Abstract
Aim
To assess endotoxemia episodes and subsequent changes in serum inflammatory biomarkers using the experimental gingivitis model
Materials and Methods
Data from 50 healthy black and white adult males and females were compared for serum concentrations of endotoxin, and serum biomarkers [neutrophil oxidative activity, interleukin (IL)-1β, IL-6, IL-8, C-reactive protein, and fibrinogen] at baseline, at 3 weeks of experimental gingivitis, and after 2 weeks of recovery. Means were compared using repeated measures ANOVA.
Results
Endotoxemia was reported in 56% of the serum samples at three weeks of induced gingivitis. At two weeks of recovery, endotoxin levels decreased to levels similar to those reported at baseline. Neutrophil oxidative activity increased significantly following three weeks of gingivitis versus baseline (p<0.05). In the endotoxin-negative group this increase was associated with the black subjects whereas in the endotoxin-positive group change in neutrophil activity was driven by the female subpopulation. Serum cytokines, CRP, and fibrinogen levels did not change during the study.
Conclusions
Experimental gingivitis was associated with endotoxemia and hyperactivity of circulating neutrophils, but not with changes in systemic levels of cytokines and acute phase proteins. This may be attributed to the mild nature and the short duration of the induced gingivitis.
Keywords: dental plaque, experimental gingivitis, inflammatory response, endotoxin, neutrophils, cytokines, acute phase proteins
Introduction
There is wide-spread interest in studying the systemic impact of oral infection. Gingivitis is universal, reversible, and rapidly inducible. Thus, it provides an appropriate model for investigation of the host response to oral infection. It can be induced experimentally by the “Experimental Gingivitis Model” (EGM) (Löe et al. 1965).
Gingivitis is associated with thinning of the sulcular epithelium and dilation of the gingival vasculature (Zoellner et al. 2002), thus facilitating invasion of the epithelial barrier by oral bacteria and/or their products into the systemic circulation. A distinctive bacterial product that can be found in blood is endotoxin, which is a cell wall component of Gram-negative bacteria. It has been shown previously that dental plaque maturation in the EGM is accompanied by a shift towards increasingly Gram-negative bacteria (Theilade et al. 1966) including periodontal pathogens of the red and orange complex species (Salvi et al. 2005). Indeed, it has been suggested that an infected periodontium is a major source of persistent endotoxemia, even as a consequence of gentle mastication (Geerts et al. 2002). Furthermore, periodontal pathogens have also been detected in atheromatous plaques and in abdominal aortic aneurysms, further supporting the occurrence of bacteremias/endotoxemias by oral bacteria (Haraszthy et al. 2000, Kurihara et al. 2004).
In addition to producing bacteremia/endotoxemia, we hypothesized that dental plaque accumulation may elicit a host systemic inflammatory response to the local dental plaque and/or bacteremia/endotoxemia. In this study potential changes in levels of serum cytokines and acute phase proteins as well as neutrophil oxidative activity as a result of gingivitis were assessed.
Peripheral blood neutrophils are the primary cells of the acute inflammatory response. Neutrophils can mediate tissue destruction in inflammatory diseases (Hansen 1995). Dental plaque accumulation and periodontal disease elicit a peripheral neutrophil response (Kowolik et al. 2001, Matthews et al. 2007, Wahaidi et al. 2009), which includes the release of cytotoxic oxidants and proteolytic enzymes, both of which possess detrimental tissue-damaging effects.
In response to infection, inflammatory cytokines promote a series of events, at the local infection site and systemically (Gabay & Kushner 1999). Dental plaque accumulation in both spontaneous and experimental gingivitis induces changes in gingival crevicular fluid cytokine levels (Ebersole et al. 1993, Offenbacher et al. 2010, Salvi et al. 2010, Trombelli et al. 2010) with potential for systemic dissemination (Ebersole et al. 1999). Human experimental endotoxemia is reported to induce changes in levels of serum cytokines (van Eijk et al. 2007, Dorresteijn et al. 2010). Systemic effects of these cytokines include production of acute phase reactants (Baumann & Gauldie 1994), leukocyte recruitment (Yoshimura et al. 1987), upregulation of endothelial adhesion factors (Munro 1993), and induction of procoagulation (Nachman et al. 1986).
An increase in serum levels of C-reactive protein (CRP) and fibrinogen occurs in response to infection including periodontal infection (Kweider et al. 1993, Noack et al. 2001, Beck & Offenbacher 2002, Mattila et al. 2005, Paraskevas et al. 2008). CRP has prothrombotic effects (Cermak et al. 1993) and activates complement (Volanakis 1982). Fibrinogen increases blood viscosity (Lowe et al. 1997), promotes platelet aggregation (Cook & Ubben 1990), and stimulates smooth muscle proliferation (Ishida & Tanaka 1982).
Systemic translocation of orally-derived endotoxin and the potential subsequent host systemic response has not been previously investigated in gingivitis, including the EGM.
This sub-study was part of a larger investigation (Subject N=128) that examined the systemic response to dental plaque accumulation, using the EGM in a population of black and white healthy adult male and female subjects (Wahaidi et al. 2009). The results of this study indicated that neutrophil activity increased in black but not white subjects in response to experimental gingivitis. No gender differences were noted. The aim of the present study was to compare data from a subpopulation (n=50) of the original study (Wahaidi et al. 2009), to determine if (1) dental plaque accumulation and the ensuing gingivitis results in endotoxemia, and (2) whether the presence of endotoxin would result in race and gender-associated differences in a subsequent host systemic response.
Materials and Methods
Study Design
The original study has been described in detail previously (Wahaidi et al. 2009). The protocol was approved by the Institutional Review Board of Indiana University Purdue University Indianapolis/Clarian Health (approval number 0405-50). All participants provided written informed consent for participation. One week after all subjects had received a professional oral prophylaxis and oral hygiene (OH) instructions, they entered the three study phases (Table 1). In the “control phase” (weeks 0–3) subjects performed optimal OH practices. In the “experimental phase” (weeks 3–6), subjects refrained from all OH measures including the use of dentifrices, mouthrinses, dental floss, and gum. At the end of the experimental phase, which was also the start of the “recovery phase” (weeks 6–9) subjects received a professional oral prophylaxis and resumed normal OH practices (Table 1). Subjects visited the clinic weekly. During each visit, oral examinations were conducted, dental plaque and gingivitis were assessed, and peripheral blood samples were collected. Serum was separated and stored for laboratory analyses. The blood samples at week 6 were collected prior to the administration of oral prophylaxis.
Table 1.
Overview of the clinical procedures performed during the study
| 7 Days prior to study | Control Phase | Experimental Phase | Recovery Phase | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week of Study | 0 | 1 | 2 | 3 a | 3 a | 4 | 5 | 6 b | 6 b | 7 | 8 | 9 | |
| Blood Draw | X | X | X | X | X | X | Xc | X | X | ||||
| Plaque and Gingivitis Assessment | X | X | X | X | X | X | X | X | X | ||||
| Dental Prophylaxis | X | X | |||||||||||
| Oral Hygiene Practices | X | X | X | X | X | X | X | ||||||
Only data from weeks 3, 6, and 8 were analyzed in this sub-study.
same day of study.
same day of study.
The blood sample at week 6 was collected prior to the administration of oral prophylaxis.
Study Population
This sub-study analyzed data received from healthy adults (n=50; whites=34; blacks=16; males=22; females=28) (mean age: 24.7, range: 18–30 years) during weeks 3 (end of the control phase and beginning of the experimental phase), 6 (end of the experimental phase and beginning of the recovery phase), and 8 (2 weeks of the recovery phase) of the study. The subjects were in possession of a minimum of 20 natural teeth and showed no signs of periodontal disease (periodontal probing depths <4mm) or gross dental caries. Exclusion criteria included the current use of tobacco products, any medication known to affect the oral soft tissue or local/systemic response, or antimicrobial products within 3 months preceding the study.
Oral Examinations
Oral examinations and assessments of dental plaque and gingivitis were performed by a single trained and calibrated examiner (weighted Kappa for intra-examiner reproducibility >0.7) using the Plaque Index (PI) (Silness and Löe, 1964) and Gingival Index (GI) (Löe and Silness, 1963), respectively. Measurements were made on six sites per tooth (distobuccal, buccal, mesiobuccal, mesiolingual, lingual, distolingual) on all teeth present, with the exception of third molars.
Laboratory Analyses
Endotoxin assay
Serum endotoxin levels were assayed using a Limulus Amebocyte Lysate commercial kit. The ENDOSAFE® Endochrome-K™ test (Charles River Laboratories, SC, USA), a kinetic colorimetric assay, was used for quantification of endotoxin levels. All samples were processed in duplicate.
Serum biomarkers
Neutophils were isolated by a density gradient centrifugation method (Wahaidi et al. 2009). Total neutrophil activity was measured via luminol-enhanced chemiluminescence using a 1251 Bio-Orbit Luminometer (Bio-Orbit, Turku, Finland) for 90 minutes (Wahaidi et al. 2009) and expressed in millivoltage.min. Samples were processed in triplicate.
Serum cytokine levels were assayed using commercial immunoassay kits. The LINCO plex™ multiplex immunoassays kit (Linco Research Inc., MO, USA) was used for quantification of IL-1β, IL-6, and IL-8. Samples were processed in duplicate.
Plasma fibrinogen levels were measured using an automated clot detection system in the Clarian Health Laboratories. A commercial kit, the Active™ C-reactive protein ELISA (Diagnostic Systems Laboratories Inc, TX), was used for quantification of CRP. Samples were processed in duplicate.
Statistical Analysis
Means and 95% confidence intervals were calculated. Comparisons between means of the endpoints of the three different phases were performed using repeated measures ANOVA, using the log-transformed data due to the skewed distributions of the measurements. The level of statistical significance was set at 0.05.
Results
Serum Endotoxin
No measurable endotoxin was detected in weeks 4 and 5 of the experimental phase. The percentage of endotoxin-positive subjects at week 6 was overall 56% (whites: 59%, blacks: 50%, males: 50%; and females: 61%). In the endotoxin positive subjects mean serum endotoxin levels (EU/ml) were significantly higher at week 6 versus week 3 (p<0.0001) (Table 2) with a mean of 0.74 EU/ml for whites, 0.78 EU/ml for blacks, 0.61 EU/ml for males and 0.81 EU/ml for females. These levels decreased significantly at week 8 to levels similar to those reported at baseline (p<0.0001).
Table 2.
Mean, 95% confidence intervals, and significance for study outcomes over the course of the study. Comparisons between means of the control and experimental phase, and the experimental and recovery phase within each group were performed using repeated measures ANOVA.
| Endotoxin-negative | Endotoxin-positive | |||||
|---|---|---|---|---|---|---|
| Weeks | 3 | 6 | 8 | 3 | 6 | 8 |
| Endotoxin (EU/ml) | <0.08 | <0.08 | <0.08 | <0.08 | 0.74***1) (0.47, 1.00) | <0.08*** |
| PI | 0.15 (0.13, 0.18) | 2.12*** (2.0, 2.24) | 0.37*** (0.32, 0.41) | 0.14 (0.12, 0.16) | 2.08*** (2.0, 2.17) | 0.40*** (0.34, 0.45) |
| GI | 0.46 (0.42, 0.49) | 1.22*** (1.16, 1.28) | 0.50*** (0.47, 0.54) | 0.41 (0.38, 0.44) | 1.16*** (1.11, 1.22) | 0.47*** (0.44, 0.51) |
| Total Neutrophil Activity (millivoltage.min) | 5.47 (4.20, 6.74) | 9.44*** (7.21, 11.7) | 8.51 (6.28, 10.8) | 5.77 (3.81, 7.74) | 8.39**2) (5.88, 10.9) | 5.54 (4.78, 6.31) |
*** p< 0.001,
** p<0.01
Plaque and Gingivitis
PI and GI increased significantly at week 6 versus week 3 (p<0.0001) in all subjects as reported previously (Wahaidi et al. 2009). This increase was independent of endotoxin status (Table 2). PI and GI decreased significantly at week 8 versus week 6 (p<0.0001), although week 8 PI values were still significantly higher than baseline (p<0.001).
Serum Biomarkers
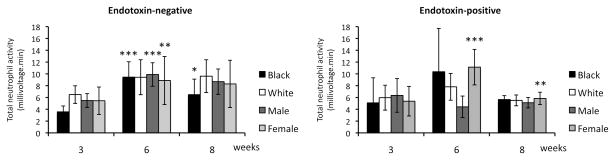
Total chemiluminescence increased significantly during the experimental phase with a p value of <0.001 in the endotoxin-negative group and 0.009 in the endotoxin-positive group (Table 2). This change was dependent on race in the endotoxin-negative group with an increase of 5.88 in blacks (p<0.001) but only 2.95 in whites (p=0.051) (Fig. 1). In the endotoxin-positive group gender influenced overall neutrophil activity with a significant increase of 5.77 in females (p<0.001) versus a decrease in activity by 1.94 in males (p=0.131) (Fig. 1). Neutrophil activity for blacks (p=0.038) and females (p=0.006) returned to baseline levels during the recovery phase.
Fig. 1.
Mean (±95% confidence interval) peripheral blood neutrophil total activity (chemiluminescence) by race and gender at weeks 3 (baseline), 6 (experimental phase), and 8 (recovery phase). Comparisons between means of the control, experimental and recovery phase within each group were performed using repeated measures ANOVA. *** p< 0.001; ** p<0.01; * p<0.05
During the course of the study, no statistically significant changes were observed in levels of the analyzed cytokines (p>0.05). Moreover, there were no significant differences in the frequency (defined as a measurable concentration versus no detection) of detection of cytokines between endotoxin-positive and endotoxin-negative subjects (p>0.05) (data not shown). High individual variability in the pattern of cytokine profiles was observed (data not shown).
Levels of serum CRP and fibrinogen did not change significantly during the study course for any of the groups (p>0.05).
Discussion
To our knowledge, this is the first report that directly links gingivitis to endotoxemia. In this study, levels of systemic endotoxin increased significantly following 21 days of dental plaque accumulation in the majority of subjects, with no differences between individuals of different race/gender. As anticipated, resolution of gingivitis following treatment resulted in the reduction of endotoxin to baseline levels.
Only endotoxemia was evaluated in this study, not bacteremia. Endotoxemia is a surrogate marker for Gram-negative bacteremia. Gram-negative bacteria comprise a significant portion of the mature subgingival plaque flora (Theilade et al. 1966). Whereas endotoxemia occurred at a high frequency in a very short period of time in the present study, only low levels of bacteremia in individuals with periodontal disease and particularly, gingivitis have been reported (Kinane et al. 2005, Forner et al. 2006). This may be due to the challenges associated with blood culture techniques.
Endotoxemia, at sufficient levels, is a potent immune and inflammatory stimulator (Guthrie et al. 1984, Lindemann et al. 1988), and may ultimately, in the long run, result in multiple organ system failure (Brandtzaeg et al. 1989). With a mean of 0.72 EU/ml, the amount of serum endotoxin detected in this study was relatively low. This may be attributed to the low severity of gingivitis as a periodontal infection. Indeed, it has been shown that levels of endotoxemia are positively associated with the severity of periodontal disease (Geerts et al. 2002). Nonetheless, although mild, as a chronic insult gingivitis may cause persistent delivery of endotoxin into the systemic circulation. In fact, a systemic endotoxin level greater than 50 pg/ml (equivalent to 0.5 EU) in individuals with chronic infections has been identified as a risk factor for early atherogenesis (Wiedermann et al. 1999).
Endotoxin is known to stimulate neutrophil activity (Guthrie et al. 1984, Fittschen et al. 1988), although the effect of endotoxin on neutrophils varies depending on whether endotoxin is free or bound. Persistent neutrophil hyperactivity plays a role in tissue destruction (Di Filippo et al. 2007; Wittkowski et al. 2007). In this study, a systemic peripheral neutrophil response was noted following 3 weeks of dental plaque accumulation. We previously reported a heightened neutrophil response in blacks versus whites following 3 weeks of experimental gingivitis in healthy adults (Wahaidi et al. 2009). Results of the current study showed that the neutrophil response following dental plaque accumulation in blacks was independent of endotoxin status. In contrast, an association between endotoxin and neutrophil activity was observed as it relates to gender. Endotoxin-positive females showed a significant increase in neutrophil activity in week 6 that dropped back to baseline levels during the recovery phase. In contrast, neutrophil activity for males decreased in the experimental phase, although the change was not significant. In accordance with our findings, gender disparity has been reported in the acute neutrophil inflammatory response. In a rat model an increase in phagocytic activity in circulating neutrophils was noted in female but not male animals in response to an endotoxin challenge (Spitzer 1999). Furthermore, van Eijk et al (2007) reported a more pronounced inflammatory response in females in a human experimental endotoxin model. Sexual dimorphism in the innate inflammatory response including neutrophil biology has been associated with the effect of sex hormones (Miyagi et al. 1992, Molloy et al. 2003).
In the present study, experimental gingivitis and the subsequent endotoxemia were not associated with changes in serum levels of cytokines and acute phase proteins. However, high individual variability in the pattern of cytokine profiles was observed. In agreement, earlier studies demonstrated no association between clinical parameters of periodontitis and serum levels of cytokines including IL-1β and IL-6, and a high variability of cytokine profiles (Chen et al. 1997, Gorska et al. 2003). Likewise, the literature supports a gingival crevicular fluid cytokine repsonse in both spontaneous and experimental gingivitis (Deinzer at al. 2007, Andriankaja et al. 2009, Salvi et al. 2010, Trombelli et al. 2010), but related changes in serum levels of cytokine have not been detected (Andriankaja et al. 2009, Trombelli et al. 2010). The inability to detect systemic cytokines may be explained by the mild nature of gingivitis (Trombelli et al. 2010) and the low level of the resultant endotoxemia. In contrast, changes in cytokine levels have been observed in induced endotoxemia (van Eijk et al. 2007, Dorresteijn et al. 2010) where serum endotoxin levels were significantly higher when compared to the present work.
Furthermore, it has been proposed that circulating levels of cytokines depend upon multiple factors including not just the type of stimulant but also the duration of exposure to the stimulant (Di Padova et al. 1991). Hence, failure to detect significant changes in serum inflammatory markers may also be attributed to the short duration of the observed endotoxemia. It is important to note that increases in serum endotoxin levels were reported at week 6 of this study, but not at week 5. In fact, it is very likely that an extension of the experimental phase by an additional week would have resulted in an increase in number of endotoxin-positive subjects and possibly endotoxin concentration. Instead, the short time-frame used in our study was probably insufficient to induce prolonged endotoxemia and an ensuing systemic response. Such a finding may be of importance in designing future studies to further understand the temporal aspect of the potential systemic response to endotoxemia following the development of gingivitis.
It should be noted that, although well-controlled, the conditions of the EGM differ from those of spontaneous gingivitis (Deinzer et al. 2007). The EGM involves meticulous OH measures, followed by complete neglect of OH for 21 days. In comparison, during spontaneous gingivitis dental plaque, in some areas, accumulates for a longer duration and some OH is practiced, which results in transient bacteremia (Silver et al. 1977). It is conceivable that endotoxin levels would have been much higher in the present study had the blood samples been collected immediately after the subjects received dental prophylaxis in week 6. These discrepancies between the EGM and spontaneous gingivitis may affect the nature and level of the resultant bacteremias/endotoxemias and the systemic host response.
Although the results from this study did not show a definitive host systemic response to dental plaque accumulation, there were findings of potential clinical relevance. The development of gingivitis was the origin of systemic endotoxemia and hyperactivity of circulating neutrophils, the latter being associated with gender in endotoxin-positive subjects. The increase in neutrophil activity observed in this study could be linked to the local oral infection (i.e. gingivitis), to the circulating bacteria/endotoxin, or to both. As a chronic insult, spontaneous gingivitis may cause persistent delivery of endotoxin into the systemic circulation. In the long-term, this “little and often” endotoxemia and inflammatory response may cumulatively cause detrimental systemic effects. The disparity in the race/gender systemic neutrophil response, in the endotoxin-positive and endotoxin-negative groups, is worthy of further investigation.
Acknowledgments
This study was supported by NIH grant # R01DEO1514501.
Footnotes
Conflict of interest
The authors declare that they have no conflicts of interest related to this study.
References
- Andriankaja OM, Barros SP, Moss K, Panagakos FS, DeVizio W, Beck J, Offenbacher S. Levels of serum interleukin (IL)-6 and gingival crevicular fluid of IL-1beta and prostaglandin E(2) among non-smoking subjects with gingivitis and type 2 diabetes. Journal of Periodontology. 2009;80:307–316. doi: 10.1902/jop.2009.080385. [DOI] [PubMed] [Google Scholar]
- Baumann H, Gauldie J. The acute phase response. Immunol Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- Beck JD, Offenbacher S. Relationships among clinical measures of periodontal disease and their associations with systemic markers. Annals of Periodontology. 2002;7:79–89. doi: 10.1902/annals.2002.7.1.79. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P, Kierulf P, Gaustad P, Skulberg A, Bruun JN, Halvorsen S, Sørensen E. Plasma endotoxin as a predictor of multiple organ failure and death in systemic meningococcal disease. Journal of Infectious Diseases. 1989;159:195–204. doi: 10.1093/infdis/159.2.195. [DOI] [PubMed] [Google Scholar]
- Cermak J, Key NS, Bach RR, Balla J, Jacob HS, Vercellotti GM. C-reactive protein induces human peripheral blood monocytes to synthesize tissue factor. Blood. 1993;82:513–520. [PubMed] [Google Scholar]
- Chen CC, Chang KL, Huang JF, Huang JS, Tsai CC. Correlation of interleukin-1 beta, interleukin-6, and periodontitis. Kaohsiung Journal of Medical Sciences. 1997;13:609–617. [PubMed] [Google Scholar]
- Cook NS, Ubben D. Fibrinogen as a major risk factor in cardiovascular disease. Trends in Pharmacological Science. 1990;11:444–451. doi: 10.1016/0165-6147(90)90125-r. [DOI] [PubMed] [Google Scholar]
- Deinzer R, Weik U, Kolb-Bachofen V, Herforth A. Comparison of experimental gingivitis with persistent gingivitis: differences in clinical parameters and cytokine concentrations. Journal of Periodontal Research. 2007;42:318–324. doi: 10.1111/j.1600-0765.2006.00951.x. [DOI] [PubMed] [Google Scholar]
- Di Filippo C, Rossi F, D’Amico M. Targeting polymorphonuclear leukocytes in acute myocardial infarction. ScientificWorld Journal. 2007;7:121–134. doi: 10.1100/tsw.2007.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Padova F, Pozzi C, Tondre MJ, Tritapepe R. Selective and early increase of IL-1 inhibitors, IL-6 and cortisol after elective surgery. Clinical and Experimental Immunology. 1991;85:137–142. doi: 10.1111/j.1365-2249.1991.tb05694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorresteijn MJ, Draisma A, van der Hoeven JG, Pickkers P. Lipopolysaccharide-stimulated whole blood cytokine production does not predict the inflammatory response in human endotoxemia. Innate Immunity. 2010;16:248–253. doi: 10.1177/1753425909339923. [DOI] [PubMed] [Google Scholar]
- Ebersole JL, Singer RE, Steffensen B, Filloon T, Kornman KS. Inflammatory mediators and immunoglobulins in GCF from healthy, gingivitis and periodontitis sites. Journal of Periodontal Research. 1993;28(6 Pt 2):543–546. doi: 10.1111/j.1600-0765.1993.tb02121.x. [DOI] [PubMed] [Google Scholar]
- Ebersole JL, Cappelli D, Mott G, Kesavalu L, Holt SC, Singer RE. Systemic manifestations of periodontitis in the non-human primate. Journal of Periodontal Research. 1999;34:358–362. doi: 10.1111/j.1600-0765.1999.tb02266.x. [DOI] [PubMed] [Google Scholar]
- Fittschen C, Sandhaus RA, Worthen GS, Henson PM. Bacterial lipopolysaccharide enhances chemoattractant-induced elastase secretion by human neutrophils. Journal of Leukcyte Biology. 1988;43:547–556. doi: 10.1002/jlb.43.6.547. [DOI] [PubMed] [Google Scholar]
- Forner L, Larsen T, Kilian M, Holmstrup P. Incidence of bacteremia after chewing, toothbrushing and scaling in individuals with periodontal inflammation. Journal of Clinical Periodontology. 2006;33:401–407. doi: 10.1111/j.1600-051X.2006.00924.x. [DOI] [PubMed] [Google Scholar]
- Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. New England Journal of Medicine. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- Geerts SO, Nys M, De MP, Charpentier J, Albert A, Legrand V, Rompen EH. Systemic release of endotoxins induced by gentle mastication: association with periodontitis severity. Journal of Periodontology. 2002;73:73–78. doi: 10.1902/jop.2002.73.1.73. [DOI] [PubMed] [Google Scholar]
- Górska R, Gregorek H, Kowalski J, Laskus-Perendyk A, Syczewska M, Madaliński K. Relationship between clinical parameters and cytokine profiles in inflamed gingival tissue and serum samples from patients with chronic periodontitis. Journal of Clinical Periodontology. 2003;30:1046–1052. doi: 10.1046/j.0303-6979.2003.00425.x. [DOI] [PubMed] [Google Scholar]
- Guthrie LA, McPhail LC, Henson PM, Johnston RB., Jr Priming of neutrophils for enhanced release of oxygen metabolites by bacterial lipopolysaccharide. Evidence for increased activity of the superoxide-producing enzyme. Journal of Experimental Medicine. 1984;160:1656–1671. doi: 10.1084/jem.160.6.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen PR. Role of neutrophils in myocardial ischemia and reperfusion. Circulation. 1995;91:1872–1885. doi: 10.1161/01.cir.91.6.1872. [DOI] [PubMed] [Google Scholar]
- Haraszthy VI, Zambon JJ, Trevisan M, Zeid M, Genco RJ. Identification of pathogens in atheromatous plaques. Journal of Periodontology. 2000;71:1554–1560. doi: 10.1902/jop.2000.71.10.1554. [DOI] [PubMed] [Google Scholar]
- Ishida T, Tanaka K. Effects of fibrin and fibrinogen-degradation products on the growth of rabbit aortic smooth muscle cells in culture. Atherosclerosis. 1982;44:161–174. doi: 10.1016/0021-9150(82)90111-3. [DOI] [PubMed] [Google Scholar]
- Kinane DF, Riggio MP, Walker KF, MacKenzie D, Shearer B. Bacteraemia following periodontal procedures. Journal of Clinical Periodontology. 2005;32:708–713. doi: 10.1111/j.1600-051X.2005.00741.x. [DOI] [PubMed] [Google Scholar]
- Kowolik MJ, Dowsett SA, Rodriguez J, De La Rosa RM, Eckert GJ. Systemic neutrophil response resulting from dental plaque accumulation. Journal of Periodontology. 2001;72:146–151. doi: 10.1902/jop.2001.72.2.146. [DOI] [PubMed] [Google Scholar]
- Kurihara N, Inoue Y, Iwai T, Umeda M, Huang Y, Ishikawa I. Detection and localization of periodontopathic bacteria in abdominal aortic aneurysms. European Journal of Vascular and Endovascular Surgery. 2004;28:553–558. doi: 10.1016/j.ejvs.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Kweider M, Lowe GD, Murray GD, Kinane DF, McGowan DA. Dental disease, fibrinogen and white cell count; links with myocardial infarction? Scottish Medical Journal. 1993;38:73–74. doi: 10.1177/003693309303800304. [DOI] [PubMed] [Google Scholar]
- Lindemann RA, Economou JS, Rothermel H. Production of interleukin-1 and tumor necrosis factor by human peripheral monocytes activated by periodontal bacteria and extracted lipopolysaccharides. Journal of Dental Research. 1988;67:1131–1135. doi: 10.1177/00220345880670081401. [DOI] [PubMed] [Google Scholar]
- Löe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontologica Scandinavica. 1963;21:533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- Löe H, Theilade E, Jensen SB. Experimental gingivitis in man. Journal of Periodontology. 1965;36:177–1787. doi: 10.1902/jop.1965.36.3.177. [DOI] [PubMed] [Google Scholar]
- Lowe GD, Lee AJ, Rumley A, Price JF, Fowkes FG. Blood viscosity and risk of cardiovascular events: the Edinburgh Artery Study. British Journal of Haematology. 1997;96:168–173. doi: 10.1046/j.1365-2141.1997.8532481.x. [DOI] [PubMed] [Google Scholar]
- Matthews JB, Wright HJ, Roberts A, Cooper PR, Chapple IL. Hyperactivity and reactivity of peripheral blood neutrophils in chronic periodontitis. Clinical & Experimental Immunology. 2007;147:255–264. doi: 10.1111/j.1365-2249.2006.03276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila KJ, Pussinen PJ, Paju S. Dental infections and cardiovascular diseases: a review. Journal of Periodontology. 2005;76(11 Suppl):2085–2088. doi: 10.1902/jop.2005.76.11-S.2085. [DOI] [PubMed] [Google Scholar]
- Munro JM. Endothelial-leukocyte adhesive interactions in inflammatory diseases. European Heart Journal. 1993;14(Suppl K):72–77. [PubMed] [Google Scholar]
- Miyagi M, Aoyama H, Morishita M, Iwamoto Y. Effects of sex hormones on chemotaxis of human peripheral polymorphonuclear leukocytes and monocytes. Journal of Periodontology. 1992;63:28–32. doi: 10.1902/jop.1992.63.1.28. [DOI] [PubMed] [Google Scholar]
- Molloy EJ, O’Neill AJ, Grantham JJ, Sheridan-Pereira M, Fitzpatrick JM, Webb DW, Watson WG. Sex-specific alterations in neutrophil apoptosis: the role of estradiol and progesterone. Blood. 2003;102:2653–2659. doi: 10.1182/blood-2003-02-0649. [DOI] [PubMed] [Google Scholar]
- Nachman RL, Hajjar KA, Silverstein RL, Dinarello CA. Interleukin 1 induces endothelial cell synthesis of plasminogen activator inhibitor. Journal of Experimental Medicine. 1986;163:1595–1600. doi: 10.1084/jem.163.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noack B, Genco RJ, Trevisan M, Grossi S, Zambon JJ, De Nardin E. Periodontal infections contribute to elevated systemic C-reactive protein level. Journal of Periodontology. 2001;72:1221–1227. doi: 10.1902/jop.2000.72.9.1221. [DOI] [PubMed] [Google Scholar]
- Offenbacher S, Barros S, Mendoza L, Mauriello S, Preisser J, Moss K, de Jager M, Aspiras M. Changes in gingival crevicular fluid inflammatory mediator levels during the induction and resolution of experimental gingivitis in humans. Journal of Clinical Periodontology. 2010;37:324–333. doi: 10.1111/j.1600-051X.2010.01543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraskevas S, Huizinga JD, Loos BG. A systematic review and meta-analyses on C-reactive protein in relation to periodontitis. Journal of Clinical Periodontology. 2008;35:277–290. doi: 10.1111/j.1600-051X.2007.01173.x. [DOI] [PubMed] [Google Scholar]
- Salvi GE, Kandylaki M, Troendle A, Persson GR, Lang NP. Experimental gingivitis in type 1 diabetics: a controlled clinical and microbiological study. Journal of Clinical Periodontology. 2005;32:310–316. doi: 10.1111/j.1600-051X.2005.00682.x. [DOI] [PubMed] [Google Scholar]
- Salvi GE, Franco LM, Braun TM, Lee A, Rutger Persson G, Lang NP, Giannobile WV. Pro-inflammatory biomarkers during experimental gingivitis in patients with type 1 diabetes mellitus: a proof-of-concept study. Journal of Clinical Periodontology. 2010;37:9–16. doi: 10.1111/j.1600-051X.2009.01500.x. [DOI] [PubMed] [Google Scholar]
- Silness J, Löe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontologica Scandinavica. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- Silver JG, Martin AW, McBride BC. Experimental transient bacteraemias in human subjects with varying degrees of plaque accumulation and gingival inflammation. Journal of Clinical Periodontology. 1977;4:92–99. doi: 10.1111/j.1600-051x.1977.tb01888.x. [DOI] [PubMed] [Google Scholar]
- Spitzer JA. Gender differences in some host defense mechanisms. Lupus. 1999;8:380–383. doi: 10.1177/096120339900800510. [DOI] [PubMed] [Google Scholar]
- Theilade E, Wright WH, Jensen SB, Löe H. Experimental gingivitis in man. II. A longitudinal clinical and bacteriological investigation. Journal of Periodontal Research. 1966;1:1–13. doi: 10.1111/j.1600-0765.1966.tb01842.x. [DOI] [PubMed] [Google Scholar]
- Trombelli L, Scapoli C, Carrieri A, Giovannini G, Calura G, Farina R. Interleukin-1 beta levels in gingival crevicular fluid and serum under naturally occurring and experimentally induced gingivitis. Journal of Clinical Periodontology. 2010;37:697–704. doi: 10.1111/j.1600-051X.2010.01573.x. [DOI] [PubMed] [Google Scholar]
- van Eijk LT, Dorresteijn MJ, Smits P, van der Hoeven JG, Netea MG, Pickkers P. Gender differences in the innate immune response and vascular reactivity following the administration of endotoxin to human volunteers. Critical Care Medicine. 2007;35:1464–1469. doi: 10.1097/01.CCM.0000266534.14262.E8. [DOI] [PubMed] [Google Scholar]
- Volanakis JE. Complement activation by C-reactive protein complexes. Annals of The New York Academy of Sciences. 1982;389:235–250. doi: 10.1111/j.1749-6632.1982.tb22140.x. [DOI] [PubMed] [Google Scholar]
- Wahaidi VY, Dowsett SA, Eckert GJ, Kowolik MJ. Neutrophil response to dental plaque by gender and race. Journal of Dental Research. 2009;88:709–714. doi: 10.1177/0022034509339019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedermann CJ, Kiechl S, Dunzendorfer S, Schratzberger P, Egger G, Oberhollenzer F, Willeit J. Association of endotoxemia with carotid atherosclerosis and cardiovascular disease: prospective results from the Bruneck Study. Journal of the American College of Cardiology. 1999;34:1975–1981. doi: 10.1016/s0735-1097(99)00448-9. [DOI] [PubMed] [Google Scholar]
- Wittkowski H, Sturrock A, van Zoelen MA, Viemann D, van der Poll T, Hoidal JR, Roth J, Foell D. Neutrophil-derived S100A12 in acute lung injury and respiratory distress syndrome. Critical Care Medicine. 2007;35:1369–1375. doi: 10.1097/01.CCM.0000262386.32287.29. [DOI] [PubMed] [Google Scholar]
- Yoshimura T, Matsushima K, Oppenheim JJ, Leonard EJ. Neutrophil chemotactic factor produced by lipopolysaccharide (LPS)-stimulated human blood mononuclear leukocytes: partial characterization and separation from interleukin 1 (IL 1) Journal of Immunology. 1987;139:788–793. [PubMed] [Google Scholar]
- Zoellner H, Chapple CC, Hunter N. Microvasculature in gingivitis and chronic periodontitis: disruption of vascular networks with protracted inflammation. Microscopy Research and Technique. 2002;56:15–31. doi: 10.1002/jemt.10009. [DOI] [PubMed] [Google Scholar]