Abstract
Congenital methemoglobinemia due to NADH-cytochrome b5 reductase 3 (CYB5R3) deficiency is an autosomal recessive disorder that occurs sporadically worldwide, although endemic clusters of this disorder have been identified in certain ethnic groups. It is present as two distinct phenotypes, type I and type II. Type I methemoglobinemia is characterized by CYB5R3 enzyme deficiency restricted to erythrocytes and is associated with benign cyanosis. The less frequent type II methemoglobinemia is associated with generalized CYB5R3 deficiency affecting all cells and is lethal in early infancy. Here we describe the molecular basis of type I methemoglobinemia due to CYB5R3 deficiency in four patients from three distinct ethnic backgrounds, Asian Indian, Mexican and Greek.
The CYB5R3 gene of three probands with type I methemoglobinemia and their relatives were sequenced revealing several putative causative mutations; in one subject multiple mutations were present. Two novel mutations, S54R and F157C, were identified and the previously described A179T, V253M mutations were also identified. All these point mutations mapped to the NADH binding domain and or the FAD binding domain. Each has the potential to sterically hinder cofactor binding causing instability of the CYB5R3 protein. Wild type CYB5R3 as well as two of these novel mutations, S54R and F157C, were amplified, cloned, and purified recombinant peptide obtained. Kinetic and thermodynamic studies of these proteins show that the above mutations lead to decreased thermal stability.
Keywords: methemoglobinemia, NADH-cytochrome b5 reductase 3, gene mutation, cyanosis, protein structure
INTRODUCTION
NADH-Cytochrome b5 reductase 3 (Diaphorase-1, also known as NADH-methemoglobin reductase) (B5R: EC 1.6.2.2:UniProt knowledgebase) is a flavin adenine dinucleotide (FAD) containing enzyme catalyzing electron transfer from NADH to cytochrome b5. A single gene located on chromosome 22q13 (GeneID: 1727) produces two protein variants. A soluble form is present predominantly in erythrocytes and a membrane-bound form is localized to the endoplasmic reticulum (ER), mitochondria, nuclei, peroxisomes and plasma membranes of somatic cells[1; 2; 3].
Deficiency of CYB5R3 leads to hereditary methemoglobinemia. This is a rare autosomal recessive disorder that presents as two distinct phenotypes, type I and type II. Type I methemoglobinemia (OMIM 250800) has CYB5R3 enzyme deficiency restricted to erythrocytes, leading to otherwise benign cyanosis that can be readily corrected by administration of methylene blue, or ascorbic acid and riboflavin. In contrast, the less frequent type II methemoglobinemia is associated with generalized CYB5R3 deficiency affecting all cells and is characterized by mild cyanosis with severe neurologic disability and numerous developmental defects [4] the affected infants usually die at an early age but an oldest reported case of type II reached the age of 23 years[5].
We studied the molecular basis of methemoglobinemia of four patients with type I methemoglobinemia from three distinct ethnic backgrounds. We determined the causative mutation in the CYB5R3 gene in 2 Asian Indian siblings, a Mexican child and an individual of Greek descent. Recombinant proteins, containing each of the mutations identified were expressed and purified. The proteins were characterized biochemically and the thermodynamic parameters for each were studied. Structural modeling of the identified mutants was performed and the predicted consequence of the mutations based on the human CYB5R crystal structure by computer modeling was attained. These investigations contribute to our understanding of the mechanism of enzyme instability of type I congenital methemoglobinemia and support the notion that most of the mutations causing sporadic autosomal recessive congenital methemoglobinemia are private mutations.
MATERIALS & METHODS
Blood sample
Institutional approved and informed consent was obtained from all subjects. Peripheral blood was collected and leukocytes were separated using the Histopaque density gradient method (Sigma-Aldrich, Inc. St.Louis MO, USA). Nucleic acids were extracted using Tri-Reagent (Molecular Research Center, Inc. Ohio, USA), and or genomic DNA (gDNA) were isolated using PUREGENE DNA purification kit (Gentra, Minneapolis, MN) according to the manufacturer’s instructions.
Polymerase chain reaction (PCR) and Sequencing
Extracted genomic DNA from each sample was PCR amplified flanking all 9 exons (see supplementary table). Amplicons were gel purified and directly sequenced in both directions. Whether the mutation was in cis or trans was determined for those with multiple mutations in amplicons of CYB5R3 coding region that were inserted into a plasmid vector and cloned using TOPO Cloning kit following the manufacturer’s instructions (Invitrogen, Carlsbad CA). Purified clones were subsequently sequenced to determine the relative position of the mutation.
Methemoglobin analysis
The methemoglobin proportion was established and globin mutations as a cause of methemoglobinemia were ruled out by standard methods [4; 6].
Cloning and Expression of Recombinant Proteins
A PCR fragment of the entire coding region of CYB5R3 harboring either wild-type, S54R, F157C or V253M sequences were cloned (Invitrogen Corp., Carlsbad, CA, USA) in frame with the 10X histidine tag into the pET16b expression vector (Novagen, EMD Chemicals, Inc., NJ, USA). Sequence fidelity and in frame fusion of all constructs was verified by sequencing both strands. Recombinant protein was expressed in E. coli and purified using 1 mL Ni-NTA affinity column chromatography. Purified protein concentrations were measured using bicinchoninic acid (BCA) method (Thermo-Fisher Scientific., IL, USA.) and were adjusted to 1mg/mL for biochemical characterization.
Enzyme assay
NADH-ferricyanide reductase (NADH:FR) activity[7; 8] was measured following oxidation of NADH at 340nm on a Synergy HT microplate reader for 10 minutes at 30 °C (Biotek Instruments, Inc., VT, USA). Reactions were monitored at 1 minute intervals and a rate was determined from the linear portion of the curve, rates were calculated using a linear regression model. Each reaction contained 0.4 ug/ul of purified protein, 2 mM potassium ferricyanide (K3Fe(CN)6), 1 mM Tris-HCl pH 8.0, 0.5 mM EDTA and 0.2 mM NADH.
Thermal Stability measurement and thermodynamics
Thermal stability profiles for normal and mutant protein were determined by measuring the decrease of NADH:FR activity at different temperatures. Samples were pre-incubated at increasing temperature from 16–55 °C for 20 minutes in a Peltier thermal cycler (MJ Research, MA, USA) and placed on ice. Residual NADH: FR activity was determined as described above. Thermodynamic parameters were calculated by rearranging the equations for the calculation of Gibbs free energy and the van’t Hoff equation as described [8].
Model of CBR mutant structure
A working structural model was built for each mutant protein by replacing Ser 54 with Arg, Phe 157 with Cys, Ala 179 with Thr and Val 253 to Met in human cytochrome B reductase (PDB:1UMK crystal structure of human erythrocyte CBR) and subjected to energy minimization using molecular visualization, modeling and simulation program, Yasara (Institute for Molecular biology, Biochemistry and Microbiology, University of Graz, Austria).
RESULTS & DISCUSSION
Clinical Studies
Four patient samples representing three distinct ethnic backgrounds were used for this study. Two siblings were Asian Indians (Kashmir Gujjar ethnicity) with clinical features of type I methemoglobinemia and methemoglobin level elevated at average of ~20%. In both cases a mutation in CYB5R3 was identified after potential mutations of the globin genes were excluded by sequencing (data not shown). Samples of whole blood were not available for measurement of enzyme activity but their DNA was referred to Salt Lake City for further study. The third subject, a 12 years old Mexican female with Turner's syndrome and apparent congenital cyanosis, was referred to our institution for evaluation. Her methemoglobin level was elevated at 18% and erythrocyte cytochrome b5 reductase was reduced (4% of the normal range). The fourth individual, a 36 year old female of Greek ancestry, had a past history of childhood cyanosis. She had an episode of cyanosis accompanied by dyspnea, syncope, and elevated methemoglobin levels at ~10%. Mutations in the globin genes was ruled out by a spectrophotometric analysis of methemoglobin[4] and this individual was subsequently referred for analysis of the CYB5R3 gene.
Identification and characterization of mutation
The patients’ genomic DNA and or cDNA were amplified and compared with the sequence of wild-type CYB5R3 (NG_012194 DNA and NM_000398 mRNA), mutations identified are depicted in Table 1. A non-synonymous heterozygous G>A transversion in exon 6 resulting in the change of amino acid 179 from alanine to threonine (p.A179T) was identified in both affected Indian siblings and not in their unaffected cousin; this mutation was first reported in a Dutch Caucasian subject [9] and then in other individuals [10; 11; 12]. Alanine 179 is located in the NADH binding domain in close proximity to the adenosine moiety; replacement by threonine has been suggested to alter the binding of the NADH coenzyme [10; 11; 12; 13; 14].
Table 1.
List of the CYB5R3 mutations found in all of the subjects evaluated.
| Exon | HGVS nomenclature for nt change*,# |
HGVS nomenclature for amino acid change |
Mutation | Ethnicity | Described in a Previous Publication |
|---|---|---|---|---|---|
| 2 | c.216 G>A | p.Pro44Pro | Silent | Greek | Not applicable |
| 3 | g.17958 C>G | p.Ser54Arg | Missense | Mexican | Not applicable |
| 6 | c.554 T>G | p.Phe157Cys | Missense | Greek | Not applicable |
| 6 | g.21783 G>A | p.Ala179Thr | Missense | Indian | Dekker et al (2001) Kugler et al (2001) Maran et al (2005) Percy et al (2005) |
| 9 | c.841 G>A | p.Val253Met | Missense | Greek | Dekker et al (2001) Kugler et al (2001) Grabowska et al (2003) Percy et al (2006) |
| 9 (3’ UTR) | c.1053 A>G | - | SBS | Greek | Not applicable |
| 9 (3’ UTR) | c.1277 C>A | - | SBS | Greek | Not applicable |
The Greek subject had V253M mutation in addition to other mutations (see below).V253M was also described in a Dutch Caucasian subject [9]. An attempt was made to determine if the mutation present in the Dutch, Indian and Greek subjects was associated with the same or a different haplotypes; i.e. were these separate denovo mutations or was a common ancestor responsible for this introduction. However, samples of DNA from the Dutch subjects were no longer available (Dr. Dekker personal communication) and this analysis could not be carried out.
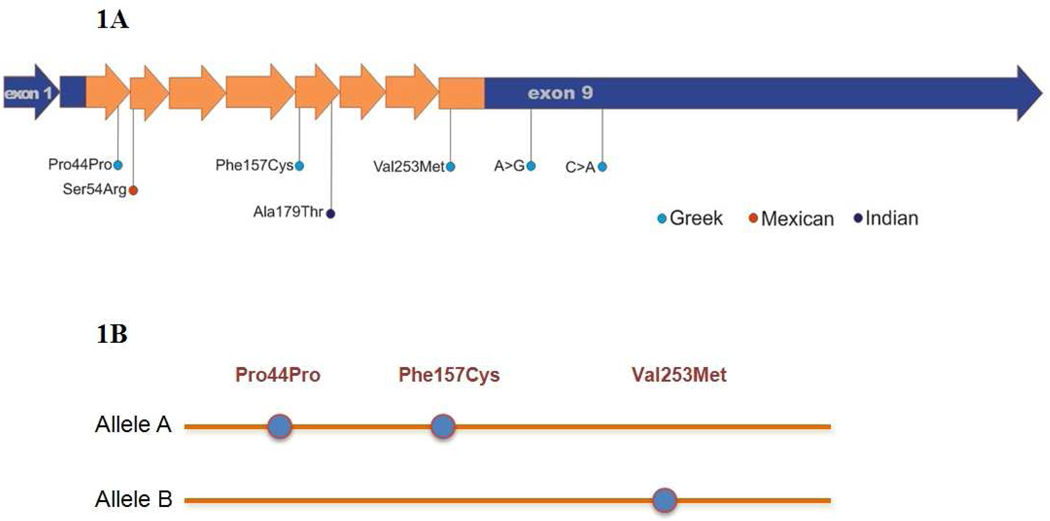
In addition to the V253M mutation four other heterozygous base changes were identified in the Greek subject. She was heterozygous for a synonymous c.216 G>A substitution (p.P44P) in exon 2, and two 3'-UTR single nucleotide polymorphisms (c.1053A>G , and c.1277 C>A) (Table 1). Lastly, a novel heterozygous c.554 T>G transversion mutation in exon 6 was found, resulting in substitution of cysteine for phenylalanine at codon 157 (p.F157C) (Figure 1a).
Figure 1.
Mutations identified in four patients with Type I Methemoglobinemia A. Schematic display of the mutations identified in CYB5R3 in the 4 subjects. The coding region is shown in orange, the 5’ and 3’ non-coding regions are shown in blue. The mutations are color coded to depict the ethnic background of the subjects. B. Allelic distribution of CYB5R3 coding region in a Greek subject with multiple mutations. P44P is in cis with F157C and in trans with V253M.
A novel homozygous mutation, in the Mexican subject, located in exon 3 (g.17958C>G transition) resulted in substitution of serine for arginine at position 54 (p.S54R). Our protein structure predictive modeling suggests that it causes disruption of hydrogen bonding between the cofactor and the protein. This negative interaction is due to steric hindrance by the bulky arginine side chain. The lack of proper cofactor assembly leads to protein instability.
Mutant CYB5R3s generate unstable proteins
To determine the effect of the identified CYB5R3 mutations on the stability of the protein as predicted by computer modeling, and their relationship to the observed phenotypes, functional tests of the methemoglobin reductase activity were conducted with purified mutant and wild type proteins. We determined the individual effect of each mutation identified in the coding region.
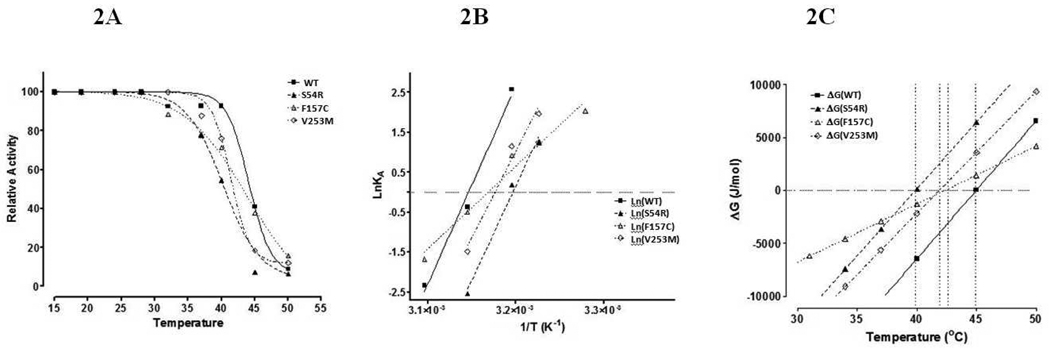
The PCR amplified CYB5R3 transcripts harboring either wild-type, S54R, V253M, and F157C substitution sequences were cloned and recombinant protein was purified for kinetic and thermodynamic studies. As shown in figure 3 the mutations at residues S54R and V253M were associated with greater thermal instability. While mutation F157C had a less stable structure according to a more positive enthalpic and entrhopic properties, it exhibited decreased sensitivity to thermal denaturation.
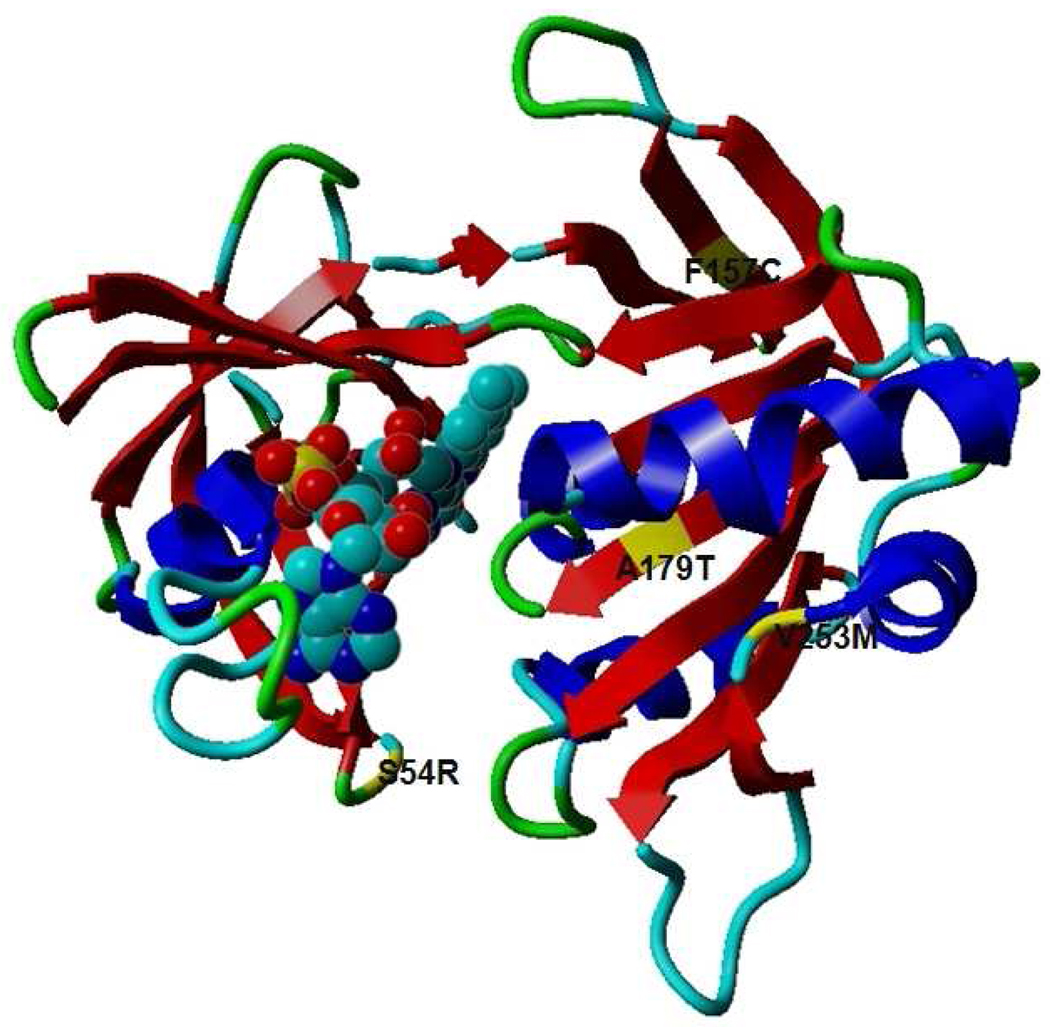
Figure 3.
Ribbon representation of three-dimensional structure of human CYB5R3. Alpha helices are shown in blue, anti-parallel β-sheets are shown in red the FAD cofactor (bubble structure) is shown. Residues in yellow indicate the locations of mutations found in this study. Figure rendered with Yasara software (YASARA Biosciences: www.yasara.org).
Changes in Gibbs stability free energy (ΔGstab), as a function of temperature, was calculated and plotted for the four recombinant proteins (Fig. 2C and Table 2). The values for ΔH and ΔS were calculated using the slope of the linear regression analysis of the data plotted in lnKA vs. 1/T (Fig. 2B, Table 2). Both thermodynamic constants ΔH and ΔS were not significantly affected by mutations S54R and V253M, while these same parameters were significantly different for the F157C mutation (Table 2). In contrast, both S54R and V253M mutations were thermally less stable than the wild-type and F157C mutations (Table 2). The lower Tm’s for S54R and V253M mutations indicate that these substitutions affect the thermal sensitivity of the protein by changing which energy well the most stable structure is placed in. In contrast, the F157C mutation is a thermally more stable mutation yet is characterized by a shallower energy well with a significant decrease in alternatively stable structural conformations. The overall free energy of stability (ΔGstab) was calculated at three different temperatures (Table 2). Further, an increase in ΔH indicates weaker internal interactions, while the change observed in ΔS points to a larger contribution of the conformational entropy versus the hydrophobic entropy for the F157C mutation.
Figure 2.
Thermostability and thermodynamics of wild-type and mutant CYB5R3. Temperature stability and thermodynamic characteristics were studied from the wild type (WT), S54A, F157C, and V253M mutants. A. Relative activity of NADH:FR after 20 minutes pre-incubation at temperatures indicated. B. Arrhenius plot used to calculate the value for ΔH and ΔS as described in methods. C. Plot of estimated and experimentally determined ΔG values as a function of temperature.
Table 2.
Thermodynamic coefficients.
| SAMPLE | SLOPE (lnKA vs. 1/T) |
ΔH (kJ/mol) | ΔS (J/mol * K−1) |
ΔGSTAB (37°C) (kJ/mol) |
Tm (°C) |
(a) R2 |
|---|---|---|---|---|---|---|
| WT | 49680 (± 5258) | −413 (± −43.7) | −1299 (± −137.5) | −10.4 (± −1.1) | 45.0 | 0.989 |
| S54R | 47415 (± 5335) | −394 (± −44.4) | −1260 (± −141.8) | −3.7 (± −0.4) | 39.9 | 0.987 |
| F157C | 20710 (± 1972) | −172 (± −16.4) | −546 (± −52.0) | −2.9 (± −0.3) | 42.4 | 0.974 |
| V253M | 43478 (± 6978) | −361 (± −58.0) | −1148 (± −184.2) | −5.6 (± −0.9) | 41.9 | 0.975 |
R2 is the fitting coefficient from the linear regression of the plot lnKA vs. 1/T.
Results are shown as MEAN (± STDEV).
Given the structural predictions based on the kinetic and thermodynamic aberrations caused by the CYB5R3 mutations we projected following model of the CYB5R3 activity. Mutant CYB5R3 leads to thermo-instability that results in impaired CYB5R3 methemoglobin reductase activity of erythrocytes (see Figure 2). The primary role of the CYB5R3 is the reduction of ferri-hemoglobin to ferro-hemoglobin by oxidation of NADH, to convert the ferri-hemoglobin into the reversible, oxygen-binding, ferro-hemoglobin. To determine if the observed phenotype is due to reduced methemoglobin reductase activity, activity rates were determined using purified recombinant proteins from wild type and mutant CYB5R3. The kinetic properties of wild type and mutant cytochrome B5 reductase were determined by measuring the oxidation of NADH at 340nm.
Using this approach any possible phenotype due to the silent mutation (P44P) found in cis to F157C, could not be evaluated, however, silent mutations were shown to alter protein expression as was first demonstrated in the MDR1 gene. In this case, the difference in a common synonymous MDR1 gene polymorphism resulted in altered cotranslational folding caused by a lower prevalence for the tRNAs of these two synonymous codons. Thus, two peptides were generated with identical primary structure but they maintained a different quaternary structure that affected MDR1 activity [15; 16]. Unfortunately, we were not able to experimentally determine whether the synonymous mutation (P44P) in CYB5R3 alters the CYB5R3 activity.
Structural studies supporting thermodynamic enzyme instability
Based on the sequence changes of the CYB5R3 gene identified in these patients, we predicted possible structural consequences resulting from these mutations. Computer structural modeling was employed to examine the tertiary structure of CYB5R3; the locations of the identified mutations are depicted in Figure 3.
These data further elaborate that type I recessive methemoglobinemia (RCM) is due to the instability of the methemoglobin reductase enzyme [8; 9; 12; 14; 17] regardless of the specific causative mutation to CYB5R3.
ACKNOWLEDGEMENTS
Supported by NIH grants: R01HL50077-14 (NHLBI) to JTP and NIH NIDDK DK020503 to JDP. Authors acknowledge Dr. Martin Steinberg help for evaluation of globin genes in the Asian Indian subject.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Borgese N, D'Arrigo A, De Silvestris M, Pietrini G. NADH-cytochrome b5 reductase and cytochrome b5 isoforms as models for the study of post-translational targeting to the endoplasmic reticulum. FEBS Lett. 1993;325:70–75. doi: 10.1016/0014-5793(93)81416-w. [DOI] [PubMed] [Google Scholar]
- 2.Borgese N, Pietrini G. Distribution of the integral membrane protein NADH-cytochrome b5 reductase in rat liver cells, studied with a quantitative radioimmunoblotting assay. Biochem J. 1986;239:393–403. doi: 10.1042/bj2390393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villalba JM, Navarro F, Gomez-Diaz C, Arroyo A, Bello RI, Navas P. Role of cytochrome b5 reductase on the antioxidant function of coenzyme Q in the plasma membrane. Mol Aspects Med. 1997;18 Suppl:S7–S13. doi: 10.1016/s0098-2997(97)00015-0. [DOI] [PubMed] [Google Scholar]
- 4.Gregg XT, Prchal JT. Red cell enzymopathies. Hematology: Basic Principles and Practice Chapter. 2009;45:611–623. [Google Scholar]
- 5.Ewenczyk C. Recessive hereditary methaemoglobinaemia, type II: delineation of the clinical spectrum. Brain. 2008:760–771. doi: 10.1093/brain/awm337. [DOI] [PubMed] [Google Scholar]
- 6.Prchal JT, Gregg XT. Red cell enzymes. Hematology Am Soc Hematol Educ Program. 2005:19–23. doi: 10.1182/asheducation-2005.1.19. [DOI] [PubMed] [Google Scholar]
- 7.Jenkins MM, Prchal JT. A novel mutation found in the 3' domain of NADH-cytochrome B5 reductase in an African-American family with type I congenital methemoglobinemia. Blood. 1996;87:2993–2999. [PubMed] [Google Scholar]
- 8.Nussenzveig RH, Lingam HB, Gaikwad A, Zhu Q, Jing N, Prchal JT. A novel mutation of the cytochrome-b5 reductase gene in an Indian patient: the molecular basis of type I methemoglobinemia. Haematologica. 2006;91:1542–1545. [PubMed] [Google Scholar]
- 9.Dekker J, Eppink MH, van Zwieten R, de Rijk T, Remacha AF, Law LK, Li AM, Cheung KL, van Berkel WJ, Roos D. Seven new mutations in the nicotinamide adenine dinucleotide reduced-cytochrome b(5) reductase gene leading to methemoglobinemia type I. Blood. 2001;97:1106–1114. doi: 10.1182/blood.v97.4.1106. [DOI] [PubMed] [Google Scholar]
- 10.Kugler W, Pekrun A, Laspe P, Erdlenbruch B, Lakomek M. Molecular basis of recessive congenital methemoglobinemia, types I and II: Exon skipping and three novel missense mutations in the NADH-cytochrome b5 reductase (diaphorase 1) gene. Hum Mutat. 2001;17:348. doi: 10.1002/humu.31. [DOI] [PubMed] [Google Scholar]
- 11.Maran J, Guan YL, Ou CN, Prchal JT. Heterogeneity of the molecular biology of methemoglobinemia: a study of eight consecutive patients. Haematologica-the Hematology Journal. 2005;90:687–689. [PubMed] [Google Scholar]
- 12.Percy MJ, Crowley LJ, Davis CA, McMullin MF, Savage G, Hughes J, McMahon C, Quinn RJ, Smith O, Barber MJ, Lappin TR. Recessive congenital methaemoglobinaemia: functional characterization of the novel D239G mutation in the NADH-binding lobe of cytochrome b5 reductase. Br J Haematol. 2005;129:847–853. doi: 10.1111/j.1365-2141.2005.05526.x. [DOI] [PubMed] [Google Scholar]
- 13.Grabowska D, Plochocka D, Jablonska-Skwiecinska E, Chelstowska A, Lewandowska I, Staniszewska K, Majewska Z, Witos I, Burzynska B. Compound heterozygosity of two missense mutations in the NADH-cytochrome b5 reductase gene of a Polish patient with type I recessive congenital methaemoglobinaemia. Eur J Haematol. 2003;70:404–409. doi: 10.1034/j.1600-0609.2003.00070.x. [DOI] [PubMed] [Google Scholar]
- 14.Percy MJ, Crowley LJ, Roper D, Vulliamy TJ, Layton DM, Barber MJ. Identification and characterization of the novel FAD-binding lobe G75S mutation in cytochrome b(5) reductase: an aid to determine recessive congenital methemoglobinemia status in an infant. Blood Cells Mol Dis. 2006;36:81–90. doi: 10.1016/j.bcmd.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Anthony V, Skach WR. Molecular mechanism of P-glycoprotein assembly into cellular membranes. Curr Protein Pept Sci. 2002;3:485–501. doi: 10.2174/1389203023380503. [DOI] [PubMed] [Google Scholar]
- 16.Kimchi-Sarfaty C, Oh JM, Kim IW, Sauna ZE, Calcagno AM, Ambudkar SV, Gottesman MM. A "silent" polymorphism in the MDR1 gene changes substrate specificity. Science. 2007;315:525–528. doi: 10.1126/science.1135308. [DOI] [PubMed] [Google Scholar]
- 17.Percy MJ, Crowley LJ, Boudreaux J, Barber MJ. Expression of a novel P275L variant of NADH:cytochrome b5 reductase gives functional insight into the conserved motif important for pyridine nucleotide binding. Arch Biochem Biophys. 2006;447:59–67. doi: 10.1016/j.abb.2005.12.015. [DOI] [PubMed] [Google Scholar]