Abstract
Objectives
To assess the performance of three risk scores from Japan that were developed to predict, in children with Kawasaki disease, resistance to intravenous immunoglobulin (IVIG) treatment.
Study design
We used data from a randomized trial of pulsed steroids for primary treatment of Kawasaki disease to assess operating characteristics of the three risk scores, and we examined whether steroid therapy lowers the risk of coronary artery abnormalities in patients prospectively classified as IVIG resistant.
Results
For comparability with published cohorts, we analyzed the data of 99 patients not treated with steroids (16% IVIG-retreated), and identified male sex, lower albumin and higher AST as independent risk factors for IVIG resistance. The Kobayashi score was similar in IVIG-resistant and responsive patients, yielding sensitivity=33% and specificity=87%. There was no interaction of high vs. low risk status by treatment received (steroid vs. placebo) using any of the three risk score algorithms.
Conclusion
Risk scoring systems from Japan have good specificity but low sensitivity for predicting IVIG resistance in a North American cohort. Primary steroid therapy did not improve coronary outcomes among patients prospectively classified as high risk for IVIG resistance.
Keywords: Kawasaki, pediatrics, inflammation, therapy
Administration of intravenous immunoglobulin (IVIG) in the first 10 days after onset of Kawasaki disease (KD) lowers the prevalence of coronary artery (CA) aneurysms. However, most studies indicate that 13% to 21% of patients have IVIG resistance, that is, recrudescent or persistent fever after completion of initial IVIG administration1–4. Children with IVIG resistance are at higher risk for development of CA aneurysms.5–6 The identification of this high-risk subset at the time of presentation might identify patients who would benefit from primary treatment regimens combining IVIG with other anti-inflammatory therapies, such as tumor necrosis factor α (TNF-α) antagonists. Recent research has focused on identification of predictors of IVIG resistance, and risk scoring algorithms have been developed to estimate a patient's likelihood of successful treatment with single, high-dose IVIG1–3,5 We used the dataset of the Pediatric Heart Network KD Trial cohort to 1) assess the operating characteristics of the published Japanese risk scoring systems in a North American population; 2) examine whether risk level defined by the scoring systems is predictive of the occurrence of CA abnormalities, and 3) assess whether primary steroid therapy can be demonstrated as effective in patients who are predicted to be at high risk of IVIG resistance based upon factors that are known at the time of presentation.
METHODS
We enrolled patients in a randomized, double-blind, placebo-controlled trial of pulsed corticosteroid therapy for primary treatment of KD from December 2002 to December 2004 at eight clinical centers in North America. The entry criteria, methods and results of the trial have been previously published.8 Enrolled patients met modified American Heart Association criteria for KD and were between days four and ten of illness9. The study was conducted in accordance with Institutional Review Board approval at each participating center. A parent or guardian of each subject provided written informed consent. The study is registered with clinicaltrials.gov: NCT00132080.
Randomization was stratified by age (< 1 year vs. ≥ 1 year) and sex, with dynamic balancing by center. Patients were randomly assigned to receive either IV methylprednisolone (IVMP), 30 mg/kg over 2–3 hours, or placebo infusion of 5% Dextrose in Water in a similar volume and over the same time period as the infusion of IVMP. Each subject, regardless of random treatment assignment, also received IVIG (2 g/kg) and aspirin, 80 to 100 mg/kg/day, until afebrile for 48 hours, then 3 to 5 mg/kg once daily until five weeks post-randomization. Subjects with fever of at least 38.3°C without another likely source at >3 6 hours after completion of the initial IVIG treatment were retreated with IVIG, 2 g/kg. A second retreatment (i.e., a third treatment) with IVIG, 2 g/kg, was administered to subjects with recrudescent or persistent fever without another source >36 hours after IVIG retreatment.
Echocardiograms and laboratory data were obtained at baseline (prior to treatment) and at one and five weeks post-randomization. All measurements were used in this analysis even if outside of the protocol-allowed measurement window. Dimensions of the left main coronary artery (LMCA), proximal left anterior coronary artery (LAD), and proximal right coronary artery (RCA) were obtained by a standardized protocol and each echocardiogram was interpreted in a core laboratory by a single observer who was blinded to subject and timing of the study.
Laboratory data included a complete blood count, erythrocyte sedimentation rate (ESR), albumin, alanine aminotransferase (ALT) and serum immunoglobulins (IgG, IgA, and IgM). Baseline sodium, total bilirubin, and aspartate aminotransferase (AST) were also collected, where available. Highly sensitive C-reactive protein (hsCRP) was measured in a core laboratory.
One subject (of 199 randomized) was excluded from analysis due to withdrawal shortly after providing informed consent. We used the Wilcoxon rank sum test to compare the continuous risk score distributions by IVIG retreatment status. We used the Fisher exact test and Mantel-Haenszel test for linear trend to compare the categorical risk score (number of points) distributions by IVIG retreatment status. Mixed model regression identified associations between CA size and risk score over time. A treatment by IVIG retreatment risk status interaction test from analysis of variance was used to assess whether baseline-adjusted treatment effect on CA outcomes (the size of the steroid minus placebo difference) varied by IVIG retreatment risk status (high vs. low). Treatment group was defined using actual treatment received; two subjects assigned to the IVMP group did not receive IVMP and were reclassified as placebo. Dimensions of the proximal LAD and proximal RCA were adjusted for body surface area and expressed in standard deviation units (z scores) to assess their size compared with the normal population and calculate the primary outcome, maximum CA z-score.10 One- and five-week changes from baseline in maximum CA raw dimension were also analyzed as outcomes. Secondary analyses excluding six patients who were randomized but discovered to have met an exclusion criterion post hoc (two diagnosed >Day 10; two with culture-proven virus; two received earlier steroid therapy) yielded inferences similar to those in this report except where noted.
RESULTS
Median time from fever onset to enrollment was 6 days (interquartile range, IQR, 6 to 8 days), and the median age was 2.9 years (IQR, 1.5 to 4.7 yrs). Fourteen percent were Asian. IVIG retreatment was administered to 27 of the 198 (14%) subjects. Subjects retreated with IVIG, compared with who were not retreated, were similar in age but were more likely to be male (82% vs. 60%, p=0.03) and in univariate analysis of baseline laboratory variables (Table I; available at www.jpeds.com), more likely to have a higher hsCRP (59% vs. 33% ≥ 10 mg/dL), percentage of neutrophils ≥80% (23% vs. 8%, p=0.03), and bilirubin ≥0.9 mg/dL (53% vs. 29%, p=0.03). Subjects retreated with IVIG also had lower platelet count (median 32.9 vs. 39.4 × 104/mm3, p=0.005) and serum albumin concentration (2.9±0.6 vs. 3.4±0.6 g/dL, p<0.001) compared with those who were not retreated.
Table 1.
Baseline Characteristics of Kawasaki Disease Trial Subjects by IVIG Retreatment Status. Mean±SD (median) or % unless otherwise specified.
| Variable | N | IVIG Retreated | N | Not IVIG- Retreated |
p-value* |
|---|---|---|---|---|---|
| Age, years | 27 | 3.7±2.5 (3.3) | 171 | 3.2±2.2 (2.8) | .30 |
| Age ≤ 1 year | 27 | 7% | 171 | 18% | .26 |
| Age < 6 months | 27 | 4% | 171 | 5% | 1.00 |
| Male | 27 | 82% | 171 | 60% | .033 |
| Hispanic | 25 | 20% | 165 | 18% | .78 |
| Race | 27 | 171 | .51 | ||
| White | 48% | 102 | 60% | ||
| Black | 26% | 30 | 18% | ||
| Asian | 19% | 22 | 13% | ||
| Other/Mixed/Unknown | 7% | 17 | 10% | ||
| Fever Days prior to Diagnosis | 27 | 6.3±1.6 (6.0) | 171 | 6.6±1.5 (6.0) | .34* |
| Fever Days ≤ 4 | 27 | 7% | 171 | 6% | .67 |
| Assigned to Steroids | 27 | 44% | 171 | 52% | .54 |
| Received Steroids | 27 | 41% | 171 | 52% | .41 |
| Baseline Echo Z-Scores | |||||
| Proximal RCA | 24 | 1.2±1.3 (0.9) | 166 | 1.2±1.4 (1.0) | .96* |
| Proximal LAD | 26 | 1.3±1.7 (1.1) | 167 | 1.1±1.5 (1.0) | .58* |
| Max Z (pRCA, pLAD) | 26 | 1.8±1.7 (1.5) | 169 | 1.6±1.5 (1.4) | .69* |
| Left Main | 26 | 1.1±1.0 (1.1) | 168 | 0.9±1.1 (0.7) | .29* |
| Baseline Laboratory Values | |||||
| C-Reactive Protein, mg/dL | 22 | 12.9±8.3 (14.3) | 116 | 9.2±8.0 (6.8) | .06 |
| C-Reactive Protein ≥ 10 mg/dL | 22 | 59% | 116 | 33% | .03 |
| ESR, mm/hr | 25 | 63±34 | 162 | 68±32 | .48* |
| White blood cell count, 103/mm3 | 27 | 12.9±5.0 | 171 | 14.0±5.5 | .30 |
| % Neutrophils | 26 | 60±25 (66) | 171 | 55±21 (59) | .13 |
| Neutrophils ≥ 80% | 26 | 23% | 171 | 8% | .03 |
| Hematocrit, % | 27 | 31±5 | 171 | 32±3 | .18* |
| Hemoglobin, g/dL | 27 | 10.6±1.5 | 171 | 11.0±2.2 | .10* |
| Platelets × 104/mm3 | 27 | 34.1±9.0 (32.9) | 171 | 40.2±14.7 (39.4) | .005* |
| Plaletets ≤ 30 × 104/mm3 | 27 | 33% | 171 | 23% | .34 |
| Albumin, g/dL | 27 | 2.9±0.6 | 155 | 3.4±0.6 | .0003* |
| IgG, median | 25 | 714 | 138 | 687 | .54 |
| IgA, median | 25 | 92 | 133 | 82 | .98 |
| IgM, median | 25 | 97 | 132 | 110 | .65 |
| ALT, IU/L, median | 20 | 70.0 | 144 | 28.5 | .06 |
| AST, IU/L, median | 20 | 39.0 | 128 | 34.0 | .40 |
| AST ≥ 100 IU/L | 20 | 10% | 128 | 10% | 1.0 |
| Sodium, mmol/L | 19 | 135±2 | 124 | 136±3 | .28 |
| Sodium < 133 mmol/L | 19 | 16% | 124 | 13% | .72 |
| Total bilirubin, mg/dL, median | 17 | 0.9 | 100 | 0.3 | .12 |
| Total bilirubin ≥ 0.9 mg/dL | 17 | 53% | 100 | 21% | .01 |
p-value for proportions from exact test; p-value for continuous variables from Wilcoxon rank sum test except where noted with an asterisk to indicate t-test N represents the total available sample size for the variable in each group.
IVIG= Intravenous immunoglobulin
RCA= Right coronary artery
LAD= Left anterior descending coronary artery
pRCA= Proximal RCA
pLAD= Proximal LAD
ESR= Erythrocyte sedimentation rate
IgG=Immunoglobulin G
IgA=Immunoglobulin A
IgM=Immunoglobulin M
ALT=Alanine aminotransferase
AST=Aspartate aminotransferase
We explored multivariate predictive models for IVIG retreatment in our dataset (Table II). In analyses including all study subjects, independent risk factors for IVIG retreatment included male sex, lower albumin, and percentage of neutrophils ≥80% (c-statistic 0.77). When we excluded the six trial-ineligible subjects who underwent randomization, only lower albumin retained statistical significance (odds ratio=0.22, p<0.001), although the odds ratios for male sex and neutrophils ≥80% both remained about three (p-values ≤0.12). Because the previously published Japanese risk scores for prediction of IVIG retreatment were derived from populations that did not receive primary steroid therapy, we also sought to develop a model under comparable conditions. Using data restricted to subjects who did not receive steroid therapy, the most consistent multivariate model included male sex, lower albumin, and higher AST as independent risk factors for IVIG retreatment (c-statistic 0.83).
Table 2.
Multivariate Predictive Models for IVIG Resistance in the Pediatric Heart Network Kawasaki Disease Trial
| Predictor | Odds Ratio | 95% Confidence Interval |
p-value |
|---|---|---|---|
| Model 1* All Subjects (N=180), c-statistic=0.77 | |||
| Male sex | 3.17 | 1.04, 10.75 | 0.04 |
| Neutrophils≥80% | 3.52 | 1.05, 11.85 | 0.04 |
| Albumin, g/dL | 0.21 | 0.08, 0.53 | 0.001 |
| Model 2† No Steroid Therapy (N=72), c-statistic=0.83 | |||
| Male sex | 13.3 | 1.30, 143 | 0.03 |
| Albumin, g/dL | 0.20 | 0.05, 0.78 | 0.02 |
| AST, IU/L | 2.24‡ | 0.97, 5.16 | 0.06 |
When six trial ineligible subjects were excluded, only albumin was a significant predictor (odds ratio=0.22, p<.001). In the three-variable model (N=174), the p-values for sex and neutrophils≥80% were 0.07 and 0.12, respectively.
*When the one trial ineligible subject who did not receive steroid therapy was excluded, the sex, albumin, and AST p-values were 0.03, 0.02, and 0.04, respectively.
per 100 IU/L increase
IVIG=intravenous immunoglobulin
AST=aspartate aminotransferase
Operating Characteristics of published scoring systems
Complete data were available to calculate Kobayashi, Egami, and Sano risk scores for 115, 149, and 107 subjects, respectively (Table III; available at www.jpeds.com). The 83, 49, and 91 subjects without a Kobayashi, Egami, or Sano score, respectively, were similar to those with a calculable score with respect to sex, age, number of illness days, baseline CA z-scores, albumin, neutrophil count, and platelet count.
Table 3.
Published Risk Scoring Systems for IVIG Resistance for Japanese Subjects
| Score Component | Point Assignment |
|---|---|
| KOBAYASHI (7 variables) | |
| N=112 Resistant, N=434 Responsive | |
| Low risk: 0–3; High Risk: ≥ 4 | |
| Sensitivity: 86%; Specificity: 67%; | |
| PPV: 43%; NPV: 95% | |
| AST ≥ 100 | 2 |
| Sodium < 133 mmol/L | 2 |
| Fever Days ≤ 4 | 2 |
| %Neutrophils ≥ 80 | 2 |
| C-Reaction protein ≥ 10 mg/dL | 1 |
| Age ≤ 1 year | 1 |
| Plaletets ≤ 30 × 104/mm3 | 1 |
| EGAMI (5 variables) | |
| N=41 Resistant, N=279 Responsive | |
| Low Risk: 0–2; High Risk: ≥ 3 | |
| Sensitivity: 78%; Specificity: 76%; | |
| PPV: 32%; NPV: 96% | |
| ALT ≥ 80 | 2 |
| Fever Days ≤ 4 | 1 |
| C-Reactive protein ≥ 8 mg/dL | 1 |
| Age < 6 months | 1 |
| Plaletets ≤ 30 × 104/mm3 | 1 |
| SANO (3 variables) | |
| N=22 Resistant, N=90 Responsive | |
| Low Risk: 0–1; High Risk=2 | |
| Sensitivity: 77%; Specificity: 86%; | |
| PPV: 59%; NPV: 94% | |
| AST ≥ 200 | 1 |
| Total Bilirubin ≥ 0.9 mg/dL | 1 |
| C-Reactive protein ≥ 7 mg/dL | 1 |
ALT=Alanine aminotransferase
AST=Aspartate aminotransferase
PPV=Positive predictive value
NPV=Negative predictive value
Previously published Japanese risk scores for prediction of IVIG retreatment were derived from populations that did not receive primary steroid therapy. Therefore, we explored the operating characteristics of these risk scores using data from the trial subjects to whom primary steroid therapy was not administered (Table IV). Within this subgroup, the median Kobayashi scores for subjects with and without IVIG retreatment were median 2.0 (interquartile range, 1.0 to 4.0) vs. 1.0 (interquartile range, 0 to 3.0), respectively (p=0.14). With high risk defined as ≥4, sensitivity was 33%, specificity was 87%, positive predictive value was 30%, and negative predictive was value 87%. Thus, for example, if the Kobayashi scoring system were applied to North American patients of mixed ethnicity to identify those who might benefit from additional primary anti-inflammatory therapy (assuming that such additional therapy was proven to have benefit), 30% of patients targeted as high risk would receive additional therapy and might benefit; 70% targeted as high risk would receive additional therapy without need, and 13% who received scores indicating low risk would not receive additional therapy from which they might have benefited. Both the Egami and Sano scores differed by IVIG retreatment status (p<0.05; Table IV), with sensitivity somewhat higher compared with that of the Kobayashi score (42% and 40%, respectively).
Table 4.
Distribution of Kobayashi, Egami, and Sano Risk Scores by IVIG Retreatment Status in Subjects who did not Receive Steroid Therapy
| All | IVIG-Retreated | Not IVIG- Retreated |
p-value* | |
|---|---|---|---|---|
| N | 62 | 9 | 53 | |
| Kobayashi Score† | 1.7±1.6 | 2.4±1.9 | 1.5±1.6 | 0.14 |
| Median score (IQR) | 1.0 (0, 3.0) | 2.0 (1.0, 4.0) | 1.0 (0, 3.0) | |
| Kobayashi Score | 0.40, 0.12 | |||
| 0 | 29% | 11% (1) | 32% (17) | |
| 1 | 31% | 33% (3) | 30% (16) | |
| 2 | 11% | 11% (1) | 11% (6) | |
| 3 | 13% | 11% (1) | 13% (7) | |
| 4 | 8% | 11% (1) | 8% (4) | |
| 5 | 7% | 22% (2) | 4% (2) | |
| 6 | 2% | 0.0% | 2% (1) | |
| Low Risk (0–3) | 84% | 67% | 87% (specificity) | |
| High Risk (≥ 4) | 16% | 33% (sensitivity) | 13% | |
| N | 78 | 12 | 66 | |
| Egami Score† | 1.3±1.3 | 2.1±1.2 | 1.1±1.3 | 0.01 |
| Median score (IQR) | 1.0 (0, 2.0) | 2.0 (1.5, 3.0) | 1.0 (0, 1.0) | |
| Egami Score | 0.01, 0.02 | |||
| 0 | 35% | 17% (2) | 38% (25) | |
| 1 | 33% | 8% (1) | 38% (25) | |
| 2 | 13% | 33% (4) | 9% (6) | |
| 3 | 13% | 33% (4) | 9% (16) | |
| 4 | 4% | 8% (1) | 3% (2) | |
| 5 | 3% | 0% | 3% (2) | |
| Low Risk (0–2) | 81% | 58% | 85% (specificity) | |
| High Risk (≥ 3) | 19% | 42% (sensitivity) | 15% | |
| N | 56 | 10 | 46 | |
| Sano Score† | 0.9±0.8 | 1.5±0.7 | 0.8±0.8 | 0.03 |
| Median score (IQR) | 1.0 (0, 1.0) | 1.0 (1.0, 2.0) | 1.0 (0, 1.0) | |
| Sano Score | 0.03, 0.01 | |||
| 0 | 32% | 0% | 39% (18) | |
| 1 | 48% | 60% (6) | 46% (21) | |
| 2 | 16% | 30% (4) | 13% (6) | |
| 3 | 4% | 10% (1) | 2% (1) | |
| Low Risk (0–1) | 80% | 60% | 85% (specificity) | |
| High Risk (2–3) | 20% | 40% (sensitivity) | 15% | |
Wilcoxon rank sum test p-value for continuous score; Exact test and Mantel-Haenszel test for linear trend p-value for categorical distribution
Mean±standard deviation
IQR = Interquartile range
Utility of risk scores in predicting coronary abnormalities
In the subjects not receiving primary steroid therapy, we examined the correlation between CA z-scores at one- and five-weeks post-randomization and the three risk scores using mixed model regression. The Kobayashi score was a significant predictor of CA z-scores for all segments (mixed model p=0.01 to 0.04, independent of time point). However, the associations were relatively weak; the largest Spearman correlation coefficient was 0.29, between maximum CA z-score at one week and Kobayashi score. The Sano and Egami scores were not associated with CA z-score. Similarly, none of the three risk scores were associated at the 0.05 level with the paired change in raw coronary artery dimensions (LMCA, proximal LAD or RCA) from baseline to one and five weeks later.
Efficacy of primary steroid therapy in reducing coronary abnormalities
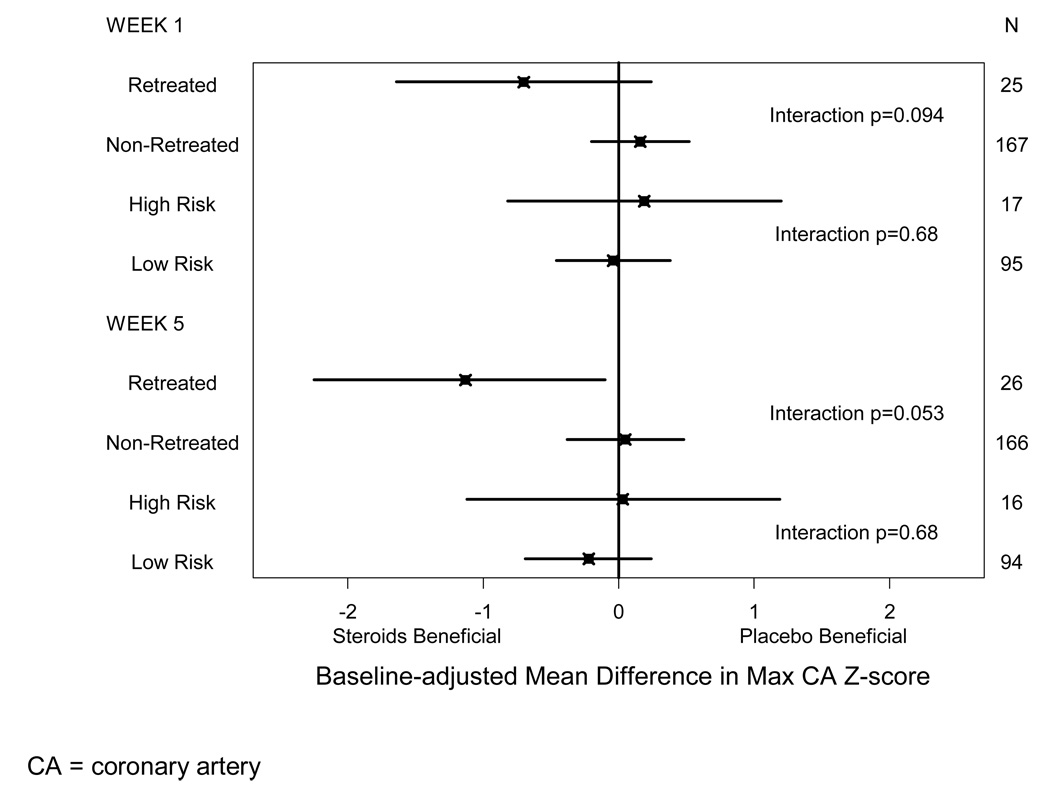
A post hoc subgroup analysis of the randomized trial suggested that, for subjects retreated with IVIG only, CA outcomes were better among those initially treated with steroids compared with those who received placebo (Figure). At five weeks post-randomization, in patients who were retreated, CA size was one standard deviation smaller for patients treated with steroids compared with those on placebo. However, in patients who were not retreated, CA size did not differ significantly for the steroid and placebo groups.
Figure 1.
Baseline-adjusted Treatment Effect Estimates and 95% Confidence Interval by Actual IVIG Retreatment Subgroup and by Estimated Kobayashi High vs. Low Risk Subgroup for Maximum Coronary Artery Z-score at One and Five Weeks Post-Randomization
We explored whether steroid treatment improved coronary outcomes in subjects whose estimated risk of retreatment at baseline was high and found no significant interaction between Kobayashi risk class (high vs. low) and steroid vs. no steroid treatment in maximum CA z-score (p=0.23 and p=0.39 at one and five weeks, respectively), maximum CA dimension, or change in maximum CA dimension from baseline. Of note, there were clinically relevant baseline differences in maximum CA z-score by steroid treatment status in each of the subgroups (high vs. low risk of IVIG resistance). Among the 17 patients classified as high risk at baseline, mean maximum CA z-score at baseline was one standard deviation lower in the steroid group compared with the placebo group. In the low-risk patients, mean baseline maximum CA z-score was 0.4 standard deviations larger in the patients treated with steroids compared with the placebo group. After adjustment for baseline differences, there was no evidence of a differential effect of steroid therapy in the high and low risk subgroups (Figure; interaction p=0.68 at one week and p=0.68 at five weeks). Similarly, when the Egami and Sano risk scores were each used to define risk status for IVIG resistance, we found no significant interaction (p≥0.18) between risk class (high vs. low) and primary treatment with steroid vs. no steroids in CA outcomes.
DISCUSSION
Using the dataset of the Pediatric Heart Network’s randomized, placebo-blind trial of pulsed corticosteroids for primary therapy for KD, we evaluated the performance of three published risk scoring systems for prediction of IVIG resistance, derived from the data of Japanese populations1–3. The three scoring systems were developed using patients who did not receive primary steroid therapy. We therefore performed our analyses both in the entire trial cohort and within the group that received placebo plus conventional primary therapy with IVIG and aspirin.
We found that sensitivity of these risk scores was low (33–42%), and specificity was moderate to high (85–87%). No differential treatment effect of steroids was found for subjects classified as high vs. low risk for IVIG resistance based on these scores. Tremoulet et al6 evaluated the Egami score using 362 children in San Diego county diagnosed with KD in 2006 and found, similar to our report, low sensitivity (38%) and good specificity (84%) to detect IVIG resistance. Our results suggest that use of Japanese scoring systems in a North American population of mixed ethnicity will exclude most patients who are at low risk, but will not capture the majority of those who may benefit from more intensive monitoring of their condition and who may be the optimum candidates for additional therapies that may interrupt the disease process.
We used our dataset directly to identify correlates of IVIG resistance to better understand why the existing Japanese risk score systems did not perform as well as expected in our North American population. Male sex and albumin were two independent risk factors in our placebo group, but none of the three existing risk scoring systems included these factors. It is possible that IVIG resistance is a sex-linked trait, and that the conflicting importance of sex as a risk factor in the Japanese datasets vs. a North American one reflects genetic differences. In a retrospective analysis of children diagnosed with KD from 2002 to 2006, Ashouri et al found low albumin, higher band counts, and a higher number of abnormal echocardiography results independently predicted IVIG resistance11. However, this single center study included a very small number of patients who were also in our analysis and cannot be considered as independent support for our finding of low albumin as a risk factor.
Because IVIG non-responsiveness is associated with poor CA outcomes, we examined whether the risk scores for IVIG resistance were correlated with maximum CA dimension adjusted for body surface area (CA z-score) and found at most modest associations. Of note, relatively few subjects in the Pediatric Heart Network trial developed clinically significant CA abnormalities, so the power for detecting a relationship between CA outcomes and risk scores was low. The reasons for the favorable CA outcomes in this cohort are unclear, but could include an aggressive protocol for retreatment of IVIG resistance or the restriction of eligibility for the trial to those with ≤10 days of fever.
The utility of a risk score for identification of the IVIG-resistant patient from baseline clinical and laboratory variables would be to identify patients who might benefit for adjunctive primary antiinflammatory therapies. The most extensively studied adjunctive therapy is corticosteroids. A recent meta-analysis of four randomized trials of primary corticosteroid treatment in addition to IVIG and aspirin suggested that such treatment decreased rates of IVIG re-treatment, although it was not shown to lower the risk of coronary aneurysms or adverse events12. Information on the role of adjunctive primary treatment with infliximab and etanercept await the results of ongoing trials.
Although primary treatment with pulsed corticosteroids did not improve CA outcomes overall in the Pediatric Heart Network trial, a post hoc subgroup analysis suggested a beneficial effect within the small group of children with IVIG resistance. In the current analysis, we sought to determine whether patients predicted to be at high risk of IVIG resistance had superior CA outcomes in response to the addition of primary corticosteroid treatment to conventional therapy. We found no significant interaction of high vs. low risk status by treatment received (steroid vs. placebo) using any of the three risk score algorithms. Thus, we found no benefit of pulsed corticosteroid therapy on maximum CA dimension in the subgroup of patients whose risk scores at presentation suggested a high likelihood of IVIG resistance, and hence might have been preemptively identified as a subgroup for whom additional treatment may have been targeted.
The results of our analyses should be considered in light of certain limitations. Depending on the risk scoring system used, 25–45% of subjects had insufficient data to calculate a score, thus reducing statistical power. However, we found that subjects with missing scores were similar to those who did have a score with respect to sex, age, number of illness days, baseline CA zscores, and several laboratory measures, suggesting that exclusion of subjects with missing scores imposed little bias. In addition, because patients were not randomly assigned to steroid vs. placebo treatment within strata of predicted risk for IVIG resistance, unmeasured factors between the low and high risk groups could account for our lack of a differential steroid treatment benefit. Finally, our study did not include an independent validation dataset to assess the reliability of the of the risk factors for IVIG resistance that we identified. Furthermore, of the Japanese risk scoring systems, only the Kobayashi score was validated.
In conclusion, risk scoring systems for IVIG resistance developed in Japan have low sensitivity (<45%) and good specificity when applied to an independent dataset of North American children. We were unable to demonstrate a benefit of primary steroid treatment with prospective classification of patients into high vs. low risk for IVIG resistance using published risk scores for IVIG resistance. Accurate prospective identification of North American patients of mixed ethnicity at high risk for IVIG retreatment remains a challenge.
Acknowledgments
Supported by U01 grants from the National Heart, Lung, and Blood Institute (HL068269, HL068270, HL068279, HL068281, HL068285, HL068292, HL068290, and HL068288).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest..
Registered with clinicaltrials.gov: NCT00132080.
REFERENCES
- 1.Kobayashi T, Inoue Y, Takeuchi K, Okada Y, Tamura K, Tomomasa T, et al. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation. 2006;113:2606–2612. doi: 10.1161/CIRCULATIONAHA.105.592865. [DOI] [PubMed] [Google Scholar]
- 2.Sano T, Kurotobi S, Matsuzaki K, Yamamoto T, Maki I, Miki K, Kogaki S, Hara J. Predition of non-responsiveness to standard high-dose gamma-globulin therapy in patients with acute Kawasaki disease before starting initial treatment. Eur J Pediatr. 2007;166:131–137. doi: 10.1007/s00431-006-0223-z. [DOI] [PubMed] [Google Scholar]
- 3.Egami K, Muta H, Ishii M, Suda K, Sugahara Y, Iemura M, Matsuishi T. Prediction of resistance to intravenous immunoglobulin treatment in patients with Kawasaki disease. J Pediatr. 2006;149:237–240. doi: 10.1016/j.jpeds.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 4.Son MF, Gauvreau K, Ma L, Baker AL, Sundel RP, Fulton DR, Newburger JW. Diagnosis and treatment of Kawasaki Disease: Analysis of 27 U.S. pediatric hospitals from 2001–2006. Pediatrics. 2009;124:1–8. doi: 10.1542/peds.2008-0730. [DOI] [PubMed] [Google Scholar]
- 5.Burns JC, Capparelli EV, Brown JA, Newburger JW, Glode MP. Intravenous gamma-globulin treatment and retreatment in Kawasaki disease. US/Canadian Kawasaki Syndrome Study Group. Pediatr Infect Dis J. 1998 Dec;17:1144–1148. doi: 10.1097/00006454-199812000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Tremoulet AH, Best BM, Song S, Wang S, Corinaldesi E, Eichenfield JR, Martin DD, Newburger JW, Burns JC. Resistance to intravenous immunoglobulin in children with Kawasaki Disease. J Pediatr. 2008;153:117–121. doi: 10.1016/j.jpeds.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muta H, Ishii M, Furui J, Nakamura Y, Matsuishi T. Risk factors associated with the need for additional intravenous gamma-globulin therapy for Kawasaki disease. Acta Paediatrica. 2006;95:189–193. doi: 10.1080/08035250500327328. [DOI] [PubMed] [Google Scholar]
- 8.Newburger J, Sleeper LA, McCrindle B, Minich L, Gersony W, Vetter V, Atz A, Li J, Takahashi M, Baker A, Colan SD, Mitchell P, Klein G, Sundel R. Randomized trial of pulse steroid therapy in Kawasaki Disease. N England J Med. 2007;356:663–675. doi: 10.1056/NEJMoa061235. [DOI] [PubMed] [Google Scholar]
- 9.Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, Shulman ST, Bolger AF, Ferrieri P, Baltimore RS, Wilson WR, Baddour LM, Levison ME, Pallasch TJ, Falace DA, Taubert KA. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110:2747–2771. doi: 10.1161/01.CIR.0000145143.19711.78. [DOI] [PubMed] [Google Scholar]
- 10.McCrindle BW, Li JS, Minich LL, Colan SD, Atz AM, Takahashi M, Vetter VL, Gersony WM, Mitchell PD, Newburger JW. Risk Factors for CA Involvement in Children with Kawasaki Disease. Circulation. 2007;116:174–179. doi: 10.1161/CIRCULATIONAHA.107.690875. [DOI] [PubMed] [Google Scholar]
- 11.Ashouri N, Takahashi M, Dorey F, Mason W. Risk factors for nonresponse to therapy in Kawasaki Disease. J Pediatr. 2008;153:365–368. doi: 10.1016/j.jpeds.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 12.Athappan G, Gale S, Ponniah T. Corticosteroid therapy for primary treatment of Kawasaki disease - weight of evidence: a meta-analysis and systematic review of the literature. Cardiovasc J Afr. 2009;20:233–236. [PMC free article] [PubMed] [Google Scholar]