Abstract
Multiple signaling molecules, including Fibroblast Growth Factor (FGF) and Wnt, induce two patches of ectoderm on either side of the hindbrain to form the progenitor cell population for the inner ear, or otic placode. Here we report that in Spry1, Spry2 compound mutant embryos (Spry1−/−; Spry2−/− embryos), the otic placode is increased in size. We demonstrate that the otic placode is larger due to the recruitment of cells, normally destined to become cranial epidermis, into the otic domain. The enlargement of the otic placode observed in Spry1−/−; Spry2−/− embryos is preceded by an expansion of a Wnt8a expression domain in the adjacent hindbrain. We demonstrate that both the enlargement of the otic placode and the expansion of the Wnt8a expression domain can be rescued in Spry1−/−; Spry2−/− embryos by reducing the gene dosage of Fgf10. Our results define a FGF-responsive window during which cells can be continually recruited into the otic domain and uncover SPRY regulation of the size of a putative Wnt inductive center.
Keywords: Sprouty1, Sprouty2, Fgf10, Wnt8a, otic placode
INTRODUCTION
The otic placode is a thickened region of cranial ectoderm that is composed of the embryonic progenitors for cells of the mature inner ear epithelium and associated auditory and vestibular neurons. Induction of these progenitors occurs in steps (reviewed in Groves, 2005; Ohyama et al., 2007; Streit, 2007). Initially, at the end of gastrulation, a broad region of ectoderm adjacent to the anterior neural plate, called the pan-placodal domain, gains competence to form any of the cranial placodes, including the otic placode. Next, a specific region of the pan-placodal domain, adjacent to the posterior hindbrain, is induced to form a restricted subset of cell types, including cells destined to become the otic placode, cranial epidermis, and epibranchial placode. This region of ectoderm, called the pre-otic field (also referred to as otic-epibranchial progenitor domain, (Freter et al., 2008), or posterior placodal area, (Ladher et al., 2010; Schlosser and Ahrens, 2004), is defined by the expression of a signature list of genes including Pax2, Dlx5, and Pax8. A subset of cells within the pre-otic field are then instructed to give rise to the otic placode itself, which becomes morphologically distinct as a defined region of pseudostratified epithelium on either side of the hindbrain. One characteristic of the otic placode is that its size is highly stereotyped, with very little variability among normal embryos in the size of the placode relative to total body size (Jayasena et al., 2008). However, little is known about how each of the steps in otic placode induction is modulated to ensure that the otic progenitor domain is neither too small nor too large.
Fibroblast Growth Factor (FGF) signaling directs formation of the otic placode at multiple distinct stages from formation of the pan-placodal domain, induction of the pre-otic field, to later patterning of the otic placode (reviewed in Schimmang, 2007; Wright and Mansour, 2003b). In the mouse, in compound mutants of Fgf3 and Fgf10 (Fgf3−/−; Fgf10−/− embryos) and of Fgf3 and Fgf8 (Fgf3−/−; Fgf8Hypomorph/− or tissue-specific inactivation of Fgf8 in an Fgf3 mutant background), the otic placodes are either completely absent or reduced in size, indicating an essential role for Fgf3, Fgf8, and Fgf10 in otic placode induction (Alvarez et al., 2003; Dominguez-Frutos et al., 2009; Ladher et al., 2005; Wright and Mansour, 2003a; Zelarayan et al., 2007). Importantly, expression of markers of the pre-otic field, such as Dlx5 and Pax2, are not detected in Fgf3, Fgf10 compound mutants, suggesting that FGF signaling is required at an early step in otic placode induction to establish the pre-otic field (Alvarez et al., 2003; Wright and Mansour, 2003a). Both tissue recombination experiments and genetic analyses in various organisms suggest that the molecular signals that induce the otic placode arise from the underlying mesoderm, endoderm, and adjacent neural ectoderm (reviewed in Groves, 2005; Ohyama et al., 2007; Streit, 2001). Members of the Fgf gene family are expressed in these tissues that are the source of otic-inductive signals in a number of diverse vertebrate organisms from amphibians to fish to mammals, although differences exist in the specific Fgf family member and the tissue sources of these FGFs between species (reviewed in Schimmang, 2007; Wright and Mansour, 2003b). In the mouse, Fgf3, Fgf10, Fgf8, and Fgf15 are expressed in partially overlapping domains in subsets of these tissues – the mesoderm, pharyngeal endoderm, neural ectoderm and surface ectoderm – during the period of otic placode induction (reviewed in Schimmang, 2007). Over-expression of various FGFs in chick, mouse, Xenopus and zebrafish result in a variety of effects including formation of extra otic vesicles and expansion of the endogenous otic vesicle, suggesting that FGFs are sufficient for otic placode induction (reviewed in Schimmang, 2007). However, in other studies, misexpression of FGFs result in reduction in size of the endogenous otic vesicle (Dominguez-Frutos et al., 2009; Hans et al., 2007), or absence of expression of later markers of the invaginating otic placode, SoHo-1 and Nkx5-1 (Freter et al., 2008), presumably due to perturbation of other functions of FGF signaling, such as formation of the pan-placodal domain and patterning of the otic placode at the end of induction.
Studies in chick (Ladher et al., 2000) and Xenopus (Park and Saint-Jeannet, 2008) indicate that FGF and Wnt function synergistically to induce the expression of markers of the otic placode. This cooperative induction of the otic placode by FGF and Wnt may be initiated by FGF signaling since in the chick, FGF beads can induce the expression of Wnt8a (previously called Wnt8c) in ectodermal explants (Ladher et al., 2000; Urness et al., 2010) and Wnt8a expression is not detected in various mutant combinations of Fgf3 and Fgf10 (Urness et al., 2010). In further support of a role of Wnt signaling in otic placode induction in the mouse, ectodermal cells in the dorsal portion of the pre-otic field, adjacent to the hindbrain, express TCF/Lef-lacZ, a Wnt signaling reporter transgene (Ohyama et al., 2006). Conditional inactivation of β-catenin, a key effector of canonical Wnt signaling, in the mouse pre-otic field (Ohyama et al., 2006) or overexpression of the Wnt inhibitor Dkk1 in chick ectoderm (Freter et al., 2008), results in smaller otocysts, with an expansion of cranial epidermis. Conversely, constitutive activation of β-catenin within the pre-otic field in the mouse results in larger otocysts at the expense of neighboring epidermis (Ohyama et al., 2006). These data suggest that Wnt signaling directs cells in the pre-otic field toward an otic vs. epidermal cell fate.
Sprouty genes encode negative intracellular regulators of signaling downstream of receptor tyrosine kinases (RTKs), including FGF signaling, and thus are good candidate modulators of otic placode induction (recently reviewed in Cabrita and Christofori, 2008; Guy et al., 2009; Kim and Bar-Sagi, 2004; Mason et al., 2006). Originally identified in Drosophila as an antagonist of FGF signaling during tracheal branching (Hacohen et al., 1998), the roles of the Spry genes (of which there are four members, Spry1-4) in vertebrate development have begun to be described through the analysis of null mutations in the mouse. These studies indicate that Spry genes function throughout embryonic development impacting kidney, tooth, enteric neuronal, craniofacial, limb, lung and inner ear development (Basson et al., 2005; Klein et al., 2008; Klein et al., 2006; Shim et al., 2005; Taketomi et al., 2005; Taniguchi et al., 2007). In all of these developmental settings, loss-of-function mutations in Spry genes result in over-activity of the RTK signaling pathways that are normally inhibited. In the postnatal inner ear, SPRY2 negatively regulates FGF8 signaling during pillar cell formation and for hearing function, such that Spry2 null mutant mice have severe hearing deficits (Shim et al., 2005). Here we demonstrate that in embryos with compound mutations in both the Spry1 and Spry2 genes, the otic placode is enlarged at a late stage of induction, around the time the otic placode becomes morphologically distinct. We show that enlargement of the otic placode occurs due to the recruitment of cells normally fated to become cranial epidermis into the otic domain. Furthermore, we find that Spry1 and Spry2 genetically antagonize FGF signaling during both formation of the otic placode and in the regulation of the size of the expression domain of Wnt8a, which encodes a candidate inducer of otic fates.
MATERIALS AND METHODS
Mouse Lines
Mouse lines carrying null or floxed alleles of Spry1 (Basson et al., 2005), Spry2 (Shim et al., 2005), Fgf10 (Min et al., 1998), and β-actin cre (Lewandoski and Martin, 1997) were maintained and genotyped a described. Spry1−/−; Spry2−/− mutant embryos were generated either by crossing Spry1−/+; Spry2−/+ parental animals (double mutants produced at an expected Mendelian frequency of 1:16) or by crossing β-actin cre/β-actin cre; Spry1−/+; Spry2−/+ males to Spry1flox/flox; Spry2flox/flox females (double mutants produced at an expected frequency of 1:4). Double mutants generated by either method were indistinguishable and were subsequently pooled. Double heterozygous embryos (Spry1−/+; Spry2−/+) were indistinguishable from CD-1 embryos and are referred to as “control” embryos.
In Situ Hybridization and E-cadherin stain
Embryos were staged such that noon on the day of vaginal plug detection was designated as embryonic (E) day 0.5. Whole-mount in situ hybridization using digoxigenin-labeled RNA probes was performed according to standard protocols, using the following probes: Dlx5, Pax2, Pax8, Spry1, Spry2, Foxi2, Fgf3, Wnt8a, Hoxb1, Krox20, and kreisler/Mafb. For hindbrain probes, photography of double mutants and controls was done at the same exposure and images were processed identically using Adobe Photoshop. Embryos were photographed using a Zeiss Discovery V.12 microscope.
For E-cadherin stains, embryos between E9 and E10 were stained in whole-mount with anti-E-cadherin antibody (Invitrogen, 1:1000 dilution) as described (Metzger et al., 2008).
Morphometric Analysis
Embryos that had been processed by in situ hybridization were embedded in plastic (JB-4, Polysciences, Warrington PA). Serial sagittal sections were cut at 6 μm thickness and stained with hematoxylin and eosin. During microtomy, 0 – 3 sections were lost per otic placode. To ensure uniformity of the plane of section among embryos, only right-side placodes were measured in 9 – 11 s embryos, and left-side placodes were measured in 12 – 13 s embryos. Anterior-to-posterior distance along the length of the otic epithelium was measured using Image J software. The otic placode was defined as the region of ectoderm at least two cells thick and by lack of Foxi2 expression or presence of Pax8 expression, as judged by examination of multiple serial sections. Cross-sectional area (mm2) of the otic placode was calculated as the sum of the A-P lengths of the otic placode in all sections multiplied by 0.006 mm (the thickness of each section). Significance of differences between genotypes was measured by Student’s unpaired t-test. Regional comparisons along the lateral-to-medial axis of the otic placode was performed as described (Jayasena et al., 2008), except with sagittal sections. Sections were grouped into three (9 – 11 s embryos) or five (12 – 13 s embryos) bins, consisting of 5 – 6 sections, in which 0% represented the most lateral section and 100% represented the most medial section closest to the hindbrain. Significance of differences between genotypes for regional comparisons was measured by one-way ANOVA.
The dorsal-to-ventral length of the forebrain was measured in every other section through the forebrain, using Image J. Cross-sectional area (mm2) was calculated by summing all D-V forebrain lengths and multiplying by 0.012 mm, and significance of difference between genotypes was measured by Student’s unpaired t-test.
For average cell density measurements, total number of nuclei per otic placode was counted for each section and the area of the otic placode was determined using Image J. Significance of differences between genotypes was measured by repeated measures ANOVA.
Quantification of cell proliferation and cell death
Embryos were fixed in 4% paraformaldehyde, 0.1% Tween-20 for 90 mins, embedded in 3.5% agarose, and cut into 40 μm-thick transverse sections. Sections were processed for immunohistochemistry as described (Shim et al., 2005). Primary antibodies were: anti-phospho-Histone-H3 (Millipore, 1:1,000 dilution), anti-PAX2 (Covance, 1:100 dilution), and anti-activated-caspase-3 (Cell Signaling, 1:100). Secondary antibodies were labeled with either Alexa-488 or Alexa-568 (Invitrogen, 1:1,000). TO-PRO-3 (far red, Invitrogen) was used to counter-stain the nuclei.
For quantification of cell proliferation, vibratome sections, positive for PAX2 staining, were placed in order from anterior to posterior, and z-stacks (2.5 μm step-size) were collected for each section by confocal microscopy. The two sections, representing 80 μm total, in the middle of the PAX2 domain (from anterior to posterior) were analyzed further for the number of individual cells, on both the left and right sides of the embryo, that co-stained for phospho-Histone-H3 and PAX2.
For quantification of cell death, four vibratome sections, representing 160 μm total, were identified that contained first pharyngeal pouch endoderm, which is located ventral to the pre-otic field. Cells staining with activated caspase-3 in the entire ectoderm on both left and right sides of the embryo were counted in z-stack (2.5 μm step-size) confocal images of these sections. Images were collected using a Zeiss LSM510 laser scanning confocal microscope. Significance of differences between genotypes was measured by Student’s unpaired t-test.
RESULTS
Expression domains of otic marker genes are enlarged in Spry1−/−; Spry2−/− double mutants
We produced compound mutants in Spry1 and Spry2 (Spry1−/−; Spry2−/− double mutants, referred to below as “double mutants”), which survive to embryonic day (E) 18.5, but die at birth. Visualization of the three-dimensional structure of the inner ear epithelium by lumenal injection of a white paint solution at a stage when epithelial morphogenesis is nearly complete (E15.5), revealed severe malformation of both the vestibular and cochlear portions of the inner ear in double mutant embryos (n = 5, Suppl. Fig. 1B, C), which were not observed in Spry1−/− or Spry2−/− single mutants alone (Suppl. Fig. 1A and (Shim et al., 2005). To determine the primary defect that ultimately resulted in malformation of the inner ear epithelium in double mutant embryos, we examined earlier stages of inner ear development, prior to E15.5. This analysis led us to the first step in inner ear development – induction of the otic placode.
Expression of the Pax8, Dlx5, and Pax2 genes mark the pre-otic field beginning at the 0 – 3 somite stage (s) or E8.0 in the mouse (Brown et al., 2005; Groves and Bronner-Fraser, 2000; Ohyama and Groves, 2004b). This expression restricts to the otic placode as it becomes morphologically distinct at 8 – 11 s or E8.5. To determine whether early steps in induction of the otic placode were disrupted in double mutants, we examined the expression of Pax8, Dlx5, and Pax2 in double mutants and Spry1−/+; Spry2−/+ embryos (referred to below as “controls”). Early expression of Pax8 in the pre-otic field at 0 – 2 s was indistinguishable from control embryos at this stage (n = 4 double mutants, n = 3 controls, data not shown). In addition, for all three marker genes examined – Pax8, Dlx5, and Pax2, no significant, reproducible differences in the size or intensity of the expression domains in the pre-otic field were observed at 3 – 7 s (compare controls, Fig. 1A, C, E with double mutants, Fig. 1B, D, F; for Pax8: n = 3 double mutants, n = 4 controls; Dlx5: n = 3 double mutants, n = 4 controls; Pax2: n = 4 double mutants, n = 5 controls).
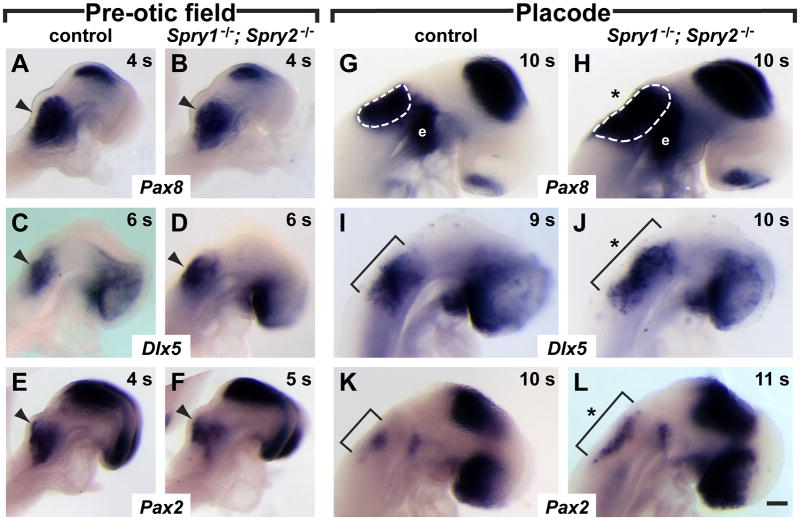
Figure 1. Expression domains of genes that mark the otic placode are expanded in Spry1−/−; Spry2−/− double mutants.
In situ hybridization analysis of Pax8, Dlx5, and Pax2 in control and double mutant embryos at pre-otic field (A – F) and placode (G – L) stages. (A – F) Arrowheads point to the expression in the pre-otic field for the genes indicated. (G, H) Otic placode staining of Pax8 is outlined (white dashed line). Epibranchial placode (e) staining adjacent to the otic domain is indicated. (I – L) Dlx5 and Pax2 expression in the otic placode is indicated (brackets). Expansion of gene expression domains is highlighted (asterisk). Anterior is to the right. Scale bar, 100 μm.
However, at the stage when the otic placode first becomes morphologically distinct (8 – 11 s), we observed an expansion in the size of otic marker expression domains. Both Pax8 and Dlx5 expression in the otic placode were increased in size in double mutants compared to controls (Fig. 1G–J). This expansion was apparent in all directions for the Pax8 marker (Fig. 1G, H), but was more pronounced in the anterior to posterior direction for Dlx5 (Fig. 1I, J). For the Pax2 gene, the difference in the size of the otic expression domain was less dramatic: compared to control embryos (n = 5), half of double mutant embryos (3 out of 6) at 8 – 12 s showed an increase in the anterior to posterior extent of Pax2 staining (Fig. 1K, L), whereas the remaining half of double mutant embryos were indistinguishable from controls (data not shown). We conclude that whereas the early establishment of the pre-otic field, visualized by Pax8, Dlx5, and Pax2 expression was unaffected in double mutants, induction of the definitive otic placode, as assayed by Pax8, Dlx5, and incompletely by Pax2 expression, was increased.
The otic placode is larger in Spry1−/−; Spry2−/− double mutants
To determine whether the expansion in otic marker expression domains corresponded with a commensurate expansion in the otic placode itself, we sagittally sectioned double mutant and control embryos and performed a morphometric analysis of placode size. The otic placode is first distinguishable as a region of pseudostratified epithelium at 9 s, coinciding with when expansions in otic marker expression domains were observed in double mutants (see Fig. 1). At this stage, (9 – 11 s), the otic placode was morphologically larger in double mutants compared to controls. Using a global measurement of otic placode size, we determined that the average cross-sectional area of the otic placode in double mutants was 0.029 ± 0.004 mm2 (mean ± st. dev., n = 5), compared to 0.016 ± 0.005 mm2 in controls, representing a statistically significant (p = 0.001), 1.8-fold increase in size of the placode. When the otic placode continues early invagination at 12 – 13 s, the otic placode remained enlarged in double mutants (compare Fig. 2C and D). The average cross-sectional area of double mutant otic placodes at 12 – 13 s was 0.057 ± 0.012 mm2 (n = 5), compared to 0.033 ± 0.013 mm2 in control embryos (n = 5), a statistically significant (p = 0.018), 1.73-fold increase in size. This increase in size of the otic placode in double mutants did not appear to be secondary to a general increase in the size of the head, since measurements of the dorsal to ventral (D-V) length of the forebrain were not significantly different between double mutants and controls. At 9 – 11 s, the average D-V cross-sectional area of the forebrain was 0.11 ± 0.01 mm2 in double mutants (n = 5) compared to 0.11 ± 0.01 mm2 (n = 5) in controls (p = 0.80); at 12 – 13 s, the average D-V cross-sectional area of the forebrain was 0.12 ± 0.05 mm2 in double mutants (n = 5) compared to 0.12 ± 0.06 mm2 (n = 5) in controls (p = 0.90).
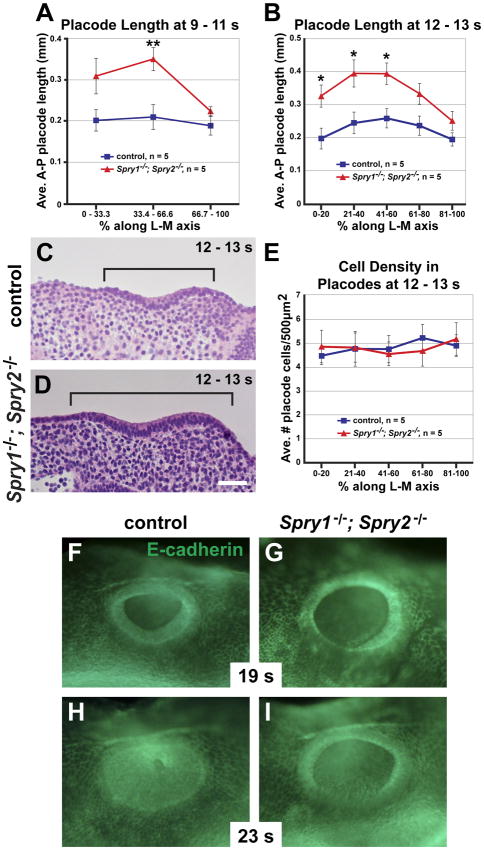
Figure 2. Morphogenetic defects in Spry1−/−; Spry2−/− double mutants.
(A, B) Average anterior to posterior lengths of the otic placode in double mutants and controls, measured on sagittal embryonic sections at 9 – 11 s and 12 – 13 s. (C, D) Sagittal sections from bin 41–60% in a 12 – 13 s control and double mutant. Anterior to posterior extent of the otic placode is bracketed. Anterior is to the left. (E) Average cell densities per bin, as in (B). (F – I) Surface views of control and double mutant embryos stained with E-cadherin antibody, at the somite stages indicated. Dorsal is at the top. *, p < 0.05. **, p = 0.01 by ANOVA; error bars represent standard error of sample means. Scale bar, C, D 50 μm.
To determine whether regional differences contributed to the overall increase in size of the otic placode in double mutants compared to controls, sections were binned along the lateral to medial axis, and anterior to posterior (A-P) lengths of the otic placode were averaged by bin. At 9 – 11 s, a significant increase in the average A-P length of the otic placode was observed in the mid-placode bin (Fig. 2A, Bin 33.34 – 66.66%, p = 0.009). Average lengths were also larger in double mutants in the most lateral bin (Fig. 2A, Bin 0 – 33.33%), however the difference was not statistically significant (p = 0.07). No difference in lengths was observed in the most medial bin, containing sections of the otic placode closest to the hindbrain (Fig. 2A, Bin 66.67 – 100%, p = 0.20). Similarly, during early invagination at 12 – 13 s, the most lateral and mid-placode bins showed a significant increase in average A-P length in double mutants compared to controls (Fig. 2B, Bin 0 – 20%, p = 0.02; Bin 21 – 40%, p = 0.02; Bin 41 – 60%, p = 0.01), but more medial, hindbrain-adjacent bins did not (Fig. 2B, Bin 61 – 80%, p = 0.06; Bin 81 – 100%, p = 0.15). Thus, overall, the enlargement of the otic placode in double mutants was due to a regional increase in length of lateral and mid-regions of the otic placode, in the absence of a change in A-P length of the hindbrain-adjacent portion of the otic placode.
Next, the same sections of 12 –13 s embryos were analyzed for cell density to determine whether the increase in size of the otic placode in double mutants resulted from decreased packing of cells within the otic epithelium. No change in cell density, across all lateral to medial bins, was observed in double mutants compared to controls (Fig. 2E, p = 1.0). Thus, the increase in size of the otic placode in double mutants was not a consequence of decreased density of an equal number of cells, but instead was presumably due to an increase in total number of otic cells.
Morphology of the otic placode in Spry1−/−; Spry2−/− double mutants
In all sections examined for the morphometric analysis above, no gross defects were observed in pseudostratification of the otic epithelium at 9 – 13 s. However, in 4 out of 10 embryos examined at 9 – 13 s, local invasions of what appeared to be the otic epithelium into the underlying mesenchyme were observed (Suppl. Fig. 2A–C). At later stages, the rim of the otic cup and surface ectoderm were visualized by staining with an E-cadherin antibody. At E9.5, the ventral rim of the otic cup was less distinct in double mutants compared to controls, and did not form the normal pointed ventral rim that is raised above the surface ectoderm (Fig. 2F, G, n = 3 double mutants, n = 3 controls). Furthermore, by E10, when all control embryos had nearly completed closure of the otic vesicle, the otic cup was still fully open in double mutants (Fig. 2 H, I, n = 3 double mutants, n = 3 controls). Complete closure was only observed in double mutants at later stages (E11.5). Thus, loss of Spry1 and Spry2 affected the normal coordinated invagination of the otic placode and delayed closure of the otic cup.
Expression of Spry1 and Spry2 during induction and invagination of the otic placode
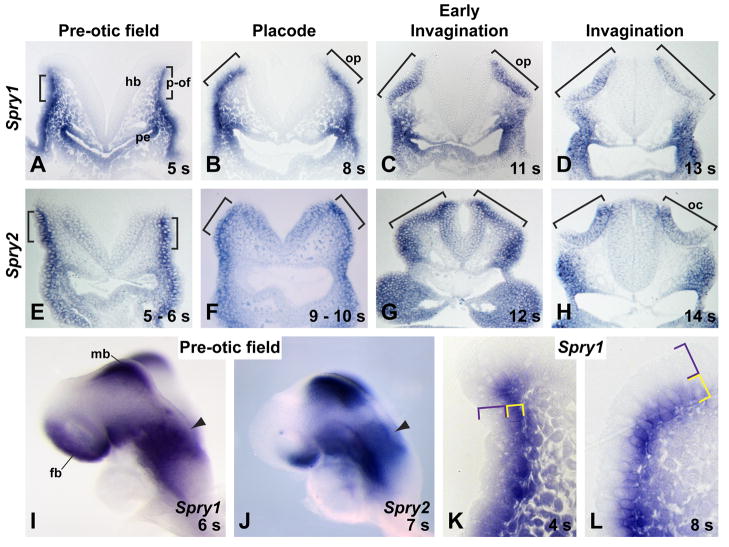
To identify the tissues in which Spry1 and Spry2 function to limit induction of the otic placode, we determined the expression pattern of these two genes in wild-type embryos from the pre-otic field stage to early invagination of the otic placode. At pre-otic field stages, Spry1 and Spry2 transcripts were detected in a broad domain lateral to the hindbrain, marking the pre-otic field (arrowheads, Fig. 3I, J). In transverse cross-sections through these embryos, Spry1 and Spry2 transcripts were detected in both the ectoderm of the pre-otic field (brackets, Fig. 3A, E) and the layer of mesenchymal cells just-underlying the ectoderm (Fig. 3A, E and see higher magnification image of Spry1 expression, Fig. 3K). When the otic placode is first apparent, Spry1 and Spry2 transcripts were detected in the placodal ectoderm (brackets, Fig. 3B, F) and in the surface ectoderm ventral to the otic placode. Whereas Spry1 transcript was no longer detected in the mesenchyme underlying the otic placode, Spry2 transcript was still detected in this tissue. Interestingly, at pre-otic field and placode stages, Spry1, but not Spry2 transcript was detected in a localized, basal region of the ectodermal cells (yellow brackets, Fig. 3K L), but was not detected in the apical region of these cells (purple brackets, Fig. 3K, L). Currently, the functional significance of this basal localization of Spry1 transcript is unknown.
Figure 3. Spry1 and Spry2 expression during induction of the otic placode.
(A – H) In situ hybridization analysis of Spry1 (A – D) and Spry2 (E – H) in transverse embryonic sections at the stages indicated. Otic tissue is bracketed. (I, J) In situ hybridization on whole mount embryos for Spry1 and Spry2 at the pre-otic field stage. (K, L) Higher-magnification images of Spry1 expression in the pre-otic field (K) and otic placode (L). The cellular region in which Spry1 transcript is observed (yellow bracket) and not observed (purple bracket) is indicated. Abbreviations: (fb) forebrain, (hb) hindbrain, (mb) midbrain, (oc) otic cup, (op) otic placode, (p-of) pre-otic field, (pe) pharyngeal endoderm. Anterior (for I, J) is to the left.
As the otic placode invaginates, Spry1 and Spry2 transcripts were still detected in the otic ectoderm, but were no longer detected in the underlying mesenchyme or in the surface ectoderm immediately ventral to the otic placode (Fig. 3C, D, H). During early invagination, both Spry1 and Spry2 transcripts were detected in all cells of the otic placode (Fig. 3 C, G), but in embryos at later stages of invagination, both Spry1 and Spry2 transcript appeared enriched in medial regions of the otic placodes, closer to the hindbrain (Fig. 3 D, H). In addition, at all stages examined, Spry1 and Spry2 transcripts were detected in the pharyngeal endoderm, the adjacent pharyngeal mesenchyme, and the overlying ventral ectoderm (Fig. 3A – H). Finally, whereas for all stages examined, Spry1 transcript was not detected in the hindbrain adjacent to the otic region, Spry2 transcript was detected in the dorsal region of the hindbrain starting at the pre-otic field stage (Fig. 3 E – H).
Mitosis and apoptosis are unaffected in the pre-otic field of Spry1−/−; Spry2−/− mutants
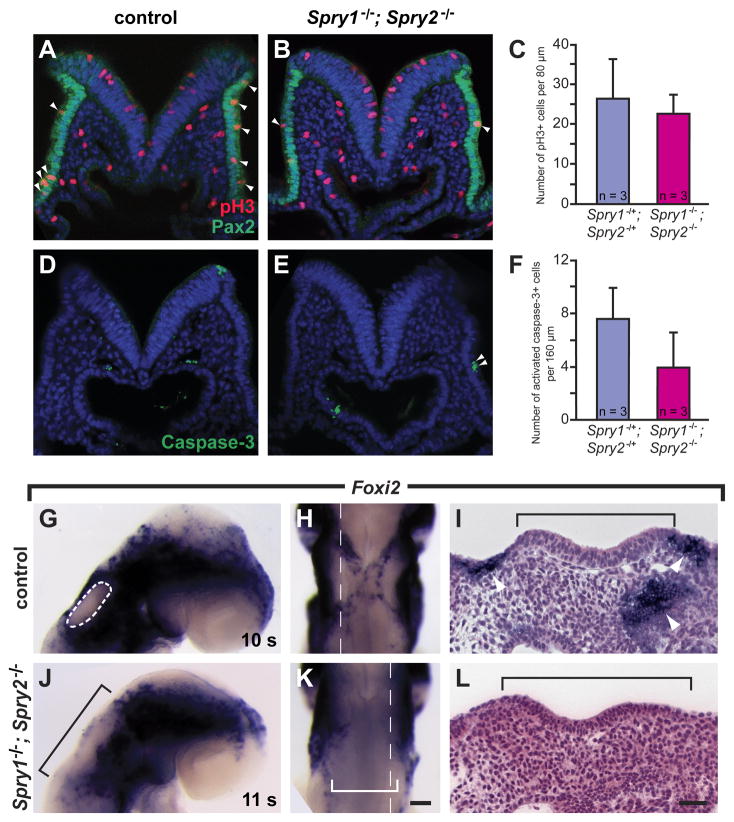
To determine whether the enlargement of the otic placode was due to increased proliferation of cells in the pre-otic ectoderm, we quantified the amount of cell proliferation at 4 – 6 s, prior to when expansions of the otic placodes were first observed. We stained transverse sections of the pre-otic field with both an antibody that detects phosphorylated Histone H3 (pH3), which is present on condensed chromosomes during mitosis, and an antibody that detects PAX2 protein, which is present in the cells in the pre-otic field (Fig. 4A, B). No statistically significant difference was found in the number of pH3+ cells within an 80 μm-thick medial region of the PAX2 domain in double mutants compared to controls (Fig. 4C, 23 ± 5 (mean ± standard deviation) pH3+ cells in double mutants vs. 26 ± 10 pH3+ cells in controls; p = 0.61, n = 3 double mutants, n = 3 controls).
Figure 4. Foxi2 expression reveals a reduction in the size of the cranial epidermal domain in Spry1−/−; Spry2−/− mutants, in the absence of changes in cell proliferation and cell death.
(A, B) Transverse embryonic sections co-immunolabeled for pH3 (red) and PAX2 (green), counterstained with a nuclear dye (blue). (C) The average number of pH3+ cells within the PAX2 domain on both sides (left and right) of the embryo over a 80 μm distance from anterior to posterior. (D, E) Transverse embryonic sections immunolabeled for activated caspase-3 (green), counterstained with a nuclear dye (blue). (F) The average number of ectodermal, activated caspase-3+ cells over a 160 μm distance from anterior to posterior, encompassing the pre-otic field. (G – L) Foxi2 expression analyzed by in situ hybridization. (G, J) Lateral views of whole embryos, anterior to the right. The otic placode, which does not express Foxi2, is outlined in the control embryo (white dashed circle) and black bracketed in the Spry1−/−; Spry2−/− mutant. (H, K) Dorsal views of the same embryos shown in G, J, respectively. The white bracket indicates the region in the Spry1−/−; Spry2−/− mutant lacking Foxi2 expression. (I, L) Sagittal sections through the otic placode in the plane indicated by a white dashed line in H, K, respectively. The otic placode is indicated with a black bracket. Arrows point to patches of cells expressing Foxi2. Anterior is the left (I and L). Error bars (C, F) represent standard deviations. Scale bar, I, L, 50 μm; G, H, J, K, 100 μm.
To determine whether otic placodes were larger in double mutants due to decreased cell death in the pre-otic field, we stained for the presence of the activated, cleaved form of the caspase-3 protease, an initiator of apoptotic cell death (Fig. 4D, E). We quantified the number of activated caspase-3+ cells in the ectoderm of a 160 μm-thick region of double mutant and control embryos that included the pre-otic field at 4 – 6 s, a stage prior to when expansion of the otic placode is first observed. Consistent with previous reports (Ohyama et al., 2006), we found very few activated caspase-3+ cells in the pre-otic field region – even in control embryos – suggesting that normally there is sparse apoptotic cell death in the pre-otic field and that a reduction in this small amount of cell death is an unlikely explanation for the expansions of the otic placodes that are observed in double mutants. Furthermore, we found no statistically significant difference in the number of activated caspase-3+ cells in double mutants compared to controls (Fig. 4F, 4.0 ± 2.6 (mean ± standard deviation) activated caspase-3+ cells in double mutants vs. 7.7 ± 2.3 activated caspase-3+ cells in controls; p = 0.14, n = 3 double mutants, n = 3 controls). Thus, we found neither a dramatic increase in the amount of cell proliferation nor a decrease in the amount of cell death that could account for the expansions of the otic placodes that were observed in double mutants.
Foxi2 expression reveals a reduction in the size of the cranial epidermal domain in Spry1−/−; Spry2−/− mutants
Another explanation for why the otic placode is larger in double mutants is that cells that normally would have become cranial epidermis, which borders the otic placode, aberrantly assume an otic fate. To determine whether cells become otic instead of epidermal, we examined the expression of the epidermally-expressed gene, Foxi2, in double mutants and controls. Foxi2 is specifically expressed in the cranial epidermis and is absent in the otic placode beginning at 10 – 12 s (Ohyama and Groves, 2004a), Fig. 4G, H). In double mutants, we detected an expansion in the Foxi2– (otic) region, coincident with an absence of neighboring Foxi2+ cells (Fig. 4 J, K, n = 4). The reduction in the Foxi2+ cellular population was particularly apparent in the regions adjacent to the dorsal, anterior and posterior sides of the otic placode, which were completely absent in double mutants. As a result, in sagittal sections through the middle of the otic placode, whereas control otic epithelium was flanked by anterior and posterior patches of Foxi2+ cells (Fig. 4I), in double mutants, no Foxi2+ cells were detected at the anterior and posterior borders of the expanded otic epithelium (Fig. 4L). We conclude that the increased size of the otic placode in double mutants was due, at least in part, to the recruitment of cells – normally fated to become cranial epidermis – into the otic cell fate.
The expression domain of Wnt8a is expanded in Spry1−/−; Spry2−/− mutants, but hindbrain patterning is not grossly disrupted
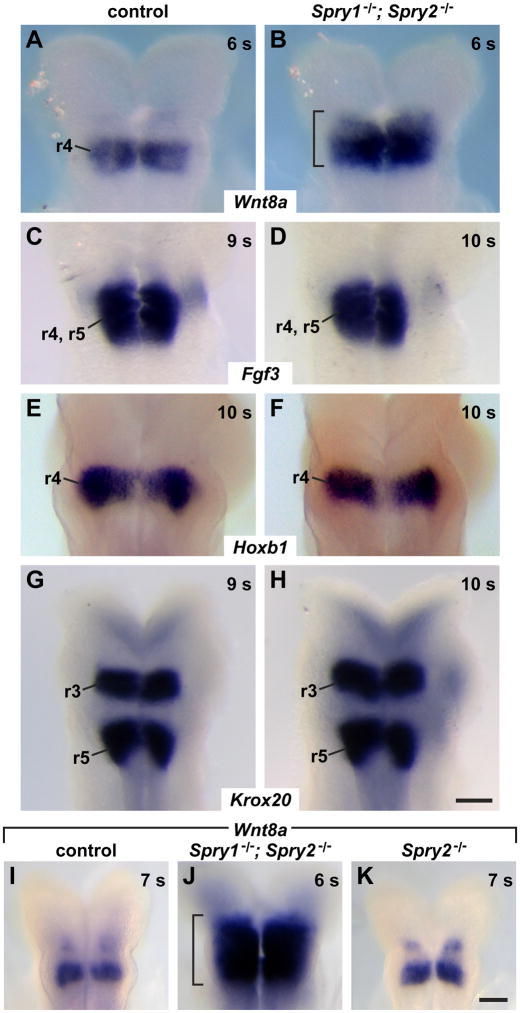
Since the hindbrain is one source of signals that induce the otic placode, it was possible that the increased size of the otic placode in double mutants was due, in part, to increased inductive signaling from the hindbrain. Both Wnt and FGF signals from the hindbrain induce the otic placode (reviewed in Ohyama et al., 2007). A gene encoding one candidate Wnt inducer, Wnt8a, is expressed in rhombomere 4 (r4), adjacent to the pre-otic field from E8.0 to E8.5, just prior to and concurrent with when expansions of the otic placode were first observed in double mutants (Ohyama et al., 2006). We detected a larger and more intensely stained Wnt8a expression domain in double mutants compared to controls (Fig. 5A, B; embryos were examined at 5 – 8 s, n = 6 controls, n = 6 double mutants). Next we examined the expression pattern of Fgf3, which is expressed in r4 and r5 and is required in combination with Fgf10 and Fgf8 for otic placode induction (Alvarez et al., 2003; Dominguez-Frutos et al., 2009; Ladher et al., 2005; Wright and Mansour, 2003a; Zelarayan et al., 2007). We detected no difference in either the size of the Fgf3 expression domain or the intensity of staining between double mutants and controls (Fig. 5C, D; embryos were examined at 6 – 10 s; n = 3 controls, n = 2 Spry1−/−; Spry2−/+ littermates, n = 1 Spry1−/+; Spry2−/− littermate, n = 6 double mutants).
Figure 5. The Wnt8a expression domain is expanded in Spry1−/−; Spry2−/− mutants, but hindbrain patterning is not grossly disrupted.
Dorsal views of whole mount embryos analyzed using in situ hybridization to visualize the rhombomeric organization of the hindbrain from rhombomeres 3 to 5 (r3 – r5) for the probes and genotypes indicated. (B, J) Bracket indicates the extent of the Wnt8a expression domain in double mutants. Scale bar, A – H, I – K 100 μm.
To determine whether the expanded Wnt8a domain was secondary to a gross expansion in r4 itself in double mutants, we examined the expression of the Hoxb1 gene, which is expressed specifically in r4. We detected no difference in the size of the Hoxb1 expression domain in double mutants compared to controls, suggesting that the size of r4 itself is not grossly changed in double mutants (Fig. 5E, F, embryos were examined at 6 – 17 s, n = 6 controls, n = 8 double mutants). To determine whether any rhombomeric segmentation of the hindbrain adjacent to the pre-otic field was disrupted, we examined the expression patterns of other genes expressed in r3 to r5. Consistent with our observation that Fgf3 expression in r4 and r5 was not disrupted in double mutants, we detected no change in the size of the Kreisler expression domain in r4 and r5 (data not shown, embryos were 6 – 9 s; n = 3 controls, n = 3 double mutants). Furthermore, we found that the Krox20 expression in r3 and r5 was not disrupted in double mutants (Fig. 5G, H; embryos were 7 – 10 s; n = 6 controls, n = 1 Spry1−/+; Spry2−/− littermate, n = 5 double mutants).
Finally, when Wnt8a expression was increased in the hindbrain, Spry2 but not Spry1 transcript was detected in the hindbrain adjacent to the pre-otic field (see Fig. 3 A, B, E, F). This raised the possibility that Spry2 alone was sufficient for restricting the Wnt8a expression domain. However, we detected no increase in Wnt8a expression in Spry2−/− single mutants compared to wild-type and Spry2−/+ littermate controls (compare Fig. 5K with Fig. 5I, n = 5 Spry2 mutants, n = 5 controls), whereas Wnt8a expression was dramatically expanded in double mutant embryos processed in parallel (Fig. 5J). Thus, both Spry1 and Spry2 are required for restricting the extent of the Wnt8a expression domain. We conclude that in the absence of gross morphological disruption of the rhombomeric segmentation pattern of the hindbrain adjacent to the pre-otic field, expression of the Wnt8a gene, which encodes a candidate otic-inductive molecule, is both increased and expanded in double mutants. Since Wnt signaling promotes otic induction in diverse organisms including chick, mouse and Medaka (Bajoghli et al., 2009; Ladher et al., 2000; Ohyama et al., 2006), the increased Wnt8a expression domain could potentially contribute to the expansion of the otic placode that is observed in double mutants.
Spry1 and Spry2 genetically antagonize FGF10 signaling
Spry1 and Spry2 were specifically expressed in tissues (the pre-otic ectoderm and underlying mesoderm, see Fig. 3A, B, E, F, I, J) that respond to FGF signaling during otic placode induction. We examined the expression of Etv4 and Etv5, two genes that are transcriptionally induced by FGF signaling in multiple organs (Brent and Tabin, 2004; Firnberg and Neubuser, 2002; Liu et al., 2003) and that genetically function downstream of Spry1 in the kidney (Michos et al., 2010). The expression domains of both Etv4 and Etv5 were expanded in double mutants (Suppl. Fig. 3A – D) at a stage (6 – 7 s, n = 5) prior to when enlargement of the otic placode was observed. This suggested a model in which SPRY1 and SPRY2 normally limit the response of tissues to FGF signals to regulate the size of the otic placode. In embryos missing the SPRY1 and SPRY2 antagonists, the ectoderm has an increased response to FGF-inductive signals, resulting in an enlargement of the otic placode. A prediction of this model is that the double mutant phenotype would be rescued by reducing FGF signaling activity. We reduced FGF signaling by producing embryos missing one functional copy of one of the Fgfs that induce the placode, the Fgf10 gene (Fgf10−/+ heterozygotes), and determined whether double mutant embryos missing one copy of Fgf10 (Spry1−/−; Spry2−/−; Fgf10−/+ embryos, referred to as “experimental” embryos) were rescued for placode size and for size of the Wnt8a expression domain.
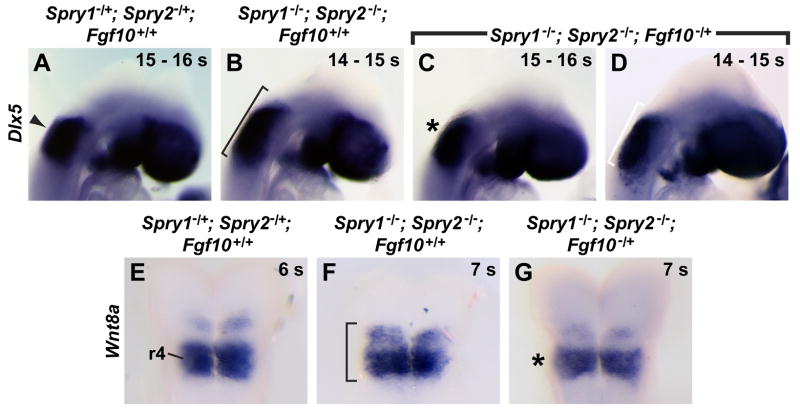
We used the size of the Dlx5 expression domain as a readout of the size of the otic placode, and compared experimental and littermate control embryos at 14 – 18 s, after the otic placode had fully formed. Among control embryos, the size and appearance of the Dlx5 expression domain was indistinguishable in Spry1−/+; Spry2−/+; Fgf10−/+ triple heterozygous embryos (n = 4, data not shown) compared to Spry1−/+; Spry2−/+ double heterozygous embryos (n = 9, Fig. 6A), suggesting that loss of one functional copy of all three genes, Spry1, Spry2, and Fgf10, by itself had no effect on induction of the otic placode. Furthermore, in littermate Spry1−/−; Spry2−/−; Fgf10+/+ double mutant embryos, the Dlx5 expression domain was enlarged (n = 6, Fig. 6B). In experimental embryos, greater than half of embryos examined (n = 3 out of 5 total experimental embryos, Fig. 6C) had a normally sized otic Dlx5 expression domain, suggesting that reducing the gene dosage of Fgf10 can rescue the enlargement of the otic placode observed in double mutants. However, in less than half of experimental embryos (n = 2 out of 5 embryos, Fig. 6D), the Dlx5 expression domain was an intermediate size, suggesting that the rescue was incomplete in these embryos.
Figure 6. Expansions of the otic placode and Wnt8a expression domain can be rescued in Spry1−/−; Spry2−/− mutants by reducing the dosage of Fgf10.
In situ hybridization analysis of Dlx5 expression to visualize the otic placode (A – D, lateral views) and of Wnt8a expression in the hindbrain (E – G, dorsal views). (A) The normal domain of Dlx5 expression is indicated with an arrowhead in a Spry1−/+; Spry2−/+; Fgf10+/+ control embryo. (B) The expanded domain of Dlx5 expression is black bracketed in a Spry1−/−; Spry2−/−; Fgf10+/+ mutant embryo. (C, D) Spry1−/−; Spry2−/−; Fgf10−/+ embryos demonstrating complete rescue of the size of the Dlx5 domain (asterisk, C) and intermediate rescue (white bracket, D). (E) The normal domain of Wnt8a expression in rhombomere 4 (r4) in a Spry1−/+; Spry2−/+; Fgf10+/+ control embryo. (F) The expanded domain of Wnt8s expression is black bracketed in a Spry1−/−; Spry2−/−; Fgf10+/+ mutant embryo. (G) The domain of Wnt8a expression in a Spry1−/−; Spry2−/−; Fgf10−/+ embryo is indicated (asterisk). (A – D) Anterior is to the right.
We also produced experimental and littermate control embryos to determine whether the expansion of the Wnt8a expression domain observed in double mutants could be rescued by loss of one copy of Fgf10. In the control group, the pattern of Wnt8a expression in the hindbrain was indistinguishable in Spry1−/+; Spry2−/+; Fgf10−/+ triple heterozygous embryos (n = 3, data not shown) compared to Spry1−/+; Spry2−/+ double heterozygous embryos (n = 6, Fig. 6E), suggesting that loss of one functional copy each of Spry1, Spry2 and Fgf10 does not have an effect on the Wnt8a expression domain. Furthermore, in littermate double mutant embryos, we detected an expected expansion of the Wnt8a expression domain (n = 5, Fig. 6F). In all experimental embryos, the expansion of Wnt8a expression in the hindbrain was rescued (n = 4, Fig. 6G), such that the size of the Wnt8a expression domain in experimental embryos was more similar to double and triple heterozygous controls. Thus, both aspects of the double mutant phenotype – the increased size of the otic placode and the expansion in Wnt8a expression in the hindbrain – is due, directly or indirectly, to the overactive response of tissues to FGF-inductive signals.
DISCUSSION
SPRY1 and SPRY2 negatively regulate FGF signaling during induction of the otic placode
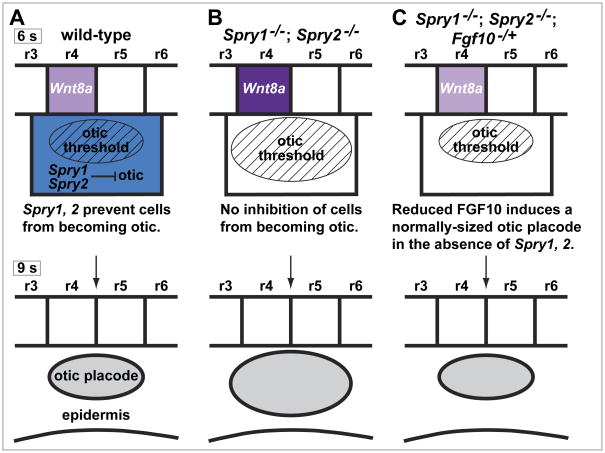
Here we provide evidence that Spry1 and Spry2 regulate the size of the otic placode by limiting FGF induction. We propose a model in which Spry1 and Spry2, expressed broadly in the pre-otic field ectoderm, limit the response of this tissue to FGF induction, so that only cells receiving the highest effective amounts of FGF signal are able to overcome Spry1, 2 inhibition to become otic (Figure 7A). In Spry1−/−; Spry2−/− mutant embryos, Spry-mediated negative regulation is absent, thus allowing more cells, including cells normally destined to become epidermis, to reach the effective threshold of FGF signaling to become an otic placode cell (Figure 7B). Consistent with this model, the expression of two transcriptional targets of FGF signaling, Etv4 and Etv5, is expanded in the pre-otic field in Spry1−/−; Spry2−/− mutant embryos (Suppl. Fig. 3), suggesting that FGF signaling is up-regulated. In addition, when FGF signaling is reduced in embryos missing one copy of the Fgf10 gene (Fgf10−/+ heterozygotes), the need for Spry1, 2 negative regulation is obviated, and normally-sized otic placodes are observed in Fgf10−/+; Spry1−/−; Spry2−/− embryos (Figure 7C).
Figure 7. Model of Spry1 and Spry2 function during otic placode induction.
(A-C) Model of Spry1 and Spry2 function in the pre-otic field for the genotypes indicated. The ectodermal region in which both Spry1 and Spry2 are expressed is indicated by blue shading; expression of Wnt8a is indicated by purple shading. The effective FGF-responsive region is indicated by the hatched oval.
In addition to the ectoderm, the underlying mesoderm also responds to FGF signal during otic placode induction, and in Fgf3−/−; Fgf8Hypomorph/− embryos, expression of Fgf10 is not maintained in the mesoderm (Ladher et al., 2005). We hypothesized that Spry1, 2 expression in the cranial mesoderm (see Figure 3A, E) limited the response of this tissue to FGF signaling, and that loss of Spry1 and Spry2 function would result in the expansion of the mesodermal Fgf10 expression domain. However, by in situ hybridization on whole mount embryos, we did not detect any dramatic differences in levels or size of the mesodermal Fgf10 expression domain in Spry1−/−; Spry2−/− embryos compared to controls (not shown). However, it is possible that differences in Fgf10 expression in double mutants compared to controls is subtle and could only be detected by more quantifiable measures than in situ hybridization.
In many but not all tissues, Spry gene expression is induced by the signaling pathway that it negatively regulates. Since Spry1 and Spry2 are expressed in the ectoderm and mesoderm, which respond to FGF signals, it is possible that Spry1, 2 expression in these tissues is FGF-regulated. Indeed, Spry1 expression is absent from the pre-otic ectoderm in Fgf3−/−; Fgf10−/− global null mutants, suggesting that Fgf3 and Fgf10 are directly or indirectly required for Spry1 expression in the ectoderm (Urness et al., 2010). Spry1 and Spry2 transcripts are also detected in the pharyngeal endoderm, a source of FGF otic-inductive signals (see Figure 3A–H). This suggests the idea that FGF signaling (either autocrine or paracrine) to the pharyngeal endoderm itself may be important in otic placode induction. Alternatively, Spry1 and Spry2 may have a distinct function in the pharyngeal endoderm, unrelated to formation of the otic placode. Further experiments involving tissue-specific inactivation of Spry1 and Spry2 in the ectoderm, mesoderm, and pharyngeal endoderm individually should help to uncover the requirements of the Spry genes in each of the these tissues during otic placode induction.
Comparison of the Spry1−/−; Spry2−/− double mutant phenotype with Fgf overexpression studies
A number of studies in diverse organisms including Medaka, zebrafish, Xenopus, chick and mouse demonstrate that ectopic overexpression of FGF can induce either supernumerary otocysts or an enlarged endogenous otocyst (reviewed in Schimmang, 2007). Consistent with our model that Spry1 and Spry2 limit the cellular response to FGF induction of the otic placode, the loss-of-function phenotype of an enlarged otic placode that we observe in Spry1−/−; Spry2−/− double mutants is comparable to this previous FGF-overexpression data. A few studies, however, report different or opposite effects upon misexpression of FGF. Freter et al. find that sustained overexpression of Fgf3 or Fgf19 in the chick suppresses the expression of Soho1 and Nkx5.1, which are normally expressed in the otic placode after it becomes morphologically distinct (Freter et al., 2008). In addition, Domingues-Frutos et al. find that ectopic misexpression of Fgf8 or Fgf10 in the chick at later stages just prior to or coinciding with the morphological appearance of an otic placode, results in a reduction in the size of or no effect on the otic vesicle, respectively (Dominguez-Frutos et al., 2009). One explanation for why different effects are observed upon ectopic overexpression of Fgfs was proposed in a study by Hans et al. in which the authors provide evidence that misexpression of Fgf at different timepoints has unique effects due to separate functions of FGF signaling during otic placode induction (Hans et al., 2007). Using a protocol in which ectopic expression of fgf8 in zebrafish could be controlled temporally by use of a heat-shock promoter, Hans et al. find that misexpression of fgf8 before gastrulation results in a severe reduction in size or complete loss of otic tissue due to loss of the pan-placodal domain. Misexpression during gastrulation up to the 4 s stage resulted in larger or duplicated vesicles, whereas misexpression at later stages had either no effect or resulted in smaller otic vesicles, presumably due to effects on patterning of the otic placode. Thus, Spry1−/−; Spry2−/− double mutant phenotypes are comparable to over-expression of Fgf after formation of the pan-placodal domain. Interestingly, Spry1 and Spry2 are expressed in both pre-otic and otic placode cells, suggesting that in addition to preventing neighboring cells from becoming otic, Spry1 and Spry2 may function in the otic placode itself. Although gross pseudostratification of the otic placode epithelium does occur in Spry1−/−; Spry2−/− double mutants, double mutants had local regions of uncoordinated invasion of the epithelium into the underlying mesenchyme (Suppl. Fig. 2). Experiments are currently underway that address whether other features of the otic placode, such as regional patterning of the placode occurs normally in Spry1−/−; Spry2−/− double mutants.
The requirement of Spry1 and Spry2 correlates with commitment of cells to an otic fate
The timing of commitment of the cranial ectoderm to an otic fate has been determined experimentally in the chick by challenging prospective otic ectoderm to form otic vesicles when grafted to a dissimilar tissue environment, including the lateral trunk, amniocardiac vesicle, anterior cranial mesenchyme and limb bud (Groves and Bronner-Fraser, 2000; Herbrand et al., 1998; Waddington, 1937). These studies all conclude that 9 s or older donor prospective otic ectoderm can form otic vesicles independent of tissue environment and thus are committed to the otic fate. We find that an early event in otic placode induction – formation of the pre-otic field at 5 s – occurs normally in Spry1−/−; Spry2−/− double mutants, since Pax8, Dlx5, and Pax2 expression in the pre-otic field are unaffected. Expansion of the Pax8, Dlx5 and Pax2 expression domains is first apparent in Spry1−/−; Spry2−/− double mutants starting at 8 – 9 s, correlating with the timing of commitment defined by studies in the chick. The FGF signaling pathway, however, is required at an earlier step to establish the pre-otic field, since in Fgf3−/−; Fgf10−/− double mutants, expression markers such as Dlx5 and Pax2 are not detected in the pre-otic field (Alvarez et al., 2003; Wright and Mansour, 2003a). Thus, our data demonstrate that the genetic requirement of Spry1 and Spry2 is of containment of FGF induction: loss of Spry1 and Spry2 is not limiting at the onset of FGF induction of the pre-otic field, but becomes essential to curtail the final extent of FGF signaling, correlating with the time of otic commitment. These data further suggest that FGF signaling occurs continuously from induction of the pre-otic field to commitment of cells within this field to an otic fate.
Although Spry1 and Spry2 are not required genetically until the presumed time of commitment of the otic cells at 8 – 9 s, both genes are expressed earlier in the pre-otic field beginning at 3 – 4 s (see Fig. 3). Therefore, it is possible that any function of Spry1 and Spry2 in the pre-otic field was not revealed by analysis of the double mutant. One additional member of the Spry gene family, Spry4, is also expressed in the mesoderm underlying the pre-otic ectoderm (A. Mahoney Rogers, unpubl. data), and thus may compensate for the loss of Spry1 and Spry2 function at this stage. This raises the possibility that inactivation of all three genes – Spry1, Spry2, and Spry4 – in the pre-otic field region may result in more severe defects that affect the pre-otic field.
SPRY1 and SPRY2 limit the size of a Wnt8a expression domain in the hindbrain
The enlargement of the otic placode that we observe in Spry1−/−; Spry2−/− mutants is similar to phenotypes observed in gain-of-function studies in the mouse in which either a constitutively active form of β-catenin (cAct) or Notch (NICD) are misexpressed in the pre-otic field (Jayasena et al., 2008; Ohyama et al., 2006). In Spry1−/−; Spry2−/− mutants, cAct and NICD embryos, the otic placode is enlarged due to the recruitment of cells normally destined to become Foxi2+ epidermis into the otic domain. Furthermore, in all three cases, no significant changes in cell proliferation or cell death are observed in the otic region. Ultimately, in Spry1−/−; Spry2−/− mutant, cAct and NICD embryos, the otic cups are delayed in closure. The commonality in the mechanisms by which the otic placode is enlarged in Spry1−/−; Spry2−/− double mutants and in embryos with constitutively active β-catenin or Notch signaling suggest that there must be signal integration between these three pathways. Indeed, we find that Spry1 and Spry2 regulate the size of the Wnt8a expression domain in the hindbrain.
Recently Urness et al. reported that Wnt8a expression is reduced or absent in Fgf3, Fgf10 compound mutants, suggesting that similar to the chick, FGF signaling induces the expression of Wnt8a in the mouse (Urness et al., 2010). Our data suggest that Spry1 and Spry2 limit the extent of FGF induction of this Wnt8a expression domain. These data raise the question of whether FGF/SPRY induction of the otic placode is direct or whether this induction is indirect via regulation of Wnt8a. The expression of Etv4, Etv5, Spry1, and Spry2 – genes whose expression are often induced by FGF signaling – in the pre-otic field suggest that FGF induction of the otic placode is direct. However, the possibility remains that FGF/SPRY and Wnt8a cooperate to induce the placode. Determination of whether Wnt8a is functionally required in otic placode induction in the mouse is a first step to address this question.
Finally, how do Spry1 and Spry2 regulate the size of the Wnt8a expression domain? If Wnt8a were a direct transcriptional target of FGF/SPRY signaling, then Spry1, Spry2, and Wnt8a should all be co-expressed in the hindbrain. By in situ hybridization, Spry2, but not Spry1, is detected in the hindbrain adjacent to the otic ectoderm (compare Fig. 3A – D to Fig. 3E – H). However, Spry2 alone is not necessary to restrict the size of the Wnt8a expression domain (Fig. 5I, K), and both Spry1 and Spry2 are required to regulate Wnt8a. Thus, two possibilities exist for how Spry1, 2 regulate Wnt8a expression. One possibility is that Spry1, expressed at undetectable levels in the hindbrain, together with Spry2, directly regulate Wnt8a expression. Alternatively, Spry1 and Spry2 may regulate Wnt8a expression indirectly from the adjacent ectoderm and mesoderm. Analysis of Wnt8a expression after tissue-specific inactivation of Spry1 and Spry2 in the hindbrain or ectoderm should help to distinguish between these possibilities.
CONCLUSIONS
Here we describe a genetic example in which loss-of-function mutations result in an enlarged otic progenitor domain: loss of Spry1 and Spry2 function results in an enlarged otic placode due to excess cells becoming otic, at the expense of cranial epidermis. We define two points at which FGF induction of the otic placode must be downregulated by Spry1 and Spry2 – first, during maintenance of Wnt8a expression in the hindbrain, and second, at a late stage of otic placode induction, correlating with commitment of cells to an otic fate. Further studies of the mechanisms by which Spry1 and Spry2 modulate induction of the otic placode should provide a picture of how and when various signals must be fine-tuned to ensure that the otic progenitor domain is the correct size.
Supplementary Material
Acknowledgments
We wish to thank Dr. Gail Martin for her valuable support and advice at the initial stages of this work; Drs. Lisa Urness, Suzi Mansour, Takahiro Ohyama, and Andy Groves for providing probes; Qun Xiang and Dr. Aniko Szabo for performing the binning and statistical analysis and for expert statistical advice; Dr. Suresh Kumar at the Children’s Research Institute imaging core for advice on confocal microscopy; and Drs. Ophir Klein, M. Albert Basson, Elena Semina and members of the Shim and Klein labs for critical reading of the manuscript. This work was supported by N.I.H. grants DC008181 and DC010387 (to K. S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez Y, Alonso MT, Vendrell V, Zelarayan LC, Chamero P, Theil T, Bosl MR, Kato S, Maconochie M, Riethmacher D, Schimmang T. Requirements for FGF3 and FGF10 during inner ear formation. Development. 2003;130:6329–38. doi: 10.1242/dev.00881. [DOI] [PubMed] [Google Scholar]
- Bajoghli B, Aghaallaei N, Jung G, Czerny T. Induction of otic structures by canonical Wnt signalling in medaka. Dev Genes Evol. 2009;219:391–8. doi: 10.1007/s00427-009-0302-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basson MA, Akbulut S, Watson-Johnson J, Simon R, Carroll TJ, Shakya R, Gross I, Martin GR, Lufkin T, McMahon AP, Wilson PD, Costantini FD, Mason IJ, Licht JD. Sprouty1 Is a Critical Regulator of GDNF/RET-Mediated Kidney Induction. Dev Cell. 2005;8:229–239. doi: 10.1016/j.devcel.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Brent AE, Tabin CJ. FGF acts directly on the somitic tendon progenitors through the Ets transcription factors Pea3 and Erm to regulate scleraxis expression. Development. 2004;131:3885–96. doi: 10.1242/dev.01275. [DOI] [PubMed] [Google Scholar]
- Brown ST, Wang J, Groves AK. Dlx gene expression during chick inner ear development. J Comp Neurol. 2005;483:48–65. doi: 10.1002/cne.20418. [DOI] [PubMed] [Google Scholar]
- Cabrita MA, Christofori G. Sprouty proteins, masterminds of receptor tyrosine kinase signaling. Angiogenesis. 2008;11:53–62. doi: 10.1007/s10456-008-9089-1. [DOI] [PubMed] [Google Scholar]
- Dominguez-Frutos E, Vendrell V, Alvarez Y, Zelarayan LC, Lopez-Hernandez I, Ros M, Schimmang T. Tissue-specific requirements for FGF8 during early inner ear development. Mech Dev. 2009;126:873–81. doi: 10.1016/j.mod.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Firnberg N, Neubuser A. FGF signaling regulates expression of Tbx2, Erm, Pea3, and Pax3 in the early nasal region. Dev Biol. 2002;247:237–50. doi: 10.1006/dbio.2002.0696. [DOI] [PubMed] [Google Scholar]
- Freter S, Muta Y, Mak SS, Rinkwitz S, Ladher RK. Progressive restriction of otic fate: the role of FGF and Wnt in resolving inner ear potential. Development. 2008;135:3415–24. doi: 10.1242/dev.026674. [DOI] [PubMed] [Google Scholar]
- Groves AK. The Induction of the Otic Placode. In: Kelley MW, Wu DK, Popper AN, Fay RR, editors. Development of the Inner Ear. Vol. 26. Springer; New York: 2005. pp. 10–42. [Google Scholar]
- Groves AK, Bronner-Fraser M. Competence, specification and commitment in otic placode induction. Development. 2000;127:3489–99. doi: 10.1242/dev.127.16.3489. [DOI] [PubMed] [Google Scholar]
- Guy GR, Jackson RA, Yusoff P, Chow SY. Sprouty proteins: modified modulators, matchmakers or missing links? J Endocrinol. 2009;203:191–202. doi: 10.1677/JOE-09-0110. [DOI] [PubMed] [Google Scholar]
- Hacohen N, Kramer S, Sutherland D, Hiromi Y, Krasnow M. Asprouty encodes a novel antagonist of FGF signaling that patterns apical branching of the Drosophila airways. Cell. 1998;92:253–63. doi: 10.1016/s0092-8674(00)80919-8. [DOI] [PubMed] [Google Scholar]
- Hans S, Christison J, Liu D, Westerfield M. Fgf-dependent otic induction requires competence provided by Foxi1 and Dlx3b. BMC Dev Biol. 2007;7:5. doi: 10.1186/1471-213X-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbrand H, Guthrie S, Hadrys T, Hoffmann S, Arnold HH, Rinkwitz-Brandt S, Bober E. Two regulatory genes, cNkx5-1 and cPax2, show different responses to local signals during otic placode and vesicle formation in the chick embryo. Development. 1998;125:645–54. doi: 10.1242/dev.125.4.645. [DOI] [PubMed] [Google Scholar]
- Jayasena CS, Ohyama T, Segil N, Groves AK. Notch signaling augments the canonical Wnt pathway to specify the size of the otic placode. Development. 2008;135:2251–61. doi: 10.1242/dev.017905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Bar-Sagi D. Modulation of signalling by Sprouty: a developing story. Nat Rev Mol Cell Biol. 2004;5:441–50. doi: 10.1038/nrm1400. [DOI] [PubMed] [Google Scholar]
- Klein OD, Lyons DB, Balooch G, Marshall GW, Basson MA, Peterka M, Boran T, Peterkova R, Martin GR. An FGF signaling loop sustains the generation of differentiated progeny from stem cells in mouse incisors. Development. 2008;135:377–85. doi: 10.1242/dev.015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein OD, Minowada G, Peterkova R, Kangas A, Yu BD, Lesot H, Peterka M, Jernvall J, Martin GR. Sprouty genes control diastema tooth development via bidirectional antagonism of epithelial-mesenchymal FGF signaling. Dev Cell. 2006;11:181–90. doi: 10.1016/j.devcel.2006.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladher RK, Anakwe KU, Gurney AL, Schoenwolf GC, Francis-West PH. Identification of synergistic signals initiating inner ear development. Science. 2000;290:1965–7. doi: 10.1126/science.290.5498.1965. [DOI] [PubMed] [Google Scholar]
- Ladher RK, O’Neill P, Begbie J. From shared lineage to distinct functions: the development of the inner ear and epibranchial placodes. Development. 2010;137:1777–85. doi: 10.1242/dev.040055. [DOI] [PubMed] [Google Scholar]
- Ladher RK, Wright TJ, Moon AM, Mansour SL, Schoenwolf GC. FGF8 initiates inner ear induction in chick and mouse. Genes Dev. 2005;19:603–13. doi: 10.1101/gad.1273605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandoski M, Martin GR. Cre-mediated chromosome loss in mice. Nat Genet. 1997;17:223–5. doi: 10.1038/ng1097-223. [DOI] [PubMed] [Google Scholar]
- Liu Y, Jiang H, Crawford HC, Hogan BL. Role for ETS domain transcription factors Pea3/Erm in mouse lung development. Dev Biol. 2003;261:10–24. doi: 10.1016/s0012-1606(03)00359-2. [DOI] [PubMed] [Google Scholar]
- Mason JM, Morrison DJ, Basson MA, Licht JD. Sprouty proteins: multifaceted negative-feedback regulators of receptor tyrosine kinase signaling. Trends Cell Biol. 2006;16:45–54. doi: 10.1016/j.tcb.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Metzger RJ, Klein OD, Martin GR, Krasnow MA. The branching programme of mouse lung development. Nature. 2008;453:745–50. doi: 10.1038/nature07005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michos O, Cebrian C, Hyink D, Grieshammer U, Williams L, D’Agati V, Licht JD, Martin GR, Costantini F. Kidney development in the absence of Gdnf and Spry1 requires Fgf10. PLoS Genet. 2010;6:e1000809. doi: 10.1371/journal.pgen.1000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min H, Danilenko DM, Scully SA, Bolon B, Ring BD, Tarpley JE, DeRose M, Simonet WS. Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to Drosophila branchless. Genes Dev. 1998;12:3156–61. doi: 10.1101/gad.12.20.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama T, Groves AK. Expression of mouse Foxi class genes in early craniofacial development. Dev Dyn. 2004a;231:640–6. doi: 10.1002/dvdy.20160. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Groves AK. Generation of Pax2-Cre mice by modification of a Pax2 bacterial artificial chromosome. Genesis. 2004b;38:195–9. doi: 10.1002/gene.20017. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Groves AK, Martin K. The first steps towards hearing: mechanisms of otic placode induction. Int J Dev Biol. 2007;51:463–72. doi: 10.1387/ijdb.072320to. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Mohamed OA, Taketo MM, Dufort D, Groves AK. Wnt signals mediate a fate decision between otic placode and epidermis. Development. 2006;133:865–75. doi: 10.1242/dev.02271. [DOI] [PubMed] [Google Scholar]
- Park BY, Saint-Jeannet JP. Hindbrain-derived Wnt and Fgf signals cooperate to specify the otic placode in Xenopus. Dev Biol. 2008;324:108–21. doi: 10.1016/j.ydbio.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmang T. Expression and functions of FGF ligands during early otic development. Int J Dev Biol. 2007;51:473–81. doi: 10.1387/ijdb.072334ts. [DOI] [PubMed] [Google Scholar]
- Schlosser G, Ahrens K. Molecular anatomy of placode development in Xenopus laevis. Dev Biol. 2004;271:439–66. doi: 10.1016/j.ydbio.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Shim K, Minowada G, Coling DE, Martin GR. Sprouty2, a mouse deafness gene, regulates cell fate decisions in the auditory sensory epithelium by antagonizing FGF signaling. Dev Cell. 2005;8:553–64. doi: 10.1016/j.devcel.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Streit A. Origin of the vertebrate inner ear: evolution and induction of the otic placode. J Anat. 2001;199:99–103. doi: 10.1046/j.1469-7580.2001.19910099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit A. The preplacodal region: an ectodermal domain with multipotential progenitors that contribute to sense organs and cranial sensory ganglia. Int J Dev Biol. 2007;51:447–61. doi: 10.1387/ijdb.072327as. [DOI] [PubMed] [Google Scholar]
- Taketomi T, Yoshiga D, Taniguchi K, Kobayashi T, Nonami A, Kato R, Sasaki M, Sasaki A, Ishibashi H, Moriyama M, Nakamura KI, Nishimura J, Yoshimura A. Loss of mammalian Sprouty2 leads to enteric neuronal hyperplasia and esophageal achalasia. Nat Neurosci. 2005 doi: 10.1038/nn1485. [DOI] [PubMed] [Google Scholar]
- Taniguchi K, Ayada T, Ichiyama K, Kohno R, Yonemitsu Y, Minami Y, Kikuchi A, Maehara Y, Yoshimura A. Sprouty2 and Sprouty4 are essential for embryonic morphogenesis and regulation of FGF signaling. Biochem Biophys Res Commun. 2007;352:896–902. doi: 10.1016/j.bbrc.2006.11.107. [DOI] [PubMed] [Google Scholar]
- Urness LD, Paxton CN, Wang X, Schoenwolf GC, Mansour SL. FGF signaling regulates otic placode induction and refinement by controlling both ectodermal target genes and hindbrain Wnt8a. Dev Biol. 2010;340:595–604. doi: 10.1016/j.ydbio.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington CH. The determination of the auditory placode in the chick. Journal of Experimental Biology. 1937;14:232–239. [Google Scholar]
- Wright TJ, Mansour SL. Fgf3 and Fgf10 are required for mouse otic placode induction. Development. 2003a;130:3379–90. doi: 10.1242/dev.00555. [DOI] [PubMed] [Google Scholar]
- Wright TJ, Mansour SL. FGF signaling in ear development and innervation. Curr Top Dev Biol. 2003b;57:225–59. doi: 10.1016/s0070-2153(03)57008-9. [DOI] [PubMed] [Google Scholar]
- Zelarayan LC, Vendrell V, Alvarez Y, Dominguez-Frutos E, Theil T, Alonso MT, Maconochie M, Schimmang T. Differential requirements for FGF3, FGF8 and FGF10 during inner ear development. Dev Biol. 2007;308:379–91. doi: 10.1016/j.ydbio.2007.05.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.