Abstract
Human arsenic methylation efficiency has been consistently associated with arsenic-induced disease risk. Interindividual variation in arsenic methylation profiles is commonly observed in exposed populations, and great effort has been put into the study of potential determinants of this variability. Among the factors that have been evaluated, body mass index (BMI) has not been consistently associated with arsenic methylation efficiency, however an underrepresentation of the upper BMI distribution was commonly observed in these studies. This study investigated potential factors contributing to variations in the metabolism of arsenic, with specific interest in the effect of BMI where more than half of the population was overweight or obese. We studied 624 adult women exposed to arsenic in drinking water from three independent populations. Multivariate regression models showed that higher BMI, arsenic (+3 oxidation state) methyltransferase (AS3MT) genetic variant 7388, and higher total urinary arsenic, were significantly associated with low percentage of urinary arsenic excreted as monomethylarsonic acid (%uMMA) or high ratio between urinary dimethylarsinic acid and uMMA (uDMA/uMMA); while AS3MT genetic variant M287T was associated with high %uMMA and low uDMA/uMMA. The association between BMI and arsenic methylation efficiency was also evident in each of the three populations when studied separately. This strong association observed between high BMI and low %uMMA and high uDMA/uMMA underscores the importance of BMI as a potential arsenic-associated disease risk factor, and should be carefully considered in future studies associating human arsenic metabolism and toxicity.
Keywords: arsenic, arsenic metabolism, BMI, body mass index, AS3MT, genetic association
INTRODUCTION
Environmental exposure to arsenic is a significant public health problem affecting millions of people worldwide. Chronic exposure to arsenic via drinking water has been associated with the development of several cancerous and non-cancerous diseases including skin cancer, diabetes, neuropathy, and cardiovascular disease (Yu et al., 2000; Tseng et al., 2002; Hafeman et al., 2005; Balakumar and Kaur, 2009). The diversity of negative health effects induced by arsenic exposure adds complexity to understanding its toxicity, and the underlying mechanism by which arsenic induces these effects is still unknown.
Arsenic in the environment is commonly found in its inorganic form, namely arsenate (As(V)) or arsenite (As(III)). In humans, ingested inorganic arsenic (InAs) follows a series of reduction and oxidative methylation reactions using arsenic (+3 oxidation state) methyltransferase (AS3MT) as the key enzyme and S-adenosylmethionine (SAM) as the methyl donor (Thomas et al., 2007). Arsenic ingested as As(V) is first reduced to As(III), and then is methylated to monomethylarsonic acid (MMA(V)), which is then reduced, forming monomethylarsonous acid (MMA(III)). A second methylation and reduction reaction follows, producing dimethylarsinic acid (DMA(V)) and dimethylarsinous acid (DMA(III)), respectively (Thomas et al., 2004; Thomas et al., 2007). Originally considered a detoxification process, it is now widely accepted that the metabolism of arsenic generates different arsenic metabolites that are readily excreted in urine and have variable degrees of toxicity. The proportion of arsenic methylated metabolites in urine has been used to evaluate the arsenic methylation efficiency in humans (Hopenhayn-Rich et al., 1996; Loffredo et al., 2003). The ratio of uDMA(V) to uMMA(V) (uDMA/uMMA) is frequently referred to as the secondary methylation index, as it spans the second methylation step. The percentage of urinary arsenic present as MMA(V) (%uMMA) is less clear in its interpretation, as it relates the MMA(V) urinary concentration to all urinary arsenic metabolites. It is reasonable to consider both a measure of methylation efficiency, as inefficient metabolism through the steps from MMA(V) to DMA(V) would be expected to raise %uMMA as well as lower uDMA/uMMA. Epidemiological studies have shown that poor methylation efficiency represented by high %uMMA and low uDMA/uMMA ratio, is associated with increased risk of developing arsenic induced diseases, including skin lesions, bladder cancer and atherosclerosis (Yu et al., 2000; Ahsan et al., 2007; Pu et al., 2007; Huang et al., 2008; Huang et al., 2009). It is speculated that the observed association between arsenic methylation efficiency and disease susceptibility relates to the fact that MMA(V) in the metabolic pathway is proximal to MMA(III), the most toxic of arsenic metabolites (Petrick et al., 2000; Styblo et al., 2000; Charoensuk et al., 2009). Substantial inter-individual variation in arsenic methylation efficiency in individuals exposed to similar concentrations of arsenic in their drinking water has been reported (Hopenhayn-Rich et al., 1996). These observations have led to the hypothesis that factors affecting arsenic methylation efficiency modulate the susceptibility to arsenic-induced diseases in exposed populations.
Currently several factors associated with arsenic methylation efficiency have been recognized, including sex, genetic variants, and nutritional status. Studies have reported that men excrete higher %uMMA than women, and are more susceptible to the development of arsenic-induced diseases (Hopenhayn-Rich et al., 1996; Steinmaus et al., 2005a; Pu et al., 2007). Similarly, genetic variations in genes involved in arsenic metabolism have also been associated with the efficiency of arsenic methylation. (Meza et al., 2005; Schlawicke Engstrom et al., 2007; Gomez-Rubio et al., 2009; Schlawicke Engstrom et al., 2009). Nutritional status has also been associated to arsenic methylation efficiency as indicated by the finding that undernourished individuals with low protein and nutrient intake have higher %uMMA excretion and higher susceptibility to skin lesions (Mitra et al., 2004; Steinmaus et al., 2005a; Heck et al., 2007).
In contrast to the widely replicated determinants of arsenic methylation efficiency, other factors such as age, smoking status, and body mass index (BMI) have been equivocal in their impact on this phenotype (Tseng, 2009). BMI, body weight adjusted for stature, is often used as a surrogate to the direct measurement of adipose tissue mass, and used as an indicator of obesity (Cole et al., 2005). Previous studies carried out in populations from Pakistan and Bangladesh that were exposed to high concentrations of arsenic in drinking water have found that underweight individuals have higher risk than normal weight and overweight individuals in developing arsenic-associated skin lesions such as melanosis and keratosis (Milton et al., 2004; Ahsan et al., 2006; Fatmi et al., 2009). A general trend has been observed that correlates BMI positively to %uDMA and uDMA/uMMA, and negatively to %uMMA, however these associations have not proven to be statistically significant in all studies. Tseng et al. in 2005 reported BMI to have a positive association to %uDMA and a negative association to %uMMA, while no statistically significant association with uDMA/uMMA was observed. Lindberg et al. (2007) reported the association between high BMI and increased arsenic methylation efficiency to be statistically significant only in men. Heck et al. (2007) used control participants from a case-control study in Bangladesh to study arsenic methylation efficiency and nutrient intake and reported that BMI had a negative association with %uMMA, and a positive association with %uDMA and uDMA/uMMA. However, a separate analysis by Ahsan et al. (2007) using the same study population with different BMI cut-points found no significant association after adjusting for variables such as age, sex and smoking status. Similarly, other studies have failed to find a statistically significant association between BMI and arsenic methylation capacity (Huang et al., 2007; Li et al., 2008; Lindberg et al., 2008). In addition to differences in analytical approaches, the lack of a robust relationship between BMI and arsenic methylation efficiency may be due to the skewed BMI distributions of the subjects in some of these studies that resulted in an underrepresentation of high BMI subjects. To address this we pooled three populations of individuals with balanced contribution from normal-weight, over-weight, and obese BMI levels, exposed to drinking water arsenic at low to moderate concentrations to examine the determinants of arsenic methylation efficiency, with particular attention to BMI. Here we report that BMI, 7388 and M287T AS3MT genetic variants, as well as total urinary arsenic are associated with arsenic methylation efficiency in adult women.
MATERIALS AND METHODS
Population description
The participants of this study were selected from three independent study populations recruited from the state of Arizona in the United States, and the state of Sonora in Mexico. Characteristics of the populations are presented below.
Sonora Arsenic Study (SAS)
A total of 405 individuals were recruited in a cross-sectional study between 2007 and 2009 in the towns of Potam, Vicam Pueblo and Vicam Estacion surrounding Ciudad Obregon, Sonora. Males and females aged ≥6 years old were recruited and not excluded based on current health status. Subjects were not screened for signs of arsenic-induced diseases but completed a general health questionnaire. Recruitments were conducted through door-to-door visits. Only one individual from each household was sampled.
Binational Arsenic Exposure Survey (BAsES)
A total of 487 individuals were recruited in a cross-sectional study between 2005 and 2007 in the communities of Ajo, New River, San Manuel and Tucson, Arizona, as well as Hermosillo, Tobarito and Guadalupe Victoria, Sonora. Men and women aged ≥18 years old that had lived at their residence at least one year were eligible. Participants were recruited via phone interview or door-to-door contact. All household participants were eligible.
Women’s Breast and Bone Density (WBBD)
Two hundred and thirty-eight women from Tucson, Arizona were enrolled in this cross-sectional study between 2001 and 2004. The study population consisted of pre and postmenopausal women aged 41–50 and 56–70 years old, respectively. Recruitment as well as inclusion/exclusion criteria have been described elsewhere (Thomson et al., 2007).
For this study we selected all adult (≥18 years old) women, as this is the only equivalent group across the three populations. The selected population totaled 678 adult women. Participants were asked to donate biological samples, and to complete lifestyle questionnaires. Anthropometric measures were recorded. Informed consent was granted by all participants prior to enrollment as approved by the Human Subjects Committee of the University of Arizona, University of Sonora (UNISON), and Instituto Tecnologico de Sonora (ITSON).
Sample collection
Buccal cells (SAS), and blood samples (BAsES and WBBD) were collected for DNA isolation. First morning urine samples and unfiltered tap water from each household (SAS and BAsES) were collected for arsenic speciation analysis. Samples from Mexico were stored at −20°C until shipment to the University of Arizona; samples were shipped at −60°C. All urine and water samples were stored at −80°C until arsenic speciation analysis.
DNA isolation and genotyping
DNA isolation of buccal cell samples from SAS was performed using a modified protocol from the Qiagen BioSprint 96 DNA Blood kit as previously described (Gomez-Rubio et al. 2009). DNA from blood samples was isolated using the Qiagen PAXgene blood DNA kit (BAsES) and Qiagen EZ1 DNA Blood 350 μL kit (WBBD) following the manufacturer’s specifications.
Genotypes for AS3MT single nucleotide polymorphisms (SNPs) rs3740393 (also called “7388”) and rs11191439 (also called “M287T”) were determined by allelic discrimination using Taqman assays (Applied Biosystems, Foster City, CA). Polymorphism rs3740393 is one member of a large cluster of linked polymorphisms in AS3MT, and thus “tags” many other genetic variants (Gomez-Rubio et al., 2009). Genomic DNA (15 ng) or water (as blank) were used to manually assemble a final reaction volume of 10 μl. Reaction mixture and thermal cycler specification were carried out as previously described (Meza et al., 2005). Genotypes were assigned using the SDS allelic discrimination software (version 2.3, Applied Biosystems). In addition to the evaluation of the water blank samples to confirm no signal, at least 10% of the samples for each polymorphism were repeat-genotyped in separate reactions, with concordance evaluated as a quality control measure.
Arsenic speciation
Arsenic species (As(III), As(V), MMA(V), DMA(V)) were determined in urine and water samples using high performance liquid chromatography (Agilent 1100 HPLC) with inductively couple plasma mass spectroscopy (Agilent 7500 ICP/MS) as previously described (Meza et al., 2005). Detection levels in μg/L for As(III), As(V), MMA(V), and DMA(V) for each set of samples were as follow: SAS 0.16, 0.41, 0.12 and 0.12; BAsES 0.12, 0.21, 0.12 and 0.12; WBBD 0.15, 0.1, 0.14, and 0.11.
Anthropometry
Weight was measured using bathroom (SAS and BAsES) and balance beam (WBBD) scales. Height was measured using stadiometers. Body mass index (BMI) was calculated as body weight (kg)/(height (m))2. The measurements were made at the time of urine sample collection.
Statistical analysis
Linear regression models were used to evaluate the relationship between arsenic methylation efficiency, measured as %uMMA and uDMA/uMMA, and potential determinants including age, smoking status, ethnicity, study (SAS, and WBBD), total urinary arsenic (TA), SNPs 7388 and M287T, and BMI. Smoking status was included as a binary variable (1 = never smoked, 2 = current and former smoker). Ethnicity was self-reported either as Hispanic or non-Hispanic. Differences between studies were tested by including dummy variables for study populations in the model (SAS and WBBD, using BAsES as reference). Total arsenic in urine and water was calculated as the sum of As(III), As(V), MMA(V), and DMA(V). TA, %uMMA and uDMA/uMMA were natural log-transformed in order to approximate a normal distribution. A dominant mode of inheritance was assumed for genetic analyses using the common homozygote (7388: G/G, M287T: T/T) as the reference group and the combined heterozygous (7388: G/C, M287T: T/C) and homozygous variant (7388: C/C, M287T: C/C) as the comparison group. Chi-square analysis was used to evaluate deviation from Hardy-Weinberg equilibrium for both polymorphisms. Arsenic metabolite concentrations below detection were replaced with one-half the detection level. A total of 196 samples had concentrations of As(V) below detection limit, while 165 samples had concentrations of As(III) below detection limit. All samples showed MMA(V) and DMA(V) concentrations above detection limit. A total of 257 subjects had at least one arsenic metabolite below detection limits. For comparison, we also performed the analysis removing all individuals with arsenic concentrations below detection in any arsenic metabolite. Adjusted %uMMA means for low and high BMI tertiles were obtained through multiple regression model of the pooled population (Table 2) by fixing age (mean of age), smoking status (non-smoker), ethnicity (Hispanic), TA (mean of TA) and genotypes (common homozygote). Adjusted %uMMA means were calculated separately for each of the three studies. Statistical analyses were performed using R software version 2.9.0. Statistical significance is considered when p≤ 0.05.
Table 2.
Multivariate linear regression models in the pooled population using %uMMA and uDMA/uMMA as dependent variables.
| Adjusted R2 | Coefficient | Standard error | p-value | |
|---|---|---|---|---|
| Model 1 | ||||
| %uMMA | 0.116 | |||
| Intercept | 2.9727 | 0.1186 | <0.0001 | |
| Age, yrs | 0.0007 | 0.0012 | 0.5818 | |
| Ever smoker | 0.0001 | 0.0476 | 0.9979 | |
| Non-Hispanic ethnicity | 0.0455 | 0.0398 | 0.2529 | |
| Study SAS | 0.0798 | 0.0508 | 0.1167 | |
| Study WBBD | −0.0529 | 0.0395 | 0.1811 | |
| Total urinary arsenic | −0.0583 | 0.0172 | 0.0007 | |
| AS3MT 7388 (G/G as reference) | −0.1221 | 0.0335 | 0.0003 | |
| AS3MT M287T (T/T as reference) | 0.0963 | 0.0414 | 0.0202 | |
| BMI | −0.0169 | 0.0027 | <0.0001 | |
| Model 2 | ||||
| uDMA/uMMA | 0.106 | |||
| Intercept | 1.1456 | 0.1436 | <0.0001 | |
| Age, yrs | 0.0003 | 0.0015 | 0.8225 | |
| Ever smoker | 0.0017 | 0.0576 | 0.9771 | |
| Non-Hispanic ethnicity | −0.042 | 0.0482 | 0.3837 | |
| Study SAS | −0.0801 | 0.0615 | 0.1934 | |
| Study WBBD | 0.1684 | 0.0478 | 0.0005 | |
| Total urinary arsenic | 0.068 | 0.0208 | 0.0012 | |
| AS3MT 7388(G/G as reference) | 0.1559 | 0.0406 | 0.0001 | |
| AS3MT M287T (T/T as reference) | −0.1019 | 0.0501 | 0.0423 | |
| BMI | 0.0208 | 0.0033 | <0.0001 | |
RESULTS
Table 1 contains the characteristics of the participants for each of the three populations. From the initial pooled population of 678 adult women, 8 did not have urinary arsenic speciation data, 20 did not have anthropometric data, and 26 had missing genotypes or smoking status, leaving a total of 624 women for the analysis. The mean and range age of the pooled population was 49 and 19–88 years old respectively. 37.5% of the participants were current or former smokers. Mean weight and height were 73.1 kg and 159.9 cm respectively. Mean BMI was 28.5. Overall, more than half of the study population was overweight (BMI between 25 and 29.9) or obese (BMI ≥30), 31.1% and 34.7% of the pooled population, respectively. Total arsenic in drinking water was not available for the WBBD study, but the mean and range of total arsenic in drinking water was 66.3 and 0.18–227.6 μg/L for SAS and 24.3 and 0.1–1004 μg/L for BAsES. Sixteen subjects from BAsES did not provide drinking water samples. Only As(III) and As(V) were detected in drinking water samples. Total urinary arsenic mean and range for pooled population were 53.6 and 1.2–1047.4 μg/L with arsenic metabolite means and ranges (μg/L) of 6.5 and 0.13–121.02 for InAs; 5.7 and 0.16–152.2 for uMMA; and 41.5 and 0.84–793.7 for uDMA.
Table 1.
Characteristics of the study populations from Arizona and Sonora.
| SAS† | BAsES† | WBBD† | This study† (pooled) | |
|---|---|---|---|---|
| Total n | 140 | 281 | 203 | 624 |
| Age◆, mean (SD) | 45 (12.8) | 48 (16.0) | 52 (8.4) | 49 (13.5) |
| Smoking, % (never/former/current) | 77.1/15.0/7.9 | 60.5/23.1/16.4 | 55.2/35.0/9.8 | 62.5/25.2/12.3 |
| Ethnicity, % (hispanic/non-hispanic) | 100/0 | 67.3/32.7 | 34.0/66.0 | 63.8/36.2 |
| SNP genotypes. % | ||||
| 7388 (GG/GC/CC) | 57.9/37.1/5.0 | 71.9/24.9/3.2 | 69.5/28.1/2.4 | 67.9/28.7/3.4 |
| M287T (TT/TC/CC) | 92.1/7.9/0.0 | 80.4/19.2/0.4 | 78.3/20.2/1.5 | 82.4/17.0/0.6 |
| Weight, mean* (SD) | 76.1 (13.2) | 72.8 (16.2) | 72.8 (15.9) | 73.1 (15.5) |
| Height, mean^ (SD) | 157.9 (5.1) | 158.9 (7.2) | 162.7 (6.2) | 159.9 (6.7) |
| Body mass index, n (%) | ||||
| underweight (≤18.4) | 0 (0.0) | 5 (1.8) | 0 (0.0) | 5 (0.8) |
| normal (18.5–24.9) | 27 (19.3) | 85 (30.2) | 77 (37.9) | 189 (30.3) |
| overweight (25–29.9) | 45 (32.1) | 77 (27.4) | 72 (35.5) | 194 (31.1) |
| obese (≥30) | 68 (48.6) | 114 (40.6) | 54 (26.6) | 236 (37.8) |
| Total arsenic in drinking water (μg/L), mean (SD) | 66.3 (38.0) | 24.3 (88.5) | NA | NA |
| Total urinary arsenic (μg/L), mean (SD) | 160.2 (160.2) | 31.9 (63.5) | 10.3 (7.6) | 53.6 (104.6) |
| InAs (μg/L), mean(SD) | 19.9 (21.7) | 4.2 (8.0) | 0.4 (0.5) | 6.5 (13.8) |
| uMMA ((μg/L), mean (SD) | 15.9 (17.7) | 3.8 (10.6) | 1.1 (0.8) | 5.7 (12.4) |
| uDMA (μg/L), mean (SD) | 124.4 (125.0) | 23.8 (45.7) | 8.7 (6.8) | 41.5 (80.5) |
| uDMA/uMMA, mean (SD) | 9.2 (7.0) | 8.2 (4.9) | 8.6 (4.6) | 8.5 (5.4) |
SD: standard deviation. NA: Not available.
All parameters presented for adult women only.
Age reported in years.
Weight reported in Kg.
Height reported in centimeters.
Repeat genotyping of the two polymorphisms in this study for quality control purposes was completely concordant between runs. Distributions of genotypes for both polymorphisms were consistent with Hardy-Weinberg equilibrium expectations.
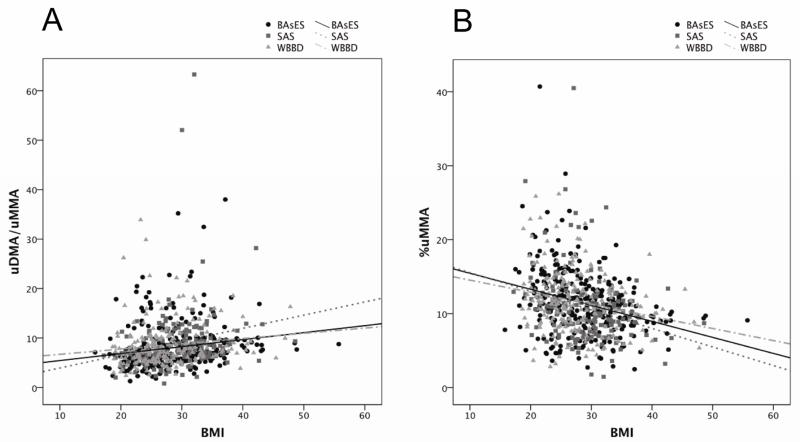
Linear regression models were constructed to explain variability in %uMMA and uDMA/uMMA, shown in Table 2. Age, smoking status, and self-reported ethnicity did not show statistically significant association with %uMMA or uDMA/uMMA. Difference between studies was only observed between WBBD and BAsES for uDMA/uMMA (p= 0.0005). Total urinary arsenic showed a negative association to %uMMA (p= 0.0007) and a positive association to uDMA/uMMA (p= 0.0012). The intronic AS3MT polymorphism 7388 showed a negative association to %uMMA (p= 0.0003) and a positive association to uDMA/uMMA (p= 0.0001), consistent with a previous reported association in the entire SAS population, where SNP 7388, along with other 45 linked polymorphisms, was found to be significantly associated with uDMA/uMMA (Gomez-Rubio et al., 2009). Genetic association analysis in WBDD and BAsES for SNP 7388 and %uMMA and uDMA/uMMA also showed significant association in both populations (WBBD p=0.001 for both variables, BAsES p=0.008 and p=0.004 respectively). SNP M287T showed a positive association with %uMMA (p= 0.02) and negative association with uDMA/uMMA (p= 0.04). Additionally, BMI was significantly associated with arsenic methylation efficiency, displaying a strong negative association with %uMMA and a positive association with uDMA/uMMA following adjustment for covariates (p<0.0001 for both). The relationship observed in the pooled population was consistent in each of the three component populations (Figure 1). This effect is further demonstrated by measuring the distribution of %uMMA in the lower and upper BMI tertiles stratifying by study population. Adjusted mean %uMMA in the low BMI tertile were 11 (BAsES), 12.2 (SAS), and 11.1 (WBBD) while adjusted mean %uMMA in the high BMI tertile were 8.8 (BAsES), 10.2 (SAS), and 9.1 (WBBD). Multiple linear regression models performed separately for each study population show that after adjustment for age, smoking status, ethnicity, TA, AS3MT 7388 and AS3MT M287T, the negative association between BMI and %uMMA is statistically significant in each of the three studies (Table 3).
Figure 1.
Scatter plot for A) uDMA/uMMA and B) %uMMA as a function of BMI in the adult women of SAS, BAsES, and WBBD study populations. Simple regression lines were fit for each study group to illustrate the trend of the association in each population. R2 for uDMA/uMMA: 0.031 BAsES, 0.042 SAS, 0.017 WBBD, and for %uMMA: 0.086 BAsES, 0.071 SAS, 0.05 WBBD.
Table 3.
Multivariate linear regression models in each of the independent study populations using %uMMA as dependent variable.
| Coefficient | Standard error | p-value | |
|---|---|---|---|
| %uMMA | |||
| BMI (BAsES) | −0.0157 | 0.0038 | <0.0001 |
| BMI (SAS) | −0.0272 | 0.0072 | 0.0002 |
| BMI (WBBD) | −0.0141 | 0.0045 | 0.0019 |
Models were adjusted for age, smoking status, ethnicity, total urinary arsenic, AS3MT 7388, and AS3MT M287T.
DISCUSSION
Understanding the determinants of human arsenic metabolic patterns is an important public health objective. While it is clear that duration-weighted exposure to consumed inorganic arsenic is the most important predictor of arsenic-associated disease, arsenic methylation efficiency has also been repeatedly associated with disease susceptibility (Huang et al., 2008). Here we report that BMI, genetic variants in AS3MT and total urinary arsenic are significantly associated with arsenic methylation efficiency measured as %uMMA and uDMA/uMMA.
Body mass index is an anthropometric measure for obesity and has also been used as a reflection of nutritional status (Gomes Fda et al., 2009). Different studies evaluating BMI as a factor affecting arsenic methylation efficiency have not arrived at consistent results. In 2007 Lindberg et al. reported an inverse association between BMI and %uMMA in a population from Central Europe, however this association was statistically significant only for men. A subsequent study by Li et al. (2008) analyzing the arsenic metabolic profile in pregnant women from Bangladesh, reported no association between BMI and arsenic metabolites (InAs, MMA, DMA, MMA/InAs, and DMA/MMA). In contrast, in the present study we report a positive association between BMI and arsenic methylation capacity in three independent populations of adult women.
The direction of the association between BMI and arsenic methylation efficiency reported here is concordant with previous studies that have shown a decrease in %uMMA and an increase in %uDMA and uDMA/uMMA with higher BMI (Ahsan et al., 2007; Huang et al., 2007; Li et al., 2008; Lindberg et al., 2007; Tseng et al., 2005). Conversely, a small number of studies have shown an inverse direction between BMI and arsenic methylation efficiency (Lindberg et al., 2008). Regardless the direction of the association, inconsistency in the significance of this association has been observed among different studies. It is possible that differences in the BMI distribution among subjects and in the analysis design between the diverse studies could account for the discrepancies observed. The previously reported non-significant association in pregnant women by Li et al. (2008) was observed in a population with an average normal weight (BMI=20), where only 5.4% of the population was overweight or obese. Similarly, Ahsan et al. (2007) and Lindberg et al. (2008) reported a lack of association in a multivariate analysis between BMI and arsenic metabolic profile in populations where more than 60% of the individuals had BMI of ≤ 20 or only about 4% of the population was overweight, respectively. Tseng et al. (2005) in a population from Taiwan showed that BMI had a significant negative association with %uMMA and a positive association with %uDMA, while uDMA/uMMA was negatively associated but not statistically significant. BMI distribution was not reported in that study. Additionally, inconsistent results have been reported in a study population that was overweight on average (Lindberg et al., 2007). In that study a significant association was observed between BMI and %uDMA (positive) and %uMMA (negative) in multiple regression analyses. However, further stratification by sex showed that BMI was only significantly associated to %uMMA and the association was restricted to men. Using a dichotomous BMI variable (<25 vs. >25) might have reduced the sensitivity of this study and thus the possibility of observing an association in women. Similarly, a lack of significance was reported in another study where individuals below and above BMI of 23 were compared by Student’s t-test (Huang et al., 2007).
In this study we found a highly significant association between elevated BMI and increased arsenic methylation efficiency in an overweight (on average) population of adult women with mean urinary total arsenic of 53.6 μg/L. Individuals with higher BMI had significantly lower %uMMA and higher uDMA/uMMA. These results are consistent with previous studies that have shown that both high %uMMA and low BMI are associated with increased incidence of arsenic-induced diseases like skin lesions (Yu et al., 2000; Ahsan et al., 2006; Ahsan et al., 2007; Pu et al., 2007; Fatmi et al., 2009). This led to the idea that poor nutritional status represented by low BMI could influence arsenic methylation efficiency and toxicity. While we are not disregarding the possibility that a nutritional effect could partially be responsible for the association between BMI and arsenic methylation efficiency, this effect could be also driven by other mechanisms associated with increased body fat content in overweight and obese individuals. To our knowledge, it has not been shown that human adipose tissue is capable of arsenic metabolism, although previous studies have demonstrated that mouse adipocytes have very low methylation capacity (Walton et al., 2004). Consequently we consider that a direct increase in arsenic metabolic efficiency by an increment in adipose tissue is unlikely to explain this association. Studies comparing obese and normal weight individuals have reported that obesity induces changes in the gene expression profile (Gomez-Ambrosi et al., 2004; Baranova et al., 2005). Recently, the gene expression profile across mouse strains was studied in livers from mice fed with standard low fat or high fat diet. It was found that about 56% of the examined genes changed expression based on diet. Additionally it was observed that among several other genes, AS3MT expression was highly correlated to body weight (Pearson r= −0.63) (Shockley et al., 2009). This correlation of high body weight with lower AS3MT expression in mice may not be an intuitive explanation of our observation of high BMI associated with higher methylation efficiency. It does, however, provide evidence that AS3MT gene expression is in a regulatory network that is impacted by body weight. The translation of these animal models to human arsenic metabolism will require further research.
In 2005 Meza et al. reported a significant positive association between a group of three linked intronic polymorphisms that included AS3MT 7388 intronic polymorphism and uDMA/uMMA. Following this report, a study bySchläwicke Engström et al. (2007) in a population of adult women from Argentina showed a similar positive association between SNP 7388 and uDMA/uMMA, as well as a significant decrease in %uMMA in the variant allele carriers. In our population of adult women, we replicated the association between this AS3MT SNP and increased methylation efficiency, where the variant allele carriers demonstrate lower %uMMA and higher uDMA/uMMA than non-variant individuals. These results confirm and highlight the importance of AS3MT genetic variants in arsenic methylation efficiency. The molecular mechanism behind this association remains unknown. Recently we have reported that the 7388 AS3MT polymorphism is in strong LD with other 45 polymorphisms in a large genomic region that includes 4 genes in addition to AS3MT (Gomez-Rubio et al., 2009). Due the lack of amino acid substitution in these polymorphisms, it seems probable that this genetic effect is driven by a regulatory polymorphism, perhaps in AS3MT. An additional non-synonymous AS3MT polymorphism M287T has previously been associated with high %uMMA excretion (Lindberg et al., 2007; Hernandez et al., 2008; Valenzuela et al., 2009). Consistent with these studies we found a significant positive association between M287T variants and %uMMA, and a significant negative association for uDMA/uMMA.
In this study we also observed a significant association between higher concentrations of TA and increased arsenic methylation capacity, represented by low %uMMA and high uDMA/uMMA. Contrary to our observations, previous studies have reported significant associations between increased concentrations of TA and decreased arsenic methylation capacity (Del Razo et al., 1997; Li et al., 2008; Lindberg et al., 2008). The statistical significance of this association however has been inconsistent from study to study (Chiou et al., 1997; Kurttio et al., 1998; Steinmaus et al., 2005b). Recently Steinmaus et al. reported a correlation between TA and %MMA and %DMA in a similar direction as our study, although the correlation was not statistically significant (Steinmaus et al., 2010). One potential weakness in our study is the lack of adjustment for urinary osmolality, which could influence total arsenic measurement. Differences in urine osmolality, however, would be expected to introduce random error and a bias to the null, and thus would not explain our findings. We considered the possibility that this association may be related to the assignment of values (one half of the detection limit) in urine samples with arsenic species below the detection limit (BDL). This possibility was not supported by the fact that in the individual component studies, the association between higher TA and lower %MMA or higher uDMA/uMMA was statistically significant in the SAS and WBBD studies, despite these studies’ wide range of subjects who had BDL arsenic values (SAS: 11.4% subjects, WBBD: 88.7% subjects) and despite the range of mean TA levels in those studies (Table 1), which is not surprisingly covariate with the BDL subject percentage. To further explore this in a study completely independent of ours, we arbitrarily chose one publicly available data set, 2005–2006, from the National Health and Nutrition Examination Survey (NHANES) for which arsenic speciation had been performed (Centers for Disease Control and Prevention, 2005–2006). Unadjusted linear regression analysis of the adult women within the NHANES data showed a highly significant association between TA and %uMMA (inversely related) and with uDMA/uMMA (positively related), consistent with our study (data not shown). This association in the NHANES data was present whether TA was calculated as the sum of the four arsenic species, as in our study, or whether measured TA was used as the dependent variable. In light of the literature reports suggesting an arsenic metabolism model based on a saturation threshold for arsenic methylation, that predicts a positive association between TA and %MMA, or a negative association between TA and uDMA/uMMA, it is possible that our population was not substantially exposed at a saturation point for arsenic methylation. Despite the many mechanistic processes that could be invoked to argue for or against this “methylation saturation threshold” hypothesis, perhaps the more important point is that this model may fail to adequately explain every human population or exposure scenario.
Previous studies have reported that age and smoking status are significantly associated with decreased arsenic methylation capacity (Heck et al., 2007; Huang et al., 2007), however these associations were not statistically significant in our study populations. Similarly, self-reported ethnicity was not associated with either %uMMA or uDMA/MMA in any of the regression models. It has been speculated previously that ethnicity might be a determinant factor in arsenic methylation efficiency. A report in 1995 showed that native Andean women had overall lower %uMMA in comparison to populations from Europe, Japan and USA (Vahter et al., 1995). Our study populations were only classified as Hispanic or Non-hispanic by self-report. However, self-reported ancestry may not capture the ancestral admixture. Future studies focusing on quantitative techniques like the use of ancestry informative markers are necessary to accurately define the potential association between ancestry and arsenic methylation capacity.
Strengths of this study include the analysis of three independently derived populations, providing an opportunity to establish the robustness of the associations. The public health implication of these findings is apparent when the BMI extremes in our pooled population are compared. The mean %uMMA in the upper and lower BMI deciles of our population were 13.8% and 9.4%, respectively, similar to the %uMMA difference reported between arsenic-associated urothelial carcinoma cases and controls (12.5% vs. 8.1%, respectively) (Chung et al., 2010). This suggests that the magnitude of the effect of BMI on arsenic methylation efficiency could be relevant to disease risk.
Limitations of this study include the lack of men, and the limitation of the cross-sectional study design in the inability to determine the causality of the observed association. Based on our findings, it is possible that the increase in BMI causes changes in the arsenic metabolic process or body distribution that result in the altered urinary metabolite profile that we observed. While this may seem more likely than the alternative, we can not rule out the possibility that an individual’s arsenic metabolism profile (e.g. high %uMMA) may be causative, or a surrogate for causation of an altered BMI.
The observation that high BMI is significantly associated to low %uMMA and high uDMA/uMMA in adult women suggests that BMI should be carefully evaluated as a potential arsenic-associated disease risk factor, and may explain previous observations that have shown arsenic-exposed individuals of lower body mass to have a higher disease risk. This association may present complexities for diseases for which obesity and arsenic exposure are independent risk factors, including vascular disease and type II diabetes. Additional studies will be necessary to understand the potential for obesity to act as both a risk factor and a protective factor in the context of arsenic toxicity.
Acknowledgments
The authors wish to acknowledge Michael Kopplin for performing the arsenic speciation analyses. PG-R was supported by a fellowship from the Mexican National Council for Science and Technology (CONACYT) under the UA-CONACyT partnership. SAS study was supported by the NIEHS Superfund Basic Research Program (ES 04940) and NIEHS Center Grant (ES 006694). BAsES study support was provided as a supplement to the University of Arizona Specialized Program of Research Excellence (SPORE) in GI Cancer (NIH/NCI CA95060). WBBD study was supported by The Susan G. Komen Breast Cancer Foundation (BCTR00-000538) and NIH SWEHSC pilot grant (ES006694).
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahsan H, Chen Y, Kibriya MG, Slavkovich V, Parvez F, Jasmine F, Gamble MV, Graziano JH. Arsenic metabolism, genetic susceptibility, and risk of premalignant skin lesions in Bangladesh. Cancer Epidemiol Biomarkers Prev. 2007;16:1270–1278. doi: 10.1158/1055-9965.EPI-06-0676. [DOI] [PubMed] [Google Scholar]
- Ahsan H, Chen Y, Parvez F, Zablotska L, Argos M, Hussain I, Momotaj H, Levy D, Cheng Z, Slavkovich V, van Geen A, Howe GR, Graziano JH. Arsenic exposure from drinking water and risk of premalignant skin lesions in Bangladesh: baseline results from the Health Effects of Arsenic Longitudinal Study. Am J Epidemiol. 2006;163:1138–1148. doi: 10.1093/aje/kwj154. [DOI] [PubMed] [Google Scholar]
- Balakumar P, Kaur J. Arsenic exposure and cardiovascular disorders: an overview. Cardiovasc Toxicol. 2009;9:169–176. doi: 10.1007/s12012-009-9050-6. [DOI] [PubMed] [Google Scholar]
- Baranova A, Collantes R, Gowder SJ, Elariny H, Schlauch K, Younoszai A, King S, Randhawa M, Pusulury S, Alsheddi T, Ong JP, Martin LM, Chandhoke V, Younossi ZM. Obesity-related differential gene expression in the visceral adipose tissue. Obes Surg. 2005;15:758–765. doi: 10.1381/0960892054222876. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS) National Health and Nutrition Examination Survey Data. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2005–2006. [Accessed Junuary, 2010]. http://www.cdc.gov/nchs/nhanes/nhanes2005-2006/lab05_06.htm. [Google Scholar]
- Charoensuk V, Gati WP, Weinfeld M, Le XC. Differential cytotoxic effects of arsenic compounds in human acute promyelocytic leukemia cells. Toxicol Appl Pharmacol. 2009 doi: 10.1016/j.taap.2009.05.016. [DOI] [PubMed] [Google Scholar]
- Chiou HY, Hsueh YM, Hsieh LL, Hsu LI, Hsu YH, Hsieh FI, Wei ML, Chen HC, Yang HT, Leu LC, Chu TH, Chen-Wu C, Yang MH, Chen CJ. Arsenic methylation capacity, body retention, and null genotypes of glutathione S-transferase M1 and T1 among current arsenic-exposed residents in Taiwan. Mutat Res. 1997;386:197–207. doi: 10.1016/s1383-5742(97)00005-7. [DOI] [PubMed] [Google Scholar]
- Chung CJ, Pu YS, Su CT, Chen HW, Huang YK, Shiue HS, Hsueh YM. Polymorphisms in one-carbon metabolism pathway genes, urinary arsenic profile, and urothelial carcinoma. Cancer Causes Control. 2010;21:1605–1613. doi: 10.1007/s10552-010-9589-3. [DOI] [PubMed] [Google Scholar]
- Cole TJ, Faith MS, Pietrobelli A, Heo M. What is the best measure of adiposity change in growing children: BMI, BMI %, BMI z-score or BMI centile? Eur J Clin Nutr. 2005;59:419–425. doi: 10.1038/sj.ejcn.1602090. [DOI] [PubMed] [Google Scholar]
- Del Razo LM, Garcia-Vargas GG, Vargas H, Albores A, Gonsebatt ME, Montero R, Ostrosky-Wegman P, Kelsh M, Cebrian ME. Altered profile of urinary arsenic metabolites in adults with chronic arsenicism. A pilot study. Arch Toxicol. 1997;71:211–217. doi: 10.1007/s002040050378. [DOI] [PubMed] [Google Scholar]
- Fatmi Z, Azam I, Ahmed F, Kazi A, Gill AB, Kadir MM, Ahmed M, Ara N, Janjua NZ. Health burden of skin lesions at low arsenic exposure through groundwater in Pakistan. Is river the source? Environ Res. 2009;109:575–581. doi: 10.1016/j.envres.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Gomes Fda S, Anjos LA, Vasconcellos MT. Influence of different body mass index cut-off values in assessing the nutritional status of adolescents in a household survey. Cad Saude Publica. 2009;25:1850–1857. doi: 10.1590/s0102-311x2009000800021. [DOI] [PubMed] [Google Scholar]
- Gomez-Ambrosi J, Catalan V, Diez-Caballero A, Martinez-Cruz LA, Gil MJ, Garcia-Foncillas J, Cienfuegos JA, Salvador J, Mato JM, Fruhbeck G. Gene expression profile of omental adipose tissue in human obesity. FASEB J. 2004;18:215–217. doi: 10.1096/fj.03-0591fje. [DOI] [PubMed] [Google Scholar]
- Gomez-Rubio P, Meza-Montenegro MM, Cantu-Soto E, Klimecki WT. Genetic association between intronic variants in AS3MT and arsenic methylation efficiency is focused on a large linkage disequilibrium cluster in chromosome 10. J Appl Toxicol. 2009 doi: 10.1002/jat.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafeman DM, Ahsan H, Louis ED, Siddique AB, Slavkovich V, Cheng Z, van Geen A, Graziano JH. Association between arsenic exposure and a measure of subclinical sensory neuropathy in Bangladesh. J Occup Environ Med. 2005;47:778–784. doi: 10.1097/01.jom.0000169089.54549.db. [DOI] [PubMed] [Google Scholar]
- Heck JE, Gamble MV, Chen Y, Graziano JH, Slavkovich V, Parvez F, Baron JA, Howe GR, Ahsan H. Consumption of folate-related nutrients and metabolism of arsenic in Bangladesh. Am J Clin Nutr. 2007;85:1367–1374. doi: 10.1093/ajcn/85.5.1367. [DOI] [PubMed] [Google Scholar]
- Hernandez A, Xamena N, Sekaran C, Tokunaga H, Sampayo-Reyes A, Quinteros D, Creus A, Marcos R. High arsenic metabolic efficiency in AS3MT287Thr allele carriers. Pharmacogenet Genomics. 2008;18:349–355. doi: 10.1097/FPC.0b013e3282f7f46b. [DOI] [PubMed] [Google Scholar]
- Hopenhayn-Rich C, Biggs ML, Smith AH, Kalman DA, Moore LE. Methylation study of a population environmentally exposed to arsenic in drinking water. Environ Health Perspect. 1996;104:620–628. doi: 10.1289/ehp.96104620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YK, Huang YL, Hsueh YM, Yang MH, Wu MM, Chen SY, Hsu LI, Chen CJ. Arsenic exposure, urinary arsenic speciation, and the incidence of urothelial carcinoma: a twelve-year follow-up study. Cancer Causes Control. 2008;19:829–839. doi: 10.1007/s10552-008-9146-5. [DOI] [PubMed] [Google Scholar]
- Huang YK, Tseng CH, Huang YL, Yang MH, Chen CJ, Hsueh YM. Arsenic methylation capability and hypertension risk in subjects living in arseniasis-hyperendemic areas in southwestern Taiwan. Toxicol Appl Pharmacol. 2007;218:135–142. doi: 10.1016/j.taap.2006.10.022. [DOI] [PubMed] [Google Scholar]
- Huang YL, Hsueh YM, Huang YK, Yip PK, Yang MH, Chen CJ. Urinary arsenic methylation capability and carotid atherosclerosis risk in subjects living in arsenicosis-hyperendemic areas in southwestern Taiwan. Sci Total Environ. 2009;407:2608–2614. doi: 10.1016/j.scitotenv.2008.12.061. [DOI] [PubMed] [Google Scholar]
- Kurttio P, Komulainen H, Hakala E, Kahelin H, Pekkanen J. Urinary excretion of arsenic species after exposure to arsenic present in drinking water. Arch Environ Contam Toxicol. 1998;34:297–305. doi: 10.1007/s002449900321. [DOI] [PubMed] [Google Scholar]
- Li L, Ekstrom EC, Goessler W, Lonnerdal B, Nermell B, Yunus M, Rahman A, El Arifeen S, Persson LA, Vahter M. Nutritional status has marginal influence on the metabolism of inorganic arsenic in pregnant Bangladeshi women. Environ Health Perspect. 2008;116:315–321. doi: 10.1289/ehp.10639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg AL, Ekstrom EC, Nermell B, Rahman M, Lonnerdal B, Persson LA, Vahter M. Gender and age differences in the metabolism of inorganic arsenic in a highly exposed population in Bangladesh. Environ Res. 2008;106:110–120. doi: 10.1016/j.envres.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Lindberg AL, Kumar R, Goessler W, Thirumaran R, Gurzau E, Koppova K, Rudnai P, Leonardi G, Fletcher T, Vahter M. Metabolism of low-dose inorganic arsenic in a central European population: influence of sex and genetic polymorphisms. Environ Health Perspect. 2007;115:1081–1086. doi: 10.1289/ehp.10026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffredo CA, Aposhian HV, Cebrian ME, Yamauchi H, Silbergeld EK. Variability in human metabolism of arsenic. Environ Res. 2003;92:85–91. doi: 10.1016/s0013-9351(02)00081-6. [DOI] [PubMed] [Google Scholar]
- Meza MM, Yu L, Rodriguez YY, Guild M, Thompson D, Gandolfi AJ, Klimecki WT. Developmentally restricted genetic determinants of human arsenic metabolism: association between urinary methylated arsenic and CYT19 polymorphisms in children. Environ Health Perspect. 2005;113:775–781. doi: 10.1289/ehp.7780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton AH, Hasan Z, Shahidullah SM, Sharmin S, Jakariya MD, Rahman M, Dear K, Smith W. Association between nutritional status and arsenicosis due to chronic arsenic exposure in Bangladesh. Int J Environ Health Res. 2004;14:99–108. doi: 10.1080/0960312042000209516. [DOI] [PubMed] [Google Scholar]
- Mitra SR, Mazumder DN, Basu A, Block G, Haque R, Samanta S, Ghosh N, Smith MM, von Ehrenstein OS, Smith AH. Nutritional factors and susceptibility to arsenic-caused skin lesions in West Bengal, India. Environ Health Perspect. 2004;112:1104–1109. doi: 10.1289/ehp.6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrick JS, Ayala-Fierro F, Cullen WR, Carter DE, Vasken Aposhian H. Monomethylarsonous acid (MMA(III)) is more toxic than arsenite in Chang human hepatocytes. Toxicol Appl Pharmacol. 2000;163:203–207. doi: 10.1006/taap.1999.8872. [DOI] [PubMed] [Google Scholar]
- Pu YS, Yang SM, Huang YK, Chung CJ, Huang SK, Chiu AW, Yang MH, Chen CJ, Hsueh YM. Urinary arsenic profile affects the risk of urothelial carcinoma even at low arsenic exposure. Toxicol Appl Pharmacol. 2007;218:99–106. doi: 10.1016/j.taap.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Schlawicke Engstrom K, Broberg K, Concha G, Nermell B, Warholm M, Vahter M. Genetic polymorphisms influencing arsenic metabolism: evidence from Argentina. Environ Health Perspect. 2007;115:599–605. doi: 10.1289/ehp.9734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlawicke Engstrom K, Nermell B, Concha G, Stromberg U, Vahter M, Broberg K. Arsenic metabolism is influenced by polymorphisms in genes involved in one-carbon metabolism and reduction reactions. Mutat Res. 2009;667:4–14. doi: 10.1016/j.mrfmmm.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Shockley KR, Witmer D, Burgess-Herbert SL, Paigen B, Churchill GA. Effects of atherogenic diet on hepatic gene expression across mouse strains. Physiol Genomics. 2009;39:172–182. doi: 10.1152/physiolgenomics.90350.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmaus C, Carrigan K, Kalman D, Atallah R, Yuan Y, Smith AH. Dietary intake and arsenic methylation in a U.S. population. Environ Health Perspect. 2005a;113:1153–1159. doi: 10.1289/ehp.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmaus C, Yuan Y, Kalman D, Atallah R, Smith AH. Intraindividual variability in arsenic methylation in a U.S. population. Cancer Epidemiol Biomarkers Prev. 2005b;14:919–924. doi: 10.1158/1055-9965.EPI-04-0277. [DOI] [PubMed] [Google Scholar]
- Steinmaus C, Yuan Y, Kalman D, Rey OA, Skibola CF, Dauphine D, Basu A, Porter KE, Hubbard A, Bates MN, Smith MT, Smith AH. Individual differences in arsenic metabolism and lung cancer in a case-control study in Cordoba, Argentina. Toxicol Appl Pharmacol. 2010;247:138–145. doi: 10.1016/j.taap.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styblo M, Del Razo LM, Vega L, Germolec DR, LeCluyse EL, Hamilton GA, Reed W, Wang C, Cullen WR, Thomas DJ. Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Arch Toxicol. 2000;74:289–299. doi: 10.1007/s002040000134. [DOI] [PubMed] [Google Scholar]
- Thomas DJ, Li J, Waters SB, Xing W, Adair BM, Drobna Z, Devesa V, Styblo M. Arsenic (+3 oxidation state) methyltransferase and the methylation of arsenicals. Exp Biol Med (Maywood) 2007;232:3–13. [PMC free article] [PubMed] [Google Scholar]
- Thomas DJ, Waters SB, Styblo M. Elucidating the pathway for arsenic methylation. Toxicol Appl Pharmacol. 2004;198:319–326. doi: 10.1016/j.taap.2003.10.020. [DOI] [PubMed] [Google Scholar]
- Thomson CA, Arendell LA, Bruhn RL, Maskarinec G, Lopez AM, Wright NC, Moll CE, Aickin M, Chen Z. Pilot study of dietary influences on mammographic density in pre- and postmenopausal Hispanic and non-Hispanic white women. Menopause. 2007;14:243–250. doi: 10.1097/01.gme.0000235362.72899.7b. [DOI] [PubMed] [Google Scholar]
- Tseng CH. A review on environmental factors regulating arsenic methylation in humans. Toxicol Appl Pharmacol. 2009;235:338–350. doi: 10.1016/j.taap.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Tseng CH, Huang YK, Huang YL, Chung CJ, Yang MH, Chen CJ, Hsueh YM. Arsenic exposure, urinary arsenic speciation, and peripheral vascular disease in blackfoot disease-hyperendemic villages in Taiwan. Toxicol Appl Pharmacol. 2005;206:299–308. doi: 10.1016/j.taap.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Tseng CH, Tseng CP, Chiou HY, Hsueh YM, Chong CK, Chen CJ. Epidemiologic evidence of diabetogenic effect of arsenic. Toxicol Lett. 2002;133:69–76. doi: 10.1016/s0378-4274(02)00085-1. [DOI] [PubMed] [Google Scholar]
- Vahter M, Concha G, Nermell B, Nilsson R, Dulout F, Natarajan AT. A unique metabolism of inorganic arsenic in native Andean women. Eur J Pharmacol. 1995;293:455–462. doi: 10.1016/0926-6917(95)90066-7. [DOI] [PubMed] [Google Scholar]
- Valenzuela OL, Drobna Z, Hernandez-Castellanos E, Sanchez-Pena LC, Garcia-Vargas GG, Borja-Aburto VH, Styblo M, Del Razo LM. Association of AS3MT polymorphisms and the risk of premalignant arsenic skin lesions. Toxicol Appl Pharmacol. 2009;239:200–207. doi: 10.1016/j.taap.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton FS, Harmon AW, Paul DS, Drobna Z, Patel YM, Styblo M. Inhibition of insulin-dependent glucose uptake by trivalent arsenicals: possible mechanism of arsenic-induced diabetes. Toxicol Appl Pharmacol. 2004;198:424–433. doi: 10.1016/j.taap.2003.10.026. [DOI] [PubMed] [Google Scholar]
- Yu RC, Hsu KH, Chen CJ, Froines JR. Arsenic methylation capacity and skin cancer. Cancer Epidemiol Biomarkers Prev. 2000;9:1259–1262. [PubMed] [Google Scholar]