Abstract
We have previously described diminished uterine progesterone response and increased uterine sensitivity to inflammation in adult female mice with a history of developmental exposure to TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin). Since parturition in mammals is an inflammatory process mediated in part by a decline in progesterone action, toxicant-mediated disruption of progesterone receptor (PR) expression at the maternal-fetal interface would likely impact the timing of birth. Therefore, in the current study, we examined pregnancy outcomes in adult female mice with a similar in utero exposure to TCDD. We also examined the impact of in utero TCDD exposure of male mice on pregnancy outcomes in unexposed females since the placenta, a largely paternally derived organ, plays a major role in the timing of normal parturition via inflammatory signaling. Our studies indicate that developmental exposure of either parent to TCDD is associated with preterm birth in a subsequent adult pregnancy due to altered PR expression and placental inflammation.
Keywords: placenta, decidua, progesterone receptor, preterm birth, TCDD, mice, inflammation
1. Introduction
Preterm birth (PTB), occurring before 37 weeks gestation, is the leading cause of perinatal mortality and morbidity in industrialized nations [1,2]. Unfortunately, despite better nutrition and prenatal care, the incidence of PTB continues to increase and now affects nearly 13% of infants in the U.S. Although as many as one-third of premature births can be linked to the presence of infection (ie, bacterial vaginosis), the reason(s) for the remaining two-thirds is often unknown [3]. Nevertheless, numerous risk factors for PTB have been identified and include smoking, infection, advanced maternal or paternal age, maternal depression or African-American heritage [4]. Although smoking during pregnancy increases the risk of prematurity in all women, exposure to tobacco smoke more profoundly affects the PTB rate among black women compared to other races [5]. These data indicate that an individual’s genetic risk for PTB may be additionally impacted by gene-environment interactions that further influence the timing of parturition. However, it must also be considered that epigenetic changes, occurring during development and early prenatal life, may equally affect the onset of labor. Thus, while adult exposure to toxicants may adversely affect an existing pregnancy, one would anticipate that fetal exposure to maternal smoking might also increase the offspring’s risk for adverse pregnancy outcomes during their own adulthood. For example, cigarette smoke is a significant source of human exposure to dioxins, PCBs and other endocrine disrupting toxicants [6] and in utero exposure to these environmental chemicals has been associated with alterations in normal reproductive tract development of the exposed offspring, resulting in life-long changes in adult steroid responses [7,8]. Importantly, we have recently demonstrated that early life exposure to an endocrine disrupting toxicant may affect pregnancy outcomes for multiple generations, especially among individuals with additional risk factors [9].
Given the potential reproductive risks to humans associated with either adult or developmental toxicant exposure, scientists have begun to develop animal models that allow for both controlled toxicant exposure during pregnancy and the opportunity to examine reproductive events as the exposed animals become sexually mature. In our laboratory, we developed a murine model of TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin or dioxin) exposure during pregnancy in order to examine the impact of this toxicant on the capacity of adult female offspring to respond appropriately to the primary ovarian steroids that support gestation. Using this model, we initially demonstrated that developmental exposure to TCDD leads to a dose-dependent reduction in uterine progesterone receptor (PR) isotype expression [10] which was associated with a poor decidualization reaction during early pregnancy [11]. Importantly, our in vivo observations using this early life murine toxicant exposure model appear to mirror our published findings in cultures of human endometrial cells exposed to TCDD in vitro. Specifically, acute exposure of adult endometrial cells to TCDD in vitro was shown to decrease progesterone action via inhibition of PR isotype expression; thereby promoting the ability of proinflammatory cytokines to disrupt key elements of cellular differentiation [10,12,13]. Taken together, our murine and human studies demonstrate that exposure to TCDD results in a disruption of progesterone-mediated uterine differentiation that affects the expression of critical factors required for successful pregnancy [10,11].
In women, normal parturition is associated with a “functional” withdrawal of progesterone via a loss of PR expression in both the decidua [14,15] and placenta [16]. In contrast, parturition in rodents is preceded by a decline in progesterone synthesis [17] while decidual and placental PR expression persist [18,19]. Despite these differences, normal parturition in either species is an inflammatory event, preceded by a disruption in progesterone action at the placental-decidual interface [20]. Not surprisingly, impairment of progesterone action prior to the end of pregnancy has been associated with PTB in both women and mice (reviewed by [20]). During normal pregnancy, progesterone inhibits expression of inflammatory cytokines by immune cells; thereby suppressing cell-mediated immunity. As pregnancy progresses, there is a gradual reduction in progesterone dominance, eventually leading to an inflammatory cascade and parturition [21, 22]. Although the precise mechanisms controlling these events are not fully known, Su et al [23] recently demonstrated that the immunosuppressive effects of progesterone were related to the ability of this steroid to suppress expression of toll-like receptor-4 (TLR-4) via inhibition of NFkappaB (nuclear factor kappa-light-chain-enhancer of activated B cells) activity. Activation of TLR-4 is an important mechanism by which bacterial infection leads to pregnancy loss [24], in part due to activation of NF-kappaB [25,26] and a concomitant decrease in PR expression [27]. Activation of TLRs has also been proposed to play a role in normal parturition via interaction with endogenously produced ligands [28]; therefore, the balance between PR and TLR-4 expression may be an important determinant in the timing of parturition. As stated above, normal term parturition is largely due to activation of an inflammatory cascade (reviewed by [29]), possibly mediated by placental inflammation [30]. Multiple TLRs have been identified within the term human placenta [31–34] when levels of placental PR expression are minimal [16]. Important to the studies described herein, it has been established that in humans and mice the placenta is largely a paternally-derived organ since fertilization of an empty oocyte with two sperm leads to development of extraembryonic tissues, but no fetal development [35,36]. Given this background, it is appropriate to consider that premature placental inflammation, occurring as a consequence of paternal TCDD exposure, might also promote PTB.
In the current study, we examined the hypothesis that developmental toxicant exposure of either parent could negatively impact progesterone responsiveness at the maternal-fetal interface, leading to a loss of immunosuppression and spontaneous PTB. Although other studies have examined the impact of maternal TCDD exposure on placental development in an existing or imminent pregnancy [37–39], to our knowledge, the impact of in utero TCDD exposure of either parent on altered placental-decidual function in a subsequent adult pregnancy has not previously been described. As previously reported [9], we demonstrated that female mice which were exposed to TCDD during their own in utero development exhibit reduced fertility while mice that become pregnant frequently deliver preterm. Significantly, we also demonstrate that a history of developmental TCDD exposure of the male is an independent risk factor for PTB in his unexposed female partner. Our studies further indicate that early life exposure of either parent leads to decreased placental PR expression concomitant with an increase in expression of inflammatory genes that have been associated with the early onset of labor in mice.
2. Material and Methods
2.1. Animals
C57BL/6 mice were purchased from Harlan Spraque-Dawley Laboratories (Indianapolis, IN). Animals were housed in the Animal Care Facility (free of mouse pathogens including MPV and MNV) according to National Institutes of Health and institutional guidelines for laboratory animals. All animals received low phytoestrogen rodent chow (Picolab 5VO2, Purina TestDiets, Richmond, IN) and water ad libitum. Animal rooms were maintained at a temperature of 22–24°C and a relative humidity of 40–50% on a 12-hour light:dark schedule. Experiments described herein were approved by Vanderbilt University Institutional Animal Care and Use Committee in accordance with the Animal Welfare Act.
2.2. Chemicals
TCDD (99%) in nonane solution was obtained from Cambridge Isotope Laboratories (Andover, MA). All other chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
2.3. In utero TCDD Exposure
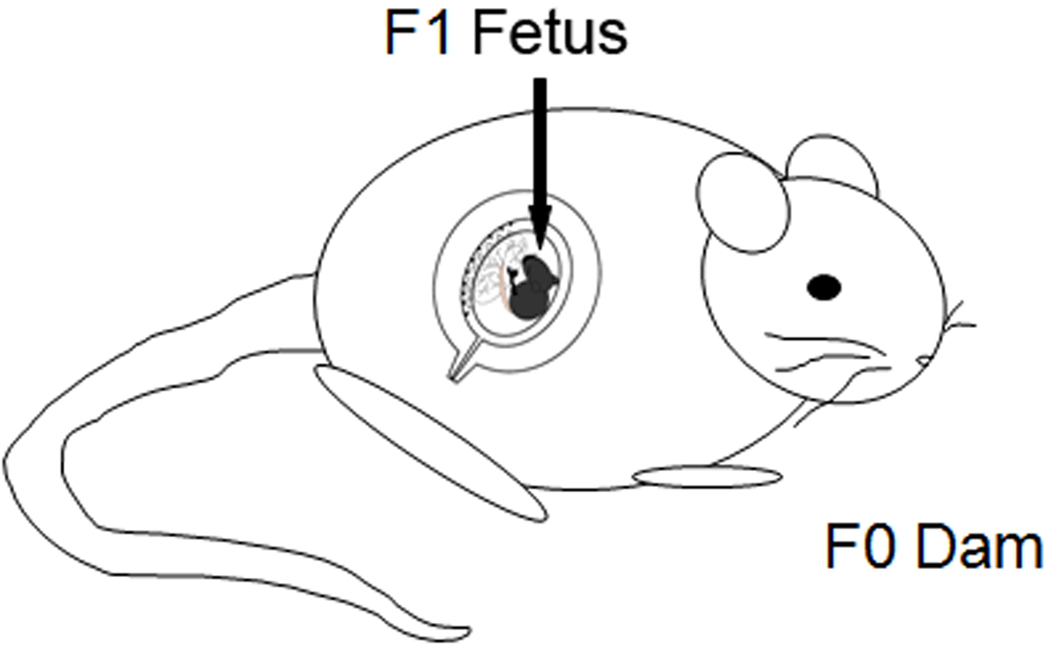
Virgin C57BL/6 females (N=10), aged 10–12 weeks, were mated with intact males of similar age. Upon observation of a vaginal plug, females were separated and termed day 0.5 of pregnancy (E0.5). Pregnant mice (F0) were exposed to TCDD (10µg/kg) or vehicle corn oil by gavage at 1100 hours CST on E15.5 (when organogenesis is complete) resulting in utero plus lactational exposure (i.e., perinatal exposure). Although this is considered a high dose of TCDD, our goal was to determine whether or not this compound could adversely affect fertility and pregnancy; examination of the toxicologic profile of TCDD was beyond the scope of this study. Importantly, the dose of TCDD used in these studies reflects the more rapid clearance of this toxicant in mice compared to humans and is well below the LD50 for adult mice of this strain (230µg/kg) [40]. TCDD given at this time and dose is not overtly teratogenic and gestation length was not affected in the F0 animals; pups (F1 mice) were typically born on E20. All studies described herein were conducted using unexposed control mice or mice which were exposed to TCDD during their own in utero development (F1 mice, Figure 1).
Figure 1.
Schematic depicting a pregnant mouse (dam) which was exposed to 10 ug/kg TCDD by gavage on gestation day 15.5 (E15.5). The exposed dam is considered the “F0”, or founding generation. Pups in utero at this time are denoted “F1” mice and, at adulthood, were utilized within the pregnancy outcomes studies described herein. Thus, F1 mice were exposed to TCDD during their own development (in utero and lactational), but received no further treatments.
2.4. Monitoring Pregnancy
A single female (control or F1 exposed) was placed with a single male (control or F1 exposed) and monitored for the presence of a vaginal plug (E0.5) each morning. Following the identification of a plug, the male was removed. Females were weighed prior to mating and again on E16.5. Females were monitored daily until delivery. Females in which a plug was observed, but which did not demonstrate signs of pregnancy (weight gain, nipple prominence) were mated with a different male 1 month after the identification of a vaginal plug (i.e., one week after parturition would have been expected). Mice which did not achieve pregnancy after 3 positive vaginal plugs were placed with a single, proven breeder male for four weeks and observed for pregnancy. Mice which were unable to become pregnant under these conditions were considered infertile. Similarly, males which produced 3 positive vaginal plugs, but no observable pregnancy, were considered infertile. Infertile male and female mice were not further analyzed in this study.
Parturition in C57BL/6 mice normally occurs 19.5 days after identification of a vaginal plug, with little variation if the timing of mating is accurate [41]. However, it is important to note that pregnancy nomenclature varies by laboratory; thus, within the literature, identification of the vaginal plug has been denoted E0, E0.5 or E1 with term pregnancy occurring on E19.5, E20 or E20.5, respectively. As stated above, for studies described herein, the day the vaginal plug was identified was considered E0.5 and parturition expected on E20. Preterm parturition in mice has been defined as spontaneous labor 12–24 hrs prior to term [42,43]. For our studies, we used a more stringent definition for PTB (spontaneous delivery more than 24 hrs prior to term). All mice were monitored twice daily for timing of delivery of the first pup.
2.5. Euthanasia and Collection of Tissues
Pregnant females were euthanized at 1400–1500 hours CST on either E18.5 or E19.5 and tissues collected. Since only a subset of TCDD exposed mice delivered preterm, all mice which were euthanized during pregnancy for assessment of placental-decidual tissues were also examined for signs of cervical ripening in order to determine the likelihood that a given mouse would have delivered early (data not shown). Only tissues from mice in the experimental groups which exhibited cervical ripening were evaluated in the current study. Notably, we also identified a single F1 female in active preterm labor on E17.5. This animal was immediately euthanized and placental-decidual tissues collected for qRT-PCR analysis as described below.
Pregnant females were weighed immediately prior to euthanasia by cervical dislocation under anesthesia. The ovaries, uterus and cervix were removed in toto and photographed. The fetuses were removed by making an incision into the mesometrial wall, opposite the placental unit. The umbilical cord was carefully cut, nearest the abdomen of the fetus, leaving it attached to the placenta. Pups were counted, weighed, and photographed. Half of the placentas were carefully dissected from the decidua then weighed. Of these, half were flash frozen and stored at −80°C, while the remainder was formalin fixed. The remaining placental-decidual units were left intact and formalin fixed. The excised uterine tissue was also divided in two and portions flash frozen for later use or formalin fixed for later histological analysis. Finally, the ovaries were removed then weighed. One was flash frozen and stored at −80°C and the other was formalin fixed, as was the cervix.
All formalin-fixed samples were stored at 4°C for 48 hours. At this time, placentas were cut in half, with the umbilical cord on one side. The cervix was also cut in half longitudinally. Samples were processed and subjected to paraffin-embedding by the Vanderbilt Histology Core Lab using standard methodology.
2.6. Histochemistry
All tissues were subjected to standard hematoxylin and eosin (H & E) staining. Placental-decidual samples were further subjected to Masson’s trichrome staining. These stains were performed by Vanderbilt’s Immunohistochemistry Core using standard methods.
All slides were viewed using an Olympus BX51 microscope system and images captured using an Olympus DP71 digital camera.
2.7 Quantitative RT-PCR analysis of Progesterone Receptor, Toll-Like Receptor-4 and p65/p105 mRNA expression
Total RNA was isolated from all frozen placental and decidual tissues with Trizol (Invitrogen, Carlsbad, CA) and purified using the RNeasy Mini Kit (Qiagen, Valencia, CA). cDNA from 1µg of total RNA was synthesized using the iScript cDNA Synthesis Kit (Bio-Rad) and random decamer primers. Reactions were performed in duplicate in a Bio-Rad CFX96 Real-time thermocycler system. As an internal control, the ribosomal protein, large, P0 (RPLP0) gene was used as an endogenous control. Results were evaluated using the delta-delta Ct method, where delta was calculated as (Target Ct)-(RPLP0 Ct), and the relative quantity of target gene expression was calculated by the delta-delta Ct as 2−[(experiment sample delta Ct)−(control sample delta Ct)].
Primers (forward and reserve) are listed in Table 1. The thermal cycling program applied on the CFX96 Real-time System was: 95°C for 30 s, 40 cycles of 95 °C for 5 s, 60 °C for 5 s, followed by a melting curve analysis to confirm product purity. Since both PR isoforms are transcribed from a single gene, primers were designed which amplify either PR-B or PR-A/B.
Table 1.
Primer sequences for RT-PCR
| Gene Name | Direction | Primer Sequence | Produce Size (bp) |
|---|---|---|---|
| p105 | FORWARD | AAATCCAACGCAGGGGTCAC | 168 |
| REVERSE | GGCGATGGGTTCCGTCTTG | ||
| p65 | FORWARD | CCAGACACAGATGATCGCCAC | 156 |
| REVERSE | GACAGAAGTTGAGTTTCGGGTAGG | ||
| RPLP0 | FORWARD | TGCCACACTCCATCATCAAT | 240 |
| REVERSE | CGAAGAGACCGAATCCCATA | ||
| TLR4 | FORWARD | GCCGGAAGGTTATTGTGGTA | 176 |
| REVERSE | AGGCGATACAATTCCACCTG | ||
| PR-A/B | FORWARD | CAGCTGTTGCTCCCTACCTC | 287 |
| REVERSE | GCAATGGGAGAGTCTTGCTC | ||
| PRB | FORWARD | CGGAGAAGGACAGCAGACTC | 122 |
| REVERSE | CCCAAAGAGACACCAGGAAG |
2.8 Statistical Analysis
Statistical analysis was performed using GraphPad Prism. All data are expressed as mean value ± SD. Statistical comparisons between two experimental groups were determined by Student’s t-test. A p value of less than 0.05 was considered statistically significant.
3. Results
3.1 Pregnancy Outcomes Following Parental Developmental Exposure to TCDD
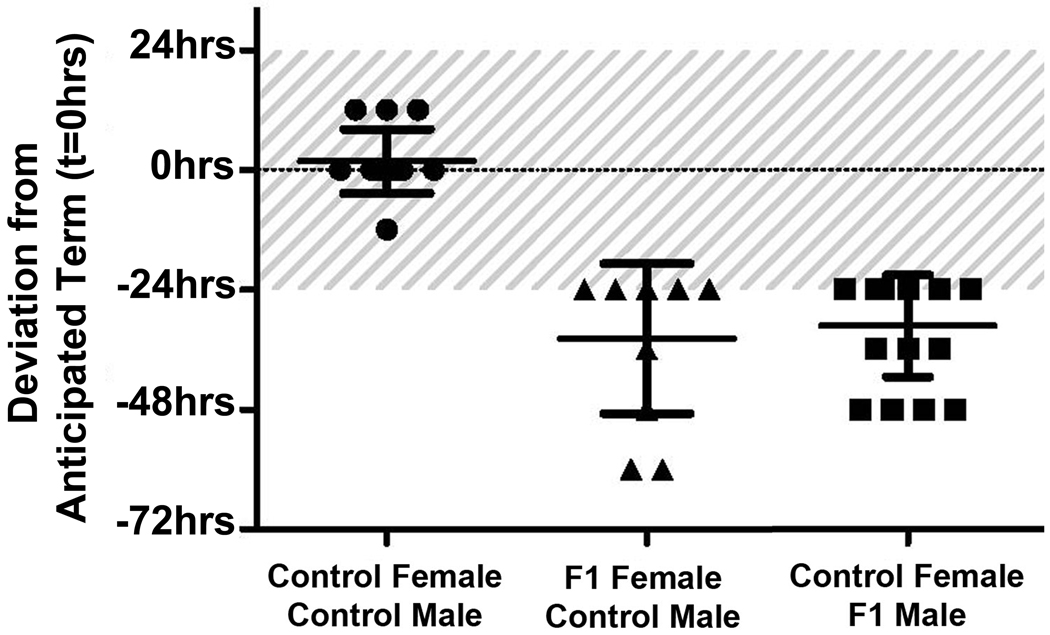
Adult C57BL/6 mice with (F1) or without (control) a history of developmental TCDD exposure were mated at 10–12 weeks of age. As shown in Table 2, all control female mice mated to control male mice achieved pregnancy and delivered at term. As expected, timing of birth in this group was minimally variable, with the majority of control mice delivering on E20. Only a single animal in this group delivered earlier, on E19.5, while three mice delivered on E20.5 (Figure 2). Female F1 mice, which were exposed to TCDD during their own in utero development, exhibited a reduced ability to become pregnant and frequently delivered >24 hours prior to term (Table 2). Interestingly, of the seven mice which delivered at term, all delivered between E19 and E19.5 and none delivered on E20 (Figure 2). Adult male F1 mice also exhibited reduced fertility, and perhaps even more important, conferred a risk of PTB to control female mice (Table 2). As with the F1 females, even among mice delivering at term, gestation length was reduced with all “term” mice delivering between E19 and E19.5 (Figure 2). Stated another way, the average gestational length of control female mice mated to control male mice was 20 days, but in either of the toxicant exposed groups, the average gestational length was only 18.5 days (or 92% of the normal gestational length, both with a p<0.001). No pregnancies were observed when F1 males were mated to F1 females, although vaginal plugs were frequently identified. It is important to note that we did not examine “infertile” animals for early pregnancy loss, a possibility which has been suggested in our other studies [11].
Table 2.
Impact of in utero TCDD Exposure on Reproductive Outcomes in Adult Mice1
| Mating Scheme | Pregnancy | Pregnancy Outcome | |
|---|---|---|---|
| Full-term | Pre-term | ||
| Control Female / Control Male | 15/15 (100%) | 15/15 | 0/15 (0%) |
| F1 Female2 / Control Male | 11/28 (39%) | 7/11 | 4/11 (36%) |
| Control Female / F1 Male3 | 18/38 (47%) | 11/18 | 7/18 (39%) |
| F1 Female2 / F1 Male3 | 0/10 (0%) | N/A | N/A |
Mice euthanized during pregnancy were excluded from table.
Pregnant mice (F0) were exposed to TCDD on E15.5. Female offspring (F1) were mated to control males or males similarly exposed to TCDD.
Pregnant mice (F0) were exposed to TCDD on E15.5. Male offspring (F1) were mated to control females or females similarly exposed to TCDD.
Figure 2.
Effect of in utero exposure to TCDD on gestation length in mice undergoing spontaneous labor and delivery. Anticipated time of delivery (ie, E20) was denoted t=0 and actual delivery times were plotted as hours deviating from that time. Each geometric symbol represents one group of mice. Control Female to Control Male (●; N=15), F1 Female to Control Male (▲, N=11), Control Female to F1 Male (■; N=18). Shaded area represents the acceptable variation in normal delivery time (±24 hrs) and which is also considered term. The horizontal lines indicate the average hours from t=0 for each group of mice.
3.2 Assessment of Placental-Decidual Samples and Fetal/Placental Weights
In order to begin to examine the potential mechanisms by which a history of TCDD exposure may be associated with PTB in adulthood, we euthanized pregnant mice during late gestation. Given that the majority of toxicant-exposed animals undergoing premature parturition delivered between E18.5 and E19, we sought to euthanize F1 female mice and control females mated to F1 males on E18.5. For comparison, control mice were euthanized on E18.5 as well as on E19.5 (just prior to anticipated term delivery).
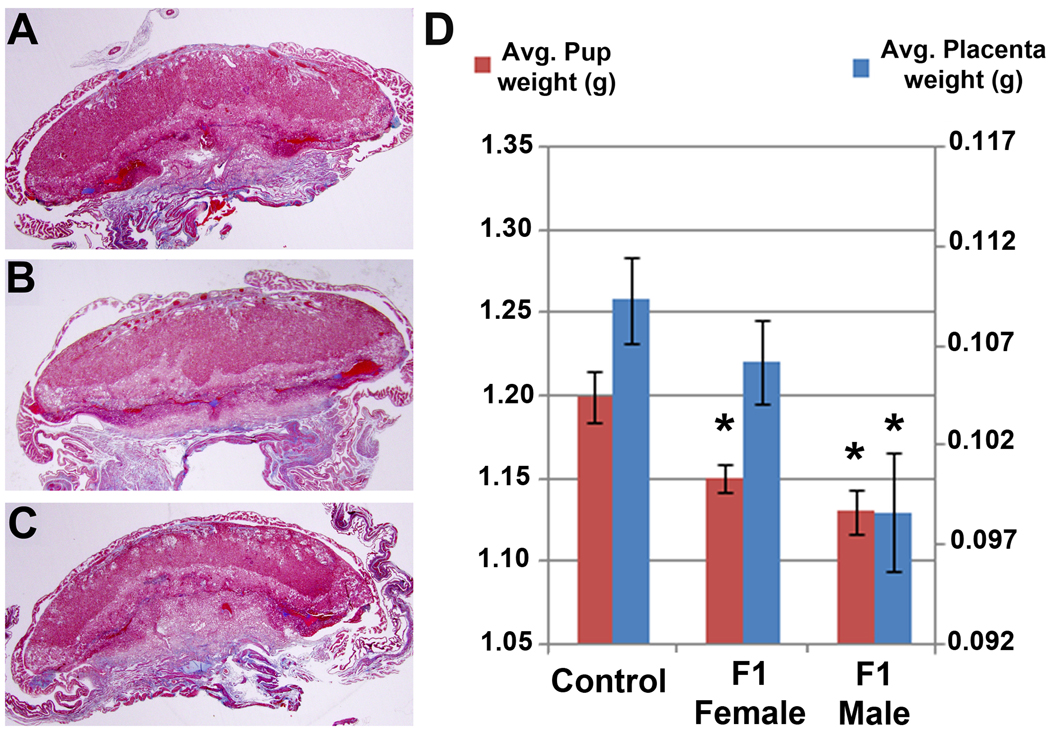
Masson’s Trichrome staining of placental-decidual samples from control mice euthanized on E18.5 revealed histologically normal tissues with readily identifiable decidual, junctional and labyrinthine zones (Figure 3A). Morphologic assessment of placentas obtained from toxicant-exposed F1 females or control females mated to toxicant-exposed F1 males revealed no clear pathology (Figure 3B–C). H and E stained sections were also examined, but were also unremarkable (data not shown).
Figure 3.
Low power photomicrographs of placental-decidual tissues stained with Masson’s trichrome from mice euthanized on E18.5. (A) Tissues from a control female mated to control male. (B) Tissues from an F1 female mated to a control male. (C) Tissues from an F1 male mated to a control female. Original magnification, 12.5×. Note: Masson’s trichrome staining results in red-blue nuclei, red cytoplasm and bright blue collagen. (D). Average placental weights (right-hand scale) and pup weights (left-hand scale) from all mice euthanized for A–C. The asterisks indicate a significant difference between the Control group and toxicant exposed animals (*, p<0.05). Photomicrographs are representative results of multiple tissues from at least 4 mice per group.
We found no significant differences between control mice and mice with a history of fetal TCDD exposure with regard to the number of pups or number of resorption sites on E18.5. However, as shown in Figure 3D, we detected a significant reduction in pup weight (p=0.0084) and a non-significant reduction in placental weight (p=0.1528) from F1 females. Examination of control females mated to F1 males revealed a significant decrease in both placental (p=0.0053) and pup (p=0.0013) weights (Figure 3D).
3.3 qRT-PCR Analysis of PR, TLR-4 and p65/p105 Expression in Placental and Decidual Tissues
Published data from our laboratory indicates that the loss of uterine progesterone response following developmental TCDD exposure is also associated with an increased uterine sensitivity to pro-inflammatory mediators [9]. Thus, we examined the expression of mRNA for PR, TLR-4 and the p65 and p105 subunits of NFkappaB in placental and decidual tissues of mice euthanized in late gestation as described above. The majority of samples from toxicant-exposed mice were obtained on E18.5, just prior to the time of anticipated preterm delivery. However, we also obtained placental and decidual tissues from one toxicant-exposed female on E17.5, when she was observed to be in active spontaneous preterm labor. Only tissues from this animal which had not yet been delivered were included in the analysis.
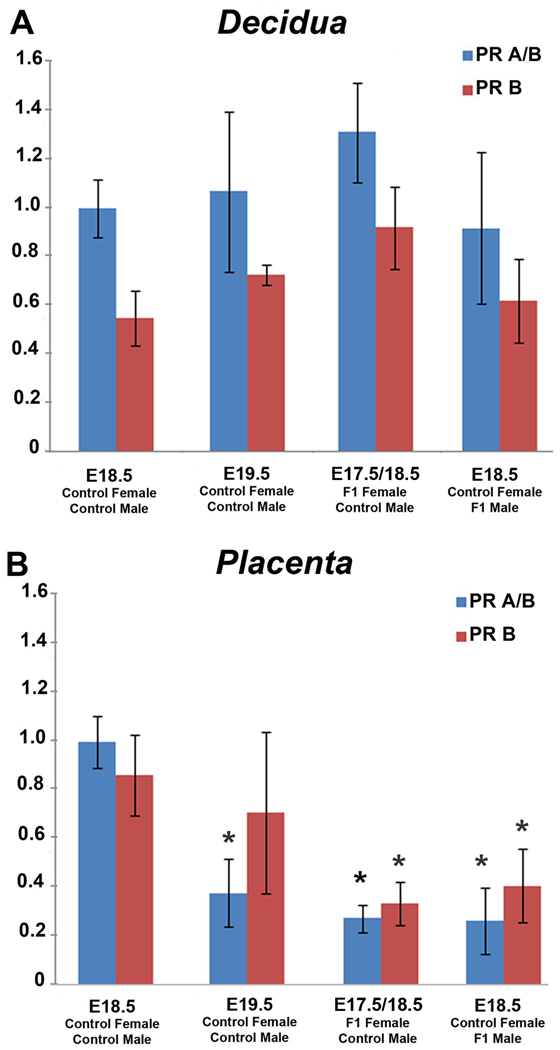
RT-PCR analysis of tissues from control mice euthanized on E18.5 revealed abundant PR mRNA expression in both the decidual and placental compartments (Figure 4) as has been previously reported by others [19]. However, control mice euthanized on E19.5, just prior to expected term parturition, revealed a significant decrease in placental PR A/B expression (p=0.0134) while the change in PR-B expression was highly variable between samples and was not significant (p=0.3485). PR mRNA expression in decidual samples from control mice euthanized on E19.5 was unremarkable. Similarly, both F1 female mice and control female mice mated to F1 males euthanized in late gestation also exhibited abundant PR mRNA within the maternal decidua (Figure 4A). However, both experimental groups exhibited a significant reduction in placental PR mRNA expression compared to control mice euthanized on E18.5 (Figure 4B). Specifically, for control females mated to F1 males, PRA/B (p=0.0078) and PR-B (p=0.0496). For F1 females mated to control males, PRA/B (p=0.0062) and PR-B (p=0.0345).
Figure 4.
Expression of PR-A/B and PR-B mRNA in decidual (A) and placental (B) tissues obtained from mice euthanized in late gestation. The mRNA level of target genes were analyzed by qRT-PCR and normalized to the expression level of the housekeeping gene RPLP0. mRNA is shown as fold changes compared with E18.5 control. Each bar represents the mean ±SD of mRNA for multiple samples from at least 5 different mice per group. Statistically significant changes (p<0.05) are denoted by *.
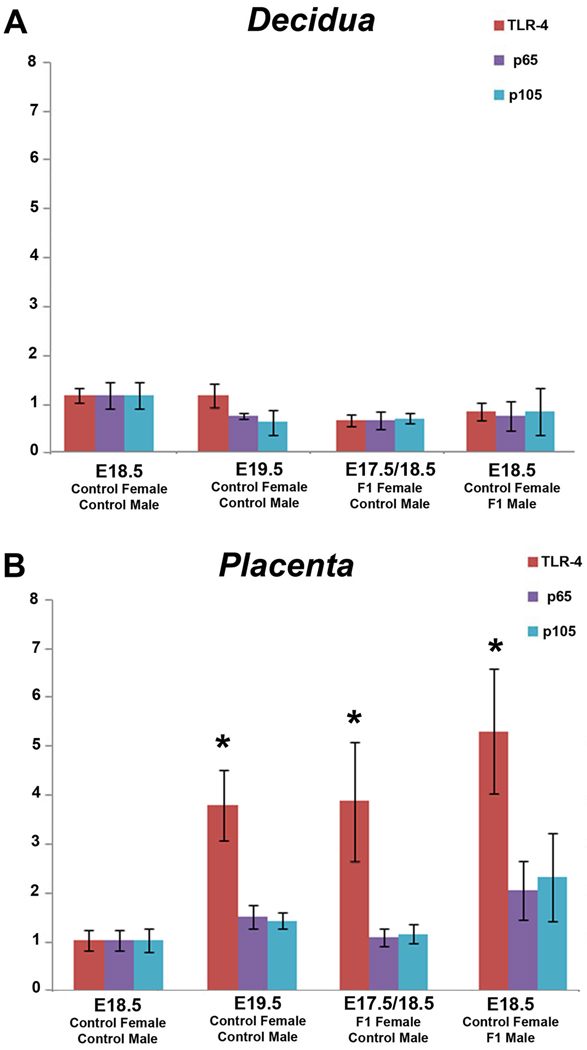
Examination of TLR-4 and p65/p105 mRNA expression in decidual (Figure 5A) and placental (Figure 5B) tissues revealed that expression levels were increased in placental tissues which also exhibited diminished PR mRNA expression. Specifically, decidual expression of TLR-4 and NF kappa B subunit expression was only moderately affected in any group (Figure 5A). Within placental tissues, expression of TLR-4 and p65/p105 mRNA was minimal in control samples obtained on E18.5. Although a significant increase in TLR-4 expression was observed in placental tissues obtained on E19.5 (p=0.0128), there was only a minimal increase in expression of either NF kappa B subunit. F1 female mice euthanized on E17.5 and E18.5 also exhibited a significantly (p=0.0210) higher level of placental TLR-4 and non-significant increases in p105/p65 mRNA (p=0.1 for both) compared to controls euthanized on E18.5. Although the highest levels of TLR-4 were observed in the placentas obtained from control females mated to F1 males (p=0.049), the increases in p65/p105 did not reach significance (p≥0.1) (Figure 5B).
Figure 5.
Expression of TLR-4 and p65/p105 mRNA in decidual (A) and placental (B) tissues obtained from mice euthanized in late gestation. The mRNA level of target genes were analyzed by qRT-PCR and normalized to the expression level of the housekeeping gene RPLP0. mRNA is shown as fold changes compared with E18.5 control. Each bar represents the mean ±SD of mRNA for multiple samples from at least 5 different mice per group. Statistically significant changes (p<0.05) are denoted by *.
4. Discussion
Humans and other animals are exposed to an ever expanding array of chemicals as a consequence of mankind’s industrial development. Importantly, toxicants such as TCDD are persistent, accumulate within our bodies and have the capacity to be transferred to the developing fetus during pregnancy [44,45]. Thus, in order to examine the potential consequence of either maternal or paternal exposure to TCDD on pregnancy outcomes, several epidemiology studies have examined accidentally-exposed human populations. In particular, a chemical plant explosion near Seveso, Italy in 1976 led to the highest known exposure of residential populations to TCDD [46], and women within this population have been carefully monitored with regard to reproductive tract disease and pregnancy outcomes [47,48]. Although to date these studies have failed to identify a significant increase in risk of PTB correlating to TCDD exposure, a non-significant increase in risk was noted in the most heavily exposed individuals [47,48]. With regard to paternal exposure, at least two studies have examined birth outcomes in women whose partners were exposed to TCDD via the herbicide known as Agent Orange [49,50]. As with the Seveso cohort, neither of these studies revealed a significant increase in PTB in the wives of men occupationally exposed to TCDD [49,50]; however, these studies examined the impact of an adult exposure on subsequent pregnancy outcomes. In the absence of life time toxicant exposure information, adult exposure studies alone may have limited value with regard to identifying an individual’s risk for experiencing an adverse reproductive outcome. Specifically, if developmental TCDD exposure of an individual confers a risk of PTB in adulthood, one would expect to observe an increased incidence of PTB in Vietnam in the decades after the American war, in the offspring of pregnant women exposed to TCDD. Although only limited information is available on birth outcomes in Vietnam, PTB is common in this country and has been correlated to population areas heavily exposed to Agent Orange [51].
Clearly, carefully controlled toxicant exposure studies related to PTB cannot be conducted in humans; thus elucidation of the mechanisms of action of any compound requires appropriate experimental animal models. To this end, we previously developed a murine model of TCDD exposure during pregnancy in order to examine the impact of early life exposure to this toxicant on uterine physiology in adult female offspring [10]. Using this model, we demonstrated that developmental exposure to TCDD leads to a dose-dependent reduction in uterine sensitivity to progesterone [10] and, in animals which were able to become pregnant, a poor decidualization reaction at the maternal-fetal interface [11]. Since appropriate decidualization under the influence of progesterone is a central player in maintaining uterine quiescence during pregnancy [29], it should not be surprising that reduced decidual response to progesterone following TCDD exposure negatively affected gestation length [9]. As noted in the current study, normal parturition in women also appears to be regulated by the action of local mediators of inflammation at the maternal-fetal interface [20]. In both women and mice, inflammatory cytokines are effective inhibitors of progesterone action [8,12]; thus, inappropriate inflammatory processes occurring at the maternal-fetal interface would also likely be associated with PTB. To this end, recent data from a number of laboratories suggest that a placental-decidual dialogue controls levels of PR expression within the inflammatory microenvironment associated with late pregnancy; thereby regulating the timing of human parturition [9,20,29,30]. Equally relevant, placental development is markedly influenced by paternally-derived genes [35,36]; thus, it is also prudent to examine the impact of early life toxicant exposure of the father on placental function in a subsequent, adult pregnancy. Therefore, in the current study we sought to determine whether a history of fetal toxicant exposure of either the male or female would negatively impact gestation length in a subsequent adult pregnancy.
In order to explore the relationship between inappropriate progesterone responsiveness and the inflammation-related timing of birth in our toxicant exposed mice, we examined placental and decidual tissues obtained during late pregnancy for PR mRNA expression as well as for selected inflammatory markers. Specifically, placental-decidual tissues from control and toxicant exposed mice were analyzed for the expression of the p65 and p105 subunits of the NF-kappa B family, which mediate numerous inflammatory responses and which have been suggested to be involved in inflammation-associated PTB [52–54]. We also examined placental-decidual expression of TLR-4 which is essential for lipopolysaccharide (LPS)-induced preterm labor in mice [55], but may also regulate normal parturition via binding of endogenous ligands [38]. Equally important, TLR-4 is abundantly expressed in the human placenta [34] and is differentially regulated by progesterone and NFkappaB [25]. Our studies of these key inflammatory markers revealed that placental tissues from control mice euthanized on E18.5 exhibit abundant PR mRNA expression and little TLR-4 or NF-kappaB subunit mRNA expression. In contrast, F1 female mice or control females mated to F1 males and euthanized in late pregnancy exhibited a marked decrease in placental PR mRNA expression coupled with a similar increase in TLR-4 mRNA expression. These changes were even more striking at the placental-decidual interface of the single F1 female undergoing spontaneous PTB at E17.5, since this animal exhibited a near complete loss of PR expression in either compartment. Supporting the use of our animal model for parturition research, the observed alterations in PR expression and inflammatory markers at the maternal-fetal interface of pregnant mice following TCDD exposure of either sex during the animal’s own in utero development are reminiscent of the expression patterns reported in women undergoing spontaneous labor at term [16,18]. Furthermore, our data suggest that a history of developmental TCDD exposure of either parent promotes the early onset of inflammatory processes normally associated with human parturition at term (Figure 6). Interestingly, advanced maternal and paternal ages have each been associated with an increased risk of PTB in women, with the greatest risk associated with the oldest parents [56,57]. Since many age related changes have been suggested to arise from a culmination of numerous inflammatory processes [58,59], it is interesting to speculate that in our mice, exposure to TCDD in utero, may result in an early onset of increased sensitivity to inflammation within the reproductive tract prior to the time that age-related changes would normally occur.
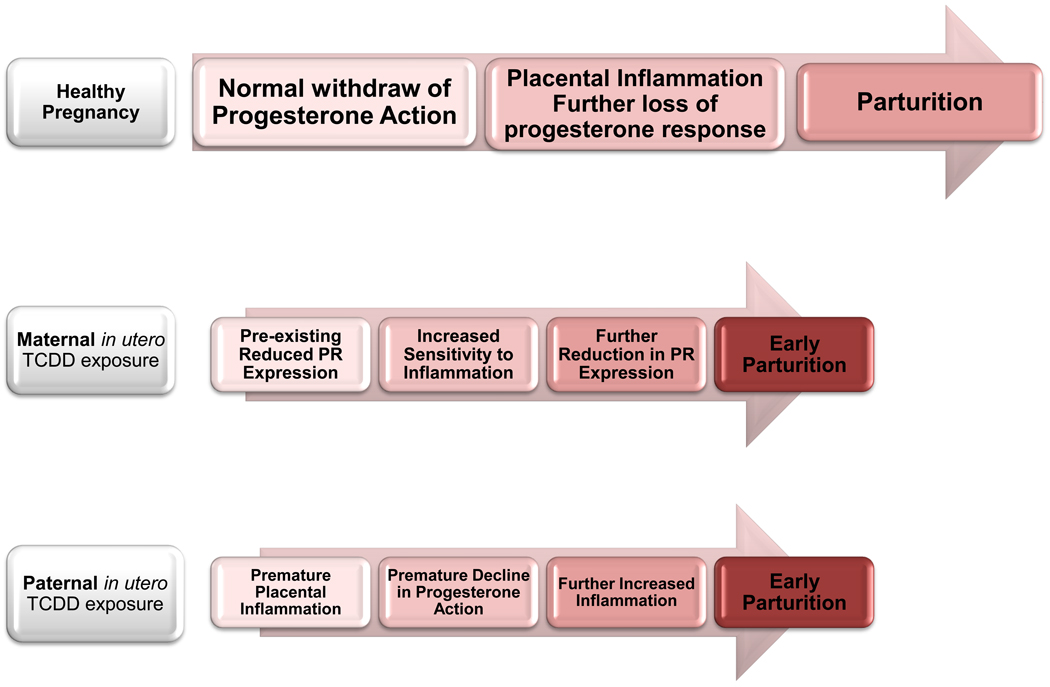
Figure 6.
Schematic diagram representing the potential influence of developmental TCDD exposure on altering the interaction between progesterone and inflammatory action at the maternal-fetal interface. Normal pregnancy proceeds under the influence of a robust progesterone environment, which begins to decline late in pregnancy due to ovarian luteolysis (mice) or decreased PR expression (women). As progesterone levels or response declines, immunosuppression is relieved and inflammatory cytokines further suppress progesterone action. Parturition occurs, at least in part, as a result of inflammation in the absence of progesterone immunosuppression.
Based on data described here and elsewhere [3,30], we propose that developmental exposure of the female leads to a lower “baseline” of progesterone response. Thus, as pregnancy proceeds, normal placental inflammation leads more quickly to a loss of progesterone associated immunosuppression and the inflammatory “threshold” required for parturition is reached too soon. When the male is exposed to TCDD during development, progesterone synthesis and response is initially normal, but premature placental inflammation also leads to reaching the inflammatory threshold required for parturition too quickly; thereby resulting in PTB.
The overall incidence of prematurity in the United States continues to increase, despite advances in prenatal care and patient awareness. We suggest that a convergence of genetic or epigenetic factors with environmental exposures influences the timing of parturition and potentially begin to explain the increased incidence of PTB as well as the difficulty in predicting women truly at risk of delivering early. Specifically, our data suggest a previously unrecognized risk of developmental toxicant exposure of either parent, which leads to PTB in adulthood. Thus, the risk of delivering preterm may be yet another disease with its origins in the fetal environment [60,61] and suggests that current preventive measures taken by clinicians near the end of pregnancy are limited in their effectiveness since they may not address the underlying causes of PTB.
Acknowledgements
We gratefully acknowledge the assistance of Dr. Jennifer L. Herington, Ms. Dana Glore and Ms. Ashley Emerson for assistance with the studies described herein and the preparation of this manuscript. Supported by NIEHS R01ES14942 (KGO), NCCAM R21AT006245-01 (KBT), Vanderbilt University Medical Center (KBT) and The Endometriosis Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Henson MC. Pregnancy maintenance and the regulation of placental progesterone biosynthesis in the baboon. Hum Reprod Update. 1998;4:389–405. doi: 10.1093/humupd/4.4.389. [DOI] [PubMed] [Google Scholar]
- 2.Kramer MS, Demissie K, Yang H, Platt RW, Sauve R, Liston R. The contribution of mild and moderate preterm birth to infant mortality. Fetal and Infant Health Study Group of the Canadian Perinatal Surveillance System. JAMA. 2000;284:843–849. doi: 10.1001/jama.284.7.843. [DOI] [PubMed] [Google Scholar]
- 3.Schindler AE. Role of progestogens for the prevention of premature birth. J Steroid Biochem Mol Biol. 2005;97:435–438. doi: 10.1016/j.jsbmb.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 4.March of Dimes website. [[cited; Aug 22, 2010]]; Available from: http://www.marchofdimes.com/
- 5.Holzman C, Eyster J, Kleyn M, Messer LC, Kaufman JS, Laraia BA, O'Campo P, Burke JG, Culhane J, Elo IT. Maternal weathering and risk of preterm delivery. Am J Public Health. 2009;99:1864–1871. doi: 10.2105/AJPH.2008.151589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitamura M, Kasai A. Cigarette smoke as a trigger for the dioxin receptor-mediated signaling pathway. Cancer Lett. 2007;252:184–194. doi: 10.1016/j.canlet.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 7.Heindel JJ. Role of exposure to environmental chemicals in the developmental basis of reproductive disease and dysfunction. Semin Reprod Med. 2006;24:168–177. doi: 10.1055/s-2006-944423. [DOI] [PubMed] [Google Scholar]
- 8.Mattison DR. Environmental exposures and development. Curr Opin Pediatr. 2010;22:208–218. doi: 10.1097/MOP.0b013e32833779bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruner-Tran KL, Osteen KG. Developmental Exposure to TCDD Reduces Fertility and Negatively Affects Pregnancy Outcomes across Multiple Generations. Reprod Toxicology. 2010 doi: 10.1016/j.reprotox.2010.10.003. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nayyar T, Bruner-Tran KL, Piestrzeniewicz-Ulanska D, Osteen KG. Developmental exposure of mice to TCDD elicits a similar uterine phenotype in adult animals as observed in women with endometriosis. Reprod Toxicol. 2007;23:326–336. doi: 10.1016/j.reprotox.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruner-Tran KL, Ding T, Osteen KG. Dioxin and endometrial progesterone resistance. Semin Reprod Med. 2010;28:59–68. doi: 10.1055/s-0029-1242995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Igarashi TM, Bruner-Tran KL, Yeaman GR, Lessey BA, Edwards DP, Eisenberg E, Osteen KG. Reduced expression of progesterone receptor-B in the endometrium of women with endometriosis and in cocultures of endometrial cells exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fertil Steril. 2005;84:67–74. doi: 10.1016/j.fertnstert.2005.01.113. [DOI] [PubMed] [Google Scholar]
- 13.Osteen KG, Bruner-Tran KL, Eisenberg E. Reduced progesterone action during endometrial maturation: a potential risk factor for the development of endometriosis. Fertil Steril. 2005;83:529–537. doi: 10.1016/j.fertnstert.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 14.Brown AG, Leite RS, Strauss JF., 3rd Mechanisms underlying "functional" progesterone withdrawal at parturition. Ann N Y Acad Sci. 2004;1034:36–49. doi: 10.1196/annals.1335.004. [DOI] [PubMed] [Google Scholar]
- 15.Zakar T, Hertelendy F. Progesterone withdrawal: key to parturition. Am J Obstet Gynecol. 2007;196:289–296. doi: 10.1016/j.ajog.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Shanker YG, Rao AJ. Progesterone receptor expression in the human placenta. Mol Hum Reprod. 1999;5:481–486. doi: 10.1093/molehr/5.5.481. [DOI] [PubMed] [Google Scholar]
- 17.Virgo BB, Bellward GD. Serum progesterone levels in the pregnant and postpartum laboratory mouse. Endocrinology. 1974;95:1486–1490. doi: 10.1210/endo-95-5-1486. [DOI] [PubMed] [Google Scholar]
- 18.Ogle TF. Progesterone-action in the decidual mesometrium of pregnancy. Steroids. 2002;67:1–14. doi: 10.1016/s0039-128x(01)00137-4. [DOI] [PubMed] [Google Scholar]
- 19.An BS, Choi KC, Lee GS, Leung PC, Jeung EB. Complex regulation of Calbindin-D(9k) in the mouse placenta and extra-embryonic membrane during mid- and late pregnancy. Mol Cell Endocrinol. 2004 Feb 12;214(1–2):39–52. doi: 10.1016/j.mce.2003.11.029. [DOI] [PubMed] [Google Scholar]
- 20.Mendelson CR. Minireview: Fetal-Maternal Hormonal Signaling in Pregnancy and Labor. Mol Endocrin. 2009;23:947–954. doi: 10.1210/me.2009-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14:353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 22.Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, 3rd, Petraglia F. Inflammation and pregnancy. Reprod Sci. 2009;16:206–215. doi: 10.1177/1933719108329095. [DOI] [PubMed] [Google Scholar]
- 23.Su L, Sun Y, Ma F, Lü P, Huang H, Zhou J. Progesterone inhibits Toll-like receptor 4-mediated innate immune response in macrophages by suppressing NF-kappaB activation and enhancing SOCS1 expression. Immunol Lett. 2009;125:151–155. doi: 10.1016/j.imlet.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Elovitz MA, Wang Z, Chien EK, Rychlik DF, Phillippe M. A New Model for Inflammation-Induced Preterm Birth: The Role of Platelet-Activating Factor and Toll-Like Receptor-4. American Journal of Pathology. 2003;163:2103–2111. doi: 10.1016/S0002-9440(10)63567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Briscoe T, Duncan J, Cock M, Choo J, Rice G, Harding R, Scheerlinck JP, Rees S. Activation of NF-kappaB transcription factor in the preterm ovine brain and placenta after acute LPS exposure. J Neurosci Res. 2006;83:567–574. doi: 10.1002/jnr.20757. [DOI] [PubMed] [Google Scholar]
- 26.Lappas M, Yee K, Permezel M, Rice GE. Lipopolysaccharide and TNF-alpha activate the nuclear factor kappa B pathway in the human placental JEG-3 cells. Placenta. 2006;27:568–575. doi: 10.1016/j.placenta.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Guo SW. Nuclear factor-kappab (NF-kappaB): an unsuspected major culprit in the pathogenesis of endometriosis that is still at large? Gynecol Obstet Invest. 2007;63:71–97. doi: 10.1159/000096047. [DOI] [PubMed] [Google Scholar]
- 28.Patni S, Flynn P, Wynen LP, Seager AL, Morgan G, White JO, Thornton CA. An introduction to Toll-like receptors and their possible role in the initiation of labour. Brit J Obstet Gynecol. 2007;114:1326–1334. doi: 10.1111/j.1471-0528.2007.01488.x. [DOI] [PubMed] [Google Scholar]
- 29.Faye-Petersen OM. The placenta in preterm birth. J Clin Pathol. 2008;61:1261–1275. doi: 10.1136/jcp.2008.055244. [DOI] [PubMed] [Google Scholar]
- 30.Houben ML, Nikkels PG, van Bleek GM, Visser GH, Rovers MM, Kessel H, de Waal WJ, Schuijff L, Evers A, Kimpen JL, Bont L. The association between intrauterine inflammation and spontaneous vaginal delivery at term: a cross-sectional study. PLoS One. 2009;4:e6572. doi: 10.1371/journal.pone.0006572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zarember KA, Godowski PJ. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol. 2002;168:554–561. doi: 10.4049/jimmunol.168.2.554. [DOI] [PubMed] [Google Scholar]
- 32.Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human toll-like receptors and related genes. Biol Pharm Bull. 2005;28:886–892. doi: 10.1248/bpb.28.886. CrossRef,PubMed. [DOI] [PubMed] [Google Scholar]; Savitz DA, Murnane P. Behavioral influences on preterm birth: a review. Epidemiology. 2010 May;21(3):291–299. doi: 10.1097/EDE.0b013e3181d3ca63. [DOI] [PubMed] [Google Scholar]
- 33.Mitsunari M, Yoshida S, Shoji T, Tsukihara S, Iwabe T, Harada T, et al. Macrophage-activating lipopeptide-2 induces cyclooxygenase-2 and prostaglandin E(2) via toll-like receptor 2 in human placental trophoblast cells. J Reprod Immunol. 2006;72:46–59. doi: 10.1016/j.jri.2006.02.003. CrossRef,PubMed. [DOI] [PubMed] [Google Scholar]
- 34.Holmlund U, Cebers G, Dahlfors AR, Sandstedt B, Bremme K, Ekström ES, Scheynius A. Expression and regulation of the pattern recognition receptors Toll-like receptor-2 and Toll-like receptor-4 in the human placenta. Immunology. 2002;107:145–151. doi: 10.1046/j.1365-2567.2002.01491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kajii T, Ohama K. Androgenetic origin of hydatidiform mole. Nature. 1977;268:633–634. doi: 10.1038/268633a0. [DOI] [PubMed] [Google Scholar]
- 36.Barton SC, Surani MA, Norris ML. Role of paternal and maternal genomes in mouse development. Nature. 1984;311:374–376. doi: 10.1038/311374a0. [DOI] [PubMed] [Google Scholar]
- 37.Ishimura R, Ohsako S, Kawakami T, Sakaue M, Aoki Y, Tohyama C. Altered protein profile and possible hypoxia in the placenta of 2,3,7,8-tetrachlorodibenzo-p-dioxin-exposed rats. Toxicol Appl Pharmacol. 2002;185:197–206. doi: 10.1006/taap.2002.9539. [DOI] [PubMed] [Google Scholar]
- 38.Ishimura R, Ohsako S, Miyabara Y, Sakaue M, Kawakami T, Aoki Y, Yonemoto J, Tohyama C. Increased glycogen content and glucose transporter 3 mRNA level in the placenta of Holtzman rats after exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol. 2002;178:161–171. doi: 10.1006/taap.2001.9333. [DOI] [PubMed] [Google Scholar]
- 39.Khera KS. Extraembryonic tissue changes induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin and 2,3,4,7,8-pentachlorodibenzofuran with a note on direction of maternal blood flow in the labyrinth of C57BL/6N mice. Teratology. 1992;45:611–627. doi: 10.1002/tera.1420450606. [DOI] [PubMed] [Google Scholar]
- 40.Vogel CF, Zhao Y, Wong P, Young NF, Matsumura F. The use of c-src knockout mice for the identification of the main toxic signaling pathway of TCDD to induce wasting syndrome. J Biochem Mol Toxicol. 2003;17:305–315. doi: 10.1002/jbt.10096. [DOI] [PubMed] [Google Scholar]
- 41.Lanman JT, Seidman L. Length of Gestation in Mice Under a 21-Hour Day. Biol Reprod. 1977;17:224–227. doi: 10.1095/biolreprod17.2.224. [DOI] [PubMed] [Google Scholar]
- 42.Wang H, Xie H, Dey SK. Loss of Cannabinoid Receptor CB1 Induces Preterm Birth. PLoS ONE. 2008;3(10):e3320. doi: 10.1371/journal.pone.0003320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roizen JD, Asada M, Tong M, Tai HH, Muglia LJ. Preterm birth without progesterone withdrawal in 15-hydroxyprostaglandin dehydrogenase hypomorphic mice. Mol Endocrinol. 2008;22:105–112. doi: 10.1210/me.2007-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Environmental Working Group. Body Burden: The Pollution in Newborns. [[Cited; Aug 6, 2010]];2007 Available from: http://www.ewg.org/reports/bodyburden2/
- 45.White SS, Birnbaum LS. An overview of the effects of dioxins and dioxin-like compounds on vertebrates, as documented in human and ecological epidemiology. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27:197–211. doi: 10.1080/10590500903310047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Homberger E, Reggiani G, Sambeth J, Wipf HK. The Seveso Accident: Its Nature, Extent and Consequences. Ann Occup Hyg. 1979;22:327–370. doi: 10.1093/annhyg/22.4.327. [DOI] [PubMed] [Google Scholar]
- 47.Eskenazi B, Mocarelli P, Warner M, Samuels S, Vercellini P, Olive D, Needham L, Patterson D, Brambilla P. Seveso Women's Health Study: a study of the effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on reproductive health. Chemosphere. 2000;40:1247–1253. doi: 10.1016/s0045-6535(99)00376-8. [DOI] [PubMed] [Google Scholar]
- 48.Eskenazi B, Mocarelli P, Warner M, Chee WY, Gerthoux PM, Samuels S, Needham LL, Patterson DG., Jr Maternal serum dioxin levels and birth outcomes in women of Seveso, Italy. Environ Health Perspect. 2003;111:947–953. doi: 10.1289/ehp.6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Michalek JE, Rahe AJ, Boyle CA. Paternal dioxin, preterm birth, intrauterine growth retardation, and infant death. Epidemiology. 1998 Mar;9(2):161–167. [PubMed] [Google Scholar]
- 50.Lawson CC, Schnorr TM, Whelan EA, Deddens JA, Dankovic DA, Piacitelli LA, Sweeney MH, Connally LB. Paternal occupational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin and birth outcomes of offspring: birth weight, preterm delivery, and birth defects. Environ Health Perspect. 2004;112:1403–1408. doi: 10.1289/ehp.7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Le TN, Johansson A. Impact of chemical warfare with agent orange on women's reproductive lives in Vietnam: a pilot study. Reprod Health Matters. 2001;v. 9:156–164. doi: 10.1016/s0968-8080(01)90102-8. [DOI] [PubMed] [Google Scholar]
- 52.Lappas M, Rice GE. Transcriptional regulation of the processes of human labour and delivery. Placenta. 2009;30:S90–S95. doi: 10.1016/j.placenta.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 53.King AE, Critchley HO, Kelly RW. The NF-kappaB pathway in human endometrium and first trimester decidua. Mol Hum Reprod. 2001;7:175–183. doi: 10.1093/molehr/7.2.175. [DOI] [PubMed] [Google Scholar]
- 54.Liu H, Redline RW, Han YW. Fusobacterium nucleatum induces fetal death in mice via stimulation of TLR4-mediated placental inflammatory response. J Immunol. 2007;179:2501–2508. doi: 10.4049/jimmunol.179.4.2501. [DOI] [PubMed] [Google Scholar]
- 55.Cookson VJ, Chapman NR. NF-kappaB function in the human myometrium during pregnancy and parturition. Histol Histopathol. 2010;25(7):945–956. doi: 10.14670/HH-25.945. [DOI] [PubMed] [Google Scholar]
- 56.Lisonkova S, Janssen PA, Sheps SB, Lee SK, Dahlgren L. The effect of maternal age on adverse birth outcomes: does parity matter? J Obstet Gynaecol Can. 2010;32:541–548. doi: 10.1016/S1701-2163(16)34522-4. [DOI] [PubMed] [Google Scholar]
- 57.Chen XK, Wen SW, Krewski D, Fleming N, Yang Q, Walker MC. Paternal age and adverse birth outcomes: teenager or 40+, who is at risk? Hum Reprod. 2008;23:1290–1296. doi: 10.1093/humrep/dem403. [DOI] [PubMed] [Google Scholar]
- 58.Mocchegiani E, Corsonello A, Lattanzio F. Frailty, ageing and inflammation: reality and perspectives. Biogerontology. 2010 Aug 18; doi: 10.1007/s10522-010-9299-y. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 59.Wolkowitz OM, Epel ES, Reus VI, Mellon SH. Depression gets old fast: do stress and depression accelerate cell aging? Depress Anxiety. 2010;27(4):327–338. doi: 10.1002/da.20686. [DOI] [PubMed] [Google Scholar]
- 60.Dolinoy DC, Weidman JR, Jirtle RL. Epigenetic gene regulation: linking early developmental environment to adult disease. Reprod Toxicol. 2007;23:297–307. doi: 10.1016/j.reprotox.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 61.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]