Abstract
Matrix Metalloproteinases (MMPs) are a group of proteinases that degrade components of the extracellular matrix (ECM). There is increasing evidence for a link between the activation of MMPs and Alzheimer’s disease (AD) pathogenesis, in which both beneficial and detrimental actions of MMPs have been suggested. It has been demonstrated that MMPs could degrade amyloid β (Aβ) and play important roles in the extracellular Aβ catabolism and clearance. On the other hand, MMPs could contribute to AD pathogenesis by compromising the blood brain barrier and promoting neurodegeneration. In the present study, we observed that oligomeric Aβ regulates MMP2 expression in a paradoxical manner. In rat primary astrocyte cultures, oligomeric Aβ down-regulated MMP2 transcription and reduced its extracellular activity. However, in a widely used mouse model for AD, immunohistochemistry demonstrated an increase of MMP2 expression in astrocytes surrounding senile plaques in APP/PS1 transgenic mice brains. Using real-time PCR, we found that the MMP2 mRNA level was elevated in APP/PS1 transgenic mice brains. In addition, elevated mRNA levels of MMP stimulating cytokines such as IL-1β and TGFβ were found in the brains of APP/PS1 mice. Our study suggests a complex regulation of MMP2 expression by oligomeric Aβ in astrocytes. While oligomeric Aβ directly down-regulates MMP2 expression and activation in astrocytes, it induces production of proinflammatory cytokines which could serve as strong stimulators for MMP2. Therefore, the ultimate outcome of the oligomeric Aβ on MMP2 activation in astrocytes might be the combination of its direct inhibitory action on astrocyte MMP2 expression and the secondary action of inducing inflammatory cytokines.
Keywords: Amyloid β, Matrix Metalloproteinase, Primary Astrocyte, Oligomeric Aβ
1. INTRODUCTION
Matrix Metalloproteinases (MMPs) are a large group of zinc-dependent proteinases with major function in degradation of the extracellular matrix (ECM). MMPs play critical roles in tissue remodeling in the normal physiological process. In addition, MMPs have been found to be involved in many pathological conditions such as inflammation and tumor progression (Birkedal-Hansen et al., 1993). MMPs are expressed in the brain and appear to be inducible under pathological conditions, with glial cells and invading inflammatory cells as their major source (Yong, 2005). On the basis of domain structure, MMPs have been classified into collagenases, gelatinases, stromelysins, and MT-MMPs, among which MMP2 (gelatinase A) and MMP9 (gelatinase B) have been most intensively studied because of their prominent role in tissue damage and repair and the ease of their identification by gelatin zymography (Rosenberg, 2009). There is increasing evidence for a link between the activation of MMPs and Alzheimer’s disease (AD) pathogenesis. Interestingly, both beneficial and detrimental actions of MMPs have been suggested in AD. Deposition of improperly processed amyloid β (Aβ) peptides has been suggested to be the main causative factor for AD. In vitro studies have demonstrated that both MMP2 and MMP9 could degrade Aβ (Backstrom et al., 1996; White et al., 2006) (Yin et al., 2006). Consistently, significant increases of Aβ have been found in the brains of MMP2 or MMP9 knockout mice (Yan et al., 2006). Therefore, it is believed that both MMP2 and MMP9 play important roles in the extracellular Aβ catabolism and clearance. On the other hand, activation of MMPs could lead to damage of the blood brain barrier, hence promoting neurodegeneration in AD (Zlokovic, 2005). In transgenic mouse models of AD, a strong association between MMP activation, oxidative stress, and cerebral amyloid angiopathy has been found (Garcia-Alloza et al., 2009).
The paradoxical actions of MMPs on AD might be the consequence of neuroinflammation given the well established actions of MMPs in neuroinflammation (Mun-Bryce et al., 2002). There is mounting evidence that neuroinflammation plays active roles in both onset and progression of AD. Astrocytes are the most abundant glial cell population in the CNS and are critical for the maintenance of CNS homeostasis. Astrocytes respond to all forms of CNS insults through a process referred to as reactive astrogliosis, which has been involved in almost all neurological diseases (Sofroniew and Vinters). Along with microglia, astrocytes are directly involved in the neuroinflammatory process of AD (Heneka and O’Banion, 2007). Mouse astrocytes have been shown to be able to degrade Aβ in vitro and in situ (Wyss-Coray et al., 2003). Furthermore, MMPs have been found to contribute to Abeta catabolism (Liao and Van Nostrand, ; Yin et al., 2006). In primary astrocyte cultures, dissimilar actions of fresh Aβ (1-40) and Aβ (1-42) on the activation of MMP2 and MMP9 have been demonstrated (Deb et al., 2003). It has been suggested that soluble oligomeric Aβ may have more critical functions in AD compared with monomeric Aβ peptides or insoluble fibril deposits (Haass and Selkoe, 2007). In the present study, we determined the action of oligomeric Aβ on MMP2 expression and activation using primary astrocyte cultures. In addition, the expression of MMP2 was assessed in a transgenic mouse model of AD.
2. RESULTS
2.1. Oligomeric Aβ downregulates MMP2 expression and activation in primary astrocytes
Synthetic amyloidogenic proteins have been widely used to study the structure, assembly, and physiological effects of both oligomeric and fibrillar forms of these proteins. However, conflicting results could arise due to the difference in the preparing of these proteins. In order to evaluate the oligomeric Aβ preparation, we analyzed the fresh Aβ and oligomeric Aβ with immunoblotting, using an Aβ specific antibody (6E10). We found that most of Aβ (1-42) exists as monomers or dimers in fresh Aβ preparations. In oligomeric Aβ preparation, high molecular weight Aβ oligomers are the predominant form. On the other hand, both fresh Aβ (1-40) and the similarly oligomeric prepared Aβ (1-40) mainly exist as monomers (Figure 1). This is consistent with a previous report (Jarrett et al., 1993) that Aβ (1-42) has much higher tendency to form large oligomers as compared with Aβ (1-40).
Figure 1.

A representative Western blot of oligomeric and fresh preparations of Aβ (1-42) and Aβ (1-40). Aβ was detected with antibody 6E10.
We treated rat primary astrocytes with Aβ (1-42) oligomeric preparation for 24 hours, and the conditioned medium was collected and evaluated for MMPs’ activities by gelatin zymography. We found that the MMP2 activity in the conditioned medium was significantly decreased after 20 μM oligomeric Aβ treatment (P < 0.05) (Figure 2A, 2B). A very low level of MMP9 activity was detected in the primary astrocyte cultures (Figure 2A, 2E), which is consistent with previous reports that astrocytes do not express a detectable level of MMP9 (Crocker et al., 2008; Muir et al., 2002). We further examined whether oligomeric Aβ affects MMP2 expression in astrocytes. Primary astrocytes were treated with increasing concentrations of oligomeric Aβ preparations and the cells were collected for analysis of MMP2 mRNA levels by real-time PCR. Consistent with the inhibitory action of oligomeric Aβ on MMP2 activation, expression of MMP2 was also decreased upon treatment of 20 μM oligomeric Aβ (Figure 2C). To further determine whether the reduction of MMP2 expression and activation is due to the cytotoxic effect of oligomic Aβ, an LDH assay was conducted in the same set of cultures. No significant increase of cell death was found in astrocyte cultures upon the treatment of oligomeric Aβ (Figure 2D). These data suggest that down-regulation of MMP2 expression in astrocytes by oligomeric Aβ is not due to the potential cytotoxic action of Aβ oligomers. Most importantly, the inhibitory action of Aβ (1-42) oligomeric preparation on MMP2 expression was not seen in either Aβ (1-40) or reverse peptide Aβ (42-1), which has much less or no propensity to form soluble oligomers compared with Aβ (1-42) (Figure 2E).
Figure 2.
Oligomeric Aβ treatment down-regulates MMP2 activity in primary rat astrocytes. (A) Gelatin zymography shows MMP2 activity in astrocyte conditioned medium treated with oligomeric Aβ at indicated concentrations (hMMP2, human MMP2 standard). (B) Relative quantification of MMP2 activity in gelatin zymography (n=4 trials). (C) Real-time PCR showing MMP2 mRNA levels after oligomeric Aβ or control treatment (n=3). (D) LDH assay of the culture medium shows no significant cell toxicity induced by oligomeric Aβ in astrocyte cultures (n=3). (E) Gelatin zymography shows MMP2 activity after treatment with 20 μM oligomeric Aβ (1-42), Aβ (1-40), and reversed peptide Aβ (42-1) as compared with DMSO control (left lane).
2.2. Clearance of oligomeric Aβ by astrocytes
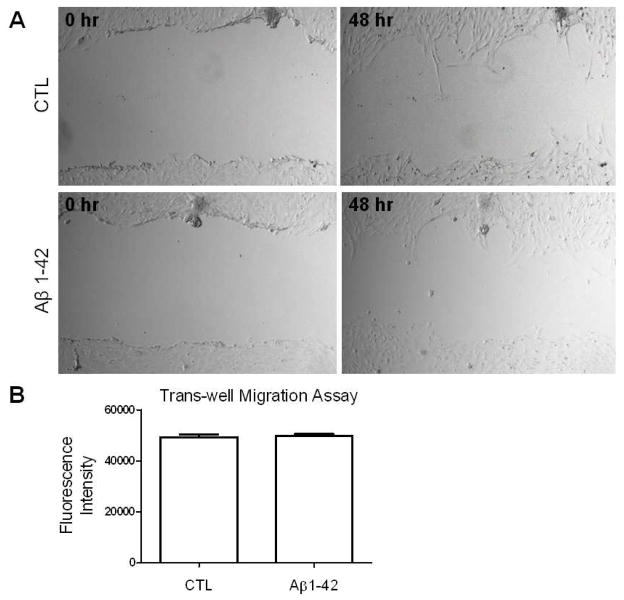
We evaluated Aβ catabolism in the astrocyte-conditioned medium. Oligomeric Aβ (1-42) or Aβ (1-40) were incubated with or without astrocyte culture. Conditioned media (10 μl) was analyzed by Western blots using antibody 6E10. We found a dramatic decrease of Aβ in the media of primary astrocyte cultures as compared to those without astrocyte cultures (Figure 3A). Consistently, an increase of Aβ in the astrocyte lysate was observed by Western blots (20 μg of total protein was loaded) (Figure 3B). We determined the effect of oligomeric Aβ preparations on the migration of primary astrocytes. No significant change in migration of the astrocytes was observed upon treatment of oligomeric Aβ using both the scratch migration assay (Figure 4A) and the trans-well migration assay (Figure 4B).
Figure 3.
Decrease of Aβ level in primary astrocytes-conditioned medium. (A) Oligomeric Aβ (1-42) and Aβ (1-40) were incubated in a 24-well plate with (astrocyte conditioned) or without primary astrocytes (control). Equal amount of medium (10 μl) was analyzed by Western blot using antibody 6E10. (B) A representative Western blot of Aβ (6E10 antibody) in astrocytes lysate (20 μg) after incubation with oligomeric Aβ (1-42) at indicated concentrations.
Figure 4.
Oligomeric Aβ has no obvious effect in primary astrocyte migration. (A) Representative images of the scratch assay of primary astrocytes treated with oligomeric Aβ (1-42) and control. (B) Quantitative analysis of the effect of oligomeric Aβ in astrocyte migration using trans-well migration assay.
2.3. Increased expression of MMP2 and proinflammatory cytokines in APP/PS1 transgenic mice brains
We determined the MMP2 expression in 4 month old APP/PS1 transgenic mouse brains. The immunohistochemistry demonstrated an increase of MMP2 positive cells with astrocyte morphology surrounding the senile plaques (Figure 5A). Consistently, the MMP2 positive cells in the brains of APP/PS1 mice were positive for the astrocyte marker, GFAP (Figure 5B). The mRNA levels of MMP2 in the brains of APP/PS1, PS1 transgenic mice and wild type litter mates were determined by real-time PCR. We found that mRNA levels of MMP2 were significantly increased in the brain of APP/PS1 transgenic mice as compared with that from wild type litter mates (Figure 6A). We evaluated the mRNA levels of MMPs stimulating cytokines (IL4, IL6, TNFα, IL-1β and TGF-β) in the brains of APP/PS1, PS1, and wild type mice by real-time PCR. We observed that the mRNA levels of IL-1β and TGF-β were significantly increased in the brains of APP/PS1 mice (Figure 6B, 6C). No significant change of IL4, IL6 or TNF-α was found.
Figure 5.
Increased expression of MMP2 surrounding senile plaques in APP/PS1 transgenic mice brains. (A) Microscopy of representative immunohistochemistry (DAB staining) of MMP2 in APP/PS1 mice brains. Left panel: 5x objective. Middle panel: 40x objective. Right panel: counter-staining of Thio-S. Arrow indicates the senile plaques. (B) Representative microscopy demonstrates the colocalization of MMP2 (green, left panel) and GFAP (red, middle panel) in APP/PS1 mice brains. Nucleus were counter-stained with DAPI (blue). Right panel: merge image of MMP2, GFAP and DAPI.
Figure 6.
Quantitative analysis of expression of MMP2 and proinflammatory cytokines IL-1β and TGFβ in APP/PS1 transgenic mice brains using real-time PCR.
3. DISCUSSION
Senile amyloid plaque has long been recognized as one of the hallmarks for AD. However, contentious debate has been raised that the extent of amyloid plaque accumulation does not correlate well with AD pathogenesis. It has been suggested that soluble oligomeric Aβ correlates better with dementia than insoluble fibrillar deposits, suggesting that oligomeric Aβ may represent the primary toxic species in AD (Haass and Selkoe, 2007). Soluble oligomers of Aβ could inhibit hippocampal long term potentiation and alter memory function, which has not been found upon the treatment of Aβ monomers, protofibrils and fibrils (Walsh et al., 2002). Differential effects of oligomeric and fibrillar Aβ (1-42) on astrocyte-mediated inflammation have been demonstrated (White et al., 2005). The previous study has found that fresh Aβ (1-40) could induce activation of both MMP2 and MMP9 in astrocytes. On the other hand, fresh prepared Aβ (1-42) only induced MMP9 activation, but not MMP2 (Deb et al., 2003). Our study suggests a different action of oligomeric Aβ on MMP2 expression. In the present study, we demonstrated that oligomeric Aβ directly inhibits MMP2 activation in primary astrocytes through its inhibition of MMP2 expression. In contrast, a very low level of active MMP9 was observed in primary astrocyte cultures and no significant effect of oligomeric Aβ on MMP9 activation was identified. Previous studies have demonstrated that astrocytes and microglia express distinct sets of MMPs with astrocytes as the major source of MMP2 (Crocker et al., 2008; Muir et al., 2002). In mouse brains, MMP2 mRNA is four- to six-fold higher than that of MMP9. Consistently, MMP2 gelatinolytic activity is significantly higher than that of MMP9. In addition, MMP2 deletion resulted in a greater Aβ accumulation compared with MMP9 knockout (Yin et al., 2006). Therefore, our finding is in agreement with that of MMP2 as the major MMP released by astrocytes and involved in extracellular Aβ catabolism.
The inhibitory action of oligomeric Aβ on MMP2 expression was not due to the cytotoxic effect of Aβ in astrocytes. It has been shown that apoptosis and cell death could be induced by 20 μM Aβ in primary neurons or mixed neuronal cultures. On the other hand, primary astrocytes are much more resistant to Aβ treatment. No obvious cell death was detected in our experiment. A similar result has been found in the previous publications, in which no obvious cell toxicity in astrocyte cultures was observed with Aβ treatment at concentrations up to 30 μM (Malchiodi-Albedi et al., 2001; Pike et al., 1993). We observed some minor changes in morphology of the astrocytes after Aβ treatment, which might be due to the astrocyte activation.
The soluble Aβ oligomers prepared in the current study are Aβ assemblies which are not pelleted from medium by high-speed centrifugation. Similar to the previous study, Western blots following SDS-PAGE indicated that this soluble oligomeric Aβ preparation is mainly comprised of large oligomers (Cerf et al., 2009). It is believed that only Aβ (1-42) peptide has a strong propensity to oligomerize in vivo (Haass and Selkoe, 2007). Consistently, oligomeric Aβ was only found in Aβ (1-42) preparations, but not in Aβ (1-40). Therefore, we predict that the large Aβ oligomers confer the inhibitory action on MMP2 expression and activation in primary astrocyte cultures. In addition, the action of oligomeric Aβ on MMP2 is independent to the potential cytotoxicity of Aβ oligomers as no cell death was found upon the treatment of Aβ oligomers.
Astrocytes play vital roles in the maintenance of CNS homeostasis. In addition, astrocytes exert migratory activity, phagocytic and proteolytic capacities, hence enabling them to internalize and degrade Aβ (Koistinaho et al., 2004; Pihlaja et al., 2008). Several Aβ-degrading proteases have been found, including tissue plasminogen activator (tPA) (Van Nostrand and Porter, 1999), angiotensin-converting enzyme (ACE) (Hu et al., 2001; Oba et al., 2005), neprilysin (NEP) (Iwata et al., 2001), insulin-degrading enzyme (IDE) (Bennett et al., 2000), MMP9 and MMP2 (Backstrom et al., 1996; White et al., 2006; Yan et al., 2006; Yin et al., 2006). In the current study, the reduction of oligomeric Aβ in the astrocyte conditioned medium is correlated with an increase of Aβ in the astrocytes, supporting that astrocytes perform important roles in the catabolism of oligomeric Aβ. Down-regulation of MMP2 expression and activation induced by oligomeric Aβ might contribute to accumulation of Aβ and formation of extracellular senile plaques in AD.
Inhibitory action of Aβ peptide on chemoattractant-induced astrocyte migration has been identified previously. Adult mouse astrocytes secrete chemoattractant protein-1 (MCP-1)-induced migration upon interaction with immobilized Aβ (1-42) (Wyss-Coray et al., 2003). Since astrocytes do not release MCP-1 in response to Aβ stimulation, it is predicted that microglia-derived MCP-1 contributes to the migration of astrocytes toward the Aβ plaques in AD (Wyss-Coray et al., 2003). Consistently, no significant effect of oligomeric Aβ in astrocytes’ migration was observed in the current study.
Contradictory to our in vitro finding, we found an increase of MMP2-expressing cells surrounding amyloid plaques in APP/PS1 mouse brains, which are positive for astrocyte marker. Given the complicated micro-environment in vivo that involves multiple cell types, it might not be a surprise that the in vitro result does not match the final outcome in vivo. We speculated that the different findings between in vivo and in vitro were due to the proinflammatory cytokines released by microglia. Microglia are the major resident immune cells in the brain which produce inflammatory factors in response to pathogen invasion or tissue damage. Elevated levels of cytokines have been found in post-mortem AD brains (Akiyama et al., 2000). Microglia activation and astrogliosis surrounding senile plaques have been found to be the major inflammatory responses in AD (Glass et al.). Studies have indicated that microglia are the major source of proinflammatory factors. Aggregates of Aβ have been shown to activate microglia and induce the production of many proinflammatory cytokines and chemokines that promote neuronal death (Kitazawa et al., 2004). Expression of proinflammatory cytokines has been observed in microglia surrounding plaques (Cartier et al., 2005). Consistently, we detected increased mRNA levels of cytokines IL1β and TGF-β in APP/PS1 mouse brains, which are potent MMP stimulating factors. Therefore, the discrepancy of the oligomeric Aβ’s effect on MMP2 expression in astrocytes between the in vitro and in vivo studies could be due to the dominant effect of proinflammatory cytokines released by microglia surrounding amyloid plaques.
In summary, our study suggests a complex regulation of the MMP2 expression and activation in astrocytes by oligomeric Aβ. While oligomeric Aβ directly down-regulates MMP2 expression and activation in astrocytes, it also induces production of proinflammatory cytokines, mainly in microglia, which serve as strong stimulators for MMP2. Therefore, the ultimate outcome of the oligomeric Aβ on MMP2 activation in astrocytes might be the combination of its direct inhibitory action on MMP2 and the secondary action of inflammatory cytokines induced by oligomeric Aβ.
4. EXPERIMENTAL PROCEDURE
4.1. Preparation of Aβ peptides
Aβ (1-42), (1-40), (42-1) peptides were purchased from AnaSpec. Aβ peptides were dissolved in DMSO at the concentration of 2 mM and stored at −80 °C. For the treatment of freshly prepared Aβ, peptides were diluted in DMEM to the indicated final concentrations and added to the astrocyte cultures. For oligomeric Aβ treatment, peptides were diluted in DMEM medium (no FBS) at the indicated concentrations and incubated at 37 °C for 24 hours. Then, the solution was centrifuged at 14,000 rpm for 5 minutes and the supernatant was collected as oligomeric Aβ to treat the astrocyte cultures.
4.2. Experimental animals and primary astrocyte culture
Sprague-Dawley rats were purchased from Charles River Laboratories, Inc. (Wilmington, MA). Hemizygous APP/PS1 (5XFAD, Jackson Laboratory) mice, PS1 mice (Duff et al., 1996), and wild type litter mates were maintained ad libitum at the animal facility. For breeding, each hemizygous transgenic mouse was bred with a wild type litter mate and the pups were genotyped by PCR as described in the vendor protocol. All animal procedures were approved by the University of North Texas Health Science Center Animal Care and Use Committee.
Primary astrocyte cultures were prepared as described previously (Xie et al.). Pups were sacrificed on postnatal day 1. The brains were harvested and the meninges and blood vessels were peeled off the parenchyma. Cortices were dissected out in a sterile 10 cm petri dish containing 5 ml phenol red-free DMEM (GIBCO) supplemented with 100 μg/ml penicillin and streptomycin. Then, cortex pieces were centrifuged at 2200 rpm for 5 minutes and supernatant was discarded. The pellet was resuspened in an equal volume of 0.25% trypsin-EDTA and incubated at 37 °C for 20 minutes with gentle agitation every 5 minutes. After digestion, tissues were spun down and the supernatant was discarded. Tissues were resuspended in an equal volume of DMEM. Cells were dissociated by pipetting up and down through fire-polished Pasteur pipettes. After dissociation, cell suspension was filtered through a 40 μm cell strainer into DMEM medium and cell number was acquired using a hemocytometer. About 10×106 cells were seeded into 400 ml flasks containing DMEM (with 10% FBS, 100 μg/ml penicillin and streptomycin). Cells were incubated for 10~14 days in a moist incubator with 5% CO2 at 37°C until cultures were confluent. Cell cultures were shaken for 6 hours at 250 rpm in the incubator (5% CO2, 37°C) to remove microglia. Adhesive astrocytes cultures were maintained and used for the experiments from passage 2 to 3.
4.3. Gelatin Zymography
Before treatment, primary astrocytes were switched to DMEM medium without FBS for 12 hours. Then, astrocytes were treated with oligomeric Aβ preparations or control for 24 hours. Cell toxicity was determined by LDH assay following manufacture’s protocol (Promega). After treatment, conditioned medium was centrifuged at 5,000 rpm for 5 minutes to get rid of cells and debris. Supernatant was collected for gelatin zymography as described previously (Liu et al.). Equal volume of sample was run at non-reducing conditions on a 10% polyacrylamide gel containing 0.1% gelatin. After electrophoresis, gels were incubated in incubation buffer (2.5% Triton X-100, 5 mMCaCl2, 1μM ZnCl2) at 37 °C overnight with gentle agitation. Then, the gels were stained with Coomassie Blue solution (0.25% Coomassie Blue, 45% MeOH, and 10% acetic acid) for 2 hours and then destained with destain solution (30% MeOH and 10% acetic acid). After the bands became clear, the gels were dried using Promega gel drying film and scanned for quantitative analysis using Image J.
4.4. Cell migration assay
The scratch assay was conducted by scratching 100% confluent rat primary astrocytes in 96-well plates cultured in DMEM (no FBS). Images of the scratch lines were obtained at 0, 24 and 48 hours after scratching for analysis. Corning 96-trans-well plates (8 μm diameter) were used for trans-well migration assay. Primary astrocytes were labeled by Calcein AM (5 ug/ml) for 15 minutes and loaded onto the 96-well trans-well insert containing DMEM (no FBS) with either oligomeric Aβ or DMSO control. The bottom well was loaded with DMEM supplemented with 10% FBS. At 24 hours after culture, cells migrated to the other side of the membrane were trypsinized and then the fluorescence of the cells was analyzed by a TECAN Infinite M200 microplate reader.
4.5. Immunohistochemistry and real-time PCR
Paraffin-embedded slides of APP/PS1 mouse brains were deparaffinized in xylene for 10 minutes, and subsequently hydrated in 100%, 95% and 70% ethanol for 10 minute each. Slides were heated in sodium citrate buffer (10 mM Citric Acid, 0.05% Tween 20, pH 6.0) for 30 minutes. After 1-hour blocking using 5% goat serum in superblock buffer (Thermo Scientific), slides were incubated with primary antibody overnight (anti-MMP2, Calbiochem, 1:100 dilution; anti-GFAP, Santa Cruz, 1: 200 dilution). After washing 3 times with PBS (with 0.05% Tween-20), HRP conjugated secondary antibody or Alexa Fluor conjugated secondary antibody (Invitrogen) were used for standard DAB staining and fluorescent staining respectively. Amyloid plaque was stained with Thio-S.
Quantitative real-time PCR was carried out using SYBR Green PCR Master Mix (Promega) and 7300 Real-Time PCR System from Applied Biosystems. Two-step real-time PCR protocol was used (95 °C for 15 sec, 60 °C for 60 sec extension and detection, 40 cycles). Sequences of primers for real-time PCR are listed as following:
IL-1β: Forward 5′ GGTGTGTGACGTTCCCATTAGA 3′
Reverse 5′ TCGTTGCTTGGTTCTCCTTGTA 3′
IL-6: Forward 5′ GACTTCCATCGAGTTGCCTTCT 3′
Reverse 5′ TTGGGAGTGGTATCCTCTGTGA 3′
TNFα: Forward 5′GCCTCTTCTCATTCCTGCTTGT 3′
Reverse 5′ CAGGCTTGTCACTCGAATTTTG 3′
TGFβ: Forward 5′ TTGCTTCAGCTCCACAGAGAAG 3′
Reverse 5′ CCAGACAGAAGTTGGCATGGTA 3′
Mouse MMP2: Forward 5′ CAGGACTCTCACAAGGTCGG 3′
Reverse 5′ TGACTGTGACCATGACCGGG 3′
Rat MMP2: Forward 5′ GATCCGTGGTGAGATCTTCTTC 3′
Reverse 5′ AGAACACAGCCTTCTCTTCCTG 3′
4.5. Statistical Analysis
All values were expressed as mean ± standard error of mean (SEM). MMP2 activity, LDH assay and real time PCR data were analyzed by one-way ANOVA. When a significant difference was detected by ANOVA, a post hoc Tukey’s test was performed to identify a specific difference between groups. Transwell assay was analyzed by student t test.
Acknowledgments
Sources of funding
This project was supported in part by NIH grants R01NS054687 (SY), R01NS054651 (SY).
Abbreviations
- Aβ
beta amyloid
- MMP
matrix metalloproteinase
- AD
Alzheimer’s disease
- ECM
extra cellular matrix
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backstrom JR, Lim GP, Cullen MJ, Tokes ZA. Matrix metalloproteinase-9 (MMP-9) is synthesized in neurons of the human hippocampus and is capable of degrading the amyloid-beta peptide (1-40) J Neurosci. 1996;16:7910–9. doi: 10.1523/JNEUROSCI.16-24-07910.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett RG, Duckworth WC, Hamel FG. Degradation of amylin by insulin-degrading enzyme. J Biol Chem. 2000;275:36621–5. doi: 10.1074/jbc.M006170200. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- Cartier L, Hartley O, Dubois-Dauphin M, Krause KH. Chemokine receptors in the central nervous system: role in brain inflammation and neurodegenerative diseases. Brain Res Brain Res Rev. 2005;48:16–42. doi: 10.1016/j.brainresrev.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Cerf E, Sarroukh R, Tamamizu-Kato S, Breydo L, Derclaye S, Dufrene YF, Narayanaswami V, Goormaghtigh E, Ruysschaert JM, Raussens V. Antiparallel beta-sheet: a signature structure of the oligomeric amyloid beta-peptide. Biochem J. 2009;421:415–23. doi: 10.1042/BJ20090379. [DOI] [PubMed] [Google Scholar]
- Crocker SJ, Frausto RF, Whitton JL, Milner R. A novel method to establish microglia-free astrocyte cultures: comparison of matrix metalloproteinase expression profiles in pure cultures of astrocytes and microglia. Glia. 2008;56:1187–98. doi: 10.1002/glia.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb S, Wenjun Zhang J, Gottschall PE. Beta-amyloid induces the production of active, matrix-degrading proteases in cultured rat astrocytes. Brain Res. 2003;970:205–13. doi: 10.1016/s0006-8993(03)02344-8. [DOI] [PubMed] [Google Scholar]
- Duff K, Eckman C, Zehr C, Yu X, Prada CM, Perez-tur J, Hutton M, Buee L, Harigaya Y, Yager D, Morgan D, Gordon MN, Holcomb L, Refolo L, Zenk B, Hardy J, Younkin S. Increased amyloid-beta42(43) in brains of mice expressing mutant presenilin 1. Nature. 1996;383:710–3. doi: 10.1038/383710a0. [DOI] [PubMed] [Google Scholar]
- Garcia-Alloza M, Prada C, Lattarulo C, Fine S, Borrelli LA, Betensky R, Greenberg SM, Frosch MP, Bacskai BJ. Matrix metalloproteinase inhibition reduces oxidative stress associated with cerebral amyloid angiopathy in vivo in transgenic mice. J Neurochem. 2009;109:1636–47. doi: 10.1111/j.1471-4159.2009.06096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 140:918–34. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–12. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- Heneka MT, O’Banion MK. Inflammatory processes in Alzheimer’s disease. J Neuroimmunol. 2007;184:69–91. doi: 10.1016/j.jneuroim.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Hu J, Igarashi A, Kamata M, Nakagawa H. Angiotensin-converting enzyme degrades Alzheimer amyloid beta-peptide (A beta ); retards A beta aggregation, deposition, fibril formation; and inhibits cytotoxicity. J Biol Chem. 2001;276:47863–8. doi: 10.1074/jbc.M104068200. [DOI] [PubMed] [Google Scholar]
- Iwata N, Tsubuki S, Takaki Y, Shirotani K, Lu B, Gerard NP, Gerard C, Hama E, Lee HJ, Saido TC. Metabolic regulation of brain Abeta by neprilysin. Science. 2001;292:1550–2. doi: 10.1126/science.1059946. [DOI] [PubMed] [Google Scholar]
- Jarrett JT, Berger EP, Lansbury PT., Jr The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer’s disease. Biochemistry. 1993;32:4693–7. doi: 10.1021/bi00069a001. [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Yamasaki TR, LaFerla FM. Microglia as a potential bridge between the amyloid beta-peptide and tau. Ann N Y Acad Sci. 2004;1035:85–103. doi: 10.1196/annals.1332.006. [DOI] [PubMed] [Google Scholar]
- Koistinaho M, Lin S, Wu X, Esterman M, Koger D, Hanson J, Higgs R, Liu F, Malkani S, Bales KR, Paul SM. Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-beta peptides. Nat Med. 2004;10:719–26. doi: 10.1038/nm1058. [DOI] [PubMed] [Google Scholar]
- Liao MC, Van Nostrand WE. Degradation of soluble and fibrillar amyloid beta-protein by matrix metalloproteinase (MT1-MMP) in vitro. Biochemistry. 49:1127–36. doi: 10.1021/bi901994d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Liu Q, He S, Simpkins JW, Yang SH. Combination therapy of 17beta-estradiol and recombinant tissue plasminogen activator for experimental ischemic stroke. J Pharmacol Exp Ther. 332:1006–12. doi: 10.1124/jpet.109.160937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malchiodi-Albedi F, Domenici MR, Paradisi S, Bernardo A, Ajmone-Cat MA, Minghetti L. Astrocytes contribute to neuronal impairment in beta A toxicity increasing apoptosis in rat hippocampal neurons. Glia. 2001;34:68–72. doi: 10.1002/glia.1041. [DOI] [PubMed] [Google Scholar]
- Muir EM, Adcock KH, Morgenstern DA, Clayton R, von Stillfried N, Rhodes K, Ellis C, Fawcett JW, Rogers JH. Matrix metalloproteases and their inhibitors are produced by overlapping populations of activated astrocytes. Brain Res Mol Brain Res. 2002;100:103–17. doi: 10.1016/s0169-328x(02)00132-8. [DOI] [PubMed] [Google Scholar]
- Mun-Bryce S, Lukes A, Wallace J, Lukes-Marx M, Rosenberg GA. Stromelysin-1 and gelatinase A are upregulated before TNF-alpha in LPS-stimulated neuroinflammation. Brain Res. 2002;933:42–9. doi: 10.1016/s0006-8993(02)02303-x. [DOI] [PubMed] [Google Scholar]
- Oba R, Igarashi A, Kamata M, Nagata K, Takano S, Nakagawa H. The N-terminal active centre of human angiotensin-converting enzyme degrades Alzheimer amyloid beta-peptide. Eur J Neurosci. 2005;21:733–40. doi: 10.1111/j.1460-9568.2005.03912.x. [DOI] [PubMed] [Google Scholar]
- Pihlaja R, Koistinaho J, Malm T, Sikkila H, Vainio S, Koistinaho M. Transplanted astrocytes internalize deposited beta-amyloid peptides in a transgenic mouse model of Alzheimer’s disease. Glia. 2008;56:154–63. doi: 10.1002/glia.20599. [DOI] [PubMed] [Google Scholar]
- Pike CJ, Burdick D, Walencewicz AJ, Glabe CG, Cotman CW. Neurodegeneration induced by beta-amyloid peptides in vitro: the role of peptide assembly state. J Neurosci. 1993;13:1676–87. doi: 10.1523/JNEUROSCI.13-04-01676.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg GA. Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol. 2009;8:205–16. doi: 10.1016/S1474-4422(09)70016-X. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nostrand WE, Porter M. Plasmin cleavage of the amyloid beta-protein: alteration of secondary structure and stimulation of tissue plasminogen activator activity. Biochemistry. 1999;38:11570–6. doi: 10.1021/bi990610f. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–9. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- White AR, Du T, Laughton KM, Volitakis I, Sharples RA, Xilinas ME, Hoke DE, Holsinger RM, Evin G, Cherny RA, Hill AF, Barnham KJ, Li QX, Bush AI, Masters CL. Degradation of the Alzheimer disease amyloid beta-peptide by metal-dependent up-regulation of metalloprotease activity. J Biol Chem. 2006;281:17670–80. doi: 10.1074/jbc.M602487200. [DOI] [PubMed] [Google Scholar]
- White JA, Manelli AM, Holmberg KH, Van Eldik LJ, Ladu MJ. Differential effects of oligomeric and fibrillar amyloid-beta 1-42 on astrocyte-mediated inflammation. Neurobiol Dis. 2005;18:459–65. doi: 10.1016/j.nbd.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Loike JD, Brionne TC, Lu E, Anankov R, Yan F, Silverstein SC, Husemann J. Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat Med. 2003;9:453–7. doi: 10.1038/nm838. [DOI] [PubMed] [Google Scholar]
- Xie L, Poteet EC, Li W, Scott AE, Liu R, Wen Y, Ghorpade A, Simpkins JW, Yang SH. Modulation of polymorphonuclear neutrophil functions by astrocytes. J Neuroinflammation. 7:53. doi: 10.1186/1742-2094-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan P, Hu X, Song H, Yin K, Bateman RJ, Cirrito JR, Xiao Q, Hsu FF, Turk JW, Xu J, Hsu CY, Holtzman DM, Lee JM. Matrix metalloproteinase-9 degrades amyloid-beta fibrils in vitro and compact plaques in situ. J Biol Chem. 2006;281:24566–74. doi: 10.1074/jbc.M602440200. [DOI] [PubMed] [Google Scholar]
- Yin KJ, Cirrito JR, Yan P, Hu X, Xiao Q, Pan X, Bateman R, Song H, Hsu FF, Turk J, Xu J, Hsu CY, Mills JC, Holtzman DM, Lee JM. Matrix metalloproteinases expressed by astrocytes mediate extracellular amyloid-beta peptide catabolism. J Neurosci. 2006;26:10939–48. doi: 10.1523/JNEUROSCI.2085-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong VW. Metalloproteinases: mediators of pathology and regeneration in the CNS. Nat Rev Neurosci. 2005;6:931–44. doi: 10.1038/nrn1807. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci. 2005;28:202–8. doi: 10.1016/j.tins.2005.02.001. [DOI] [PubMed] [Google Scholar]