Abstract
Lysophosphatidic acid (LPA) is a common product of glycerophospholipid metabolism and an important mediator of signal transduction. Aberrantly high LPA concentrations accompany multiple disease states. One potential approach for treatment of these diseases, therefore, is the therapeutic application of antibodies that recognize and bind LPA as their antigen. We have determined the x-ray crystal structure of an anti-LPA antibody (LT3015) Fab fragment in its antigen-free form to 2.15 Å resolution and in complex with two LPA isotypes (14:0 and 18:2) to resolutions of 1.98 and 2.51 Å, respectively. The variable complementarity determining region (CDR) loops at the antigen binding site adopt nearly identical conformations in the free and antigen-bound crystal structures. The crystallographic models reveal that the LT3015 antibody employs both heavy and light chain CDR loops to create a network of eight hydrogen bonds with the glycerophosphate head group of its LPA antigen. The head group is almost completely excluded from contact with solvent, while the hydrocarbon tail is partially solvent exposed. In general, mutation of amino acid residues at the antigen binding site disrupts LPA binding. However, the introduction of particular mutations chosen strategically based upon the structures can positively influence LPA binding affinity. Finally, these structures elucidate the exquisite specificity demonstrated by an anti-lipid antibody for binding a structurally simple and seemingly unconstrained target molecule.
Keywords: antibodies, cancer therapies, lipid signal transduction, lysophosphatidic acid, x-ray crystallography
INTRODUCTION
In addition to their role as integral components of biological membranes, many lipids also function as potent signaling molecules that influence a variety of cellular processes. Some examples of lipids with biological activity include cholesterol, sphingomyelin, and the glycerophospholipids as well as many of their modification and hydrolysis products1. Lysophosphatidic acid (LPA) is one example of a signaling lipid for which abnormally high concentrations are associated with several disease pathologies. LPA levels increase near sites of inflammation and are observed in inflammatory diseases such as rheumatoid arthritis2. Moreover, LPA has been linked to a multitude of physiological processes including angiogenesis and chemotaxis3,4. With respect to cancer, higher than normal LPA concentrations are associated with several carcinomas and contribute to tumor cell growth and metastasis5,6.
Antibody-mediated therapy relies upon antibodies that have the ability to bind specific antigens with high affinity. Antibodies have several advantages as therapeutics: they can be produced in large amounts, are stable when delivered intravenously, they can be extremely selective, and they are typically cleared by the body’s normal immunological processes after binding their target. One recent example of successful use of an antibody as a therapeutic is Bevacizumab (Avastin), a humanized monoclonal antibody that is directed against the protein VEGF and that is used to treat diverse metastatic tumors7. Additional antibody-mediated therapies include targeting proteins such as the insulin-like growth factor receptor and HER28,9. One possible weakness of such therapeutic approaches is that it is possible for the protein epitope to mutate, thus rendering the therapy less effective. Since LPA is a signaling lipid and, therefore, not likely subject to mutation it may not suffer the limitations of antibodies raised against proteins10.
As part of a larger effort to develop new therapies aimed at treating disease by influencing the levels of specific lipids in the plasma of patients, we have produced a humanized monoclonal antibody (LT3015) against diverse biologically active forms of the lipid LPA. In order to gain insight into its mode of antigen recognition and binding, we determined x-ray crystal structures of the LT3015 Fab fragment alone and in complex with two different LPA isotypes and validated the models by site-directed mutagenesis and in vitro binding experiments.
RESULTS
LPA binding by LT3015
In order to better understand the molecular mechanism by which LT3015 recognizes LPA antigens, we prepared and purified LT3015 antibody whole IgG and Fab fragments and tested their binding to different LPA isotypes in vitro (Figure 1a). The two forms of the LT3015 antibody display comparable binding affinities toward a biotinylated stearic acid (18:0)-containing LPA. Neither whole IgG nor Fab fragment versions of the LT1009 antibody that recognizes the closely related biologically active lipid sphingosine-1-phosphate (S1P) interacts with LPA in this assay (Figure 1b). LT3015 binding to two LPA isoforms containing either myristic acid (14:0) or linoleic acid (18:2) was next assayed based upon the ability of free LPA to compete with the biotinylated LPA for binding to either the whole IgG or the isolated Fab fragment (Figure 1c). This study yielded equilibrium dissociation constants (Ki) that range between 50–250 nM. In general, we observe that both whole IgG and Fab preparations of LT3015 bind with nanomolar affinity to diverse LPA isotypes in vitro.
Figure 1.
The LT3015 antibody binds LPA. (a) Two LPA isotypes. The first contains an esterified saturated myristic acid (14:0) moiety and the second contains the polyunsaturated fatty acid linoleic acid (18:2). Numbering the of carbon atoms corresponds with that of their respective atomic coordinate files. (b) Saturation binding assay of biotinylated-LPA (18:0) to surface-anchored LT3015 and LT1009 antibodies and Fab fragments. (c) Competition assays in which unlabeled LPA (14:0) or (18:2) were used to displace biotinylated-LPA (18:0) from LT3015 whole antibody (IgG) or Fab fragment (Fab) complexes.
LT3015 Fab x-ray crystal structure
We next produced single crystals of the free LT3015 Fab fragment and determined its x-ray crystal structure by molecular replacement. The resulting model was refined against diffraction data to a resolution limit of 2.15 Å (Table 1). The structure reveals the familiar immunoglobin folds of the antibody light and heavy chains (Figure 2A). The elbow angle measures 165.0°, a typical value for Fab fragment structures containing κ light chains11. An elongated loop CDR-L1 adopts an extended conformation that stretches into solvent while CDR-H3 folds down over a vast hydrophobic cavity in a manner reminiscent of the homologous region of the LT1009 anti-S1P antibody12.
Table 1.
Data collection and refinement statistics
| LT3015 Fab | LT3015 Fab: LPA (14:0) | LT3015 Fab: LPA (18:2) | |
|---|---|---|---|
| Data collection | |||
| X-ray source | ALS 8.2.2 | NSLS X25 | NSLS X25 |
| Wavelength (Å) | 1.0000 | 1.0000 | 1.0000 |
| Space group | P43212 | C2221 | C2221 |
| Unit cell (Å) | |||
| a | 84.52 | 86.53 | 86.98 |
| b | 84.52 | 184.35 | 183.13 |
| c | 145.28 | 130.00 | 127.50 |
| Molecules/asymm. unit | 1 | 2 | 2 |
| Resolution range (Å)1 | 50.0 – 2.15 (2.23 – 2.15) | 50.0 – 1.98 (2.01 – 1.98) | 50.0 – 2.51 (2.54 – 2.51) |
| Rsym (%) | 6.5 (64.5) | 6.1 (48.2) | 12.2 (47.5) |
| Observations | 331,182 | 536,235 | 257,208 |
| Unique reflections | 29,461 | 72,518 | 34,955 |
| Completeness (%) | 100 (100) | 99.9 (99.9) | 100 (100) |
| Redundancy | 11.2 (10.1) | 7.4 (7.2) | 7.4 (7.4) |
| <I/σ> | 36.0 (3.4) | 29.9 (3.6) | 16.9 (4.3) |
| Refinement | |||
| Number of reflections | 26,305 | 68,835 | 33,177 |
| Rcryst (%) | 0.238 (0.290) | 0.222 (0.247) | 0.215 (0.272) |
| Rfree (%)2 | 0.287 (0.377) | 0.252 (0.309) | 0.266 (0.381) |
| Protein atoms | 3,343 | 6,686 | 6,686 |
| LPA/Ion/PGE/H2O atoms | 0/10/10/160 | 50/0/0/284 | 58/0/0/97 |
| R.m.s.d. | |||
| Bond lengths (Å) | 0.012 | 0.010 | 0.010 |
| Bond angles (°) | 1.33 | 1.29 | 1.27 |
| Mean B factors(Å2) | |||
| Protein atoms | 44.36 | 22.50 | 27.98 |
| LPA | - | 23.10 | 31.16 |
| Ion | 54.40 | - | - |
| PGE | 53.69 | - | - |
| H2O | 45.12 | 26.96 | 23.30 |
| Ramachandran plot3 | |||
| Favored | 97.22 | 97.56 | 96.06 |
| Allowed | 2.78 | 2.32 | 3.94 |
| Disallowed | 0.00 | 0.124 | 0.00 |
| PDB accession code | 3QCT | 3QCU | 3QCV |
Data in parentheses are for highest resolution shell
Calculated against a cross-validation set of 5.1% of data selected at random prior to refinement.
Calculated from MOLPROBITY34.
ProH41 exhibits a disallowed combination of phi/psi angles.
Figure 2.
The LT3015 anti-LPA antibody Fab x-ray crystal structure. (a) A ribbon diagram representation of the Fab fragment with the heavy chain colored bottle green and the light chain in spring green. Variable and constant domains are labeled. Two sulfate ions and a triethylene glycol moiety that are bound near the antigen binding site in the crystal are shown in ball-and-stick representation and the side chains of several amino acids from this region are also depicted. (b) A closeup view of the antigen binding site with 2|FO|−|FC| difference electron density (in blue) about the two sulfate ions (SO4) and triethylene glycol (PGE) calculated at 1.1 σ. The extended CDR-L1 loop is also labeled. (c) The same closeup view of the LT3015 antigen binding site as in panel b after 90° rotation about the x-axis. (d) The electrostatic surface potential of the LT3015 Fab antigen binding site calculated in APBS and plotted from −20 (red) to +20 (blue) kBT/e. Note that the two sulfate ions and the triethylene glycol were not included in the calculation and that the molecule is oriented as in panel c.
Electron density for two sulfate ions is clearly observable at the antigen binding site within the LT3015 Fab crystal structure (Figure 2b, c). One ion resides at the interface between the heavy and light chains while the second is more surface-exposed and mediates contacts with a close-packed neighbor in the crystal. The presence of sulfate in the x-ray structure is not surprising as the crystal grew after equilibration against 1.75 M ammonium sulfate. Calculation and modeling of the electrostatic surface potential on the LT3015 Fab crystal structure reveals strongly electropositive pockets at both the sites where the two sulfates are bound (Figure 2d). Another strong peak of elongated electron density was refined as triethylene glycol (PGE). As PGE was not a component of the crystallization or crystal stabilization solutions, it is likely that this represents a relatively short, ordered portion of a larger polyethylene glycol (PEG) polymer. Additive amounts of PEG 400 were necessary for LT3015 Fab crystallization. The position occupied by the PGE molecule partially fills a hydrophobic pocket created by CDR-L3 and the three heavy chain CDR loops and mediates contact with the CH1 domain of a close-packed neighbor in the crystal.
LT3015 Fab:LPA complex crystal structure
In order to directly observe the interactions that mediate specific and high affinity binding of LPA by LT3015, we next crystallized and determined the 1.98 Å x-ray crystal structure of the LT3015 Fab in complex with its LPA antigen. The version of LPA used in this experiment contained the fully saturated 14-carbon myristic acid (14:0) esterified to glycerol at the carbon-1 position. The complex crystallized in a centered orthorhombic space group with two complexes in the asymmetric unit allowing for independent refinement of two LT3015 Fab:LPA complex structures in slightly different chemical environments. The complex crystal structure reveals that LT3015 binds LPA through an intricate network of hydrogen bonds and hydrophobic interactions involving amino acids from loops in both the light and heavy chains (Figure 3a, b). The refined electron density is excellent for LPA at the antigen binding site in both complexes in the asymmetric unit.
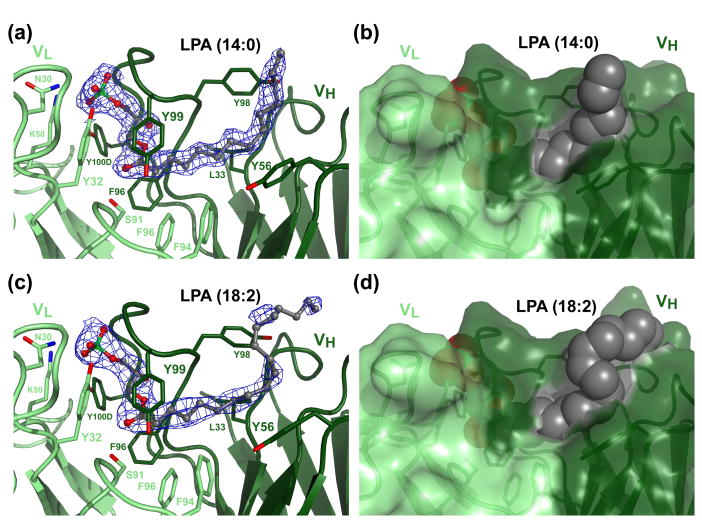
Figure 3.
LPA in the LT3015 antigen binding site. (a) Electron density about the LPA (14:0) antigen (ball-and-stick rendering) from refined 2|FO|−|FC| difference maps is contoured in blue at 1.2 σ. Amino acid side chains that contact the antigen are rendered as sticks and labeled. Electron density from the other complex in the asymmetric unit is practically identical. (b) The extent to which the antigen is shielded from solvent is illustrated by the LT3015 Fab:LPA (14:0) complex crystal structure oriented as in panel a and depicted with the Fab as a semi-transparent surface and the antigen as cpk spheres. (c) Antigen electron density in the LT3015 Fab:LPA (18:2) complex crystal structure oriented and labeled as in panel a. (d) Semi-transparent surface rendering of the complex between LT3015 Fab and the lipid LPA (18:2).
Comparison of the two symmetrically unrelated LT3015 Fab:LPA complex structures reveals slight, but noticeable differences. For example, the side chain of TyrH98 presents a different rotomer and the tail ends of the LPA antigen adopt slightly different conformations in the two structures (the Kabat and Wu numbering convention is used throughout this paper and the letters “L” and “H” immediately prior to amino acid numbers indicates that they derive from light or heavy chains, respectively)13. It is likely that these differences result from the involvement of these atoms in close packing of the two complexes of the crystallographic asymmetric unit.
LT3015 Fab:LPA (18:2) complex crystal structure
The LPA (14:0) isotype is not commonly found in biological samples and is likely not as potent a signaling molecule as LPA that contains longer chain fatty acids with varying degrees of unsaturation14. One of the most abundant and potent LPA isotypes contains linoleic acid, the 18-carbon di-unsaturated (18:2) fatty acid, esterified to glycerol at carbon-115,16. In order to determine how LT3015 binds LPA (18:2) we next crystallized and solved the 2.51 Å x-ray crystal structure of the LT3015 Fab:LPA (18:2) complex.
The complex was prepared in a manner similar to the LPA (14:0) complex and crystallized under similar conditions in the same space group (Table 1). The structure reveals only minor differences between the two complex structures. Electron density about the LPA (18:2) ligand is not as well defined as for the saturated version (Figure 3c, d). This is particularly noticeable in the region of the hydrocarbon tail where slightly higher values for thermal factors were generated during B factor refinement. Interestingly, the terminal portion (ω1-ω5 carbons) of LPA (18:2) hovers over an intramolecular hydrogen bond formed between the side chains of AsnH52 and SerH54. This interaction, along with the position of the TyrH98 side chain, projects the terminal portion of the hydrophobic tail away from the antibody and out into solvent. Otherwise, the antibody:antigen interactions observed in the LPA (14:0) are preserved in the structure of the complex between LT3015 and the unsaturated LPA (18:2).
Formation of the complex between the LT3015 Fab and its LPA antigen buries between 60 (18:2) and 67% (14:0) of the LPA molecular surface area (Table 2). For LPA (18:2), this places it squarely in line with other small molecule antigens whose structures in complex with antibodies are known. LPA (14:0), on the other hand, is among the top small molecule antigens when ranked based on the ratio of percent buried surface/molecular surface area17. The extent to which the LPA (14:0) is shielded from solvent by LT3015 is similar to that observed in the complex of the LT1009 antibody Fab fragment and its S1P antigen12. However, the nature of the interactions in the two antibody:antigen complexes differ drastically. LT1009 effectively buries the hydrocarbon tail portion of S1P while contacting its head group through two bridging Ca2+ ions that leave the phosphate relatively solvent exposed. In contrast, LT3015 almost completely buries the LPA phosphoglycerol head group while leaving significant portions of the hydrocarbon tail uncovered (Figure 3b, d).
Table 2.
Molecular surface area (in Å2)
| Molecule A | Molecule B | Average | |
|---|---|---|---|
| LPA(14:0) | |||
| Free lipid | 412.12 | 412.52 | 412.32 |
| Bound lipid | 133.22 | 137.12 | 135.17 |
| Percent buried | 67.7 | 66.8 | 67.2 |
| LPA(18:2) | |||
| Free lipid | 475.48 | 474.12 | 474.80 |
| Bound lipid | 183.97 | 187.99 | 185.96 |
| Percent buried | 61.3 | 60.3 | 60.8 |
Analysis was carried out using Surface Racer38
Overall, the conformation of the LT3015 antibody is the same with or without antigen bound. Elbow angles for the two Fab:LPA complexes are 167.5° and 166.9° compared to 165.0° for the free Fab 11. Interestingly, the CDR loop conformations are also nearly perfectly shared by the Fab in its LPA complex and free crystal structures (Figure 4). Superposition of the free Fab on to the two Fab:LPA complexes leads to a root-mean-squared deviation (RMSD) of only 0.84 Å for all Cα positions. Remarkably, the value of this difference is precisely the same whether the RMSD is calculated for Cα positions throughout the entire Fab fragment, throughout the two variable domains only, or for just the six CDR loops.
Figure 4.
Superposition of the free and LPA-bound LT3015 Fab structures. (a) The LT3015 Fab:LPA (14:0) complex (green) and free Fab (sand) structures are overlaid and viewed as in Figure 3. Ligands are depicted in ball-and-stick representations and contact amino acids are shown as sticks and labeled. (b) The same overlay from part A rotated 90° about the x-axis.
LT3015:LPA interactions
LT3015 employs eight amino acids from both its light and heavy chains to bind the LPA glycerophosphate head group through an intricate hydrogen bonding network (Figure 5). The AsnL30 and TyrL32 side chains from CDR-L1 each contribute one hydrogen atom to the same phosphate oxygen on LPA. Furthermore, additional amino acids from the extended CDR-L1 pack closely to the heavy chain nearly completely occluding the phosphate from solvent and likely increasing the stability of the buried hydrogen bonds. LysL50 from CDR-L2 participates in a hydrogen bond with phosphate while SerL91 from CDR-L3 shares a hydrogen with the acyl oxygen of the esterified myristic acid moiety. The only hydrogen bond mediated by an amino acid side chain from the antibody heavy chain is between TyrH100D from CDR-H3 and phosphate. The three remaining hydrogen bonds rely upon amide nitrogens from CDR-H3 glycine residues. GlyH100 and GlyH100B from CDR-H3 each contact phosphate oxygens while GlyH97 hydrogen bonds with the hydroxyl group at the carbon-2 position of glycerol. Two ordered water molecules occupy positions over the only surface of the LPA phosphate moiety that is accessible to solvent bringing the total to ten hydrogen bonds to LPA in the LT3015 Fab:LPA complex crystal structure.
Figure 5.
Hydrogen bonding in the LT3015 Fab:LPA (14:0) complex crystal structure. Variable and heavy chains are colored as in previous figures and LPA is rendered in ball-and-stick representation. Hydrogen bonds are shown as black dashed lines. For clarity, the side chain of amino acid residue TyrH99 is not displayed.
The remaining contacts between LT3015 antibody and its LPA antigen are dominated by closely packed hydrophobic interactions. The LPA aliphatic tail is contacted by PheL94 and PheL96 from CDR-L3 as well as LeuH33 (CDR-H1), TyrH56 (CDR-H2), and PheH96, TyrH98, and TyrH99 (CDR-H3). TyrH99 passes over the glycerol portion of LPA and appears to stabilize the CDR-H3 conformation by fastening to the light chain through interactions with the side chains of HisL27D and PheL96. A similar “restraint” role is played by the identical TyrH98 amino acid in the anti-S1P LT1009 Fab:S1P complex crystal structure, suggesting that this interaction may be a conserved mechanism for stabilizing antibody-lipid complexes12.
Site-directed mutagenesis
In order to validate the crystallographic models and assess the contribution of individual amino acid positions to overall complex stability, we performed site-directed mutagenesis and in vitro binding assays. Whole LT3015 IgG containing specific mutations were expressed in mammalian cells, purified to homogeneity, and assayed for binding to biotinylated LPA (18:0). Mutation on the CDR-H3 loop of TyrH99 to Ala completely abrogates LPA binding (Figure 6a). This suggests a more important role for this residue than simply contacting the glycerol head group of LPA. It seems likely that by passing over the bound LPA and fastening against the light chain, TyrH99 might position the CDR-H3 loop such that four hydrogen bonds (mediated by GlyH97, GlyH100, GlyH100B, and TyrH100D) can be created. Mutation of TyrH100D, also from CDR-H3, to Asn severely weakens LPA binding affinity. This suggests that exclusion of water by the bulky TyrH100D side chain is at least as important to complex stability as is its ability to form hydrogen bonds with the LPA glycerophosphate head group. We arrived upon a similar conclusion after TyrL32 from loop CDR-L1 was mutated to Arg and the resulting protein was observed to bind LPA extremely weakly.
Figure 6.
Site-directed mutagenesis and LPA binding assays of LT3015. (a) LPA binding affinity measured as in Figure 1B for native LT3015 (WT) and three LT3015 single point mutations. (b) In comparison to native LT3015 (WT), the introduction of mutations in the LT3015 heavy chain (AsnH52Tyr/SerH52Tyr) or light chain (AnsL30Arg) does not significantly increase LPA affinity. However, their combination (AsnH52Tyr/SerH54Tyr/AsnL30Arg) results in a modified version of the antibody with increased LPA binding affinity.
Based upon the LT3015 Fab:LPA complex crystal structures, mutations were introduced at two positions in the antibody that contact either the phosphate group (AsnL30) or the terminal end of the fatty acid tail (AsnH52 and SerH54). AsnL30 was mutated to Arg based upon the assumption that the longer, basic amino acid side chain could better contact the LPA phosphate. AsnH52 and SerH54 were both mutated to Tyr in an effort to augment interactions between LT3015 and the hydrocarbon tail of LPA. When introduced separately, neither mutated antibody exhibits significant alteration of its binding affinity for biotinylated LPA (18:0). However, the introduction of the mutations at both sites results in a mutated LT3015 antibody with significantly (roughly 5-fold) improved LPA binding affinity (Figure 6b).
AsnL30 contributes one hydrogen bond to the phosphate group of LPA. We suspect that replacement of this residue with Arg might better shield the LPA phosphate head group while maintaining or improving the ability of the antibody hydrogen bond with phosphate. Substitution of AsnH52 and SerH54 to Tyr disrupts an intramolecular hydrogen bond within the antigen binding site and may result in a more favorable surface for hydrophobic interactions with the LPA fatty acid tail. As the murine antibody from which LT3015 was generated by immunizing mice with an LPA adduct that contained the short lauric acid (12:0), it is possible that the CDR-H2 did not recognize the lipid and, therefore, was not optimized for contacting LPA isoforms with longer fatty acid hydrocarbon chains.
Lipid specificity and cross-reactivity
The ImmuneY2™ technology developed at Lpath has lead to the generation of antibodies that recognize bioactive lipid targets with a high degree of specificity10. This is illustrated through in vitro competition binding assays in which a broad range of phospholipids compete with biotinylated LPA (18:0) for binding to LT3015. A gallery of the structures of each of the lipids used in this experiment is available as Supplemental Data. The experiment reveals that the closely related lipid sphingosine-1-phosphate (S1P) fails to effectively compete with LPA for LT3015 binding (Figure 7a). The LT3015 Fab:LPA crystal structures suggest that the unique amino alcohol head group of S1P would disrupt the LT3015 hydrogen bonding network and that the addition of choline would interfere with the close packing between the LT3015 light chain CDR loops, the GlyH100-SerH100A-GlyH100B hairpin turn from loop CDR-H3, and the lipid phosphate. The choline-containing lysophophatidylcholine (LPC) also fails to bind LT3015 competitively. Attachment of choline to phosphate in the head group of LPA likely disrupts the close packing of loops CDR-H3 and CDR-L1 and breaks the network of hydrogen bonds. This also explains the inability LT3015 to cross-react with other lysophospholipids that contain modified phosphate head groups such as lysophosphatidylserine, lysophosphatidylinositol, and lysophosphatidylglycerol (data not shown). Furthermore, as illustrated by the lipids phosphatidic acid (PA) and phosphatidylcholine (PC), the addition of fatty acids at the sn-2 position of glycerol disrupt LT3015 binding. This substitution likely disrupts the ability of LT3015 loop CDR-H3 to adopt the conformation observed in the LT3015 crystal structures. The combination of these factors likely contributes to observed inability of LT3015 to bind the potent signaling lipid platelet-activating factor (PAF).
Figure 7.
Antigen binding specificity of LT3015. (a) Competition binding assays were carried out on surface-anchored biotin-LPA (18:0):LT3015 complexes with the lipids: sphingosine-1-phosphate (S1P), lysophosphatidylcholine (18:0 LPC), phosphatidic acid (PA), phosphatidiylcholine (PC), and platelet-activating factor (PAF) in comparison with LPA (18:1 LPA). (b) Competition binding assays were carried out on surface-anchored biotin-LPA (18:0):LT3015 complexes with the lipids: 1-alkyl-lysophosphatidic acid (C18:1 ether LPA), monoacylglyceral (18:1 sn-1 ester MG), and lysophosphatidic acid cyclic phosphodiester (18:1 ester cLPA) in comparison with LPA (18:1 ester LPA). Other than native LPA, only cLPA shows appreciable binding to LT3015.
The strict specificity of LT3015 is further borne out upon comparison of its behavior in competition assays with lipids that are more closely structurally related to LPA. Alteration of the linkage between the fatty acid and glycerol from ester to ether yields 1-alkyl-lysophosphatidic acid (C18:1 ether LPA) and removal of LPA phosphate converts LPA to monoacyl glycerol (18:1 sn-1 ester MG). Neither of these more subtle changes generates lipids with LT3015 binding affinities that are comparable to LPA (Figure 7b). The only lipid tested by this assay that binds even weakly to the LT3015 antibody is the cyclic phosphodiester version of LPA (18:1 ester cLPA) in which one phosphate moiety is covalently attached to both glycerol carbons-3 and -2. This alters the position and fixes the orientation of the phosphate relative to the rest of the head group. Modeling cLPA into the LT3015 antigen binding site suggests that TyrL32 and LysL55 side chains and the backbone amide of G100B amide remain poised to interact with oxygen atoms in the cyclic phosphate ring.
Comparison of anti-LPA and anti-S1P antibody structures
Like LPA, the signaling molecule S1P has been shown to play a critical role in several important cellular processes including proliferation, migration, and cell survival18,19. However, little is known about the manner by which specific phospholipids are recognized by binding proteins20. We recently reported the x-ray crystal structure of the humanized monoclonal antibody (LT1009) Fab fragment bound to S1P. The structure revealed that LT1009 relies on two bridging Ca2+ ions to bind its S1P antigen12.
Comparison of the amino acid sequences of the LT3015 and LT1009 CDR loops reveals a high degree of similarity in two of the three heavy chain hypervariable regions (CDR-H1 and CDR-H2) as well as light chain CDR-L3. As a consequence of the insertion of a phenylalanine residue at position 96 of CDR-H3 and the numbering convention of Kabat and Wu, which relies on the position of CDR residues relative to conserved landmarks within the sequence of the antibody scaffold, several conserved residues within this loop are numbered differently in the LT3015 and LT1009 antibodies13. TyrH99 of LT3015, for example, occupies the same position in sequence as the identical residue TyrH98 in LT1009 (Figure 8). The sequences of the two antibodies differ significantly in CDR-L1 and -L2. This is notable as three neighboring Asp residues in CDR-L1 of LT1009 (AspL30-32) and AspL92 from the LT1009 CDR-L2 loop are involved in coordinating a pair of calcium ions that bridge the antibody and its S1P antigen12. LT3015 lacks these metal-coordinating residues. It is not surprising, therefore, that the affinity of LPA binding by LT3015 is independent of metal ion concentration. Finally, the LT3015 CDR-L1 loop contains an insertion of five additional amino acids.
Figure 8.
Comparison of antigen binding modes by LT3015 and LT1009 (PDB ID: 3I9G). (a) Ribbon diagram representation of the LT3015 Fab:LPA complex crystal structure with the heavy and light chains colored in green shades as in previous figures and the LPA antigen rendered in ball-and-stick representation. (b) The same model from part A superimposed upon the LT1009 Fab:S1P complex crystal structure. The LT1009 heavy chain is colored brown and the light chain is gold. S1P is shown as a ball-and-stick model. Two Ca2+ ions are depicted as light grey spheres labeled “Ca”. (c) The LT1009 Fab:S1P complex crystal structure with the same coloring and orientation as in panel B. (d–f) Each of panels a, b, and c, respectively, are rotated 90° about the horizontal axis.
Comparison of the LT1009 and LT3015 complex structures reveals that they employ drastically different chemistries in recognizing and binding to their S1P and LPA antigens, respectively. Therefore, it is somewhat surprising that the two antibodies share significant sequence and structural homology within their CDR loops. Principal differences are in loops CDR-L1 and CDR-H3. Whereas, in LT1009 the heavy chain primarily contributes hydrophobic residues for burying the S1P hydrophobic tail, the LT3015 heavy chain appears to also be critical for antigen binding specificity.
Superposition of the two antibody Fab:lipid complex crystal structures illustrates their similarities and differences (Figure 8). The LT1009 CDR-L1 contains an AspL30-AspL31-Asp32 motif that coordinates two Ca2+ ions that, in turn, contact phosphate from the head group of S1P. The analogous loop in LT3015 contributes a pair of hydrogen bonds to the phosphate and possesses an insertion of five amino acid residues that shield the LPA phosphate group from contact with solvent. As a direct consequence of these differences, the phosphate head group of LPA binds to both the LT3015 light and heavy chains and is significantly more buried than the S1P phosphate group, which is bound (through Ca2+) entirely by LT1009 light chain atoms and is largely solvent exposed. These differences in the lipid head group positions lead to drastic differences in the roles played by the closely related CDR-H3 loops from the two antibodies. As discussed earlier, the LT3015 CDR-H3 loop contributes four hydrogen bonds to the head group of LPA, three of which contact phosphate. The same loop from LT1009 forms two hydrogen bonds to the carbon-3 hydroxyl group of S1P. Both employ a conserved Tyr residue (TyrH99 in LT3015 and TyrH98 in LT1009) to pass over their respective antigens and contact the light chain. However, TyrH99 in LT3015 covers the LPA head group while TyrH98 of LT1009 lies squarely over the center of the S1P hydrocarbon tail.
DISCUSSION
The biologically active lipids lysophosphatidic acid (LPA) and sphingosine-1-phosphate (S1P) are ubiquitous in mammals. Both lipids can be generated through the phosphodiesterase enzyme autotaxin, which in soluble form is known as lysoPLD18,21,22. LPA and S1P interact with G protein-coupled receptors activating both unique and overlapping pathways23. Both lipids can promote cellular migration, which may contribute to cancer cell metastasis, as well as stimulate angiogenesis, which is a key factor in tumor development and growth2,4,10,19. The extent to which LPA promotes angiogenesis is currently being determined. S1P, on the other hand, has been compared with known potent angiogenic molecules VEGF and bFGF24.
As part of an effort to treat diseases by influencing the concentration and identity of signaling lipids in patients, scientists at Lpath have developed technology to generate antibodies that bind with high affinity and specificity to individual biologically active lipids. Here we report the x-ray crystal structures of the Fab fragment of LT3015, a humanized monoclonal antibody developed to specifically recognize and bind LPA, in its free form and in complex with LPA (14:0) and (18:2) isotypes. It is important to note that LT3015 does not necessarily represent the lead candidate in Lpath’s program to develop a therapeutic antibody that targets LPA.
The LT3015 Fab crystal structures reveal minimal differences in the CDR loop conformations of the free and antigen-bound antibodies. Even CDR-H3, which often displays conformational variability upon antigen binding, is identical in each of three unique chemical environments (one free and two antigen-bound monomers) from two significantly different crystallization conditions. The presence of two bound sulfate ions, one occupying the same site as the phosphate of LPA, and an ordered portion of a PEG molecule over an antigen binding site hydrophobic groove in the antigen-free LT3015 Fab structure could contribute to this similarity. However, it is possible that the CDR loops of LT3015 are highly constrained and that only a small number of alternative stable conformations are available to the antibody. Such an antibody with a more rigidly structured antigen binding site might be better suited to accommodate binding to antigens that exhibit more conformational flexibility, as is expected with lipids such as LPA. Antigen binding site stability could also explain the remarkable specificity of the LT3015 antibody for LPA over other closely related lipids as CDR loop conformational flexibility has been proven to support induced-fit binding of diverse antigens and antibody multispecificity25–27.
In comparison with the anti-S1P antibody LT1009, LT3015 employs a drastically different chemistry to recognize its small molecule biologically active lipid antigen. Whereas, LT1009 relies on coordination of two Ca2+ ions to bind to phosphate and buries the hydrophobic tail of S1P, LT3015 employs multiple hydrogen bonds and bulky aromatic amino acids to bury the LPA head group while only partially shielding the hydrocarbon tail. While the differences observed within the vicinity of the phospho-head groups result from variability in the amino acid sequence of the antibody light chain CDR loops and heavy chain CDR-H1, we observe an overall similarity within the amino acid sequences of the LT1009 and LT3015 lipid tail-contacting CDR-H2 and CDR-H3 loops. This suggests that these heavy chain loops are capable of mediating interactions with the hydrocarbon components of diverse lipids.
The two x-ray crystal structures of LT3015 Fab in complex with saturated myristic acid (14:0) and di-unsaturated linoleic acid (18:2) LPA isotypes do not immediately suggest an explanation for the small but measurable differences in affinity of LT3015 for the two lipids. The slightly higher affinity (lower Ki) of LT3015 toward LPA containing the shorter myristic acid compared to linoleic acid correlates with a larger percentage of lipid surface area buried in the crystal structures suggesting one determinant of lipid binding affinity. Along these lines, the increased surface exposure of the linoleic acid (18:2) hydrophobic tail upon binding likely imposes a larger entropic penalty that contributes to the observed difference in binding. However, preliminary binding data reveal that LT3015 binds to LPA bearing the saturated stearic acid (18:0) with an even weaker affinity than to LPA (18:2). This phenomenon of preferential LPA isotype binding also exists for LPA agonists binding to their cognate G protein-coupled receptors14. Like LT3015, most receptors favor binding to unsaturated LPA isotypes. However, in contrast to LT3015 the G protein-coupled receptors display weaker binding for LPA species with shorter hydrocarbon tails. Therefore, it appears that a combination of factors involving the number and type of conformations available to the lipid hydrocarbon tail as well as the amount of solvent exposure ultimately dictate preferential binding of individual LPA isotypes.
Taken together, our x-ray structure analyses on antibodies that selectively bind two such similar molecules as LPA and S1P suggest how, despite their relative dearth of potentially reactive chemical groups and their closely related structures, the two lipids are capable of binding selectively to diverse macromolecules. This specificity is central to their respective functions as unique mediators of signal transduction and it further validates targeting with monoclonal antibodies as a viable approach for therapeutic intervention.
MATERIALS AND METHODS
Production and humanization of an anti-LPA monoclonal antibody
Murine anti-LPA antibodies were isolated by immunizing mice with a lauric acid (12:0) ester LPA covalently linked to a carrier protein. The hypervariable loop sequences from one of the hybridomas was grafted on to a humanized scaffold giving rise to LT3015 (manuscript in preparation).
Large-scale antibody expression and purification
The full-length LT3015 antibody was produced from stable CD-CHO cells and purified using protein-A affinity chromatography as previously described for the anti-sphingosine-1-phosphate (S1P) antibody LT100912.
Fab preparation
Purified, full-length LT3015 was incubated with slow rocking for 3 hours at 37°C in a 100:1 ratio with activated papain (Worthington; treat 10 mg/mL papain in 5.5 mM cysteine-HCl, 1.1 mM EDTA, 67 μM 2-mercaptoethanol for 0.5 h at 37°C) in digestion buffer (50 mM sodium phosphate pH 7.2, 2 mM EDTA). The reaction was quenched with 50 mM iodoacetamide, diluted 6-fold in 50 mM Tris-HCl pH 7.5, 150 mM NaCl, and loaded onto a pre-equilibrated Pro-Sep-vA Ultra column (Millipore). The column flow through containing the LT3015 Fab was concentrated at 4°C using a Centricon-30 centrifugal concentrator (Millipore) at 2000 × g and loaded into a Superdex 75 10/300 size exclusion column equilibrated with 50 mM Tris-HCl pH 7.5, 150mM NaCl. Peak fractions were combined and concentrated by Centricon-30 to a final concentration of 12 mg/mL (determined by absorption at 280 nm).
Complex formation
One mL of 50 mg/mL 14:0 LPA (Avanti Polar Lipids) suspension in chloroform/methanol/water was dried in a 12 × 75 mm borosilicate glass tube under low vacuum for 90 minutes. Separately, 50 mg of the dried 14:0 LPA and 50 mg 18:2 LPA (Echelon Biosciences) were resuspended to 50 mg/mL in 50 mM Tris-HCl pH 7.5, 150 mM NaCl by incubation in a sonic bath for 10 minutes and then added in 10-fold molar excess to purified LT3015 Fab and incubated for 5 days at 4°C. The resulting ~10 mg/mL LPA:LT3015 Fab emulsions were filtered through 0.22 μm Costar Spin-X centrifugal cellulose acetate filters (Corning), transferred to glass screw top vials, and stored at 4°C.
Crystallization
All crystals were grown at room temperature by the hanging drop, vapor diffusion method. One μL of 12 mg/mL LT3015 Fab was mixed with 1 μL of reservoir solution containing 0.1 M HEPES sodium pH 7.5, 2% PEG 400 (v/v), and 1.75 M ammonium sulfate. Crystals grew to a final size of 0.1 × 0.1 × 0.1 mm in 3 days. Both the 14:0 and 18:2 LPA:LT3015 Fab complex co-crystals were grown by incubating 1 μL of ~10 mg/mL LPA:LT3015 Fab complex with 1 μL of reservoir solution containing 0.095 M sodium citrate pH 5.6, 19% (v/v) isopropanol, 19% (w/v) PEG 4000, and 5% (v/v) glycerol. The crystals grew to a final size of 0.1 × 0.1 × 0.1 mm in 8 days.
X-ray diffraction data collection
LT3015 Fab crystals were harvested with nylon loops and immersed in mother liquor supplemented with 15% glycerol for 1 minute. They were then removed and flash cooled directly in liquid nitrogen. The 14:0 and 18:2 LPA:LT3015 Fab complex crystals were cryo-cooled by direct transfer to liquid nitrogen in their mother liquor solution. Initial x-ray diffraction data were collected at 100 K on an R-Axis-IV++ image plate detector (Rigaku) at the San Diego State University Macromolecular x-ray Crystallography Facility. Synchrotron data were collected on ADSC Q315 CCD detectors at the Advanced Light Source Beamline 8.2.2 Berkeley National Laboratory and National Synchrotron Light Source Beamline X25 Brookhaven National Laboratory. Data processing was carried out using HKL200028. Data collection statistics are presented in Table 1.
Structure solution and refinement
Molecular replacement was performed in PHASER using as a probe the LT1009 Fab (PDB ID code 3I9G) with ions, antigen, and solvent molecules removed and allowing the constant and variable domains to move relative to one another12,29. Rigid body refinement by maximum likelihood was run in REFMAC530. The resulting model was rebuilt in the program COOT before further refinement in PHENIX31,32. Coordinates for triethylene glycol (PGE) and sulfate ion were obtained from the HIC-Up server33. Stereochemical analysis and final adjustments to the model were directed by MOLPROBITY34,35. The 14:0 LPA:LT3015 Fab complex structure was solved by molecular replacement in PHASER using as a probe the LT3015 Fab crystal structure. Maximum likelihood refinement in REFMAC5 revealed clear electron density for LPA in the antigen binding site. Coordinates for LPA were prepared using the PRODRG server36. Model building was carried out in COOT, further refined in PHENIX, and analyzed by MOLPROBITY. The 18:2 LPA:LT3015 Fab complex structure was solved by molecular replacement in PHASER with the 14:0 LPA:LT3015 Fab complex structure as probe, built in COOT, refined in PHENIX, and analyzed by MOLPROBITY. Statistics on the refined models are presented in Table 1.
Structure analysis
Electrostatic surface potential was calculated using the Poisson-Boltzmann equation to continuously model solvent within the program APBS37. Analysis of ligand molecular surface area buried upon complex formation was carried out using the program Surface Racer with a 1 Å probe38. Modeling and superposition of Fab crystallographic models was carried out in PyMol and root-mean-square deviations were calculated by the rms_cur command. All structure figures were created using PyMol39.
Mutagenesis
Gene constructs harboring mutations in the variable domains of LT3015 were synthesized and cloned into separate heavy and light chain expression vectors by GeneArt AG. Full-length LT3015 variants were produced from transiently transfected HEK 293F cells and purified by Protein A chromatography as described previously12.
LPA-binding ELISAs
Biotinylated LPA was prepared by coupling biotin to a stearic acid (18:0) LPA derivative, 1-(18-mercaptooctadecanoyl)-2-hydroxy-/sn/-glycero-3-phosphate (Echelon), using thiol-reactive maleimide chemistry. In separate vials, maleimide-(PEG)2-biotin (Pierce) and the LPA derivative were dissolved in DMSO to final concentrations of 100 mM and 5 mM, respectively, by sonication and vortex mixing until both solutions were clear and particulate-free. Equal volume aliquots of each solution were added to 3-fold excess 1× phosphate buffered saline, pH 7.4 (PBS) and incubated 4 hours at 25 °C. The final concentration of biotinylated LPA was assumed to be 1 mM.
Direct binding to biotinlyated LPA (LPA-biotin) was measured using an enzyme-linked immunosorbent assay (ELISA) as follows. Fcγ specific anti-human IgG (Jackson Immunoresearch) was diluted to 1 μg/mL in 0.1 M carbonate buffer pH 9.5 and 25 μL/well was used to coat 96 well, half-area plates (Greiner) for 1 hour at 37 °C. Each well was blocked by adding 100 μL of PBS, 0.01%Tween-20 containing 1% BSA (blocking buffer) and incubated for 1 hour at room temperature followed by 3 washes with PBS. Antibody samples were diluted to 50 ng/mL with blocking buffer, loaded onto the plate (25 μL/well), and incubated for 1 hour at room temperature and washed 3 times with PBS. Two-fold serial dilutions of LPA-biotin were prepared in blocking buffer and 100 μL were added to the captured antibody and incubated for 3–4 hours. The unbound lipid was removed by washing the plate 3 times with PBS. The antibody-bound LPA was detected by adding 25 μL of 1:60,000 dilution of horseradish peroxidase (HRP) conjugated streptavidin (Jackson Immunoresearch), incubating for 15 minutes, washing 3 times with PBS, and adding 25 μL of cold tetramethylbenzidine substrate (Sigma), and quenching by the addition of 1 M H2SO4. The optical density (OD) was measured at 450 nm using a Thermo Multiskan EX and the data was plotted using Graphpad Prism software.
Competition binding experiments were performed as described above except the antigen samples contained a fixed concentration of LPA-biotin (100 nM, Figure 1c; 20 nM, Figure 7) in the presence of two-fold diluted concentrations of native, unlabeled (Competitor) lipids and 50 μL was added to LT3015 IgG or Fab captured on a 96 well plate (Figure 1c) or 15 μL was added LT3015 captured on a 384 well plate (Figure 7). The plates were incubated for 4 hours, washed with PBS, and the bound LPA-biotin was detected as described above. Equilibrium dissociation constants (Ki) were calculated by fitting the binding isotherms in Figures 1b and 1c with one-site models and using the equation of Cheng and Prusoff40.
Supplementary Material
Acknowledgments
The authors thank K. Moreno, V. Nguyen, J. Cruz, and B. Shestowsky at Lpath Inc. for valuable technical support and C. Ralston, A. Héroux, and C. Whalen for support during synchrotron data collection. The Advanced Light Source is supported by the Director, Office of Science, Office of Basic Energy Sciences, of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231. Use of the National Synchrotron Light Source, Brookhaven National Laboratory, was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-98CH10886. This research is supported by a phase 1 SBIR grant (1 R43 GM 088956-01) from the NIH to JMW. TH is the recipient of an American Cancer Society grant RSG-08-287-01-GMC. Biochemistry research at SDSU is supported in part by the California Metabolic Research Foundation.
Abbreviations
- bFGF
basic fibroblast growth factor
- CDR
complementarity determining region
- IgG
immunoglobulin G
- LPA
lysophosphatidic acid
- LPC
lysophosphatidylcholine
- PA
phosphatidic acid
- PAF
platelet-activating factor
- PC
phosphatidylcholine
- PEG
polyethylene glycol
- PGE
triethylene glycol
- S1P
sphingosine-1-phosphate
- VEGF
vascular endothelial growth factor
Footnotes
ACCESSION NUMBERS
Coordinates and structure factors have been deposited in the Protein Data Bank and assigned PDB ID: 3QCT, 3QCU, and 3QCV.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eyster KM. The membrane and lipids as integral participants in signal transduction: lipid signal transduction for the non-lipid biochemist. Adv Physiol Educ. 2007;31:5–16. doi: 10.1152/advan.00088.2006. [DOI] [PubMed] [Google Scholar]
- 2.Song HY, Lee MJ, Kim MY, Kim KH, Lee IH, Shin SH, Lee JS, Kim JH. Lysophosphatidic acid mediates migration of human mesenchymal stem cells stimulated by synovial fluid of patients with rheumatoid arthritis. Biochim Biophys Acta. 2010;1801:23–30. doi: 10.1016/j.bbalip.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Birgbauer E, Chun J. New developments in the biological functions of lysophospholipids. Cell Mol Life Sci. 2006;63:2695–701. doi: 10.1007/s00018-006-6155-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rivera-Lopez CM, Tucker AL, Lynch KR. Lysophosphatidic acid (LPA) and angiogenesis. Angiogenesis. 2008;11:301–10. doi: 10.1007/s10456-008-9113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Umezu-Goto M, Kishi Y, Taira A, Hama K, Dohmae N, Takio K, Yamori T, Mills GB, Inoue K, Aoki J, Arai H. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J Cell Biol. 2002;158:227–233. doi: 10.1083/jcb.200204026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mills GB, Moolenaar WH. The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer. 2003;3:582–591. doi: 10.1038/nrc1143. [DOI] [PubMed] [Google Scholar]
- 7.Motzer RJ, Bukowski RM. Targeted therapy for metastatic renal cell carcinoma. J Clin Oncol. 2006;24:5601–5608. doi: 10.1200/JCO.2006.08.5415. [DOI] [PubMed] [Google Scholar]
- 8.Atzori F, Traina TA, Ionta MT, Massidda B. Targeting insulin-like growth factor type 1 receptor in cancer therapy. Target Oncol. 2009;4:255–266. doi: 10.1007/s11523-009-0123-z. [DOI] [PubMed] [Google Scholar]
- 9.Larbouret C, Robert B, Bascoul-Mollevi C, Penault-Llorca F, Ho-Pun-Cheung A, Morisseau S, Navarro-Teulon I, Mach JP, Pelegrin A, Azria D. Combined cetuximab and trastuzumab are superior to gemcitabine in the treatment of human pancreatic carcinoma xenografts. Ann Oncol. 2010;21:98–103. doi: 10.1093/annonc/mdp496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Visentin B, Vekich JA, Sibbald BJ, Cavalli AL, Moreno KM, Matteo RG, Garland WA, Lu Y, Yu S, Hall HS, Kundra V, Mills GB, Sabbadini RA. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell. 2006;9:225–238. doi: 10.1016/j.ccr.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 11.Stanfield RL, Zemla A, Wilson IA, Rupp B. Antibody elbow angles are influenced by their light chain class. J Mol Biol. 2006;357:1566–1574. doi: 10.1016/j.jmb.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 12.Wojciak JM, Zhu N, Schuerenberg KT, Moreno K, Shestowsky WS, Hiraiwa M, Sabbadini R, Huxford T. The crystal structure of sphingosine-1-phosphate in complex with a Fab fragment reveals metal bridging of an antibody and its antigen. Proc Natl Acad Sci USA. 2009;106:17717–17122. doi: 10.1073/pnas.0906153106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kabat EA, Wu TT, Perry HM, Gottesman KS, Foeller C. Sequences of Proteins of Immunological Interest. 5. National Institutes of Health; Bethesda, MD: 1991. [Google Scholar]
- 14.Tigyi G. Aiming drug discovery at lysophosphatidic acid targets. Br J Pharmacol. 2010;161:241–270. doi: 10.1111/j.1476-5381.2010.00815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moolenaar WH. Lysophosphatidic acid, a multifunctional phospholipid messenger. J Biol Chem. 1995;270:12949–12952. doi: 10.1074/jbc.270.22.12949. [DOI] [PubMed] [Google Scholar]
- 16.Sano T, Baker D, Virag T, Wada A, Yatomi Y, Kobayashi T, Igarashi Y, Tigyi G. Multiple mechanisms linked to platelet activation result in lysophosphatidic acid and sphingosine 1-phosphate generation in blood. J Biol Chem. 2002;277:21197–21206. doi: 10.1074/jbc.M201289200. [DOI] [PubMed] [Google Scholar]
- 17.Pozharski E, Moulin A, Hewagama A, Shanafelt AB, Petsko GA, Ringe D. Diversity in hapten recognition: structural study of an anti-cocaine antibody M82G2. J Mol Biol. 2005;349:570–582. doi: 10.1016/j.jmb.2005.03.080. [DOI] [PubMed] [Google Scholar]
- 18.Moolenaar WH, van Meeteren LA, Giepmans BN. The ins and outs of lysophosphatidic acid signaling. Bioessays. 2004;26:870–881. doi: 10.1002/bies.20081. [DOI] [PubMed] [Google Scholar]
- 19.Takabe K, Paugh SW, Milstien S, Spiegel S. “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev. 2008;60:181–195. doi: 10.1124/pr.107.07113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones JA, Rawles R, Hannun YA. Identification of a novel phosphatidic acid binding domain in protein phosphatase-1. Biochemistry. 2005;44:13235–13245. doi: 10.1021/bi0505159. [DOI] [PubMed] [Google Scholar]
- 21.Tokumura A, Majima E, Kariya Y, Tominaga K, Kogure K, Yasuda K, Fukuzawa K. Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J Biol Chem. 2002;277:39436–39442. doi: 10.1074/jbc.M205623200. [DOI] [PubMed] [Google Scholar]
- 22.Murph M, Mills GB. Targeting the lipids LPA and S1P and their signalling pathways to inhibit tumour progression. Expert Rev Mol Med. 2007;9:1–18. doi: 10.1017/S1462399407000476. [DOI] [PubMed] [Google Scholar]
- 23.Moolenaar WH. Bioactive lysophospholipids and their G protein-coupled receptors. Exp Cell Res. 1999;253:230–238. doi: 10.1006/excr.1999.4702. [DOI] [PubMed] [Google Scholar]
- 24.Lee MJ, Thangada S, Claffey KP, Ancellin N, Liu CH, Kluk M, Volpi M, Sha’afi RI, Hla T. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 1999;99:301–312. doi: 10.1016/s0092-8674(00)81661-x. [DOI] [PubMed] [Google Scholar]
- 25.James LC, Roversi P, Tawfik DS. Antibody multispecificity mediated by conformational diversity. Science. 2003;299:1362–1367. doi: 10.1126/science.1079731. [DOI] [PubMed] [Google Scholar]
- 26.Mariuzza RA. Multiple paths to multispecificity. Immunity. 2006;24:359–361. doi: 10.1016/j.immuni.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 27.Eisen HN, Chakraborty AK. Evolving concepts of specificity in immune reactions. Proc Natl Acad Sci USA. 2010;107:22373–22380. doi: 10.1073/pnas.1012051108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Otwinowski Z, Minor W. Processing of X-ray Diffraction data Collected in Oscillation Mode. In: Carter CW Jr, Sweet RM, editors. Macromolecular Crystallography, part A. Vol. 276. Academic Press; New York: 1997. pp. 307–326. [DOI] [PubMed] [Google Scholar]
- 29.McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. Phaser crystallographic software. J Appl Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 31.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kleywegt GJ, Jones TA. Databases in protein crystallography. Acta Crystallogr D. 1998;54:1119–1131. doi: 10.1107/s0907444998007100. [DOI] [PubMed] [Google Scholar]
- 34.Davis IW, Leaver-Fay A, Chen VB, Block JN, Kapral GJ, Wang X, Murray LW, Arendall WB, 3rd, Snoeyink J, Richardson JS, Richardson DC. MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 2007;35:W375–W383. doi: 10.1093/nar/gkm216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen VB, Arendall WB, 3rd, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, Murray LW, Richardson JS, Richardson DC. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr D Biol Crystallogr. 2010;66:12–21. doi: 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schuttelkopf AW, van Aalten DM. PRODRG: a tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallogr D Biol Crystallogr. 2004;60:1355–1363. doi: 10.1107/S0907444904011679. [DOI] [PubMed] [Google Scholar]
- 37.Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA. Electrostatics of nanosystems: application to microtubules and the ribosome. Proc Natl Acad Sci USA. 2001;98:10037–10041. doi: 10.1073/pnas.181342398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsodikov OV, Record MT, Jr, Sergeev YV. Novel computer program for fast exact calculation of accessible and molecular surface areas and average surface curvature. J Comput Chem. 2002;23:600–609. doi: 10.1002/jcc.10061. [DOI] [PubMed] [Google Scholar]
- 39.DeLano WL. The PyMOL Molecular Graphics System. DeLano Scientific; Palo Alto, CA, USA: 2002. [Google Scholar]
- 40.Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.