Abstract
Pulmonary arterial hypertension (PAH) is a chronic lung disease with poor diagnosis and limited therapeutic options. The currently available therapies are ineffective in improving the quality of life and reducing mortality rates. There exists a clear unmet medical need to treat this disease, which necessitates the discovery of novel therapeutic targets/agents for safe and successful therapy. An altered renin–angiotensin system (RAS) has been implicated as a causative factor in the pathogenesis of PAH. Angiotensin II (Ang II), a key effector peptide of the RAS, can exert deleterious effects on the pulmonary vasculature resulting in vasoconstriction, proliferation, and inflammation, all of which contribute to PAH development. Recently, a new member of the RAS, angiotensin converting enzyme 2 (ACE2), was discovered. This enzyme functions as a negative regulator of the angiotensin system by metabolizing Ang II to a putative protective peptide, angiotensin-(1–7). ACE2 is abundantly expressed in the lung tissue and emerging evidence suggests a beneficial role for this enzyme against lung diseases. In this review, we focus on ACE2 in relation to pulmonary hypertension and provide proof of principle for its therapeutic role in PAH.
Introduction
Pulmonary arterial hypertension (PAH) is a debilitating chronic disorder of the lungs characterized by sustained elevation of blood pressure in the pulmonary vasculature. The normal mean pulmonary arterial pressure in a healthy adult is about 14 mm Hg at rest. However, in PAH, the resting mean pulmonary arterial pressure measures over 25 mm Hg (and greater than 30 mm Hg during exercise) [1]. PAH can either be of idiopathic origin with unknown etiology or, it can arise due to secondary medical conditions such as collagen vascular diseases, heart malformation or viral infections. Genetic and epigenetic risk factors have also been linked to PAH pathogenesis [2]. Regardless of the cause, PAH is associated with endothelial dysfunction, vasoconstriction, and remodeling of the pulmonary vessels. Endothelial dysfunction is believed to be an early component of the pulmonary hypertensive process, creating an imbalance between vasodilator (nitric oxide (NO) and prostacyclin) and vasoconstrictor substances (endothelin, thromboxane A2 and serotonin) [3•]. This imbalance leads to a cascade of events resulting in hyper-proliferation of pulmonary smooth muscle cells, activation of lung fibroblasts, induction of thrombotic mediators and release of inflammatory cytokines, all of which increase vascular resistance and pressure. One of the causal factors for endothelial dysfunction and vascular impairment is the renin–angiotensin system (RAS) [4]. Activation of the classic ACE-AngII-AT1R axis of the RAS, comprising of angiotensin converting enzyme (ACE), the vasoactive peptide angiotensin II (Ang II), and its receptor AT1R, adversely affects pulmonary hemodynamics to cause PAH [5]. Conversely, ACE2, the recently discovered homologue of ACE has been shown to oppose the detrimental effects of the ACE-AngII-AT1R axis on the cardio-pulmonary system [6••]. This review focuses on the role of ACE2 in the pathobiology of pulmonary hypertension and provides proof of concept that targeting of this enzyme can prove to be an effective therapeutic strategy for the treatment of PAH.
Intrapulmonary renin–angiotensin system (RAS) — a critical component in PAH
The RAS is composed of a series of enzymatic reactions that lead to the generation of several angiotensin peptides. The cascade begins with the proteolytic cleavage of angiotensinogen by renin to yield the inactive precursor angiotensin I. Angiotensin I is further acted upon by ACE to form Ang II, a biologically active vasopeptide that mediates its effects via two distinct receptor subtypes — AT1R and AT2R. Stimulation of the AT1R causes vasoconstriction, cell proliferation, inflammation, and fibrosis, while activation of AT2R regulates opposing effects [7]. Several new members of the RAS have recently been identified, which has expanded our understanding of this biological system. These new members include (pro) renin receptor [8], angiotensin-(1–12) [9], ACE2 [10, 11], angiotensin-(1–7) [12], and the Mas receptor [13]. All the major components of the RAS including renin, angiotensinogen, ACE, ACE2, AT1R, AT2R, and the Mas receptor are expressed in the lung tissue [5]. It is relevant to point out that polymorphism in the genes encoding for angiotensinogen [14], and ACE [15], has been linked to the development of PAH. With regard to the ACE gene, an insertion–deletion polymorphism of 287 base pairs in intron 16 results in three distinct genotypes; DD(homozygous deletion allele), II(homozygous insertion allele), and ID heterozygotes [16]. The deletion allele (DD) is associated with increased ACE activity, while the insertion allele (II) is associated with decreased ACE activity [17]. The ACE DD genotype, accompanied by elevated circulating levels of Ang II, has been implicated in the development of PAH [18]. Interestingly, the lungs of pulmonary hypertensive patients as well as animals with PAH express high levels of ACE, suggesting a causative role for this enzyme in disease pathogenesis [19]. ACE is the primary enzyme responsible for Ang II production in the lungs. This Ang II peptide is a potent pulmonary vasoconstrictor with mitogenic actions [20]. Both animal and clinical studies have shown that stimulation of the pulmonary smooth muscle cells with Ang II produces migratory, hypertrophic, and proliferative effects [21]. Furthermore, Ang II initiates inflammatory processes and generates cellular reactive oxygen species (ROS), factors that contribute to the pathophysiology of PAH [22, 23]. Accumulating evidence also suggests that Ang II induces apoptosis of the lung parenchymal cells, leading to pulmonary hypertension [24]. Collectively, these findings clearly indicate an active role for RAS in the pulmonary hypertensive process. However, pharmacological blockade with ACE inhibitors or AT1R antagonists has produced mixed results against PAH [25, 26, 27, 28, 29, 30, 31], thus rendering their medical use somewhat controversial. Nevertheless, several lines of emerging evidence strongly suggest that the recently discovered ACE homologue, ACE2, either by itself or through its catalytic product Ang-(1–7) opposes the proliferative, hypertrophic, and fibrotic effects of Ang II [32] in many organs, including the lungs, suggesting a plausible protective role against PAH.
Pharmacology and tissue distribution of ACE2
ACE2 was discovered as a close homologue of ACE about a decade ago, by two independent research groups using distinct methodologies [10, 11]. It contains 805 amino acids and shares 42% sequence homology with the active site of ACE. However, unlike ACE, which is a dipeptidyl-peptidase, ACE2 is a mono-carboxypeptidase that cleaves a single amino acid from the C-terminal of its substrates. Ang II appears to be the main substrate for ACE2, and is effectively hydrolyzed to angiotensin-(1–7) [Ang-(1–7)]. Apelin-13, Apelin 36, Neurotensin 1–8, Dynorphin A (1–13), [des-Arg9]-Bradykinin, and [Lys-des-Arg9]-Bradykinin are the other known peptide targets for ACE2. ACE2 exists as a membrane-bound protein in the lungs, stomach, spleen, intestine, bone-marrow, kidney, liver, and the brain [33, 34]. Particularly, in the lungs, it is expressed on the vascular endothelium, type I and type II alveolar epithelial cells, the smooth muscle cells of the pulmonary vasculature, and in bronchial epithelia [35]. Though, ACE2 exists primarily as a membrane-bound protein, a soluble form has been detected in both plasma and urine [36].
Contribution of ACE2 to lung pathophysiology
ACE2 has been suggested to play an important role in lung pathophysiology. Evidence for this comes from the following observations: first, decreased expression of ACE2 is associated with lung fibrosis in both human and experimental animals [37]; second, lung overexpression of ACE2 attenuates monocrotaline-induced pulmonary hypertension [38] and bleomycin-induced pulmonary fibrosis [39]; third, ACE2 protects murine lungs from acute injury [40] and respiratory distress syndrome [41]; fourth, mutant ACE2 mice exhibit enhanced vascular permeability with declining lung function [42]; fifth, reduced pulmonary ACE2 expression due to SARS-CoV infection results in lung failure [43]; sixth, administration of recombinant ACE2 prevents the development of lung failure in ACE2 knockout mice [42]; and seventh, ACE2 treatment reversed established PAH in BMPR2R899X mice (JA Johnson et al., abstract in Am J Respir Crit Care Med 2010, 181:A6327). A recent report by Takahashi et al. [44], also suggests that auto-antibodies to ACE2 may predispose patients with connective tissue diseases to PAH. All these observations substantiate the importance of ACE2 in lung pathophysiology.
Protective role of ACE2 against PAH-induced cardiac remodeling
Remodeling of the right ventricle, characterized by myocyte hypertrophy and fibrosis, is often observed in pulmonary hypertensive patients [45]. During the early stages of PAH, the remodeling process represents an adaptive response to compensate for the increased right ventricular workload. However, over time the right ventricle becomes thickened, enlarged, and dysfunctional, which can culminate in heart failure. In fact, right ventricular failure is the primary cause of death in patients with PAH [46]. Zisman et al., were the first investigators to report increased ACE2 activity and elevated angiotensin-(1–7) levels in the ventricles of PAH patients [47]. It appears that upregulation of ACE2 and the subsequent increase in Ang-(1–7) levels may be a compensatory response to protect against tissue injury. This view is supported by several experimental studies, wherein administration of ACE2 or Ang-(1–7) exerted cardio-protective effects [48, 49, 50]. Conversely, ACE2 deficiency resulted in increased incidence of cardiac death due to chronic pressure overload [51]. Of particular relevance to pulmonary therapeutics is that lung overexpression of ACE2 prevented the development of right ventricular hypertrophy in animal models of PAH and lung fibrosis (Figure 1 ). Similar beneficial effects were also observed with endogenous activation of ACE2 using XNT (1-[(2-dimethylamino) ethylamino]-4-(hydroxymethyl)-7-[(4-methylphenyl) sulfonyl oxy]-9H-xanthene-9-one) [6••]. Part of the protective mechanism of ACE2 may be related to decrease in the levels of Ang II and/or the ensuing increase in Ang-(1–7). Besides this, modulation of matrix metalloproteinases (MMPs) by ACE2, especially that of MMP-2 and MMP-9 may also be responsible for the anti-remodeling effects [52]. It is well known that MMPs contribute to cardiac remodeling in PAH [53]. Furthermore, ACE2 overexpression has been shown to inhibit hypoxia-induced collagen production by cardiac fibroblasts, highlighting the anti-fibrotic actions of ACE2 [54].
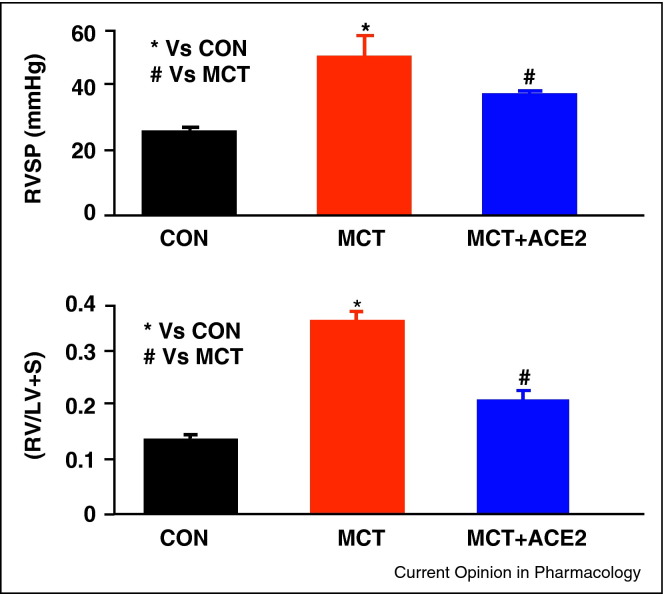
Figure 1.
Lentiviral mediated lung overexpression of ACE2 attenuates monocrotaline (MCT)-induced increase in right ventricular systolic pressure (RVSP) and prevents the development of right ventricular hypertrophy, expressed as the ratio of right ventricular weight (RV) to left ventricular + septum weight (LV + S).
Anti-proliferative effects on vascular smooth muscle cells
All the layers of the pulmonary vessel wall undergo structural and functional changes during PAH [55]. These changes include proliferation of the vascular smooth muscle cells, intimal thickening and extension of smooth muscle into previously non-muscularized arterioles. Vascular remodeling reduces lumen diameter, thereby increasing pulmonary vascular resistance and pressure. In vitro studies have shown that decreased ACE2 level is associated with hyper-proliferation and enhanced migration of pulmonary smooth muscle cells, suggesting a vasoprotective role for ACE2 [56]. These in vitro data were further confirmed by in vivo studies, wherein lung overexpression of ACE2 not only prevented vessel wall thickening but also attenuated muscularization of the pulmonary vessels [38, 39]. Administration of XNT, the synthetic ACE2 activator, also exerted similar beneficial effects on the pulmonary vasculature in an experimental model of PAH [6••].
Effect on endothelial dysfunction, oxidative stress, and inflammation
Reduced bioavailability of NO, increased formation of ROS, and inflammation are hallmarks of PAH [57]. Improvement of the aforementioned factors is essential for effective pulmonary hypertensive therapy. ACE2 is predominantly localized to the vascular endothelium and exerts vasodilatory actions [58]. On the contrary, deficiency in ACE2 impairs endothelium-dependent vasorelaxation [59], which underscores the importance of ACE2 in endothelial function. A stoichiometric balance between NO and superoxide is critical for maintaining normal vascular tone. It is well established that Ang II increases superoxide production by upregulating endothelial nicotinamide adenine dinucleotide phosphate oxidase (NADPH oxidase) resulting in vascular impairment. On the other hand, ACE2 has been shown to reduce Ang II mediated superoxide production [60], thereby improving vascular function. Inflammatory cytokines (e.g., IL-1, IL-6, TNF-α and MCP-1) and growth factors (TGF-β) substantially contribute to the development and/or progression of PAH. In this regard, treatment with ACE2 or XNT has been shown to decrease the expression of pro-inflammatory cytokines in the lungs [6••, 38]. Also, this decrease in pro-inflammatory cytokine levels was accompanied by a concomitant increase in the anti-inflammatory cytokine, IL-10. Taken together, experimental evidence indicates a protective role for ACE2 against endothelial dysfunction, oxidative stress, and inflammation.
Conclusions
In light of the limited efficacy of currently available treatment options for PAH therapy, development of novel therapeutic strategies is needed. In this regard, discovery of ACE2 seems to be relevant. As discussed above, ACE2 has been shown to exert a host of beneficial effects on the cardio-pulmonary system, resulting in the prevention of PAH (Figure 2 ). Thus, it appears that genetic approaches to increase ACE2 activity and/or the use of synthetic ACE2 activators may represent potential new therapies for effectively treating PAH.
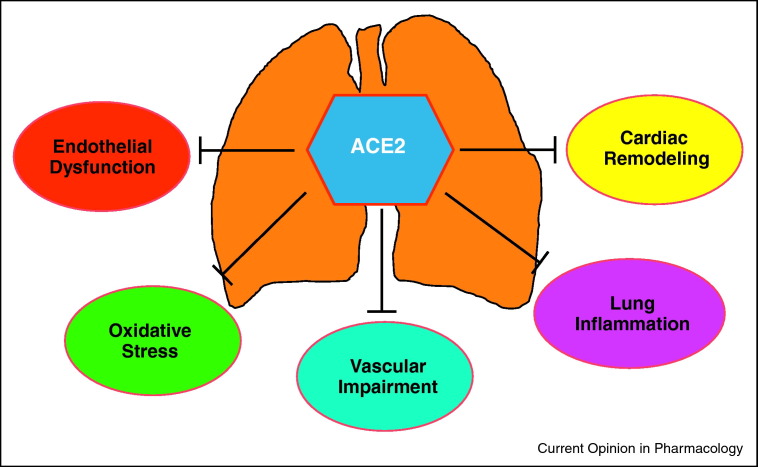
Figure 2.
ACE2 exerts a host of actions on the cardio-pulmonary system that include prevention of endothelial dysfunction, reduction in pulmonary oxidative stress, attenuation of vascular impairment, anti-inflammatory, and anti-cardiac remodeling effects. All these properties are responsible for the protective role of ACE2 against pulmonary arterial hypertension.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgement
This work was supported by N.I.H Grants HL 99980 and HL102033.
References
- 1.Galie N., Torbicki A., Barst R., Dartevelle P., Haworth S., Higenbottam T., Olschewski H., Peacock A., Pietra G., Rubin L.J. Guidelines on diagnosis and treatment of pulmonary arterial hypertension. The Task Force on Diagnosis and Treatment of Pulmonary Arterial Hypertension of the European Society of Cardiology. Eur Heart J. 2004;25:2243–2278. doi: 10.1016/j.ehj.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Loscalzo J. Genetic clues to the cause of primary pulmonary hypertension. N Engl J Med. 2001;345:367–371. doi: 10.1056/NEJM200108023450511. [DOI] [PubMed] [Google Scholar]
- 3•.Morrell N.W., Adnot S., Archer S.L., Dupuis J., Jones P.L., MacLean M.R., McMurtry I.F., Stenmark K.R., Thistlethwaite P.A., Weissmann N. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S20–S31. doi: 10.1016/j.jacc.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article comprehensively reviews the pathophysiological mechanisms involved in PAH.
- 4.Luscher T.F. Endothelial dysfunction: the role and impact of the renin–angiotensin system. Heart. 2000;84(Suppl. 1):i20–i22. doi: 10.1136/heart.84.suppl_1.i20. [discussion i50] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marshall R.P. The pulmonary renin–angiotensin system. Curr Pharm Des. 2003;9:715–722. doi: 10.2174/1381612033455431. [DOI] [PubMed] [Google Scholar]
- 6••.Ferreira A.J., Shenoy V., Yamazato Y., Sriramula S., Francis J., Yuan L., Castellano R.K., Ostrov D.A., Oh S.P., Katovich M.J., Raizada M.K. Evidence for angiotensin-converting enzyme 2 as a therapeutic target for the prevention of pulmonary hypertension. Am J Respir Crit Care Med. 2009;179:1048–1054. doi: 10.1164/rccm.200811-1678OC. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first study that describes the use of a synthetic activator of ACE2 can prevent the development of PAH and associated cardio-pulmonary remodeling.
- 7.Stroth U., Unger T. The renin–angiotensin system and its receptors. J Cardiovasc Pharmacol. 1999;33(Suppl. 1):S21–S28. doi: 10.1097/00005344-199900001-00005. [discussion S41–3] [DOI] [PubMed] [Google Scholar]
- 8.Nguyen G., Delarue F., Burckle C., Bouzhir L., Giller T., Sraer J.D. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109:1417–1427. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagata S., Kato J., Sasaki K., Minamino N., Eto T., Kitamura K. Isolation and identification of proangiotensin-12, a possible component of the renin–angiotensin system. Biochem Biophys Res Commun. 2006;350:1026–1031. doi: 10.1016/j.bbrc.2006.09.146. [DOI] [PubMed] [Google Scholar]
- 10.Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N., Donovan M., Woolf B., Robison K., Jeyaseelan R. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res. 2000;87:E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 11.Tipnis S.R., Hooper N.M., Hyde R., Karran E., Christie G., Turner A.J. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275:33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 12.Schiavone M.T., Santos R.A., Brosnihan K.B., Khosla M.C., Ferrario C.M. Release of vasopressin from the rat hypothalamo-neurohypophysial system by angiotensin-(1–7) heptapeptide. Proc Natl Acad Sci USA. 1988;85:4095–4098. doi: 10.1073/pnas.85.11.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santos R.A., Simoes e Silva A.C., Maric C., Silva D.M., Machado R.P., de Buhr I., Heringer-Walther S., Pinheiro S.V., Lopes M.T., Bader M. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci USA. 2003;100:8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solari V., Puri P. Genetic polymorphisms of angiotensin system genes in congenital diaphragmatic hernia associated with persistent pulmonary hypertension. J Pediatr Surg. 2004;39:302–306. doi: 10.1016/j.jpedsurg.2003.11.008. [discussion 302–6] [DOI] [PubMed] [Google Scholar]
- 15.Kanazawa H., Okamoto T., Hirata K., Yoshikawa J. Deletion polymorphisms in the angiotensin converting enzyme gene are associated with pulmonary hypertension evoked by exercise challenge in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162:1235–1238. doi: 10.1164/ajrccm.162.4.9909120. [DOI] [PubMed] [Google Scholar]
- 16.Niu T., Chen X., Xu X. Angiotensin converting enzyme gene insertion/deletion polymorphism and cardiovascular disease: therapeutic implications. Drugs. 2002;62:977–993. doi: 10.2165/00003495-200262070-00001. [DOI] [PubMed] [Google Scholar]
- 17.Rigat B., Hubert C., Alhenc-Gelas F., Cambien F., Corvol P., Soubrier F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. 1990;86:1343–1346. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abraham W.T., Raynolds M.V., Badesch D.B., Wynne K.M., Groves B.M., Roden R.L., Robertson A.D., Lowes B.D., Zisman L.S., Voelkel N.F. Angiotensin-converting enzyme DD genotype in patients with primary pulmonary hypertension: increased frequency and association with preserved haemodynamics. J Renin Angiotensin Aldosterone Syst. 2003;4:27–30. doi: 10.3317/jraas.2003.003. [DOI] [PubMed] [Google Scholar]
- 19.Orte C., Polak J.M., Haworth S.G., Yacoub M.H., Morrell N.W. Expression of pulmonary vascular angiotensin-converting enzyme in primary and secondary plexiform pulmonary hypertension. J Pathol. 2000;192:379–384. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH715>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 20.Lipworth B.J., Dagg K.D. Vasoconstrictor effects of angiotensin II on the pulmonary vascular bed. Chest. 1994;105:1360–1364. doi: 10.1378/chest.105.5.1360. [DOI] [PubMed] [Google Scholar]
- 21.Morrell N.W., Upton P.D., Kotecha S., Huntley A., Yacoub M.H., Polak J.M., Wharton J. Angiotensin II activates MAPK and stimulates growth of human pulmonary artery smooth muscle via AT1 receptors. Am J Physiol. 1999;277:L440–L448. doi: 10.1152/ajplung.1999.277.3.L440. [DOI] [PubMed] [Google Scholar]
- 22.Cheng Z.J., Vapaatalo H., Mervaala E. Angiotensin II and vascular inflammation. Med Sci Monit. 2005;11:RA194–RA205. [PubMed] [Google Scholar]
- 23.Schiffrin E.L., Touyz R.M. Inflammation and vascular hypertrophy induced by angiotensin II: role of NADPH oxidase-derived reactive oxygen species independently of blood pressure elevation? Arterioscler Thromb Vasc Biol. 2003;23:707–709. doi: 10.1161/01.ATV.0000069907.12357.7E. [DOI] [PubMed] [Google Scholar]
- 24.Wang R., Zagariya A., Ibarra-Sunga O., Gidea C., Ang E., Deshmukh S., Chaudhary G., Baraboutis J., Filippatos G., Uhal B.D. Angiotensin II induces apoptosis in human and rat alveolar epithelial cells. Am J Physiol. 1999;276:L885–L889. doi: 10.1152/ajplung.1999.276.5.L885. [DOI] [PubMed] [Google Scholar]
- 25.Cassis L.A., Rippetoe P.E., Soltis E.E., Painter D.J., Fitz R., Gillespie M.N. Angiotensin II and monocrotaline-induced pulmonary hypertension: effect of losartan (DuP 753), a nonpeptide angiotensin type 1 receptor antagonist. J Pharmacol Exp Ther. 1992;262:1168–1172. [PubMed] [Google Scholar]
- 26.Nguyen L., Ward W.F., Ts’ao C.H., Molteni A. Captopril inhibits proliferation of human lung fibroblasts in culture: a potential antifibrotic mechanism. Proc Soc Exp Biol Med. 1994;205:80–84. doi: 10.3181/00379727-205-43681. [DOI] [PubMed] [Google Scholar]
- 27.Kreutz R., Fernandez-Alfonso M.S., Ganten D., Paul M. Effect of losartan on right ventricular hypertrophy and cardiac angiotensin I-converting enzyme activity in pulmonary hypertensive rats. Clin Exp Hypertens. 1996;18:101–111. doi: 10.3109/10641969609082610. [DOI] [PubMed] [Google Scholar]
- 28.Molteni A., Moulder J.E., Cohen E.F., Ward W.F., Fish B.L., Taylor J.M., Wolfe L.F., Brizio-Molteni L., Veno P. Control of radiation-induced pneumopathy and lung fibrosis by angiotensin-converting enzyme inhibitors and an angiotensin II type 1 receptor blocker. Int J Radiat Biol. 2000;76:523–532. doi: 10.1080/095530000138538. [DOI] [PubMed] [Google Scholar]
- 29.Nadrous H.F., Ryu J.H., Douglas W.W., Decker P.A., Olson E.J. Impact of angiotensin-converting enzyme inhibitors and statins on survival in idiopathic pulmonary fibrosis. Chest. 2004;126:438–446. doi: 10.1378/chest.126.2.438. [DOI] [PubMed] [Google Scholar]
- 30.Otsuka M., Takahashi H., Shiratori M., Chiba H., Abe S. Reduction of bleomycin induced lung fibrosis by candesartan cilexetil, an angiotensin II type 1 receptor antagonist. Thorax. 2004;59:31–38. doi: 10.1136/thx.2003.000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molteni A., Wolfe L.F., Ward W.F., Ts’ao C.H., Molteni L.B., Veno P., Fish B.L., Taylor J.M., Quintanilla N., Herndon B., Moulder J.E. Effect of an angiotensin II receptor blocker and two angiotensin converting enzyme inhibitors on transforming growth factor-beta (TGF-beta) and alpha-actomyosin (alpha SMA), important mediators of radiation-induced pneumopathy and lung fibrosis. Curr Pharm Des. 2007;13:1307–1316. doi: 10.2174/138161207780618777. [DOI] [PubMed] [Google Scholar]
- 32.Santos R.A., Ferreira A.J., Simoes E., Silva A.C. Recent advances in the angiotensin-converting enzyme 2-angiotensin(1–7)-Mas axis. Exp Physiol. 2008;93:519–527. doi: 10.1113/expphysiol.2008.042002. [DOI] [PubMed] [Google Scholar]
- 33.Gembardt F., Sterner-Kock A., Imboden H., Spalteholz M., Reibitz F., Schultheiss H.P., Siems W.E., Walther T. Organ-specific distribution of ACE2 mRNA and correlating peptidase activity in rodents. Peptides. 2005;26:1270–1277. doi: 10.1016/j.peptides.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soler M.J., Barrios C., Oliva R., Batlle D. Pharmacologic modulation of ACE2 expression. Curr Hypertens Rep. 2008;10:410–414. doi: 10.1007/s11906-008-0076-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Epelman S., Tang W.H., Chen S.Y., Van Lente F., Francis G.S., Sen S. Detection of soluble angiotensin-converting enzyme 2 in heart failure: insights into the endogenous counter-regulatory pathway of the renin–angiotensin–aldosterone system. J Am Coll Cardiol. 2008;52:750–754. doi: 10.1016/j.jacc.2008.02.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li X., Molina-Molina M., Abdul-Hafez A., Uhal V., Xaubet A., Uhal B.D. Angiotensin converting enzyme-2 is protective but downregulated in human and experimental lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;295:L178–L185. doi: 10.1152/ajplung.00009.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamazato Y., Ferreira A.J., Hong K.H., Sriramula S., Francis J., Yamazato M., Yuan L., Bradford C.N., Shenoy V., Oh S.P. Prevention of pulmonary hypertension by angiotensin-converting enzyme 2 gene transfer. Hypertension. 2009;54:365–371. doi: 10.1161/HYPERTENSIONAHA.108.125468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shenoy V., Ferreira A.J., Qi Y., Fraga-Silva R.A., Diez-Freire C., Dooies A., Jun J.Y., Sriramula S., Mariappan N., Pourang D. The angiotensin-converting enzyme 2/angiogenesis-(1–7)/Mas axis confers cardiopulmonary protection against lung fibrosis and pulmonary hypertension. Am J Respir Crit Care Med. 2010;182:1065–1072. doi: 10.1164/rccm.200912-1840OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Imai Y., Kuba K., Penninger J.M. The discovery of angiotensin-converting enzyme 2 and its role in acute lung injury in mice. Exp Physiol. 2008;93:543–548. doi: 10.1113/expphysiol.2007.040048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Imai Y., Kuba K., Penninger J.M. Angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Cell Mol Life Sci. 2007;64:2006–2012. doi: 10.1007/s00018-007-6228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., Yang P., Sarao R., Wada T., Leong-Poi H. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takahashi Y., Haga S., Ishizaka Y., Mimori A. Autoantibodies to angiotensin-converting enzyme 2 in patients with connective tissue diseases. Arthritis Res Ther. 2010;12:R85. doi: 10.1186/ar3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bronicki R.A., Baden H.P. Pathophysiology of right ventricular failure in pulmonary hypertension. Pediatr Crit Care Med. 2010;11(2 Suppl.):S15–S22. doi: 10.1097/PCC.0b013e3181c7671c. [DOI] [PubMed] [Google Scholar]
- 46.Bogaard H.J., Abe K., Vonk Noordegraaf A., Voelkel NF The right ventricle under pressure: cellular and molecular mechanisms of right-heart failure in pulmonary hypertension. Chest. 2009;135:794–804. doi: 10.1378/chest.08-0492. [DOI] [PubMed] [Google Scholar]
- 47.Zisman L.S., Asano K., Dutcher D.L., Ferdensi A., Robertson A.D., Jenkin M., Bush E.W., Bohlmeyer T., Perryman M.B., Bristow M.R. Differential regulation of cardiac angiotensin converting enzyme binding sites and AT1 receptor density in the failing human heart. Circulation. 1998;98:1735–1741. doi: 10.1161/01.cir.98.17.1735. [DOI] [PubMed] [Google Scholar]
- 48.Huentelman M.J., Grobe J.L., Vazquez J., Stewart J.M., Mecca A.P., Katovich M.J., Ferrario C.M., Raizada M.K. Protection from angiotensin II-induced cardiac hypertrophy and fibrosis by systemic lentiviral delivery of ACE2 in rats. Exp Physiol. 2005;90:783–790. doi: 10.1113/expphysiol.2005.031096. [DOI] [PubMed] [Google Scholar]
- 49.Der Sarkissian S., Grobe J.L., Yuan L., Narielwala D.R., Walter G.A., Katovich M.J., Raizada M.K. Cardiac overexpression of angiotensin converting enzyme 2 protects the heart from ischemia-induced pathophysiology. Hypertension. 2008;51:712–718. doi: 10.1161/HYPERTENSIONAHA.107.100693. [DOI] [PubMed] [Google Scholar]
- 50.Grobe J.L., Mecca A.P., Lingis M., Shenoy V., Bolton T.A., Machado J.M., Speth R.C., Raizada M.K., Katovich M.J. Prevention of angiotensin II-induced cardiac remodeling by angiotensin-(1–7) Am J Physiol Heart Circ Physiol. 2007;292:H736–H742. doi: 10.1152/ajpheart.00937.2006. [DOI] [PubMed] [Google Scholar]
- 51.Yamamoto K., Ohishi M., Katsuya T., Ito N., Ikushima M., Kaibe M., Tatara Y., Shiota A., Sugano S., Takeda S. Deletion of angiotensin-converting enzyme 2 accelerates pressure overload-induced cardiac dysfunction by increasing local angiotensin II. Hypertension. 2006;47:718–726. doi: 10.1161/01.HYP.0000205833.89478.5b. [DOI] [PubMed] [Google Scholar]
- 52.Kassiri Z., Zhong J., Guo D., Basu R., Wang X., Liu P.P., Scholey J.W., Penninger J.M., Oudit G.Y. Loss of angiotensin-converting enzyme 2 accelerates maladaptive left ventricular remodeling in response to myocardial infarction. Circ Heart Fail. 2009;2:446–455. doi: 10.1161/CIRCHEARTFAILURE.108.840124. [DOI] [PubMed] [Google Scholar]
- 53.Ohbayashi H. Matrix metalloproteinases in lung diseases. Curr Protein Pept Sci. 2002;3:409–421. doi: 10.2174/1389203023380549. [DOI] [PubMed] [Google Scholar]
- 54.Grobe J.L., Der Sarkissian S., Stewart J.M., Meszaros J.G., Raizada M.K., Katovich M.J. ACE2 overexpression inhibits hypoxia-induced collagen production by cardiac fibroblasts. Clin Sci (Lond) 2007;113:357–364. doi: 10.1042/CS20070160. [DOI] [PubMed] [Google Scholar]
- 55.Tuder R.M., Marecki J.C., Richter A., Fijalkowska I., Flores S. Pathology of pulmonary hypertension. Clin Chest Med. 2007;28:23–42. doi: 10.1016/j.ccm.2006.11.010. vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang R., Wu Y., Zhao M., Liu C., Zhou L., Shen S., Liao S., Yang K., Li Q., Wan H. Role of HIF-1alpha in the regulation ACE and ACE2 expression in hypoxic human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2009;297:L631–L640. doi: 10.1152/ajplung.90415.2008. [DOI] [PubMed] [Google Scholar]
- 57.Budhiraja R., Tuder R.M., Hassoun P.M. Endothelial dysfunction in pulmonary hypertension. Circulation. 2004;109:159–165. doi: 10.1161/01.CIR.0000102381.57477.50. [DOI] [PubMed] [Google Scholar]
- 58.Rentzsch B., Todiras M., Iliescu R., Popova E., Campos L.A., Oliveira M.L., Baltatu O.C., Santos R.A., Bader M. Transgenic angiotensin-converting enzyme 2 overexpression in vessels of SHRSP rats reduces blood pressure and improves endothelial function. Hypertension. 2008;52:967–973. doi: 10.1161/HYPERTENSIONAHA.108.114322. [DOI] [PubMed] [Google Scholar]
- 59.Lovren F., Pan Y., Quan A., Teoh H., Wang G., Shukla P.C., Levitt K.S., Oudit G.Y., Al-Omran M., Stewart D.J. Angiotensin converting enzyme-2 confers endothelial protection and attenuates atherosclerosis. Am J Physiol Heart Circ Physiol. 2008;295:H1377–H1384. doi: 10.1152/ajpheart.00331.2008. [DOI] [PubMed] [Google Scholar]
- 60.Gwathmey T.M., Pendergrass K.D., Reid S.D., Rose J.C., Diz D.I., Chappell M.C. Angiotensin-(1–7)-angiotensin-converting enzyme 2 attenuates reactive oxygen species formation to angiotensin II within the cell nucleus. Hypertension. 2010;55:166–171. doi: 10.1161/HYPERTENSIONAHA.109.141622. [DOI] [PMC free article] [PubMed] [Google Scholar]