Abstract
The increased activity of intrarenal renin–angiotensin system (RAS) in a setting of elevated arterial pressure elicits renal vasoconstriction, increased sodium reabsorption, proliferation, fibrosis and renal injury. Increases in intrarenal and interstitial angiotensin (Ang) II levels are due to increased AT1 receptor mediated Ang II uptake and stimulation of renal angiotensinogen (AGT) mRNA and protein expression. Augmented proximal tubule AGT production increases tubular AGT secretion and spillover of AGT into the distal nephron and urine. Increased renin formation by principal cells of the collecting ducts forms Ang I from AGT thus increasing Ang II. The catalytic actions of renin and prorenin are enhanced by prorenin receptors (PRRs) on the intercalated cells. The resultant increased intrarenal Ang II levels contribute to the genesis of chronic hypertension.
Introduction
The intrarenal renin–angiotensin system (RAS) regulates a diversity of renal hemodynamic and transport processes which contribute to sodium balance and blood pressure homeostasis [1]. Angiotensin II (Ang II), the most potent component of the RAS, exerts pleotropic actions on the renal microvascularture, the tubular network and the interstitium. Although there are two major receptor subtypes responsive to Ang II (AT1 and AT2), the AT1 receptor is primarily responsible for the hypertensinogenic actions of the RAS. Through its effects on AT1 receptors, Ang II regulates vascular tone of the afferent and efferent arterioles and the glomerular filtration coefficient [2]. It also exerts major influences on several tubule transporters including the Na+/H+ exchanger and the Na+/HCO3− co-transporter in proximal tubules and the amiloride sensitive sodium channel (ENaC) and Na+/Cl− co-transporter in distal nephron segments [3]. Ang II modulates the sensitivity of the tubuloglomerular feedback mechanism and regulates the medullary microvasculature by directly constricting the pericytes in the vasa recta [2,4]. These multiple actions of Ang II act in a synergistic manner to increase the capability of the kidneys to conserve sodium and maintain blood pressure under conditions of sodium depletion, loss of extracellular fluid volume and hypotension. When inappropriately activated, however, the intrarenal RAS leads to excessive sodium retention coupled with increased pressor activity and the development of Ang II dependent hypertension [1,5].
Angiotensin dependent hypertension
There are many models of Ang II dependent hypertension including the 2-kidney, 1-clip (2K1C) Goldblatt model [6], the chronic Ang II infusion model [7] and transgenic rat and mouse models of hypertension [5,8–10]. In these Ang II dependent hypertensive models, intrarenal Ang II content increases progressively to levels that cannot be explained on the basis of simple equilibration with plasma Ang II concentrations [11]. The increased intrarenal Ang II content results from both AT1 receptor mediated uptake of circulating Ang II and de novo intrarenal Ang II generation as a consequence of local augmentation of intrarenal angiotensinogen (AGT) produced and secreted by proximal tubule cells [12,13]. These mechanisms lead to increased intrarenal, interstitial, and intratubular Ang II concentrations even under conditions where plasma renin activity (PRA) is markedly suppressed [14–17].
Intrarenal angiotensinogen
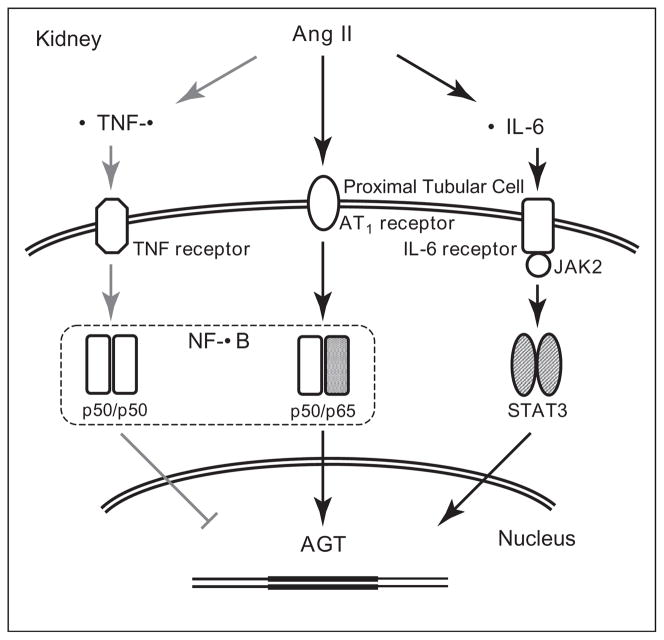
Chronic Ang II infusions resulting in moderate increases in circulating Ang II stimulate intrarenal AGT mRNA and protein in proximal tubule cells [1,12]. Ang II infusion increases intrarenal NF-κB activity [18]. Activation of NF-κB plays an important role in the stimulation of AGT expression in cultured proximal tubule cells [19]. Moreover, as shown in Figure 1, Ang II elicits intrarenal pro-inflammatory cytokine expression such as interleukin-6 (IL-6) [20••,21••]. As indicated in Figure 2, IL-6 contributes to the increase in AGT expression via activation of a JAK-STAT pathway [19]. These results indicate that Ang II stimulates AGT expression via both direct and indirect mechanisms mediated by NF-κB and cytokines in renal proximal tubular cells. IL-6 knockout reduces the activation of intrarenal JAK-STAT pathway and the severity of the hypertension [22•,23•]. In contrast, tumor necrosis factor α, which is also an Ang II-induced pro-inflammatory factor in the kidney, suppresses AGT expression through the formation of p50/p50 complex (Figure 2) in cultured renal proximal tubular cells [24]. This action serves to counteract or limit Ang II-induced AGT augmentation in renal proximal tubular cells which may explain how higher Ang II doses into mice fail to stimulate intrarenal AGT levels [7]. Interestingly, while chronic Ang II infusions tend to downregulate AT1 receptors in vascular smooth muscle cells, the AT1 receptors in tubular cells are either upregulated or maintained [1,25], thus allowing sustained actions on proximal tubule AGT as well as stimulation of sodium reabsorption. In Ang II dependent hypertension, there are associated increases in Ang II levels in the renal interstitial fluid which can contribute to increased renal microvascular tone and increased tubular reabsorption [15].
Figure 1.
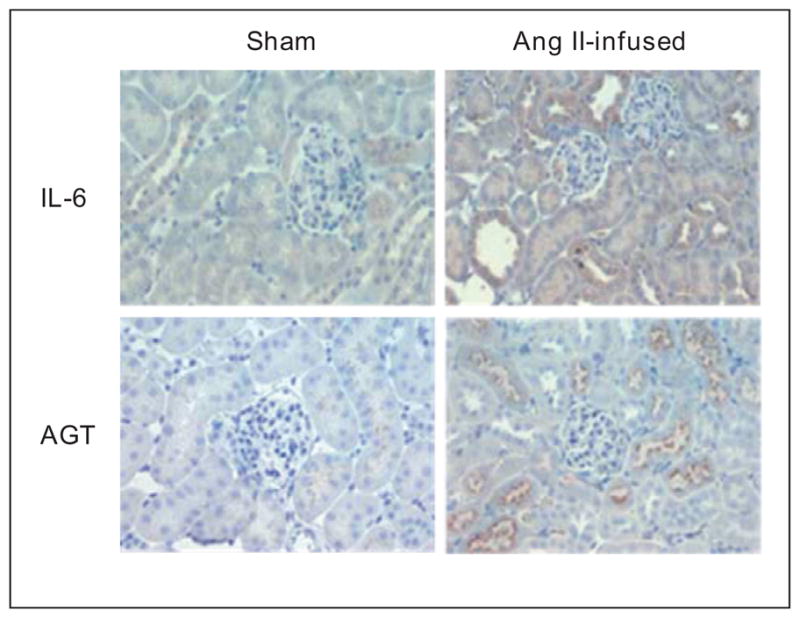
Stimulation of intrarenal IL-6 and AGT levels in Ang II-infused mice. Chronic Ang II infusion increases the expression of intrarenal IL-6 and AGT in mice. Ang II was infused at a dose of 400 ng/kg/min for two weeks via osmotic minipump.
Figure 2.
Schematic summary of regulation of AGT expression in renal proximal tubular cells. The black arrows indicate mechanisms of augmentation of AGT expression. The gray arrows indicate a mechanism of suppression of AGT expression. Derived from Satou et al. [19,24].
AGT is secreted into the proximal tubular lumen where it gives rise to Ang I and Ang II formation at the level of the proximal tubule thereby stimulating proximal sodium reabsorption rate [26]. Furthermore, the increased AGT expression and secretion in Ang II dependent hypertension lead to spillover into distal nephron segments leading to increased urinary excretion of AGT [27]. Thus, AGT secreted into the lumen of proximal tubules traverses through the distal nephron segments and provides substrate for further downstream Ang I and Ang II generation. Importantly, in the presence of oxidative stress, which exists in hypertension and other vascular diseases, the AGT molecule undergoes a subtle conformational rearrangement to a form that more effectively releases Ang I when exposed to renin [28••], thus facilitating increased generation of Ang II. The increased urinary AGT is derived from the enhanced proximal tubule AGT secretion as well as increased filtration of AGT due to slight increases in glomerular permeability [29,30•]. Nevertheless, several studies have demonstrated that urinary AGT provides an index of the intrarenal Ang II levels in Ang II-infused rats [1,27,31]. To extend these studies to human subjects, an assay was developed to quantitatively measure urinary AGT in humans [32]. Studies using this human AGT ELISA, demonstrated elevated urinary AGT in hypertensive subjects not treated with blockers or inhibitors of the RAS [33] and also found that urinary AGT is correlated with blood pressure in humans participating in the Bogalusa Heart Study [34].
Collecting duct renin and Ang II
Renin from juxtaglomerular apparatus (JGA) cells is released primarily into the interstitium but JGA renin is suppressed in chronic Ang II infused hypertensive rats [17]. Thus, the source of intratubular renin available to act on intratubular AGT has remained unclear. It is now recognized, however, that renin is also expressed by the principal cells of connecting tubules and cortical and medullary collecting ducts (CD) from rat, mouse and human kidneys [13,35••,36]. In distal nephron segments, renin resides in principal cells co-localizing with aquaporin 2 [36]. Importantly, renin in distal nephron segments is differentially regulated from renin in JGA cells. In response to chronic Ang II infusions, renin mRNA and protein levels in principal cells are stimulated leading to increased distal nephron renin expression during Ang II-dependent hypertension [36]. This effect is an AT1 receptor-mediated process since treatment with an AT1 receptor blocker prevents the stimulation of distal nephron renin mRNA and protein levels [37], a response distinct from the well known effect of AT1 receptor blockade to stimulate JGA renin levels. These results indicate that the regulation of renin in principal cells of the CD is different from that of JGA cells, and help to explain the marked reduction in sodium excretion and impairment in pressure natriuresis that occurs with chronic Ang II infusions [8,38,39].
The augmentation of CD renin in chronic Ang II-infused rats does not distinguish between the direct effects of Ang II versus those possibly due to the effects of chronic elevations in arterial blood pressure. To distinguish between these effects, renin gene expression in the CD of clipped and non-clipped kidneys from 2K1C Goldblatt hypertensive rats was measured three weeks after clipping one renal artery [40]. We observed increases in distal nephron renin expression in both clipped and non-clipped kidneys indicating that the stimulation occurs independently of blood pressure [40]. The results suggest that in 2K1C Goldblatt hypertensive rats, there is a direct positive effect exerted by intrarenal Ang II to stimulate renin expression in CD cells. In further studies, we observed enhancement of ACE and inhibition of ACE2 in both kidneys associated with substantial decreases in intrarenal Ang 1–7 levels suggesting that reductions in ACE2 activity provide another mechanism for increased Ang II levels [41]. Collectively, the results provide further support to the hypothesis that the increased AGT spillover into distal nephron segments leads to increased distal intratubular Ang I formation and subsequent conversion to Ang II, which increases CD Ang II concentration. Increases in Ang II concentrations in CD, as reflected by increases in urinary Ang II concentrations, have been shown in studies in rats [16,17] and mice [39,42•]. In particular, rats infused chronically with Val5-Ang II show a progressive increase in urinary concentration of endogenous Ang II (Ile5-Ang) indicating increased tubular production of Ang II [17]. Furthermore, AT1 receptor blockers prevented this progressive increase indicating that chronic augmentation requires AT1 receptor activation [16].
Prorenin receptor
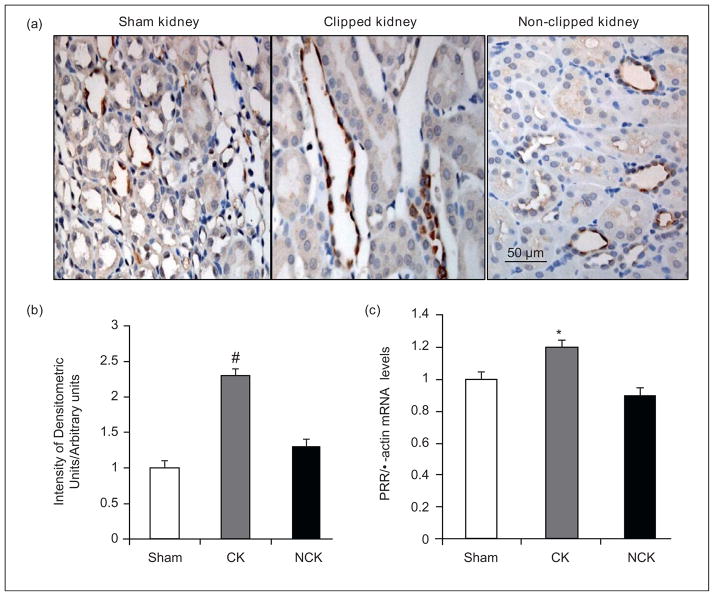
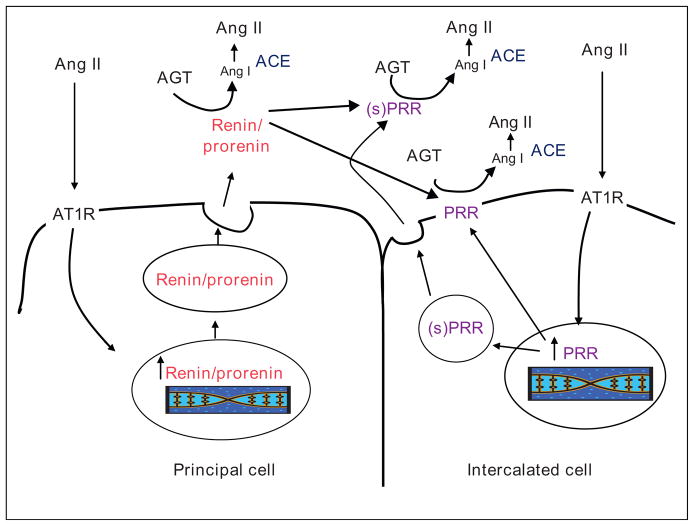
Recently, a prorenin receptor (PRR), which binds both renin and prorenin and activates prorenin, was cloned and found to be localized to lung, brain, placenta and kidneys [43••,44••]. In the kidney, the PRR is present in mesangial cells, podocytes, renal arteries and tubules. In particular, the PRR is present on intercalated cells of the CDs [44••]. These results suggest the intriguing hypothesis that prorenin or renin formed by principal cells and secreted into the distal tubular fluid is anchored by the PRR on the apical surface of the intercalated cells thus increasing the catalytic activity for Ang I generation and reducing washout of prorenin or renin into the urine [45,46]. Indeed, we recently showed increases in PRR mRNA levels and specific immunoreactivity in the medullary tissues from clipped kidney (CK) but not in the non-clipped kidneys of 2K1C Goldblatt hypertensive rats (NCK) (Figure 3). In addition, increased levels of the PRR transcript and the soluble form of the PRR in renal medullary tissues and urine of chronic Ang II-infused rats and Cyp1a1Ren2 transgenic rats with Ang II-dependent malignant hypertension have been reported [47,48]. It is possible that the soluble form of PRR activates prorenin secreted by CD cells and, together with the upregulation of ACE, further enhance the formation of Ang II in the distal nephron segments [41,49]. The increased Ang II directly stimulates sodium reabsorption in CD cells [50] which contributes to the suppression of the pressure-natriuretic response to elevations in arterial blood pressure in Ang II infused rats [38] and the non-clipped kidney of 2K1C Goldblatt rats [51]. As depicted in Figure 4, the presence of prorenin, renin and PRR in distal nephron segments [36,40,48] may provide a critical final mechanism for intratubular Ang I and Ang II formation and, together with the augmentation of proximal tubule AGT expression, play a major role in the genesis and maintenance of chronic hypertension [45].
Figure 3.
Prorenin receptor (PRR) immunoreactivity and mRNA in collecting duct cells. (a) Specific PRR immunoreactivity (brown, DAB chromogen) is shown in the collecting duct cells of sham (left panel), clipped (middle panel) and non-clipped (right panel) kidney medullary regions. (b) Densitometric analysis of PRR intensity immunoreactivity in collecting duct cells of both kidneys (CK and NCK) of Goldblatt hypertensive rats was performed. Sham rats (n = 5), 2K1C rats (n = 6). (c) PRR mRNA levels were quantified in the renal medullary tissues of sham kidneys, and CK and NCK of Goldblatt hypertensive rats by qRT-PCR using samples in triplicate and values expressed relative to β-actin in arbitrary units. Values are mean ± S.E. *P < 0.05 versus sham rats. PRR (Abcam 5959) antibody dilution used 1:200. CK: clipped kidney; NCK: non-clipped kidney. #P < 0.05 clipped kidney versus non-clipped kidney. IDU: integrated densitometric units.
Figure 4.
Renin and prorenin receptor interaction in the collecting duct. Ang II mediated stimulation of renin and prorenin in the principal cells of the collecting ducts may increase intrarenal and intratubular Ang I and consequently Ang II content. The presence of prorenin receptor at the surface of the intercalated cells increases the catalytic activity for Ang I generation and may anchor renin and prorenin secreted by the principal cells to reduce washout into the urine. The availability of ACE in distal nephron segments along with reduction in ACE2 facilitates subsequent enhanced formation of Ang II. Ang: angiotensin; AGT: angiotensinogen; ACE: angiotensin converting enzyme; AT1R: angiotensin II type 1 receptor; PRR: prorenin receptor; (s)PRR: soluble form of the prorenin receptor.
Conclusion
Experimental findings within recent years have demonstrated the complexity of the mechanisms regulating renal interstitial and tubular Ang II concentrations. An initial inappropriate increase in Ang II can lead to a positive augmentation of intratubular AGT and CD renin activity which exacerbates the hypertension. Thus it is essential to recognize the importance of blocking the intrarenal RAS in order to achieve good blood pressure control and restoration of normal renal function. Toward this end, measurements of urinary AGT provide one means of determining the efficacy of the therapeutic regimen. The available data have identified the key components responsible for the augmented intrarenal RAS activity, but their specific quantitative contributions to the genesis and maintenance of chronic hypertension remain to be determined.
Acknowledgments
The authors thank Debbie Olavarrieta for her assistance in preparing the manuscript. Research support to the authors includes grants from National Institutes of Health (RO1HL-26371, RO1DK-072408, P20RR-017659, and K12HD043451) and from the American Heart Association (09BGIA2280440 and 10GRNT3020018).
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin–angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 2.Navar LG, Arendshorst WJ, Pallone TL, Inscho EW, Imig JD, Bell PD. The renal microcirculation. In: Tuma RF, Duran WN, Ley K, editors. Handbook of physiology: microcirculation. Academic Press; 2008. pp. 550–683. [Google Scholar]
- 3.Navar LG, Prieto-Carrasquero MC, Kobori H. Molecular aspects of the renal renin–angiotensin system. In: Re R, DiPette DJ, Schiffrin EL, Sowers JR, editors. Molecular mechanisms in hypertension. Taylor & Francis Group; 2006. pp. 3–14. [Google Scholar]
- 4.Pallone TL, Huang JM. Control of descending vasa recta pericyte membrane potential by angiotensin II. Am J Physiol Renal Physiol. 2002;282:F1064–F1074. doi: 10.1152/ajprenal.00306.2001. [DOI] [PubMed] [Google Scholar]
- 5.Coffman TM, Crowley SD. Kidney in hypertension: guyton redux. Hypertension. 2008;51:811–816. doi: 10.1161/HYPERTENSIONAHA.105.063636. [DOI] [PubMed] [Google Scholar]
- 6.Cervenka L, Vaneckova I, Huskova Z, Vanourkova Z, Erbanova M, Thumova M, Skaroupkova P, Opocensky M, Maly J, Chabova VC, et al. Pivotal role of angiotensin II receptor subtype 1A in the development of two-kidney, one-clip hypertension: study in angiotensin II receptor subtype 1A knockout mice. J Hypertens. 2008;26:1379–1389. doi: 10.1097/HJH.0b013e3282fe6eaa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez-Villalobos RA, Seth DM, Satou R, Horton H, Ohashi N, Miyata K, Katsurada A, Tran DV, Kobori H, Navar LG. Intrarenal angiotensin II and angiotensinogen augmentation in chronic angiotensin II-infused mice. Am J Physiol Renal Physiol. 2008;295:F772–F779. doi: 10.1152/ajprenal.00019.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell KD, Bagatell SJ, Miller CS, Mouton CR, Seth DM, Mullins JJ. Genetic clamping of renin gene expression induces hypertension and elevation of intrarenal Ang II levels of graded severity in Cyp1a1-Ren2 transgenic rats. J Renin Angiotensin Aldosterone Syst. 2006;7:74–86. doi: 10.3317/jraas.2006.013. [DOI] [PubMed] [Google Scholar]
- 9.Smithies O. A mouse view of hypertension. Hypertension. 1997;30:1318–1324. doi: 10.1161/01.hyp.30.6.1318. [DOI] [PubMed] [Google Scholar]
- 10.Kobori H, Ozawa Y, Satou R, Katsurada A, Miyata K, Ohashi N, Hase N, Suzaki Y, Sigmund CD, Navar LG. Kidney-specific enhancement of ANG II stimulates endogenous intrarenal angiotensinogen in gene-targeted mice. Am J Physiol Renal Physiol. 2007;293:F938–F945. doi: 10.1152/ajprenal.00146.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Navar LG, Harrison-Bernard LM, Nishiyama A, Kobori H. Regulation of intrarenal angiotensin II in hypertension. Hypertension. 2002;39:316–322. doi: 10.1161/hy0202.103821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobori H, Prieto-Carrasquero MC, Ozawa Y, Navar LG. AT1 receptor mediated augmentation of intrarenal angiotensinogen in angiotensin II-dependent hypertension. Hypertension. 2004;43:1126–1132. doi: 10.1161/01.HYP.0000122875.91100.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lalouel JM, Rohrwasser A. Genetic susceptibility to essential hypertension: insight from angiotensinogen. Hypertension. 2007;49:597–603. doi: 10.1161/01.HYP.0000257145.20363.9c. [DOI] [PubMed] [Google Scholar]
- 14.Zou LX, Imig JD, Von Thun AM, Hymel A, Ono H, Navar LG. Receptor-mediated intrarenal angiotensin II augmentation in angiotensin II-infused rats. Hypertension. 1996;28:669–677. doi: 10.1161/01.hyp.28.4.669. [DOI] [PubMed] [Google Scholar]
- 15.Nishiyama A, Seth DM, Navar LG. Angiotensin II type 1 receptor-mediated augmentation of renal interstitial fluid angiotensin II in angiotensin II-induced hypertension. J Hypertens. 2003;21:1897–1903. doi: 10.1097/00004872-200310000-00017. [DOI] [PubMed] [Google Scholar]
- 16.Shao W, Seth DM, Navar LG. Angiotensin II type 1 receptor-mediated augmentation of urinary excretion of endogenous angiotensin II in Val5-angiotensin II-infused rats. Hypertension. 2010;56:378–383. doi: 10.1161/HYPERTENSIONAHA.110.153106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shao W, Seth DM, Navar LG. Augmentation of endogenous intrarenal angiotensin II levels in Val5-ANG II-infused rats. Am J Physiol Renal Physiol. 2009;296:F1067–F1071. doi: 10.1152/ajprenal.90596.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozawa Y, Kobori H, Suzaki Y, Navar LG. Sustained renal interstitial macrophage infiltration following chronic angiotensin II infusions. Am J Physiol Renal Physiol. 2007;292:F330–F339. doi: 10.1152/ajprenal.00059.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Satou R, Gonzalez-Villalobos RA, Miyata K, Ohashi N, Katsurada A, Navar LG, Kobori H. Costimulation with angiotensin II and interleukin 6 augments angiotensinogen expression in cultured human renal proximal tubular cells. Am J Physiol Renal Physiol. 2008;295:F283–F289. doi: 10.1152/ajprenal.00047.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20••.Ruiz-Ortega M, Ruperez M, Lorenzo O, Esteban V, Blanco J, Mezzano S, Egido J. Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney. Kidney Int Suppl. 2002;82:S12–S22. doi: 10.1046/j.1523-1755.62.s82.4.x. The authors demonstrated that Ang II infusion stimulates intrarenal proinflammatory factors including TNF-α, IL-6 and MCP-1, and activates intrarenal NF-κB. These findings suggest that Ang II plays an active role in the inflammatory response in renal diseases. [DOI] [PubMed] [Google Scholar]
- 21••.Brasier AR, Recinos A, III, Eledrisi MS. Vascular inflammation and the renin–angiotensin system. Arterioscler Thromb Vasc Biol. 2002;22:1257–1266. doi: 10.1161/01.atv.0000021412.56621.a2. In this review, the authors provide evidence that Ang II has significant proinflammatory actions in the vascular wall, inducing the production of reactive oxygen species, inflammatory cytokines, and adhesion molecules. Furthermore, they propose an important role of Ang II-IL-6-JAK/STAT-AGT positive feedback system in the development of vascular inflammation. [DOI] [PubMed] [Google Scholar]
- 22•.Brands MW, Banes-Berceli AK, Inscho EW, Al Azawi H, Allen AJ, Labazi H. Interleukin 6 knockout prevents angiotensin II hypertension: role of renal vasoconstriction and janus kinase 2/signal transducer and activator of transcription 3 activation. Hypertension. 2010;56:879–884. doi: 10.1161/HYPERTENSIONAHA.110.158071. This paper reports that Ang II infusion decreases renal blood flow in both wild-type and IL-6 knockout mice although the increase in blood pressure caused by Ang II infusion was attenuated in IL-6 knockout mice. They also report that activation of JAK2/STAT3 pathway by the Ang II infusion in renal cortex was prevented in IL-6 knockout mice indicating that activation of the renal JAK2/STAT3 pathway plays a role in Ang II hypertension but not in the effect of Ang II to decrease total RBF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23•.Lee DL, Sturgis LC, Labazi H, Osborne JB, Jr, Fleming C, Pollock JS, Manhiani M, Imig JD, Brands MW. Angiotensin II hypertension is attenuated in interleukin-6 knockout mice. Am J Physiol Heart Circ Physiol. 2006;290:H935–H940. doi: 10.1152/ajpheart.00708.2005. The authors demonstrated that the increase in blood pressure by Ang II infusion is attenuated in IL-6 knockout mice. The finding indicates that IL-6 contributes to the development of Ang II-dependent hypertension. [DOI] [PubMed] [Google Scholar]
- 24.Satou R, Miyata K, Katsurada A, Navar LG, Kobori H. Tumor necrosis factor-{alpha} suppresses angiotensinogen expression through formation of a p50/p50 homodimer in human renal proximal tubular cells. Am J Physiol Cell Physiol. 2010;299:C750–C759. doi: 10.1152/ajpcell.00078.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrison-Bernard LM, Zhuo J, Kobori H, Ohishi M, Navar LG. Intrarenal AT1 receptor and ACE binding in angiotensin II-induced hypertensive rats. Am J Physiol Renal Physiol. 2002;282:F19–F25. doi: 10.1152/ajprenal.00335.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Navar LG, Harrison-Bernard LM, Wang C-T, Cervenka L, Mitchell KD. Concentrations and actions of intraluminal angiotensin II. J Am Soc Nephrol. 1999;10:S189–S195. [PubMed] [Google Scholar]
- 27.Kobori H, Nishiyama A, Harrison-Bernard LM, Navar LG. Urinary angiotensinogen as an indicator of intrarenal angiotensin status in hypertension. Hypertension. 2003;41:42–49. doi: 10.1161/01.hyp.0000050102.90932.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28••.Zhou A, Carrell RW, Murphy MP, Wei Z, Yan Y, Stanley PL, Stein PE, Pipkin FB, Read RJ. A redox switch in angiotensinogen modulates angiotensin release. Nature. 2010;468:108–111. doi: 10.1038/nature09505. By evaluation of the crystal structures of the renin–AGT complex, the authors show the existence of two different structures of AGT dependent on an oxidative switch which may alter reactivity to renin. Moreover, they demonstrated that a ratio of these AGT forms is different in pre-eclampsia and suggest that the redox-response transition of angiotensinogen allows it to more readily release Ang II. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milani CJ, Kobori H, Mullins JJ, Mitchell KD. Enhanced urinary angiotensinogen excretion in Cyp1a1-Ren2 transgenic rats with inducible ANG II-dependent malignant hypertension. Am J Med Sci. 2010;340:389–394. doi: 10.1097/MAJ.0b013e3181eabd28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30•.Pohl M, Kaminski H, Castrop H, Bader M, Himmerkus N, Bleich M, Bachmann S, Theilig F. Intrarenal renin angiotensin system revisited: role of megalin-dependent endocytosis along the proximal nephron. J Biol Chem. 2010;285:41935–41946. doi: 10.1074/jbc.M110.150284. The authors reveal distributions of components of renin–angiotensin system in renal proximal tubular cells indicating that angiotensinogen mRNA expression is primarily localized to the latter segments of the proximal tubules. In addition, they demonstrate that AGT protein is taken up into renal proximal tubular cells via the megalin receptor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int. 2002;61:579–585. doi: 10.1046/j.1523-1755.2002.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katsurada A, Hagiwara Y, Miyashita K, Satou R, Miyata K, Ohashi N, Navar LG, Koboir H. Novel sandwich ELISA for human angiotensinogen. Am J Physiol Renal Physiol. 2007;293:F956–F960. doi: 10.1152/ajprenal.00090.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobori H, Alper AB, Jr, Shenava R, Katsurada A, Saito T, Ohashi N, Urushihara M, Miyata K, Satou R, Hamm LL, Navar LG. Urinary angiotensinogen as a novel biomarker of the intrarenal renin–angiotensin system status in hypertensive patients. Hypertension. 2009;53:344–350. doi: 10.1161/HYPERTENSIONAHA.108.123802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kobori H, Urushihara M, Xu JH, Berenson GS, Navar LG. Urinary angiotensinogen is correlated with blood pressure in men (Bogalusa Heart Study) J Hypertens. 2010;28:1422–1428. doi: 10.1097/HJH.0b013e3283392673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35••.Rohrwasser A, Morgan T, Dillon HF, Zhao L, Callaway CW, Hillas E, Zhang S, Cheng T, Inagami T, Ward K, Terreros DA, Lalouel JM. Elements of a paracrine tubular renin–angiotensin system along the entire nephron. Hypertension. 1999;34:1265–1274. doi: 10.1161/01.hyp.34.6.1265. This seminal paper provides evidence of synthesis and secretion of angiotensinogen by polarized proximal tubule cells through its apical side. The protein can be detected in urine as a function of dietary sodium. Furthermore, renin is expressed and secreted in the connecting tubule, also in a sodium-dependent fashion. It is suggested that the paracrine RAS along the entire nephron may contribute to long-term arterial pressure regulation by integrating distinct tubular sodium-reabsorbing functions. [DOI] [PubMed] [Google Scholar]
- 36.Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, Ozawa Y, Hering-Smith KS, Hamm LL, Navar LG. Enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Hypertension. 2004;44:223–229. doi: 10.1161/01.HYP.0000135678.20725.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prieto-Carrasquero MC, Kobori H, Ozawa Y, Gutierrez A, Seth D, Navar LG. AT1 receptor-mediated enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Am J Physiol Renal Physiol. 2005;289:F632–F637. doi: 10.1152/ajprenal.00462.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang C-T, Chin SY, Navar LG. Impairment of pressure-natriuresis and renal autoregulation in ANG II-infused hypertensive rats. Am J Physiol Renal Physiol. 2000;279:F319–F325. doi: 10.1152/ajprenal.2000.279.2.F319. [DOI] [PubMed] [Google Scholar]
- 39.Zhao D, Seth DM, Navar LG. Enhanced distal nephron sodium reabsorption in chronic angiotensin II-infused mice. Hypertension. 2009;54:120–126. doi: 10.1161/HYPERTENSIONAHA.109.133785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prieto-Carrasquero MC, Botros FT, Pagan J, Kobori H, Seth DM, Casarini DE, Navar LG. Collecting duct renin is upregulated in both kidneys of 2-kidney, 1-clip goldblatt hypertensive rats. Hypertension. 2008;51:1590–1596. doi: 10.1161/HYPERTENSIONAHA.108.110916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prieto MC, Gonzalez-Villalobos RA, Botros FT, Martin VL, Pagan J, Sato R, Lara LS, Feng Y, Fernandez F, Kobori H, et al. Reciprocal changes in renal ACE/Ang II and ACE2/Ang 1–7 are associated with enhanced collecting duct renin in Goldblatt hypertensive rats. Am J Physiol Renal Physiol. 2011 doi: 10.1152/ajprenal.00383.2009. in press, [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42•.Gonzalez-Villalobos RA, Billet S, Kim C, Satou R, Fuchs S, Bernstein KE, Navar LG. Intrarenal angiotensin-converting enzyme induces hypertension in response to angiotensin I infusion. J Am Soc Nephrol. 2011;22 doi: 10.1681/ASN.2010060624. in press, [ahead of print]In this study, the authors used a special mouse model which expresses angiotensin-converting enzyme only in renal tubules. They demonstrated that chronic Ang I infusion increases blood pressure and only renal Ang II content, indicating that intrarenal Ang II formation plays an important role in development of hypertension. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43••.Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109:1417–1427. doi: 10.1172/JCI14276. The authors report the expression cloning of the human renin receptor complementary DNA encoding a 350-amino acid protein with a single transmembrane domain. In transfected cells stably expressing the receptor, specific binding for renin and prorenin is demonstrated. The binding of renin induced a fourfold increase of the catalytic efficiency of angiotensinogen conversion to angiotensin I and induced an intracellular signal with phosphorylation of serine and tyrosine residues associated to an activation of MAP kinases ERK1 and ERK2. High levels of the receptor mRNA were detected in the heart, brain, placenta, and lower levels in the kidney and liver. This discovery opened new perspectives on the tissue renin–angiotensin system and on renin effects independent of angiotensin II. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44••.Advani A, Kelly DJ, Cox AJ, White KE, Advani SL, Thai K, Connelly KA, Yuen D, Trogadis J, Herzenberg AM, et al. The (Pro)renin receptor: site-specific and functional linkage to the vacuolar H+-ATPase in the kidney. Hypertension. 2009;54:261–269. doi: 10.1161/HYPERTENSIONAHA.109.128645. The expression of the (P)RR is shown predominantly at the apical side of the acid-secreting cells in the collecting duct. The co-localization and homology of the (P)RR with an accessory protein of the vacuolar H(+)-ATPase, suggests that the (P)RR may function primarily in distal nephron H(+) transport, recently noted to be, at least in part, an angiotensin II-dependent phenomenon. [DOI] [PubMed] [Google Scholar]
- 45.Prieto MC, Navar LG. Collecting duct renin: a critical link in angiotensin II-dependent hypertension. In: Frohlich ED, Re RN, editors. The local cardiac renin–angiotensin aldosterone system. New York: Springer Science + Business Media, LLC; 2009. pp. 133–141. [Google Scholar]
- 46.Prieto-Carrasquero MC, Botros FT, Kobori H, Navar LG. Collecting duct renin: a major player in angiotensin II-dependent hypertension. J Am Soc Hypertens. 2009;3:96–104. doi: 10.1016/j.jash.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prieto MC, Williams DE, Liu L, Kavanagh KL, Mullins JJ, Mitchell KD. Enhancement of renin and prorenin receptor in the collecting ducts of Cyp1a1-Ren2 rats contribute to development and progression of malignant hypertension. Am J Physiol Renal Physiol. 2011;300:F581–F588. doi: 10.1152/ajprenal.00433.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gonzalez AA, Lara LS, Seth DM, Prieto MC. The soluble form of the prorenin receptor [S(PRR)] is augmented in the collecting ducts and in the urine of angiotensin II (Ang II)-dependent hypertensive rats. Hypertension. 2011:57. doi: 10.1161/HYPERTENSIONAHA.110.167957. in press, [ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gonzalez-Villalobos RA, Satou R, Seth DM, Semprun-Prieto LC, Katsurada A, Kobori H, Navar LG. Angiotensin-converting enzyme-derived angiotensin II formation during angiotensin II-induced hypertension. Hypertension. 2009;53:351–355. doi: 10.1161/HYPERTENSIONAHA.108.124511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephrol. 2002;13:1131–1135. doi: 10.1097/01.asn.0000013292.78621.fd. [DOI] [PubMed] [Google Scholar]
- 51.Navar LG, Ploth DW. Pathophysiology of renovascular hypertension. In: Izzo JL, Black HR, Sica DA, editors. Hypertension primer: the essentials of high blood pressure. Philadelphia: Lippincott Williams & Wilkins; 2008. pp. 162–165. [Google Scholar]