Abstract
Since cord blood (CB) has become a commonly used source of transplantable hematopoietic stem (HSC) and hematopoietic progenitor cells (HPC), there has been a need to overcome the limited HSC and HPC numbers available to transplant from a single CB, especially for adult recipients. Our laboratory previously demonstrated that Rheb2 overexpression significantly impaired the repopulating ability of HSC. Since overexpression of Rheb2 leads to increased signaling through mTOR, we examined the effect of the mTOR inhibitor rapamycin ex vivo on cytokine expanded CD34+ CB cells for the engraftment of these cells in non-obese diabetic, severe combined immunodeficient, IL-2 receptor γ chain null (NSG) mice. We observed significant enhancement in engraftment of the CB treated ex vivo with cytokines in the presence of rapamycin prior to transplant, effects seen in primary as well as secondary transplants. These pre-clinical results suggest a positive role for rapamycin during ex vivo culture of CB SCID repopulating cells/HSC.
Keywords: cord blood, ex vivo expansion, mTOR, rapamycin, CD34+ cell engraftment
Introduction
Understanding how HSCs balance self-renewal, quiescence and proliferation is essential for improving transplantation, ex vivo expansion, and other clinical uses of these cells. Mammalian target of rapamycin (mTOR), a serine/threonine kinase [1–3], is important in hematopoiesis, affecting both benign and malignant hematopoietic cell growth, differentiation and survival [4–10]. Interestingly, hyperactivation of the mTOR pathway leads to exhaustion of HSC/HPC repopulating cells in many mouse models including the conditional phosphatase and tension homolog located on chromosome 10 (PTEN) knockout mouse, the tuberlous sclerosis complex (TSC) knockout mouse, the constitutively activated Akt mouse and the Foxp3 knockout mouse [11–14]. Our laboratory examined the effects of forced overexpression of the known mTOR activator Rheb2 that has been shown to be preferentially expressed in HSC populations [15]. In our previous study using a transplant model with gene transduced mouse bone marrow cells, we observed positive effects on hematopoietic progenitor cell (HPC) colony formation, growth, and survival; however, overexpression of Rheb2 significantly impaired the repopulating ability of HSCs [15].
Since cord blood (CB) was first used for hematopoietic stem cell (HSC) transplantation in 1988, its use as a source of transplantable HSC and hematopoietic progenitor cells (HPCs) has steadily increased [16; 17]. There are several advantages to using CB, as opposed to bone marrow (BM) or peripheral blood (PB), as a source of transplantable HSC: reduced risk of graft-versus-host disease, lower histocompatability requirement, and widespread availability due to CB banking, [18; 19]. However, an area of concern for CB is delayed recovery of neutrophils and platelets compared to BM and mobilized peripheral blood cells [20]. Also, limited numbers of cells from a single CB, as well as the amount of CB collected, have resulted in the need to increase the transplant efficiency of CB HSC populations. Amongst a number of potentially promising attempts to enhance the engrafting capability of limiting numbers of CB cells [19; 21; 22], ex vivo stem cell expansion procedures have been evaluated in an effort to generate increased numbers of HSC and HPC; however, these attempts have had limited success [19; 23]. Transplantation of expanded CD34+ CB cells into nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice suggest that even though expanded CB cells may maintain long-term repopulating capability, there is a loss or delay in engraftment as compared to unexpanded CD34+ CB cells [24; 25]. However, the combination of various growth factors (e.g. stem cell factor (SCF), thrombopoietin (TPO) and Flt3-ligand (FL)) has been shown to efficiently expand numbers of HPCs and SCID repopulating cells (SRCs) in an ex vivo setting [26; 27], indicating that expansion may in the future be able to provide a more potent unit of transplantable HSC. Since increased mTOR signaling leads to impaired HSC repopulating activity as previously described, we hypothesized that by blocking mTOR using rapamycin in ex vivo growth factor expanded human cord blood CD34+ cultures, we could enhance the efficiency of their repopulating activity. In the present study, we evaluated the effect of rapamycin on the ex vivo culture of SRCs in CD34+ CB cells in the presence of SCF, TPO, and FL. We found that CB cells treated with rapamycin displayed an increase in repopulating ability compared to control treated cells transplanted into NSG mice.
Materials and Methods
Mice
For the repopulation assay, NSG (NOD.Cg-Prkdscid IL2rgtm1Wjl/Sz) mice of approximately 6–8 weeks old were obtained from an on-site core breeding colony and used. The Institutional Animal Care and Use Committee of the Indiana University School of Medicine approved all experimental procedures.
Preparation of CD34+ human cord blood cells
Normal human CB was collected with institutional approval and mononuclear cells (MNCs) were isolated by density gradient centrifugation over Ficoll-Paque Plus (GE Healthcare Life Sciences, Piscataway, NJ). CD34+ cells were isolated from MNCs using direct CD34+ magnetic bead separation over two sequential columns (Miltenyi Biotec, Auburn, CA). CD34+ cells were then cultured for 72 hours at 37°C in RPMI-1640 + 10% FBS + 100 ng/ml each of SCF, TPO, and FL with 50 nM Rapamycin (Cell Signaling Technology, Inc, Danvers, MA) or a methanol-diluent control.
Short- and long-term engraftment
Transplantation of human cells into NOD/SCID mice is a well-established protocol [28]. We utilized a newer variant of this model, NSG mice that had an IL-2 receptor gamma chain null genotype, and that is better at accepting engraftment of human CD34+ cells than NOD/SCID mice [29]. To perform these experiments, recipient mice were sublethally irradiated (350 cGy; 137Cs source, single dose) and were transplanted by tail vein injection with various concentrations of CD34+ 24 hours after irradiation. Peripheral blood (PB) was collected by tail-vein bleeding into heparinized microcapillary tubes (Fisher Scientific, Pittsburg, PA). Red blood cells (RBCs) in the PB were lysed by incubating with RBC lysis buffer (0.155 M NH4Cl, 0.01 M KHCO3, 0.1 mM EDTA in H2O and filter sterilized) and samples were washed in PBS + 2% BSA. Flow cytometric analysis of PB was performed with a human-specific APC (allophycocyanin) anti-CD45 monoclonal antibody (BD Bioscience) at various time points after transplantation and analyzed using a FACSCalibur with APC [15]. Peripheral blood from an untransplanted NSG mouse was used as a negative control. Data analysis was done using FCS Express V3 software (De Novo Software, Ontario, Canada). Data are presented as the mean ± SEM of the percent of cells in the PB of recipient mice that are expressing human CD45 (CD45+).
Statistical analysis
Data were analyzed statistically using the Student’s t test. A P value less than 0.05 was considered statistically significant.
Results and Discussion
CD34+ cells were isolated from human CB and evaluated for CD34 content by flow cytometric analysis. The percentage of CD34+ cells collected from the CD34 enrichment protocol averaged 97.8 ± 2.2% (mean ±1SEM). We also counted cell numbers before and after culturing in stem cell expansion media in the presence or absence of rapamycin. Even though the starting number of CD34+ cells was the same for both rapamycin and control treatment groups, we observed that numbers of control treated CD34+ cells expanded during the three day culture period (2.8 ± 0.3 fold increase). However, rapamycin treated CD34+ cells did not expand to the same extent as the control treated cells (1.4 ± 0.2 fold increase).
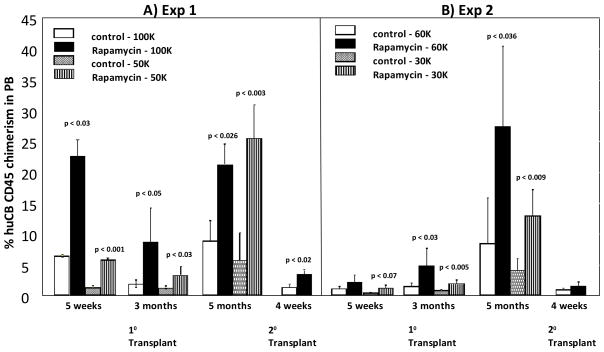
Short-term and long-term engraftment were measured by flow cytometric analysis of the human CD45+ cell content in PB of NS2 mice at various time points after transplantation of rapamycin or control treated CD34+ CB cells. For the first experiment, we examined the engraftment of 100,000 or 50,000 CD34+ CB cells treated with rapamycin or vehicle control. For the second experiment, we examined the engraftment of 60,000 or 30,000 CD34+ CB cells treated with rapamycin or vehicle control. The first and second experiments were performed with CD34+ cells from two different CB units. For the first experiment, we observed significantly increased engraftment in both the 100,000 and 50,000 rapamycin treatment group compared to the control treated group at all time points examined (Figure 1A). This increase in engraftment was also observed in the secondary transplant in which bone marrow cells from the primary mice were injected intravenously into secondary mice. For the second experiment, we observed significantly increased engraftment in both the 60,000 and 30,000 rapamycin treatment group compared to the control treated group at all time points examined, except at the 5 week time point for the 60,000 rapamycin group (Figure 1B). The increase in secondary irradiated NSG mice transplanted with bone marrow from primary transplant recipients in the second experiment did not reach statistical significance for the secondary transplant. Interestingly, the percentage of CD34+ cells within the expanded cultures did not dramatically differ between control and rapamycin treated cells (89% vs. 83% in control and rapamycin treated cells, respectively in experiment one; 64% vs. 69% in control and rapamycin treated cells, respectively in experiment two), suggesting that our observations cannot be explained simply by differences in transplanted numbers of CD34+ cells between these groups.
Figure 1. Effect of Rapamycin treatment ex-vivo on human CB CD34+ engraftment of sublethaly-irradiated NSG mice.
NSG mice were transplanted with either (A) 100,000 or 50,000 or (B) 60,000 or 30,000 rapamycin or control treated CD34+ CB cells 24 hours after sublethal irradiation. After 5 months, bone marrow from primary recipients of CB were transplanted into secondary recipients. Peripheral blood was collected at different time points after transplantation and assessed for percentage of human CD45+ cells present. Results are shown for 3–5 primary recipients each and 5 recipients for secondary transplants using pooled BM cells from the primary recipients. Data, for each of the two different experiments with different CB collections, were analyzed statistically using the Student’s t test. A P value less than 0.05 was considered statistically significant. Significance compared to control treated cells for that number of transplanted cells.
Limiting numbers of HSC and HPC still present a problem for the use of CB in the clinical transplant setting. Current practices suggest the need for a minimum of 2 × 107 nucleated cells/kg recipient body weight (which contains approximately 2×105 CD34+ cells/kg) for successful transplantation, though fewer cells have been used for successful transplants [30]. Therefore, research efforts have focused on ways to overcome the limited number of cells in a single CB unit. Different efforts in this context have been repeated [21; 22]. Herein, we tested the ability of ex vivo mTOR inhibition (rapamycin for 72 hours in culture) to alter the engraftment of CB CD34+ cells in the NSG mouse model. Our data show that by treating CD34+ CB cells with rapamycin prior to transplantation, we are able to increase the efficiency of engraftment of ex vivo-expanded cells. Importantly, our study followed engraftment into secondary transplant animals after long-term engraftment in primary mice, suggesting that these differences impacted long-term repopulating HSC. These findings implicate mTOR signaling as a negative regulator of the repopulating ability of ex vivo expanded HSCs from human CB. Our data could also be interpreted to suggest that ex vivo expansion of human CB HSCs negatively affects their repopulating ability, and inhibiting mTOR under these conditions prevents this loss of function. In addition to enhancing the engrafting capabilities of limiting numbers of CB cells, these findings could also have important clinical uses in fields such as gene transduction that rely on preserving HSC functions while they are manipulated and treated ex vivo.
Footnotes
These studies were supported by Public Health Service grants R01 HL67384, R01 HL56416, and a project in P01 HL53586 to HEB.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tee AR, Blenis J, Proud CG. Analysis of mTOR signaling by the small G-proteins, Rheb and RhebL1. FEBS Lett. 2005;579:4763–4768. doi: 10.1016/j.febslet.2005.07.054. [DOI] [PubMed] [Google Scholar]
- 2.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 3.Fingar DC, Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene. 2004;23:3151–3171. doi: 10.1038/sj.onc.1207542. [DOI] [PubMed] [Google Scholar]
- 4.Cruz R, Hedden L, Boyer D, Kharas MG, Fruman DA, Lee-Fruman KK. S6 kinase 2 potentiates interleukin-3-driven cell proliferation. J Leukoc Biol. 2005;78:1378–1385. doi: 10.1189/jlb.0405225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raslova H, Baccini V, Loussaief L, Comba B, Larghero J, Debili N, Vainchenker W. Mammalian target of rapamycin (mTOR) regulates both proliferation of megakaryocyte progenitors and late stages of megakaryocyte differentiation. Blood. 2006;107:2303–2310. doi: 10.1182/blood-2005-07-3005. [DOI] [PubMed] [Google Scholar]
- 6.Bertrand FE, Spengemen JD, Shelton JG, McCubrey JA. Inhibition of PI3K, mTOR and MEK signaling pathways promotes rapid apoptosis in B-lineage ALL in the presence of stromal cell support. Leukemia. 2005;19:98–102. doi: 10.1038/sj.leu.2403560. [DOI] [PubMed] [Google Scholar]
- 7.Xu Q, Thompson JE, Carroll M. mTOR regulates cell survival after etoposide treatment in primary AML cells. Blood. 2005;106:4261–4268. doi: 10.1182/blood-2004-11-4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu RH, Pelicano H, Zhang H, Giles FJ, Keating MJ, Huang P. Synergistic effect of targeting mTOR by rapamycin and depleting ATP by inhibition of glycolysis in lymphoma and leukemia cells. Leukemia. 2005;19:2153–2158. doi: 10.1038/sj.leu.2403968. [DOI] [PubMed] [Google Scholar]
- 9.Follo MY, Mongiorgi S, Bosi C, Cappellini A, Finelli C, Chiarini F, Papa V, Libra M, Martinelli G, Cocco L, Martelli AM. The Akt/mammalian target of rapamycin signal transduction pathway is activated in high-risk myelodysplastic syndromes and influences cell survival and proliferation. Cancer Res. 2007;67:4287–4294. doi: 10.1158/0008-5472.CAN-06-4409. [DOI] [PubMed] [Google Scholar]
- 10.Drayer AL, Olthof SG, Vellenga E. Mammalian target of rapamycin is required for thrombopoietin-induced proliferation of megakaryocyte progenitors. Stem Cells. 2006;24:105–114. doi: 10.1634/stemcells.2005-0062. [DOI] [PubMed] [Google Scholar]
- 11.Yilmaz OH, Valdez R, Theisen BK, Guo W, Ferguson DO, Wu H, Morrison SJ. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- 12.Kharas MG, Okabe R, Ganis JJ, Gozo M, Khandan T, Paktinat M, Gilliland DG, Gritsman K. Constitutively active AKT depletes hematopoietic stem cells and induces leukemia in mice. Blood. 2010;115:1406–1415. doi: 10.1182/blood-2009-06-229443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen C, Liu Y, Zheng P. Mammalian target of rapamycin activation underlies HSC defects in autoimmune disease and inflammation in mice. J Clin Invest. 2010;120:4091–4101. doi: 10.1172/JCI43873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen C, Liu Y, Liu R, Ikenoue T, Guan KL, Zheng P. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med. 2008;205:2397–2408. doi: 10.1084/jem.20081297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell TB, Basu S, Hangoc G, Tao W, Broxmeyer HE. Overexpression of Rheb2 enhances mouse hematopoietic progenitor cell growth while impairing stem cell repopulation. Blood. 2009;114:3392–3401. doi: 10.1182/blood-2008-12-195214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broxmeyer HE, Douglas GW, Hangoc G, Cooper S, Bard J, English D, Arny M, Thomas L, Boyse EA. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci U S A. 1989;86:3828–3832. doi: 10.1073/pnas.86.10.3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gluckman E, Broxmeyer HA, Auerbach AD, Friedman HS, Douglas GW, Devergie A, Esperou H, Thierry D, Socie G, Lehn P, et al. Hematopoietic reconstitution in a patient with Fanconi’s anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989;321:1174–1178. doi: 10.1056/NEJM198910263211707. [DOI] [PubMed] [Google Scholar]
- 18.Wagner JE, Kernan NA, Steinbuch M, Broxmeyer HE, Gluckman E. Allogeneic sibling umbilical-cord-blood transplantation in children with malignant and non-malignant disease. Lancet. 1995;346:214–219. doi: 10.1016/s0140-6736(95)91268-1. [DOI] [PubMed] [Google Scholar]
- 19.Broxmeyer HE, Smith FO. Cord Blood Hematopoietic Cell Transplantation. In: Appelbaum F, Forman S, Negrin R, Blume K, editors. Thomas’ Hematopoieitic Cell Transplantation. Wiley-Blackwell; West Sussex, United Kingdom: 2009. pp. 559–576. [Google Scholar]
- 20.Broxmeyer HE, Kurtzberg J, Gluckman E, Auerbach AD, Douglas G, Cooper S, Falkenburg JH, Bard J, Boyse EA. Umbilical cord blood hematopoietic stem and repopulating cells in human clinical transplantation. Blood Cells. 1991;17:313–329. [PubMed] [Google Scholar]
- 21.Rocha V, Broxymeyer H. New approaches for improving engraftment after Cord Blood Transplantation. Biol Blood Marrow Transplant. 2009 doi: 10.1016/j.bbmt.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Shpall EJ, Bollard CM, Brunstein C. Novel cord blood transplant therapies. Biol Blood Marrow Transplant. 2011;17:S39–45. doi: 10.1016/j.bbmt.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelly SS, Sola CB, de Lima M, Shpall E. Ex vivo expansion of cord blood. Bone Marrow Transplant. 2009;44:673–681. doi: 10.1038/bmt.2009.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu R, Reems JA. Umbilical cord blood progeny cells that retain a CD34+ phenotype after ex vivo expansion have less engraftment potential than unexpanded CD34+ cells. Transfusion. 2001;41:213–218. doi: 10.1046/j.1537-2995.2001.41020213.x. [DOI] [PubMed] [Google Scholar]
- 25.Guenechea G, Segovia JC, Albella B, Lamana M, Ramirez M, Regidor C, Fernandez MN, Bueren JA. Delayed engraftment of nonobese diabetic/severe combined immunodeficient mice transplanted with ex vivo-expanded human CD34(+) cord blood cells. Blood. 1999;93:1097–1105. [PubMed] [Google Scholar]
- 26.Piacibello W, Sanavio F, Garetto L, Severino A, Bergandi D, Ferrario J, Fagioli F, Berger M, Aglietta M. Extensive amplification and self-renewal of human primitive hematopoietic stem cells from cord blood. Blood. 1997;89:2644–2653. [PubMed] [Google Scholar]
- 27.Piacibello W, Sanavio F, Severino A, Dane A, Gammaitoni L, Fagioli F, Perissinotto E, Cavalloni G, Kollet O, Lapidot T, Aglietta M. Engraftment in nonobese diabetic severe combined immunodeficient mice of human CD34(+) cord blood cells after ex vivo expansion: evidence for the amplification and self-renewal of repopulating stem cells. Blood. 1999;93:3736–3749. [PubMed] [Google Scholar]
- 28.Bock TA, Orlic D, Dunbar CE, Broxmeyer HE, Bodine DM. Improved engraftment of human hematopoietic cells in severe combined immunodeficient (SCID) mice carrying human cytokine transgenes. J Exp Med. 1995;182:2037–2043. doi: 10.1084/jem.182.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y, Hangoc G, Campbell TB, Goodman M, Tao W, Pollok K, Srour EF, Broxmeyer HE. Identification of parameters required for efficient lentiviral vector transduction and engraftment of human cord blood CD34+ NOD/SCID Repopulating cells. Exp Hematol. 2008;36:947–956. doi: 10.1016/j.exphem.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gluckman E, Rocha V, Arcese W, Michel G, Sanz G, Chan KW, Takahashi TA, Ortega J, Filipovich A, Locatelli F, Asano S, Fagioli F, Vowels M, Sirvent A, Laporte JP, Tiedemann K, Amadori S, Abecassis M, Bordigoni P, Diez B, Shaw PJ, Vora A, Caniglia M, Garnier F, Ionescu I, Garcia J, Koegler G, Rebulla P, Chevret S. Factors associated with outcomes of unrelated cord blood transplant: guidelines for donor choice. Exp Hematol. 2004;32:397–407. doi: 10.1016/j.exphem.2004.01.002. [DOI] [PubMed] [Google Scholar]