Abstract
Adult male quail show high levels of aromatase activity in the preoptic area-hypothalamus (POA-HYP), which parallels the high number of aromatase-immunoreactive cells and elevated mRNA concentrations detected in this brain region by in situ hybridization. Interestingly, females display considerably lower aromatase activity than males but have nearly equal numbers of aromatase-immunoreactive cells and express similar levels of aromatase mRNA. Aromatase activity in the male POA-HYP can be rapidly regulated by calcium-dependent phosphorylations, in the absence of changes in enzyme concentration. We investigated here whether aromatase activity is differentially regulated by phosphorylations in males and females. A linear increase in accumulation of aromatization products was observed in both sexes as a function of time but the rate of conversion was slower in females. Saturation analysis confirmed the lower maximum velocities (Vmax) in females but indicated a similar affinity (Km) in both sexes. Aromatase activity in females reacted differentially to manipulations of intracellular calcium. In particular, chelating calcium with EGTA resulted in a larger increase of enzymatic activity in males than in females, especially in the presence of ATP. A differential reaction to kinase inhibitors was also observed between males and females (larger increase in aromatase activity in females than in males after exposure to specific inhibitors). These findings suggest that the nature of aromatase is conserved between the sexes but the control of its activity by calcium appears to be different. Additional characterizations of intracellular calcium in both sexes would therefore be in order to better understand aromatase regulation.
Keywords: aromatase activity, sex differences, protein kinases, preopic area, hypothalamus
INTRODUCTION
In many avian and mammalian species the preoptic aromatase activity (transformation of testosterone in oestradiol) plays a key-limiting role in the activation by testosterone of male sexual behaviour (1–3). This effect has been particularly well documented in Japanese quail (Coturnix japonica), a species in which inhibition of brain aromatase blocks almost completely the activating effects of testosterone on male behaviour expression (4). Interestingly, in quail, testosterone treatment activates in castrated males the expression of the male-typical copulatory sequence but the steroid is completely unable to activate these behaviours in females (4–6).
Given the key role of testosterone aromatization in behavioural activation in males, it was suggested that the absence of behavioural reaction to testosterone in females could be due to their relative inability to aromatize testosterone into oestradiol, the active metabolite. Some support for this idea was obtained by the demonstration that aromatase activity is higher in sexually mature males than in females (7, 8). However, this difference cannot be sufficient alone to explain the different behavioural responses to testosterone in males and females. This conclusion is supported by two arguments. On the one hand, it was shown that in gonadectomised birds treated with a same dose of testosterone, brain aromatase activity is sometimes higher in males than in females (8) but this difference is not always reproducible (sometimes no significant sex difference in enzymatic activity is found) whereas the sex difference in behavioural response to testosterone is extremely robust and suffers no exception (9). On another hand, injections of ovariectomised females with an oestrogen, a treatment that should bypass the putative enzymatic limiting step related to aromatase, is not sufficient to activate male-typical copulatory behaviour while the same treatment is effective in males (10). Other mechanisms are thus required to explain the behavioural sex dimorphism in quail and multiple experimental studies have been and still are dedicated to their identification (11).
Independently of its behavioural significance, the sex difference in brain aromatase activity has also been the subject of intense research. Multiple studies showed that, in sexually mature gonadally intact birds, the enzymatic activity is significantly higher (two to three fold) in males than in females (7, 8). However, when antibodies that allow a visualization of the enzymatic protein by immunohistochemistry became available (12), they revealed, somewhat surprisingly, that the numbers of aromatase-immunoreactive neurons are only slightly larger in the preoptic area of males compared to females. A significant difference was reported in a few studies but its magnitude was far smaller (10 to 30 %) than the difference in enzymatic activities (13, 14). Furthermore we reported more recently that the sex difference in aromatase activity observed in the preoptic area-hypothalamus of sexually mature gonadally intact males and females is not associated with any major difference in the density of the aromatase mRNA in the corresponding brain areas as observed by quantitative in situ hybridization histochemistry (15). A reverse sex difference in mRNA density (females > males) was even detected in the medial part of the bed nucleus of the stria terminalis (BSTM) (15) where males have a higher aromatase activity than females (16). This suggested that the sex difference in enzymatic activity does not result primarily from a differential transcription of the corresponding mRNA in males and females and thus presumably results from sexually differentiated post-transcriptional or even post-translational events.
Analyses of the biochemical mechanisms controlling brain aromatase activity in male quail revealed that the enzyme activity in brain homogenates can be inhibited within minutes by phosphorylating conditions such as the addition of high but physiological concentrations of adenosine triphosphate (ATP), magnesium (Mg2+) and calcium (Ca2+) (17, 18). In the presence of Mg2+ and Ca2+ acting as co-factors, the terminal phosphate group of ATP can be transferred to the serine, tyrosine, or threonine residues of proteins such as aromatase and in this way modulate their functional (enzymatic) properties (see (19, 20)). Pharmacological studies confirmed that the enzymatic inhibition results from protein phosphorylation since the inhibitory effects of ATP, Mg2+ and Ca2+ are blocked by the addition of kinase inhibitors (18). Similarly, activation of glutamatergic receptors of the AMPA and kainate sub-type rapidly (within 5 min) and reversibly inhibited aromatase activity in preoptic explants maintained in vitro (21). This enzymatic activity can thus be modulated rapidly by mechanisms such as neurotransmitter activity or calcium-dependent phosphorylations in a time frame that is obviously not compatible with changes in enzyme concentrations that would result from a de novo synthesis of the enzyme. Accordingly, no change in concentration of the enzymatic protein has been detected following these manipulations that rapidly affect enzyme activity (Charlier T.D. and Balthazart J., unpublished data).
In the present studies, we tested whether these mechanisms of rapid control of aromatase activity could be implicated in the control of the sex differences in enzyme activity. It is indeed conceivable that a chronic differential exposure to calcium or a differential degree of phosphorylation of the enzymatic protein could be at the basis of the sex difference in aromatase activity even if the enzyme concentration is similar in both sexes as suggested by the absence of sex difference in mRNA expression. Detailed kinetic studies of the aromatase enzyme in male and female brains were therefore carried out and the effects of phosphorylating conditions and of kinase inhibitors were compared in both sexes. Differential effects of these treatments were observed in males and females, suggesting indeed that these mechanisms could contribute to the enzymatic sex difference that has been previously described and was repeatedly confirmed in all these experiments.
MATERIAL AND METHODS
Subjects
Experiments were carried out on adult (>8 week old) sexually mature male and female Japanese quail (Coturnix japonica) that were obtained from a local breeder in Belgium and housed as previously described with food and water constantly available at libitum (22, 23). Housing, manipulation and sacrifice of animals was done according to the Principles of Laboratory Animal Care (NIH of the USA), and the relevant Belgian laws on the protection of animals. All experimental procedures were approved by the Ethics Committee for the Use of Animals at the University of Liège. Birds were killed by rapid decapitation in order to avoid potential effects of anaesthetics on aromatase activity.
Tissue Collection
Immediately after sacrifice, brains were carefully removed from the skull and the preoptic area-hypothalamic (POA-HYP) block dissected out, weighed and stored at −80°C until assayed. The POA-HYP was dissected out by two coronal cuts at the level of the tractus septopallio-mesencephalicus (rostral edge of the POA) and the oculomotor nerves (caudal edge of the hypothalamus), two parasagittal cuts placed approximately 2 mm lateral to the brain midline and one horizontal cut about 2 mm above the floor of the brain.
Aromatase Activity Assays
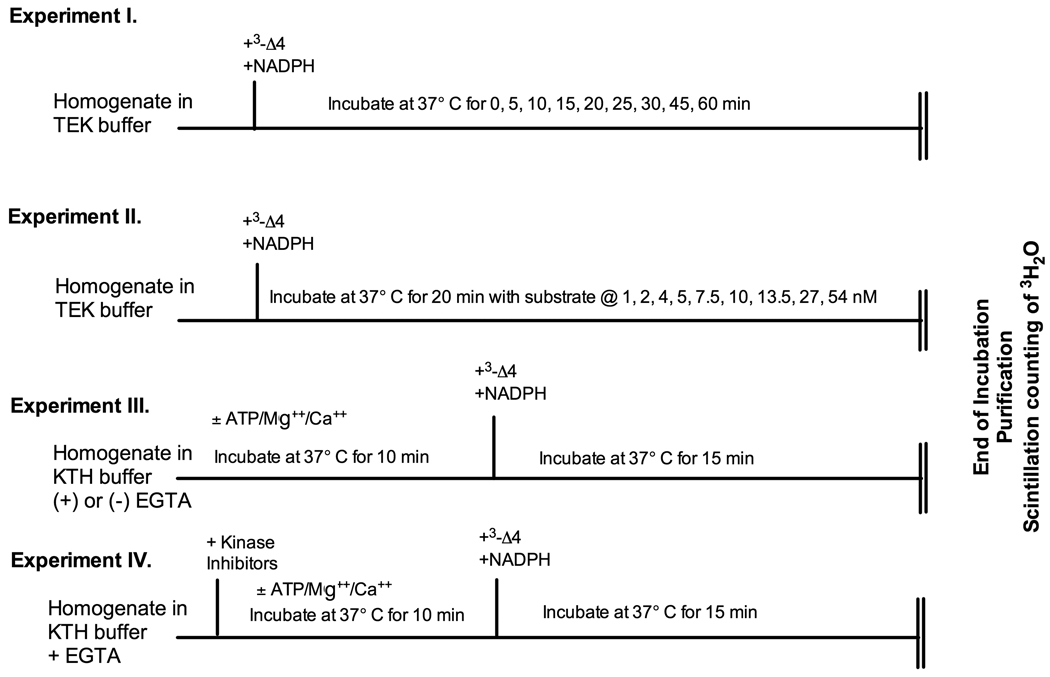
Aromatase activity was quantified by measuring the release of tritiated water during aromatization of [1β-3H]androstenedione, as described by Roselli and Resko (24), with minor modifications as previously described (18) and validated for use in the quail brain (25). Four independent experiments were carried out using specific protocols that are schematically presented in figure 1.
Figure 1.
Schematic presentation of the experimental protocols for experiments 1 to 4. The sequential treatments applied to the tissue homogenates are indicated above the vertical lines and the incubation duration and temperature on the horizontal line. Δ4= androstenedione
Experiment 1: Time-course of in vitro accumulation of aromatase products
Frozen POA-HYP blocks were homogenized at a concentration of approximately 20 mg/ml in ice cold buffer containing 10 mM Tris-Hepes pH 7.2, 1 mM EDTA and 150 mM KCl (TEK). Homogenates from two POA-HYP blocks were pooled (separately for males and females) in order to obtain a sufficient sample volume for each time point and kept in an ice bath during all procedures until the incubation at 37°C. Assays were performed in triplicate in one sample of pooled POA-HYP blocks for each sex. Aliquots (50 µl) of homogenate were added to 50 µl of TEK buffer and 50 µl of 100 nM [1β-3H]androstenedione. The final concentration of radioactive substrate during the assays was 25 nM (final incubation volume=200 µl), which represent a saturating concentration based on previous experiments with tissue from male quail (25).
To initiate the assay, 50 µl of 4.8 nM NADPH in TEK was added to reach a final concentration of 1.2 mM. All tubes were quickly capped and incubated at 37 °C in a water bath for 0, 5, 10, 15, 20, 25, 30, 45, or 60 min. To stop the reaction, tubes were quickly placed on ice and 400 µl of ice-cold 10% trichloroacetic acid containing 2% charcoal was added to each tube. The tritiated water produced was purified by centrifugation followed by Dowex cation exchange column separation and quantified as previously described (25). Enzyme activity was expressed as fmol of products (tritiated water or oestrone) produced in the entire POA-HYP block. This was shown repeatedly to provide more reliable and reproducible results than a calculation of fmol produced per mg protein or per mg fresh weight. This is easy to understand if one considers that, as demonstrated by our previous anatomical studies (12, 14, 15), the micro-dissection of the POA-HYP always contains the entire population of aromatase-expressing cells in the preoptic area and hypothalamus but includes slightly variable amounts of negative tissue. If results were expressed per mg fresh weight or per mg protein they would be affected in a variable manner by this inconsistent amount of extraneous tissue and this would generate additional unexplained variance in the data (See (18)).
Experiment 2: Characterization of the affinity and maximum velocity of aromatase activity
This experiment was designed to determine comparatively in males and females the aromatase enzyme kinetics. The experimental protocol was the same as above except that: 1) based on the results of Experiment 1, a 20 min duration was selected for the incubation at 37°C in order to determine enzyme characteristics during the linear phase of product accumulation and 2) homogenate samples were incubated with increasing concentrations of substrate to reach a final concentration during incubations of either 1, 2, 4, 5, 7.5, 10, 13.5, 27, or 54 nM. Assays were performed in triplicate in 4 different samples of pooled POA-HYP blocks for each sex; each sample consisted of 2 POA-HYP blocks for each sex. Results were expressed in fmol/h for the entire POA-HYP blocks.
Experiment 3: Aromatase inhibition by phosphorylating conditions
The frozen POA-HYP blocks were first homogenized in ice cold buffer containing 150 mM KCl and 10 mM Tris-Hepes pH 7.2 (KTH; i.e., TEK without EDTA) at a tissue concentration twice higher than in previous experiments. Aliquots of the homogenate were then further diluted (1:1) with KTH buffer (0 EGTA) or with 1 mM EGTA (ethylene glycol tetraacetic acid) in KTH yielding a final EGTA concentration of 0.5 mM. This concentration was selected based on preliminary experiments using a range of concentrations (final concentrations of 0, 1, 2, and 4 nM) demonstrating that 0.5 mM represents a minimal concentration maximally increasing aromatase activity in otherwise untreated brain homogenates of both sexes. Aliquots (50 µl) of these samples were then pre-incubated for 10 min at 37°C in a water bath with 50 µl KTH buffer in the presence or absence of 1 mM ATP + 1 mM Mg2+ + 1 mM Ca2+ (ATP/Mg2+/Ca2+) (Initial concentrations that were halved during the pre-incubations due to addition of an equal volume of homogenate).
Following this pre-incubation, tubes were placed on ice; substrate (50µl tritiated androstenedione to reach a final concentration of 25 nM) and NADPH (50 µl, final concentration 1.2 mM) were quickly added to the homogenates. Samples were then returned to the water bath for an additional incubation at 37°C for 15 min (duration selected to ascertain as much as possible the linearity of product accumulation as a function of time). The assay then proceeded as described in Experiment 2. The assay was performed in triplicate in 7 (males) or 8 (females) different samples for each sex during two independent assays.
Experiment 4: Effects of kinase inhibitors
Frozen POA-HYP blocks were homogenized in ice cold buffer containing 150 mM KCl and 10 mM Tris-Hepes pH 7.2 (KTH) + 0.5 mM EGTA. Aliquots (50 µl) of these homogenates were then added to 50 µl KTH buffer or to 50 µl KTH buffer containing 1 mM ATP + 1 mM Mg2+ + 1 mM Ca2+ (ATP/Mg2+/Ca2+; initial concentrations that were halved during the pre-incubations due to addition of an equal volume of homogenate) with or without various kinase inhibitors. The inhibitors tested in this experiment included: Staurosporine (10 µM, final concentration, a general serine/threonine kinase [PKC] inhibitor), Genistein (50 µM, final concentration, a general tyrosine kinase inhibitor), H89 (10 µM, final concentration, a protein kinase A inhibitor) and Bisindolylmaleimide (10 µM, final concentration, a protein kinase C inhibitor). All inhibitors were purchased from Calbiochem (Distributed by Merck, Nottingham UK). These inhibitors were used previously in our studies of brain aromatase in male quail and provided results in agreement with their pharmacological properties in mammals (see (18) for discussion). Their specificity of action in avian tissue has, however, not been confirmed independently.
Samples were pre-incubated for 10 min at 37°C in a water bath. Radioactive substrate and NADPH were then added and the remainder of the assay was carried out as previously described for experiment 3. The assay was performed in triplicate in four different samples for males and females; each sample was made by pooling POA-HYP blocks of two same sex animals.
Data Analysis
Data were analyzed by linear and non-linear regressions, by t-tests (Vmax and Km), or by multi-factorial analyses of variance (ANOVA) adapted to the experimental designs for analysis of the effects of phosphorylating conditions and kinase inhibitors. The origin of significant interactions was researched by appropriate post-hoc tests based on the ANOVA of smaller sets of samples. When appropriate, non-parametric Mann Whitney U tests were also used to compare two sets of data.
All assays were performed in triplicate (assay reproducibility) on multiple (pools of) brains (biological reproducibility, except for experiment 1). The results of triplicates were always averaged and their mean entered in statistical analyses. The measures of variance presented here only reflect the biological reproducibility (differences between independent samples). The number of data points used in statistical analyses is thus always the numbers of independent brain (or pools of brains) that were analyzed. Effects and differences were considered significant for p<0.05. All data are expressed as means ± standard errors of the mean.
RESULTS
Experiment 1: time-course study
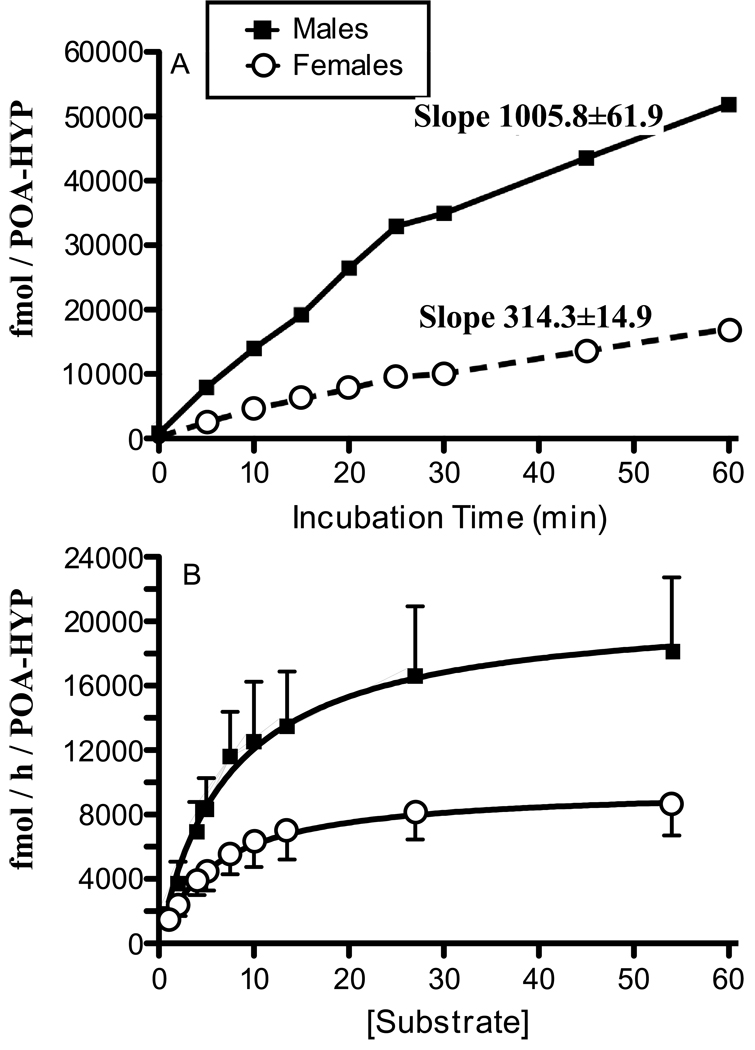
Aromatase activity was first assayed comparatively in males and females as a function of the incubation time. Incubating quail POA-HYP homogenates for 0–60 min yielded increasing productions of tritiated water in both sexes for periods up to 60 min (see Fig 2A). The runs tests indicated that these two sets of data (males and females) did not deviate significantly from linearity despite the apparent inflexion in product accumulation in males after the 30 min time point. Analysis by linear regression of these data (forcing the regression line through the origin of both axes) indicated that the slope of product accumulation in males was markedly higher in males (1005.8±61.9 fmol/POA-HYP/min) than in females (314.3±14.9 fmol/POA-HYP/min). The 95% confidence intervals of these slopes did not overlap (862.9–1148.0 f in males vs. 279.9–348.6 fmol/POA-HYP/min in females).
Figure 2.
Kinetic characterization of aromatase activity in the male and female quail POA-HYP. A) Measure of aromatase activity as a function of incubation time (Conditions: pH 7.2, 25 nM radioactive substrate, 1 mg wet weight per assay). B) Effect of substrate concentration on aromatase activity in the POA-HYP of the male and female quail (Conditions: pH 7.2, 20 min incubation time, 1 mg wet weight per assay). The time-course shown in Fig. 2A was studied in only one pool of brains for each sex and no measure of biological variation is therefore available for these data.
Analysis of the initial rate of accumulation taking only data points up to 25 min (when accumulation rate apparently slows down slightly in males) similarly indicated a higher enzymatic activity in males than in females with no overlap between the confidence intervals of the slopes (males: 1325.4±23.0 fmol/POA-HYP/min, confidence interval: 1266.2–1384.6; females: 395.8±12.4 fmol/POA-HYP/min, confidence interval: 364.0–427.6). While still linear between 30 and 60 min; accumulation of aromatase products took place at a reduced rate in both sexes (slopes, males: 562.0±6.3 vs. female: 234.0±1.28 fmol/POA-HYP/min). The confidence intervals of these slops still did not overlap (males: 482.2–642.0; females: 217.8–250.4) and rates of enzymatic activity were still significantly lower in females.
Experiment 2: affinity and maximum velocity of aromatase in males and females
During the second experiment, analysis in 4 males and 4 females of tritiated water accumulation during 20 min at various concentrations of substrate revealed an enzyme activity in close agreement with Michaelis-Menten kinetics. Upon closer examination of the data, we noticed that results for one female were aberrant with absolute measures of enzymatic activity being much more than 3 standard deviations above the group mean. Final analyses were therefore performed on data from 4 males and 3 females, excluding this atypical dataset, and results are presented in figure 2B.
Non linear regression analysis indicated that aromatase affinity for its substrate was similar in males and females (male Km of 7.37 ± 3.20 and female Km 5.82 ± 2.14 nM) while the maximum velocity of the enzymatic reaction was much higher in males than in females (males: 20962 ±3312; females: 9682±1199 fmol/h/POA-HYP). There was no overlap between the 95% confidence intervals for these estimates of maximum velocity (males: 14228–27696 vs. females: 7212–12152 fmol/h/POA-HYP) while confidence intervals for the Km were extensively overlapping (males: 0.86–13.88 vs. females: 1.42–10.22 nM). Student's t-test, however, did detect a significant difference in Vmax between males and females (t= 1.693, df= 5, 2p=0.1513).
Experiment 3: phosphorylations differentially affect aromatase activity in male and female brains
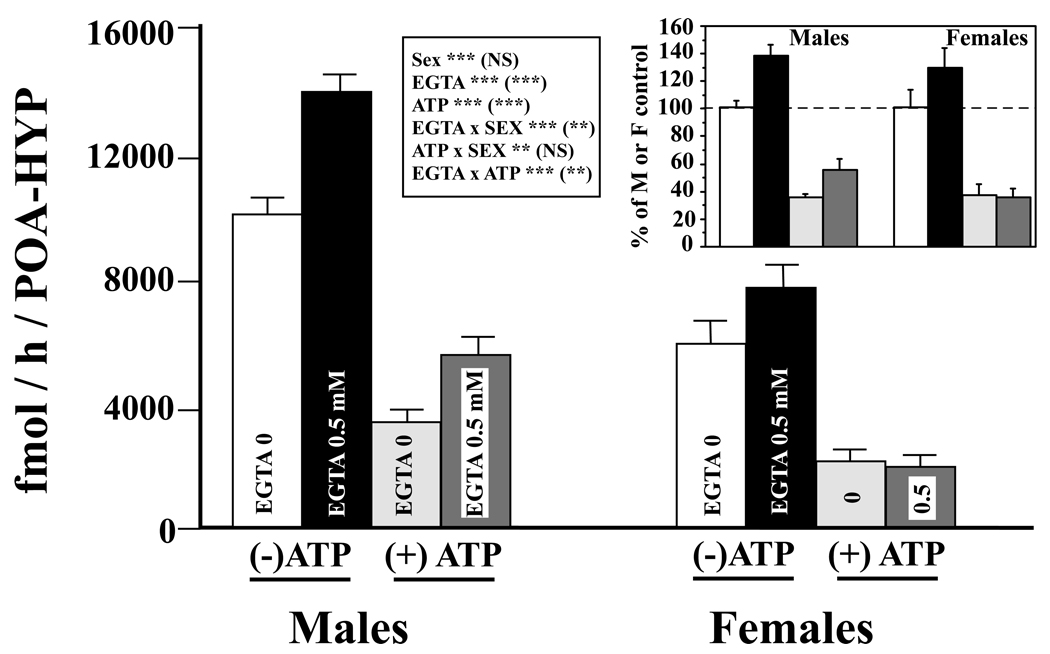
The effects on aromatase activity of phosphorylating conditions and of a calcium chelating agent (EGTA) were analyzed by a three way ANOVA with the sex of the samples considered as an independent factor and the presence/absence of ATP/Mg2+/Ca2+ or of EGTA (0 vs. 0.5 mM) as two repeated factors (Figure 3). This analysis first confirmed that, overall, aromatase activity is significantly lower in females than males (F1,13 = 27.31; p<0.002). We also found an effect of ATP/Mg2+/Ca2+ (F1,13 = 206.42; p<0.0001), with aromatase activity being significantly reduced by its addition. Likewise, the analysis revealed a main effect of EGTA (F1,13 = 113.50; p<0.0001), confirming that removal of endogenous calcium increases aromatase activity.
Figure 3.
Calcium-dependent changes in aromatase activity in the male and female quail POA-HYP. Males and female tissue homogenates were exposed to 0 or 0.5 mM EGTA combined with the presence or absence of ATP/Mg2+/Ca2+, thus creating four different experimental conditions. The insert presents the same data after transformation of all individual enzymatic activities as percentage of the average sex-typical activity in the absence of EGTA and of ATP/Mg2+/Ca2+. The grey coding of the bars is the same as in the main part of the figure. The box summarizes the results of the three-way ANOVAs used to analyze these data with results on each line referring first to data expressed in fmol/h/POA-HYP and then in parentheses to data expressed as percentage. **= p<0.01, ***= p<0.001, NS= not significant; ATP = ATP/Mg2+/Ca2+.
The analysis also detected a significant interaction between the presence/absence of ATP/Mg2+/Ca2+ and Sex (F1,13 = 11.02; p<0.0055). Plotting this interaction revealed that the effect of was more prominent in males than in females (sharper decrease in Fig. 4A). Additionally, the EGTA-induced increase in aromatase activity was more apparent in males than females (Fig. 4B), a finding supported by a significant interaction between EGTA and Sex (F1,13 = 36.37; p<0.0001). Finally, the analysis revealed a weaker but still significant interaction between ATP/Mg2+/Ca2+ and EGTA (F1,13 = 6.47; p<0.0244), caused by the fact that the EGTA-induced increase in aromatase activity was blunted in the ATP-exposed groups (increase without ATP to 135.16±5.70%, with ATP to 122.95±12.69%).
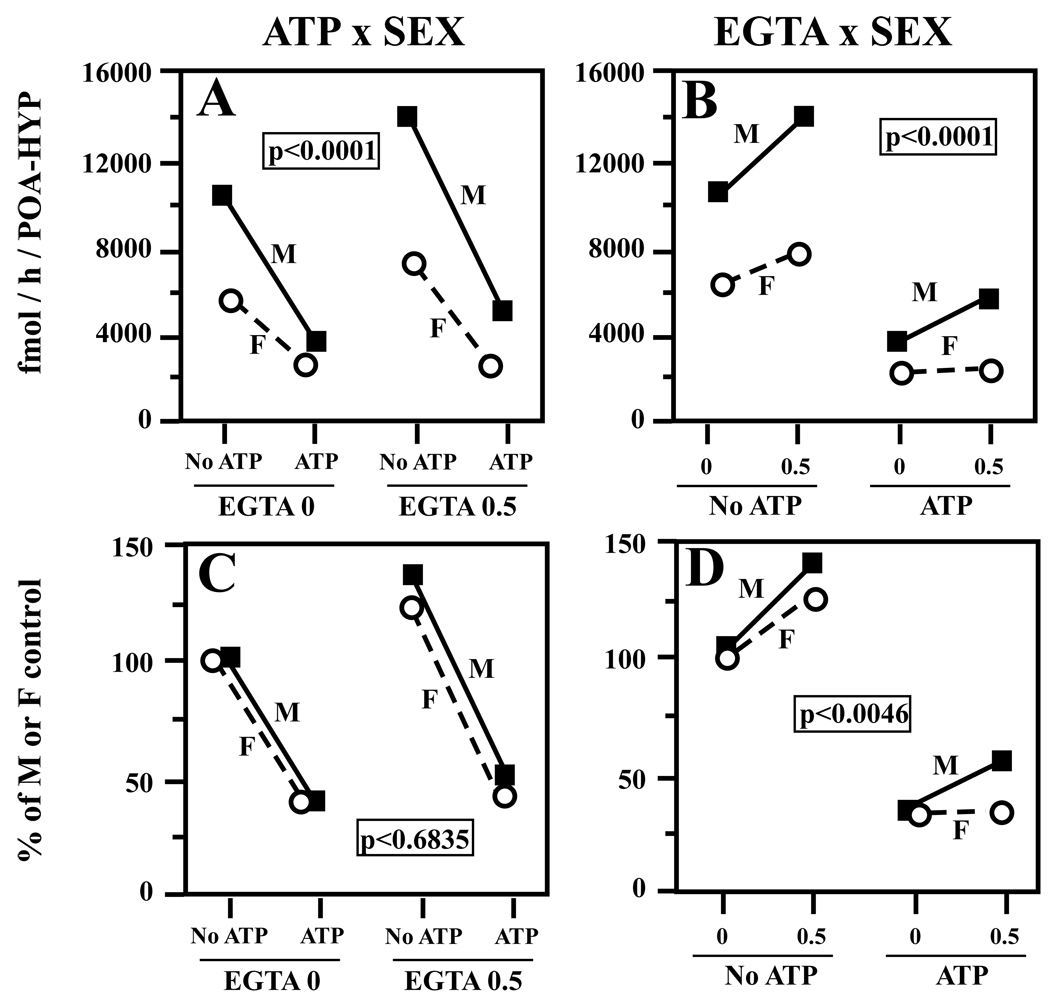
Figure 4.
Graph illustrating the interactions between the sex of the experimental subjects and the effects of ATP/Mg2+/Ca2+ (A, C) or of EGTA (B, D) on brain aromatase activity in the data presented in figure 3. The top two panels (A,B) represent interactions as detected in raw data (in fmol/h/POA-HYP) while the bottom panels (C, D) represent these same interactions with data expressed as percentage of the average sex-typical activity in the absence of EGTA and of ATP/Mg2+/Ca2+. No ATP/ATP = absence/presence of ATP/Mg2+/Ca2+; 0/0.5 = concentrations of EGTA in mM.
The two interactions including the sex factor detected by this analysis (ATP × Sex and EGTA × Sex) could result either from a difference in the absolute or in the relative magnitude of changes in males and females, given that the initial activity before any treatment was much higher in males (e.g., a decrease from 5000 fmol to 2000 in males and from 2000 fmol to 600 in females represents in both sexes a decrease of 60% but will appear as a sharper decline in males). To discriminate between these two interpretations, the raw data for each male and female were transformed as percentage of the average values observed in males or in females in the absence of ATP and of EGTA (see Fig. 3 insert). These transformed data were then re-analyzed by the same three-way ANOVA model. No effect of sex was obviously seen here but the transformation did not affect the two other main factors that remained significant (presence/absence of ATP/Mg2+/Ca2+: F1,13 = 190.03, p<0.0001; presence/absence of EGTA: F1,13 = 90.09, p<0.0001). The interaction between ATP/Mg2+/Ca2+ and Sex was no longer significant (F1,13 = 0.17, p = 0.6835; Fig. 4C: male and female lines are essentially parallel) but the interaction of EGTA and Sex remained present (F1,13 = 11.63, p<0.0046; Fig. 4D) and resulted from the existence of a more prominent effect of EGTA in males than in females. Additionally, this effect of EGTA had a larger amplitude in the presence than in the absence of ATP/Mg2+/Ca2+ thus resulting in a significant ATP × EGTA interaction (F1,13 = 10.57, p<0.0063).
The ANOVA of data transformed into percentages violates to some extent the basic assumptions of this statistical model and in this particular case, data did not obviously comply with the homoscedasticity requirements (e.g. significantly larger variance in several groups of data compared to the male data in the presence of ATP but not EGTA). Although the ANOVA is usually quite resistant to such violations, we tried nevertheless to confirm the existence of the two interactions described above by more conservative non-parametric analyses that had no pre-requirements concerning the nature of the data. For these analyses, we subtracted percentage-transformed data obtained in the presence of 0 mM of EGTA from those obtained in the presence of 0.5 mM EGTA to obtain a measure of the increase in activity observed by adding EGTA. This was done separately for the assays performed in the absence or in the presence of ATP and then these differences were compared in males and females by two Mann Whitney U-tests (in the presence or absence of ATP). This analysis confirmed that the EGTA-induced increases in aromatase activity is larger in males than in females but this sex difference was present only in assays performed in the presence of ATP (n males=7, n females= 8; with ATP: U=10, p=0.0372, no ATP: U=24, p=0.6434). This non-parametric approach thus fully confirms and reinforces the conclusions of the more questionable parametric ANOVA, in particular the presence of interactions between Sex and EGTA and between ATP and EGTA.
Experiment 4: kinase inhibitors differentially affect aromatase activity in males and females
The last experiment explored in a comparative manner in males and females the effects of kinase inhibitors on aromatase activity. As a first step, results of this experiment were analyzed with a two-way ANOVA with sex of the subjects as an independent factor and the six experimental conditions (KTH control, ATP/Mg2+/Ca2+ associated or not with one of 4 kinase inhibitors) as a repeated factor (Fig. 5). Once again, we identified a major overall sex difference in aromatase activity (F1,6 = 31.92; p<0.005), with lower levels in females. Furthermore, the analysis revealed an overall effect of treatments (F5,30 = 57.74; p<0.001) and an interaction between treatments and sex (F5, 30 = 19.85; p<0.001).
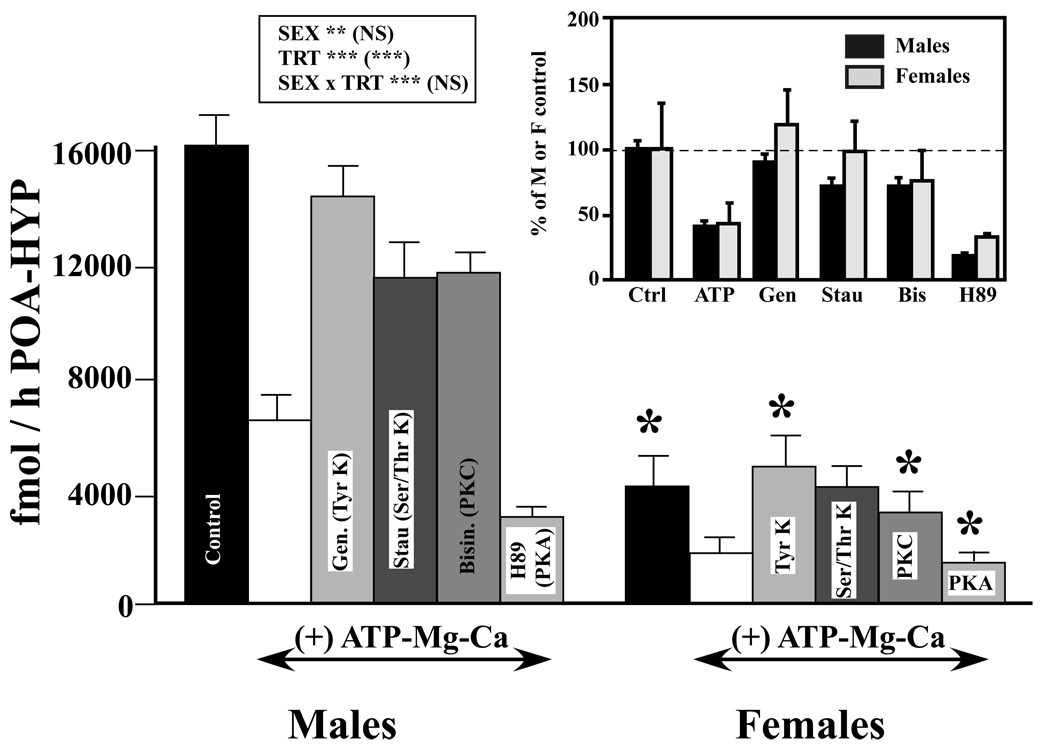
Figure 5.
Effects of various kinase inhibitors on the ATP/Mg2+/Ca2+-induced reduction in aromatase activity in male and female quail POA-HYP. All groups except Controls were treated with ATP/Mg2+/Ca2+. In addition, they were exposed to buffer or to one of the following kinase inhibitors: Genistein (Gen), a tyrosine kinase inhibitor (Tyr K), Staurosporin (Stau), a general serine/threonine kinase inhibitor (Ser/Thr K), Bisindolylmaleimide (Bisin), a protein kinase C inhibitor (PKC) or H89, a protein kinase A inhibitor (PKA). The insert presents the same data after transformation of all individual enzymatic activities as percentage of the average sex-specific activity in control (KTH) conditions. The box summarizes the results of the two-way ANOVAs used to analyze these data with results on each line referring first to data expressed in fmol/h/POA-HYP and then in parentheses to data expressed as percentage. **= p<0.01, ***= p<0.001, NS= not significant. The asterisks above the female bars in the main part of the figure refer to results of the two-way ANOVA that were performed to compare specifically in males and females the effect of each condition compared to the ATP/Mg2+/Ca2+ with no kinase inhibitor condition (see text).
We then researched the origins of this significant interaction by performing additional two-way ANOVAs comparing in males and females each experimental condition versus the ATP/Mg++/Ca++ condition. In each of these 5 analyses, an overall sex difference in aromatase activity was detected with males displaying a higher enzymatic activity than females (p≤0.0048). Each analysis also indicated that the experimental treatment had significantly affected the overall enzymatic activity. ATP/Mg2+/Ca2+ significantly reduced aromatase activity as compared to the KTH buffer control condition (F1,6=131.55, p<0.0001), the first three kinase inhibitors that were tested (Genistein, Staurosporine and Bisindolylmaleimide) significantly increased the activity by comparison with the ATP/Mg2+/Ca2+ condition (F1,6≥44.02; p≤0.0006; in fact they blocked the decrease induced by ATP/Mg2+/Ca2+) and somewhat surprisingly, H89, the PKA inhibitor, further reduced the overall aromatase activity as compared to the ATP/Mg2+/Ca2++ condition (F1,6=19.34, p<0.0048).
These effects were, however, not observed equally in both sexes. In 4 out of 5 analyses, a significant interaction was detected between the effect of treatment and the sex of the subjects (KTH vs. ATP/Mg2+/Ca2+, more decrease in males: F1,6=48.60, p<0.0004; vs. Genistein: F1,6=21.03, p<0.0037, more increase in females; vs. Bisindolylmaleimide: F1,6=29.21, p<0.0017, more increase in females; vs. H89: F1,6=11.67, p<0.0142, more decrease in males). This interaction was additionally close to significance for the last treatment (ATP/Mg2+/Ca2+ vs. Staurosporine: F1,6=5.66, p=0.0548, more increase in females).
Qualitative inspection of these data indicated that these interactions could, like in experiment 3, result from the sex difference in basal enzymatic activity (much higher activity in males so that in absolute terms, changes potentially have a larger magnitude) or alternatively reveal the existence of a sex difference in the relative response to ATP/Mg2+/Ca2+ and to kinase inhibitors (different percentage of changes). To discriminate between these possibilities, all raw data for each male and female were here also transformed as percentage of the average values observed in males or in females respectively in the absence of any treatment (control KTH condition). These data are plotted in the insert of figure 5. Their analysis by the same two-way ANOVA model, continued to indicate the presence of a very significant effect of the treatments (F5,30 = 19.37, p<0.0001). As expected, there was, however, no longer any sex difference between these two sets of data (F1,6 = 0.346, p=0.5775) and importantly there was no interaction between these two factors (F5, 30 = 0.77, p=0.5804). Data in the insert of figure 5 suggest that there was a numerically larger increase in aromatase activity in females compared to males after treatment with Genistein or Staurosporin. This could have contributed to the interactions observed during the analysis of raw data, but in the data transformed as percentages, these interactions are far from significant (p=0.3123 and 0.1677 respectively). Non-parametric Mann-Whitney tests that are distribution-free and therefore not affected by potential violations of the ANOVA model similarly did not detect any sex difference in the percentage of changes in activity induced by the addition of ATP or of any of the kinase inhibitors (p≥ 0.34, data not shown).
DISCUSSION
This paper clearly confirms the presence of a reliable sex difference affecting aromatase activity in the preoptic area/hypothalamus of Japanese quail. The data indicate that the larger accumulation of aromatase products in males as compared to females results from a sex difference in enzymatic activity that is present for the whole duration of the incubations such that initial enzymatic velocities are actually different. Saturation analysis further indicated that the numerically higher maximum velocity is not associated with a difference in the apparent affinity of the enzyme for its substrate (similar Km) suggesting that the properties and presumably nature of the enzyme is similar in both sexes. Consequently, the difference in activity results either from a higher concentration of enzymatic protein in males or from a sex specific regulation of the enzyme that does not affect its apparent affinity.
Although significantly less active in females than in males, aromatase could also play a significant biological role in females. It is indeed well established that significant concentrations of testosterone are circulating in females (6, 26). If the average concentration of this androgen is lower in females than in males, there is still a substantial amount of overlap between values observed in the two sexes. Potentially, brain aromatase could thus contribute substantially to the pool of estrogens that is present in the female brain. One unresolved question is, however, what could be the specific role of locally produced estrogens in the female brain when large amounts of this steroid are present in the general circulation. We have previously speculated that local production could result in high local concentrations in the male brain that would activate biological responses that could not be triggered by the lower systemic concentrations (27). The same could be true in females but this notion is currently awaiting experimental testing.
Because in situ hybridization studies had suggested that at the mRNA level no major sex difference appears to affect aromatase expression (15) suggesting that the amount of enzyme is the same in both sexes (unless a same amount of message is translated into a larger quantity of enzymatic protein), we wondered whether a differential regulation of the activity of a same amount of enzyme could explain the sex difference in total aromatase activity. Some support for this hypothesis was collected here. In particular, effects of EGTA were more pronounced in males than in females both in raw data as well as in data expressed as a percentage of the average sex-specific activity. Several aspects of these results have important consequences for the understanding of sex differences in aromatase activity and they deserve additional explanations.
Based on statistical criteria, the time-course study assessing accumulation of aromatization products (the tritiated water and thus the estrogens produced in equivalent molar ratio) over time suggested that the enzymatic reaction proceeds in a linear fashion for at least 60 min. Some numerical deviation from linearity was, however, observed after 25 min of incubation in males. This relatively minor decrease in enzymatic rate could be the result of a decrease in substrate concentration due to aromatase activity, of the use of substrate by other enzymes or finally of the accumulation of enzymatic products that would inhibit the reaction. The first of these interpretations appears currently unlikely. The radioactive substrate, androstenedione was indeed present in the incubation tubes at a concentration of 25 nM. After 25–30 min, the amount of aromatization products that had been synthesized (and thus of substrate metabolized) was approximately 30,000 fmoles for a whole POA-HYP block or approximately 750 fmoles per tube (POA-HYP blocks of approximately 40 mg were homogenized at a concentration of 20 mg/ml of which 50 µl, i.e. 1 mg equivalent tissue, were used for each assay). The production of 750 fmoles of products (i.e., 0.75 pmole) therefore used up only a small fraction of the substrate (initial concentration of 25 nM = 5 pmole/200 µl, the assay volume) and substrate concentration decrease probably cannot be invoked to explain the deviation from linearity of product accumulation.
It is therefore likely that the slight deviation from linearity in the reaction resulted from the activity of other enzymes or from an enzymatic inhibition caused by the accumulation of androstenedione metabolites. Testosterone and androstenedione can indeed be transformed into 5β-reduced products by the enzyme 5β-reductase that is widespread and very active in the quail brain including in the preoptic area and hypothalamus (7, 28). Previous work demonstrated that 5β-dihydrotestosterone (DHT), the first and most abundant metabolite produced by this enzyme inhibits aromatase activity in quail brain homogenates (29). Thus the decrease in substrate concentration related to 5β-reductase activity (not measured here) or the accumulation of 5β-reduced metabolites could explain the slight inhibition of aromatase activity at longer incubation time. Alternatively, oestradiol itself or its hydroxylated metabolites (catecholoestrogens) could inhibit its own production. Such an inhibition has been suggested in quail brain homogenates but was taking place only at high non physiological (10−4 M) concentrations (25). Furthermore, addition of 100 µM oestradiol to preoptic in vitro explants acutely inhibits (within 5 min) the rate of aromatization measured in these explants (Baillien M. and Balthazart J., unpublished data). These doses are, however, still much higher than physiological circulating concentrations and cannot explain in the present case the inhibition of enzymatic activity that took place in the second part of the incubations.
Saturation analysis identified a similar apparent affinity of the enzyme aromatase in both males and females (respectively 7.37 and 5.82 nM). The observed values are in the range of affinities previously measured in quail and other avian (25, 28, 30) and mammalian species (31–33) that denote a very high affinity of the enzyme for its substrate. These data also indirectly confirm a previous study from our laboratory indicating that the higher aromatase activity observed in testosterone-treated castrated males as compared to testosterone-treated ovariectomized females does not result from a different apparent affinity of the enzyme for its substrate (8). This suggests that the sex difference in Vmax is either caused by a higher concentration of active enzyme in males or a non-competitive inhibition of enzymatic activity in females (a competitive inhibition would affect the apparent Km). However, there is currently no data indicating the presence of such a non-competitive inhibitor in females specifically. Additionally, the in situ hybridization data mentioned above (15) suggest that the synthesis of the enzymatic protein is similar in males and in females, at least if no sex-specific regulation takes place at the translational level. This prompted us to investigate whether a differential phosphorylation status of the aromatase protein in males and females could be responsible for the sexually differentiated enzymatic activity.
We indeed showed in recent years that conditions promoting protein phosphorylation profoundly inhibit aromatase activity in brain homogenates of male quail, a phenomenon that is partially or completely blocked by a calcium-chelating agent such as EGTA or by protein kinase inhibitors (17, 18). Based on the fact that consensus phosphorylation sites are present in the aromatase sequence and that phosphorylated amino acids have been detected in Western blot on aromatase protein that had been partially purified by immunoprecipitation (18), we have assumed so far that the control of aromatase activity results from the phosphorylation of the aromatase protein itself. Studies are currently in progress to test this interpretation.
The last two experiments reported here fully confirmed these effects observed so far in males. Addition of the calcium-chelating agent EGTA increased and ATP/Mg2+/Ca2+ decreased aromatase activity in male brain homogenates whereas the effects of ATP/Mg2+/Ca2+ were largely blocked by the simultaneous addition of a variety of protein kinase inhibitors. Similar effects were observed in female quail homogenates but, in some cases, their magnitude differed significantly from what was observed in males. This resulted in significant interactions in the statistical analyses whose interpretation is not trivial based on current knowledge. A few possibilities are discussed below.
Blocking Ca2+ action with EGTA increased aromatase activity proportionally less in females than in males; no increase in activity at all was even observed in female homogenates that contained ATP/Mg2+/Ca2+ (Figs. 3 and 4B). This difference was still present when raw data for males and females were transformed as percentage of the sex-specific mean activity in control conditions (Fig. 4D). The difference (larger magnitude of aromatase activity increase after release from Ca2+-induced inhibition) is therefore not only the result of the absolute magnitude of changes that are present in males, but represents a true differential reaction of the enzyme in the two sexes. These results suggest that in physiological conditions either the enzymatic activity is inhibited more in males than in females or that the female enzyme and its molecular environment is less sensitive to the calcium-chelating action of EGTA.
If the first of these interpretations is true, the sex difference in reaction to EGTA is then in the opposite direction to what could have been originally predicted and the difference cannot be invoked to explain the lower enzymatic activity measured in females as compared to males in physiological conditions. The alternative interpretation, in contrast, assumes that, for some unidentified reason, the female enzyme is chronically inhibited by Ca2+ in a manner that cannot be reversed by chelating Ca2+ with EGTA. This could potentially explain why aromatase activity is lower in females than in males even if the concentration of the enzyme and of Ca2+ are similar in both sexes. It must also be added that the physiological intracellular calcium concentration in the vicinity of aromatase has never been investigated (in males and females comparatively) and it is therefore impossible to know whether these concentrations are sexually differentiated. This could obviously also be a factor contributing to the sex difference in aromatase activity.
Analysis of the raw data of experiment 3 also indicated that addition of ATP/Mg2+/Ca2+ inhibited aromatase activity more in males than in females suggesting that the enzyme present in male homogenates is more sensitive to these phosphorylating conditions (Fig. 4A). However, this statistically significant interaction disappeared when data were transformed as percentages of the average activity in each sex (Fig. 4C). Comparison of these two analyses thus indicates that the interaction reflects a differential absolute magnitude of ATP/Mg2+/Ca2+-induced changes but that these changes are proportionally similar in males and females and thus nearly identical when a same enzymatic activity (same number of enzymatic molecules?) is considered. The interpretation of such an interaction is therefore complex. A similar effect must take place at the molecular level in males and in females (presumably activation of protein kinases that inhibit the enzymatic activity) but it remains than in physiological conditions the magnitude of changes in oestrogen production induced by ATP/Mg2+/Ca2+ will be much larger in males than in females.
Conversely, the last experiment demonstrated that as observed previously in males, several protein kinase inhibitors almost completely block the inhibition of aromatase activity by ATP/Mg2+/Ca2+. This general effect was shown here to be significantly more prominent in females than in males (significant interaction in the general ANOVA). Partial analyses also showed that the significant interaction with sex was affecting the action of two of the inhibitors tested: one general tyrosine kinase inhibitor, Genistein and one protein kinase C inhibitor, Bisindolylmaleimide. A trend in the same direction (p=0.0548) was also present for the serine/threonine kinase inhibitor, Staurosporin. In contrast, the protein kinase A inhibitor, H89 further accentuated the inhibitory effects of ATP/Mg2+/Ca2+ and did so more extensively in males than in females. This inhibitor was presumably used at a concentration that was too high to reveal an increase in aromatase activity following blockade of the ATP/Mg2+/Ca2+ effects. Our previous work indeed showed that somewhere between 1 and 100 µM the effects of H89 on aromatase activity shifts from being stimulatory (or more correctly from blocking the ATP/Mg2+/Ca2+-induced decrease) to inhibitory (18). Alternatively, it is also possible that H89 displays atypical pharmacological properties in avian as compared to mammalian brain tissue. Additional experiments testing various doses of this compound should therefore be carried out to assess the true effect of PKA inhibition on aromatase activity in males and females.
As observed in the analysis of effects of ATP/Mg2+/Ca2+ during experiment 3, this differential reaction to kinase inhibitors was no longer present when data were transformed as percentages of average sex-specific values in enzymatic activity. Taken together, data of these two experiments thus show that activating with ATP/Mg2+/Ca2+ has a more profound effect on the total aromatase activity in males than in females but blocking phosphorylations with kinase inhibitors increases enzyme activity more in females as compared to males. The biochemical/molecular bases of the sexually differentiated reaction of the aromatase enzyme are not known at present but will presumably relate to the different cellular environment of the enzyme rather than to differentiated properties of the enzymatic protein itself (same Km in both sexes). The extent to which these sexually differentiated responses contribute to explain the sex difference in enzymatic activity that has been consistently reported will require further investigation.
It is finally interesting to note that the sex differences in reaction to ATP/Mg2+/Ca2+ and to kinase inhibitors systematically disappeared when data within each sex were transformed as a percentage of the sex-typical enzymatic activity whereas the interaction between EGTA action and sex remained fully significant in these conditions. This obviously suggests the existence of different underlying mechanisms. In the former case, manipulations presumably affect protein phosphorylation. In the latter case, chelating calcium could similarly modulate protein kinase activity but other calcium-dependent mechanisms might also be affected. Previous work in our lab identified effects of calcium that are not mediated via phosphorylation and seem to result from direct interactions of calmodulin with aromatase (34). Different biochemical mechanisms could therefore be involved in mediating sexually differentiated responses of brain aromatase to these experimental treatments. It must also be noted that these different mechanisms do not necessarily need to co-exist in the same aromatase-expressing neurons that are already known to be quite heterogeneous. It has, for example, been demonstrated that castration and treatment with exogenous testosterone do not affect in the same manner aromatase expression in different parts of the medial preoptic nucleus. While castration markedly decreases the number of these neurons in most of the nucleus, a population of very small aromatase-immunoreactive neurons increase in number following castration in the most medial part of the nucleus (14).
In conclusion, these studies indicate that, in both male and female quail, brain aromatase activity is regulated in the short term by complex mechanisms including interactions with calcium and with various kinases. These mechanisms are differentially active in males and females and could potentially contribute to explain the sex difference in enzymatic activity that is well established not only in quail (7, 8) but also in a variety of other avian and mammalian species (3, 35). The sex differences in aromatase regulation that were observed here have, however, a limited magnitude and do not seem to explain by themselves the discordance that has been reported between the large sex difference affecting enzyme activity and the more limited sex difference detected at the level of the enzymatic protein or its messenger. Other mechanisms might thus be implicated such as a differential subcellular distribution of the enzyme in males and females. It is indeed well established that a substantial part of the brain aromatase activity is present in presynatic boutons in a variety of species (quail: (36), zebra finch: (37); rat: (38)) and that, in zebra finches at least, the distribution of aromatase between perikarya and synaptic boutons is different in males and in females (39). It is therefore conceivable: a) that in vivo these different cellular compartments are differentially affected by phosphorylating conditions and this sex difference would disappear in homogenized tissue or b) that the relative lack of a sex difference in the amount of enzymatic protein as detected by immunohistochemistry relates to the differential subcellular distribution of the enzyme making part of it less (not) visible in one sex. Note that this would not explain the lack of sex difference in the concentration of the corresponding mRNA. The present data clearly indicate that more research is warranted to investigate comparatively in males and females not only the reactions of aromatase to its changing neurochemical environment but also the mechanisms controlling calcium homeostasis in the sub-cellular compartment(s) that contain(s) the active enzyme and how these effects are differentially modulated for the enzyme present in various subcellular compartments.
Acknowledgements
This work was supported by grants from the National Institutes of Health (R01 NIH/MH50388) to Gregory F. Ball and JB and from the Belgian FRFC (2.4537.09) and the University of Liège (Fonds spéciaux 2009) to JB and an NSERC PDF to ATMK. We thank Drs. Charlotte A. Cornil and Thierry D. Charlier (University of Liège) for fruitful discussions and suggestions concerning the analysis and interpretation of these data.
References
- 1.Balthazart J. Steroid metabolism and the activation of social behavior. In: Balthazart J, editor. Advances in Comparative and Environmental Physiology, vol 3. 1 ed. Berlin: Springer Verlag; 1989. pp. 105–159. [Google Scholar]
- 2.Lephart ED. A review of brain aromatase cytochrome P450. Brain Res Rev. 1996;22:1–26. [PubMed] [Google Scholar]
- 3.Boon WC, Chow JD, Simpson ER. The multiple roles of estrogens and the enzyme aromatase. Prog Brain Res. 2010;181:209–232. doi: 10.1016/S0079-6123(08)81012-6. [DOI] [PubMed] [Google Scholar]
- 4.Balthazart J, Baillien M, Cornil CA, Ball GF. Preoptic aromatase modulates male sexual behavior: slow and fast mechanisms of action. Physiol Behav. 2004;83:247–270. doi: 10.1016/j.physbeh.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 5.Adkins EK, Adler NT. Hormonal control of behavior in the Japanese quail. J Comp Physiol Psychol. 1972;81:27–36. doi: 10.1037/h0033315. [DOI] [PubMed] [Google Scholar]
- 6.Balthazart J, Schumacher M, Ottinger MA. Sexual differences in the Japanese quail: behavior, morphology and intracellular metabolism of testosterone. Gen Comp Endocrinol. 1983;51:191–207. doi: 10.1016/0016-6480(83)90072-2. [DOI] [PubMed] [Google Scholar]
- 7.Schumacher M, Balthazart J. Sexual dimorphism of the hypothalamic metabolism of testosterone in the Japanese quail (Coturnix coturnix japonica) Prog Brain Res. 1984;61:53–61. doi: 10.1016/S0079-6123(08)64428-3. [DOI] [PubMed] [Google Scholar]
- 8.Schumacher M, Balthazart J. Testosterone-induced brain aromatase is sexually dimorphic. Brain Res. 1986;370:285–293. doi: 10.1016/0006-8993(86)90483-x. [DOI] [PubMed] [Google Scholar]
- 9.Balthazart J. Correlation between the sexually dimorphic aromatase of the preoptic area and sexual behavior in quail: effects of neonatal manipulations of the hormonal milieu. Arch Int Physiol Bioch. 1989;97:465–481. doi: 10.3109/13813458909075078. [DOI] [PubMed] [Google Scholar]
- 10.Schumacher M, Balthazart J. The effects of testosterone and its metabolites on sexual behavior and morphology in male and female Japanese quail. Physiol Behav. 1983;30:335–339. doi: 10.1016/0031-9384(83)90135-x. [DOI] [PubMed] [Google Scholar]
- 11.Balthazart J, Tlemçani O, Ball GF. Do sex differences in the brain explain sex differences in the hormonal induction of reproductive behavior? What 25 years of research on the Japanese quail tells us. Horm Behav. 1996;30:627–661. doi: 10.1006/hbeh.1996.0066. [DOI] [PubMed] [Google Scholar]
- 12.Balthazart J, Foidart A, Harada N. Immunocytochemical localization of aromatase in the brain. Brain Res. 1990;514:327–333. doi: 10.1016/0006-8993(90)91428-j. [DOI] [PubMed] [Google Scholar]
- 13.Foidart A, De Clerck A, Harada N, Balthazart J. Aromatase-immunoreactive cells in the quail brain: Effects of testosterone and sex dimorphism. Physiol Behav. 1994;55:453–464. doi: 10.1016/0031-9384(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 14.Balthazart J, Tlemçani O, Harada N. Localization of testosterone-sensitive and sexually dimorphic aromatase-immunoreactive cells in the quail preoptic area. J Chem Neuroanat. 1996;11:147–171. doi: 10.1016/0891-0618(96)00149-4. [DOI] [PubMed] [Google Scholar]
- 15.Voigt C, Ball GF, Balthazart J. Neuroanatomical specificity of sex differences in expression of aromatase mRNA in the quail brain. J Chem Neuroanat. 2007;33:75–86. doi: 10.1016/j.jchemneu.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Cornil CA, Balthazart J. New insights in sex differences in quail preoptic/hypothalamic aromatase activity. Abst Soc Behav Neuroendo. 2008;120 [Google Scholar]
- 17.Balthazart J, Baillien M, Ball GF. Rapid and reversible inhibition of brain aromatase activity. J Neuroendocrinol. 2001;13:63–73. doi: 10.1046/j.1365-2826.2001.00598.x. [DOI] [PubMed] [Google Scholar]
- 18.Balthazart J, Baillien M, Charlier TD, Ball GF. Calcium-dependent phosphorylation processes control brain aromatase in quail. Eur J Neurosci. 2003;17:1591–1606. doi: 10.1046/j.1460-9568.2003.02598.x. [DOI] [PubMed] [Google Scholar]
- 19.Nestler EJ, Greengard P. Protein phosporylation and the regulation of neuronal function. In: Siegel GJ, Agranoff BW, Albers RW, Molinoff PB, editors. Basic neurochemistry. New York, NY: Raven Press; 1989. pp. 373–398. [Google Scholar]
- 20.Nestler EJ, Greengard P. Serine and threonine phosphorylation. In: Siegel GJ, Agranoff BW, Albers RW, Fisher SK, Uhler MD, editors. Basic Neurochemistry Molecular, Cellular and Medical Aspects. 6 ed. Philadelphia: Lippincott-Raven; 1999. pp. 471–495. [Google Scholar]
- 21.Balthazart J, Baillien M, Ball GF. Rapid control of brain aromatase activity by glutamatergic inputs. Endocrinology. 2006;147:359–366. doi: 10.1210/en.2005-0845. [DOI] [PubMed] [Google Scholar]
- 22.Schumacher M, Balthazart J. The postnatal demasculinization of sexual behavior in the Japanese quail. Horm Behav. 1984;18:298–312. doi: 10.1016/0018-506x(84)90018-7. [DOI] [PubMed] [Google Scholar]
- 23.Charlier TD, Ball GF, Balthazart J. Rapid action on neuroplasticity precedes behavioral activation by testosterone. Horm Behav. 2008;54:488–495. doi: 10.1016/j.yhbeh.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roselli CE, Resko JA. In vitro assay of aromatase activity in the central nervous system. In: Greenstein B, editor. Neuroendocrine research methods, Vol 2. Chur, Switzerland: Harwood Academic Publishers; 1991. 937-951-0. [Google Scholar]
- 25.Baillien M, Balthazart J. A direct dopaminergic control of aromatase activity in the quail preoptic area. J Steroid Biochem Mol Biol. 1997;63:99–113. doi: 10.1016/s0960-0760(97)00080-0. [DOI] [PubMed] [Google Scholar]
- 26.Balthazart J, Delville Y, Sulon Y, Hendrick JC. Plasma levels of luteinizing hormone and of five steroids in photostimulated, castrated and testosterone-treated male and female Japanese quail (Coturnix coturnix japonica) General Endocrinol (Life SciAdv) 1987;5:31–36. [Google Scholar]
- 27.Cornil CA, Ball GF, Balthazart J. Functional significance of the rapid regulation of brain estrogen action: Where do the estrogens come from? Brain Res. 2006;1126:2–26. doi: 10.1016/j.brainres.2006.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schumacher M, Contenti E, Balthazart J. Partial characterization of testosterone-metabolizing enzymes in the quail brain. Brain Res. 1984;305:51–59. doi: 10.1016/0006-8993(84)91118-1. [DOI] [PubMed] [Google Scholar]
- 29.Schumacher M, Hutchison RE, Hutchison JB. Inhibition of hypothalamic aromatase activity by 5 beta-dihydrotestosterone. J Neuroendocrinol. 1991;3:221–226. doi: 10.1111/j.1365-2826.1991.tb00266.x. [DOI] [PubMed] [Google Scholar]
- 30.Steimer T, Hutchison JB. Androgen increases formation of behaviourally effective oestrogen in dove brain. Nature. 1981;292:345–347. doi: 10.1038/292345a0. [DOI] [PubMed] [Google Scholar]
- 31.Roselli CE, Ellinwood WE, Resko JA. Regulation of brain aromatase activity in rats. Endocrinology. 1984;114:192–200. doi: 10.1210/endo-114-1-192. [DOI] [PubMed] [Google Scholar]
- 32.Hutchison RE, Hutchison JB, Steimer T, Steel E, Powers JB, Walker AP, Herbert J, Hastings MH. Brain aromatization of testosterone in the male Syrian hamster: Effects of androgen and photoperiod. Neuroendocrinol. 1991;53:194–203. doi: 10.1159/000125718. [DOI] [PubMed] [Google Scholar]
- 33.Wozniak A, Hutchison RE, Hutchison JB. Localisation of aromatase activity in androgen target areas of the mouse brain. Neurosci Lett. 1992;146:191–194. doi: 10.1016/0304-3940(92)90075-i. [DOI] [PubMed] [Google Scholar]
- 34.Balthazart J, Baillien M, Charlier TD, Ball GF. Effects of calmodulin on aromatase activity in the preoptic area. J Neuroendocrinol. 2005;17:664–671. doi: 10.1111/j.1365-2826.2005.01355.x. [DOI] [PubMed] [Google Scholar]
- 35.Roselli CE, Resko JA. Aromatase activity in the rat brain: Hormonal regulation and sex differences. J Steroid Biochem Mol Biol. 1993;44:499–508. doi: 10.1016/0960-0760(93)90254-t. [DOI] [PubMed] [Google Scholar]
- 36.Schlinger BA, Callard GV. Localization of aromatase in synaptosomal and microsomal subfractions of quail (Coturnix coturnix japonica) brain. Neuroendocrinol. 1989;49:434–441. doi: 10.1159/000125149. [DOI] [PubMed] [Google Scholar]
- 37.Peterson RS, Yarram L, Schlinger BA, Saldanha CJ. Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proc Biol Sci. 2005;272:2089–2096. doi: 10.1098/rspb.2005.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roselli CE. Subcellular localization and kinetic properties of aromatase activity in rat brain. J Steroid Biochem Mol Biol. 1995;52:469–477. doi: 10.1016/0960-0760(94)00192-o. [DOI] [PubMed] [Google Scholar]
- 39.Rohmann KN, Schlinger BA, Saldanha CJ. Subcellular compartmentalization of aromatase is sexually dimorphic in the adult zebra finch brain. Dev Neurobiol. 2007;67:1–9. doi: 10.1002/dneu.20303. [DOI] [PubMed] [Google Scholar]