Abstract
Perception of the temporal structure of acoustic signals contributes critically to vocal signaling. In the aquatic clawed frog Xenopus laevis, calls differ primarily in the temporal parameter of click rate, which conveys sexual identity and reproductive state. We show here that an ensemble of auditory neurons in the laminar nucleus of the torus semicircularis (TS) of X. laevis specializes in encoding vocalization click rates. We recorded single TS units while pure tones, natural calls, and synthetic clicks were presented directly to the tympanum via a vibration-stimulation probe. Synthesized click rates ranged from 4 to 50 Hz, the rate at which the clicks begin to overlap. Frequency selectivity and temporal processing were characterized using response-intensity curves, temporal-discharge patterns, and autocorrelations of reduplicated responses to click trains. Characteristic frequencies ranged from 140 to 3,250 Hz, with minimum thresholds of −90 dB re 1 mm/s at 500 Hz and −76 dB at 1,100 Hz near the dominant frequency of female clicks. Unlike units in the auditory nerve and dorsal medullary nucleus, most toral units respond selectively to the behaviorally relevant temporal feature of the rate of clicks in calls. The majority of neurons (85%) were selective for click rates, and this selectivity remained unchanged over sound levels 10 to 20 dB above threshold. Selective neurons give phasic, tonic, or adapting responses to tone bursts and click trains. Some algorithms that could compute temporally selective receptive fields are described.
Keywords: advertisement calls, animal communication, anura, inferior colliculus, temporal processing
the communicative value of social signals can be conveyed by a number of different physical properties. Anurans, a group specializing in vocal signaling, can use temporal parameters, alongside spectral ones, to discriminate between calls (Capranica 1965; Gerhardt 1988). Temporal features that are important as cues include pulse-repetition rate (Blair 1964; Gerhardt 1978; Loftus-Hills and Littlejohn 1971), number of pulses per call (Fouquette 1975), individual pulse duration (Narins and Capranica 1978), envelope rise time (Gerhardt and Doherty 1988), and bout rate (Pauly et al. 2006).
One temporal feature in particular, click rate, plays a primary role for the aquatic anuran Xenopus laevis (X. laevis) (Daudin 1802). Xenopus is adapted to underwater hearing in shallow-water ponds (Christensen-Dalsgaard and Elliott 2008), and the special acoustical characteristics of this habitat, such as multiple reflecting surfaces, may constrain the temporal processing of communication signals. Calls are made up of trains of clicks, and each call type of males and females is distinguished by characteristic click rates (Kelley and Tobias 1999). Behavioral studies of acoustically evoked calling in males have shown that click rate suffices as a female sex recognition cue (Vignal and Kelley 2007), and for discrimination of the two female calls, ticking and rapping (Elliott and Kelley 2007). We wish to determine at which stage of the auditory pathway selectivity in the time domain arises and to uncover the kinds of algorithms that compute temporally selective receptive fields.
In a systematic approach, we have shown first that auditory units, both in the periphery and in the hindbrain, provide an accurate representation of click rate by means of neural-discharge patterns that temporally couple to the pattern of clicks in the sound waveform, without any temporal selectivity (Elliott et al. 2007). Here we extend our investigation to the torus semicircularis (TS), the midbrain target of medullary auditory neurons, and characterize basic frequency and intensity representations, along with the selectivity for recurrence rates of natural or synthetic clicks. Selectivity for the rates of repeated clicks could underlie social behaviors that require discrimination between conspecific calls differing primarily in click rate. The auditory midbrain is an especially attractive target for research because neurons in the torus show specializations for calculating relative time variables (Edwards et al. 2002; Rose and Gooler 2006).
Temporal features of sounds are important not only for animal communication but also for human speech and for music appreciation. Investigation of neural response properties in an animal model in which temporal cues are paramount, such as X. laevis, may lead to further understanding of how neural circuitry extracts time information from repetitive sound signals and uses it to inform behavioral decisions.
MATERIALS AND METHODS
Animals
Nine male and one female adult X. laevis were obtained either commercially (Nasco, Fort Atkinson, WI; Xenopus One, Ann Arbor, MI) or from the August Krogh Institute of the University of Copenhagen in Denmark. Animal care and use for this study was approved both by the Institutional Animal Care and Use Committee at Columbia University (AC-AAAA1401) and the Danish Animal Experimentation Board (Dyreforsøgstilsynet). The experiments are legal in both the United States and Denmark, and comply with the Principles of Animal Care, publication no. 86–23, of the National Institute of Health.
Recordings
Frogs were anesthetized by a subcutaneous injection of tricaine methanesulphonate (MS-222, 2.6%, 0.5–1.0 ml; Sigma-Aldrich, Brøndby, Denmark) and placed on ice. A flap of skin was folded to expose the tympanum and the top of the head, and the skull was thinned with a drill dorsal to the midbrain. The hole was then plugged with a gelatin sponge overnight (Gelfoam; Pharmacia & Upjohn, Kalamazoo, MI). Frogs usually recovered from surgery in 3 h, after which we applied lidocaine topically (5%, DD427; AstraZeneca, Albertslund, Denmark). The following day we again applied lidocaine, immobilized the frog (with d-turbocurarine chloride, 12 μg/g body wt; Carl Roth, Karlsruhe, Germany), took out the sponge, and removed the dura from the midbrain.
Recording sessions lasted up to 12 h. Neural responses were isolated using an epoxy-insulated tungsten microelectrode (127 μm, 6 MΩ; A-M Systems, Carlsborg, WA) positioned by a piezo manipulator, amplified (3000 extracellular amplifier, A-M Systems) and recorded as previously described (Elliott et al. 2007). Units were isolated by aural and visual monitoring of the recording trace through a loudspeaker and a digital storage oscilloscope (PM3370 Fluke). Spike traces were saved for later sorting of possible multiunit activity, but in practice success at single-unit isolation precluded inspection of the spike-amplitude files. Spike events were identified from the single-unit recordings as threshold crossings (Tucker-Davis Technologies SD1, Alachua, FL), and the crossings were logged with microsecond accuracy (Tucker-Davis Technologies ET1).
Stimuli and Experimental Procedure
To avoid distortions of underwater sound presentations in the confines of a small space, the tympanum was vibrated using a calibrated vibration-exciter probe attached directly to the cartilaginous tympanic disk (details in Elliott et al. 2007) because the large plate-like vibrations of the tympanic disk during underwater sound broadcast have been previously measured (Christensen-Dalsgaard and Elepfandt 1995). This vibration stimulation corresponds to closed-coupler stimulation in terrestrial frogs. Neither this method nor direct vibration entirely reproduces natural stimulation because free-field sound may enter the inner ear through extratympanic pathways (Christensen-Dalsgaard 2005). Extratympanic sensitivity has only been measured in terrestrial frog species as sensitivity to low-frequency sounds (<400 Hz). However, both in terrestrial frogs and in Xenopus, tympanic stimulation produces excellent low-frequency sensitivity (Elliott et al. 2007; Wilczynski et al. 1987), so low-frequency sensitivity is not underestimated by closed-coupler stimulation or direct vibration. Furthermore, in the special habitat of Xenopus (shallow water), low frequencies do not transmit well, and, therefore, the high-frequency tympanic sensitivity is probably most important for sound communication (dominant frequencies of all call types are above 1 kHz). Stimuli consisted of pure tone bursts, synthetic click trains, and recorded calls. The digital sampling rate of synthetic and recorded stimuli was 22 kHz.
Synthetic click trains and pure tones.
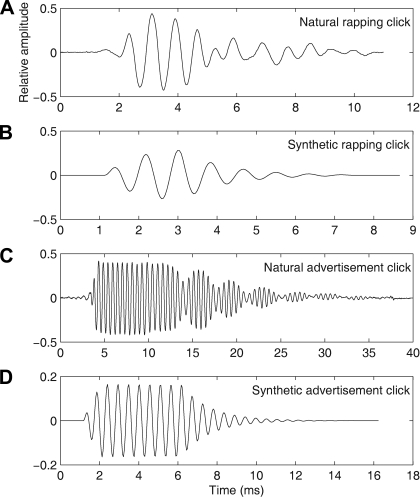
To vary pulse rates in the calls systematically, we used trains of synthetic clicks that differed in interclick intervals (ICIs) and resembled actual recorded male and female clicks in rise time, decay, and spectral representation (Fig. 1). The synthetic male and female clicks we used are effective at stimulating male advertisement calling when presented at appropriate click rates (Vignal and Kelley 2007). The synthetic click designed to resemble clicks in recorded female rapping had a fundamental frequency of 1,200 Hz, a rise time of 1.95 ms, and a fall time of 3.53 ms, with exponential time constants krise = −0.00143 s−1 and kfall = −0.000898 s−1, respectively (Fig. 1B). The synthetic female ticking click also had a fundamental frequency of 1,200 Hz but a rise time of 0.7709 ms and a fall time of 4.72 ms, with exponential time constants krise = −0.0026 s−1 and kfall = −0.0008496 s−1. The synthetic male click had a fundamental frequency of 1,850 Hz, a rise time of 1 ms, a plateau of 4 ms, and a fall of 7 ms (Fig. 1D). Because of our interest in male responses to female calls and because there were minimal differences in response thresholds to the three kinds of clicks, synthetic female rapping clicks were presented first to most cells. We also included for analysis six cells (out of 139) in which responses to synthetic male clicks were the only good-quality recordings.
Fig. 1.
Oscillograms of 370 ms recorded natural clicks together with their synthetic click counterparts used in synthetic-click-train stimuli. A: natural female rapping click. B: synthetic female rapping click. C: natural click from the slow trill of the male advertisement call. D: synthetic version of the male advertisement click.
Depending on the experiment, the duration of tone pulses was 50–150 ms and the rise time was 10 ms. Frequency and synthetic click rates were generated on a digital signal-processing board (Tucker-Davis Technologies System 2, clock rate 22 kHz) controlled by a custom-made PC program (FrogMasterTV) and passed through digital attenuators (PA4, Tucker-Davis; and type 2125, Hatfield Instruments, Hatfield, PA) and a custom-built 10× amplifier.
Recorded calls.
Figure 1 shows example recorded and synthetic female (Fig. 1, A and B) and male clicks (Fig. 1, C and D) that we used as stimuli. Digitized underwater recordings of X. laevis calls were obtained from the field (Tobias et al. 1998) and from laboratory tanks using a Cornell Bioacoustics Program hydrophone [Ithaca, NY; output sensitivity −163 dB (SD 3) at 1 V/μPa, frequency sensitivity 0.015–10.000 kHz] and a Marantz cassette tape recorder (PMD430) and digitally sampled at 22.05 kHz, 16 bit. Small segments (370 ms) of these calls were stored in a “call library” so that all cells could be tested with identical call segments (Elliott et al. 2007). Call signals were normalized in amplitude so that the peak energy of the unattenuated signal was 0 dB re 1 mm/s. All stimuli were presented through the A/D converter (AD2, Tucker-Davis Technologies), filtered, digitally attenuated, and finally amplified as described for the synthetic stimuli above.
INITIAL CHARACTERIZATION OF NEURONS.
Search stimuli consisted of a frequency sweep (60-3,200 Hz) interleaved with a series of clicks at rates from 4–50 Hz. A custom-made frequency-tuning curve (FTC) program determined the spontaneous rate, minimum threshold, and characteristic frequency (CF) of single units in response to 50-ms tone bursts.
Rate-intensity curves and poststimulus time histograms (PSTHs) were obtained from responses to a series of 100-ms tones (rise-fall time 10 ms, 8 levels in 4-dB steps) as described in Elliott et al. (2007). Response area plots consisted of spike counts recorded during systematic variation in the frequency and level of 50-ms tone bursts, presented in pseudorandom order (Elliott et al. 2007).
CLICK-RATE SPIKE-RATE EXPERIMENTS.
Eight synthetic click trains were presented with increasingly fast click rates: (in Hz) 4, 7, 10, 14, 19, 24, either 30 or 35, and either 2 or 50. (We could not present synthetic clicks as fast as the fast trill in the male advertisement call segment because at that rate male synthetic clicks would overlap as they do in recorded calls.) At the beginning of each experiment, click-train duration was set to a constant value, 1,000–1,200 ms. Vibration velocities were set at 20 dB above threshold, then at 10 dB and at other levels as possible, with 10 repetitions of each click-rate series at each level setting. As explained above, experiments selected for analysis used female rapping clicks except for 6 out of 139 cells.
Statistical Analysis
Data analysis used custom-made software (RetrieveMaster) and a vector-processing programming toolbox (OptiVec; Martin Sander, Schriesheim, Germany).
CF was determined as the frequency at which minimum threshold was measured by an adaptive staircase threshold-tracking technique as previously described (Elliott et al. 2007). Response threshold was determined at a 5% increase over spontaneous rate by fitting (using least squares) a cumulative normal distribution to the spike-rate stimulus-response curve measured from the rate-intensity experiments, as previously described (Elliott et al. 2007; Kanneworff 2004). Dynamic range was calculated as the sound-level range one standard deviation around mean spike rate on the fitted rate-intensity curve. Median latency of the first spike after stimulus onset was estimated by least-squares fit to an exponential decay model, as described previously (Elliott et al. 2007; Imaizumi and Pollack 2001; Kanneworff 2004).
The phasic or tonic nature of the response to pure tones was quantified by the index (S1 − S2)/(S1 + S2) where S1 is the spike count in the first 40 ms after tone onset, and S2 is the steady-state spike count in the last 40 ms of the tone (Hall and Feng 1988; Megela and Capranica 1981). Using this index, 1 represents a phasic response with spiking in only the initial 40 ms; 0 represents an ideally tonic response; and −1 represents a response in the last 40 ms but none in the first.
Measurement of response selectivity.
Cells that did not show selectivity up to 50 Hz, the upper limit of click rate that our stimuli could include because of click duration, are referred to here as nonselective.
CALCULATION OF DISCHARGE RATE.
To gauge differences in firing rate in response to stimuli with different click rates, we counted the total spikes during the 1,460-ms duration of the record and divided by the number of clicks in the train to obtain spikes per click.
COINCIDENCE RATE CALCULATED FROM SHUFFLED RESPONSES.
We quantified preferences of a neuron for stimuli with different click rates by calculating a spike coincidence rate (CR) between the responses of the neuron to repeated stimulus presentations, to account for both changes in spike rate per click and in spike timing.
Briefly, for each unit, we counted the number of coincident spikes within 1-ms time bins between 10 spike trains from the 10 presentations of each synthetic click stimulus, which yielded 45 pairs of nonidentical spike trains. The “shuffling” underlying this measure is performed between spike trains from a single neuron in response to a repeated stimulus, rather than comparisons of the same response signal with itself. To emphasize the temporal pattern of firing, we controlled for the overall discharge count by scaling the ordinate of the CR to make the bin values independent of number of presentation (N = 10) pairs, choice of bin width (Δτ = 1 ms), and the stimulus duration T [specifically, we divided by N (N−1)ΔτT/2]. The resulting CR has dimensionless units. Bin widths of 1 ms appeared to reveal relevant periodicities.
The CR is more appropriate than firing rate as a selectivity measure because responses to stimuli with different dynamics such as different click rates are expected to differ in overall spike rate. Using spike rates alone as an indicator of selectivity could mask selection by means of local spike-rate codes, such as time-varying spike-rate codes or other correlation codes (Dayan and Abbott 2001; Shadlen 2002). Because of its temporal sensitivity, an analysis relying on the CR quantifies rate selectivity more completely than an analysis of spike rate alone can do. The CR gauges not only the overall time course of spike rate but the reproducibility of spikes at precise times (Joris et al. 2006; Louage et al. 2004).
To compare CRs of responses of toral units to recorded calls with those of units previously recorded in the auditory nerve (Elliott et al. 2007), we used a repeated-measures ANOVA at the P < 0.05 level and made post hoc comparisons with a Bonferroni/Dunn correction.
Histology
We attempted to record auditory-evoked responses throughout the posterior, dorsal midbrain, a region that includes the three nuclei of the TS, namely the laminar nucleus and the principal nucleus dorsomedially, and the magnocellular nucleus ventrolaterally (Edwards and Kelley 2001). Recordings proceeded systematically, starting at the midline and extending to within 100 μm of the lateral edge of the tissue. When the last cell was recorded along the final penetration in 6 of the 10 animals, a lesion was made by passing current (1–10 s, 100 μA). Animals were overdosed with MS-222 (subcutaneous, 2.6%, 2 ml; Sigma-Aldrich) and transcardially perfused with artificial frog cerebrospinal fluid (20 ml) (Luksch et al. 1996) followed by 4% paraformaldehyde. The brain was removed and cryoprotected in a solution of 10% sucrose in 4% paraformaldehyde until it sank. It was frozen in embedding medium (Tissue-tek 4583) and sectioned horizontally at 30 μm on a cryostat (5030 Microtome; Bright Instrument, Huntingdon, England, supplied by Hacker Instruments, Fairfield, NJ). After dehydration, sections were stained with cresyl violet to facilitate brain nuclei identification and coverslipped with cytoseal (VWR, West Chester, PA).
RESULTS
Overview
We examined the preferences of TS units for click rates (in synthetic click trains or recorded natural-call stimuli) using a coincidence analysis of spike times and rate in responses of a single unit to a repeated stimulus (the CR described in materials and methods; Joris et al. 2006). Most neurons were selectively responsive to click rate; their CRs were maximal for certain click rates within the range of those we presented. For neurons without preferences in this range of stimuli (nonselective cells), CRs increased as the number of clicks in the stimulus rose. These nonselective responses resemble those previously recorded in the auditory nerve (n.VIII) and the dorsal medullary nucleus (DMN), and we thus compare basic features of auditory responsiveness in the TS to those in the nerve and the medulla.
Anatomical Location of Electrophysiological Recording Sites
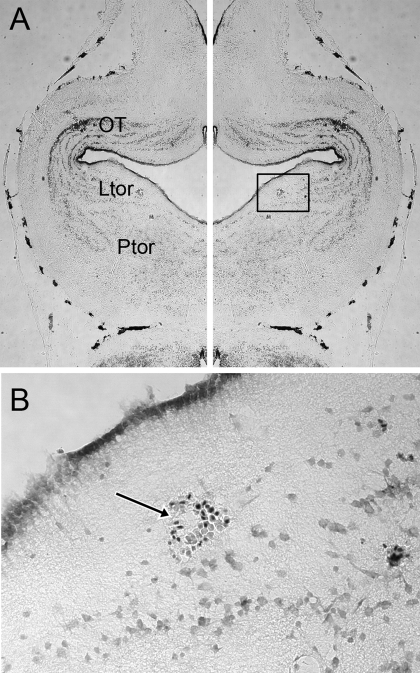
Direct-current lesions at the end of our experimental recordings in the six brains prepared for histology revealed that all recording sites with auditory responses lay within the laminar nucleus of the TS, (Ltor, Fig. 2). There were no auditory responses recorded in either the principal or the magnocellular nucleus. In X. laevis, the laminar nucleus consists of three sheets of cells extending from dorsal to ventral, just caudal to the tectal ventricle. The laminar nucleus extends laterally from the caudal margin of the tectum to the midline (Edwards and Kelley 2001). Our recording tracks passed largely within the plane of the nucleus and terminated in the laminar nucleus. Three lesion sites were in lateral Ltor, one was medial, and two were midlateral.
Fig. 2.
A: cresyl-violet-stained horizontal section through a brain that was lesioned by direct current at the end of the final ventral-going electrode track, marking the recording site of a cell nonselective for click rate. The left half of the figure is a reverse of the right, for the purposes of nuclei identification. Penetration was perpendicular to the plane of section. Ltor, laminar nucleus of the torus semicircularis (TS); OT, optic tectum; Ptor, principal nucleus of the torus semicircularis. B: enlargement of the box in A showing red blood cells that mark the lesion location in the laminar nucleus of the TS.
Synthetic Call Filtering
To determine how TS cells process repeated clicks like those in conspecific calls, a battery of synthetic click trains at eight click rates, from 4 to 50 Hz, was presented to 139 cells. Response patterns fell into two broad categories: rate selective and non-rate selective. Rate selectivity was evident either as changes in spikes per click over the course of the click train (e.g., failing to respond after later clicks in trains with click rates faster than some cutoff rate) or by the unreliability with which spikes coupled to clicks (e.g., when spike times were more irregular and irreproducible during faster click trains). Both manifestations of selectivity are captured by the CR, a measure of both spike number and the consistency in spike timing between reduplicated responses, as explained below. In 122 of 125 rate-selective cells, evaluations of selectivity based on spikes per click and those based on CR were in good agreement; responses to a click rate other than the fastest presented, 50 Hz (i.e., one between 4 and 35 Hz), resulted in the maximum CR.
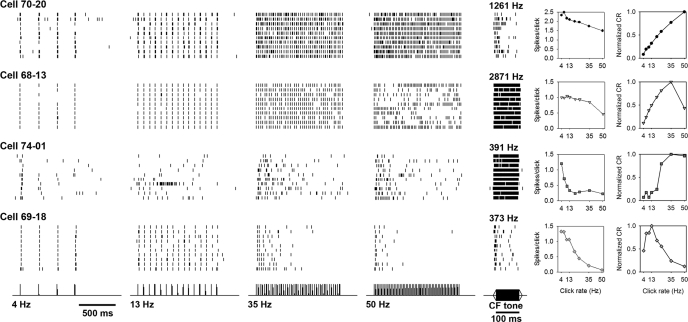
Rate-selective neurons were of three types according to temporal firing pattern. A low-pass type with nonadapting, locally time-varying spike rates reliably followed click rates up to a specific rate but responded only sporadically to click rates exceeding that upper boundary (between 35 and 50 Hz for cell 68–13; Fig. 3 second row). Up to the boundary rate, the spikes per click in the response of these cells increased regularly as click rate increased. Above the boundary rate, spike trains instead followed the clicks for only brief episodes; these responses were otherwise silent for hundreds of milliseconds during various portions of the 1,200-ms stimulus. The spike absences occurred during different portions of the response each time the above-boundary stimulus was repeated. As a result, both spikes per click and spikes per second were lower overall in response to 50-Hz than to 35-Hz click trains. Selective cells of a second type, the always-adapting type, followed clicks at the beginning of every click train, from 4 to 50 Hz, but then fired less often (and at more variable intervals) over the course of each click train. For example, cell 74–01 (Fig. 3, third row) discharged following many of the first clicks, but the number of spikes following subsequent clicks decreased as the 35-Hz and 50-Hz click trains continued. A third type of rate-specific cells (adapting exclusively at higher click rates) fired at a short latency after every click only for the slow trains (4 and 13 Hz). During faster-click trains (35 and 50 Hz), spikes tracked just a few of the first clicks in the train (e.g., cell 69–18, Fig. 3, bottom row). Some cells in this category showed an offset response at the end of the click train (data not shown), suggesting that their preference for slow click rates was not merely a matter of adaptation. To group cells into these categories of selectivity type for subsequent analysis, raster plots were visually inspected for irregular absences of firing across presentations (at high click rates: type 1 time-varying selectivity), in addition to inspection of PSTHs for repeatable decreases in spike counts after clicks later in the train (at all click rates: type 2) and spike counts for a drop in spikes per click (at all click rates: type 2; or at high rates only: type 1 or type 3 selectivity).
Fig. 3.
Raster plots showing the spike record from 4 representative cells (rows) responding to 10 presentations of 4 different 1,200-ms synthetic click trains 20 dB re 1 mm/s above threshold (left 4 columns) and pure characteristic frequency (CF) tones at 10 dB above threshold (fifth column). The order of the responses is from bottom to top in each plot. Cell 70–20 is nonselective for click rate and gives a phasic burst to the CF tone. Cell 68–13 fires consistently throughout synthetic click trains at lower rates but fires inconsistent bouts of spikes to 50-Hz clicks. Its CF was 2,871 Hz at a threshold of 21 dB re 1 mm/s. Cell 74–01 increases its spikes per click correspondingly with increasing click rates up to 35 Hz, but it adapts early to click trains at any rate. Its CF was 391 Hz and threshold 26 dB re 1 mm/s. Cell 69–18 (same cell as in Fig. 5) is band pass for click rate, firing throughout the duration of click trains below 19 Hz (19 Hz not shown) but giving only a few spikes to click trains at higher rates. Responses to pure tones at CF are shown in the fifth column. Spikes per click in the sixth column steadily decrease with higher click rates regardless of the temporal patterns in cell responses. However, the nonmonotonicity (i.e., deviation from a monotonically increasing diagonal) of the coincidence rate (CR) curves of repeated responses (column seven) indicates the selectivity of these cells for click rate (right-most column). The click rate eliciting responses with a peak CR (plots show the CR normalized to its maximum over 8 synthetic click rates) is the preferred rate in each of the 4 example cells. Based on CR maxima, cell 70–20 is nonselective for click rate within the range tested, whereas cells 74–01 and 68–13 are selective for 35-Hz clicks; and cell 69–18 is selective for 13-Hz clicks.
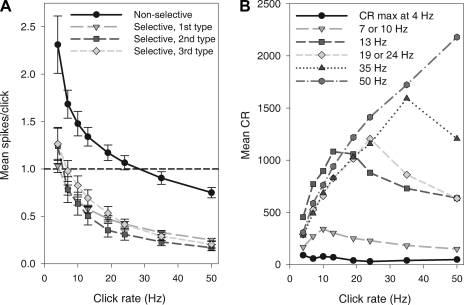
Nonselective neurons (e.g., cell 70–20, Fig. 3, top row) fired with smaller percentage decreases in spikes per click to increasing click rates, indicating no selection for click rate (Fig. 4A). Despite small decreases in spikes per click on average in this category, interspike intervals indicated that units could follow fast ICIs (data not shown). The CR increased regularly with click rate (Fig. 4B, dark gray hexagons), reflecting relatively consistent firing throughout all click trains.
Fig. 4.
Characteristics of selectivity for click rate in the responses to 8 synthetic click rates (in ∼1,200-ms trains) presented to a group of 139 cells, 20 dB above threshold. A: cells grouped by visual inspection of raster-plot discharge patterns into 4 click-rate-selectivity types (symbols) all show a similar decrease in spikes per click averaged across 10 presentations. Error bars indicate standard error. B: cells are grouped according to the click rate that evoked the highest CR.
Ninety percent of cells stimulated with synthetic clicks were rate selective; only 14 of 139 cells (10%) belonged to the nonselective category. A majority (68%) of responses fit the nonadapting rate-selective type. Ten cells (7%) were rate selective and adapted at all rates, and 21 cells (15%) were low pass with adaptation at high click rates. The responses of some uncategorized cells shared characteristics with more than one of these broad response types. For example, incomplete adaptation might be combined with the hallmark intermittently time-varying spike rates of the low-pass type, as in the initial overall decrease in spike rate and the subsequent irregular firing of cell 74–01 (Fig. 3, third row) at click rates from 13 to 50 Hz. One cell out of the 139 had a high spontaneous rate and was completely inhibited during click trains at rates faster than 4 Hz.
The spiking levels of the responses of the 139 cells to synthetic clicks are summarized in Fig. 4A (mean spikes per click ± SE). Nonselective cells (Fig. 4A) gave more spikes per click to 50-Hz trains than cells in any other category, even though like other cells their spike rate was less than one spike per click at the highest click rates. In selective cells of all three types (Fig. 4A), the response to most click rates averaged less than one spike per click. Although their responses to fast click trains depend on the cutoff and adaptation involved in the preference of each cell for slow click trains (as indicated by the CR, see below), in absolute terms the decrease in spikes per click with increasing click rate is no greater than in nonselective cells (Fig. 4A), implying that selectivity may be exhibited locally (in spike time patterns) rather than in overall spike rate. However, when normalized by number of clicks, the percentage spike rate decrease with increasing click rate is greatest in the responses of selective neurons; in nonselective neurons, the spikes per click in response to 50 Hz decreased on average 62% from the spikes per click in response to 4 Hz, whereas the average decrease was 77% for rate-selective neurons (as in cells 68–13, 74–01, and 69–18 in Fig. 3, sixth column).
Neurons differed markedly in click-rate tuning as revealed by the CR (Fig. 3, right). Both cells 68–13 and 74–01 had a maximum CR in response to 35-Hz clicks although cell 68–13 was more sharply tuned than cell 74–01, which had a high CR for both 35- and 50-Hz clicks. This difference in tuning reflected different combinations of temporal discharge patterns: cell 68–13 was tonic at low click rates and produced irregular bursts at high rates, whereas cell 74–01 gave bursts at the train onset of all click rates. Cell 69–18 was one of the most selective, with a narrow CR peak at 13 Hz. The comparatively steady rise of CR with click rate in cell 70–20 indicates that this cell was nonselective over the click rates tested.
Accord between temporal spike pattern and CR evaluation of selectivity was seen. Of the 14 cells that we identified as nonselective by the aforesaid visual inspection of the steady time course of their spike times within the raster plots (like cell 70–20 in Fig. 3), two cells actually showed maximum CRs in response to 35 Hz. Among selective cells of the first type (low pass with nonadapting, time-varying local spike rates), 64% (60 of the 94 cells) had CRs tuned to 35 or 50 Hz. In this type, the 28 cells with 50-Hz CR peaks had response PSTHs with characteristically declining spike counts, after an initial period of spikes precisely locked to each of the first clicks of the fast click trains. The maximal CRs of the 10 cells with the second type of selective responses (always adapting) were distributed approximately evenly across the battery of click rates. Half the cells (12 out of 21) with the third type of selective response (adaptation exclusively at higher click rates) had peak CRs at 10 or 13 Hz. Although categorization based on CR compares well to visual categorization, three of the cells categorized as type 3 by visual inspection of PSTHs (because, like in the 50-Hz response of cell 69–18 in Fig. 5, spikes occurred mainly toward the beginning of the 50-Hz train) produced their highest CRs at 50 Hz (due to the consistent spike latency after clicks at the beginning of the train). When cells with similar preferred rates are grouped, the pass-band tuning widths are fairly broad (Fig. 4B) although some cells are clearly narrowly tuned (Fig. 3, cell 69–18).
Fig. 5.
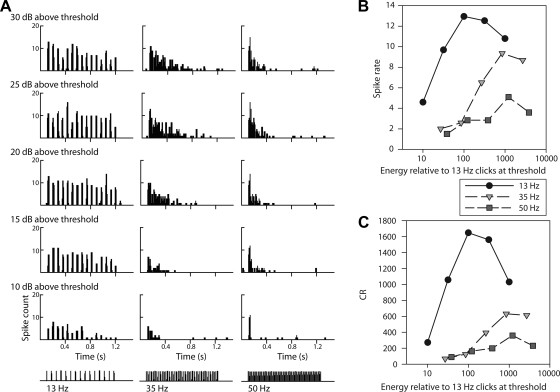
Rate selectivity persists across sound energy levels. Cell 69–18 was low pass for click rate as judged by A, the responses shown in poststimulus time histograms (PSTHs). B: spike rates of the same responses plotted against relative total stimulus energy. C: CR against stimulus energy. Three synthetic click rates shown at bottom of A (13, 35, and 50 Hz; columns in A and symbols in B and C) were presented 10 times at 5 different sound intensities (10, 15, 20, 25, and 30 dB above threshold; rows in A, converted to relative energy in B). Peak spike rates in B and CRs in C indicate a low-pass preference for rate (13 Hz), rather than a consistent low-pass preference for total energy (e.g., 100 times the 13-Hz train at threshold) regardless of click temporal pattern.
Click-Rate Selectivity Across Sound Level
The click train stimuli we have analyzed thus far differ concomitantly in a second acoustic parameter, the total sound energy over the click-rate period. To examine the possibility of selection for overall energy differences between the stimuli, we compared the CR of the responses of 14 selective cells to their preferred click rate 20 dB above threshold vs. the CR to 50-Hz clicks 10 dB above threshold. If selection was for stimulus energy below a cutoff level, rather than click rates slower than a cutoff rate, then CRs should be higher for the softer 50-Hz stimulus. To the contrary, without exception, CRs were higher for the preferred rate (paired t-test, P < 0.005). Band-pass tuning for energy level (i.e., peaking at the energy of the preferred click train 20 dB above threshold) could not explain (except in 1 cell out of 14) the CR decrease for the softer 50-Hz stimulus because CRs in responses to a still softer stimulus, the preferred rate 10 dB above threshold, were also higher than to the 50 Hz at 10 dB (paired t-test, P < 0.05). As an example, we show the responses of a cell that was low pass for click rate (cell 69–18) when presented with the same series of click rates at five different sound pressure levels, which differed accordingly in energy (Fig. 5). If the cell were selecting for lower mean-stimulus power, rather than for a slower temporal pattern of click rate, the spike rate would increase as a 50-Hz click train became attenuated. A slight spike count increase did occur in the 50-Hz response as the stimulus intensity dropped from 30 dB above threshold to 25 dB above. However, this low-pass cell responded with even more spikes to the louder 35-Hz train (30 dB above threshold) than to the softer 50-Hz train (25 dB above threshold), even though that loud 35-Hz train contained 2.2 times more total energy (at a 5-dB increase in sound level, individual clicks contain 3.2 times the energy, but 35-Hz trains have 70% of the clicks that 50-Hz trains do) (Fig. 5B). As judged by CR curves (Fig. 5C), click-rate selection persisted in this cell across a 20-dB increase in sound-pressure level, corresponding to a 100-fold change in energy. Thus selective cells remain selective across sound-intensity levels.
Interspike Intervals and Synchronization to Click Trains
One parameter that contributes to CR is the temporal precision of spikes in response to individual clicks. To determine whether selective cells had lower CRs at higher click rates attributable not to decreased firing but to increased jitter (imprecision in locking spikes at characteristic delays), we examined interspike interval histograms. In case not all clicks resulted in a spike, we also created phase histograms with the ICI as a time base (here the click rate is analogous to the stimulus cycle of a pure tone in classical phase histograms). In weak responses, the locking of neural activity remained strong. Low-pass cells had a maximum count of ISIs corresponding to the ICI of the stimulus period down to 20 ms. The phase histograms were very peaked even for the low-pass cell whose 50-Hz response had few spikes, cell 69–18. Thus relatively low CRs are low neither by virtue of jitter in spike latencies nor due to recovery processes preventing any ISIs as short as the ICIs.
Individual clicks can stimulate cells in any category to fire fast bursts of spikes, with interspike intervals of 2–4 ms. In some cells, bursts were associated with a particular range of click rates. Half the units (71 out of 139) responded with at least eight pairs of spikes (2–4 ms apart) over the course of 10 presentations to stimuli at both the slowest and fastest click rates (4 Hz and 50 Hz). Units that produced the fast spike pairs had somewhat higher spontaneous rates (2.3 vs. 1.1 spikes/s, unpaired t-test P < 0.005) than other units.
Natural Call Filtering
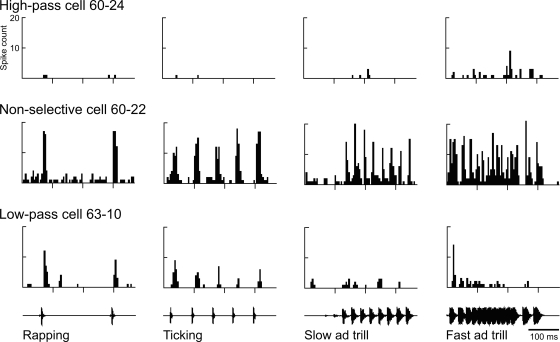
Twenty-eight cells were tested with natural call stimuli in addition to a pilot version of our synthetic stimuli. Half of the cells appeared selective for click rate, whereas the other half was nonselective. The 14 nonselective cells fired along with the clicks for all click rates (as exemplified by cell 60–22, Fig. 6), and the PSTHs of call responses in these nonselective cells reflected the call envelope. In these cells, the threshold to natural call stimuli was prefigured by thresholds in the pure tone FTCs at the relevant frequencies. The broadband spectral contents of the male and female clicks in the call recordings overlapped but differed in peak frequency by ∼0.5 kHz (female click peak at 1.2 kHz and male at ∼1.7 kHz).
Fig. 6.
Call responses in a cell that was nonselective for click rate (60–22, middle), a rare cell high pass for click rate (60–24, top), and a low-pass cell (63–10, bottom). PSTHs show the responses of 3 cells (rows) to recorded natural call segments presented 10 times at 20 dB above threshold. The 370-ms call stimuli are shown below the histograms (the ticking is female; ad, male advertisement).
In contrast, the selective 14 cells responded regularly to only particular natural-call stimuli (Fig. 6, high- and low-pass cells), consistent with a preference for click rate. Over ten presentations, the example low-pass cell (63–10, Fig. 6) responded reliably to most click-train onsets but fired throughout only the slower trains, the two female calls. Across the trials, we tallied spikes over periods having equal numbers of clicks within the two advertisement-trill stimuli. Twelve out of 14 selective cells were low pass, giving higher spike counts to lower click rates in the slow advertisement trill, compared with the fast trill (Fig. 6, right two columns). The remaining two cells were high pass (Fig. 6, top), firing consistently only to the fast trill of the male advertisement call, with the highest PSTH peak occurring at the end of the series of overlapping clicks in that stimulus (Fig. 6, cell 60–24, right). Some selective cells were difficult to drive with pure tones, as indicated by irregular FTC shapes (not shown).
The last-recorded 12 cells out of these 28 were presented with both recorded calls and synthetic click trains having rates similar to the calls, 4 to 30 Hz (data not shown). We found little difference between responses to male and female synthetic clicks. In all 12 cells, the patterns of responses to natural recordings were consistent with those to synthetic stimuli: spikes per click in response to ticking, rapping, and the slow trill of male advertisement call corresponded well with spikes per click in response to 4, 13, and 30 Hz, respectively.
Characterization and Comparison of Other Response Properties
The average spontaneous firing rate, measured over 2 s before each tone in the FTC experiments, was 2.3 spikes per second, with a range from 0.5 spikes per second (the minimum detectable) to 35.6. Mean minimum latency to pure tones at CF was 12.8 ms (SD 6.2).
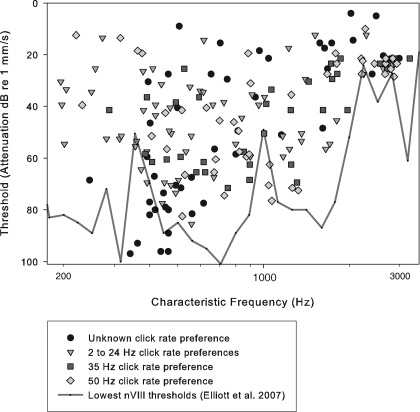
CFs ranged from 140 to 3,250 Hz (Fig. 7). FTCs were generally broad and included v-shapes and peaks with shoulders. The tuning quality factor Q10 (the CF/bandwidth of the FTC 10 dB above threshold) varied from 0.6 to 13.0, with a mean of 2.0 (SD 2.1). The distribution of CFs across the population had two modes close to 400 and 1,100 Hz (not shown). Minimum thresholds at 1,100 kHz were −76 dB re 1 mm/s, whereas thresholds at 500 Hz were at levels below −90 dB (Fig. 7). The tympanic vibrations with which we stimulated are approximately equivalent to a sound pressure of 0.1 mm*s−1 / Pa (Elliott et al. 2007). Cells with different CRs, and thus different click-rate preferences, did not vary systematically in frequency tuning or threshold (Fig. 7). Dynamic ranges averaged 39.0 dB (SD 24.3), with a maximum dynamic range of 100 dB. The dynamic range tended to be small for the few units having spontaneous rates above five spikes per second (data not shown).
Fig. 7.
CF and thresholds from frequency-tuning curves in 191 TS cells. Minimum eighth nerve thresholds are overlain (black line) (Elliott et al. 2007). The categorization of cells based on click-rate selectivity is explained in the results from synthetic stimuli (see Figs. 3 and 4).
Temporal response patterns varied from phasic to tonic firing at CF (Fig. 3, fifth column). A phasic-tonic index quantifying these discharge patterns spanned the whole possible range from −1 (spikes occurring only during the last 40 ms of the tone) to 1 (phasic spiking limited to 40 ms after tone onset), with a mean (0.31, SD ± 0.47) not far from an ideally tonic response (data not shown). The index showed no systematic relation to CF or to threshold (data not shown). Cells in the four categories of click-rate-response patterns did not significantly differ in phasic-tonic index (see tone PSTHs in Fig. 3, second to right column). Neither did the index differ between cells with CR maxima below 50 Hz (98 cells; mean 0.35, SD ± 0.48) and nonselective cells with CR preferences for 50 Hz (43 cells; mean 0.41 SD ± 0.42). Selective and nonselective cell categories did not differ significantly either in the minimum response latency to CF tones or in the CF itself (data not shown; all comparisons by ANOVA, P > 0.05).
DISCUSSION
We have shown that neurons selective for behaviorally important click rates are found in the auditory midbrain of X. laevis. All auditory responses were located in the laminar nucleus of the TS (homolog of the mammalian inferior colliculus), which is, on the basis of our evidence, specialized for the temporal processing of clicks in this species. We evaluated rate selectivity using two response parameters: the average spike rate per click and the overall patterns of spike-rate modulation. Using these criteria, the overwhelming majority of laminar cells are rate selective, whereas some other neurons in the nucleus are nonselective for click rates (over the range tested) like responses in the auditory nerve and medulla (Elliott et al. 2007). We begin by discussing the features of rate selectivity in the laminar nucleus and how well these data are supported by a model consisting of two neurons, one excitatory and the other inhibitory. Then we compare the basic auditory responses and temporal processing in the midbrain to that in terrestrial frogs.
Midbrain Processing of Click Rate
Although some laminar neurons (26 of 168 cells presented with natural recordings or synthetic clicks) were nonselective for click rates, following increasingly fast click rates with regularly increasing spike rates up to 50 Hz, the majority (85%) displayed some form of rate selectivity. Most selective cells were low pass, with more precise and reliable responses to clicks at rates below a cutoff value, but 4 of 168 were high pass (e.g., Fig. 6, cell 60–24). Selectivity in about 20% of the rate-tuned cells resulted partially from adaptation during trains of all, or only the high, click rates. Offset responses in some of these cells suggested that inhibition, in addition to adaptation, was involved in shaping the selectivity. The preference for slow click rates in 14 cells was independent of total sound energy over the stimulus duration and thus reflected the temporal structure of sound stimuli, rather than merely a preference for relatively low stimulus power overall. The contrast between low-pass responses with different cutoffs could suffice in distinguishing click rates that differ between natural calls (Straughan 1975).
Transformation of Temporal Information Between the Hindbrain and Midbrain
The majority of TS units were rate selective and responded with reliable latency only to clicks at low rates, and additionally a few units were selective for high click rates (Fig. 6). TS cells thus differ substantially from primary afferents and cells in the DMN, which synchronize to click envelopes as high as the upper limit of click rates in the Xenopus call repertoire (100 Hz in growling) (Elliott et al. 2007).
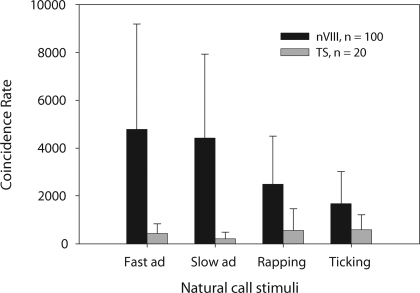
We can directly compare the natural call responses of 20 midbrain cells (Fig. 8) with those of 100 auditory nerve fibers presented with the same four natural-call stimuli (Elliott et al. 2007). Nerve responses showed higher spike rates, and their CRs were significantly higher than those of TS responses (repeated-measures ANOVA, P < 0.0001, Bonferroni/Dunn). An unusually high number of the TS cells in this comparison happened to be low pass when categorized by visual inspection of the PSTHs (16 cells). The high proportion of these cells explains the lower CRs in toral responses to male than to female call stimuli, which contain fewer clicks within the same stimulus duration (Fig. 8, gray bars, all four comparisons P < 0.0001, repeated-measures ANOVA, Bonferroni/Dunn).
Fig. 8.
CRs of responses to 10 presentations of the 4 natural-call stimuli, from 100 auditory nerve fibers and 20 TS units. Sound levels were 10–30 dB above the threshold at CF. CRs were significantly higher in the nerve responses than in the TS (repeated-measures ANOVA, P < 0.0001). Male calls (the slow and fast advertisement trills, left-hand gray bars) produced significantly lower CRs in the TS than female calls did (rapping and ticking, right-hand gray bars).
Processing of time-varying signals in the midbrain is thus achieved through a transformation of the representations in primary afferents and the medulla. Synchronization of eighth nerve and DMN units to temporal modulations in the signal produces a time-varying spike “rate code”, in which firing rate scales proportionally with the modulation rate of the stimulus. In contrast, the representation in most neurons in the laminar nucleus of the TS constitutes a “correlation code”, which is based on spike rate and spike timing correlations between neurons (in other words, synchrony between spike trains). In these units, firing rates (in spikes per modulation) peak for intermediate temporal modulation rates and spike times do not phase lock to modulation rates above some limit, as reflected in the CR of the responses.
The auditory midbrains of all anurans examined to date include neurons that specialize in processing the temporal characteristics of vocal communication signals. Just as frequency tuning becomes increasingly selective along the ascending auditory pathway, temporal selectivity increases for the whole variety of temporal patterns required to evoke behavioral responses, including the rate of pulses and their number (Alder and Rose 2000; Edwards et al. 2002; Eggermont and Epping 1986; Feng and Schellart 1999; Gooler and Feng 1992). This selectivity is apparent not only at the level of the TS but also in thalamic targets (Hall and Feng 1986).
The temporal processing in the TS of other frog species is summarized in Rose and Gooler (2006). Amplitude modulation (AM)-selective cells have been found in the TS of all the terrestrial frogs studied (ranids, bufonids and hylids), and most cells are band pass selective (when stimulated by sinusoidal AM noise or tones). In contrast, most cells in this study were low pass selective for click rates. With the caveat that the type of stimulation likely influences the proportion of band-pass-selective units (Rose and Gooler 2006), we think that this difference reflects a different organization of temporal processing in the Xenopus TS.
Differences in responses to natural-call stimuli may depend on spectral features in addition to temporal features. As in the ranid midbrain, a minority of TS units in our recordings responded better to broadband clicks than to pure tones (Fuzessery and Feng 1982), opening the possibility that the TS may be the first auditory nucleus in the ascending pathway of Xenopus in which neurons require a combination of spectral features.
Three toral nuclei are generally recognized: the principal, the laminar, and the magnocellular. The sensory sources of input to the torus are auditory, vestibular, and, in both tadpoles and adult Xenopus, lateral line (Edwards and Kelley 2001). The principal nucleus is the main auditory recipient in terrestrial frogs (Feng and Lin 1991). In contrast, previous anatomical and metabolic marker studies show that in Xenopus the laminar nucleus receives the major ascending auditory projections from the DMN (Edwards and Kelley 2001; Kelley 1980; Paton et al. 1982); auditory responses have also been demonstrated neurophysiologically (Behrend et al. 2006). The specialization of the laminar nucleus for auditory processing in Xenopus may reflect the retention of the lateral line system in adult stages and the concomitant requirement for retained neural processing of lateral line information in the principal nucleus.
In terrestrial frogs the laminar nucleus has a rostrocaudal orientation, but in Xenopus it has a dorsoventral orientation of its longest axis. In the present study, auditory responses were found to be confined to the laminar nucleus. These neurons showed a CF range that overlaps that of the eighth nerve (Elliott et al. 2007), but there were many fewer toral units with CFs below 300 Hz. Because, in terrestrial frogs, low frequencies are better represented in the center of the principal nucleus and higher frequencies towards the periphery (Feng and Schellart 1999), more extensive sampling within the laminar nucleus of X. laevis might uncover neurons driven by lower frequencies.
Neural Mechanisms for Encoding Rate Selectivity
Metrics of the response strength of neurons presuppose principally two sorts of information-processing strategies (Staude et al. 2008). In the first, neuronal assemblies depend on contributions of consistent firing-rate levels by individual neurons. The second kind of information processing by populations requires the temporal coordination of spikes across neurons, possibly in addition to shifts of overall firing-rate level related to changes of stimulus parameters with time. Metrics such as the CR that compare temporal coordination of spikes across multiple responses have an important advantage over crosscorrelations between stimulus and response; the former require no a priori knowledge about the information in the stimulus (Rieke et al. 1999).
We used a coincidence analysis (Joris et al. 2006) along with spikes per click to measure tuning for click rate. Responses with a deteriorated CR could be combined by a detector receiving them as input to encode increased click rates in the following way: changes in spike correlation reflected in the CR (which scales with the spike rate as well as the consistency of spike times between repeated responses) could provide the basis for a correlation code in which spike times correlated between similar neurons offer more temporal information than can be gleaned from their mean-spike rates considered independently (Dayan and Abbott 2001). Additionally, our evidence from short call segments suggests the potential for distinct average spike-rate encoding of the fast and slow trills of the advertisement call. These changes in mean spike counts in a minority of cells when responding to different click rates could be termed a rate code in the sense of overall spike rate (Shadlen 2002).
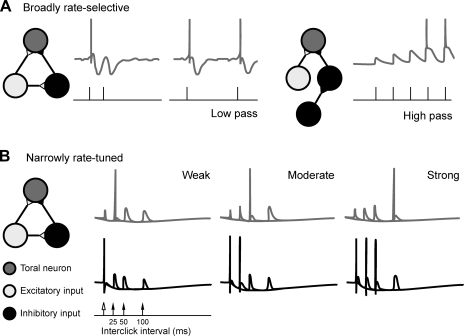
The computational underpinnings of temporal selectivity in the midbrain and forebrain have been thought to rely on three mechanisms including the following: coincidence detection (Carr 1993; Narins and Capranica 1980), short-term synaptic plasticity (Fig. 9A; Atzori et al. 2001; Oswald et al. 2006), and the interplay of inhibitory and excitatory connections (Fig. 9B; Buonomano 2000; Casseday et al. 1994). In Fig. 9A, plasticity in the form of long-lasting inhibition (Fig 9A, left) leads to low-pass selectivity for click rate, whereas facilitation (right) leads to high-pass filtering of click rate. The resulting firing pattern of the former (Fig. 9A, left) resembles selective cells of type 3 (like cell 69–18 in Fig. 3) or low-pass cell 63–10 (Fig. 6); the latter response pattern (Fig. 9A, right) resembles high-pass cells like 60–24 (Fig. 6). Alternatively, as in Fig. 9B, the timing and strength of excitatory (light grey) and inhibitory (black) inputs could lead to spikes (three dark-grey traces are overlaid; only one response shows a spike at the interval corresponding to the preferred ICI). Such a mechanism could explain the response of the high-pass cell 60–24 (Fig. 6, top, right), which reliably spiked about 200 ms into the response, after integrating over most of the clicks in the fast trill of the male advertisement call. However, it was uncommon for low-pass-rate-selective cells to fail to spike for the first click in the train (cells 68–13, 74–01, and 69–18, Fig. 3). Neurons in the cochlear nucleus are often characterized by a long hyperpolarizing poststimulus recovery (Wang and Manis 2008), which could explain low-pass preferences by extending the refractory periods of the neurons. In contrast, in our data, even quickly adapting low-pass cells often burst in response to single clicks within a fast click train, so their sensitivity cannot be due to an extended refractory period but may involve instead a mechanism such as delayed or slowly integrating inhibitory input.
Fig. 9.
A 3-neuron model for generating broadly or narrowly rate-selective neurons in the TS (cells in dark gray). The selectivity of the TS neurons relies on the circuitry: a monosynaptic excitatory input (cells in light gray) coupled to a disynaptic inhibitory input (cells in black) and various characteristics of short-term plasticity (e.g., facilitation) at the synapses in this circuit. A: generating broadly rate-selective neurons. Neurons selective for slow click rates (e.g., 6 Hz; stimulus amplitude in black and voltage in dark gray) are prevented from responding to faster rates by relatively long-lasting inhibition that follows initial excitation (left trace, after Oswald et al. 2006). Other neurons that select for fast click rates rely on facilitation after several clicks at a threshold rate (e.g., 35 Hz; right trace) and the absence of activity in the inhibitory pathway (due to a difference either in circuitry or in disinhibition). B: generating narrowly rate-tuned neurons. Selectivity for click rate can be generated by varying the strength of inhibitory (black traces) and excitatory input (not depicted; a spike locked to each click) to toral neurons (dark gray traces). Three traces are overlaid for three different interclick intervals. Weak input synapses cause the TS neuron to exhibit paired pulse facilitation that peaks at 25 ms (left). With a moderate increase of both inputs, a fast inhibitory postsynaptic potential prevents the TS neuron from firing until the 50-ms pulse (middle). Further strengthening of the input synapses leads to a response from the TS neuron exclusively to the second pulse at 100 ms attributable similarly to the recruitment of a slower inhibitory postsynaptic potential input (right). After Buonomano (2000).
Edwards et al. (2007) examined excitation, inhibition, and a possible role for short-term plasticity in the highly selective midbrain neurons of Hyla regilla and of Rana pipiens (Alder and Rose 2000). Intracellular recordings in interval-counting neurons revealed a profound inhibition produced by the first pulse in a train. This inhibition could be overcome by the excitatory effects of additional pulses, a recovery effect that occurs only at specific rates and produces subthreshold changes in membrane potential until threshold for action potential production is achieved. This latter feature accounts for the ability of these neurons to “count” pulses, and for the result that longer interpulse intervals reset the membrane potential. Rate-dependent depression of inhibition (a form of short-term plasticity) was not apparent; the hyperpolarization produced by a single pulse was equivalent to that produced by a train of pulses at the most effective intervals for inhibition. Classical presynaptic facilitation (another form of short-term plasticity) cannot explain the ability of a single nonoptimal interval to reset the membrane potential below threshold. Instead the interplay of excitation and inhibition, generally, and the rate dependence of excitation appear largely responsible for rate selectivity.
High-pass cells might rely on a similar mechanism with specificity determined by the temporal parameters of excitation. The few high-pass cells in this study responded only weakly to the beginning of click trains but after some time (possibly for integration) responded reliably to subsequent clicks. As an alternative to the models involving inhibition (Fig. 9A, right and 9B, left), this could result from a selection mechanism involving neural delays and coincidence detectors in which input responsive to the beginning of the click train coincides with input in response to later clicks.
Spike rate and consistency of spike timing represented by the CR could be extracted by a hypothetical coincidence detector receiving inputs from two cells having identical response properties (Joris et al. 2006). The curve of each cell of relative CRs across stimuli, normalized to the maximum CR, would determine the maximal effect one neuron might have on a postsynaptic target receiving multiple inputs. The effect of neurons on their postsynaptic targets depends on both timing and rate. The combination of timing and rate in the CR is analogous to combinations of vector strength with spike rate that have been used in other analyses of AM selectivity (Liang et al. 2002; Rees and Palmer 1989), and analyses of this nature could be used in the future to evaluate correlation-code models.
Neural selectivity for click rate could also be achieved by selectivity for the intervals of silence between clicks, which could be experimentally controlled for by altering the duration of clicks while holding click rate constant.
From Hearing to Utterance
Temporal filtering in the responses to synthetic stimuli parallels that to the short segments of recorded natural calls. Male X. laevis produce a repertoire of six call types ranging in click rate from about 2 Hz (amplectant call) to 100 Hz (growling) (Tobias et al. 2004); females produce a 5-Hz call (ticking) and a 12-Hz call (rapping) (Tobias et al. 1998). The diversity of neuronal responses illustrated in Fig. 3 could be useful in discriminating fast calls (male advertisement, answer calling, chirping, and growling) from female calls (rapping and ticking) and the male calls with the slowest click rates (male ticking and male amplectant calling).
Cells whose CR peaked sharply at low click rates (like the 13 Hz peak of cell 69–18, Fig. 3, bottom) could be used in discriminating female ticking from faster rapping, as well as from faster male calls. Although rate tuning as narrow as this cell is not widespread among the TS cells, more narrowly tuned coding of click rates might emerge at the circuit level or elsewhere in the auditory system (see Fig. 9 above for a three-neuron model). Selectivity for click rate in the responses of all cells shows finer tuning in the CR vs. click-rate functions than when responses are plotted as spikes per click. Our inability to present nonoverlapping synthetic clicks at sufficiently high rates hindered our ability to judge the click-rate-tuning bandwidth of cells preferring click rates at 35 Hz and above.
The two female calls of X. laevis depend on ICIs to convey essential information on sexual receptivity. Each has a distinct effect on male vocal behavior (Tobias et al. 1998). The rapid trill, rapping, acts as an acoustic aphrodisiac to stimulate male calling, whereas the slow trill, ticking, suppresses calling (Elliott and Kelley 2007). The neural discrimination of click rate by cells like the one in the third row of Fig. 3 (cell 74–01) matches a male's behavioral responses to rate-manipulated click trains in the sense that the CR of the neural response changes steeply, but not categorically, over a limited range of boundary click rates (Elliott and Kelley 2007). In female-evoked calling experiments, males perceive click rates intermediate between the two female calls, rapping and ticking, as ambiguous. A habituation-dishabituation paradigm showed that males do not respond differentially to click rates within the ticking category (Elliott and Kelley 2007). Thus behavioral experiments suggest that male preference for female click rates occurs only across boundaries distinguishing the calls.
In males, the advertisement call is responsible for vocal dominance (Tobias et al. 2004). The call also evokes phonotaxis and rapping in sexually receptive females (Tobias et al. 1998), and perception of this call may also rely on neurons that respond selectively to fast click rates. In the neurophysiological studies described here, two toral cells responded consistently only to the fast trill of the male advertisement call, exhibiting their highest spike rate toward the end of the fast trill portion of the call (Fig. 6), and these might contribute to the representation of this call type in both males and females (Tobias et al. 2004). In contrast, another cell (68–13, Fig. 3, second row) responded selectively to synthetic clicks at the 35-Hz click rate contained in the slow trill portion of the advertisement call.
Information used to discriminate calls within the auditory system must be passed on to vocal, locomotory, autonomic, and attentional centers. Distributed centers in the ascending auditory pathway (such as the thalamus) could further process the significance-bearing features of acoustic communication signals, but these pathways must ultimately converge on the same premotor targets responsible for sound-mediated behaviors. In all frogs, the laminar nucleus is the major source of projections to the auditory areas of the dorsal thalamus, the posterior, and central thalamic nuclei (Butler and Hodos 2005; Edwards and Kelley 2001). In Xenopus, the ventral striatum is the exclusive telencephalic target of projections from the central thalamic nucleus (Marin et al. 1997). The ventral striatum is also the major source of synaptic input to the hindbrain vocal pattern generator (Brahic and Kelley 2003) and is thus a strong candidate for serving as an interface between the auditory representation of calls and the distinct vocal responses that each evokes.
Conclusion
Temporal processing of click rate, a feature that gives X. laevis calls communicative significance, arises in the laminar nucleus of the TS. Although processing of click rate by single cells is less narrowly tuned than pulse-rate tuning described in terrestrial frogs (Alder and Rose 2000), precise information about click rate could emerge as a circuit property through the combination of inputs from low-pass and high-pass filtering cells with different click-rate cutoffs and could contribute to the matching of auditory information to appropriate vocal responses.
GRANTS
Research and international travel was supported by a National Science Foundation graduate fellowship, a National Institute of Deafness and Communication Disorders NRSA graduate fellowship, and the Danish National Science Foundation. Research at Columbia was supported by a grant from the National Institute of Neurological Diseases and Stroke. Animal care and use for this study was approved both by the Institutional Animal Care and Use Committee at Columbia University (AC-AAAA1401) and the Danish Animal Experimentation Board (Dyreforsøgstilsynet), grant 1999/561-169.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the help of Christian Brandt, Catherine Carr, Chris Edwards, Patrick Gill, Josina Goense, Morten Kanneworff, Elizabeth Olson, Daniel Salzman, David Vicario, Sarah M. Woolley, and Erik Zornik. Martha Tobias made the underwater call recordings used as stimuli.
REFERENCES
- Alder TB, Rose GJ. Integration and recovery processes contribute to the temporal selectivity of neurons in the midbrain of the northern leopard frog, Rana pipiens. J Comp Physiol A 186: 923–937, 2000 [DOI] [PubMed] [Google Scholar]
- Atzori M, Lei S, Evans DI, Kanold PO, Phillips-Tansey E, McIntyre O, McBain CJ. Differential synaptic processing separates stationary from transient inputs to the auditory cortex. Nat Neurosci 4: 1230–1237, 2001 [DOI] [PubMed] [Google Scholar]
- Behrend O, Branoner F, Zhivkov Z, Ziehm U. Neural responses to water surface waves in the midbrain of the aquatic predator Xenopus laevis laevis. Eur J Neurosci 23: 729–744, 2006 [DOI] [PubMed] [Google Scholar]
- Blair WF. Isolating mechanisms and interspecies interactions in anuran amphibians. Q Rev Biol 39: 334–344, 1964 [DOI] [PubMed] [Google Scholar]
- Brahic CJ, Kelley DB. Vocal circuitry in Xenopus laevis: telencephalon to laryngeal motor neurons. J Comp Neurol 464: 115–130, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonomano DV. Decoding temporal information: A model based on short-term synaptic plasticity. J Neurosci 20: 1129–1141, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AB, Hodos W. Comparative Vertebrate Neuroanatomy. Hoboken, New Jersey: John Wiley & Sons, 2005, p. 715 [Google Scholar]
- Capranica RR. Evoked vocal response of the bullfrog: a study of communication in anurans. In: Research Monographs. Cambridge, MA: MIT Press, 1965, p. 73–75 [Google Scholar]
- Carr CE. Processing of temporal information in the brain. Annu Rev Neurosci 16: 223–243, 1993 [DOI] [PubMed] [Google Scholar]
- Casseday JH, Ehrlich D, Covey E. Neural tuning for sound duration: role of inhibitory mechanisms in the inferior colliculus. Science 264: 847–850, 1994 [DOI] [PubMed] [Google Scholar]
- Christensen-Dalsgaard J. Directional hearing in the non-mammalian tetrapods. In: Sound Source Localization, edited by Popper AN, Fay RR. New York: Springer-Verlag, 2005, p. 67–123 [Google Scholar]
- Christensen-Dalsgaard J, Elepfandt A. Biophysics of underwater hearing in the clawed frog, Xenopus laevis. J Comp Physiol A 176: 317–324, 1995 [DOI] [PubMed] [Google Scholar]
- Christensen-Dalsgaard J, Elliott TM. Amphibian underwater hearing: biophysics and neurophysiology. Bioacoustics 17: 60–62, 2008 [Google Scholar]
- Dayan P, Abbott LF. The Neural Code. In: Theoretical Neuroscience: Computational and Mathematical Modeling of Neural Systems. Cambridge, MA: The MIT Press, 2001, p. 34–39 [Google Scholar]
- Edwards CJ, Alder TB, Rose GJ. Auditory midbrain neurons that count. Nat Neurosci 5: 934–936, 2002 [DOI] [PubMed] [Google Scholar]
- Edwards CJ, Kelley DB. Auditory and lateral line inputs to the midbrain of an aquatic anuran: neuroanatomic studies in Xenopus laevis. J Comp Neurol 438: 148–162, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards CJ, Leary CJ, Rose GJ. Counting on inhibition and rate-dependent excitation in the auditory system. J Neurosci 27: 13384–13392, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermont JJ, Epping WJ. Sensitivity of neurons in the auditory midbrain of the grassfrog to temporal characteristics of sound. III. Stimulation with natural and synthetic mating calls. Hear Res 24: 255–268, 1986 [DOI] [PubMed] [Google Scholar]
- Elliott TM, Christensen-Dalsgaard J, Kelley DB. Tone and call responses of units in the auditory nerve and dorsal medullary nucleus of Xenopus laevis. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 193: 1243–1257, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott TM, Kelley DB. Male discrimination of receptive and unreceptive female calls by temporal features. J Exp Biol 210: 2836–2842, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng AS, Lin WY. Differential innervation patterns of three divisions of frog auditory midbrain (torus semicircularis). J Comp Neurol 306: 613–630, 1991 [DOI] [PubMed] [Google Scholar]
- Feng AS, Schellart NAM. Central auditory processing in fish and amphibians. In: Comparative Hearing: Fish and Amphibians, edited by Fay RR, Popper AN. New York: Springer-Verlag, 1999, p. 218–268 [Google Scholar]
- Fouquette MJ. Speciation in Chorus Frogs.1. Reproductive character displacement in Pseudacris-nigrita complex. Systematic Zoology 24: 16–23, 1975 [Google Scholar]
- Fuzessery ZM, Feng AS. Frequency selectivity in the anuran auditory midbrain: single unit responses to single and multiple tone stimulation. J Comp Physiol A 146: 471–484, 1982 [Google Scholar]
- Gerhardt HC. Acoustic properties used in call recognition by frogs and toads. In: The Evolution of the Amphibian Auditory System, edited by Fritzsch B, Hetherington TE, Ryan MJ, Wilczynski W, Walkowiak W. New York: John Wiley & Sons, 1988, p. 455–483 [Google Scholar]
- Gerhardt HC. Discrimination of intermediate sounds in a synthetic call continuum by female green tree frogs. Science 199: 1089–1091, 1978 [DOI] [PubMed] [Google Scholar]
- Gerhardt HC, Doherty JA. Acoustic communication in the gray treefrog, Hyla versicolor: evolutionary and neurobiological implications. J Comp Physiol A 162: 261–278, 1988 [Google Scholar]
- Gooler DM, Feng AS. Temporal coding in the frog auditory midbrain: the influence of duration and rise-fall time on the processing of complex amplitude-modulated stimuli. J Neurophysiol 67: 1–22, 1992 [DOI] [PubMed] [Google Scholar]
- Hall J, Feng AS. Neural analysis of temporally patterned sounds in the frog's thalamus: processing of pulse duration and pulse repetition rate. Neurosci Lett 63: 215–220, 1986 [DOI] [PubMed] [Google Scholar]
- Hall JC, Feng AS. Influence of envelope rise time on neural responses in the auditory system of anurans. Hear Res 36: 261–276, 1988 [DOI] [PubMed] [Google Scholar]
- Imaizumi K, Pollack GS. Neural representation of sound amplitude by functionally different auditory receptors in crickets. J Acoust Soc Am 109: 1247–1260, 2001 [DOI] [PubMed] [Google Scholar]
- Joris PX, Louage DH, Cardoen L, van der Heijden M. Correlation Index: A new metric to quantify temporal coding. Hear Res 216–217: 19–30, 2006 [DOI] [PubMed] [Google Scholar]
- Kanneworff M. Neural processing of directional cues in the frog dorsal medullary nucleus. In: Center for Sound Communication, Institute of Biology, Faculty of Science and Engineering. Odense, Denmark: University of Southern Denmark, 2004, p. 100 [Google Scholar]
- Kelley DB. Auditory and vocal nuclei in the frog brain concentrate sex hormones. Science 207: 553–555, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley DB, Tobias ML. Vocal communication in Xenopus laevis. In: The Design of Animal Communication, edited by Hauser MD, Konishi M. Boston, MA: MIT Press, 1999, p. 9–35 [Google Scholar]
- Liang L, Lu T, Wang X. Neural representations of sinusoidal amplitude and frequency modulations in the primary auditory cortex of awake primates. J Neurophysiol 87: 2237–2261, 2002 [DOI] [PubMed] [Google Scholar]
- Loftus-Hills JJ, Littlejohn MJ. Pulse repetition rate as the basis for mating call discrimination by two sympatric species of Hyla. Copeia 1971: 154–156, 1971 [Google Scholar]
- Louage DH, van der Heijden M, Joris PX. Temporal properties of responses to broadband noise in the auditory nerve. J Neurophysiol 91: 2051–2065, 2004 [DOI] [PubMed] [Google Scholar]
- Luksch H, Walkowiak W, Munoz A, Donkelaar HJ. The use of in vitro preparations of the isolated amphibian central nervous system in neuroanatomy and electrophysiology. J Neurosci Methods 70: 91–102, 1996 [DOI] [PubMed] [Google Scholar]
- Marin O, Gonzalez A, Smeets WJ. Basal ganglia organization in amphibians: afferent connections to the striatum and the nucleus accumbens. J Comp Neurol 378: 16–49, 1997 [DOI] [PubMed] [Google Scholar]
- Megela AL, Capranica RR. Response patterns to tone bursts in peripheral auditory system of anurans. J Neurophysiol 46: 465–478, 1981 [DOI] [PubMed] [Google Scholar]
- Narins PM, Capranica RR. Communicative significance of the two-note call of the treefrog Eleutherodactylus coqui. J Comp Physiol A 127: 1–9, 1978 [Google Scholar]
- Narins PM, Capranica RR. Neural adaptations for processing the two-note call of the Puerto Rican treefrog, Eleutherodactylus coqui. Brain Behav Evol 17: 48–66, 1980 [DOI] [PubMed] [Google Scholar]
- Oswald AM, Schiff ML, Reyes AD. Synaptic mechanisms underlying auditory processing. Curr Opin Neurobiol 16: 371–376, 2006 [DOI] [PubMed] [Google Scholar]
- Paton JA, Kelley DB, Sejnowski TJ, Yodlowski ML. Mapping the auditory central nervous system of Xenopus laevis with 2-deoxyglucose autoradiography. Brain Res 249: 15–22, 1982 [DOI] [PubMed] [Google Scholar]
- Pauly GB, Bernal XE, Rand AS, Ryan MJ. The vocal sac increases call rate in the Tungara frog Physalaemus pustulosus. Physiol Biochem Zool 79: 708–719, 2006 [DOI] [PubMed] [Google Scholar]
- Rees A, Palmer AR. Neuronal responses to amplitude-modulated and pure-tone stimuli in the guinea pig inferior colliculus, and their modification by broadband noise. J Acoust Soc Am 85: 1978–1994, 1989 [DOI] [PubMed] [Google Scholar]
- Rieke F, Warland D, De Ruyter van Steveninck R, Bialek W. Spikes: Exploring the Neural Code. Cambridge, MA: The MIT Press, 1999, p. 416 [Google Scholar]
- Rose GJ, Gooler DM. Function of the amphibian central auditory system. In: Hearing and Sound Communication in Amphibians, edited by Narins PM, Feng AS. New York: Springer, 2006, p. 250–290 [Google Scholar]
- Shadlen MN. Rate versus temporal coding of information in the cerebral cortex. In: Encyclopedia of Cognitive Science. New York: Macmillan, 2002, p. 819–825 [Google Scholar]
- Staude B, Rotter S, Grün S. Can spike coordination be differentiated from rate covariation? Neural Comput 20: 1973–1999, 2008 [DOI] [PubMed] [Google Scholar]
- Straughan IR. An analysis of the mechanisms of mating call discrimination in the frogs Hyla regilla and H. cadaverina. Copeia 3: 415–424, 1975 [Google Scholar]
- Tobias ML, Barnard C, O'Hagan R, Horng SH, Rand M, Kelley DB. Vocal communication between male Xenopus laevis. Anim Behav 67: 353–365, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias ML, Viswanathan SS, Kelley DB. Rapping, a female receptive call, initiates male-female duets in the South African clawed frog. Proc Natl Acad Sci USA 95: 1870–1875, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignal C, Kelley DB. Significance of temporal and spectral acoustic cues for sexual recognition in Xenopus laevis. Proc R Soc Lond B Biol Sci 274: 479–488, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Manis PB. Short-term synaptic depression and recovery at the mature mammalian endbulb of Held synapse in mice. J Neurophysiol 100: 1255–1264, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilczynski W, Resler C, Capranica RR. Tympanic and extratympanic sound transmission in the leopard frog. J Comp Physiol A 161: 659–669, 1987 [DOI] [PubMed] [Google Scholar]