Abstract
The striatum is one of the major forebrain regions that strongly express muscarinic and nicotinic cholinergic receptors. This article reviews the current knowledge and our new findings about the striatal cholinoceptive organization and its role in a variety of cognitive functions. Pharmacological and genetic manipulations have indicated that the cholinergic and dopaminergic system in the striatum modulate each other’s function. In addition to modulating the dopaminergic system, nicotinic cholinergic receptors facilitate GABA release, whereas muscarinic receptors attenuate GABA release. The striatal cholinergic system has also been implicated in various cognitive functions including procedural learning and intradimensional set shifting. Together, these data indicate that the cholinergic system in the striatum is involved in a diverse set of cognitive functions through interactions with other neurotransmitter systems including the dopaminergic and GABAergic systems.
Keywords: Cognitive strategy, T-maze, set-shifting, reversal learning, place navigation, nicotinic, muscarinic, striatum, aging, basal ganglia
1. Introduction
The basal ganglia are a group of nuclei situated at the base of the forebrain. The main components of the basal ganglia are the striatum (the largest component), pallidum, substantia nigra and subthalamic nucleus. The striatum is the main input processing unit of the basal ganglia, extremely rich in acetylcholine (ACh) and its associated enzymes Acetylcholinesterase (AChE; the ACh degrading enzyme), Cholineacetyltransferase (ChAT; the ACh synthesizing enzyme), and cholinergic receptors (muscarinic and nicotinic; mAChRs and nAChRs, respectively). The striatum receives input from virtually all areas of the cerebral cortex. Once the cortical information is integrated at the striatal level, it is conveyed to basal ganglia output nuclei (e.g., the globus pallidus) via the striatal medium spiny neurons (MSNs). The integration is strongly modulated by striatal ACh interacting with dopamine (DA). It has long been recognized that the striatal cholinergic system, together with dopaminergic circuitry within the striatum, plays a key role in voluntary movement. In addition, it is widely accepted that the striatal cholinergic system contributes to the cognitive functions of the striatum, which is the focus of this review.
2. Anatomical organization of the striatum
The rodent striatum can be divided into a dorsal and ventral portion based on connectivity and function. The dorsal striatum (or neostriatum) consists of the caudate putamen, and the ventral striatum includes the ventral conjunction of the caudate putamen, the nucleus accumbens, and portions of the olfactory tubercle (Figure 1A). All regions of the neocortex send afferents to the neostriatum in a topographic fashion, and these corticostriatal axons target the major striatal cell type, the GABAergic MSNs, which comprise roughly 95.0 % of the neurons in the rat striatum [150]. These cells have dendrites densely covered with dendritic spines; hence their name. The cortical projections form mainly asymmetrical (excitatory) synapses to MSNs [80,160]. The MSNs send axons to the output nuclei, such as the globus pallidus, also referred to as paleostriatum. These projection neurons project to the internal segment of the globus pallidus, forming the so-called direct, monosynaptic pathway. Other striatal MSNs project to the external segment of the globus pallidus multisynaptically, via intermediate connections, forming the indirect pathway. However, these two pathways are not strictly separated, as some MSNs project to the internal segment of the globus pallidus and also send axon collaterals to the external segment of the globus pallidus [82,115]. The thalamus is another major input region of the neostriatum, with glutamatergic thalamostriatal neurons. The response of the MSNs to cortical and other inputs is key to the functions of the basal ganglia [170]. The neostriatum mainly serves motor related functions [50], and the cortical areas related to sensorimotor functions project to this subdivision [63]. The ventral striatum receives its major glutamatergic input from the prefrontal cortex, hippocampus and amygdala [61,63]. This ventral subdivision serves mainly as the limbic-motor or motivation-action interface and plays a key role in reward-based learning and addiction. For a review of the striatal projections see [161].
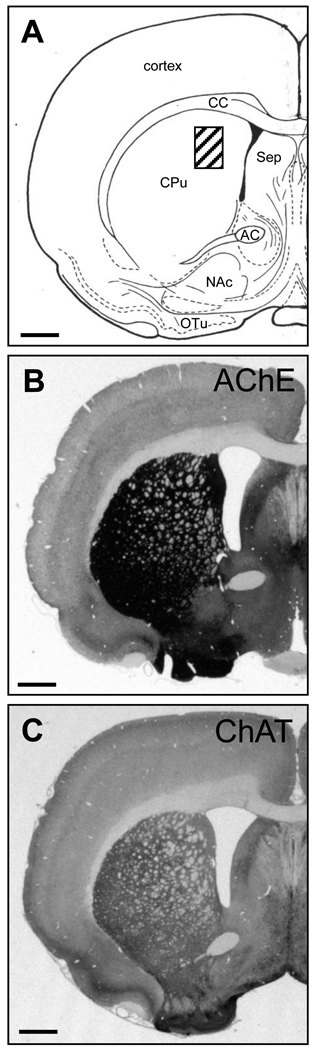
Figure 1.
A schematic drawing of a coronal section of the striatum of the rat adapted from [87] (A). The expression of acetylcholinesterase (AChE; B) and choline acetyltransferase (ChAT; C) is very high in the striatum. The striped box in A depicts the location of the photomicrographs of figure 2. AC = anterior commissure; CC = corpus callosum; CPu = caudate putamen; NAc = nucleus accumbens; OTu = olfactory tubercle; Sep = septum. Scale bar = 900µm
Four types of striatal interneurons have been defined [79]. Besides the cholinergic interneurons (see" section 3), three other largely overlapping subtypes of GABAergic interneurons are recognized: 1) interneurons expressing nitric oxide synthase, somatostatin (SS) or neuropeptide Y, 2) interneurons that contain the calcium binding protein parvalbumin (PARV), and 3) interneurons that contain calretinin. These GABAergic interneurons make up approximately 5 % of the neuronal population in the striatum. Nevertheless, these GABAergic interneurons have large spheres of influence. They are placed strategically to integrate and modulate cortical information flows. Notably the PARV-positive interneurons receive cortical input [96], but the other subtypes receive considerable cortical input as well [171].
3. Cholinergic innervation of the striatum
It is known for a long time that the striatum is extremely rich in cholinergic innervation as can be seen in figure 1B for AChE and figure 1C for ChAT [152]. Only a minor cholinergic projection from the pedunculopontine tegmental area to the neostriatum has been described as an afferent source [198]; other brainstem areas project via monoaminergic afferents to the neostriatum. Hence, the neostriatal cholinergic innervation arises almost exclusively from the intrinsic, relatively large, cholinergic aspiny interneurons. These cells, with smooth dendrites, are distributed in a distinctive spatiotemporal pattern in the different compartments of the neostriatum [162,186]. These neurons most likely correspond to the tonically-active neurons recorded in vivo [5,196]. Cholinergic axons are often characterized by small varicosities [32]. In contrast to the very dense neostriatal cholinergic innervation, relatively few cholinergic synapses have been found [6,32]. This indicates that ACh acts mainly via non-synaptic (paracrine or non-junctional) and diffuse (volume) transmission, released by the varicosities, in addition to synaptic transmission (for review see [42]). This would explain why cholinergic receptors expressed by non-neuronal elements in the neostriatum (e.g. astrocytes and endothelial cells) can be functional in the absence of axonal termination onto these cells.
The neostriatum is characterized by a very high content of AChE (Figure 1B). It could be that this high content of AChE serves to keep ambient ACh levels within physiological limits besides the classical role of eliminating overspill of synaptically released ACh from the extracellular space. The basal levels of ACh in the striatum [40] seems high enough to continuously activate mAChRs and nAChRs [91,137], establishing a baseline and tonic level of cholinergic neurotransmission. The position of the cholinergic varicosities can undergo dynamic changes, by which their exact position in relation to cholinergic receptor-expressing elements (for example releasing more massively ACh in a distal or proximal part of the dendritic tree of an neuron together with local differences in cholinergic receptor densities over the dendritic tree) shifts thereby altering their functional influence [32], adding to functional plasticity within the striatum.
4. The cholinoceptive neural substrate of the striatum
The expression of striatal mAChRs (G-protein-coupled receptors acting primarily on either phospholipase c/Protein Kinase C (PKC) and cAMP pathways) and nAChRs (which form ion channels) has traditionally been studied with autoradiography using tritriated agonists. These studies made clear that the striatum is richly endowed with both classes of cholinergic receptors [15,23,62,188,190,191]. Due to the relatively poor anatomical resolution of autogradiographic images, this field of research moved forward by employing poly- and monoclonal antibodies for receptor protein detection. Here we will briefly review these studies.
4.1. Muscarinic receptors
Originally, the immunocytochemical distribution of mAChRs was first described using a monoclonal antibody named M35 recognizing all five receptor subtypes with equal affinity [21,184]. M35 staining gives a good match between cholinergic innervation patterns and mAChR detection, both in brain and peripheral organs [176,182]. Several types of striatal interneurons express mAChRs as determined by M35 staining (Fig. 2 A, B). Numerous MSNs are mAChR-positive, with labeling density varying from moderate to relatively high (Fig. 2B). Cholinergic interneurons are in general more densely stained for mAChRs than the MSNs. This feature differs somewhat from other cholinergic cells that typically express low numbers of mAChRs [179]. The m2 subtype is known to be preferentially expressed by the cholinergic interneurons [3], and the strong mAChR expression suggests an important cholinergic regulation of ACh release via autoreceptors. Striatal SS- and PARV-positive interneurons also express mAChRs as revealed by colocalization studies (data not shown), but less dense than the cholinergic cells and not as abundant as the Striatal SS- and PARV-positive interneurons in the hippocampus [178,181]. The mAChRs in these interneurons can function postsynaptically and/or presynaptically, regulating intracellular signaling cascades or modulating transmitter release, respectively. These staining patterns suggest that mAChRs play a more dominant role in the regulation of ACh release than regulating GABA release in the striatum, whereas the opposite is more often found in other brain regions. The functional impact of ACh release is discussed below.
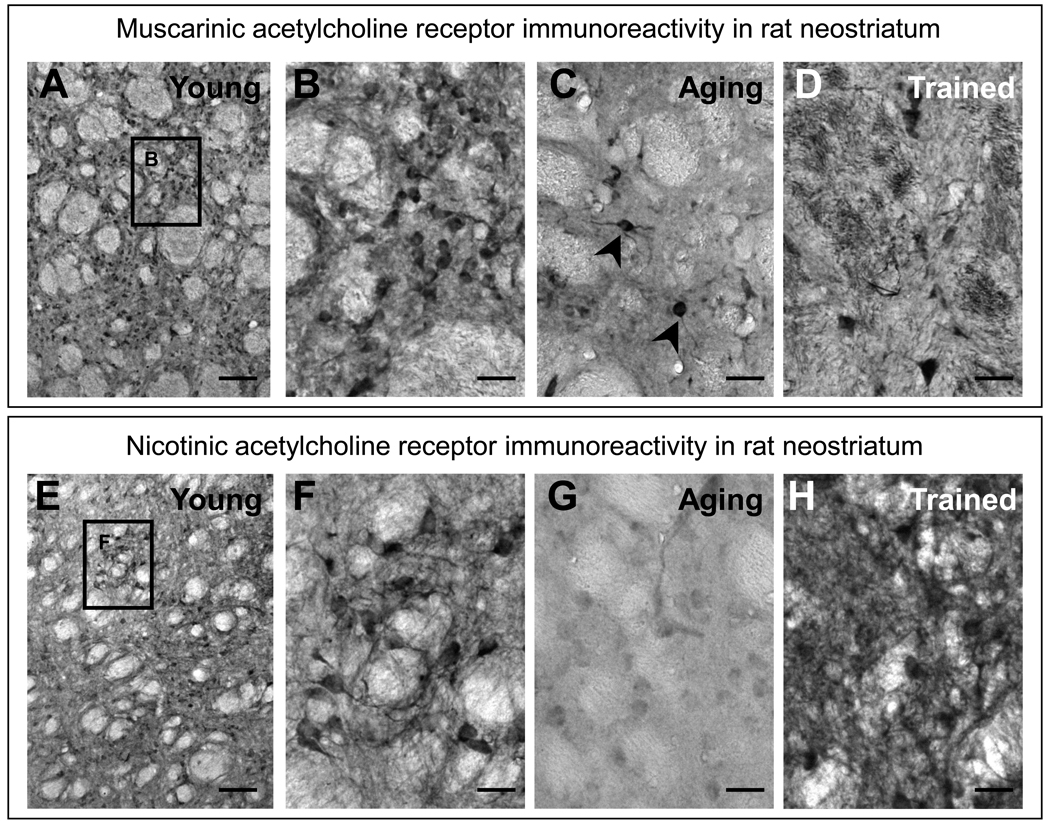
Figure 2.
Cholinergic receptor immunoreactivity in the rat neostriatum (caudate putamen; see striped box in Figure 1A for location). Expression of mAChRs (upper panels) and nAChRs (lower panels) in young (A, B, E, F; 3 mo of age), aged (C, D; 32 mo of age), and holeboard-trained rats (D, H; 3 mo of age) are shown. The boxes in A and E are enlarged in B and F, respectively. Scale bars in A and F = 100 µm; in B–D and F–H =50 µm.
In aged rats, the expression of mAChRs decreases most strongly in the MSNs, and somewhat less so in the putative cholinergic interneurons (identified based on size, distribution pattern, and morphology which was confirmed; arrowheads in Fig. 2C). Apparently, mAChR control over ACh release is less aging-sensitive than mAChR control over GABA release. Occasionally, and in contrast to the young striatum, mAChR-positive astrocytes were found in the aging striatum, a feature also found in some other brain regions and species [154,182,185]. Interestingly, food-rewarded learning tasks (e.g. holeboard learning; [11,177]) cause a characteristic alteration in mAChR expression (Fig. 2D). The large cholinergic interneurons remain densely stained, whereas the MSNs are reduced in labeling. In contrast, a considerable increase in mAChR-positive striato-pallidal fiber bundles is seen throughout the neostriatum. This could hint at changes in the presynaptic functioning of cholinergic heteroreceptors, and possibly reflects a stronger striatal output and/or stronger synchronization among MSNs contributing to the formation of striatal memory traces (Figure 3).
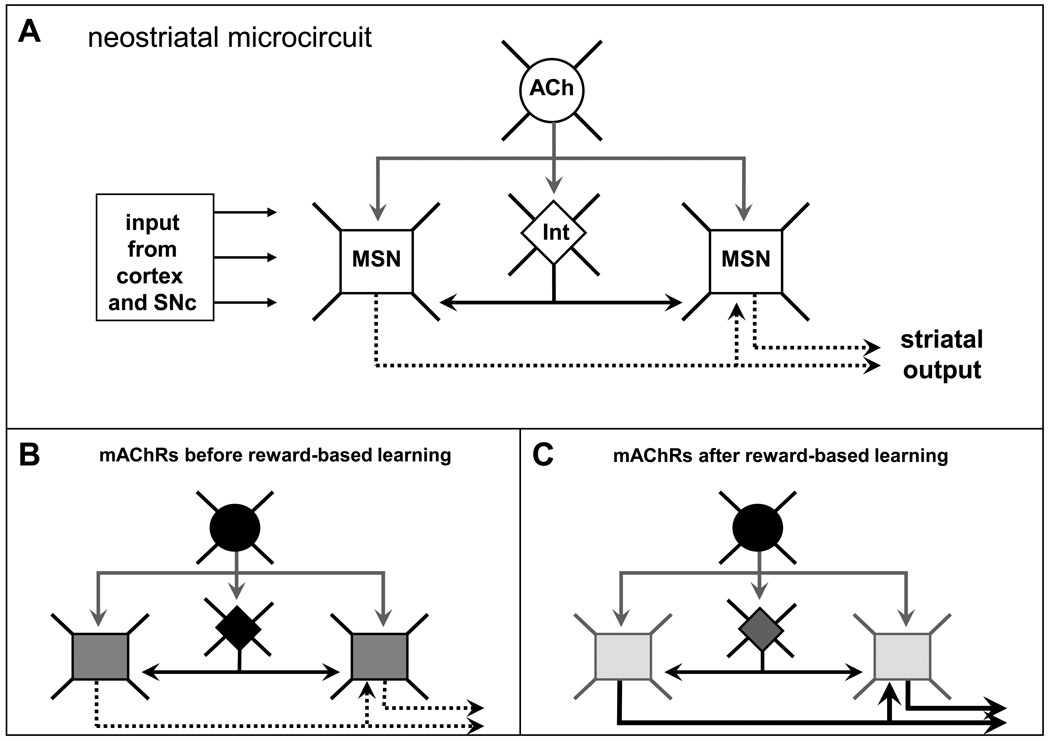
Figure 3.
A schematic representation of the canonical microcircuit (A) of a cholinergic neuron (ACh), two medium spiny neurons (MSN), and an interneuron (Int). The MSNs and Int receive massive glutamatergic input from the cortex and dopaminergic input from the substantia nigra compacta (SNc). The MSNs provide the output of the microcircuit. The cholinergic neurons innervate the Int at the MSNs. The density of mAChR immunoreactivity in the neostriatal microcircuit, as detected with the monoclonal antibody M35, is indicated in B and C. B represents the mAChR situation of experimentally naive (home cage) control rats (black = high immunoreactivity; dark grey = moderate immunoreactivity, and light grey = low immunoreactivity). C represents the situation after a spatial learning task (holeboard) has been completed. It should be noted that the rats performed at 90% correct choices for several days [11]. Low M35 staining indicates a relative open circuit, with free, functional mAChRs located in the cell membrane ready for processing cholinergic input. High M35 staining indicates activated and subsequently internalized mAChRs, reflecting a relative closed circuit, internally processing acquired information [182]. The hippocampus of these holeboard-trained animals showed dense staining for mAChRs already halfway training while the neostriatum still resembles that of control rats (unpublished observations and [11]; for mice see [177]) This suggests that the neostriatum starts processing holeboard task information in a later phase, and/or continues processing this information longer than the hippocampus does. Thickness of the arrows corresponds to the level of output activity.
Learning and memory-induced increases and decreases in mAChR immunoreactivity have been described in various other brain regions (for review [182]). An increase in M35 immunoreactivity reflects internalization of activated (phosphorylated) mAChRs, usually as a consequence of ACh stimulation or otherwise indirectly by kinase activity (phosphorylating mAChRs) induced by non-cholinergic activity (most notably glutamate) [179,182]. Enhanced and prolonged cholinergic stimulation in the striatum results in internalized mAChRs and redistribution towards intracellular organelles [103]. A decrease in M35 immunoreactivity suggests higher numbers of functional, membrane-incorporated mAChRs. The relatively high staining intensity in experimentally naïve animals is in line with the tonically high level of ACh release in the striatum. The cholinergic cells receive excitatory synaptic input from the thalamus (although a smaller cortical projection is also present; [95,147]), and respond to rewarding and salient stimuli [34]. Reward-based learning apparently alters the dynamics of the otherwise tonic ACh release such that mAChRs become functionally available to respond to the increased but temporally changed overall ACh release to process task-specific information. The functional interpretation of these characteristic striatal alterations awaits further investigation, but it at least demonstrates that certain aspects of performing and mastering a spatial learning task are accompanied by robust alterations in the cholinoceptive organization of the neostriatum. These aspects could be the reward, the formation of a procedural-memory related trace, enhanced locomotor activity, or a combination of these. Locomotor activity, not related to a memory task, can also induce massive changes in striatal mAChR expression [11].
After the initial studies with M35 antibody that recognizes all subtypes, subtype-selective antibodies became available. These studies confirmed the earlier data, and extended the neuroanatomical knowledge of subtype specific location of action of the five mAChR subclasses [101]. The detailed distribution of mAChRs added significantly to our understanding of distinct populations of striatal neurons and cholinergic/cholinoceptive microcircuitry.
The subtype-specific antibodies revealed that m1 is widely expressed by small to medium sized striatal neurons (Calbindin-positive MSNs; [3]) and also on glutamatergic corticostriatal terminals acting as heteroreceptors (modulating glutamate release via ACh). The m2 subtype is seen in most large neurons (cholinergic interneurons; [3,70,159] and to a lesser degree in SS/NPY-positive interneurons [14,159]. The m2 subtype appeared to be concentrated in the cholinergic axon terminals [149]. Immunostaining for m3 is barely detected but if so, apparent in MSNs, whereas m4 is seen within the neuropil in a patchy distribution throughout the striatum [70]. A comparison between mAChR protein expression and mRNA expression revealed a good match with a ranking of m1 > m2 >> m3 > m4 [71]. It is clear that striatal mAChRs act presynaptically (either as autoreceptors or heteroreceptors) besides their postsynaptic localization at the aforementioned types of striatal cells. The m1, m3, and m5 subtypes are functionally coupled to mobilization of intracellular calcium, and they have the additional potential to couple to the activation of phospholipase A2, C, and D, and tyrosine kinase. The m2 and m4 subtypes are functionally coupled to the inhibition of adenyl cyclase and they play an additional role in the augmentation of phospholipase A2 (for review see [52])
The current detailed knowledge of mAChR subtype expression within different compartments of the striatum makes it clear how the cholinergic striatal system can interact with several other transmitter systems in different ways. It explains the wide range of functional consequences of cholinergic striatal neurotransmission, and also makes it clear that the differential position of the mAChR subtypes in the different intrinsic and extrinsic circuits needs to be taken into account to achieve a desired functional alteration of the striatum via cholinergic pharmacological manipulation. Moreover, the plastic effects of learning and memory or motor activity on the expression and localization of mAChRs and shifts in presynaptic activity adds to the complexity and dynamics of striatal cholinergic neurotransmission.
4.2. Nicotinic receptors
The immunocytochemical distribution of nAChRs was first described with monoclonal antibodies directed against the main immunogenic region of nAChRs of Torpedo electric organ membranes [43]. In this study, striatal neuropil and some scattered neurons notably in the lateral aspect of the neostriatum were densely labeled. Most likely, the scattered neurons are the cholinergic interneurons. Later on, the antibody mAb270 was used, raised against nAChRs from chicken brain [167]. With this antibody, the striatum appeared to be moderately stained.
Soon thereafter, the monoclonal antibody WF6 recognizing the α-subunit of the Torpedo nAChR became available. In contrast to mAChRs, the nAChR-positive MSNs detected with the antibody WF6 were not distributed as evenly throughout the neostriatum but were instead more often observed in patches (Fig. 2E, F). Besides staining in the cell bodies and dendrites, small thin fiber-like structures could be encountered within and between these patches. The staining intensity for nAChRs did not differ strikingly between the large cholinergic interneurons and the other cell types of the neostriatum. In aged rats, a dramatic decrease in immunoreactivity was seen in all striatal elements (Fig. 2G). This decrease exceeded the general decrease seen in other brain regions, suggesting selective vulnerability of the striatal nAChR system in senescence. A strong increase in nAChR-immunoreactivity was seen in all striatal elements after spatial learning (Fig. 2H; holeboard spatial learning; see [11] for procedural details), as is seen for mAChRs.
Using an antibody raised against the β2-subunit of the nAChR, Hill and coworkers reported that sparsely distributed large neurons (possibly corresponding to the cholinergic interneurons) were intensely stained. Weaker labeling was observed in many MSNs [74]. The entire striatal region appeared to be extremely rich in delicate neuronal processes, which is in line with the presence of nAChRs on axon terminals. The results with this β2-subunit antibody in general parallels our observations with WF6 antibody (Fig. 2 E–H).
4.3. Striatal microcircuitry and cholinergic neurotransmission
A number of presynaptic and postsynaptic neuronal mechanisms are controlled by nAChRs and mAChRs. The presence of presynaptic nAChRs and mAChRs as autoreceptors on cholinergic cells has been reviewed in [17,34,203] and as heteroreceptors in [17,34,51,93,203]. These pre- and postsynaptic mechanisms regulate cholinergic release, glutamatergic afferents from the cortex and thalamus, and dopaminergic input from the brainstem regions. See Nakano [114] for a schematic overview of the primate basal ganglia-thalamo-cortical connections. Regarding the dopaminergic innervation, the neostriatum receives dense innervation primarily from the substantia nigra pars compacta (SNc), and to a lesser degree from the ventral tegmental area (VTA) [63]. The ventral striatum receives dopaminergic input primarily from the VTA and to a lesser degree from the SNc. The cholinergic microcircuitry is depicted in figure 3A. The canonical microcircuit of the neostriatum consists of two MSNs, a cholinergic interneuron and a GABAergic interneuron [170]. Except for the cholinergic interneuron, these cells are innervated by glutamatergic input primarily from the cortex and dopaminergic input primarily from the SNc. The functional modulation of MSNs through the striatal cholinergic system will make them more synchronous, enhancing network synchrony [20]. The expression of mAChRs in this circuit and the changes herein following reward-based learning are depicted in figure 3B and C.
Cholinergic interneurons are tonically active [34], responding to rewarding and salient stimuli (see below). These interneurons can significantly change striatal output and functions, due both to their tonic activity and their dense local innervation [83,204]. Interestingly, a novel microcircuit in the striatum has been suggested, in which the cholinergic interneurons are connected and communicate to one another through GABAergic interneurons [164]. This connection depends on the activation of nAChRs and this microcircuit exerts powerful control over the firing activity of cholinergic interneurons. The transient depression of tonic firing of these cells is critical for conditioning involving processing of sensory and motor information [5,146,196].
Many of the striatal mAChR-regulated functions are mediated by protein kinase C (PKC). Nearly all MSNs express PKCβII, PKCγ, and PKCε, whereas the cholinergic interneurons express PKCα [41,200]. As such, PKCα may be important for controlling the release of ACh that influences the basal ganglia circuit and maintaining cholinergic tone [41]. Notably PKCγ is known to be essential for synaptic plasticity and memory formation in many brain regions [47,168,180,183], but whether this holds true for the striatum is less well known although mAChR-mediated modulation of different calcium channels in neostriatal neurons by PKC has been described [131]. Interestingly, part of the cholinergic striatal neurotransmission via mAChR may depend on A-kinase anchoring protein (AKAP) 150 [174], which is highly expressed in the striatum [121]. AKAPs compartmentalize cAMP signaling by forming complexes of kinases, phosphatases and substrates [157]. It has recently been shown that AKAP150 is critically involved in learning and memory [118,121,174]. AKAP150 could link mAChR activation to potassium channel regulation [72,75]. This provides another way by which the cholinergic and dopaminergic systems may interact (as discussed below) in the regulation of potassium currents [165]; AKAP150 anchors PKC regulated via ACh whereas PKA anchoring is regulated via dopamine.
5. Interaction of the cholinergic and dopaminergic system
The dopaminergic and cholinergic system are the primary and" secondary largest neuromodulatory systems in the striatum and their interaction plays a key role in coordinating striatum-mediated behavioral responses. A large body of data suggests that these systems can bi-directly modulate one another’s function. Initial studies suggested that activation of the dopaminergic system generally inhibits the release of ACh [100]. However, these findings were biased by the fact that the originally developed DA-receptor agonists preferentially modulated dopamine receptor 2 (D2) activity rather than dopamine receptor 1 (D1) activity [92]. Indeed, later studies showed that D2 activation reduces whereas activation of the D1 receptor using specific agonists facilitates ACh release [1,16,29,31,35,36,45], for review see [46].
In addition to the dopaminergic system modulating the cholinergic system, the cholinergic system also affects activity of the striatal dopaminergic system. Although it has been widely accepted that presynaptic mAChRs can modulate DA release, whether or not mAChRs facilitate or inhibit this process remains a matter of debate. The studies by De Belleroche and Bradford [37] indicated that muscarinic receptor-evoked DA release could be facilitated by using high levels of the muscarinic antagonist atropine. Other studies described similar findings [44,90]. In contrast, Giorguieff and colleagues [57] showed that application of the muscarinic antagonist atropine blocked ACh-mediated DA release indicating that presynaptic mAChRs facilitate DA release, a finding confirmed by others [69,99,144]. It was suggested that the original findings of De Belleroche and Bradford [37] were confounded by the use of inappropriately high levels of agonists and antagonists. In addition to pharmacological studies aimed at determining the modulatory role of the presynaptic mAChRs, genetic approaches have been undertaken to elucidate the role of the cholinergic system in striatal DA release. Using mutant mice lacking AChE, Hrabovska et al [76] showed that this manipulation resulted in a marked decrease in D1 and D2 receptors besides the previously-described reduction in m1, m2 and m4 mAChRs in the brain [189]. The reduction in D1 and D2 receptor expression in the AChE knockout mice was hypothesized to be a consequence of increased DA release due to mAChR overstimulation. This critical dopamine-acetylcholine misbalance has recently been suggested to underlie the cognitive dysfunctions in Parkinson’s disease [18]. Furthermore, selective loss of m1 cholinergic receptors resulted in upregulation of dopaminergic transmission that was accompanied by increased locomotor activity, and stronger activation of the dopaminergic system in response to amphetamine treatment [56]. These studies confirmed the previous pharmacological findings described above suggesting that blocking muscarinic cholinergic activity facilitates rather than inhibits dopaminergic transmission indicating that the cholinergic system acts as a constraint on the dopaminergic system. This antagonistic function of the dopaminergic and cholinergic system was further strengthened by the fact that both facilitating dopaminergic signaling or inhibiting cholinergic signaling rescued the motor dysfunctions seen with Parkinson’s disease [8,134].
nAChRs, as discussed previously, are located on pre-synaptic axon terminals [78], and can directly facilitate or inhibit DA release [148,201,202]. To date, 5 nAChR subtypes are known to be expressed on dopaminergic nerve terminals [59]. Three of these subunits contain the α6 subunit (α4α6β2β3, α6β2β3, α6β2) whereas the other two contain the α4-subunit (α4β2, α4α5β2) with the later being more numerous than the α6* containing subtypes and the α4* containing subtypes having the highest affinity for nicotine [59]. ACh released by cholinergic interneurons activate these nAChRs which maintain the background DA levels [202]. However these same nAChRs restrict DA release in response to burst firing of dopaminergic neurons since a reduction of nAChR activity facilitates this process [148,201]. A recent study by Drenan et al [49] indicated that transgenic overexpression of α6-channels hypersensitive to endogenous ACh or exogenous nicotine resulted in greatly augmented DA release and increased DA neuron firing.
6. Interaction of the cholinergic and GABAergic system
Because most of the striatal neurons are GABAergic, it is fair to assume that the cholinergic system also mediates GABA release in addition to modulating DA release [17]. Activation of postsynaptically localized mAChRs on striatal projection neurons have been suggested to inhibit GABA-mediated synaptic potentials [17]. Besides postsynaptically-mediated alteration of GABAergic signaling, the cholinergic system may also presynaptically mediate GABA release. Both nicotinic and mAChRs have been reported to be expressed in GABA-releasing presynaptic terminals [60,88,102,105,106,163], with the α4β2-containing subtypes of nicotinergic cholinergic receptors being the major modulators of GABA release based on studies using genetic [132], or pharmacological approaches [105]. A recent study by Grilli et al [60] showed that mAChRs and nAChRs (of the α4β2 subtype) co-exist on GABAergic nerve terminals and that nicotinergic-mediated release of GABA was prevented by activation of M4 mAChRs (that are strongly expressed in the striatum (see figure 2, and also [135]), suggesting an antagonistic function of mAChRs and nAChRs regarding GABA release from presynaptic terminals.
As mentioned above, a novel microcircuit in the striatum is suggested in which the cholinergic interneurons are connected and communicate to one another through GABAergic interneurons [164]. This interaction between the striatal cholinergic system and GABAergic signal transduction could be pivotal for proper striatal functioning.
7. Associative memory functions and the striatal system
Initial studies on the function of the striatum in learning and memory indicated that the striatum is a critical region for specific forms of learning. For example, lesions of the striatum impaired avoidance learning [4,81,112,116,136,197] and performance in the cued version of the Morris water maze where a visual mark indicated the location of the platform [193,194]. Packard and colleagues [124] tested the effect of either bilateral caudate nucleus or bilateral fimbria-fornix lesions in two versions of the radial-arm maze. The first version was a ‘win-shift’ version in which each of the eight arms was baited once and the number of revisits to a previously baited arm was measured. This working memory version of the task was used to assess the capability of the animals to remember which arm(s) were already visited and which arms were not. Memory was indicated by no revisits. The" second version of the task was a ‘win-stay’ version of the same task in which the location of 4 randomly selected baited arms was signaled by a light at the entrance to each arm. Rats had to learn to selectively visit the arms signaled by light, a form of habit or skill learning. Fimbria-fornix lesions impaired learning in the working memory-dependent ‘win-shift’ task, but enhanced performance in the win-stay version of the task. In contrast, lesions of the caudate nucleus did not affect learning in the win-shift version of the task, but markedly impaired performance in the win-stay version of the task indicating that the striatum plays a crucial role in habit learning. This study together with other studies indicated that different memory systems exist in the brain. A similar habit memory or ‘skill’ learning deficit was later demonstrated for amnesic patients and patients with Parkinson’s disease [84]. More detailed analyses indicated that the dorsal striatum and ventral striatum have different functions. The ventral striatum is indirectly involved in driving instrumental responses by allowing cues associated with reward (for instance cocaine) to exert a general motivational influence on responding [19,155,192,195]. The dorsal striatum, on the other hand seems to play a crucial role in adaptive motor control and procedural memory [54,89,199]. The latter will be discussed in more detail below.
8. Place navigation and the striatal cholinergic system
A general theme in behavioral neuroscience is that distinct “memory systems” mediate dissociable aspects of memory. In place navigation, these distinct memory system mediate different navigational strategies [58,77,113,122]. These systems predominantly rely on specific brain regions, such as the hippocampus, the striatum and the amygdala. The existence of distinguishable neuronal systems is supported by many studies in various species including rats [119,124,126,151], mice [98], monkeys [205,206] and humans [25,66,84]. For example, Packard and McGaugh [126] demonstrated that rats with hippocampal or fimbria/fornix lesions are impaired in place learning which depends on the use of a configuration of extra-maze cues to locate a submerged escape platform, although they are not impaired in a cued version of the task in which a visible platform directs the rat’s escape. In contrast, rats with lesions of the dorsal striatum are impaired in the ‘visible platform’ version of the task, while performance was not affected the in spatial version of the task where the rats had to use extra-maze cues to find the platform. These findings indicate that the hippocampus, but not the dorsal striatum, plays a crucial role in spatial learning (also known as allocentric learning; making responses based on external cues). In contrast, rats with dorsal striatal lesions were impaired in the cued version of the water maze task, but not in the spatial version suggesting that the dorsal striatum is important for response learning (i.e. “egocentric” learning; making responses based on their own body orientation in space). The neuronal systems underlying the different behavioral strategies used for place navigation have been further studied using other behavioral paradigms including a modified version of the T maze also known as cross maze [28,127], originally described by Tolman [172,173]. For instance, rats in which the striatum was inactivated were impaired in a right-left discrimination paradigm [33], as well as non-spatial versions of the Morris water maze and modified version of the T-maze [28,127]. Likewise, glutamate injections into the striatum was shown to facilitate response learning [123], whereas blocking the activity of N-Methyl-D-Aspartate (NMDA) receptors in the dorsal striatum impaired response learning, leaving place learning unaffected [38,128].
The role of the cholinergic system in striatum-mediated egocentric learning has also been determined. Kobayashi and Iwasaki [85] showed that striatal lesion of the cholinergic system selectively impaired egocentric learning, but not allocentric learning. Interestingly, McIntyre and colleagues [109] showed that the profile of ACh release in the dorsal striatum relative to the hippocampus indicates the preferred behavioral strategy used by a particular rat; high ratios of striatal versus hippocampal ACh release were found in individuals that preferentially used a dorsal striatal-dependent response strategy, rather than a hippocampus-dependent spatial strategy. In line with these observations, Vetreno and colleagues [187] showed that preferential use of an egocentric response facilitated ACh release in the striatum. ACh levels were found to be enhanced in both hippocampus and striatum, when rats were trained in either a place or response version of the task. However, the increase in striatal ACh release was significantly higher in the task in which rats could selectively use a response strategy. This parallel increase in ACh release in both hippocampus and striatum suggested that in case of the cholinergic system, both systems are activated in parallel, but that the striatum predominates the hippocampus under specific conditions, for example when a striatum-dependent response strategy is required, as a result of stronger cholinergic activation. The principle of multiple parallel memory systems has been described previously and under certain conditions these systems can complement or compete with each other. The findings described in the paragraphs above suggest that the cholinergic system in both hippocampus and striatum are activated in parallel but that if the striatal activation is stronger it can overrule the hippocampal system.
Several studies have indicated that during acquisition in a place navigation task both humans and rodents preferentially use a hippocampus-dependent spatial strategy rather than a striatum-dependent response strategy. With extended training, humans as well as rodents switch to using a response-strategy [22,120,123,127]. It is interesting to note that this shift in behavioral strategy is paralleled by a transition in ACh release from hippocampus to striatum. Chang and Gold [22] showed that with ongoing training in a T-maze reference task, rats shifted from using a spatial to a response strategy, and that this shift was accompanied by a gradual increase in ACh release from the striatum. ACh release from the hippocampus did not decrease but remained high, indicating that the striatal was activated at the time rats started to use the striatum-dependent response strategy. The authors argued that the hippocampus remains active, but that the striatum can override hippocampal activity when fully engaged by repeated training. In addition to the different levels of activation of the striatal and hippocampal cholinergic system, similar observations have been made using general activity markers including c-fos, phospho-CREB and c-Jun [26,27,169].
9. Cholinergic involvement in intra- and extradimensional set shifting
The basal ganglia play a crucial role in motor planning, procedural learning, non-declarative forms of memory and motivation [55,73,89,117,125,199]. In addition to these functions, the dorsomedial region of the striatum is involved in the adaptation of previously acquired behavioral responses (e.g., behavioral flexibility) [65,86,104,133,140,141] for review [138]. It has strong connections with the orbitofrontal cortex and pre-limbic area [13], two prefrontal cortex areas both known to be critically involved in behavioral flexibility [139].
One of the various behavioral paradigms used to study the molecular mechanisms underlying the adaptation of previously learned responses is (place) reversal learning, also known as intradimensional set shifting [7,39,130,145]. In a symmetrical T or Y maze, rodents are initially trained to retrieve a food reward that is located in either of two accessible goal arms. After the animals have learned which of the two arms is baited, indicated by a strong significant preference to visit the baited arm, the food reward is relocated to the previously non-baited arm. Initially the animals will tend to visit the previously-baited arm, but gradually they will learn that the previously non-baited arm is now baited, indicated by a strong preference to visit that arm. The latter is referred to as reversal learning. Previous studies have indicated that place learning and reversal learning differentially impact hippocampal plasticity [67,68]. Similar to the hippocampus, the dorsomedial striatum has been shown to be differentially involved in both processes; lesions or electrical stimulation of the dorsomedial striatum results in impaired spatial reversal learning [65,86,104,156], as well as non-spatial reversal learning [12,24,133]. These findings were confirmed by Ragozzino and others which locally infused anesthetics to temporally inactivate the dorsomedial striatum (for review see [138]).
Because the cholinergic system is a key modulatory system in the dorsomedial striatum, the question that remained to be answered was whether the striatal cholinergic system also had a prominent role in intradimensional and extradimensional reversal learning, for example through alterations in striatal ACh release. For this purpose, Ragozzino and Choi [140] subjected rats to training and reversal training in a spatial discrimination task and measured ACh release from the dorsomedial striatum. They found that ACh levels were increased during spatial reversal learning, but not during the initial training suggesting that the medial-striatal cholinergic system is differently involved in spatial learning and spatial reversal learning. Similar observations were made by Palencia and Ragozzino [129]. In parallel with these findings that intradimensional reversal learning induces changes in ACh levels in the dorsomedial striatum, pharmacological studies have been undertaken to determine the role of specific cholinergic receptors in reversal learning. Ragozzino et al [141] delivered muscarinic or nicotinic cholinergic antagonists into the dorsomedial striatum during training and reversal training in a response discrimination task. They found that the muscarinic cholinergic blocker scopolamine did not interfere with the initial acquisition of response learning, but did impair reversal learning of the previously acquired response. In contrast, infusion of the nicotinic cholinergic antagonist mecamylamine did not affect either the acquisition or reversal learning, suggesting that specifically mAChRs in the dorsomedial striatum play a crucial role in the reversal of a previously acquired response. To determine whether the M1-type or M4-type mAChR was involved in place reversal learning, the laboratory of Michael Ragozzino did similar experiments, using m1-type or m4-type specific mAChR and demonstrated that specific blockage of the m1-type receptor impairs reversal learning, but not acquisition [108,175].
In addition to the fact that the dorsomedial striatum is important for intradimensional set shifting, as described above, the dorsomedial striatum has shown to be involved in extradimensional set shifting, the switching from using a spatial strategy to using a response strategy and vice versa in a cross maze reference task. Ragozzino et al [142] showed that temporal inactivation of the dorsomedial striatum using tetracaine did not impair acquisition of egocentric learning or allocentric learning. However, it markedly impaired the shifting from using one strategy to the other, and vice versa. However, to our knowledge, no experiments have been conducted to determine whether the cholinergic system in the dorsomedial striatum is critically involved in extra-dimensional set shifting as it is in intra-dimensional set shifting.
10. Behavioral flexibility and the striatal cholinergic system
Loss of behavioral flexibility (impairment of intra- or extradimensional reversal learning) is one of the first hallmarks of non-pathological ageing and the development of major dementing illnesses like Alzheimer’s disease [94,107] for review [2], and Parkinson’s disease [48,53]. Similar observations have been made in rodents. For instance, two-year old mice perform similarly as young mice during acquisition in a two-arm reference memory task, but are impaired during intra-dimensional reversal learning (Figure 4). As such, the training and reversal training Y-maze paradigm is very suitable to detect aging-specific deficits besides the functional interaction between the striatum and hippocampus [64,67,68]. Other studies have also reported that the ability to adapt previously acquired responses is the first to be affected by ageing [110,111,143]. In terms of the cholinergic system, the deficits in behavioral flexibility may be due, in part, to a selective loss of mAChRs in the striatum [158], see also Figure 2. Likewise, several studies reported alterations in the balance of ACh release in the dorsomedial striatum and hippocampus with aging [30,153] and development of neurodegenerative diseases. Changes in ACh release have been observed with the development of Alzheimer’s disease and Huntington’s disease [10,166]. Lazari and colleagues [97] showed that aging-accompanied impairments in procedural memory could be rescued through intra-striatal delivery an M2-type muscarinic receptor antagonist. Future studies are needed to determine whether manipulation of ACh release from the dorsomedial striatum (and hippocampus) is sufficient to overcome the reduced behavioral flexibility and other cognitive deficits observed with pathological and non-pathological aging.
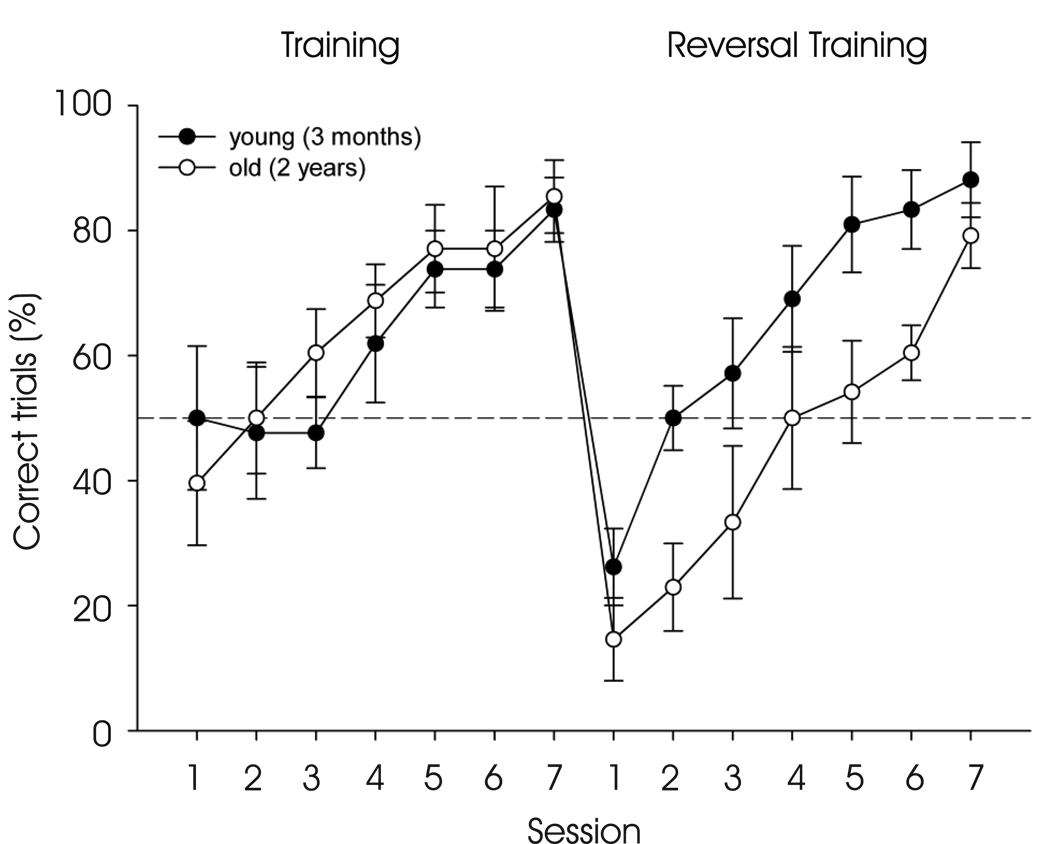
Figure 4.
Aged mice are impaired in intra-dimensional reversal learning Performance of young (n=7) and aged C57Bl6/j mice (n=8) in the Y maze during training and reversal training. Both groups gradually learned to locate the baited arm (ANOVA F6,78 = 8.880, P < 0.001). Aging did not affect the rate of acquisition during training (ANOVA F < 1). Although both young and aged mice both improved their performance during reversal training (ANOVA F6,78 = 22.564 P < 0.001), rate of acquisition during reversal training was reduced in aged mice (ANOVA F1,13 = 8.208, P <0.05).
11. Conclusion and future directions
The striatum robustly expresses both muscarinic and nicotinic cholinergic receptors that modulate the function of striatal dopaminergic and GABAergic systems. Behavioral studies have shown that the striatal cholinergic system is important for procedural learning and intradimensional set-shifting although a role in extradimensional set-shifting remains to be elucidated. It will be of great value to determine whether aging-induced changes in ACh release from the hippocampus and dorsomedial striatum are paralleled by changes in the preferred behavioral strategy used by young and aged animals in a Y or T-maze reference task. For instance, the ratio of ACh release from the hippocampus and dorsomedial striatum may predict the behavioral strategy used in aged animals as was demonstrated for young animals by McIntyre and colleagues [109]. This is of great significance since Barnes and colleagues [9] showed that aged rats preferentially use a striatum-dependent strategy, in contrast to young rats that prefer to use a hippocampus-dependent strategy. Altogether, these studies would indicate whether a shift in the preferentially used behavioral strategy is due to alterations in the balance of hippocampal and striatal cholinergic activity with pathological and non-pathological aging. Understanding how the balance between striatal and hippocampal cholinergic activity controls behavior and memory may result in novel approaches to rescue aging-related cognitive decline.
Acknowledgements
We thank Joshua Hawk and Dr. Sara Aton for valuable comments on a previous version of this manuscript. We thank Jan Keijser for his help with making the photomicrographs. This work was supported by The Netherlands Organization for Scientific Research (NWO-Vernieuwingsimpuls E.A.V.d.Z. (Grant 016.021.017)) and by P50 AG 017628 (A. I. Pack, PI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Acquas E, Fibiger HC. Dopaminergic regulation of striatal acetylcholine release: the critical role of acetylcholinesterase inhibition. J Neurochem. 1998;70:1088–1093. doi: 10.1046/j.1471-4159.1998.70031088.x. [DOI] [PubMed] [Google Scholar]
- 2.Albert MS. Cognitive and neurobiologic markers of early Alzheimer disease. Proc Natl Acad Sci U S A. 1996;93:13547–13551. doi: 10.1073/pnas.93.24.13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alcantara AA, Mrzljak L, Jakab RL, Levey AI, Hersch SM, Goldman-Rakic PS. Muscarinic m1 and m2 receptor proteins in local circuit and projection neurons of the primate striatum: anatomical evidence for cholinergic modulation of glutamatergic prefronto-striatal pathways. J Comp Neurol. 2001;434:445–460. doi: 10.1002/cne.1186. [DOI] [PubMed] [Google Scholar]
- 4.Allen JD, Davison CS. Effects of caudate lesions on signaled and nonsignaled Sidman avoidance in the rat. Behav Biol. 1973;8:239–250. doi: 10.1016/s0091-6773(73)80023-9. [DOI] [PubMed] [Google Scholar]
- 5.Apicella P. Leading tonically active neurons of the striatum from reward detection to context recognition. Trends Neurosci. 2007;30:299–306. doi: 10.1016/j.tins.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Aznavour N, Mechawar N, Watkins KC, Descarries L. Fine structural features of the acetylcholine innervation in the developing neostriatum of rat. J Comp Neurol. 2003;460:280–291. doi: 10.1002/cne.10660. [DOI] [PubMed] [Google Scholar]
- 7.Bannerman DM, Deacon RM, Seeburg PH, Rawlins JN. GluR-A-Deficient mice display normal acquisition of a hippocampus-dependent spatial reference memory task but are impaired during spatial reversal. Behav Neurosci. 2003;117:866–870. doi: 10.1037/0735-7044.117.4.866. [DOI] [PubMed] [Google Scholar]
- 8.Barbeau A. The pathogenesis of Parkinson's disease: a new hypothesis. Can Med Assoc J. 1962;87:802–807. [PMC free article] [PubMed] [Google Scholar]
- 9.Barnes CA, Nadel L, Honig WK. Spatial memory deficit in senescent rats. Can J Psychol. 1980;34:29–39. doi: 10.1037/h0081022. [DOI] [PubMed] [Google Scholar]
- 10.Bartus RT, Dean RL, 3rd, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 11.Beldhuis HJ, Everts HG, Van der Zee EA, Luiten PG, Bohus B. Amygdala kindling-induced seizures selectively impair spatial memory. 2. Effects on hippocampal neuronal and glial muscarinic acetylcholine receptor. Hippocampus. 1992;2:411–419. doi: 10.1002/hipo.450020408. [DOI] [PubMed] [Google Scholar]
- 12.Bellebaum C, Koch B, Schwarz M, Daum I. Focal basal ganglia lesions are associated with impairments in reward-based reversal learning. Brain. 2008;131:829–841. doi: 10.1093/brain/awn011. [DOI] [PubMed] [Google Scholar]
- 13.Berendse HW, Galis-de Graaf Y, Groenewegen HJ. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J Comp Neurol. 1992;316:314–347. doi: 10.1002/cne.903160305. [DOI] [PubMed] [Google Scholar]
- 14.Bernard V, Laribi O, Levey AI, Bloch B. Subcellular redistribution of m2 muscarinic acetylcholine receptors in striatal interneurons in vivo after acute cholinergic stimulation. J Neurosci. 1998;18:10207–10218. doi: 10.1523/JNEUROSCI.18-23-10207.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernard V, Normand E, Bloch B. Phenotypical characterization of the rat striatal neurons expressing muscarinic receptor genes. J Neurosci. 1992;12:3591–3600. doi: 10.1523/JNEUROSCI.12-09-03591.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertorelli R, Consolo S. D1 and D2 dopaminergic regulation of acetylcholine release from striata of freely moving rats. J Neurochem. 1990;54:2145–2148. doi: 10.1111/j.1471-4159.1990.tb04922.x. [DOI] [PubMed] [Google Scholar]
- 17.Calabresi P, Centonze D, Gubellini P, Pisani A, Bernardi G. Acetylcholine-mediated modulation of striatal function. Trends Neurosci. 2000;23:120–126. doi: 10.1016/s0166-2236(99)01501-5. [DOI] [PubMed] [Google Scholar]
- 18.Calabresi P, Picconi B, Parnetti L, Di Filippo M. A convergent model for cognitive dysfunctions in Parkinson's disease: the critical dopamine-acetylcholine synaptic balance. Lancet Neurol. 2006;5:974–983. doi: 10.1016/S1474-4422(06)70600-7. [DOI] [PubMed] [Google Scholar]
- 19.Carelli RM. Nucleus accumbens cell firing during goal-directed behaviors for cocaine vs. 'natural' reinforcement. Physiol Behav. 2002;76:379–387. doi: 10.1016/s0031-9384(02)00760-6. [DOI] [PubMed] [Google Scholar]
- 20.Carrillo-Reid L, Tecuapetla F, Tapia D, Hernandez-Cruz A, Galarraga E, Drucker-Colin R, Bargas J. Encoding network states by striatal cell assemblies. J Neurophysiol. 2008;99:1435–1450. doi: 10.1152/jn.01131.2007. [DOI] [PubMed] [Google Scholar]
- 21.Carsi-Gabrenas JM, Van der Zee EA, Luiten PG, Potter LT. Non-selectivity of the monoclonal antibody M35 for subtypes of muscarinic acetylcholine receptors. Brain Res Bull. 1997;44:25–31. doi: 10.1016/s0361-9230(96)00422-4. [DOI] [PubMed] [Google Scholar]
- 22.Chang Q, Gold PE. Switching memory systems during learning: changes in patterns of brain acetylcholine release in the hippocampus and striatum in rats. J Neurosci. 2003;23:3001–3005. doi: 10.1523/JNEUROSCI.23-07-03001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clarke DJ, Bjorklund A. Restoration of cholinergic circuitry in the hippocampus by foetal grafts. Exs. 1989;57:275–287. doi: 10.1007/978-3-0348-9138-7_27. [DOI] [PubMed] [Google Scholar]
- 24.Clarke HF, Robbins TW, Roberts AC. Lesions of the medial striatum in monkeys produce perseverative impairments during reversal learning similar to those produced by lesions of the orbitofrontal cortex. J Neurosci. 2008;28:10972–10982. doi: 10.1523/JNEUROSCI.1521-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen NJ, Squire LR. Preserved learning and retention of pattern-analyzing skill in amnesia: dissociation of knowing how and knowing that. Science. 1980;210:207–210. doi: 10.1126/science.7414331. [DOI] [PubMed] [Google Scholar]
- 26.Colombo PJ. Learning-induced activation of transcription factors among multiple memory systems. Neurobiol Learn Mem. 2004;82:268–277. doi: 10.1016/j.nlm.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Colombo PJ, Brightwell JJ, Countryman RA. Cognitive strategy-specific increases in phosphorylated cAMP response element-binding protein and c- Fos in the hippocampus and dorsal striatum. J Neurosci. 2003;23:3547–3554. doi: 10.1523/JNEUROSCI.23-08-03547.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Compton DM. Behavior strategy learning in rat: effects of lesions of the dorsal striatum or dorsal hippocampus. Behav Processes. 2004;67:335–342. doi: 10.1016/j.beproc.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Consolo S, Girotti P, Russi G, Di Chiara G. Endogenous dopamine facilitates striatal in vivo acetylcholine release by acting on D1 receptors localized in the striatum. J Neurochem. 1992;59:1555–1557. doi: 10.1111/j.1471-4159.1992.tb08473.x. [DOI] [PubMed] [Google Scholar]
- 30.Consolo S, Sieklucka M, Fiorentini F, Forloni G, Ladinsky H. Frontal decortication and adaptive changes in striatal cholinergic neurons in the rat. Brain Res. 1986;363:128–134. doi: 10.1016/0006-8993(86)90664-5. [DOI] [PubMed] [Google Scholar]
- 31.Consolo S, Wu CF, Fiorentini F, Ladinsky H, Vezzani A. Determination of endogenous acetylcholine release in freely moving rats by transstriatal dialysis coupled to a radioenzymatic assay: effect of drugs. J Neurochem. 1987;48:1459–1465. doi: 10.1111/j.1471-4159.1987.tb05686.x. [DOI] [PubMed] [Google Scholar]
- 32.Contant C, Umbriaco D, Garcia S, Watkins KC, Descarries L. Ultrastructural characterization of the acetylcholine innervation in adult rat neostriatum. Neuroscience. 1996;71:937–947. doi: 10.1016/0306-4522(95)00507-2. [DOI] [PubMed] [Google Scholar]
- 33.Cook D, Kesner RP. Caudate nucleus and memory for egocentric localization. Behav Neural Biol. 1988;49:332–343. doi: 10.1016/s0163-1047(88)90338-x. [DOI] [PubMed] [Google Scholar]
- 34.Cragg SJ. Meaningful silences: how dopamine listens to the ACh pause. Trends Neurosci. 2006;29:125–131. doi: 10.1016/j.tins.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 35.Damsma G, Fibiger HC. The effects of anaesthesia and hypothermia on interstitial concentrations of acetylcholine and choline in rat striatum. Life Sci. 1991;48:2469–2474. doi: 10.1016/0024-3205(91)90383-m. [DOI] [PubMed] [Google Scholar]
- 36.Damsma G, Tham CS, Robertson GS, Fibiger HC. Dopamine D1 receptor stimulation increases striatal acetylcholine release in the rat. Eur J Pharmacol. 1990;186:335–338. doi: 10.1016/0014-2999(90)90456-g. [DOI] [PubMed] [Google Scholar]
- 37.De Belleroche J, Bradford HF. Biochemical evidence for the presence of presynaptic receptors on dopaminergic nerve terminals. Brain Res. 1978;142:53–68. doi: 10.1016/0006-8993(78)90176-2. [DOI] [PubMed] [Google Scholar]
- 38.De Leonibus E, Oliverio A, Mele A. A study on the role of the dorsal striatum and the nucleus accumbens in allocentric and egocentric spatial memory consolidation. Learn Mem. 2005;12:491–503. doi: 10.1101/lm.94805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deacon RM, Bannerman DM, Kirby BP, Croucher A, Rawlins JN. Effects of cytotoxic hippocampal lesions in mice on a cognitive test battery. Behav Brain Res. 2002;133:57–68. doi: 10.1016/s0166-4328(01)00451-x. [DOI] [PubMed] [Google Scholar]
- 40.DeBoer P, Abercrombie ED, Heeringa M, Westerink BH. Differential effect of systemic administration of bromocriptine and L-dopa on the release of acetylcholine from striatum of intact and 6-OHDA-treated rats. Brain Res. 1993;608:198–203. doi: 10.1016/0006-8993(93)91459-6. [DOI] [PubMed] [Google Scholar]
- 41.Deng P, Pang ZP, Lei Z, Xu ZC. Excitatory roles of protein kinase C in striatal cholinergic interneurons. J Neurophysiol. 2009;102:2453–2461. doi: 10.1152/jn.00325.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Descarries L, Gisiger V, Steriade M. Diffuse transmission by acetylcholine in the CNS. Prog Neurobiol. 1997;53:603–625. doi: 10.1016/s0301-0082(97)00050-6. [DOI] [PubMed] [Google Scholar]
- 43.Deutch AY, Holliday J, Roth RH, Chun LL, Hawrot E. Immunohistochemical localization of a neuronal nicotinic acetylcholine receptor in mammalian brain. Proc Natl Acad Sci U S A. 1987;84:8697–8701. doi: 10.1073/pnas.84.23.8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dewey SL, Smith GS, Logan J, Brodie JD, Simkowitz P, MacGregor RR, Fowler JS, Volkow ND, Wolf AP. Effects of central cholinergic blockade on striatal dopamine release measured with positron emission tomography in normal human subjects. Proc Natl Acad Sci U S A. 1993;90:11816–11820. doi: 10.1073/pnas.90.24.11816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Chiara G, Carboni E, Morelli M, Cozzolino A, Tanda GL, Pinna A, Russi G, Consolo S. Stimulation of dopamine transmission in the dorsal caudate nucleus by pargyline as demonstrated by dopamine and acetylcholine microdialysis and Fos immunohistochemistry. Neuroscience. 1993;55:451–456. doi: 10.1016/0306-4522(93)90514-g. [DOI] [PubMed] [Google Scholar]
- 46.Di Chiara G, Morelli M, Consolo S. Modulatory functions of neurotransmitters in the striatum: ACh/dopamine/NMDA interactions. Trends Neurosci. 1994;17:228–233. doi: 10.1016/0166-2236(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 47.Douma BR, Van der Zee EA, Luiten PG. Translocation of protein kinase Cgamma occurs during the early phase of acquisition of food rewarded spatial learning. Behav Neurosci. 1998;112:496–501. doi: 10.1037//0735-7044.112.3.496. [DOI] [PubMed] [Google Scholar]
- 48.Downes JJ, Roberts AC, Sahakian BJ, Evenden JL, Morris RG, Robbins TW. Impaired extra-dimensional shift performance in medicated and unmedicated Parkinson's disease: evidence for a specific attentional dysfunction. Neuropsychologia. 1989;27:1329–1343. doi: 10.1016/0028-3932(89)90128-0. [DOI] [PubMed] [Google Scholar]
- 49.Drenan RM, Grady SR, Whiteaker P, McClure-Begley T, McKinney S, Miwa JM, Bupp S, Heintz N, McIntosh JM, Bencherif M, Marks MJ, Lester HA. In vivo activation of midbrain dopamine neurons via sensitized, high-affinity alpha 6 nicotinic acetylcholine receptors. Neuron. 2008;60:123–136. doi: 10.1016/j.neuron.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duvoisin RC. Cholinergic-anticholinergic antagonism in parkinsonism. Arch Neurol. 1967;17:124–136. doi: 10.1001/archneur.1967.00470260014002. [DOI] [PubMed] [Google Scholar]
- 51.Exley R, Cragg SJ. Presynaptic nicotinic receptors: a dynamic and diverse cholinergic filter of striatal dopamine neurotransmission. Br J Pharmacol. 2008;153 Suppl 1:S283–S297. doi: 10.1038/sj.bjp.0707510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Felder CC. Muscarinic acetylcholine receptors: signal transduction through multiple effectors. Faseb J. 1995;9:619–625. [PubMed] [Google Scholar]
- 53.Flowers KA, Robertson C. The effect of Parkinson's disease on the ability to maintain a mental set. J Neurol Neurosurg Psychiatry. 1985;48:517–529. doi: 10.1136/jnnp.48.6.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frank MJ, Claus ED. Anatomy of a decision: striato-orbitofrontal interactions in reinforcement learning, decision making, and reversal. Psychol Rev. 2006;113:300–326. doi: 10.1037/0033-295X.113.2.300. [DOI] [PubMed] [Google Scholar]
- 55.Gabrieli JD, Brewer JB, Poldrack RA. Images of medial temporal lobe functions in human learning and memory. Neurobiol Learn Mem. 1998;70:275–283. doi: 10.1006/nlme.1998.3853. [DOI] [PubMed] [Google Scholar]
- 56.Gerber DJ, Sotnikova TD, Gainetdinov RR, Huang SY, Caron MG, Tonegawa S. Hyperactivity, elevated dopaminergic transmission, and response to amphetamine in M1 muscarinic acetylcholine receptor-deficient mice. Proc Natl Acad Sci U S A. 2001;98:15312–15317. doi: 10.1073/pnas.261583798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Giorguieff MF, Le Floc'h ML, Glowinski J, Besson MJ. Involvement of cholinergic presynaptic receptors of nicotinic and muscarinic types in the control of the spontaneous release of dopamine from striatal dopaminergic terminals in the rat. J Pharmacol Exp Ther. 1977;200:535–544. [PubMed] [Google Scholar]
- 58.Gold PE. Coordination of multiple memory systems. Neurobiol Learn Mem. 2004;82:230–242. doi: 10.1016/j.nlm.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 59.Grady SR, Salminen O, Laverty DC, Whiteaker P, McIntosh JM, Collins AC, Marks MJ. The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum. Biochem Pharmacol. 2007;74:1235–1246. doi: 10.1016/j.bcp.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grilli M, Zappettini S, Raiteri L, Marchi M. Nicotinic and muscarinic cholinergic receptors coexist on GABAergic nerve endings in the mouse striatum and interact in modulating GABA release. Neuropharmacology. 2009;56:610–614. doi: 10.1016/j.neuropharm.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 61.Groenewegen HJ, Wright CI, Beijer AV, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Ann N Y Acad Sci. 1999;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- 62.Guttman M. Receptors in the basal ganglia. Can J Neurol Sci. 1987;14:395–401. doi: 10.1017/s0317167100037793. [DOI] [PubMed] [Google Scholar]
- 63.Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hagewoud R, Havekes R, Novati A, Keijser JN, EA VDZ, Meerlo P. Sleep deprivation impairs spatial working memory and reduces hippocampal AMPA receptor phosphorylation. J Sleep Res. 2009 doi: 10.1111/j.1365-2869.2009.00799.x. [DOI] [PubMed] [Google Scholar]
- 65.Hannon R, Bader A. A comparison of frontal pole, anterior median and caudate nucleus lesions in the rat. Physiol Behav. 1974;13:513–521. doi: 10.1016/0031-9384(74)90282-0. [DOI] [PubMed] [Google Scholar]
- 66.Hartley T, Maguire EA, Spiers HJ, Burgess N. The well-worn route and the path less traveled: distinct neural bases of route following and wayfinding in humans. Neuron. 2003;37:877–888. doi: 10.1016/s0896-6273(03)00095-3. [DOI] [PubMed] [Google Scholar]
- 67.Havekes R, Nijholt IM, Luiten PG, Van der Zee EA. Differential involvement of hippocampal calcineurin during learning and reversal learning in a Y-maze task. Learn Mem. 2006;13:753–759. doi: 10.1101/lm.323606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Havekes R, Timmer M, Van der Zee EA. Regional differences in hippocampal PKA immunoreactivity after training and reversal training in a spatial Y-maze task. Hippocampus. 2007;17:338–348. doi: 10.1002/hipo.20272. [DOI] [PubMed] [Google Scholar]
- 69.Hernandez-Lopez S, Gongora-Alfaro JL, Martinez-Fong D, Aceves J. A cholinergic input to the substantia nigra pars compacta increases striatal dopamine metabolism measured by in vivo voltammetry. Brain Res. 1992;598:114–120. doi: 10.1016/0006-8993(92)90174-8. [DOI] [PubMed] [Google Scholar]
- 70.Hersch SM, Gutekunst CA, Rees HD, Heilman CJ, Levey AI. Distribution of m1–m4 muscarinic receptor proteins in the rat striatum: light and electron microscopic immunocytochemistry using subtype-specific antibodies. J Neurosci. 1994;14:3351–3363. doi: 10.1523/JNEUROSCI.14-05-03351.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hersch SM, Levey AI. Diverse pre- and post-synaptic expression of m1–m4 muscarinic receptor proteins in neurons and afferents in the rat neostriatum. Life Sci. 1995;56:931–938. doi: 10.1016/0024-3205(95)00030-a. [DOI] [PubMed] [Google Scholar]
- 72.Higashida H, Hoshi N, Zhang JS, Yokoyama S, Hashii M, Jin D, Noda M, Robbins J. Protein kinase C bound with A-kinase anchoring protein is involved in muscarinic receptor-activated modulation of M-type KCNQ potassium channels. Neurosci Res. 2005;51:231–234. doi: 10.1016/j.neures.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 73.Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev. 2000;80:953–978. doi: 10.1152/physrev.2000.80.3.953. [DOI] [PubMed] [Google Scholar]
- 74.Hill JA, Jr, Zoli M, Bourgeois JP, Changeux JP. Immunocytochemical localization of a neuronal nicotinic receptor: the beta 2-subunit. J Neurosci. 1993;13:1551–1568. doi: 10.1523/JNEUROSCI.13-04-01551.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hoshi N, Zhang JS, Omaki M, Takeuchi T, Yokoyama S, Wanaverbecq N, Langeberg LK, Yoneda Y, Scott JD, Brown DA, Higashida H. AKAP150 signaling complex promotes suppression of the M-current by muscarinic agonists. Nat Neurosci. 2003;6:564–571. doi: 10.1038/nn1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hrabovska A, Farar V, Bernard V, Duysen EG, Brabec J, Lockridge O, Myslivecek J. Drastic decrease in dopamine receptor levels in the striatum of acetylcholinesterase knock-out mouse. Chem Biol Interact. 183:194–201. doi: 10.1016/j.cbi.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 77.Johnson A, van der Meer MA, Redish AD. Integrating hippocampus and striatum in decision-making. Curr Opin Neurobiol. 2007;17:692–697. doi: 10.1016/j.conb.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jones IW, Bolam JP, Wonnacott S. Presynaptic localisation of the nicotinic acetylcholine receptor beta2 subunit immunoreactivity in rat nigrostriatal dopaminergic neurones. J Comp Neurol. 2001;439:235–247. doi: 10.1002/cne.1345. [DOI] [PubMed] [Google Scholar]
- 79.Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC. Striatal interneurones: chemical, physiological and morphological characterization. Trends Neurosci. 1995;18:527–535. doi: 10.1016/0166-2236(95)98374-8. [DOI] [PubMed] [Google Scholar]
- 80.Kemp JM, Powell TP. The termination of fibres from the cerebral cortex and thalamus upon dendritic spines in the caudate nucleus: a study with the Golgi method. Philos Trans R Soc Lond B Biol Sci. 1971;262:429–439. doi: 10.1098/rstb.1971.0105. [DOI] [PubMed] [Google Scholar]
- 81.Kirkby RJ, Polgar S. Active avoidance in the laboratory rat following lesions of the dorsal and ventral caudate nucleus. Physiological Psychology. 1974:301–306. [Google Scholar]
- 82.Kita H. Globus pallidus external segment. Prog Brain Res. 2007;160:111–133. doi: 10.1016/S0079-6123(06)60007-1. [DOI] [PubMed] [Google Scholar]
- 83.Kitabatake Y, Hikida T, Watanabe D, Pastan I, Nakanishi S. Impairment of reward-related learning by cholinergic cell ablation in the striatum. Proc Natl Acad Sci U S A. 2003;100:7965–7970. doi: 10.1073/pnas.1032899100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996;273:1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- 85.Kobayashi T, Iwasaki T. Functional dissociation of striatal and hippocampal cholinergic systems in egocentric and allocentric localization: effect of overtraining. Nihon Shinkei Seishin Yakurigaku Zasshi. 2000;20:113–121. [PubMed] [Google Scholar]
- 86.Kolb B. Studies on the caudate-putamen and the dorsomedial thalamic nucleus of the rat: implications for mammalian frontal-lobe functions. Physiol Behav. 1977;18:237–244. doi: 10.1016/0031-9384(77)90128-7. [DOI] [PubMed] [Google Scholar]
- 87.König J, KLippel RA. The Rat Brain - A stereotaxic Atlas of the Forebrain and Lower Parts of the Brain Stem. USA: The Williams & Wilkins Company, Waverly Press, Inc.; 1963. [Google Scholar]
- 88.Koos T, Tepper JM. Dual cholinergic control of fast-spiking interneurons in the neostriatum. J Neurosci. 2002;22:529–535. doi: 10.1523/JNEUROSCI.22-02-00529.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kreitzer AC, Malenka RC. Striatal plasticity and basal ganglia circuit function. Neuron. 2008;60:543–554. doi: 10.1016/j.neuron.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kudernatsch M, Sutor B. Cholinergic modulation of dopamine overflow in the rat neostriatum: a fast cyclic voltammetric study in vitro. Neurosci Lett. 1994;181:107–112. doi: 10.1016/0304-3940(94)90571-1. [DOI] [PubMed] [Google Scholar]
- 91.Kurosaki T, Fukuda K, Konno T, Mori Y, Tanaka K, Mishina M, Numa S. Functional properties of nicotinic acetylcholine receptor subunits expressed in various combinations. FEBS Lett. 1987;214:253–258. doi: 10.1016/0014-5793(87)80065-0. [DOI] [PubMed] [Google Scholar]
- 92.Laduron . In: Dopamine receptors. Kaiser C, Kebabian JW, editors. American Chemical society; 1983. pp. 46–52. [Google Scholar]
- 93.Laduron PM. Presynaptic heteroreceptors in regulation of neuronal transmission. Biochem Pharmacol. 1985;34:467–470. doi: 10.1016/0006-2952(85)90176-5. [DOI] [PubMed] [Google Scholar]
- 94.Lafleche G, Albert M. Executive function deficits in mild Alzheimer's disease. Neuropsychology. 1995;9:313–320. [Google Scholar]
- 95.Lapper SR, Bolam JP. Input from the frontal cortex and the parafascicular nucleus to cholinergic interneurons in the dorsal striatum of the rat. Neuroscience. 1992;51:533–545. doi: 10.1016/0306-4522(92)90293-b. [DOI] [PubMed] [Google Scholar]
- 96.Lapper SR, Smith Y, Sadikot AF, Parent A, Bolam JP. Cortical input to parvalbumin-immunoreactive neurones in the putamen of the squirrel monkey. Brain Res. 1992;580:215–224. doi: 10.1016/0006-8993(92)90947-8. [DOI] [PubMed] [Google Scholar]
- 97.Lazaris A, Cassel S, Stemmelin J, Cassel JC, Kelche C. Intrastriatal infusions of methoctramine improve memory in cognitively impaired aged rats. Neurobiol Aging. 2003;24:379–383. doi: 10.1016/s0197-4580(02)00067-2. [DOI] [PubMed] [Google Scholar]
- 98.Lee AS, Duman RS, Pittenger C. A double dissociation revealing bidirectional competition between striatum and hippocampus during learning. Proc Natl Acad Sci U S A. 2008;105:17163–17168. doi: 10.1073/pnas.0807749105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lehmann J, Langer SZ. Muscarinic receptors on dopamine terminals in the cat caudate nucleus: neuromodulation of [3H]dopamine release in vitro by endogenous acetylcholine. Brain Res. 1982;248:61–69. doi: 10.1016/0006-8993(82)91147-7. [DOI] [PubMed] [Google Scholar]
- 100.Lehmann J, Langer SZ. The striatal cholinergic interneuron: synaptic target of dopaminergic terminals? Neuroscience. 1983;10:1105–1120. doi: 10.1016/0306-4522(83)90102-1. [DOI] [PubMed] [Google Scholar]
- 101.Levey AI, Kitt CA, Simonds WF, Price DL, Brann MR. Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J Neurosci. 1991;11:3218–3226. doi: 10.1523/JNEUROSCI.11-10-03218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Limberger N, Spath L, Starke K. A search for receptors modulating the release of gamma-[3H]aminobutyric acid in rabbit caudate nucleus slices. J Neurochem. 1986;46:1109–1117. doi: 10.1111/j.1471-4159.1986.tb00625.x. [DOI] [PubMed] [Google Scholar]
- 103.Liste I, Bernard V, Bloch B. Acute and chronic acetylcholinesterase inhibition regulates in vivo the localization and abundance of muscarinic receptors m2 and m4 at the cell surface and in the cytoplasm of striatal neurons. Mol Cell Neurosci. 2002;20:244–256. doi: 10.1006/mcne.2001.1083. [DOI] [PubMed] [Google Scholar]
- 104.Livesey PJ, Muter V. Functional differentiation within the neostriatum of the rat using electrical (blocking) stimulation during discrimination learning. J Comp Physiol Psychol. 1976;90:203–211. doi: 10.1037/h0077200. [DOI] [PubMed] [Google Scholar]
- 105.Lu Y, Grady S, Marks MJ, Picciotto M, Changeux JP, Collins AC. Pharmacological characterization of nicotinic receptor-stimulated GABA release from mouse brain synaptosomes. J Pharmacol Exp Ther. 1998;287:648–657. [PubMed] [Google Scholar]
- 106.Marchi M, Sanguineti P, Raiteri M. Muscarinic receptors mediate direct inhibition of GABA release from rat striatal nerve terminals. Neurosci Lett. 1990;116:347–351. doi: 10.1016/0304-3940(90)90099-u. [DOI] [PubMed] [Google Scholar]
- 107.Marschner A, Mell T, Wartenburger I, Villringer A, Reischies FM, Heekeren HR. Reward-based decision-making and aging. Brain Res Bull. 2005;67:382–390. doi: 10.1016/j.brainresbull.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 108.McCool MF, Patel S, Talati R, Ragozzino ME. Differential involvement of M1-type and M4-type muscarinic cholinergic receptors in the dorsomedial striatum in task switching. Neurobiol Learn Mem. 2008;89:114–124. doi: 10.1016/j.nlm.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McIntyre CK, Marriott LK, Gold PE. Patterns of brain acetylcholine release predict individual differences in preferred learning strategies in rats. Neurobiol Learn Mem. 2003;79:177–183. doi: 10.1016/s1074-7427(02)00014-x. [DOI] [PubMed] [Google Scholar]
- 110.McMonagle-Strucko K, Fanelli RJ. Enhanced acquisition of reversal training in a spatial learning task in rats treated with chronic nimodipine. Pharmacol Biochem Behav. 1993;44:827–835. doi: 10.1016/0091-3057(93)90013-j. [DOI] [PubMed] [Google Scholar]
- 111.Means LW, Holsten RD. Individual aged rats are impaired on repeated reversal due to loss of different behavioral patterns. Physiol Behav. 1992;52:959–963. doi: 10.1016/0031-9384(92)90377-e. [DOI] [PubMed] [Google Scholar]
- 112.Mitcham JC, Thomas RK., Jr Effects of substantia nigra and caudate nucleus lesions on avoidance learning in rats. J Comp Physiol Psychol. 1972;81:101–107. doi: 10.1037/h0033323. [DOI] [PubMed] [Google Scholar]
- 113.Mizumori SJ, Yeshenko O, Gill KM, Davis DM. Parallel processing across neural systems: implications for a multiple memory system hypothesis. Neurobiol Learn Mem. 2004;82:278–298. doi: 10.1016/j.nlm.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 114.Nakano K. Neural circuits and topographic organization of the basal ganglia and related regions. Brain Dev. 2000;22 Suppl 1:S5–S16. doi: 10.1016/s0387-7604(00)00139-x. [DOI] [PubMed] [Google Scholar]
- 115.Nambu A. Globus pallidus internal segment. Prog Brain Res. 2007;160:135–150. doi: 10.1016/S0079-6123(06)60008-3. [DOI] [PubMed] [Google Scholar]
- 116.Neill DB, Grossman SP. Behavioral effects of lesions or cholinergic blockade of the dorsal and ventral caudate of rats. J Comp Physiol Psychol. 1970;71:311–317. doi: 10.1037/h0029110. [DOI] [PubMed] [Google Scholar]
- 117.Nicola SM. The nucleus accumbens as part of a basal ganglia action selection circuit. Psychopharmacology (Berl) 2007;191:521–550. doi: 10.1007/s00213-006-0510-4. [DOI] [PubMed] [Google Scholar]
- 118.Nijholt IM, Ostroveanu A, de Bruyn M, Luiten PG, Eisel UL, Van der Zee EA. Both exposure to a novel context and associative learning induce an upregulation of AKAP150 protein in mouse hippocampus. Neurobiol Learn Mem. 2007;87:693–696. doi: 10.1016/j.nlm.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 119.O'Keefe J, Conway DH. Hippocampal place units in the freely moving rat: why they fire where they fire. Exp Brain Res. 1978;31:573–590. doi: 10.1007/BF00239813. [DOI] [PubMed] [Google Scholar]
- 120.Orban P, Rauchs G, Balteau E, Degueldre C, Luxen A, Maquet P, Peigneux P. Sleep after spatial learning promotes covert reorganization of brain activity. Proc Natl Acad Sci U S A. 2006;103:7124–7129. doi: 10.1073/pnas.0510198103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ostroveanu A, Van der Zee EA, Dolga AM, Luiten PG, Eisel UL, Nijholt IM. A-kinase anchoring protein 150 in the mouse brain is concentrated in areas involved in learning and memory. Brain Res. 2007;1145:97–107. doi: 10.1016/j.brainres.2007.01.117. [DOI] [PubMed] [Google Scholar]
- 122.Packard MG. Anxiety, cognition, and habit: a multiple memory systems perspective. Brain Res. 2009;1293:121–128. doi: 10.1016/j.brainres.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 123.Packard MG. Glutamate infused posttraining into the hippocampus or caudate-putamen differentially strengthens place and response learning. Proc Natl Acad Sci U S A. 1999;96:12881–12886. doi: 10.1073/pnas.96.22.12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Packard MG, Hirsh R, White NM. Differential effects of fornix and caudate nucleus lesions on two radial maze tasks: evidence for multiple memory systems. J Neurosci. 1989;9:1465–1472. doi: 10.1523/JNEUROSCI.09-05-01465.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Packard MG, Knowlton BJ. Learning and memory functions of the Basal Ganglia. Annu Rev Neurosci. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- 126.Packard MG, McGaugh JL. Double dissociation of fornix and caudate nucleus lesions on acquisition of two water maze tasks: further evidence for multiple memory systems. Behav Neurosci. 1992;106:439–446. doi: 10.1037//0735-7044.106.3.439. [DOI] [PubMed] [Google Scholar]
- 127.Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem. 1996;65:65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- 128.Packard MG, Teather LA. Double dissociation of hippocampal and dorsal-striatal memory systems by posttraining intracerebral injections of 2-amino-5-phosphonopentanoic acid. Behav Neurosci. 1997;111:543–551. doi: 10.1037//0735-7044.111.3.543. [DOI] [PubMed] [Google Scholar]
- 129.Palencia CA, Ragozzino ME. The effect of N-methyl-D-aspartate receptor blockade on acetylcholine efflux in the dorsomedial striatum during response reversal learning. Neuroscience. 2006;143:671–678. doi: 10.1016/j.neuroscience.2006.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Palencia CA, Ragozzino ME. The influence of NMDA receptors in the dorsomedial striatum on response reversal learning. Neurobiol Learn Mem. 2004;82:81–89. doi: 10.1016/j.nlm.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 131.Perez-Burgos A, Perez-Rosello T, Salgado H, Flores-Barrera E, Prieto GA, Figueroa A, Galarraga E, Bargas J. Muscarinic M(1) modulation of N and L types of calcium channels is mediated by protein kinase C in neostriatal neurons. Neuroscience. 2008;155:1079–1097. doi: 10.1016/j.neuroscience.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 132.Picciotto MR, Zoli M, Lena C, Bessis A, Lallemand Y, Le Novere N, Vincent P, Pich EM, Brulet P, Changeux JP. Abnormal avoidance learning in mice lacking functional high-affinity nicotine receptor in the brain. Nature. 1995;374:65–67. doi: 10.1038/374065a0. [DOI] [PubMed] [Google Scholar]
- 133.Pisa M, Cyr J. Regionally selective roles of the rat's striatum in modality-specific discrimination learning and forelimb reaching. Behav Brain Res. 1990;37:281–292. doi: 10.1016/0166-4328(90)90140-a. [DOI] [PubMed] [Google Scholar]
- 134.Pisani A, Bonsi P, Centonze D, Gubellini P, Bernardi G, Calabresi P. Targeting striatal cholinergic interneurons in Parkinson's disease: focus on metabotropic glutamate receptors. Neuropharmacology. 2003;45:45–56. doi: 10.1016/s0028-3908(03)00137-0. [DOI] [PubMed] [Google Scholar]
- 135.Potter LT, Flynn DD, Liang JS, McCollum MH. Studies of muscarinic neurotransmission with antimuscarinic toxins. Prog Brain Res. 2004;145:121–128. doi: 10.1016/S0079-6123(03)45008-5. [DOI] [PubMed] [Google Scholar]
- 136.Prado-Alcala RA, Grinberg ZJ, Arditti ZL, Garcia MM, Prieto HG, Brust-Carmona H. Learning deficits produced by chronic and reversible lesions of the corpus striatum in rats. Physiol Behav. 1975;15:283–287. doi: 10.1016/0031-9384(75)90095-5. [DOI] [PubMed] [Google Scholar]
- 137.Quirion R, Araujo D, Regenold W, Boksa P. Characterization and quantitative autoradiographic distribution of [3H]acetylcholine muscarinic receptors in mammalian brain. Apparent labelling of an M2-like receptor sub-type. Neuroscience. 1989;29:271–289. doi: 10.1016/0306-4522(89)90057-2. [DOI] [PubMed] [Google Scholar]
- 138.Ragozzino ME. Acetylcholine actions in the dorsomedial striatum support the flexible shifting of response patterns. Neurobiol Learn Mem. 2003;80:257–267. doi: 10.1016/s1074-7427(03)00077-7. [DOI] [PubMed] [Google Scholar]
- 139.Ragozzino ME. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Ann N Y Acad Sci. 2007;1121:355–375. doi: 10.1196/annals.1401.013. [DOI] [PubMed] [Google Scholar]
- 140.Ragozzino ME, Choi D. Dynamic changes in acetylcholine output in the medial striatum during place reversal learning. Learn Mem. 2004;11:70–77. doi: 10.1101/lm.65404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ragozzino ME, Jih J, Tzavos A. Involvement of the dorsomedial striatum in behavioral flexibility: role of muscarinic cholinergic receptors. Brain Res. 2002;953:205–214. doi: 10.1016/s0006-8993(02)03287-0. [DOI] [PubMed] [Google Scholar]
- 142.Ragozzino ME, Ragozzino KE, Mizumori SJ, Kesner RP. Role of the dorsomedial striatum in behavioral flexibility for response and visual cue discrimination learning. Behav Neurosci. 2002;116:105–115. doi: 10.1037//0735-7044.116.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rahner-Welsch S, Frolich L, Stoll S, Hoyer S. Decline and preservation of reversal learning abilities and acquisition in the course of senescence. Neurosci Lett. 1995;194:121–123. doi: 10.1016/0304-3940(95)11712-6. [DOI] [PubMed] [Google Scholar]
- 144.Raiteri M, Marchi M, Maura G. Presynaptic muscarinic receptors increase striatal dopamine release evoked by "quashi-physiological" depolarization. Eur J Pharmacol. 1982;83:127–129. doi: 10.1016/0014-2999(82)90296-5. [DOI] [PubMed] [Google Scholar]
- 145.Reisel D, Bannerman DM, Schmitt WB, Deacon RM, Flint J, Borchardt T, Seeburg PH, Rawlins JN. Spatial memory dissociations in mice lacking GluR1. Nat Neurosci. 2002;5:868–873. doi: 10.1038/nn910. [DOI] [PubMed] [Google Scholar]
- 146.Reynolds JN, Hyland BI, Wickens JR. Modulation of an afterhyperpolarization by the substantia nigra induces pauses in the tonic firing of striatal cholinergic interneurons. J Neurosci. 2004;24:9870–9877. doi: 10.1523/JNEUROSCI.3225-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Reynolds JN, Wickens JR. The corticostriatal input to giant aspiny interneurons in the rat: a candidate pathway for synchronising the response to reward-related cues. Brain Res. 2004;1011:115–128. doi: 10.1016/j.brainres.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 148.Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci. 2004;7:583–584. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- 149.Rouse ST, Thomas TM, Levey AI. Muscarinic acetylcholine receptor subtype, m2: diverse functional implications of differential synaptic localization. Life Sci. 1997;60:1031–1038. doi: 10.1016/s0024-3205(97)00044-1. [DOI] [PubMed] [Google Scholar]
- 150.Rymar VV, Sasseville R, Luk KC, Sadikot AF. Neurogenesis and stereological morphometry of calretinin-immunoreactive GABAergic interneurons of the neostriatum. J Comp Neurol. 2004;469:325–339. doi: 10.1002/cne.11008. [DOI] [PubMed] [Google Scholar]
- 151.Salinas JA, White NM. Contributions of the hippocampus, amygdala, and dorsal striatum to the response elicited by reward reduction. Behav Neurosci. 1998;112:812–826. doi: 10.1037//0735-7044.112.4.812. [DOI] [PubMed] [Google Scholar]
- 152.Satoh K, Staines WA, Atmadja S, Fibiger HC. Ultrastructural observations of the cholinergic neuron in the rat striatum as identified by acetylcholinesterase pharmacohistochemistry. Neuroscience. 1983;10:1121–1136. doi: 10.1016/0306-4522(83)90103-3. [DOI] [PubMed] [Google Scholar]
- 153.Scali C, Vannucchi MG, Pepeu G, Casamenti F. Peripherally injected scopolamine differentially modulates acetylcholine release in vivo in the young and aged rats. Neurosci Lett. 1995;197:171–174. doi: 10.1016/0304-3940(95)11914-i. [DOI] [PubMed] [Google Scholar]
- 154.Schroder H, Giacobini E, Struble RG, Luiten PG, van der Zee EA, Zilles K, Strosberg AD. Muscarinic cholinoceptive neurons in the frontal cortex in Alzheimer's disease. Brain Res Bull. 1991;27:631–636. doi: 10.1016/0361-9230(91)90038-l. [DOI] [PubMed] [Google Scholar]
- 155.Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cereb Cortex. 2000;10:272–284. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- 156.Schwartzbaum JS, Donovick PJ. Discrimination reversal and spatial alternation associated with septal and caudate dysfunction in rats. J Comp Physiol Psychol. 1968;65:83–92. doi: 10.1037/h0025394. [DOI] [PubMed] [Google Scholar]
- 157.Scott JD. Compartmentalized cAMP signalling: a personal perspective. Biochem Soc Trans. 2006;34:465–467. doi: 10.1042/BST0340465. [DOI] [PubMed] [Google Scholar]
- 158.Sherman KA, Friedman E. Pre- and post-synaptic cholinergic dysfunction in aged rodent brain regions: new findings and an interpretative review. Int J Dev Neurosci. 1990;8:689–708. doi: 10.1016/0736-5748(90)90063-8. [DOI] [PubMed] [Google Scholar]
- 159.Smiley JF, Levey AI, Mesulam MM. Infracortical interstitial cells concurrently expressing m2-muscarinic receptors, acetylcholinesterase and nicotinamide adenine dinucleotide phosphate-diaphorase in the human and monkey cerebral cortex. Neuroscience. 1998;84:755–769. doi: 10.1016/s0306-4522(97)00524-1. [DOI] [PubMed] [Google Scholar]
- 160.Smith AD, Bolam JP. The neural network of the basal ganglia as revealed by the study of synaptic connections of identified neurones. Trends Neurosci. 1990;13:259–265. doi: 10.1016/0166-2236(90)90106-k. [DOI] [PubMed] [Google Scholar]
- 161.Smith Y, Bevan MD, Shink E, Bolam JP. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience. 1998;86:353–387. doi: 10.1016/s0306-4522(98)00004-9. [DOI] [PubMed] [Google Scholar]
- 162.Staines WA, Nagy JI, Vincent SR, Fibiger HC. Neurotransmitters contained in the efferents of the striatum. Brain Res. 1980;194:391–402. doi: 10.1016/0006-8993(80)91220-2. [DOI] [PubMed] [Google Scholar]
- 163.Sugita S, Uchimura N, Jiang ZG, North RA. Distinct muscarinic receptors inhibit release of gamma-aminobutyric acid and excitatory amino acids in mammalian brain. Proc Natl Acad Sci U S A. 1991;88:2608–2611. doi: 10.1073/pnas.88.6.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sullivan MA, Chen H, Morikawa H. Recurrent inhibitory network among striatal cholinergic interneurons. J Neurosci. 2008;28:8682–8690. doi: 10.1523/JNEUROSCI.2411-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Surmeier DJ, Kitai ST. D1 and D2 dopamine receptor modulation of sodium and potassium currents in rat neostriatal neurons. Prog Brain Res. 1993;99:309–324. doi: 10.1016/s0079-6123(08)61354-0. [DOI] [PubMed] [Google Scholar]
- 166.Suzuki M, Desmond TJ, Albin RL, Frey KA. Vesicular neurotransmitter transporters in Huntington's disease: initial observations and comparison with traditional synaptic markers. Synapse. 2001;41:329–336. doi: 10.1002/syn.1089. [DOI] [PubMed] [Google Scholar]
- 167.Swanson LW, Lindstrom J, Tzartos S, Schmued LC, O'Leary DD, Cowan WM. Immunohistochemical localization of monoclonal antibodies to the nicotinic acetylcholine receptor in chick midbrain. Proc Natl Acad Sci U S A. 1983;80:4532–4536. doi: 10.1073/pnas.80.14.4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tanaka C, Nishizuka Y. The protein kinase C family for neuronal signaling. Annu Rev Neurosci. 1994;17:551–567. doi: 10.1146/annurev.ne.17.030194.003003. [DOI] [PubMed] [Google Scholar]
- 169.Teather LA, Packard MG, Smith DE, Ellis-Behnke RG, Bazan NG. Differential induction of c-Jun and Fos-like proteins in rat hippocampus and dorsal striatum after training in two water maze tasks. Neurobiol Learn Mem. 2005;84:75–84. doi: 10.1016/j.nlm.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 170.Tepper JM, Bolam JP. Functional diversity and specificity of neostriatal interneurons. Curr Opin Neurobiol. 2004;14:685–692. doi: 10.1016/j.conb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 171.Thomas TM, Smith Y, Levey AI, Hersch SM. Cortical inputs to m2-immunoreactive striatal interneurons in rat and monkey. Synapse. 2000;37:252–261. doi: 10.1002/1098-2396(20000915)37:4<252::AID-SYN2>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 172.Tolman EC RB, Kalish D. Studies in spatial learning. II. Place learning versus response learning. Journal of Experimental Psychology. 1946:221–229. doi: 10.1037/h0060262. [DOI] [PubMed] [Google Scholar]
- 173.Tolman EC RB, Kalish D. Studies in spatial learning. V. Response learning versus place learning by the noncorrection method. Journal of Experimental Psychology. 1947:285–292. doi: 10.1037/h0057434. [DOI] [PubMed] [Google Scholar]
- 174.Tunquist BJ, Hoshi N, Guire ES, Zhang F, Mullendorff K, Langeberg LK, Raber J, Scott JD. Loss of AKAP150 perturbs distinct neuronal processes in mice. Proc Natl Acad Sci U S A. 2008;105:12557–12562. doi: 10.1073/pnas.0805922105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tzavos A, Jih J, Ragozzino ME. Differential effects of M1 muscarinic receptor blockade and nicotinic receptor blockade in the dorsomedial striatum on response reversal learning. Behav Brain Res. 2004;154:245–253. doi: 10.1016/j.bbr.2004.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Van der Zee EA, Buwalda B, Strubbe JH, Strosberg AD, Luiten PG. Immunocytochemical localization of muscarinic acetylcholine receptors in the rat endocrine pancreas. Cell Tissue Res. 1992;269:99–106. doi: 10.1007/BF00384730. [DOI] [PubMed] [Google Scholar]
- 177.Van der Zee EA, Compaan JC, Bohus B, Luiten PG. Alterations in the immunoreactivity for muscarinic acetylcholine receptors and colocalized PKC gamma in mouse hippocampus induced by spatial discrimination learning. Hippocampus. 1995;5:349–362. doi: 10.1002/hipo.450050408. [DOI] [PubMed] [Google Scholar]
- 178.van der Zee EA, de Jong GI, Strosberg AD, Luiten PG. Parvalbumin-positive neurons in rat dorsal hippocampus contain muscarinic acetylcholine receptors. Brain Res Bull. 1991;27:697–700. doi: 10.1016/0361-9230(91)90048-o. [DOI] [PubMed] [Google Scholar]
- 179.Van der Zee EA, Douma BR, Bohus B, Luiten PG. Passive avoidance training induces enhanced levels of immunoreactivity for muscarinic acetylcholine receptor and coexpressed PKC gamma and MAP-2 in rat cortical neurons. Cereb Cortex. 1994;4:376–390. doi: 10.1093/cercor/4.4.376. [DOI] [PubMed] [Google Scholar]
- 180.Van der Zee EA, Kronforst-Collins MA, Maizels ET, Hunzicker-Dunn M, Disterhoft JF. gamma Isoform-selective changes in PKC immunoreactivity after trace eyeblink conditioning in the rabbit hippocampus. Hippocampus. 1997;7:271–285. doi: 10.1002/(SICI)1098-1063(1997)7:3<271::AID-HIPO3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 181.Van der Zee EA, Luiten PG. GABAergic neurons of the rat dorsal hippocampus express muscarinic acetylcholine receptors. Brain Res Bull. 1993;32:601–609. doi: 10.1016/0361-9230(93)90161-4. [DOI] [PubMed] [Google Scholar]
- 182.van der Zee EA, Luiten PG. Muscarinic acetylcholine receptors in the hippocampus, neocortex and amygdala: a review of immunocytochemical localization in relation to learning and memory. Prog Neurobiol. 1999;58:409–471. doi: 10.1016/s0301-0082(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 183.Van der Zee EA, Luiten PG, Disterhoft JF. Learning-induced alterations in hippocampal PKC-immunoreactivity: a review and hypothesis of its functional significance. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:531–572. doi: 10.1016/s0278-5846(97)00017-1. [DOI] [PubMed] [Google Scholar]
- 184.van der Zee EA, Matsuyama T, Strosberg AD, Traber J, Luiten PG. Demonstration of muscarinic acetylcholine receptor-like immunoreactivity in the rat forebrain and upper brainstem. Histochemistry. 1989;92:475–485. doi: 10.1007/BF00524759. [DOI] [PubMed] [Google Scholar]
- 185.van der Zee EA, Streefland C, Strosberg AD, Schroder H, Luiten PG. Colocalization of muscarinic and nicotinic receptors in cholinoceptive neurons of the suprachiasmatic region in young and aged rats. Brain Res. 1991;542:348–352. doi: 10.1016/0006-8993(91)91590-w. [DOI] [PubMed] [Google Scholar]
- 186.van Vulpen EH, van der Kooy D. Striatal cholinergic interneurons: birthdates predict compartmental localization. Brain Res Dev Brain Res. 1998;109:51–58. doi: 10.1016/s0165-3806(98)00012-1. [DOI] [PubMed] [Google Scholar]
- 187.Vetreno RP, Anzalone SJ, Savage LM. Impaired, spared, and enhanced ACh efflux across the hippocampus and striatum in diencephalic amnesia is dependent on task demands. Neurobiol Learn Mem. 2008;90:237–244. doi: 10.1016/j.nlm.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Vilaro MT, Wiederhold KH, Palacios JM, Mengod G. Muscarinic cholinergic receptors in the rat caudate-putamen and olfactory tubercle belong predominantly to the m4 class: in situ hybridization and receptor autoradiography evidence. Neuroscience. 1991;40:159–167. doi: 10.1016/0306-4522(91)90181-m. [DOI] [PubMed] [Google Scholar]
- 189.Volpicelli-Daley LA, Hrabovska A, Duysen EG, Ferguson SM, Blakely RD, Lockridge O, Levey AI. Altered striatal function and muscarinic cholinergic receptors in acetylcholinesterase knockout mice. Mol Pharmacol. 2003;64:1309–1316. doi: 10.1124/mol.64.6.1309. [DOI] [PubMed] [Google Scholar]
- 190.Waelbroeck M, Gillard M, Robberecht P, Christophe J. Kinetic studies of [3H]-N-methylscopolamine binding to muscarinic receptors in the rat central nervous system: evidence for the existence of three classes of binding sites. Mol Pharmacol. 1986;30:305–314. [PubMed] [Google Scholar]
- 191.Weiner I. Neural substrates of latent inhibition: the switching model. Psychol Bull. 1990;108:442–461. doi: 10.1037/0033-2909.108.3.442. [DOI] [PubMed] [Google Scholar]
- 192.Wheeler RA, Carelli RM. Dissecting motivational circuitry to understand substance abuse. Neuropharmacology. 2009;56 Suppl 1:149–159. doi: 10.1016/j.neuropharm.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Whishaw IQ, Kolb B. Decortication abolishes place but not cue learning in rats. Behav Brain Res. 1984;11:123–134. doi: 10.1016/0166-4328(84)90135-9. [DOI] [PubMed] [Google Scholar]
- 194.Whishaw IQ, O'Connor WT, Dunnett SB. Disruption of central cholinergic systems in the rat by basal forebrain lesions or atropine: effects on feeding, sensorimotor behaviour, locomotor activity and spatial navigation. Behav Brain Res. 1985;17:103–115. doi: 10.1016/0166-4328(85)90023-3. [DOI] [PubMed] [Google Scholar]
- 195.Williams GV, Rolls ET, Leonard CM, Stern C. Neuronal responses in the ventral striatum of the behaving macaque. Behav Brain Res. 1993;55:243–252. doi: 10.1016/0166-4328(93)90120-f. [DOI] [PubMed] [Google Scholar]
- 196.Wilson CJ, Chang HT, Kitai ST. Firing patterns and synaptic potentials of identified giant aspiny interneurons in the rat neostriatum. J Neurosci. 1990;10:508–519. doi: 10.1523/JNEUROSCI.10-02-00508.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Winocur G. Functional dissociation within the caudate nucleus of rats. J Comp Physiol Psychol. 1974;86:432–439. doi: 10.1037/h0036152. [DOI] [PubMed] [Google Scholar]
- 198.Woolf NJ, Butcher LL. Cholinergic systems in the rat brain: III. Projections from the pontomesencephalic tegmentum to the thalamus, tectum, basal ganglia, and basal forebrain. Brain Res Bull. 1986;16:603–637. doi: 10.1016/0361-9230(86)90134-6. [DOI] [PubMed] [Google Scholar]
- 199.Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- 200.Yoshihara C, Saito N, Taniyama K, Tanaka C. Differential localization of four subspecies of protein kinase C in the rat striatum and substantia nigra. J Neurosci. 1991;11:690–700. doi: 10.1523/JNEUROSCI.11-03-00690.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhang H, Sulzer D. Frequency-dependent modulation of dopamine release by nicotine. Nat Neurosci. 2004;7:581–582. doi: 10.1038/nn1243. [DOI] [PubMed] [Google Scholar]
- 202.Zhou FM, Liang Y, Dani JA. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat Neurosci. 2001;4:1224–1229. doi: 10.1038/nn769. [DOI] [PubMed] [Google Scholar]
- 203.Zhou FM, Wilson C, Dani JA. Muscarinic and nicotinic cholinergic mechanisms in the mesostriatal dopamine systems. Neuroscientist. 2003;9:23–36. doi: 10.1177/1073858402239588. [DOI] [PubMed] [Google Scholar]
- 204.Zhou FM, Wilson CJ, Dani JA. Cholinergic interneuron characteristics and nicotinic properties in the striatum. J Neurobiol. 2002;53:590–605. doi: 10.1002/neu.10150. [DOI] [PubMed] [Google Scholar]
- 205.Zola-Morgan S, Squire LR. Preserved learning in monkeys with medial temporal lesions: sparing of motor and cognitive skills. J Neurosci. 1984;4:1072–1085. doi: 10.1523/JNEUROSCI.04-04-01072.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zola-Morgan S, Squire LR, Mishkin M. The neuroanatomy of amnesia: amygdala-hippocampus versus temporal stem. Science. 1982;218:1337–1339. doi: 10.1126/science.6890713. [DOI] [PubMed] [Google Scholar]