Abstract
Despite being one of the leading causes of maternal death and a major contributor of maternal and perinatal morbidity, the mechanisms responsible for the pathogenesis of preeclampsia are unclear. One important initiating event in preeclampsia is thought to be reduced placental perfusion leading to the production of a variety of factors that cause widespread dysfunction of the maternal vasculature. The major objective of this review is to discuss the potential role of a novel agonistic autoantibody to the angiotensin II type I receptor (AT1-AA) in mediating hypertension during pregnancy. Although animal studies suggest that increasing plasma AT1-AA concentration in pregnant rats to levels observed in preeclamptic women or placental ischemic rats result in significant increases in arterial pressure, the quantitative importance of AT1-AA in the pathophysiology of preeclampsia in humans has yet to be fully elucidated.
Introduction
Preeclampsia is estimated to affect 5-7% of all pregnancies in the U.S. (1-4). Despite being one of the leading causes of maternal death and a major contributor of maternal and perinatal morbidity, the mechanisms responsible for the pathogenesis of preeclampsia are unclear. Hypertension associated with preeclampsia develops during the third trimester of pregnancy and remits after delivery, implicating the placenta as a central culprit in the disease. One important initiating event in preeclampsia is thought to be reduced placental perfusion leading to the production of a variety of factors that cause widespread dysfunction of the maternal vasculature (1-4). The major objective of this review is to discuss the potential role of a novel agonistic autoantibody to the angiotensin II type I receptor (AT1-AA) in mediating hypertension during pregnancy.
Angiotensin II type 1 receptor agonistic autoantibodies are present in preeclamptic women
The renin-angiotensin system (RAS) plays an important role in normal pregnancy and in preeclampsia. Normal pregnancy is associated with increased activation of RAS components but reduced vascular responsiveness to angiotensin II (5-10). In contrast, women with preeclampsia, have suppressed angiotensinogen, plasma renin acitivity and angiotensin II, but markedly enhanced vascular responsiveness to angiotensin II. A number of recent studies have also indicated that women with preeclampsia produce a novel agonistic autoantibody to the angiotensin II type I receptor (10-13). Dechend and colleagues previously reported that sera from preeclamptic women contain an IgG (type 3) autoantibody that reacts with the AT1 receptor. The binding of the AT1-AA to the seven amino acid stretch of the second extracellular loop of the angiotensin II type 1 receptor stimulates a chronotropic response from rat neonatal cardiomyocytes which can be attenuated with administration of an AT1 receptor antagonist, which is the basis of the bioassay primarily used for the detection of the autoantibody (11, 14).
A number of recent studies have demonstrated increased concentrations of AT1-AA in serum from women with preeclampsia compared to serum from women with normal pregnancies (11-18). AT1-AA was also detectable in women with a history of preeclampsia up to 18 months after delivery (14). While the importance of the AT-AA in these women is unclear, the AT1-AA could be involved in the increase risk for cardiovascular diseases in women with a history of preeclampsia.
While one study has reported that pregnant patients with abnormal uterine artery Doppler flows had increases in the AT1-AA, whether preeclampsia was present or not (19-22), the levels of AT1-AA observed in these patients may have not been enough to elicit a chronic blood pressure response. Interestingly, patients with malignant hypertension who experienced humoral kidney transplant rejection also have high circulating levels of the AT1-AA (23). Fu et al., found that 33% of patients with malignant secondary hypertension due to renovascular disease had increased AT1-AA, thus suggesting that the AT1-AA is not limited to only preeclampsia (24).
The AT1-AA has been purified and investigators have shown that AT1-AA signaling, via the AT1 receptor, results in a variety of physiological effects. AT1-AA induces signaling in vascular cells and trophoblasts including transcription factor activation. The signaling results in tumor necrosis factor alpha and reactive oxygen species generation, both of which have been implicated in preeclampsia (12-18). Although these novel findings implicate AT1-AA in the pathogenesis of hypertension during preeclampsia, the specific mechanisms that lead to excess production and the mechanisms where by AT1-AA increases blood pressure during pregnancy remain unclear.
Placental ischemia is an important stimulus for AT1-AA production
Utilizing the bioassay for AT1-AA described above, we recently determined that reductions in placental perfusion in pregnant rats had a profound effect on stimulating AT1-AA production (11,14,16-18). In contrast, the AT1-AA was not detected in normal pregnant rats (Figure 1). In addition, we have been able to suppress the production of AT1-AA in the reduced uterine perfusion pressure (RUPP) model of placental ischemia by administration of CD20 blockade which acts through B lymphocyte depletion and therefore suppressed secretion of antibody (25). AT1-AA suppression via B cell depletion results in a blunted blood pressure response to placental ischemia (25). Collectively, these novel findings suggest that a reduction in placental perfusion may be an important stimulus for AT1-AA production in preeclampsia (See Figure 2).
Figure 1.
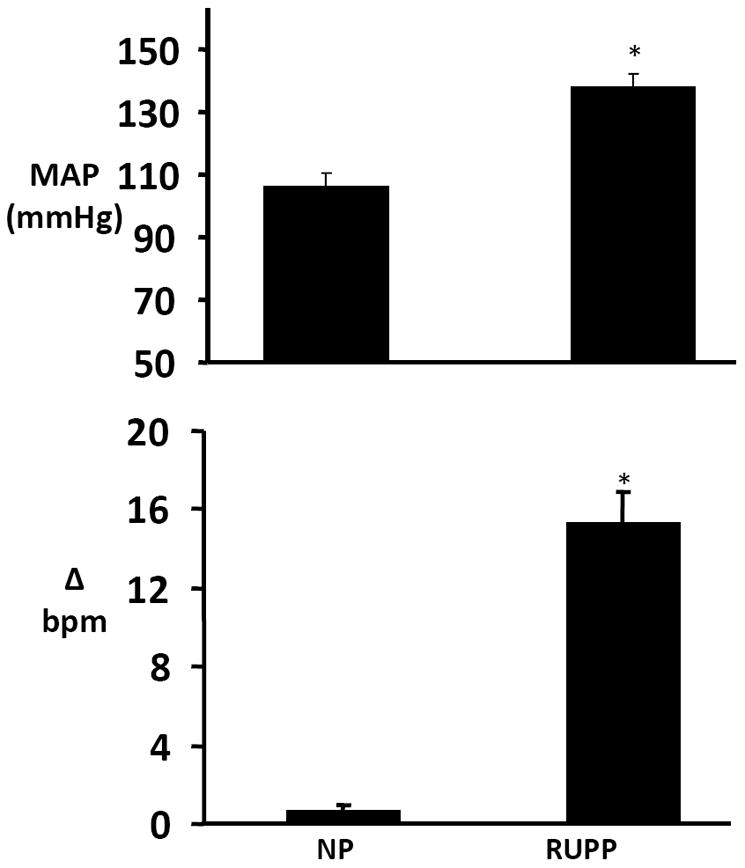
Top. Changes in mean arterial pressure (MAP) in response to placental ischemia in pregnant rats. Reduction in Uterine Perfusion (RUPP); Normal Pregnant (NP) rats. (* P<0.001 vs control groups). All data are expressed as mean ±SEM.
Bottom. Angiotensin type 1 (AT1) autoantibodies in RUPP and normal pregnant (NP) rats as detected by the chronotropic responses (bpm) to AT1 receptor–mediated stimulation of cultured neonatal rat cardiomyocytes (* P<0.05 vs NP rats; †P<0.05 vs RUPP). All data are expressed as mean ±SEM.
Figure 2.
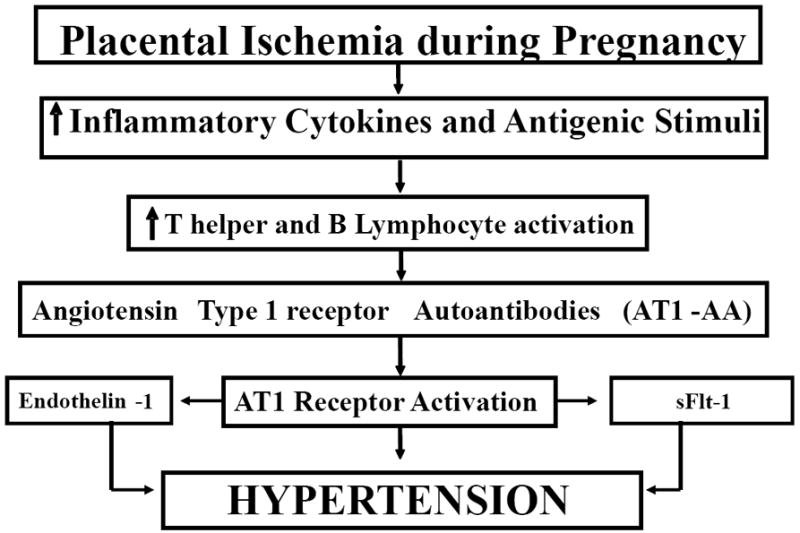
Hypertension in response to placental ischemia is associated with elevated proinflammatory cytokines, activated T and B lymphocytes and production of agonistic autoantibodies to the angiotensin II type I receptor (AT1-AA) AT1-AA in stimulate the vasoconstrictor peptide (endothelin 1) and antiangiogenic peptide, soluble fms-like tyrosine kinase-1 (sFlt-1), to cause hypertension during pregnancy.
In addition to placental ischemia as a stimulus for the AT1-AA we have shown that elevations of tumor necrosis factor-alpha (TNF-α) are also associated with increased production of the AT1-AA (26). Moreover, we have shown that chronic infusion of TNF-α in pregnant rats results in AT1-AA production. This would suggest the immune mechanisms stimulated in response to placental ischemia may play an important role in AT1-AA production (see Figure 2).
Antigenic stimuli for the production AT1-AA are still unknown
The specific stimulus for the production of the AT1-AA during preeclampsia remains unknown. While the AT1-AA is implicated in preeclampsia and animal models of preeclampsia, it has also been found in other pathophysiological disorders such as patients with abnormal uterine artery Doppler flow and malignant hypertensives that had humoral kidney transplant rejection (22-24). These data suggests that the AT1-AA is not limited to only preeclampsia but yet in none of these diseases has the antigenic stimulus for the AT1-AA been found.
Interestingly, a recent study by Herse and colleagues found that the protein alignment of the binding site for AT1-to be highly homologous to the capsid protein VP2 of parvovirus B19 (27). Parvovirus B19 is a single-stranded DNA virus known to cause erythema infectiosum, mild childhood exanthema, having a seroprevalence of 80% in the general population. Many viral infections including Parvovirus B19 infection are implicated in various autoimmune diseases (28). Molecular mimicry to parvovirus B19 proteins can contribute to the appearance of autoimmune antibodies, such as antiphospholipid and antineutrophil cytoplasmic antibodies (28). The IgG against parvovirus B19 VP2-protein showed a positive reaction in the AT1-AA bioassay, which was blocked by an AT1 receptor blocker, as well as by the seven amino acid epitope binding sequence (27). Herse et al performed a prospective, nested, case-control study of 30 gestational age–matched women with preeclampsia and 30 normotensive pregnant women in which they measured AT1-AA and serum IgG against parvovirus B19 proteins (27). AT1-AAs were present in 70% of preeclamptic patients but absent in 80% of control subjects. The authors detected significantly more AT1-AA in women with an immune response corresponding with parvovirus B19 infection. Therefore they concluded that the presence of B19-specific antibodies directed against VP2 epitopes could play an important role in the molecular mimicry for the generation of AT1-AAs.
Moreover, we are now learning of the characteristics shared between preeclamptic women with autoimmune patients. For example the autoimmune associated T helper 17 lymphocyte has recently found to be elevated in the plasma of preeclamptic women (29). Further evidence implicates an imbalance between regulatory (Treg) and effector T cells, subclasses of CD4+ T lymphocytes, in preeclamptic women (29-31). Th17 secrete Interleukin 17, and play an important role in the development and progression of chronic inflammatory and autoimmune diseases such as lupus, allograft rejection, autoimmune arthritis, psoriasis and multiple sclerosis (32,33). Interleukin 17 induces the synthesis of other cytokines such as IL-6, granulocyte colony stimulating factor, TNF alpha, IL-8 and macrophage colony stimulating factor, all of which are associated with preeclampsia and we have previously shown to be associated with hypertension in response to reductions in uterine placental perfusion in pregnant rats (34-36).
The importance of AT1-AA in causing hypertension in response to placental ischemia
While the agonistic autoantibody to the Ang II type 1 receptor is elevated in preeclamptic women and in pregnant rats with reduced uterine perfusion or chronic elevations in circulating levels of TNF alpha, the importance of Ang II type 1 receptor activation in causing hypertension in preeclampsia or in RUPP or TNF alpha-induced hypertension in pregnant rats has yet to be fully elucidated. However, we recently reported that treatment with a selective angiotensin type 1 receptor antagonist, Losartan, markedly attenuated the hypertension produced during placental ischemia as well as in response to chronic TNF excess in pregnant rats (16). Although these findings indicate that AT1 receptor activation plays an important role in mediating the increase in blood pressure in these animal models, our results do not quantify the relative importance of the AT1-AA in activating the AT1 receptor. It is important to note that a previous study from our laboratory indicated that chronic inhibition of endogenous angiotensin II formation, via oral administration of an converting enzyme inhibitor, decreased MAP to a similar extent in pregnant rats with reduced uterine perfusion pressure and normal pregnant rats. In addition, we also reported that plasma renin activity was not different between RUPP and normal pregnant rats. These data suggest that endogenous angiotensin II does not play a major role in mediating the hypertension produced by chronic reductions in uterine perfusion pressure in pregnant rats.
We also recently reported that infusion of purified rat AT1-AA, isolated from serum collected from a pregnant transgenic rat overproducing components of the renin angiotensin system, into pregnant rats from day 12 to day 19 of gestation, increased serum AT1-AA and blood pressure (17). Recent studies from Zhou et al also demonstrated that immunoglobulin isolated from preeclamptic women increases systolic pressure in pregnant mice (7,12,13). This phenotype was ameliorated with co-injection of an AT1 receptor antagonist or the 7 amino acid peptide that selectively blocks the actions of the AT1-AA. While these collective findings suggest that AT1-AA do have important hypertensive actions, the contribution of AT1-AA in the pathophysiology of hypertension in response to placental ischemia or preeclampsia remains to be an important area of investigation.
Mechanisms involved in AT1-AA induced hypertension during pregnancy
While the AT1-AA causes hypertension by direct activation of AT1 receptor, additional pathways involving endothelin and anti-angiogenic factors may also be involved during pregnancy (See Figure 1). We have recently reported that infusion of purified rat AT1-AA isolated from the transgenic rat model, (18) into normal pregnant rats increased serum AT1-AA, blood pressure, and tissue levels of preproendothelin (17). AT1-AA-induced hypertension in pregnant rats was attenuated by either oral administration of the AT1R antagonist losartan or an ET type A receptor antagonist. In addition, the increase in ET-1 transcript in response to AT1-AA induced hypertension was abolished by administration of an AT1R antagonist. These data suggest that AT1-AA induced hypertension during pregnancy is in part due to activation of the endothelin system.
We also recently reported that both circulating anti-angiogenic factors, sFlt-1 and sEndoglin (sEng), are significantly elevated in AT1-AA-induced hypertensive pregnant rats(26). We examined the media of placental explants from AT1-AA induced hypertensive pregnant rats and found significant increases in sFlt-1, again indicating the placenta as a potential source of excess sFlt-1. However, when we examined soluble sEng from placental explant media, there was no difference when compared to the media collected from placentas of NP control rats, indicating that there is another cellular source for the sEng production. These data support the hypothesis that chronic immune activation in association with placental ischemia leads to sFlt-1 and sEng over expression via stimulation of the AT1-receptor possibly by production of the AT1-AA. These studies demonstrate an important interaction between inflammatory and angiogenic markers found to be produced excessively in response to placental ischemia.
Summary
Recent studies have demonstrated increased concentrations of AT1-AA in serum from women with preeclampsia compared to serum from women with normal pregnancies. Results from animal studies indicate that placental ischemia may be an important stimulus for AT1-AA production during pregnancy. Generation of AT1-AA in response to reduced placental perfusion however may be secondary to inflammatory factors during preeclampsia. Several laboratories have demonstrated that chronic administration of AT1-AA in animal models elicits many of the phenotypic characteristic seen in preeclamptic women. In addition, suppression of AT1-AA production decreases blood pressure in response to placental ischemia in pregnant rats. These data suggest an important role for AT1-AA in the hypertension in response to placental ischemia.
Although animal studies suggest that increasing plasma AT1-AA concentration in pregnant rats to levels observed in preeclamptic women or placental ischemic rats result in significant increases in arterial pressure, the quantitative importance of AT1-AA in the pathophysiology of preeclampsia in humans has yet to be fully elucidated. Clinical studies utilizing specific antagonist of the AT1-AA or studies that block the formation of AT1-AA in preeclamptic women are the only approaches to truly determine the role of AT-AA in preeclampsia.
Acknowledgments
Sources of Funding This work was supported by AHA SDG0835472N; NIH grants HL78147 and HL51971.
Footnotes
There are no relationships to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review have been highlighted as:
-
◦
of special interest
-
◦◦
of outstanding interest
- 1.Roberts JM, Pearson G, Cutler J, Lindheimer M. Summary of the NHLBI Working Group on Research on Hypertension During Pregnancy. Hypertension. 2003 March;41(3):437–45. doi: 10.1161/01.HYP.0000054981.03589.E9. [DOI] [PubMed] [Google Scholar]
- 2.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005 February 26;365(9461):785–99. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 3.Sibai BM, Caritis S, Hauth J. What we have learned about preeclampsia. Semin Perinatol. 2003 June;27(3):239–46. doi: 10.1016/s0146-0005(03)00022-3. [DOI] [PubMed] [Google Scholar]
- 4.Roberts JM, Lain KY. Recent Insights into the pathogenesis of pre-eclampsia. Placenta. 2002 May;23(5):359–72. doi: 10.1053/plac.2002.0819. [DOI] [PubMed] [Google Scholar]
- 5.Alexander BT, Kassab SE, Miller MT, Abram SR, Reckelhoff JF, Bennett WA, Granger JP. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension. 2001;37:1191–1195. doi: 10.1161/01.hyp.37.4.1191. [DOI] [PubMed] [Google Scholar]
- ◦◦6.LaMarca BB, Gilbert J, Granger JP. Recent Progress Toward the Understanding of the Pathophysiology of Hypertenssion During Preeclampsia Hypertension. 2008 Apr;51(4):982–8. doi: 10.1161/HYPERTENSIONAHA.107.108837.. A recent review of the preeclampsia research literature
- ◦7.Zhou CC, Zhang Y, Irani RA, Zhang H, Mi T, Popek EJ, Hicks MJ, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat Med. 2008 August;14(8):855–62. doi: 10.1038/nm.1856.. Paper showing that overexpression of AT1-AA in mice causes preeclampsia like phenotype
- 8.Alexander BT, Rinewalt AN, Cockrell KL, Massey MB, Bennett WA, Granger JP. Endothelin type a receptor blockade attenuates the hypertension in response to chronic reductions in uterine perfusion pressure. Hypertension. 2001 February;37(2 Part 2):485–9. doi: 10.1161/01.hyp.37.2.485. [DOI] [PubMed] [Google Scholar]
- 9.Shah DM. Role of the renin-angiotensin system in the pathogenesis of preeclampsia. Amj Physiol Renal Physiol. 2005 April;288(4):F614–F625. doi: 10.1152/ajprenal.00410.2003. [DOI] [PubMed] [Google Scholar]
- 10.Xia Y, Ramin SM, Kellems RE. Potential roles of angiotensin receptor-activating autoantibody in the pathophysiology of preeclampsia. Hypertension. 2007 August;50(2):269–75. doi: 10.1161/HYPERTENSIONAHA.107.091322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ◦◦11.Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jupner A, et al. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. Journal of Clinical Investigation. 1999;103:945–952. doi: 10.1172/JCI4106.. Original finding that patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor
- 12.Xia Y, Wen H, Bobst S, Day M, Kellems R. Maternal autoantibodies from preeclamptic patients activate angiotensin receptors on human trophoblast cells. Reproductive Sciences. 2003;10:82–93. doi: 10.1016/s1071-5576(02)00259-9. [DOI] [PubMed] [Google Scholar]
- 13.Xia Y, Ramin S, Kellems R. Potential roles of angiotensin receptor-activating autoantibody in the pathophysiology of preeclampsia. Hypertension. 2007;50:269–275. doi: 10.1161/HYPERTENSIONAHA.107.091322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hubel C, Wallukat G, Wolf M, Herse F, Rajakumar A, Roberts J, et al. Agonistic Angiotenin II Type I Receptor Autoantibodies in Postpartum Women with a History of Preeclampsia. Hypertension. 2007;49:612–617. doi: 10.1161/01.HYP.0000256565.20983.d4. [DOI] [PubMed] [Google Scholar]
- 15.LaMarca B, Bennett W, Alexander B, Cockrell K, Granger J. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of tumor necrosis factor-alpha. Hypertension. 2005;46:1022–1025. doi: 10.1161/01.HYP.0000175476.26719.36. [DOI] [PubMed] [Google Scholar]
- ◦◦16.Lamarca B, Wallukat G, Llinas M, Herse F, Dechend R, Granger J. Autoantibodies to the angiotensini type I receptor in response to placental ischemia and tumor necrosis factor alpha in pregnant rats. Hypertension. 2008;52:1168–1172. doi: 10.1161/HYPERTENSIONAHA.108.120576.. Study showing that placental ischemia and tumor necrosis factor alpha are important stimuli of autoantibodies to the angiotensini type I receptor in pregnant rats
- 17.LaMarca B, Parrish M, Ray L, Murphy S, Roberts L, Glover P, et al. Hypertension in response to autoantibodies to the angiotensin II type I receptor (AT1-AA) in pregnant rats: a role of endothelin-1. Hypertension. 2009;54:905–909. doi: 10.1161/HYPERTENSIONAHA.109.137935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dechend R, Viedt C, Muller DN, Ugele B, Brandes RP, Wallukat G, Park JK, Janke J, Barta P, Theuer J, Fiebeler A, Homuth V, Dietz R, Haller H, Kreuzer J, Luft FC. AT1 receptor agonistic antibodies from preeclamptic patients stimulate NADPH oxidase. Circulation. 2003;107:1632–1639. doi: 10.1161/01.CIR.0000058200.90059.B1. [DOI] [PubMed] [Google Scholar]
- 19.Dechend R, Homuth V, Wallukat G, Müller DN, Krause M, Dudenhausen J, Haller H, Luft FC. Agonistic antibodies directed at the angiotensin II, AT1 receptor in preeclampsia. J Soc Gynecol Investig. 2006;13:79–86. 8. doi: 10.1016/j.jsgi.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Thway TM, Shlykov SG, Day MC, Sanborn BM, Gilstrap LC, III, Xia Y, Kellems RE. Antibodies from preeclamptic patients stimulate increased intracellular Ca2_ mobilization through angiotensin receptor activation. Circulation. 2004;110:1612–1619. doi: 10.1161/01.CIR.0000142855.68398.3A. [DOI] [PubMed] [Google Scholar]
- 21.Dechend R, Gratze P, Wallukat G, Shagdarsuren E, Plehm R, Brasen J, et al. Agonistic autoantibodies to the AT1 receptor in a transgeneic rat model of preeclampsia. Hypertension. 2005;45:742–746. doi: 10.1161/01.HYP.0000154785.50570.63. [DOI] [PubMed] [Google Scholar]
- 22.Walther T, Wallukat G, Jank A, Bartel S, Schultheiss H, Faber R, et al. Angiotensin II Type 1 Receptor Agonistic antibodies reflect fundamental alterations in the uteroplacental vasculature. Hypertension. 2005;46:1275–1279. doi: 10.1161/01.HYP.0000190040.66563.04. [DOI] [PubMed] [Google Scholar]
- 23.Dragun D, Muller D, Brasen J, Fritsche L, Nieminen-Kelha M, Dechend R, et al. Angiontensin II type-1 receptor activating anitbodies in renal-allograft rejection. New England Journal of Medicine. 2005;352:558–569. doi: 10.1056/NEJMoa035717. [DOI] [PubMed] [Google Scholar]
- 24.Fu M, Herlitz H, Schulze W, Wallukat G, Micke P, Eftekhari P, et al. Autoantibodies against the angiotensin receptor (AT1) in patients with hypertension. Journal of Hypertension. 2000;18:945–953. doi: 10.1097/00004872-200018070-00017. [DOI] [PubMed] [Google Scholar]
- 25.LaMarca B, Wallace K, Herse F, Wallukat G, Weimer A, Dechend R. Hypertension in response to placental ischemia during pregnancy: Role of agonistic autoantibodies to the angiotensin II type I receptor (AT1-AA) Hypertension. 2010 Dec;56(5):e56. [Google Scholar]
- 26.Parrish MR, Murphy SR, Rutland S, Wallace K, Wenzel K, Wallukat G, Keiser S, Ray LF, Dechend R, Martin JN, Granger JP, LaMarca B. The Effect of Immune Factors, Tumor Necrosis Factor-alpha, and Agonistic Autoantibodies to the Angiotensin II Type I Receptor on Soluble fms-Like Tyrosine-1 and Soluble Endoglin Production in Response to Hypertension During Pregnancy. Am J Hypertens. 2010 Aug;23(8):911–6. doi: 10.1038/ajh.2010.70. Epub 2010 Apr 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herse F, Verlohren S, Wenzel K, Pape J, Muller DN, Modrow S, Wallukat G, Luft FC, Redman CWG, Dechend R. Prevalence of Agonistic Autoantibodies Against the Angiotensin II Type 1 Receptor and Soluble fms-Like Tyrosine Kinase 1 in a Gestational Age–Matched Case Study. Hypertension. 2009;53:393–398. doi: 10.1161/HYPERTENSIONAHA.108.124115. [DOI] [PubMed] [Google Scholar]
- 28.Broliden K, Tolfvenstam T, Norbeck O. Clinical aspects of parvovirus B19 infection. J of Int Med. 2006;260:285–304. doi: 10.1111/j.1365-2796.2006.01697.x. [DOI] [PubMed] [Google Scholar]
- 29.Santner-Nanan B, Peek M, Khanam R, Richarts L, Zhu E, Fazekas de St G, et al. Systemic increase in the ration between FoxP3+ and IL-17 producing CD4+ T cells in healthy pregnancy but not in preeclampsia. Journal of Immunology. 2009;183:7023–7030. doi: 10.4049/jimmunol.0901154. [DOI] [PubMed] [Google Scholar]
- 30.Sargent I, Borzychowski A, Redman C. Immunoregulation in normal pregnancy and preeclampsia: an overview. Reproductive Biomed Online. 2006;13:680–686. doi: 10.1016/s1472-6483(10)60659-1. [DOI] [PubMed] [Google Scholar]
- 31.Prins J, Boelens H, Heimweg J, Van der Heide S, Dubois A, Van Oosterhout A, et al. Preeclampsia is associated with lower percentages of regulatory T cells in maternal blood. Hypertension in Pregnancy. 2009;28:300–311. doi: 10.1080/10641950802601237. [DOI] [PubMed] [Google Scholar]
- 32.Abbus A. Cellular and Molecular Immunology. 5. Philadelphia: Elsevier; 2005. [Google Scholar]
- 33.Cruse J, Lewis R. Illustrated Dictionary of Immunology. 3. CRC Press; 2009. [Google Scholar]
- 34.Alexander B, Kassab S, Miller M, Abram S, Reckelhoff J, Bennett W, et al. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension. 2001;37:1191–1195. doi: 10.1161/01.hyp.37.4.1191. [DOI] [PubMed] [Google Scholar]
- 35.Granger J, LaMarca B, Cockrell K, Sedeek M, Balzi C, Chandler D, et al. Reduced uterine perfusion pressure (RUPP) model for studying cardiovascular-renal dysfunction in response to placental ischemia. Methods in Molecular Medicine. 2006;122:383–392. doi: 10.1385/1-59259-989-3:381. [DOI] [PubMed] [Google Scholar]
- 36.LaMarca B. The role of immune activation in contributing to vascular dysfunction and the pathophysiology of hypertension during preeclampsia. Minerva Ginecol. 2010;62:105–120. [PMC free article] [PubMed] [Google Scholar]


