Abstract
The p53 transcription factor is activated by various types of cell stress or DNA damage, and induces the expression of genes that control cell growth and inhibit tumor formation. Analysis of mice that express mutant forms of p53 suggest that inappropriate p53 activation can alter tissue homeostasis and life span, connecting p53 tumor suppressor functions with accelerated aging. However, other mouse models that display increased levels of wildtype p53 in various tissues fail to corroborate a link between p53 and aging phenotypes, possibly due to the retention of signaling pathways that negatively regulate p53 activity in these models. In this present study, we have generated mice lacking Mdm2 in the epidermis. Deletion of Mdm2, the chief negative regulator of p53, induced an aging phenotype in the skin of mice, including thinning of the epidermis, reduced wound healing, and a progressive loss of fur. These phenotypes arise due to an induction of p53-mediated senescence in epidermal stem cells and a gradual loss of epidermal stem cell function. These results reveal that activation of endogenous p53 by ablation of Mdm2 can induce accelerated aging phenotypes in mice.
Keywords: Mdm2, p53, epithelial stem cell, aging
Introduction
The p53 transcription factor activates the expression of numerous genes involved in cell cycling, senescence, and apoptosis (Vousden and Lu, 2002). These p53 effectors regulate the growth of cells exposed to certain types of DNA damage or metabolic insult, and mutation of p53 or other genes encoding regulators of the p53 signaling pathway are the most common genetic alterations observed in human cancers (Soussi et al., 2001). In keeping with a key role for p53 in tumor suppression, mice either heterozygous or homozygous-deficient for functional p53 develop tumors either spontaneously (Donehower et al., 1992) or following exposure to various genotoxic agents (Kemp et al., 1994).
The oncoprotein Mdm2 is a well-established negative regulator of p53 activity. Mdm2 complexes with the amino-terminal portion of p53 and interferes with the ability of p53 to transactivate target genes by sterically hindering the NH2-terminal activation domain of the p53 protein (Momand et al., 1992; Chen et al., 1995) and by shuttling p53 from the nucleus to the cytoplasm (Freedman and Levine, 1998; Geyer et al., 2000). Furthermore, Mdm2 can function as an E3 ligase to ubiquitinate p53 (Honda et al., 1997) and induce p53 degradation in the 26S proteasome (Haupt et al., 1997; Kubbutat et al., 1997; Li et al., 2003). Studies of Mdm2-mutant mice have highlighted the fundamental importance of Mdm2 in inhibiting p53 stability and function in development (Jones el al., 1995; Montes de Oca Luna et al., 1995; Itahana et al., 2007).
Recently, analysis of several p53 mouse models has suggested that p53 must also be negatively regulated in adult mice in order to facilitate homeostatic regulation of normal tissues and to prevent accelerated organismal aging. We have reported previously that mice heterozygous for a mutated p53 allele (m allele) encoding an amino-truncated p53 display a reduced incidence of cancer relative to p53 heterozygous mice and an early aging-like phenotype that includes osteoporosis, a reduced mass of internal organs, a thinning of the dermis, and deficiencies in hair re-growth (Tyner et al., 2002). Since expression of the m-allele was found to increase the transcriptional activity of wildtype p53 molecules in vitro, it was proposed that the m-allele bearing (p53 +/m) mice had a slight increase in the level of p53 activity that reduced stem cell proliferation in the affected tissues, leading to the loss of tissue cellularity and an accelerated aging-like phenotype in this model (Tyner et al., 2002). More recently, the Scrable lab performed an analysis of transgenic mice expressing a short, naturally occurring, p53 splice isoform lacking sequences encoding the amino terminal portion of the p53 protein. The variant p53 protein (p44) encoded by this transgene induced p53 hyperactivity in the transgenic mice and a reduced cancer incidence. In agreement with a role for p53 activity in aging, the p44 mice displayed an increase in aging-associated insulin growth factor signaling and accelerated aging (Maier et al., 2004).
In contrast to these findings, other mouse models that contain increased amounts of p53 activity fail to present with accelerated aging-like phenotypes. The “super-p53” transgenic mice harbor extra copies of a wildtype p53 gene and display upregulated p53 activities and increased cancer resistance, yet these mice have normal life span and normal skeletal structure, hair growth, and skin thickness (Garcia-Cao et al., 2002). Likewise, mice bearing reduced levels of Mdm2 activity due to the presence of a hypomorphic Mdm2 allele (Mdm2puro) were found to have increased levels of p53 activity and increased resistance to tumor formation, but did not possess any of the features characteristic of accelerated aging (Mendrysa et al., 2006).
One possible explanation for the differences in the aging phenotypes in these various mouse models is that the p53 proteins encoded by the m-allele and the p44 transgene lack the amino-terminal portion of p53 containing the p53-Mdm2 interaction domain. Therefore, these variant p53 proteins would not be subject to Mdm2 regulation, whereas Mdm2 would negatively regulate the wildtype, full-length p53 proteins encoded in the super p53 transgenic mice. And although there is reduced Mdm2 function and increased p53 activity in the Mdm2-hypomorphic model, the endogenous wildtype p53 proteins encoded in these mice are still subject to Mdm2 binding and regulation, albeit at a reduced level. Thus, it is possible that the ability (or inability) of Mdm2 to fully regulate p53 may underlie the presence or absence of any accelerated aging phenotypes in these various mouse models. An alternate explanation for the difference in aging-associated phenotypes in these models is that the amino-truncated forms of p53 encoded by the m-allele or the p44 transgene might be inducing an early aging phenotype not by altering p53 activity per se, but by also altering the activity of p63, a p53 family member that has been recently demonstrated to regulate senescence in fibroblasts and epithelial cells in a p53-independent manner (Guo et al., 2009; Flores and Blasco, 2009) and to induce premature aging phenotypes in the skin of mice (Flores and Blasco, 2009). Therefore, the role of p53 in regulating accelerated aging in mice remains a matter of much debate (Poyurovsky and Prives, 2006; Vijg and Hasty, 2005; Gentry and Venkatachalam, 2005).
In this present study, we have utilized an Mdm2-conditional mouse model to determine whether epidermal-specific loss of Mdm2-p53 signaling in newborn mice can induce a premature aging phenotype in the skin. Such an approach allows us to avoid the embryonic lethality associated with ubiquitous or tissue-specific inactivation of Mdm2 during development (Jones el al., 1995; Montes de Oca Luna et al., 1995; Itahana et al., 2007; Lengner et al., 2006; Xiong et al., 2006). Furthermore, in contrast to p53, the stability and transcriptional activity of the p63 protein is not regulated by Mdm2 (Wang et al., 2001; Little and Jochemsen et al., 2001). Therefore, ablation of Mdm2 permits a specific assessment of the effects of the endogenous p53 protein in organismal aging.
Our results reveal that deletion of Mdm2 activity in mouse skin epithelium results in increased p53 levels and increased expression of p53 target genes that are specifically involved in regulating cellular senescence. These Mdm2-ablated mice display hallmarks of accelerated aging in their skin, including a thinning of the epidermal layer, reduced integrity of the skin, and widespread and progressive loss of fur. Analysis of the epidermis in these mice indicates increased cellular senescence in the follicular bulge region and a reduction in skin epidermal stem cell numbers and functions, as determined by wound healing and hair growth assays. These findings document a role for Mdm2-p53 signaling in the maintenance of the epidermal stem cell compartment, and demonstrate that increased activity of endogenous p53 can induce early aging phenotypes in mouse skin.
Materials and Methods
Mice and mouse genotyping
The generation of both the Mdm2 conditional mice (Steinman and Jones, 2002) and the K5-Cre transgenic mice (Zhou et al., 2002) has been described previously. Rosa26 Flox-Stop-β-Geo reporter mice (Stock#: 003310) were obtained from Jackson Laboratories. All experimental and littermate control mice used in this study were on a mixed 129Sv X C57Bl/6 background. Genomic PCR was used to identify inheritance of the K5-Cre transgene (primers 5’-CGGTCGATGCAACGAGTGAT-3’ and 5’-CCACCGTCAGTACGTGAGAT-3’), to identify inheritance of the conditional Mdm2 allele (primers 5’-GGTCTTCCCATTTATGTATGT-3’ and 5’-AAGAGTCTGTATCGCTTTCT-3’), and to verify Mdm2 allelic excision (primers 5’-GGTCTTCCCATTTATGTATGT-3’ and 5’-TCCACCTCTTTCTTCTTCCTG-3’). Animals were maintained and used in accordance with federal guidelines and those established by the Institutional Animal Care and Use Committee at the University of Massachusetts Medical School.
Western analysis
Protein lysates were collected using a modified protocol described previously (Balasubramanian et al., 1998). A 10-mm2 dorsal section of skin tissue was homogenized in 1 ml NP-40 lysis buffer [50 mM Tris-HCl, pH 7.5; 150 mM NaCl; 0.5% NP-40; 20% Glycerol; 1X protease inhibitor cocktail (Roche); 1X phosphatase inhibitor cocktail (Roche)]. Samples were placed on ice for 15 minutes, vortexed, and centrifuged at 13000 rpm for 20 minutes. Supernatants were collected, and 50µg of each sample loaded onto a 10% SDS-polyacrylamide gel. Antibodies against p53 (1:500 of both Ab-1 and Ab-3 from Calbiochem) and α-tubulin (1:4000 T5168 from Sigma) were diluted in 5% non-fat milk.
Gene expression analysis
Total mRNA from skin tissue was isolated according to the Trizol Reagent (Invitrogen) protocol, and cDNA was generated using the SuperScript II First Strand Synthesis Kit (Invitrogen). The following primers were used in real time PCR reactions to determine relative gene expression:
p21: 5’-TGAGGAGGAGCATGAATGGAGACA-3’ and
5’-AACAGGTCGGACATCACCAGGATT-3’,
Pai-1: 5’-GCTGCACCCTTTGAGAAAGA-3’ and
5’-GCCAGGGTTGCACTAAACAT-3’,
Pai-2: 5’-GGGCTTTATCCTTTCCGTGT-3’ and
5’-GTGTGTCTTTGCTGATCCAC-3’,
Puma: 5’-CCTGGAGGGTCATGTACAATCT-3’ and
5’-TGCTACATGGTGCAGAAAAAGT-3’,
Noxa: 5’-CCACCTGAGTTCGCAGCTCAA-3’ and
5’-GTTGAGCACACTCGTCCTTCAA-3’,
p16: 5’-ATCTGGAGCAGCATGGAGTC-3’ and
5’-TCGAATCTGCACCGTAGTTG-3’,
GAPDH: 5’-TGGCAAAGTGGAGATTGTTGCC-3’ and
5’-AAGATGGTGATGGGCTTCCCG-3’.
Histology
Skin tissue was fixed in 10% formalin overnight. Skin sections (5µm) were stained with hematoxylin and eosin (H&E), anti-cytokeratin 15 (K15) antibody from Neomarkers (Freemont, CA), or anti-Lhx2 from Santa Cruz (sc-19342) as performed by the UMMS DERC Morphology Core. TUNEL staining of tissue sections to detect apoptotic cells was performed using the In Situ Cell Death Detection Kit, POD (Roche, 11684817910).
SA-β-Galactosidase staining
Fresh skin tissue was washed twice with PBS, followed by briefly fixing in 2% formaldehyde/0.2% glutaraldehyde for 5 minutes. The tissue was rinsed twice in PBS and then completely submerged in staining solution [all diluted in 40 mM citrate/sodium phosphate buffer (pH 6): 5 mM potassium ferricyanide; 5 mM potassium ferrocyanide; 2 mM MgCl2; 150 mM NaCl; 1mg/ml X-gal] for 4 hours in the dark at 37 °C. After two washes with PBS, the tissue was fixed overnight in 10% formalin and paraffin embedded. Sections were counterstained with either H&E or Nuclear Fast Red.
Isolation of bulge stem cells
Epidermal bulge stem cells were isolated according to a previous protocol (Nowak and Fuchs, 2009). Whole skin was treated with 0.25% trypsin overnight, which allowed complete segregation of the epidermis from the dermis. The resulting epidermal cell suspensions were washed with media, resuspended in staining buffer (2% fetal bovine serum in sterile PBS), and stained with the following antibodies: phycoerythrin-conjugated rat anti-human CD49f [integrin α6 chain] (clone GoH3) from BD Pharmingen; biotin-conjugated rat anti-mouse CD34 (clone RAM34) from eBioscience; strepavidin-allophycocyanin conjugate from BD Pharmingen). Cells were stained with 7-aminoactinomycin D (7-AAD, Cat. No. 00–6993-50) from eBioscience to determine cell viability. FACS was performed by the UMASS Medical School Flow Cytometry Core Facility.
Wound healing assay
The wound healing procedure was modified from a previously described protocol (Tyner et al., 2002). The dorsal surface of anesthetized mice (0.023 cc/gram body weight, 1.2% Avertin) was completely shaved with an electric razor and disinfected with Betadine (Cardinal Health) and 75% ethanol. A 3-mm punch biopsy was used to introduce a single wound on the dorsum of each mouse. The wounds were imaged each day and the diameter of each wound was measured. Healing was defined as the decrease in wound diameter over time, and was expressed as the percentage of the day 0 wound diameter.
Hair growth assay
The procedure for this assay was modified from a previously described protocol (Tyner et al., 2002). A 2-cm2 dorsal section of skin on age-matched mice was shaved with an electric razor at day 0. A 0.5-cm2 square grid was used to measure hair re-growth, which was defined as the percentage of the total number of squares that are covered with over 50% new hair. Measurements were taken every 3–4 days.
Results
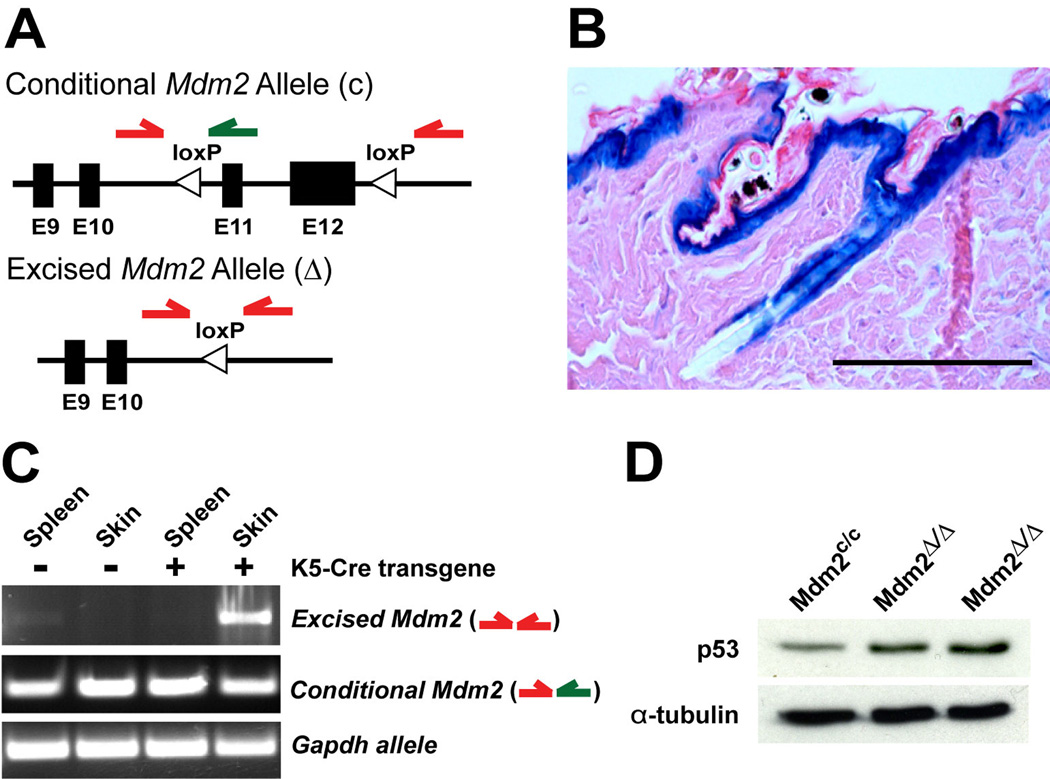
To study the role of Mdm2 in regulating p53-mediated effects on premature aging phenotypes, Mdm2-conditional mice (Mdm2c/c) (Steinman and Jones, 2002) were mated with transgenic mice bearing the Cre recombinase gene under the transcriptional control of Keratin 5 promoter sequences (K5-Cre) (Zhou et al., 2002). Since the K5 promoter is active in early postnatal development in epidermal progenitor cells of the skin and hair follicles, induction of Cre expression should excise exons 11 and 12 in the conditional Mdm2 allele, resulting in loss of functional Mdm2 in the epidermis (Fig. 1A).
Figure 1.
Deletion of Mdm2 in epidermis upregulates p53 protein levels. (A) Exons 11 and 12 of the conditional Mdm2 allele (c) are flanked by loxP sites (top). Cre recombinase deletes these floxed exons, generating an Mdm2-null allele (Δ, bottom). Green arrows represent PCR primers used to identify the conditional Mdm2 allele and red arrows represent PCR primers used to detect genomic rearrangement. (B) LacZ staining in Rosa26 Flox-Stop-β-Geo reporter mice expressing the K5-Cre transgene. Scale bar, 100 µm. (C) PCR on genomic DNA indicates excision of the Mdm2 allele specifically in the skin of Mdm2c/c mice that also inherited the K5-Cre transgene. Primers to Gapdh were used as a positive control for the PCR reactions. (D) Western blot of p53 protein levels in skin from 5-month-old mice. α-Tubulin was used as a loading control for the western.
To confirm robust Cre recombinase function in the epidermis of K5-Cre mice, Rosa26 Flox-Stop-β-Geo reporter (R26R) mice were crossed with K5-Cre mice and the pattern of reporter gene activity was examined in these compound heterozygous mice. Expression of β–galactosidase was detected in the interfollicular epidermis and hair follicles of 4-week old K5-Cre+, R26Rmice, indicating robust and widespread Cre mediated excision of floxed alleles in epidermal cells (Fig. 1B). To document excision of the floxed exons in the conditional Mdm2 mice, genomic DNA was isolated from tissues of Mdm2c/c and Mdm2c/c, K5-Cre+ mice (Mdm2Δ/Δ), and PCR was performed to determine the status of the Mdm2 alleles. PCR primers to the glyceraldehyde-3-phosphate dehydrogenase (Gapdh) gene were used as a positive control. Loss of functional Mdm2 alleles was observed in the skin of Mdm2Δ/Δ mice, but not in various control tissues, including spleen (Fig. 1C), liver, lung, kidney or small bowel (data not shown). To confirm that Cre-excision of the floxed Mdm2 exons in the epidermis resulted in loss of Mdm2 function, we examined p53 levels in the epidermis of Mdm2Δ/Δ mice at 5 months of age. Western blot analysis revealed that endogenous p53 protein levels are slightly increased (3–4 fold by densitometry) in the skin of Mdm2Δ/Δ mice (Fig. 1D).
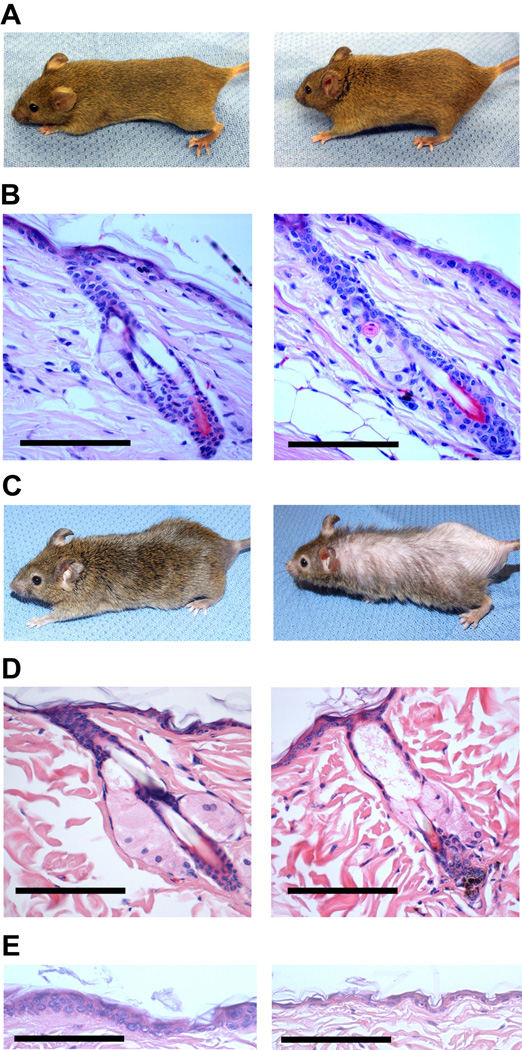
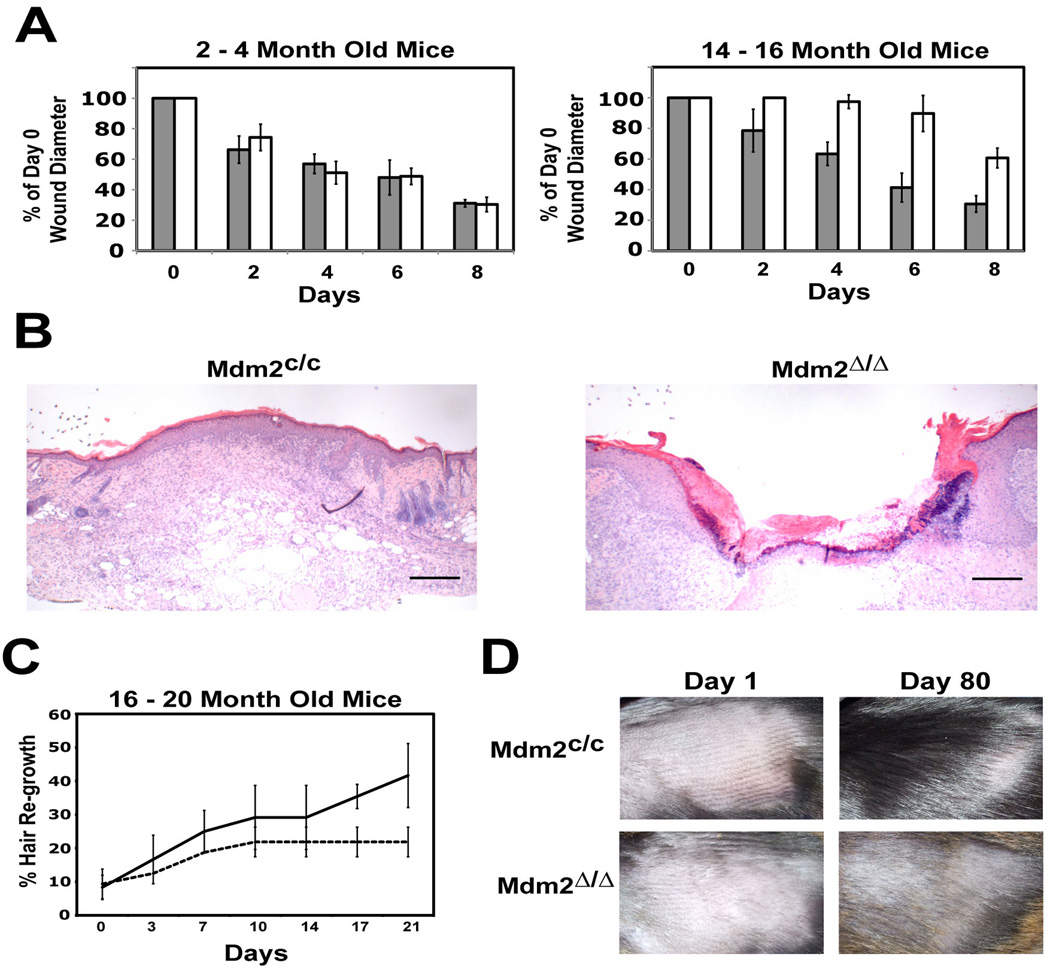
Loss of Mdm2 function has been associated with an increase in p53-mediated cell growth arrest or apoptosis in a number of mouse models, resulting in aberrant tissue function (Jones el al., 1995; Montes de Oca Luna et al., 1995; Itahana et al., 2007; Mendrysa et al., 2006; Lengner et al., 2006; Xiong et al., 2006; Grier et al., 2006). Surprisingly, Mdm2Δ/Δ mice younger than 10 months display no overt phenotype, despite the loss of Mdm2 function and increased p53 stabilization in the epidermis (Figs. 2A and 2B). However, all Mdm2Δ/Δ mice displayed progressive hair loss and decreased skin elasticity as they continued to age (Fig. 2C). Analysis of hematoxylin & eosin (H&E) - stained sections of skin from older (14–15 month old) Mdm2Δ/Δ mice revealed there are fewer hair follicles per millimeter (fl/mm) of dorsal skin in Mdm2Δ/Δ mice (0.70 fl/mm) than in control Mdm2c/c mice (1.4 fl/mm) (p=0.003). In addition, 27.1% of Mdm2Δ/Δ mouse follicles display an abnormal phenotype compared to 7.0% of the follicles in age-matched Mdm2c/c controls (p=0.004). These abnormal Mdm2Δ/Δ follicles were dilated, debris-laden, devoid of a hair shaft, cellularly atrophic, and morphologically disorganized (Fig. 2D).
Figure 2.
Mdm2Δ/Δ mice exhibit abnormal hair loss and a thinning of the epidermal layer after 12 months of age. (A) Younger Mdm2c/c (left) and Mdm2Δ/Δ mice (right) reveals no overt phenotypic difference. (B) H&E staining of representative hair follicles from young Mdm2c/c (left) and Mdm2Δ/Δ mice (right). Scale bars, 100 µm. (C) Phenotype of older Mdm2Δ/Δ mice (right) includes increased hair loss in aged mice deleted for Mdm2 compared to older Mdm2c/c (left) mice. (D) H&E staining of representative hair follicles from older Mdm2c/c (left) and Mdm2Δ/Δ mice (right). Scale bars, 100 µm. (E) H&E staining of interfollicular epidermis from older Mdm2c/c (left) and Mdm2Δ/Δ mice (right). Scale bars, 100 µm.
In addition to defects in fur maintenance, approximately 10% of all Mdm2Δ/Δ mice developed severe open skin wounds between 18–24 months of age. As these affected mice were housed individually, the skin lesions were likely caused by self-grooming. Similar wounding was not observed in age-matched, Mdm2c/c control mice or in older K5-Cre transgenic mice. To further examine the epidermal defects in these mice, skin biopsies were taken from Mdm2Δ/Δ mice and Mdm2c/c control mice at 16 months of age. There was an obvious decrease in the thickness of the interfollicular epidermis in older Mdm2Δ/Δ mouse relative to age-matched controls, accounting for the diminished integrity of the epidermal layer in older Mdm2Δ/Δ mice (Fig. 2E). These phenotypes are not present in young Mdm2Δ/Δ mice (Figs. 2A and 2B), suggesting a gradual degeneration of epidermal skin and hair follicle morphology in Mdm2Δ/Δ mice over time.
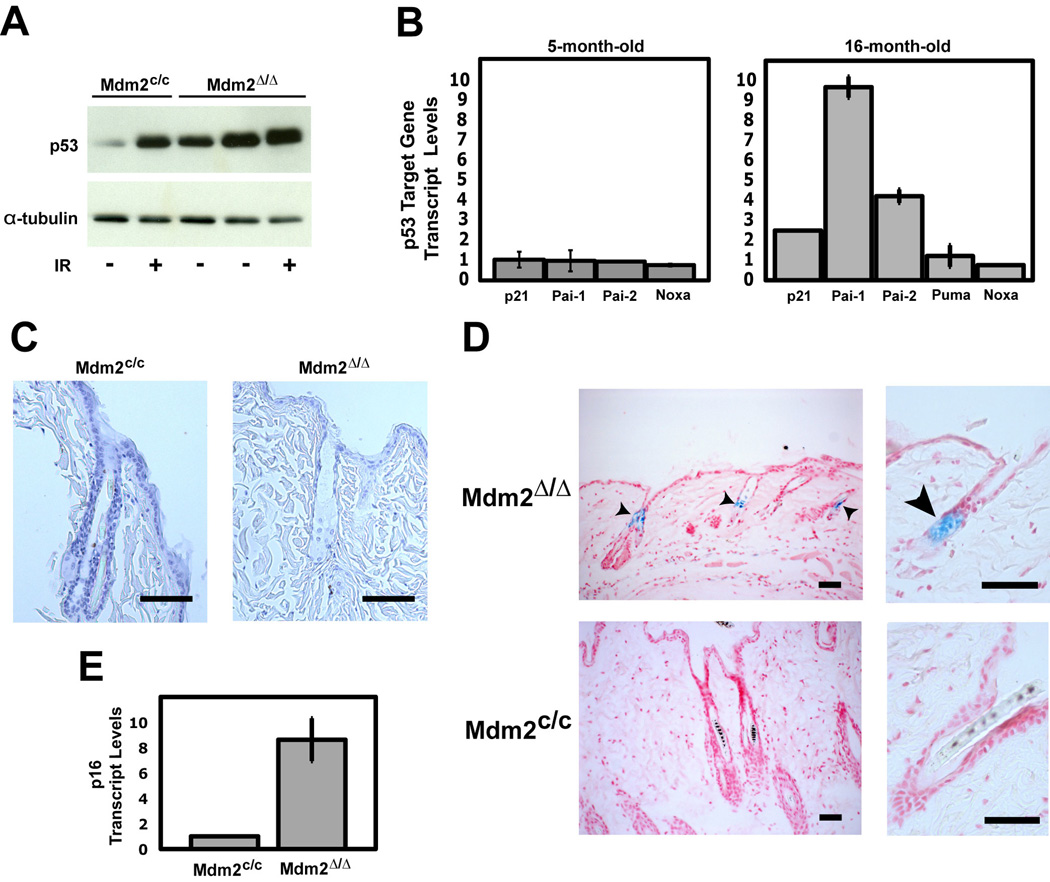
To examine epidermal p53 levels in older mice, we performed western blot analysis using skin protein lysates isolated from 20–23 month old Mdm2Δ/Δ and Mdm2c/c mice, as well as lysates isolated from γ–irradiated (3Gy) control mice (Fig. 3A). The amount of p53 present in older Mdm2Δ/Δ skin was far greater than the level of p53 detected in age-matched control Mdm2c/c skin, and was comparable to p53 levels observed in irradiated Mdm2c/c controls. To determine if the highly elevated p53 levels in older Mdm2Δ/Δ mice correlated with increased p53 activity, we examined the expression levels of select p53 target genes in the skin of 5-month old mice and 16-month old mice (Fig. 3B). Little or no elevation of the expression levels of p53 target genes was observed in the younger mouse skin (left panel). In contrast, the large increase in p53 protein levels in the older mouse skin correlated with an increase in p53 target gene activation (right panel). However, only a subset of p53 target genes displayed increased expression in older Mdm2Δ/Δ skin samples. Interestingly, these upregulated p53 targets included genes associated with cell cycle arrest and senescence (p21, Pai-1 and Pai-2) but not apoptosis (Puma and Noxa).
Figure 3.
Older Mdm2Δ/Δ mice have elevated p53 activity in the skin, leading to increased senescence but not apoptosis. (A) Western blot of p53 protein levels in skin from untreated and γ-irradiated (3 Gy, 15 hours) 20- to 23-month-old mice. α-Tubulin was used as a loading control. (B) Relative mRNA levels of p53 target genes in untreated skin from young (left) and older (right) mice determined by qRT-PCR. Values represent the fold difference in Mdm2Δ/Δ mice compared to Mdm2c/c mice (n=3 per genotype). All levels were normalized to Gaphd expression levels. Standard deviation indicated by error bars. (C) TUNEL staining of skin from an aged Mdm2c/c mouse (left), and Mdm2Δ/Δ mouse (right). Scale bars, 100 µm. (D) Senescence-associated-β-galactosidase activity detected in skin of aged Mdm2Δ/Δ mice (top) but not in Mdm2c/c mice (bottom). Sections were counterstained with Nuclear Fast Red. Scale bars, 50 µm. Arrowheads denote β-gal positive stain. (E) Relative p16 mRNA levels in skin from older mice determined by qRT-PCR (n=3 per genotype). Levels were normalized to Gaphd expression level. Standard deviation indicated by error bars.
To confirm that ablation of Mdm2 and upregulation of p53 activity in the skin of Mdm2Δ/Δ mice did not induce apoptosis in this tissue, we performed terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining on skin sections of 14–16 month old Mdm2Δ/Δ and Mdm2c/c mice. TUNEL staining was readily detected at the base of those follicles undergoing remodeling (catagen-phase) in both Mdm2Δ/Δ and Mdm2c/c tissues (data not shown). This staining was anticipated, because normal fur remodeling is ongoing even in older mice and appears to be regulated primarily by Bcl2 and not by p53 (Stenn and Paus, 2001). However, TUNEL positive cells were not observed in either the follicular or interfollicular epidermis of older Mdm2c/c or Mdm2Δ/Δ mice (Fig. 3C) or in younger Mdm2c/c or Mdm2Δ/Δ mice (data not shown), indicating that increased p53 activity in the skin of Mdm2Δ/Δ mice did not correlate with an increased number of apoptotic cells. These negative results correlate with the lack of increased apoptotic gene expression seen in the skin of Mdm2Δ/Δ mice, confirming that the loss of fur and thinning of the epidermis seen in the Mdm2Δ/Δ mice is unlikely to be due to p53-mediated apoptosis in the skin.
Increased cellular senescence has been linked to aging phenotypes in other mouse models (Rodier et al., 2007). Since upregulation of p53 activity in Mdm2Δ/Δ mice resulted in the activation of p53 target genes primarily involved in cell senescence, we examined the epidermis of older Mdm2Δ/Δ mice for senescent cells. Skin sections of 16-month old Mdm2Δ/Δ mice and Mdm2c/c mice were fixed and stained for senescence-associated β–galactosidase (SA-β-Gal) activity. SA-β-Gal activity was undetectable in the follicular epithelium of Mdm2c/c control mice (Fig. 3D–bottom panels), whereas 21% (±3.6%) of the hair follicles in Mdm2Δ/Δ mice stained positive for SA-β-Gal activity (Fig. 3D– top panels). Interestingly, SA-β-Gal staining was particularly acute in the follicular bulge, the region of the follicle containing the epidermal stem cell compartment (Tumbar et al., 2004). To confirm senescence of epidermal cells in Mdm2Δ/Δ mice, we examined expression of the p16INK4A tumor suppressor, a gene whose expression is not governed by p53 and that has been previously reported to be increased in senescent and aging skin cells of older mice and humans (Jenkins, 2002; Ressler et al., 2006; Campisi, 2005). A significant increase in p16INK4A expression was detected by qPCR in the skin of older Mdm2Δ/Δ mice, relative to expression levels in age-matched Mdm2c/c skin (Fig. 3E), confirming that loss of Mdm2-p53 signaling induces an established molecular marker of senescence and accelerated aging in the epidermis. Assays of age-matched, younger Mdm2Δ/Δ mice and control Mdm2c/c mice (5–6 months) revealed no differences SA-β-Gal staining or in p16INK4A expression in the skin (data not shown).
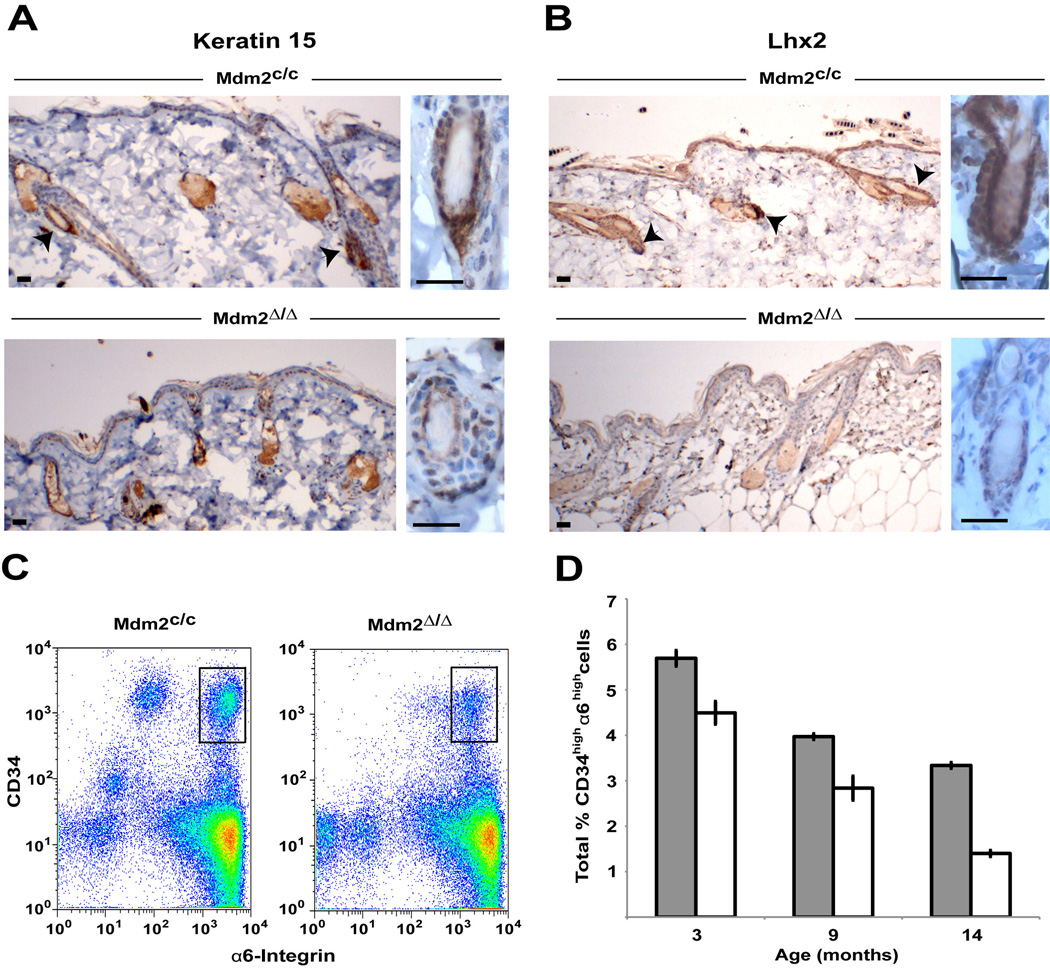
SA-β-Gal staining of the follicular bulge region and upregulation of p16 expression indicates premature cell senescence in the epidermal stem cell compartment of Mdm2Δ/Δ mice. To confirm that the defect in Mdm2Δ/Δ mice is due to the reduced numbers of epidermal stem cells, we examined Mdm2Δ/Δ follicular bulge cells for keratin 15 (K15) and LIM homeobox protein 2 (Lhx2) staining, specific markers of bulge stem cells in mice (Liu et al., 2003; Rhee et al., 2006). Staining of skin cross sections of older Mdm2Δ/Δ and control Mdm2c/c mice revealed a decrease in K15-positive (Fig. 4A) and Lhx2-positive (Fig. 4B) cells in Mdm2Δ/Δ mice. Typical follicles in Mdm2Δ/Δ mice have very faint K15 and Lhx2 staining (Fig. 4A and B-bottom panels) compared to age-matched control follicles (Fig. 4A and B-top panels), and staining is absent in the all of the abnormal follicles examined in Mdm2Δ/Δ mice (data not shown). To confirm a reduction in numbers of epidermal stem cells in mice ablated for Mdm2, we performed FACS analysis of epidermal cells harvested from age-matched Mdm2Δ/Δ and control Mdm2c/c mice and documented the numbers of cells expressing high levels of both CD34 and α6-integrin, well-established epidermal stem cells markers (Nowak and Fuchs, 2009). In agreement with our K15 and Lhx2 staining results, fewer CD34/α6-intgrin double-positive cells are present in the skin of Mdm2Δ/Δ mice compared to the numbers of double positive cells in Mdm2c/c control mice (Fig. 4C and D). Interestingly, while fewer epidermal stem cells are seen in the skin of Mdm2Δ/Δ mice at 3 months and 9 months of age (before the skin phenotype is observed in mice- Fig. 2A), a much larger decrease in epidermal stem cell numbers is seen in 14-month old Mdm2Δ/Δ mice (Fig. 4D), when the phenotype of the Mdm2Δ/Δ mice is readily apparent (Fig. 2C).
Figure 4.
Hair follicle stem cell markers are diminished in older Mdm2Δ/Δ mice. (A) Follicular keratin 15 staining in older Mdm2c/c (top) and Mdm2Δ/Δ (bottom) mice. Arrowheads denote specific staining. Scale bars, 25 µm. (B) Follicular Lhx2 staining in older Mdm2c/c (top) and Mdm2Δ/Δ (bottom) mice. Arrowheads denote specific staining. Scale bars, 25 µm. (C) Representative FACS plots for 9-month-old Mdm2c/c (left) and Mdm2Δ/Δ (right) mice. Bulge stem cells (CD34high/α6-Integrinhigh) are shown in black boxes. (D) Summary of the total percentage of CD34high/α6-Integrinhigh cells determined by FACS analysis in Mdm2c/c (grey bars) and Mdm2Δ/Δ (white bars) mice at different ages (n=3 per genotype for each age group). Standard deviation indicated by error bars.
Loss of tissue regenerative potential and disrupted organ homeostasis are hallmarks of normal aging in mice and are often exacerbated in mouse models of accelerated aging (Rodier et al., 2007; Campisi, 2005). In skin, follicular bulge stem cells produce transiently amplifying epidermal progenitor cells that migrate to wounded areas of the interfollicular epidermis and coordinate the wound healing response (Tumbar et al., 2004; Taylor et al., 2000). To confirm that epidermal stem cell function is compromised in older Mdm2Δ/Δ mice, we compared the decrease in diameter of an introduced 3mm2 dorsal wound in Mdm2Δ/Δ mice and Mdm2c/c control mice. Wounds in younger (2 to 4-month old) Mdm2Δ/Δ and Mdm2c/c mice healed at a similar rate (Fig. 5A left panel), with an average 65% wound closure in mice of both genotypes at 8 days. Wounds in older (14 to 16-month old) Mdm2c/c control mice (Figure 5A-right panel) closed at a rate similar to that seen in the younger mice. In contrast, wounds in the older Mdm2Δ/Δ mice closed at a much slower rate, consistent with a decrease in epidermal stem cell function. After 8 days, the wound sites in control Mdm2c/c mice were fully healed, whereas the wound sites in age-matched Mdm2Δ/Δ mice remained mostly open (Fig. 5B). These difference were not due to different rates of epidermal apoptosis at the site of wounding, as no differences in TUNEL staining were observed in Mdm2Δ/Δ and Mdm2c/c tissue sections (data not shown).
Figure 5.
Bulge stem cell function is impaired in older Mdm2Δ/Δ mice. (A) Wound healing assay of 2- to 4-month old (left) and 14- to 16-month old (right) Mdm2c/c (grey bars) and Mdm2Δ/Δ mice (white bars). The decrease in diameter of a dorsal punch biopsy wound was measured over time (n=3 per genotype). Standard deviation indicated by error bars. (B) H&E staining of wounds from a Mdm2c/c mouse (left) and a Mdm2Δ/Δ mouse (right) at day 8 of the wound healing assay. Scale bars, 250 µm. (C) Hair growth in 16- to 20-month-old mice. The percentage hair growth for Mdm2c/c mice (solid line) and Mdm2Δ/Δ mice (dotted line) was plotted over time (n=3 per genotype). Standard deviation indicated by error bars. (F) Pictures of an aged Mdm2c/c mouse (top) and Mdm2Δ/Δ mouse (bottom) at day 1 and day 80 post-shaving.
Epidermal stem cells are also required to promote the anagen-telogen-catagen hair cycle (Tumbar et al., 2004; Morris and Potten, 1999), and the rate of fur regrowth after shaving of mice is another direct measure of follicular stem cell function. To further confirm loss of epidermal stem cell-mediated functions in older Mdm2Δ/Δ mice, we shaved the fur from a 4cm2 dorsal area on Mdm2Δ/Δ mice and Mdm2c/c control mice and measured fur re-growth over time (Fig. 5C). While there is no difference in the growth of fur in younger Mdm2Δ/Δ mice and Mdm2c/c mice (data not shown), fur growth in older (14–16 month old) Mdm2Δ/Δ mice is significantly delayed relative to control mice in the 3-week interval after shaving (Fig. 5C). In addition, older Mdm2Δ/Δ mice display reduced fur growth even 80 days post-shaving (Fig. 5D)
Discussion
The Mdm2Δ/Δ mice present a premature aging phenotype in the epidermis that is highly similar to the accelerated aging phenotype that we observed previously in the skin of p53 m-allele mice, including a thinning of the epidermal layer, reduced wound healing, and a reduced capacity to re-grow fur. As deletion of Mdm2 results in an increase in endogenous p53 levels and upregulation of select p53 target gene expression in the epidermis of Mdm2Δ/Δ mice, our results corroborate that an increase in p53 activity in p53 m-allele mice is responsible for the accelerated aging phenotypes observed previously in this model and in certain other p53 models (Tyner et al., 2002; Maier et al., 2004). However, not all mouse models bearing increased levels of p53 display premature aging, and retention of some degree of Mdm2-p53 signaling in these models may well account for the lack of accelerated aging-like phenotypes in these mice (Garcia-Cao et al., 2002; Mendrysa et al., 2006).
It is interesting to note that ablation of Mdm2 and upregulation of p53 in the epidermis did not result in p53 activation of pro-apoptotic genes and immediate cell death as seen in other settings (Lengner et al., 2006; Xiong et al., 2006; Martins et al., 2006). Instead, disruption of Mdm2-p53 signaling induced SA-β-Gal staining of the follicular bulge region and upregulation of p16 expression, indicating cellular senescence within the epidermal stem cell compartment in Mdm2Δ/Δ mice. Thus, the ability of Mdm2 to properly regulate p53-mediated senescence in this tissue determines the presence or absence of p53-associated aging phenotypes. Activation of p53-mediated cell senescence in epidermal stem cells and altered wound healing has also been observed in mice bearing shortened telomeres (Flores and Blasco, 2009), supporting a role for p53 activation in regulating epidermal stem cell function. However, as fur loss and reduced skin integrity was not reported in these mice, the differences in the level of p53 activation or the timing of p53-induced senescence in the epidermis may also underlie the presence or absence of aging-like phenotypes in the skin.
The results of our study reveal that loss of Mdm2, the concomitant upregulation of p53, and the subsequent induction of p53-mediated senescence induces an accelerated aging (but not progeria-like) phenotype in the skin. Although increased p53 levels can be detected in the skin of younger Mdm2Δ/Δ mice (Fig. 1D), these mice do not display upregulated levels of p53-target genes in their skin (Fig. 2B), and the Mdm2Δ/Δ mice appear unaffected up to 10 months (Figs. 2A and 2B). However, after 10 months of age fur loss and epidermal thinning becomes readily apparent in these mice. As stem cells in the epidermal bulge region are generated during late embryogenesis in the mouse (Stenn and Paus, 2001), the postnatal ablation of Mdm2 induced by the K5-Cre in our model may be governing epidermal stem cell renewal and not the functional differentiation of pre-existing epidermal stem cells. In keeping with this possibility, it has been established previously that follicle bulge cells are slow cycling, with an average of 14 months in the mouse (Morris and Potten, 1999). Thus, the pre-established epidermal stem cell compartment in Mdm2Δ/Δ mice likely retain cells that are still capable of differentiating into committed progenitor cells that form the follicular and inter-follicular epidermis, but have lost the capacity to self-renew the epidermal stem cell compartment, leading to exhaustion of the stem cell population with age. The precise role of p53-induced senescence in regulating self-renewal in epidermal stem cells will be a matter for further investigation.
Conclusions
Our findings reveal that activation of p53 due to loss of Mdm2 in the skin of Mdm2-conditional mice induces p53-mediated senescence within the epidermal stem cell compartment and a reduction in epithelial stem cell numbers and functions in older mice. In addition, Mdm2-mediated inhibition of p53 activity in epithelial cells of the skin is essential for maintenance of skin integrity and fur as mice age. Thus, Mdm2-p53 signaling governs certain rapid-aging phenotypes in skin. The ability of Mdm2 to properly downregulate p53 in other p53 mouse models may therefore account for the presence or absence of aging-like phenotypes in these models.
Acknowledgements
We thank Dennis Roop (Baylor College of Medicine) for kindly providing us with K5-Cre mice, Qichang Shen and Kathleen Hoover for assistance with breeding and genotype analysis of the mouse lines, and Zdenka Matijasevic for comments on the manuscript. Core facilities were partly supported by grant DK32520 from the NIDDK, and SNJ is a member of the UMMS Diabetes and Endocrinology Research Center. This research was supported by grant CA077735 from the National Cancer Institute to SNJ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balasubramanian S, Ahmad N, Jeedigunta S, Mukhtar H. Alterations in cell cycle regulation in mouse skin tumors. Biochem Biophys Res Commun. 1998;243(3):744. doi: 10.1006/bbrc.1998.8172. [DOI] [PubMed] [Google Scholar]
- Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120(4):513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Chen J, Lin J, Levine AJ. Regulation of transcription functions of the p53 tumor suppressor by the mdm-2 oncogene. Mol Med. 1995;1(2):142–152. [PMC free article] [PubMed] [Google Scholar]
- Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, Butel JS, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356(6366):215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- Freedman DA, Levine AJ. Nuclear export is required for degradation of endogenous p53 by MDM2 and human papillomavirus E6. Mol Cell Biol. 1998;18(12):7288–7293. doi: 10.1128/mcb.18.12.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores I, Blasco MA. A p53-dependent response limits epidermal stem cell functionality and organismal size in mice with short telomeres. PLoS One. 2009;4(3):e4934. doi: 10.1371/journal.pone.0004934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cao I, Garcia-Cao M, Martin-Caballero J, Criado LM, Klatt P, Flores JM, Weill JC, Blasco MA, Serrano M. “Super p53” mice exhibit enhanced DNA damage response, are tumor resistant and age normally. EMBO J. 2002;21(22):6225–6235. doi: 10.1093/emboj/cdf595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry A, Venkatachalam S. Complicating the role of p53 in aging. Aging Cell. 2005;4(3):157–160. doi: 10.1111/j.1474-9726.2005.00154.x. [DOI] [PubMed] [Google Scholar]
- Geyer RK, Yu ZK, Maki CG. The MDM2 RING-finger domain is required to promote p53 nuclear export. Nat Cell Biol. 2000;2(9):569–573. doi: 10.1038/35023507. [DOI] [PubMed] [Google Scholar]
- Grier JD, Xiong S, Elizondo-Fraire AC, Parant JM, Lozano G. Tissue-specific differences of p53 inhibition by Mdm2 and Mdm4. Mol Cell Biol. 2006;26(1):192–198. doi: 10.1128/MCB.26.1.192-198.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Keyes WM, Papazoglu C, Zuber J, Li W, Lowe SW, Vogel H, Mills AA. TAp63 induces senescence and suppresses tumorigenesis in vivo. Nat Cell Biol. 2009;11(12):1451–1457. doi: 10.1038/ncb1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387(6630):296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- Honda R, Tanaka H, Yasuda H. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 1997;420(1):25–27. doi: 10.1016/s0014-5793(97)01480-4. [DOI] [PubMed] [Google Scholar]
- Itahana K, Mao H, Jin A, Itahana Y, Clegg HV, Lindstrom MS, Bhat KP, Godfrey VL, Evan GI, Zhang Y. Targeted inactivation of Mdm2 RING finger E3 ubiquitin ligase activity in the mouse reveals mechanistic insights into p53 regulation. Cancer Cell. 2007;12:355–366. doi: 10.1016/j.ccr.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Jenkins G. Molecular mechanisms of skin ageing. Mech Ageing Dev. 2002;123(7):801–810. doi: 10.1016/s0047-6374(01)00425-0. [DOI] [PubMed] [Google Scholar]
- Jones SN, Roe AE, Donehower LA, Bradley A. Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature. 1995;378(6553):206–208. doi: 10.1038/378206a0. [DOI] [PubMed] [Google Scholar]
- Kemp CJ, Wheldon T, Balmain A. p53-deficient mice are extremely susceptible to radiation-induced tumorigenesis. Nat Genet. 1994;8(1):66–69. doi: 10.1038/ng0994-66. [DOI] [PubMed] [Google Scholar]
- Kubbutat MH, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature. 1997;387(6630):299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- Lengner CJ, Steinman HA, Gagnon J, Smith TW, Henderson JE, Kream BE, Stein GS, Lian JB, Jones SN. Osteoblast differentiation and skeletal development are regulated by Mdm2-p53 signaling. J Cell Biol. 2006;172(6):909–921. doi: 10.1083/jcb.200508130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Brooks CL, Wu-Baer F, Chen D, Baer R, Gu W. Mono- versus polyubiquitination: differential control of p53 fate by Mdm2. Science. 2003;302(5652):1972–1975. doi: 10.1126/science.1091362. [DOI] [PubMed] [Google Scholar]
- Little NA, Jochemsen AG. Hdmx and Mdm2 can repress transcription activation by p53 but not by p63. Oncogene. 2001;20(33):4576–4580. doi: 10.1038/sj.onc.1204615. [DOI] [PubMed] [Google Scholar]
- Liu Y, Lyle S, Yang Z, Cotsarelis G. Keratin 15 promoter targets putative epithelial stem cells in the hair follicle bulge. J Invest Dermatol. 2003;121(5):963–968. doi: 10.1046/j.1523-1747.2003.12600.x. [DOI] [PubMed] [Google Scholar]
- Maier B, Gluba W, Bernier B, Turner T, Mohammad K, Guise T, Sutherland A, Thorner M, Scrable H. Modulation of mammalian life span by the short isoform of p53. Genes Dev. 2004;18(3):306–319. doi: 10.1101/gad.1162404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins CP, Brown-Swigart L, Evan GI. Modeling the therapeutic efficacy of p53 restoration in tumors. Cell. 2006;127(7):1323–1334. doi: 10.1016/j.cell.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Mendrysa SM, O’Leary KA, McElwee MK, Michalowski J, Eisenman RN, Powell DA, Perry ME. Tumor suppression and normal aging in mice with constitutively high p53 activity. Genes Dev. 2006;20(1):16–21. doi: 10.1101/gad.1378506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momand J, Zambetti GP, Olson DC, George D, Levine AJ. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69(7):1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- Montes de Oca Luna R, Wagner DS, Lozano G. Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature. 1995;378(6553):203–206. doi: 10.1038/378203a0. [DOI] [PubMed] [Google Scholar]
- Morris RJ, Potten CS. Highly persistent label-retaining cells in the hair follicles of mice and their fate following induction of anagen. J Invest Dermatol. 1999;112(4):470–475. doi: 10.1046/j.1523-1747.1999.00537.x. [DOI] [PubMed] [Google Scholar]
- Nowak JA, Fuchs E. Isolation and Culture of Epithelial Stem Cells. In: Audet J, Stanford WL, editors. Stem Cells in Regenerative Medicine: Methods and Protocols. Vol. 482. Humana Press; 2009. pp. 215–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyurovsky MV, Prives C. Unleashing the power of p53: lessons from mice and men. Genes Dev. 2006;20(2):125–131. doi: 10.1101/gad.1397506. [DOI] [PubMed] [Google Scholar]
- Rhee H, Polak L, Fuchs E. Lhx2 maintains stem cell character in hair follicles. Science. 2006;312(5782):1946–1949. doi: 10.1126/science.1128004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler S, Bartkova J, Niederegger H, Bartek J, Scharffetter-Kochanek K, Jansen-Durr P, Wlaschek M. p16INK4A is a robust in vivo biomarker of cellular aging in human skin. Aging Cell. 2006;5(5):379–389. doi: 10.1111/j.1474-9726.2006.00231.x. [DOI] [PubMed] [Google Scholar]
- Rodier F, Campisi J, Bhaumik D. Two faces of p53: aging and tumor suppression. Nucleic Acids Res. 2007;35(22):7475–7484. doi: 10.1093/nar/gkm744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soussi T, Beroud C. Assessing TP53 status in human tumours to evaluate clinical outcome. Nat Rev Cancer. 2001;1(3):233–240. doi: 10.1038/35106009. [DOI] [PubMed] [Google Scholar]
- Steinman HA, Jones SN. Generation of an Mdm2 conditional allele in mice. Genesis. 2002;32(2):142–144. doi: 10.1002/gene.10035.abs. [DOI] [PubMed] [Google Scholar]
- Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81(1):449–494. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 2000;102(4):451–461. doi: 10.1016/s0092-8674(00)00050-7. [DOI] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303(5656):359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyner SD, Venkatachalam S, Choi J, Jones S, Ghebranious N, Igelmann H, Lu X, Soron G, Cooper B, Brayton C, Hee Park S, Thompson T, Karsenty G, Bradley A, Donehower LA. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415(6867):45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- Vijg J, Hasty P. Aging and p53: getting it straight. A commentary on a recent paper by Gentry and Venkatachalam. Aging Cell. 2005;4(6):331–333. doi: 10.1111/j.1474-9726.2005.00179.x. [DOI] [PubMed] [Google Scholar]
- Vousden KH, Lu X. Live or let die: the cell’s response to p53. Nat Rev Cancer. 2002;2(8):594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- Wang X, Arooz T, Siu WY, Chiu CH, Lau A, Yamashita K, Poon RY. MDM2 and MDMX can interact differently with ARF and members of the p53 family. FEBS Lett. 2001;490(3):202–208. doi: 10.1016/s0014-5793(01)02124-x. [DOI] [PubMed] [Google Scholar]
- Xiong S, Van Pelt CS, Elizondo-Fraire AC, Liu G, Lozano G. Synergistic roles of Mdm2 and Mdm4 for p53 inhibition in central nervous system development. Proc Natl Acad Sci U S A. 2006;103(9):3226–3231. doi: 10.1073/pnas.0508500103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Wang D, Wang XJ, Roop DR. In utero activation of K5.CrePR1 induces gene deletion. Genesis. 2002;32(2):191–192. doi: 10.1002/gene.10064. [DOI] [PubMed] [Google Scholar]