projection from locus coeruleus to the olfactory bulb in the rat. Brain Res 329: 294–299, 1985 [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA. Molecular biology of early olfactory memory. Learn Mem 10: 1–4, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombley PQ. Norepinephrine inhibits calcium currents and EPSPs via a G-protein-coupled mechanism in olfactory bulb neurons. J Neurosci 12: 3992–3998, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombley PQ, Shepherd GM. Noradrenergic inhibition of synaptic transmission between mitral and granule cells in mammalian olfactory bulb cultures. J Neurosci 12: 3985–3991, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban NN. Lateral inhibition in the olfactory bulb and in olfaction. Physiol Behav 77: 607–612, 2002 [DOI] [PubMed] [Google Scholar]
- Urban NN, Arevian AC. Computing with dendrodendritic synapses in the olfactory bulb. Ann NY Acad Sci 1170: 264–269, 2009 [DOI] [PubMed] [Google Scholar]
- Vankov A, Herve-Minvielle A, Sara SJ. Response to novelty and its rapid habituation in locus coeruleus neurons of the freely exploring rat. Eur J Neurosci 7: 1180–1187, 1995 [DOI] [PubMed] [Google Scholar]
- Veyrac A, Nguyen V, Marien M, Didier A, Jourdan F. Noradrenergic control of odor recognition in a nonassociative olfactory learning task in the mouse. Learn Mem 14: 847–854, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse BD, Azizi SA, Burne RA, Woodward DJ. Modulation of rat cortical area 17 neuronal responses to moving visual stimuli during norepinephrine and serotonin microiontophoresis. Brain Res 514: 276–292, 1990 [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Mouradian R, Sessler FM, Lin RC. Differential modulatory effects of norepinephrine on synaptically driven responses of layer V barrel field cortical neurons. Brain Res 868: 39–47, 2000 [DOI] [PubMed] [Google Scholar]
- Winzer-Serhan UH, Raymon HK, Broide RS, Chen Y, Leslie FM. Expression of α2 adrenoreceptors during rat brain development-I α2A messenger RNA expression. Neuroscience 76: 241–260, 1997 [DOI] [PubMed] [Google Scholar]
- Woo CC, Leon M. Distribution and development of beta-adrenergic receptors in the rat olfactory bulb. J Comp Neurol 352: 1–10, 1995 [DOI] [PubMed] [Google Scholar]
- Yokoi M, Mori K, Nakanishi S. Refinement of odor molecule tuning by dendrodendritic synaptic inhibition in the olfactory bulb. Proc Natl Acad Sci USA 92: 3371–3375, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]