Abstract
Peroxisome Proliferator-Activated Receptors (PPAR)-γ belongs to the nuclear hormone receptor superfamily of ligand-dependent transcription factors. It is a mediator of adipocyte differentiation, regulates lipid metabolism and macrophage function. The ligands of PPAR-γ have long been in the clinic for the treatment of type II diabetes and have a very low toxicity profile. Activation of PPAR-γ was shown to modulate various hallmarks of cancer through its pleiotropic affects on multiple different cell types in the tumor microenvironment. An overwhelming number of preclinical-studies demonstrate the efficacy of PPAR-γ ligands in the control of tumor progression through their affects on various cellular processes, including cell proliferation, apoptosis, angiogenesis, inflammation and metastasis. A variety of signaling pathways have been implicated as potential mechanisms of action. This review will focus on the molecular basis of these mechanisms; primarily PPAR-γ cross-regulation with other signaling pathways and its relevance to lung cancer therapy will be discussed.
Keywords: PPAR, Lung cancer therapy, Synergistic drug interactions, tumor microenvironment, TZDs, PPAR-gamma ligands
Introduction
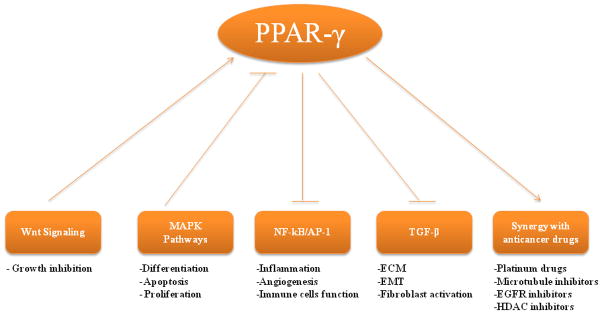
Lung cancer is the leading cause of cancer death, [1] and every year 1.2 million new cases are diagnosed worldwide. Despite improvements in diagnostic imaging, surgery, radiotherapy and chemotherapy, the overall survival for lung cancer remains poor with only 14% of patients surviving 5 years from the date of diagnosis. This underscores the desperate need for novel strategies for early detection, prevention and treatment of this disease. PPAR-γ is known to be expressed in both human SCLC and NSCLC cell lines [2, 3]. The expression status of PPAR-γ was shown to correlate with differentiation status and survival in the lung cancer patients [4, 5]. Many PPAR-γ ligands were shown to inhibit tumor growth and progression in preclinical models of lung cancer, by modulating various cellular processes in cancer cells, stromal cells and tumor microenvironment. They do so by influencing various signaling pathways in a PPAR-γ-dependent manner (Figure 1). In addition, these ligands also employ other novel mechanisms including PPAR-γ-independent mechanisms to exert their anti-neoplastic affects.
Figure 1.
Mutual cross-regulation of PPAR-γ and other signaling pathways and its implications
PPARs are members of the nuclear hormone receptor superfamily that includes receptors for steroids, thyroid hormone, vitamin D, and retinoic acid [6]. PPARs are transcription factors that upon ligand binding regulate both target gene expression and repression [7]. To date, three isotypes of PPARs called PPAR-α, PPAR-γ and PPAR-δ have been identified [8, 9]. Each of these three subtypes display differential tissue distribution and mediate specific functions such as early development, cell proliferation, differentiation, apoptosis and metabolic homeostasis [10]. PPAR-γ is highly expressed in adipose tissue and it is a master regulator of adipocyte differentiation [11, 12]. PPAR-γ is also expressed in multiple other tissues, such as breast, colon, lung, ovary, prostate and thyroid [13]. A single PPAR-γ gene is transcribed into three isoforms namely PPAR-γ1, PPAR-γ2, PPAR-γ3 and PPAR-γ4 utilizing four different promoters [8]. However PPAR-γ1 and γ3 transcripts both translate into the same PPAR-γ1 protein. PPAR-γ2 protein contains an additional 30 aminoacids at N-terminus compared to PPAR-γ1, which contribute to its constitutive transcription activation function that is 5–6-fold greater than PPAR-γ1 [14].
PPAR-γ receptors are activated by several lipophilic ligands, including long-chain polyunsaturated fatty acids and several eicosanoids. The cyclopentone prostaglandin J2, was suggested to be the most potent endogenous ligand for the PPAR-γ receptor and is the most commonly used naturally occurring PPAR-γ agonist [15, 16]. A wide range of synthetic PPAR-γ ligands have been developed. The most widely used synthetic agents belong to the thiazolidinedione class of antidiabetic drugs, that include ciglitazone, troglitazone, pioglitazone and rosiglitazone (also referred to as glitazones or TZDs). Some of the glitazones were already in clinical use as insulin sensitizers in type 2 diabetic patients [17]. Non-thiozolidinedione compounds such as isoxazolidinedione JTT-501 [18] and the tyrosine-based GW7845 [19] are also identified as PPAR-γ ligands along with several plant derived compounds. [20–22].
Activation of PPAR-γ plays an inhibitory role in cell proliferation and growth by virtue of its differentiation inducing ability. This property makes PPAR-γ activation by natural and synthetic ligands an attractive tool in cancer treatment and prevention. The precise mechanism(s) linking modulation of PPAR-γ with cancer growth inhibition is been elucidated. PPAR-γ ligands exert their effects through both PPAR-γ dependent and independent pathways, often triggering crosstalk with other signaling pathways (Figure 1). Better understanding of the biological role of PPAR-γ and its ability to trigger crosstalk with other cell signaling pathways would allow rational development of selective PPAR-γ modulators, and for targeting aspects of PPAR-γ biology that are implicated in tumor progression. This review will focus on the mechanisms of cross-regulation between PPAR-γ activation and signaling pathways that regulate hallmarks of a cancer cell and will discuss the implications of this cross-talk for lung cancer therapy.
Mode of Action
Similar to other nuclear hormone receptors, PPARs contain five distinct regions A/B, C, D, E and F [23]. C and E domains contain a highly conserved DNA-binding domain (DBD) and a moderately conserved ligand-binding domain (LBD), respectively. The amino-terminal A/B domain contains the AF1 domain which is implicated in ligand-independent activation. F region contains a ligand-dependent activation domain, AF2. The D domain harbors a variable hinge region and acts as docking site for co-factors. PPAR-γ binds to its response elements (PPRE) in the promoter region of target genes through its DBD [24]. LBD is responsible for ligand specificity and activation of PPAR-γ binding to PPRE. Recruitment of co-factors occurs on AF2, located in the F region. The E/F domain also includes a region involved in dimerization with the partner nuclear receptor, RXR [25].
Similar to other members of the nuclear hormone receptor superfamily, PPAR-γ functions as a heterodimer with the retinoid X receptor (RXR). PPAR binds to 5′repeat unit of PPRE as a heterodimer with RXR bound to the 3′ repeat. PPRE contain repeats of the sequence AGGTCA separated by one or two nucleotides (known as DR-1 or DR-2 response elements, respectively) and has been found in the promoter regions of most PPAR-γ target genes [26]. In the absence of ligand activation PPAR/RXR bind to various transcription co-repressors (nuclear receptor co-repressor/silencing mediator for retinoid and thyroid hormone receptors, NCoR/SMRT) and histone deacetylases (HDACs), preventing the binding of PPAR-γ/RXR to DNA [26]. After ligand binding PPARs undergo conformational change, recruit certain co-activator complexes (p300/CBP, p160, etc.) to displace co-repressors and the heterodimer binds to DNA on PPRE of the target genes. This results in the recruitment of additional factors (TRAP, DRIP, etc.), disruption of nucleosomes, and chromatin reorganization, facilitating the entry of general transcriptional machinery such as RNA Pol II to promote transcription [27].
PPAR-γ can also negatively regulate expression of some pro-inflammatory genes by a mechanism that does not involve PPAR-γ binding to its response elements, which is termed as transrepression [28–30]. No unifying model was established to account for transrepression activity of PPARs. The proposed models include direct protein – protein interactions with other transcription factors, competing for co-activators, interacting with co-repressors and regulation by kinase activity [26]. Specific examples and the proposed mechanisms will be discussed in the subsequent sections of the review.
Regulation of PPAR-γ Function by Growth Factor Signaling
Several studies have implicated a role for growth factor induced mitogen-activated protein kinase (MAPK) activation in the regulation of PPAR-γ function. It was shown that ERK, JNK and p38 MAPKs phosphorylate a consensus-MAPK motif (PXSPP) located in N-terminal AF-1 domain of PPAR-γ and thereby significantly inhibit its ligand-independent and ligand-dependent transcriptional activation [31–33]. Growth factor activated MAPKs phosphorylate Ser84 (Ser 82 in mouse) on PPAR-γ1 or Ser114 (Ser 112 in mouse) on PPAR-γ2 in humans [34, 35]. Consistently, mutating Ser84 or Ser112 prevents PPAR-γ phosphorylation as well as the growth factor-mediated transcriptional repression [32]. Furthermore, deletion of consensus MAPK phosphorylation motif in PPAR-γ confers enhanced transcriptional activity [36]. It was also shown that N-terminal phosphorylation results in reduced ligand-binding affinity through inter domain communication between the phosphorylated AF-1 domain and the ligand-binding pocket, resulting in the negative regulation of PPAR-γ activity [37]. Apart from this-on mitogenic stimulation, phospho-MEK directly interacts with PPAR-γ. This leads to rapid export of PPAR-γ-MEK complex from nucleus to cytoplasm through the Nuclear Export Signal (NES) of MEK, thus reducing transcriptional activity of PPAR-γ. MEK does not significantly phosphorylate PPAR-γ [35, 36].
Various in vivo studies have established that growth factor activated MAPK cascades regulate PPAR-γ function to control the balance between proliferation and differentiation in certain cell types. During adipogenic differentiation of mesenchymal stem cells, co-operation between PPAR-γ and MEK1 facilitates the adipogenic program by MEK1-dependent induction of the C/EBPα gene [38]. Consistently, inhibition of MEK attenuates high glucose enhanced adipogenesis and PPAR-γ expression in bone marrow-derived mesenchymal stem cells [39]. Mice with the knocked-in S82/112A mutant allele of PPAR-γ exhibit resistance to diet-induced obesity. Furthermore PPAR-γ phosphorylation on Ser 112 by ERK in Dok1 knockout embryonic fibroblasts exhibit defective adipogenic differentiation [40]. Interestingly, MAPK signaling can also modulate PPAR-γ functions by regulating the expression of co-factors needed for PPAR-γ transcriptional activation [41]. From the above examples, though the significance of PPAR-γ regulation by MAPKs is evident in normal physiology, its role in the cancer cell survival is not well understood. In cancer cells where MAPK signaling is elevated due to enhanced growth factor signaling, it is assumed that differentiation promoting functions of PPAR-γ are attenuated. Therefore, reactivation of PPAR-γ, by its ligands was used as a therapeutic approach to promote differentiation and growth inhibition of cancer cells. However, in certain instances MAPK activation is known to co-operate in mediating the biological effects of PPAR-γ. Troglitazone induced a sustained ERK1/2 activation, concurrent with growth inhibition in lung cancer cells, suggesting that in some cell types, PPAR-γ ligands utilize ERK-pathway to promote growth inhibition [2, 42]. Consistently, there are reports demonstrating that sustained ERK activation can induce apoptosis and differentiation in cancer cells [42, 43].
Inhibition of Pro-inflammatory Pathways by PPAR-γ Activation
Anti-inflammatory activity is one of the first non-diabetic functions attributed to PPAR-γ and its ligands. PPAR-γ agonists rosiglitazone, troglitazone and 15d-PGJ2 were shown to abrogate the expression of pro-inflammatory genes such as nitric oxide synthase (iNOS), matrix metalloproteinase 9 (MMP-9), and scavenger receptor A in murine macrophages [44] and TNF-α, IL-1β, and IL-6 in human monocytes [45]. Anti-inflammatory actions of PPAR-γ are dependent on its ability to antagonize the transcriptional regulation of NF-κB, AP-1 and STAT [44, 46]. However, rather than having a broader effect, PPAR-γ lignads were reported to selectively inhibit only a subset of genes driven by above transcription factors. For example, rosiglitazone inhibits LPS-induced MMP-9 expression, but not the LPS-induced IL-8 expression [47]. In another mechanism, PPAR-γ is proposed to mediate transrepression of a subset of LPS induced inflammatory genes in macrophages by preventing the clearance of co-repressor complexes from their promoters. Under basal conditions, iNOS gene promoter is occupied with NCoR/HDAC3/TBL/TAB2 complexes [48] and following LPS stimulation the NCoR and HDAC3 components is cleared from iNOS promoter by ubiquitin ligases. Interestingly, on agonist binding SUMOylated PPAR-γ was shown to localize to NCoR complexes on the iNOS promoter and prevents its removal by ubiquitination-dependent mechanism. Mutataion of K365 SUMOylation site on PPAR-γ prevents its ability to repress iNOS promoter [30]. Similar results were obtained for additional endogenous LPS-target genes including Ccl3, Ccl7, Cxcl10 and Tgtp, indicating that this mechanism of transrepression is not specific for the iNOS promoter. In addition, repression of only a subset of LPS-target genes by NCoR complexes indicates a PPAR-γ-specific repression rather than a general repression of all LPS target genes [30].
NF-κB is the master regulator of inflammatory responses. PPAR-γ is able to attenuate NF-κB function either by directly interfering with the transcription activating capacity of the NF-κB complex [49] or indirectly, by regulating proteins that suppress activation of NF-κB or by competing for the proteins that are essential for NF-κB function through a process known as squelching [50, 51]. Ciglitazone block LPS-induced IL-12 production in murine macrophages and promote apoptosis in HT-29 cells by inhibiting the activity of NF-κB through direct protein-protein interaction between NF-κB subunits and PPAR-γ [49, 52]. In another mechanism, PPAR-γ was shown to prevent the up regulation of p53 as well as its effector p21 in MCF-7 cells by replacing the NF-κB from its binding sites on the p53 promoter [53]. Alternatively, PPAR-γ inhibits NF-κB activity indirectly either by inducing the expression of IκBα [54], an inhibitor of NF-κB or inhibiting the IKK activity, which prevents IκBα degradation [55, 56]. Even though regulation of IκBα by PPAR-γ was not demonstrated, activation of PPAR-α in human smooth muscle cells was shown to increase IκBα levels by transcriptional activation [57]. The other common indirect mechanism of inhibition employed by PPARs, is competing for the limited pool of transcriptional co-activators such as p300, CREB-binding protein, C/EBPb and GRIP-1/TIF-2) [58, 59]. However, in many cases the role of co-regulators in transrepression by PPAR-γ were deduced from studies utilizing over-expression systems and transient transfections experiments, which do not necessarily reflect the actual scenario of transrepression observed at naturally occurring levels of coregulators. Therefore, there is a need to address these issues using appropriate experimental model systems. Similarly, direct and indirect mechanisms are implicated in PPAR-γ-mediated inhibition of other pro-inflammatory transcription factors including AP-1, C-JUN, STATs, and NFAT [58].
Activation of PPAR-γ Antagonizes TGF-β Signaling
Transforming growth factor (TGF-β) is a multifunctional cytokine involved in the regulation of cell proliferation, differentiation and extracellular matrix production. Upon TGF-β binding, the type II (TGFβRII) receptor heterodimerizes with type I (TGFβRI) TGF-β receptor at the cell surface and that results in phosphorylation of R-Smads (Smad2 and Smad3). R-Smads heterodimerize with Smad4, and translocate into the nucleus to induce transcription of target genes [60, 61]. During early stages when response to TGF-β in cells is normal, it inhibits tumorogenesis. Whereas, in later stages of tumor progression when genetic or epigenetic alterations in multiple pathways overcome the tumor suppressor activity, resulting in the pleiotropic tumor promoting roles for TGF-β [62]. Although the exact mechanism remains to be defined, mutual interference between PPAR-γ and TGF-β signaling pathways has been reported at multiple levels including phosphorylation of PPAR-γ, repression of PPAR-γ gene expression, and the interaction of PPAR-γ and Smad3 [63].
In hepatobiliary cells PPAR-γ activation inhibits the tumor suppressive activity of TGF-β by inhibiting Smad transcriptional activity. In these cells, TGF-β treatment simultaneously activates Smad-mediated gene transcription and phosphorylation of cPLA2α, wherein phosphorylation of cPLA2α initiates two signaling pathways that counteract Smad-mediated growth inhibition, including activation of its G-protein-coupled receptor EP1 through PGE2 and activation of PPAR-γ. Antisense inhibition of cPLA2α or siRNA-mediated depletion of PPAR-γ enhances TGF-β-mediated Smad activation and partially restores the growth inhibition by TGF-β [64]. On the other hand, PPAR-γ activity was inhibited by TGF-β, during adipogenic differentiation, by decreasing the expression of both C/EBPα and C/EBPβ, which are important co-regulators of PPAR-γ [65]. The basis for such a mutually antagonistic affect between PPAR-γ and TGF-β is not clear. Recently it has been demonstrated that Cited2 (CREB-binding protein/p300-interacting transactivator with ED-rich tail 2) protein functions as a transcriptional co-activator for both TGF-β [66] and PPAR-γ [67] and competing for the shared common transcriptional co-activator may result in mutual antagonism. Contrary to above examples, in vascular smooth muscle cells (VSMCs) PPAR-γ activation by pioglitazone was reported to exert direct anti-atherosclerotic and anti-restenotic effects by inducing apoptosis through increased TGF-β levels and translocation of phospho-Smad2 into nucleus. This effect was blocked either by using PPAR-γ antagonist GW9662 or anti-TGF-β1 antibody or activin receptor-like kinase inhibitor (SB-431442), demonstrating the TGF-β-dependent regulation of PPAR-γ signaling [68].
Accumulating evidence suggests that the activation of PPAR-γ can also interfere with the TGF-β signaling in the tumor microenvironment. In advanced stages of cancer, TGF-β is known to induce activation of fibroblasts to myofibroblasts [69]. Myofibroblasts serve as major stromal source of extracellular matrix proteins, especially fibrillar collagens, fibronectin, proteoglycans, MMPs, cytokines and chemokines that are involved in chemoresistance, angiogenesis, tumor migration, invasion and metastasis. Myofibroblasts affect the cancer progression by secreting and organizing altered ECM within the tumor stroma [70]. Both natural and synthetic PPAR-γ agonists are reported to suppress the activation of fibroblasts into myofibroblasts [71]. PPAR-γ ligands 15d-PGJ2, ciglitazone and rosiglitazone inhibited TGF-β-driven myofibroblast differentiation as well as type I collagen production in human lung fibroblasts without affecting their viability [71]. Pioglitazone attenuates the induction of fibronectin and its spliced variant EDA+FN by TGF-β in human mesangial cells [72]. Similarly pioglitazone counteracts fibronectin activated invasion of breast carcinoma through the suppression of TGF-β signaling. TGF-β is also a potent inducer of the process known as EMT which is implicated in the dissemination of individual cancer cells to distant organs for metastasis [69, 73]. Activation of PPAR-γ was shown to inhibit EMT at least in the context of fibrosis [74]. We have recently shown that activation of PPAR-γ inhibits tumor metastasis in lung cancer cells by antagonizing Smad3-mediated EMT [63].
Implications for Lung Cancer Therapy
Several independent studies including ours, demonstrated that various ligands of PPAR-γ induce differentiation of lung cancer cells and inhibit their growth. Transcriptional activation of PPAR-γ by ciglitazone or PGJ2 was shown to induce general (gelsolin, PPAR-γ, Mad, and p21) as well as lineage specific (MUC1, SP-A, CC10, and HTI56) differentiation markers in lung cancer cells making them less tumorigenic [75]. Interestingly, this differentiation response was observed only in the presence of serum in the culture medium where PPAR-γ ligands inhibited cell growth to promote differentiation. In the absence of serum, the same ligands induced apoptosis in the lung cancer cells, at a 5-fold lesser concentration than what is required for inducing differentiation in the presence of serum [4]. Consistently, using two different TZDs we showed inhibition of tumor cell growth both in-vitro and in-vivo, by promoting differentiation but did not induce apoptosis. In addition, we demonstrated that sustained Erk1/2 activation mediated troglitazone-induced differentiation of lung cancer cells [2].
PPAR-γ ligands inhibit growth and induce apoptosis of lung cancer cells by different mechanisms depending on the growth conditions and the ligands used. Ciglitazone and PGJ2 were shown to induce p21 expression transcriptionally by enhancing the binding of transcriptional factors SP1 and C/EBP-α to the promoter of p21 gene. [76]. Similarly, troglitazone treatment can activate the promoter activity of DNA damage inducible gene, GADD153 and inhibit growth and induce apoptosis in NSCLC cells [77]. Rosiglitazone was shown to effect lung cancer growth by modulating mTOR signaling [78]. In addition pathways such as cPLA2, Cox2, PGE2, 15-PGDH, and Wnt7a were also implicated in the PPAR-γ ligands induced growth inhibition of lung cancer cells [79] [80] [81]. In another interesting study, overexpression of PPAR-γ cDNA alone was sufficient to inhibit tumor growth in-vivo, cellular migration and invasion in-vitro in lung cancer cells [82]. This study clearly demonstrates the direct anti-neoplastic affects of PPAR-γ and suggests potential presence of an endogenous PPAR-γ ligand.
In addition to affecting cancer cells, PPAR-γ ligands also influence tumor progression by modulating various aspects of tumor microenviroment as described above in lung cancers, including angiogenesis, ECM components, immune cell function, and fibroblast activation. Rosiglitazone was shown to inhibit mouse lung tumor cell growth and metastasis in-vivo through direct and indirect anti-angiogenic effects [83]. Similarly, A549 cell xenografts from SCID mice that were treated with pioglitazone or troglitazone showed significant reduction in blood vessel density. Consistently, treatment of A549 cells, in-vitro with troglitazone or transfected with a constitutively active PPAR-γ blocked the production of angiogenic chemokines IL-8, ENA-78, and Gro-α by inhibiting NF-κB transcriptional activity that regulates their expression [84]. Among the ECM components, PPAR-γ ligands were shown to inhibit fibronectin expression by antagonizing transcription factors that regulate its expression. In addition, these ligands were also reported to inhibit the expression of α5 integrin resulting in the reduction of a fibronectin receptor, α5β1. Together, these results suggest that by inhibiting fibronectin and its receptor, PPAR-γ ligands disrupt tumor cell and ECM interactions essential for tumor cell proliferation [85, 86]. With respect to the effects on immune cell functions in tumor microenvironment, PPAR-γ ligands reverse the antitumor cytotoxic T-lymphocyte suppressive activity and the M2 phenotype of tumor associated macrophages [87]. PPAR-γ ligands are also known to inhibit the expression of several cytokines and chemokines produced by most of the major immune cell types present in the tumor microenvironment. As described earlier these ligands can inhibit activation of lung fibroblasts into myofibroblasts, a phenotype similar to that of tumor-associated fibroblasts [71]. Together, these observations suggest that PPAR-γ might be an important target for modulating lung tumor microenvironment.
PPAR-γ ligands, apart from their direct activity, also demonstrate a synergistic interaction with other cytotoxic as well as targeted anti-cancer agents. Combining platinum based chemo therapeutic drugs including cisplatin and carboplatin with PPAR-γ ligands such as rosiglitazone and GW1929, demonstrate a potent synergistic activity against lung cancer cells in-vitro and in-vivo [88, 89]. Further analysis revealed that PPAR-γ ligands induce a downregulation of metallothionins which sequester platinum drugs and prevent their cytotoxicity [89]. We observed a similar synergistic interaction between PPAR-γ ligands (troglitazone and pioglitazone) and chemotherapeutic drugs cisplatin and paclitaxel, in spite of their two different modes of action. Interestingly, this synergy was observed only when the treatment of PPAR-γ ligands is preceded by the treatment with cisplatin or paclitaxel. This sequence specific synergy was suggested to be due to induction of PPAR-γ expression by cisplatin and paclitaxel [90]. Among the targeted agents, PPAR-γ ligands facilitated the antiproliferative effects of gefitinib (rosiglitazone), an inhibitor of the epidermal growth factor receptor signaling [91], potentiated the effect of the HDAC inhibitor, phenyl butyrate ( Ciglitazone) [75], and demonstrated synergy with lovastatin (troglitazone) [92].
In summary, despite such a wide range of potential anti-tumor affects and overwhelming amount of preclinical data, demonstrating the efficacy of PPAR-γ ligands, so far there are no clinical trials testing the efficacy of these ligands in oncology, with the exception of one ongoing clinical trial in lung cancer. Though less relevant to oncology use, recent findings of potential liver and cardio toxicities associated with PPAR-γ ligands has partly tempered the enthusiasm. However, a retrospective analysis of more than 80,000 individuals revealed a 33% reduction in lung cancer risk among TZD users compared to nonusers after adjusting for confounding variables [93]. This observation, together with the relatively low toxicity profile of TZDs that are currently in clinic justify prospective, randomized, clinical studies to determine the true effect of PPAR-γ ligands, at least in lung cancer.
Acknowledgments
This work is funded by NIH/NCI (CA132571-01), and American Cancer Society (RSG - CSM-116801) grants to V.G.K.
Abbreviations
- EGFR
Epidermal growth factor receptor
- HDAC
Histone deacetylases
- IKK
IκB kinase
- IL-1β
Interleukin 1 β
- MAPK
Mitogen activated protein kinase
- MMP
Matrix metallo protease
- NES
Nuclear export signal
- PGC1α
PPARγ coactivator-1
- PGJ2
Prostaglandin J2
- PGE
Prostaglandin E
- PPAR
Peroxisome Proliferators-Activated Receptors
- PPRE
Peroxisome proliferators’ response elements
- RXR
Retinoid X receptor
- SRC-1
Steroid receptor coactivator 1
- TGF-β
Transforming growth factor β
- TNF-α
Tumor necrosis factor α
- TZD
Thiazolidinediones
Footnotes
Conflict of interest:
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000;50:7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- 2.Keshamouni VG, Reddy RC, Arenberg DA, Joel B, Thannickal VJ, Kalemkerian GP, Standiford TJ. Peroxisome proliferator-activated receptor-gamma activation inhibits tumor progression in non-small-cell lung cancer. Oncogene. 2004;23:100–108. doi: 10.1038/sj.onc.1206885. [DOI] [PubMed] [Google Scholar]
- 3.Li M, Lee TW, Mok TS, Warner TD, Yim AP, Chen GG. Activation of peroxisome proliferator-activated receptor-gamma by troglitazone (TGZ) inhibits human lung cell growth. J Cell Biochem. 2005;96:760–774. doi: 10.1002/jcb.20474. [DOI] [PubMed] [Google Scholar]
- 4.Chang TH, Szabo E. Induction of differentiation and apoptosis by ligands of peroxisome proliferator-activated receptor gamma in non-small cell lung cancer. Cancer Res. 2000;60:1129–1138. [PubMed] [Google Scholar]
- 5.Inoue K, Kawahito Y, Tsubouchi Y, Yamada R, Kohno M, Hosokawa Y, Katoh D, Bishop-Bailey D, Hla T, Sano H. Expression of peroxisome proliferator-activated receptor (PPAR)-gamma in human lung cancer. Anticancer Res. 2001;21:2471–2476. [PubMed] [Google Scholar]
- 6.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zieleniak A, Wojcik M, Wozniak LA. Structure and physiological functions of the human peroxisome proliferator-activated receptor gamma. Arch Immunol Ther Exp (Warsz) 2008;56:331–345. doi: 10.1007/s00005-008-0037-y. [DOI] [PubMed] [Google Scholar]
- 8.Fajas L, Auboeuf D, Raspe E, Schoonjans K, Lefebvre AM, Saladin R, Najib J, Laville M, Fruchart JC, Deeb S, Vidal-Puig A, Flier J, Briggs MR, Staels B, Vidal H, Auwerx J. The organization, promoter analysis, and expression of the human PPARgamma gene. J Biol Chem. 1997;272:18779–18789. doi: 10.1074/jbc.272.30.18779. [DOI] [PubMed] [Google Scholar]
- 9.Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 10.Misra P, Owuor ED, Li W, Yu S, Qi C, Meyer K, Zhu YJ, Rao MS, Kong AN, Reddy JK. Phosphorylation of transcriptional coactivator peroxisome proliferator-activated receptor (PPAR)-binding protein (PBP). Stimulation of transcriptional regulation by mitogen-activated protein kinase. J Biol Chem. 2002;277:48745–48754. doi: 10.1074/jbc.M208829200. [DOI] [PubMed] [Google Scholar]
- 11.Spiegelman BM. PPAR-gamma: adipogenic regulator and thiazolidinedione receptor. Diabetes. 1998;47:507–514. doi: 10.2337/diabetes.47.4.507. [DOI] [PubMed] [Google Scholar]
- 12.Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 13.Lambe KG, Tugwood JD. A human peroxisome-proliferator-activated receptor-gamma is activated by inducers of adipogenesis, including thiazolidinedione drugs. Eur J Biochem. 1996;239:1–7. doi: 10.1111/j.1432-1033.1996.0001u.x. [DOI] [PubMed] [Google Scholar]
- 14.Werman A, Hollenberg A, Solanes G, Bjorbaek C, Vidal-Puig AJ, Flier JS. Ligand-independent activation domain in the N terminus of peroxisome proliferator-activated receptor gamma (PPARgamma). Differential activity of PPARgamma1 and -2 isoforms and influence of insulin. J Biol Chem. 1997;272:20230–20235. doi: 10.1074/jbc.272.32.20230. [DOI] [PubMed] [Google Scholar]
- 15.Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 16.Willson TM, Brown PJ, Sternbach DD, Henke BR. The PPARs: from orphan receptors to drug discovery. J Med Chem. 2000;43:527–550. doi: 10.1021/jm990554g. [DOI] [PubMed] [Google Scholar]
- 17.Kletzien RF, Clarke SD, Ulrich RG. Enhancement of adipocyte differentiation by an insulin-sensitizing agent. Mol Pharmacol. 1992;41:393–398. [PubMed] [Google Scholar]
- 18.Shibata T, Matsui K, Nagao K, Shinkai H, Yonemori F, Wakitani K. Pharmacological profiles of a novel oral antidiabetic agent, JTT-501, an isoxazolidinedione derivative. Eur J Pharmacol. 1999;364:211–219. doi: 10.1016/s0014-2999(98)00832-2. [DOI] [PubMed] [Google Scholar]
- 19.Suh N, Wang Y, Williams CR, Risingsong R, Gilmer T, Willson TM, Sporn MB. A new ligand for the peroxisome proliferator-activated receptor-gamma (PPAR-gamma), GW7845, inhibits rat mammary carcinogenesis. Cancer Res. 1999;59:5671–5673. [PubMed] [Google Scholar]
- 20.Hwang BY, Lee JH, Nam JB, Kim HS, Hong YS, Lee JJ. Two new furanoditerpenes from Saururus chinenesis and their effects on the activation of peroxisome proliferator-activated receptor gamma. J Nat Prod. 2002;65:616–617. doi: 10.1021/np010440j. [DOI] [PubMed] [Google Scholar]
- 21.Kuroda M, Mimaki Y, Sashida Y, Mae T, Kishida H, Nishiyama T, Tsukagawa M, Konishi E, Takahashi K, Kawada T, Nakagawa K, Kitahara M. Phenolics with PPAR-gamma ligand-binding activity obtained from licorice (Glycyrrhiza uralensis roots) and ameliorative effects of glycyrin on genetically diabetic KK-A(y) mice. Bioorg Med Chem Lett. 2003;13:4267–4272. doi: 10.1016/j.bmcl.2003.09.052. [DOI] [PubMed] [Google Scholar]
- 22.Xu J, Fu Y, Chen A. Activation of peroxisome proliferator-activated receptor-gamma contributes to the inhibitory effects of curcumin on rat hepatic stellate cell growth. Am J Physiol Gastrointest Liver Physiol. 2003;285:G20–30. doi: 10.1152/ajpgi.00474.2002. [DOI] [PubMed] [Google Scholar]
- 23.Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999;20:649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- 24.AIJ, Jeannin E, Wahli W, Desvergne B. Polarity and specific sequence requirements of peroxisome proliferator-activated receptor (PPAR)/retinoid X receptor heterodimer binding to DNA. A functional analysis of the malic enzyme gene PPAR response element. J Biol Chem. 1997;272:20108–20117. doi: 10.1074/jbc.272.32.20108. [DOI] [PubMed] [Google Scholar]
- 25.Kliewer SA, Umesono K, Noonan DJ, Heyman RA, Evans RM. Convergence of 9-cis retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature. 1992;358:771–774. doi: 10.1038/358771a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daynes RA, Jones DC. Emerging roles of PPARs in inflammation and immunity. Nat Rev Immunol. 2002;2:748–759. doi: 10.1038/nri912. [DOI] [PubMed] [Google Scholar]
- 27.Rochette-Egly C. Nuclear receptors: integration of multiple signalling pathways through phosphorylation. Cell Signal. 2003;15:355–366. doi: 10.1016/s0898-6568(02)00115-8. [DOI] [PubMed] [Google Scholar]
- 28.Standiford TJ, Keshamouni VG, Reddy RC. Peroxisome proliferator-activated receptor-{gamma} as a regulator of lung inflammation and repair. Proc Am Thorac Soc. 2005;2:226–231. doi: 10.1513/pats.200501-010AC. [DOI] [PubMed] [Google Scholar]
- 29.Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 30.Pascual G, Fong AL, Ogawa S, Gamliel A, Li AC, Perissi V, Rose DW, Willson TM, Rosenfeld MG, Glass CK. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature. 2005;437:759–763. doi: 10.1038/nature03988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu E, Kim JB, Sarraf P, Spiegelman BM. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARgamma. Science. 1996;274:2100–2103. doi: 10.1126/science.274.5295.2100. [DOI] [PubMed] [Google Scholar]
- 32.Camp HS, Tafuri SR. Regulation of peroxisome proliferator-activated receptor gamma activity by mitogen-activated protein kinase. J Biol Chem. 1997;272:10811–10816. doi: 10.1074/jbc.272.16.10811. [DOI] [PubMed] [Google Scholar]
- 33.Adams M, Reginato MJ, Shao D, Lazar MA, Chatterjee VK. Transcriptional activation by peroxisome proliferator-activated receptor gamma is inhibited by phosphorylation at a consensus mitogen-activated protein kinase site. J Biol Chem. 1997;272:5128–5132. doi: 10.1074/jbc.272.8.5128. [DOI] [PubMed] [Google Scholar]
- 34.Burgermeister E, Seger R. PPARgamma and MEK Interactions in Cancer. PPAR Res. 2008;2008:309469. doi: 10.1155/2008/309469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burgermeister E, Seger R. MAPK kinases as nucleo-cytoplasmic shuttles for PPARgamma. Cell Cycle. 2007;6:1539–1548. doi: 10.4161/cc.6.13.4453. [DOI] [PubMed] [Google Scholar]
- 36.Burgermeister E, Chuderland D, Hanoch T, Meyer M, Liscovitch M, Seger R. Interaction with MEK causes nuclear export and downregulation of peroxisome proliferator-activated receptor gamma. Mol Cell Biol. 2007;27:803–817. doi: 10.1128/MCB.00601-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shao D, Rangwala SM, Bailey ST, Krakow SL, Reginato MJ, Lazar MA. Interdomain communication regulating ligand binding by PPAR-gamma. Nature. 1998;396:377–380. doi: 10.1038/24634. [DOI] [PubMed] [Google Scholar]
- 38.Prusty D, Park BH, Davis KE, Farmer SR. Activation of MEK/ERK signaling promotes adipogenesis by enhancing peroxisome proliferator-activated receptor gamma (PPARgamma ) and C/EBPalpha gene expression during the differentiation of 3T3-L1 preadipocytes. J Biol Chem. 2002;277:46226–46232. doi: 10.1074/jbc.M207776200. [DOI] [PubMed] [Google Scholar]
- 39.Chuang CC, Yang RS, Tsai KS, Ho FM, Liu SH. Hyperglycemia enhances adipogenic induction of lipid accumulation: involvement of extracellular signal-regulated protein kinase 1/2, phosphoinositide 3-kinase/Akt, and peroxisome proliferator-activated receptor gamma signaling. Endocrinology. 2007;148:4267–4275. doi: 10.1210/en.2007-0179. [DOI] [PubMed] [Google Scholar]
- 40.Hosooka T, Noguchi T, Kotani K, Nakamura T, Sakaue H, Inoue H, Ogawa W, Tobimatsu K, Takazawa K, Sakai M, Matsuki Y, Hiramatsu R, Yasuda T, Lazar MA, Yamanashi Y, Kasuga M. Dok1 mediates high-fat diet-induced adipocyte hypertrophy and obesity through modulation of PPAR-gamma phosphorylation. Nat Med. 2008;14:188–193. doi: 10.1038/nm1706. [DOI] [PubMed] [Google Scholar]
- 41.Coll T, Jove M, Rodriguez-Calvo R, Eyre E, Palomer X, Sanchez RM, Merlos M, Laguna JC, Vazquez-Carrera M. Palmitate-mediated downregulation of peroxisome proliferator-activated receptor-gamma coactivator 1alpha in skeletal muscle cells involves MEK1/2 and nuclear factor-kappaB activation. Diabetes. 2006;55:2779–2787. doi: 10.2337/db05-1494. [DOI] [PubMed] [Google Scholar]
- 42.Cagnol S, Chambard JC. ERK and cell death: mechanisms of ERK-induced cell death--apoptosis, autophagy and senescence. FEBS J. 277:2–21. doi: 10.1111/j.1742-4658.2009.07366.x. [DOI] [PubMed] [Google Scholar]
- 43.Kim J, Adam RM, Freeman MR. Activation of the Erk mitogen-activated protein kinase pathway stimulates neuroendocrine differentiation in LNCaP cells independently of cell cycle withdrawal and STAT3 phosphorylation. Cancer Res. 2002;62:1549–1554. [PubMed] [Google Scholar]
- 44.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 45.Hinz B, Brune K, Pahl A. 15-Deoxy-Delta(12,14)-prostaglandin J2 inhibits the expression of proinflammatory genes in human blood monocytes via a PPAR-gamma-independent mechanism. Biochem Biophys Res Commun. 2003;302:415–420. doi: 10.1016/s0006-291x(03)00195-5. [DOI] [PubMed] [Google Scholar]
- 46.Chinetti G, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors (PPARs): nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm Res. 2000;49:497–505. doi: 10.1007/s000110050622. [DOI] [PubMed] [Google Scholar]
- 47.Shu H, Wong B, Zhou G, Li Y, Berger J, Woods JW, Wright SD, Cai TQ. Activation of PPARalpha or gamma reduces secretion of matrix metalloproteinase 9 but not interleukin 8 from human monocytic THP-1 cells. Biochem Biophys Res Commun. 2000;267:345–349. doi: 10.1006/bbrc.1999.1968. [DOI] [PubMed] [Google Scholar]
- 48.Straus DS, Glass CK. Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol. 2007;28:551–558. doi: 10.1016/j.it.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 49.Chen F, Wang M, O’Connor JP, He M, Tripathi T, Harrison LE. Phosphorylation of PPARgamma via active ERK1/2 leads to its physical association with p65 and inhibition of NF-kappabeta. J Cell Biochem. 2003;90:732–744. doi: 10.1002/jcb.10668. [DOI] [PubMed] [Google Scholar]
- 50.Castrillo A, Diaz-Guerra MJ, Hortelano S, Martin-Sanz P, Bosca L. Inhibition of IkappaB kinase and IkappaB phosphorylation by 15-deoxy-Delta(12,14)-prostaglandin J(2) in activated murine macrophages. Mol Cell Biol. 2000;20:1692–1698. doi: 10.1128/mcb.20.5.1692-1698.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Straus DS, Pascual G, Li M, Welch JS, Ricote M, Hsiang CH, Sengchanthalangsy LL, Ghosh G, Glass CK. 15-deoxy-delta 12,14-prostaglandin J2 inhibits multiple steps in the NF-kappa B signaling pathway. Proc Natl Acad Sci U S A. 2000;97:4844–4849. doi: 10.1073/pnas.97.9.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chung SW, Kang BY, Kim SH, Pak YK, Cho D, Trinchieri G, Kim TS. Oxidized low density lipoprotein inhibits interleukin-12 production in lipopolysaccharide-activated mouse macrophages via direct interactions between peroxisome proliferator-activated receptor-gamma and nuclear factor-kappa B. J Biol Chem. 2000;275:32681–32687. doi: 10.1074/jbc.M002577200. [DOI] [PubMed] [Google Scholar]
- 53.Bonofiglio D, Aquila S, Catalano S, Gabriele S, Belmonte M, Middea E, Qi H, Morelli C, Gentile M, Maggiolini M, Ando S. Peroxisome proliferator-activated receptor-gamma activates p53 gene promoter binding to the nuclear factor-kappaB sequence in human MCF7 breast cancer cells. Mol Endocrinol. 2006;20:3083–3092. doi: 10.1210/me.2006-0192. [DOI] [PubMed] [Google Scholar]
- 54.Vanden Berghe W, Vermeulen L, Delerive P, De Bosscher K, Staels B, Haegeman G. A paradigm for gene regulation: inflammation, NF-kappaB and PPAR. Adv Exp Med Biol. 2003;544:181–196. doi: 10.1007/978-1-4419-9072-3_22. [DOI] [PubMed] [Google Scholar]
- 55.Boyault S, Bianchi A, Moulin D, Morin S, Francois M, Netter P, Terlain B, Bordji K. 15-Deoxy-delta(12,14)-prostaglandin J(2) inhibits IL-1beta-induced IKK enzymatic activity and IkappaBalpha degradation in rat chondrocytes through a PPARgamma-independent pathway. FEBS Lett. 2004;572:33–40. doi: 10.1016/j.febslet.2004.06.090. [DOI] [PubMed] [Google Scholar]
- 56.Rossi A, Kapahi P, Natoli G, Takahashi T, Chen Y, Karin M, Santoro MG. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IkappaB kinase. Nature. 2000;403:103–108. doi: 10.1038/47520. [DOI] [PubMed] [Google Scholar]
- 57.Delerive P, Gervois P, Fruchart JC, Staels B. Induction of IkappaBalpha expression as a mechanism contributing to the anti-inflammatory activities of peroxisome proliferator-activated receptor-alpha activators. J Biol Chem. 2000;275:36703–36707. doi: 10.1074/jbc.M004045200. [DOI] [PubMed] [Google Scholar]
- 58.Ricote M, Glass CK. PPARs and molecular mechanisms of transrepression. Biochim Biophys Acta. 2007;1771:926–935. doi: 10.1016/j.bbalip.2007.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li M, Pascual G, Glass CK. Peroxisome proliferator-activated receptor gamma-dependent repression of the inducible nitric oxide synthase gene. Mol Cell Biol. 2000;20:4699–4707. doi: 10.1128/mcb.20.13.4699-4707.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Attisano L, Wrana JL. Signal transduction by the TGF-beta superfamily. Science. 2002;296:1646–1647. doi: 10.1126/science.1071809. [DOI] [PubMed] [Google Scholar]
- 61.Nakao A, Imamura T, Souchelnytskyi S, Kawabata M, Ishisaki A, Oeda E, Tamaki K, Hanai J, Heldin CH, Miyazono K, ten Dijke P. TGF-beta receptor-mediated signalling through Smad2, Smad3 and Smad4. EMBO J. 1997;16:5353–5362. doi: 10.1093/emboj/16.17.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Massague J. TGFbeta in Cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reka AK, Kurapati H, Narala VR, Bommer G, Chen J, Standiford TJ, Keshamouni VG. Peroxisome proliferator-activated receptor-gamma activation inhibits tumor metastasis by antagonizing Smad3-mediated epithelial-mesencymal transition. Mol Cancer Ther. 2010;9 (12):1–12. doi: 10.1158/1535-7163.MCT-10-0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Han C, Demetris AJ, Liu Y, Shelhamer JH, Wu T. Transforming growth factor-beta (TGF-beta) activates cytosolic phospholipase A2alpha (cPLA2alpha)-mediated prostaglandin E2 (PGE)2/EP1 and peroxisome proliferator-activated receptor-gamma (PPAR-gamma)/Smad signaling pathways in human liver cancer cells. A novel mechanism for subversion of TGF-beta-induced mitoinhibition. J Biol Chem. 2004;279:44344–44354. doi: 10.1074/jbc.M404852200. [DOI] [PubMed] [Google Scholar]
- 65.Choy L, Derynck R. Transforming growth factor-beta inhibits adipocyte differentiation by Smad3 interacting with CCAAT/enhancer-binding protein (C/EBP) and repressing C/EBP transactivation function. J Biol Chem. 2003;278:9609–9619. doi: 10.1074/jbc.M212259200. [DOI] [PubMed] [Google Scholar]
- 66.Chou YT, Wang H, Chen Y, Danielpour D, Yang YC. Cited2 modulates TGF-beta-mediated upregulation of MMP9. Oncogene. 2006;25:5547–5560. doi: 10.1038/sj.onc.1209552. [DOI] [PubMed] [Google Scholar]
- 67.Tien ES, Davis JW, Vanden Heuvel JP. Identification of the CREB-binding protein/p300-interacting protein CITED2 as a peroxisome proliferator-activated receptor alpha coregulator. J Biol Chem. 2004;279:24053–24063. doi: 10.1074/jbc.M401489200. [DOI] [PubMed] [Google Scholar]
- 68.Redondo S, Ruiz E, Santos-Gallego CG, Padilla E, Tejerina T. Pioglitazone induces vascular smooth muscle cell apoptosis through a peroxisome proliferator-activated receptor-gamma, transforming growth factor-beta1, and a Smad2-dependent mechanism. Diabetes. 2005;54:811–817. doi: 10.2337/diabetes.54.3.811. [DOI] [PubMed] [Google Scholar]
- 69.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shimoda M, Mellody KT, Orimo A. Carcinoma-associated fibroblasts are a rate-limiting determinant for tumour progression. Semin Cell Dev Biol. 21:19–25. doi: 10.1016/j.semcdb.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Burgess HA, Daugherty LE, Thatcher TH, Lakatos HF, Ray DM, Redonnet M, Phipps RP, Sime PJ. PPARgamma agonists inhibit TGF-beta induced pulmonary myofibroblast differentiation and collagen production: implications for therapy of lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1146–1153. doi: 10.1152/ajplung.00383.2004. [DOI] [PubMed] [Google Scholar]
- 72.Maeda A, Horikoshi S, Gohda T, Tsuge T, Maeda K, Tomino Y. Pioglitazone attenuates TGF-beta(1)-induction of fibronectin synthesis and its splicing variant in human mesangial cells via activation of peroxisome proliferator-activated receptor (PPAR)gamma. Cell Biol Int. 2005;29:422–428. doi: 10.1016/j.cellbi.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 73.Moustakas A, Heldin CH. Signaling networks guiding epithelial-mesenchymal transitions during embryogenesis and cancer progression. Cancer Sci. 2007;98:1512–1520. doi: 10.1111/j.1349-7006.2007.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tan X, Dagher H, Hutton CA, Bourke JE. Effects of PPAR gamma ligands on TGF-beta1-induced epithelial-mesenchymal transition in alveolar epithelial cells. Respir Res. 11:21. doi: 10.1186/1465-9921-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chang TH, Szabo E. Enhanced growth inhibition by combination differentiation therapy with ligands of peroxisome proliferator-activated receptor-gamma and inhibitors of histone deacetylase in adenocarcinoma of the lung. Clin Cancer Res. 2002;8:1206–1212. [PubMed] [Google Scholar]
- 76.Han S, Sidell N, Fisher PB, Roman J. Up-regulation of p21 gene expression by peroxisome proliferator-activated receptor gamma in human lung carcinoma cells. Clin Cancer Res. 2004;10:1911–1919. doi: 10.1158/1078-0432.ccr-03-0985. [DOI] [PubMed] [Google Scholar]
- 77.Satoh T, Toyoda M, Hoshino H, Monden T, Yamada M, Shimizu H, Miyamoto K, Mori M. Activation of peroxisome proliferator-activated receptor-gamma stimulates the growth arrest and DNA-damage inducible 153 gene in non-small cell lung carcinoma cells. Oncogene. 2002;21:2171–2180. doi: 10.1038/sj.onc.1205279. [DOI] [PubMed] [Google Scholar]
- 78.Han S, Roman J. Rosiglitazone suppresses human lung carcinoma cell growth through PPARgamma-dependent and PPARgamma-independent signal pathways. Mol Cancer Ther. 2006;5:430–437. doi: 10.1158/1535-7163.MCT-05-0347. [DOI] [PubMed] [Google Scholar]
- 79.Han S, Roman J. Suppression of prostaglandin E2 receptor subtype EP2 by PPARgamma ligands inhibits human lung carcinoma cell growth. Biochem Biophys Res Commun. 2004;314:1093–1099. doi: 10.1016/j.bbrc.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 80.Hazra S, Batra RK, Tai HH, Sharma S, Cui X, Dubinett SM. Pioglitazone and rosiglitazone decrease prostaglandin E2 in non-small-cell lung cancer cells by up-regulating 15-hydroxyprostaglandin dehydrogenase. Mol Pharmacol. 2007;71:1715–1720. doi: 10.1124/mol.106.033357. [DOI] [PubMed] [Google Scholar]
- 81.Winn RA, Van Scoyk M, Hammond M, Rodriguez K, Crossno JT, Jr, Heasley LE, Nemenoff RA. Antitumorigenic effect of Wnt 7a and Fzd 9 in non-small cell lung cancer cells is mediated through ERK-5-dependent activation of peroxisome proliferator-activated receptor gamma. J Biol Chem. 2006;281:26943–26950. doi: 10.1074/jbc.M604145200. [DOI] [PubMed] [Google Scholar]
- 82.Bren-Mattison Y, Van Putten V, Chan D, Winn R, Geraci MW, Nemenoff RA. Peroxisome proliferator-activated receptor-gamma (PPAR(gamma)) inhibits tumorigenesis by reversing the undifferentiated phenotype of metastatic non-small-cell lung cancer cells (NSCLC) Oncogene. 2005;24:1412–1422. doi: 10.1038/sj.onc.1208333. [DOI] [PubMed] [Google Scholar]
- 83.Panigrahy D, Singer S, Shen LQ, Butterfield CE, Freedman DA, Chen EJ, Moses MA, Kilroy S, Duensing S, Fletcher C, Fletcher JA, Hlatky L, Hahnfeldt P, Folkman J, Kaipainen A. PPARgamma ligands inhibit primary tumor growth and metastasis by inhibiting angiogenesis. J Clin Invest. 2002;110:923–932. doi: 10.1172/JCI15634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Keshamouni VG, Arenberg DA, Reddy RC, Newstead MJ, Anthwal S, Standiford TJ. PPAR-gamma activation inhibits angiogenesis by blocking ELR+CXC chemokine production in non-small cell lung cancer. Neoplasia. 2005;7:294–301. doi: 10.1593/neo.04601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Han S, Ritzenthaler JD, Rivera HN, Roman J. Peroxisome proliferator-activated receptor-gamma ligands suppress fibronectin gene expression in human lung carcinoma cells: involvement of both CRE and Sp1. Am J Physiol Lung Cell Mol Physiol. 2005;289:L419–428. doi: 10.1152/ajplung.00002.2005. [DOI] [PubMed] [Google Scholar]
- 86.Han S, Rivera HN, Roman J. Peroxisome proliferator-activated receptor-gamma ligands inhibit alpha5 integrin gene transcription in non-small cell lung carcinoma cells. Am J Respir Cell Mol Biol. 2005;32:350–359. doi: 10.1165/rcmb.2004-0345OC. [DOI] [PubMed] [Google Scholar]
- 87.Van Ginderachter JA, Meerschaut S, Liu Y, Brys L, De Groeve K, Hassanzadeh Ghassabeh G, Raes G, De Baetselier P. Peroxisome proliferator-activated receptor gamma (PPARgamma) ligands reverse CTL suppression by alternatively activated (M2) macrophages in cancer. Blood. 2006;108:525–535. doi: 10.1182/blood-2005-09-3777. [DOI] [PubMed] [Google Scholar]
- 88.Girnun GD, Chen L, Silvaggi J, Drapkin R, Chirieac LR, Padera RF, Upadhyay R, Vafai SB, Weissleder R, Mahmood U, Naseri E, Buckley S, Li D, Force J, McNamara K, Demetri G, Spiegelman BM, Wong KK. Regression of drug-resistant lung cancer by the combination of rosiglitazone and carboplatin. Clin Cancer Res. 2008;14:6478–6486. doi: 10.1158/1078-0432.CCR-08-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Girnun GD, Naseri E, Vafai SB, Qu L, Szwaya JD, Bronson R, Alberta JA, Spiegelman BM. Synergy between PPARgamma ligands and platinum-based drugs in cancer. Cancer Cell. 2007;11:395–406. doi: 10.1016/j.ccr.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reddy RC, Srirangam A, Reddy K, Chen J, Gangireddy S, Kalemkerian GP, Standiford TJ, Keshamouni VG. Chemotherapeutic drugs induce PPAR-gamma expression and show sequence-specific synergy with PPAR-gamma ligands in inhibition of non-small cell lung cancer. Neoplasia. 2008;10:597–603. doi: 10.1593/neo.08134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee SY, Hur GY, Jung KH, Jung HC, Kim JH, Shin C, Shim JJ, In KH, Kang KH, Yoo SH. PPAR-gamma agonist increase gefitinib’s antitumor activity through PTEN expression. Lung Cancer. 2006;51:297–301. doi: 10.1016/j.lungcan.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 92.Yao CJ, Lai GM, Chan CF, Cheng AL, Yang YY, Chuang SE. Dramatic synergistic anticancer effect of clinically achievable doses of lovastatin and troglitazone. Int J Cancer. 2006;118:773–779. doi: 10.1002/ijc.21361. [DOI] [PubMed] [Google Scholar]
- 93.Govindarajan R, Ratnasinghe L, Simmons DL, Siegel ER, Midathada MV, Kim L, Kim PJ, Owens RJ, Lang NP. Thiazolidinediones and the risk of lung, prostate, and colon cancer in patients with diabetes. J Clin Oncol. 2007;25:1476–1481. doi: 10.1200/JCO.2006.07.2777. [DOI] [PubMed] [Google Scholar]