Abstract
Cytochrome c (Cytc) is essential in mitochondrial electron transport and intrinsic type II apoptosis. Mammalian Cytc also scavenges reactive oxygen species (ROS) under healthy conditions, produces ROS with the co-factor p66Shc, and oxidizes cardiolipin during apoptosis. The recent finding that Cytc is phosphorylated in vivo underpins a model for the pivotal role of Cytc regulation in making life and death decisions. An apoptotic sequence of events is proposed involving changes in Cytc phosphorylation, increased ROS via increased mitochondrial membrane potentials or the p66Shc pathway, the oxidation of cardiolipin by Cytc, and its release from the mitochondria. Cytc regulation in respiration and cell death is discussed in a human disease context including neurodegenerative and cardiovascular diseases, cancer, and sepsis.
Keywords: Aging, apoptosis, cardiolipin, cell signaling, cytochrome c, oxidative phosphorylation, reactive oxygen species
1. Introduction
Mitochondria are unique cellular organelles that possess their own DNA, encoding thirteen subunits of the respiratory complexes in mammals as well as twenty-two tRNAs and two rRNAs (Chan, 2006). Mitochondria are the site of the citric acid cycle and utilize the substrates from this process to drive the electron transport chain (ETC) and the production of cellular energy in the form of adenosine triphosphate (ATP). As the main (>90%) producers of ATP, mitochondria satisfy the energy requirements of the entire cell. Mitochondria serve a vital function by maintaining a balance of cellular homeostasis which is not limited to energy level maintenance, but includes such diverse factors as calcium and reactive oxygen species (ROS) (Jezek and Hlavata, 2005; Lee and Tse, 2005; Samavati et al., 2008; Wang et al., 2003a). The balance between ROS and ATP production is preserved by integrating multiple cellular signals. When the equilibrium is shifted towards ROS production, major disturbances in cell function and viability occur. Such perturbations are seen in an increasing number of human diseases including such diverse conditions as neurodegenerative diseases, diabetes, cancer, and sepsis (Duvigneau et al., 2008; Kadenbach et al., 2004b; Samavati et al., 2008; Sheehan et al., 1997). In addition, mitochondria play a key role in aging, which is accompanied by decreased mitochondrial capacity to produce ATP and accumulation of damaged mitochondria over time, including mtDNA mutations (reviewed in Mammucari and Rizzuto, 2010). The ETC produces the majority of cellular ROS, and it has been estimated that up to 1-2% of oxygen consumed can be converted into superoxide anions (O2•–) (Richter et al., 1988). Consequently, the underlying processes that dictate the cell's fate are important for cellular development and maintenance and must be tightly regulated in order to ensure overall tissue and organism health. When stress signals outweigh the benefits of sustaining viability, various signals can initiate the programmed cell death process through intrinsic (mitochondrial) type II apoptosis. These two seemingly contradictory functions, i.e., life-sustaining energy production and apoptosis, converge on cytochrome c (Cytc).
Cytc is an evolutionarily conserved nuclear-encoded mitochondrial protein, which contains 104 amino acids in mammals. It is highly positively charged with a pI of 9.6. Cytc is essential for aerobic energy production and Cytc knockout mice die around midgestation (Li et al., 2000a). Until that developmental stage energy production relies only on 5% aerobic metabolism, then switches to aerobic energy metabolism relying 95% on OxPhos after gestation day 11 (Morriss and New, 1979).
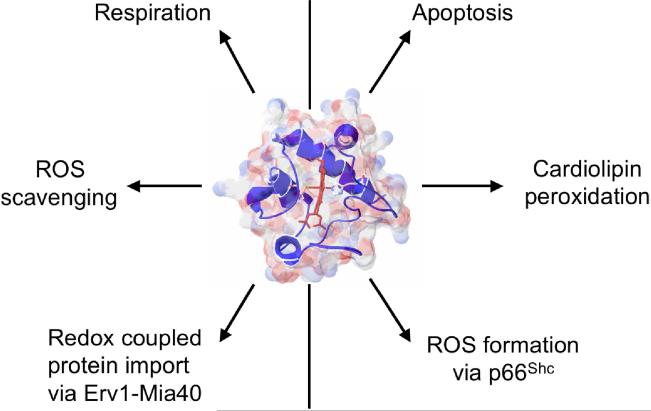
Cytc is a multi-functional enzyme that is involved in life and death decisions of the cell (Fig. 1). It participates in electron transfer as part of the mitochondrial electron transport chain (ETC) and is thus an indispensable part of the energy production process. It is also essential for the formation of the apoptosome and the progression of apoptosis. Recent discoveries of additional functions of Cytc, including its activity as a cardiolipin peroxidase (Kagan et al., 2004; Kagan et al., 2005), and the detection of four phosphorylation sites on Cytc (Lee et al., 2006; Yu et al., 2008; Zhao et al., 2010), suggest that its multiple functions are regulated by cell signaling pathways. Derivation of the specific pathways that operate these regulatory mechanisms and their effects may become an important avenue for therapeutic targeting of various human illnesses including neurodegenerative diseases, congestive heart failure, and cancer. Below, we first discuss the diverse functions of Cytc and conclude with a model that proposes regulation of Cytc via phosphorylation as the central mechanism integrating and regulating the functions of Cytc.
Figure 1.
Summary of the main functions of cytochrome c. The left side of the figure highlights its involvement in life-sustaining functions, whereas its involvement in cellular death functions is shown on the right side.
2. Structure of cytochrome c
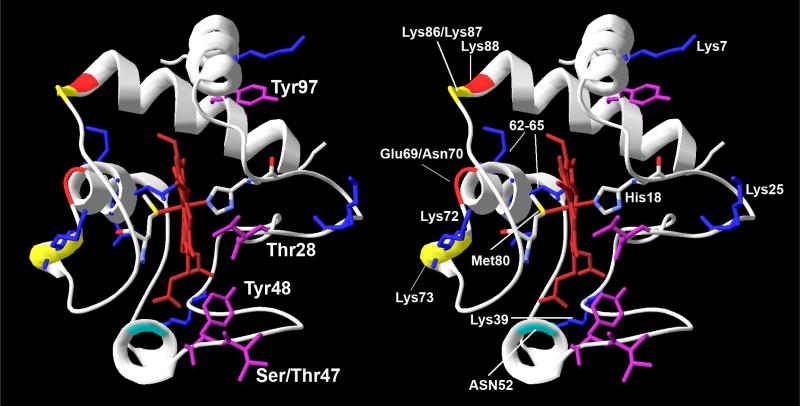
Cytc was one of the first mammalian proteins subjected to X-ray crystallography, and the first 4Å resolution structure was obtained from oxidized horse heart Cytc in the late sixties (Dickerson et al., 1967). Higher resolution structures of Cytc from horse (Bushnell et al., 1990) and other organisms were subsequently published allowing a more detailed view of its basic properties (Fig. 2). The heme group is covalently linked to the Cytc peptide chain through thioether bonds with cysteine residues 14 and 17 (amino acid numbering is based on the mature peptide, which lacks the N-terminal methionine, Fig. 3). The heme iron is in a hexacoordinate configuration with His18 and Met80 as amino acid ligands (Fig. 2). The heme iron-Met80 bond causes the weak 695 nm absorption band in the spectrum of Cytc in the oxidized state. The presence of aliphatic and aromatic amino acid side chains places the heme group in a very hydrophobic environment, which together with the iron ligands His18 and Met80 was proposed to explain the high redox potential of Cytc of about 260 mV in mammals (Salemme, 1977). Of the entire heme surface only 7.5% are solvent-exposed as part of the frontal edge of the heme group (Bushnell et al., 1990). This is the site used to transfer electrons from bc1 complex to cytochrome c oxidase (CcO), which was confirmed by co-crystallization of Cytc with bc1 complex from yeast (Lange and Hunte, 2002) and computer prediction of the Cytc-CcO complex based on the bovine CcO crystal structure (Roberts and Pique, 1999).
Figure 2.
Stereo view of the cytochrome c structure in the conventional orientation. Crystallographic data from horse Cytc was used (Sanishvili et al., 1995) and processed with the program Swiss PDB viewer (version 3.7). Residues Thr28, Ser/Thr47, Tyr48, and Tyr97 that can be phosphorylated in mammals are highlighted in purple (Lee et al., 2006; Yu et al., 2008; Zhao et al., 2010). A-side residues Lys72, Lys73, and Lys87 (yellow ribbon) and C-side residue 52 (turquoise ribbon) proposed to be involved in phospho-lipid binding through electrostatic interaction with the negatively charged lipid head group (Kagan et al., 2009) are highlighted. Amino acids Glu69, Asn70, and Lys88 indicated in red ribbon form an ATP binding pocket together with C-side residues Lys72, Lys86, and Lys87 (yellow ribbon) (McIntosh et al., 1996). Key residues required for Apaf-1 binding and caspase activation are highlighted in blue sticks. Phosphorylation sites localize to the right side of the molecule whereas amino acids involved in binding of phospholipids and ATP are on the left side.
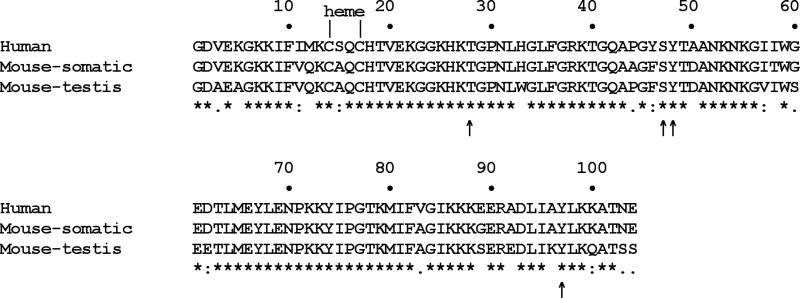
Figure 3. Cytochrome c alignment.
Human (NM_018947), mouse somatic (CAA25899) and testis (NP_034119) cytochrome c show 81% sequence identity with individual comparisons revealing 91% (human-mouse somatic), 82% (human-mouse testis), and 86% (mouse somatic-mouse testis) conservation. Thr28, Ser47, Tyr48, and Tyr97 which can be phosphorylated in vivo are indicated with an arrow. Note that human Cytc contains an additional fifth tyrosine residue, Tyr46, which is immediately adjacent to the phospho-epitope containing Ser47 and Tyr48. Future work should explore if this residue is targeted for human-specific regulation of Cytc via phosphorylation.
3. Role of cytochrome c in oxidative phosphorylation
ATP generation is the purpose of the oxidative phosphorylation (OxPhos) machinery, which is housed in the inner mitochondrial membrane and consists of the electron transport chain (ETC) and ATP synthase. Oxidative phosphorylation is fueled by several substrates that feed electrons into the ETC, including NADH and FADH2. While transferring these electrons, the ETC generates the mitochondrial membrane potential (ΔΨm). ETC complexes I (NADH dehydrogenase), III (bc1 complex), and IV (cytochrome c oxidase, CcO) pump protons from the matrix into the intermembrane space. The mitochondrial membrane potential is utilized by ATP synthase (complex V) to drive its operation in the final step, the production of ATP. ATP synthase is a rotary nano motor converting the membrane potential into rotational and chemical energy by combining a phosphate and an ADP molecule into ATP (Stock et al., 1999).
Cytc is located in the mitochondrial intermembrane space and functions as a single electron carrier from the bc1 complex to CcO in the final step of the ETC. In this step reduced Cytc transfers an electron to CcO and four such electron transfers are required to reduce an oxygen molecule to water. In intact mammalian cells this reaction is the proposed rate-limiting step of the ETC under physiological conditions (Acin-Perez et al., 2003; Dalmonte et al., 2009; Piccoli et al., 2006; Villani and Attardi, 1997; Villani et al., 1998). The electron transfer reaction from Cytc to oxygen via CcO leads to the conversion of oxygen to water with a free energy of ΔGo’= -100 kJ/mol, which is about twice as high compared to the reactions catalyzed by complexes I and III (Hinkle et al., 1991). Since this is an essentially irreversible reaction, we and others have proposed that this step of the ETC should be tightly regulated. This hypothesis is supported by the fact that all major regulatory mechanisms are present, including allosteric regulation of CcO and Cytc (e.g., by the ATP/ADP ratio), the expression of tissue-specific isoforms of Cytc and six subunits of CcO, and reversible post-translational modifications, in particular phosphorylations, which have been identified in all mammalian OxPhos components (for reviews see Hüttemann et al., 2007; Hüttemann et al., 2008).
4. Cytochrome c and Erv1-Mia40-coupled protein import
Since the majority of mitochondrial proteins are nuclear encoded, their transport into mitochondria is a crucial process. This is mainly accomplished through the work of translocases of the inner and outer membranes, TIMs and TOMs, respectively (Rehling et al., 2001). Import of the precursor proteins is followed by additional folding and/or assembly into functional proteins. Often this involves enzymes to assist in converting a protein from its apo- to holo-form as in the case of Cytc import.
Cytc is imported into mitochondria as apocytochrome c, a process that is independent of the mitochondrial membrane potential, ATP, or the Mia40-dependent pathway involving TOM40 (Diekert et al., 2001). The holo-form of Cytc is formed by heme addition, catalyzed by the enzyme Cytc heme lyase, which is also known as holocytochrome-c synthase (Dumont et al., 1991).
Cytc is involved in redox-coupled import of proteins containing twin CX3C and CX9C motifs into the mitochondrial intermembrane space in connection with the proteins Erv1 and Mia40. Small TIMs represent a prominent group of proteins imported into the mitochondria via this pathway (reviewed in Riemer et al., 2010). This import occurs via TOMs and similarly requires post-import modification, i.e., alternative folding introduced via the creation of disulfide bonds between cysteine residues. These reactions are dependent upon oxidoreductase systems. Specifically, the import and assembly of TIMs has been shown to be contingent upon Mia40 oxidation of the imported protein and the formation of disulfide bonds that alter the protein's tertiary structure (Chacinska et al., 2004). Reactivation of the Mia40 protein subsequently relies on oxidation by Erv1, which is dependent upon the activity of Cytc for its re-oxidation (Allen et al., 2005). This mechanism of import and cascade of redox reactions provides a route for the transfer of electrons from the Mia40 import pathway to Cytc and thus into the ETC.
5. Regulation of cytochrome c and cytochrome c oxidase by adenine nucleotides
Anions in general have been demonstrated to affect the physical interaction between Cytc and CcO, but ATP is particularly noteworthy. ATP, the end product of OxPhos, regulates the rate of electron transport as a feedback inhibitor of the interaction between Cytc and CcO through binding to both proteins (Arnold and Kadenbach, 1999; Ferguson-Miller et al., 1976; Napiwotzki et al., 1997). The allosteric inhibition exhibited by ATP has been shown to act in an uncompetitive manner, altering the high-affinity Cytc-CcO binding site to a low-affinity site (Ferguson-Miller et al., 1976). Arg91 has been identified as the key amino acid of Cytc involved in the binding of ATP. When this site is occupied by ATP, electron-transfer activity of Cytc is significantly downregulated (Craig and Wallace, 1995; Tuominen et al., 2001). Presumably this effect is due to structural changes of both proteins as well as electrostatic changes when negatively charged ATP binds to Cytc and CcO. As a result, ETC activity is adjusted to energy demand through the ATP/ADP ratio as an intrinsic measure of the energetic state of the cell. However, it should be noted that the phosphorylation state of CcO (and perhaps also Cytc) plays an overarching role by enabling allosteric regulation, because after in vitro dephosphorylation of cow liver CcO no inhibitory effect of ATP was detectable (Hüttemann et al., 2008). Therefore, allosteric regulation should be considered in conjunction with the phosphorylation state of CcO and Cytc.
6. Regulation of cytochrome c through tyrosine phosphorylation
Cell signaling targeting the mitochondrial OxPhos proteins is a new research area. It may transform the traditional thinking about the regulation of OxPhos, which is mainly derived from studies in bacteria (see also section 9). More than 20 phosphorylation sites have been mapped on mammalian OxPhos proteins (Hüttemann et al., 2007). However, for most of them the corresponding signaling pathways as well as kinases and phosphatases remain unknown or unclear. Tyrosine phosphorylation is very specific and mainly found in higher organisms. It is this regulatory mechanism that affects Cytc in a distinct tissue-specific manner.
In the past, commonly used isolation methods of Cytc have not taken into account the possibility of Cytc phosphorylation in vivo. The result has been isolation of dephosphorylated protein, including those available from commercial sources, and therefore its potential as an integral target of cellular signaling was overlooked. In order to preserve in vivo phosphorylation, our isolation methods include the use of nonspecific phosphatase inhibitors, fluoride and vanadate.
Preserving the physiological in vivo phosphorylation state, we isolated Cytc from cow heart and liver tissue without manipulating cell signaling pathways. Western analysis with an anti-phosphoSer/Thr/Tyr antibodies indicated tyrosine phosphorylation in both experiments. Interestingly, mass spectrometry unambiguously revealed that the phosphorylated residues were distinct, suggesting that cell signaling targets Cytc in a tissue-specific manner. Cytc isolated from cow heart was phosphorylated on Tyr97 (Lee et al., 2006), whereas cow liver Cytc revealed phosphorylation on Tyr48 (Fig. 2) (Yu et al., 2008). Both tyrosines are highly conserved among eukaryotes (Fig. 3), suggesting their potential importance as targets of cell signaling pathways.
Spectral analysis of oxidized Cytc shows a weak but specific 695 nm absorption band which is the result of the Met80-heme iron bond. This band was called an “indicator of trouble” because its absence implies a dysfunctional and denatured conformation of Cytc (Dickerson and Timkovich, 1975). Tyr97- but not Tyr48-phosphorylated Cytc showed a shift of the 695 nm absorption band to 687 nm suggesting that Tyr97-phosphorylation influences the heme environment despite being localized to the periphery of the molecule (Fig. 2) (Lee et al., 2006).
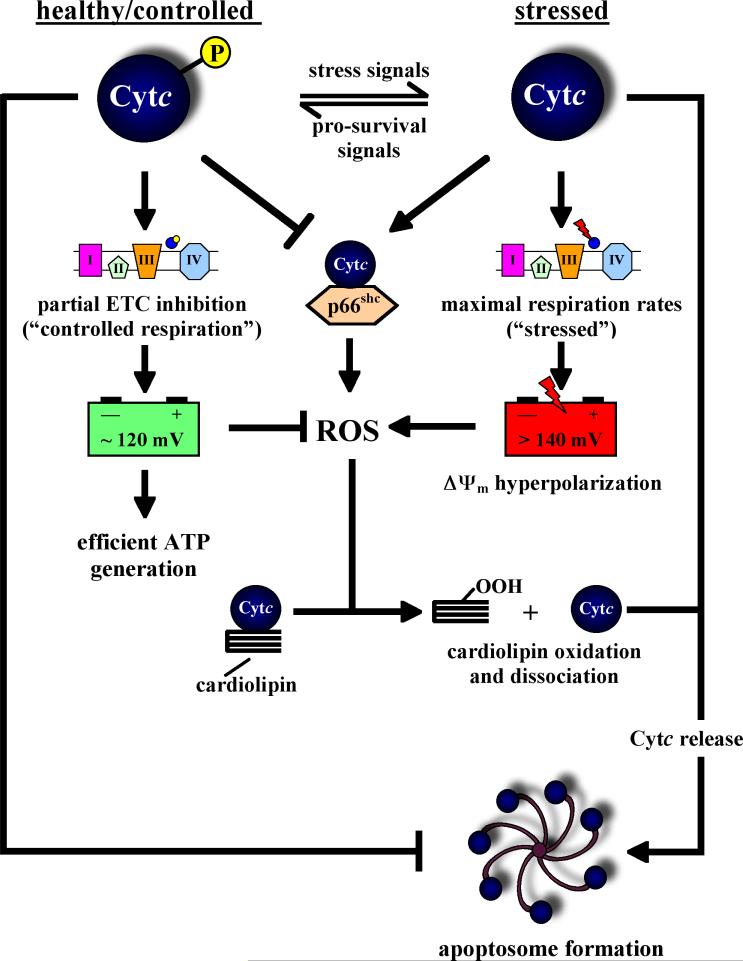
Both tyrosine phosphorylations lead to an inhibition in the reaction with isolated CcO. Tyr97-phosphorylated Cytc showed enhanced sigmoidal kinetics in the reaction with CcO, and half-maximal turnover was observed at a Cytc substrate concentration of 5.5 μM compared to 2.5 μM for unphosphorylated Cytc (Lee et al., 2006). For Tyr48-phosphorylated Cytc the kinetics were hyperbolic, similar to unphosphorylated Cytc. However, maximal turnover was more than 50% reduced for the phosphorylated form (Yu et al., 2008). These observations of partial inhibition of mitochondrial respiration as a consequence of Cytc phosphorylation fits our model that under healthy conditions mammalian OxPhos runs at reduced activity (‘controlled state,’ Fig. 4) to avoid high mitochondrial membrane potentials, which are known to lead to excessive ROS production, a hypothesis that we will revisit in section 9. The effects of tyrosine phosphorylation on the other functions of Cytc, as far as they are known, are discussed below.
Figure 4. Regulation of mitochondrial respiration, ROS production, and apoptosis through phosphorylation of cytohrome c.
Our model proposes changes in the phosphorylation state of Cytc during cellular stress as a switch for the execution of the cell death program: Under healthy unstressed conditions Cytc is phosphorylated leading to partial inhibition of mitochondrial respiration and healthy mitochondrial membrane potentials ΔΨm in the range of 80-140 mV. These membrane potentials are ideal and sufficient to drive efficient ATP generation, and importantly, no significant amounts of ROS are produced. In addition, under healthy conditions Cytc functions as a ROS scavenger (not shown). Under stressed conditions, including the presence of apoptotic stimuli and excessive calcium, a phosphatase dephosphorylates Cytc, which has multiple consequences. First, maximal ETC flux is now possible resulting in membrane potentials >140 mV. This hyperpolarization triggers excessive ROS production at complexes I and III due to backup of electrons in the ETC and increased half-lives of the semiquinole free radicals. Second, it is the unphosphorylated form of Cytc which catalyzes the oxidation of cardiolipin, a step which is required to dissociate Cytc from the inner mitochondrial membrane and thus to release it from the mitochondria during apoptosis. Cardiolipin oxidation requires ROS (e.g., peroxide equivalents), which are provided by ΔΨm hyperpolarization-mediated ROS production. Third, p66shc might also contribute to ROS production through the interaction with unphosphorylated Cytc. Finally, Cytc is released from the mitochondria, and it is the unphosphorylated form that is required for apoptosome formation, another safeguard mechanism for regulating this committing step as part of the death program. There is strong evidence that the other components of the OxPhos system are also regulated by phosphorylation, but Cytc appears to be the central player due to its dual active involvement in both respiration and apoptosis.
Two additional phosphorylation sites on Cytc have recently been reported. Mitochondria isolation from human skeletal muscle followed by high throughput phospho-proteomic mass-spectroscopy analysis revealed phosphorylation of Thr28 and Ser47 on Cytc (Figs. 2 and 3) (Zhao et al., 2010), suggesting that Cytc in this tissue is targeted for phosphorylation by yet another signaling pathway. The extent of those phosphorylations, i.e., the ratio of phosphorylated versus unphosphorylated Cytc, and their functional consequences remain unknown.
7. The Good, the Bad and the Ugly – reactive oxygen species and the role of cytochrome c as a radical scavenger and producer
The most common reactive oxygen species include superoxide (O2• –), hydrogen peroxide (H2O2), and hydroxyl radicals (•OH). The term reactive oxygen species (ROS) applies to any of the numerous oxygen containing molecules that have a strong propensity to fill their outer orbital shells by extracting an electron from an alternate source (e.g., the “free radical” •OH) or because – in the presence of transition metals – they are unstable molecules (e.g., H2O2, which is not a free radical). These molecules are highly reactive and if given the opportunity will damage DNA and other cellular structures. They also initiate oxidation cascades that regenerate radical species and perpetuate their damage.
ROS are generated in mitochondria as a direct result of the very nature of the electron transport chain. The electron transfer reactions that occur at the ETC complexes expose the redox intermediates to side reactions that can result in transfer of electrons directly to oxygen (Cadenas and Davies, 2000; Kadenbach et al., 2004b). The main ROS production sites are complex I, complex III, and the reduced ubiquinol pool. Complex I generates and releases ROS on the matrix site whereas complex III can release ROS into the matrix and the intermembrane space (Han et al., 2003; Han et al., 2001; Turrens et al., 1985). These side reactions cause cellular damage, and a buildup of ROS, if unregulated, will result in apoptosis (Aoki et al., 2002).
7.1. Cytochrome c as a ROS scavenger
Since ROS are unavoidable, cells have developed a battery of tools to neutralize these threats. These radical scavengers function as enzymes and are therefore restricted by their kinetic reaction rates. Superoxide dismutase (SOD) reduces superoxide molecules to hydrogen peroxide that is subsequently detoxified by catalase. However, ROS such as hydroxyl radicals are much too reactive to be effectively neutralized by an enzymatic pathway due to a half-life of only about one nanosecond. In contrast superoxide can be neutralized due to a half-life of about one millisecond.
It was shown that free Cytc also functions as a radical scavenger within the inner-membrane space by removing unpaired electrons from superoxide thus regenerating O2 (Korshunov et al., 1999; Pereverzev et al., 2003). The extracted electron can subsequently be used in energy production via transfer to CcO, thereby regenerating the oxidized form of Cytc. In addition, it was demonstrated that Cytc operates as a hydrogen peroxide scavenger (Wang et al., 2003c). In contrast to neutralization of superoxide, which requires oxidized Cytc, conversion of hydrogen peroxide will be effective in both reduced and oxidized states of Cytc. Since Cytc constantly undergoes oxidation/reduction cycles in a respiring cell, both detoxification reactions can take place. Therefore, coupling of ROS neutralization with the redox capabilities of Cytc makes the latter an ideal antioxidant for the cell.
7.2. Cytochrome c and p66shc
p66shc is a splice variant of the growth factor adapter Shc and found throughout the cell with a subset localizing to the mitochondria where it affects mitochondrial function (Nemoto et al., 2006). p66shc is regulated via reversible phosphorylation which involves the protein kinase Cβ pathway (Pinton et al., 2007). Downstream phosphorylation of Ser36 appears to be an essential regulator of p66shc function and phosphorylation of this site increases with age in several organs including lung, liver, and skin, leading to higher ROS production rates and accumulation of oxidative damage as was demonstrated in aged mice (Lebiedzinska et al., 2009). Under stress conditions, mitochondrial p66shc oxidizes, i.e., receives electrons from Cytc and subsequently produces hydrogen peroxide (Giorgio et al., 2005). This pathway represents a means of ROS production which can be regulated.
Cell lines null for p66shc have lower levels of ROS (Nemoto and Finkel, 2002) and decreased oxygen consumption rates compared to wild-type cells, and transfection with a p66shc expression vector restored normal levels of oxygen consumption (Nemoto et al., 2006). p66shc null cells also show a decreased response to oxidative damage after treatment with H2O2 or UV light (Migliaccio et al., 1999). Treatment with X-rays resulted in cell-proliferation arrest in both wild-type and null cell lines, suggesting that the p66shc protein functions specifically in the apoptotic response to oxidative damage (Migliaccio et al., 1999). p66shc null cells also have slower metabolic rates and switch to glycolytic ATP production, which is accompanied by decreased ROS generation (Nemoto et al., 2006). The decrease in oxidative stress, coupled with a disruption of the cellular response to oxidative stress, results in an almost 30% increase in lifespan of p66shc null mice (Migliaccio et al., 1999).
The increase in ROS production via p66shc as part of the apoptotic pathway would provide Cytc with peroxide equivalents necessary for the oxidation of cardiolipin, a process that is required for its release from the mitochondria as discussed below. Indeed, release of Cytc correlates with the H2O2 production activity of p66shc (Sun et al., 2010). This – along with inability of Cytc/cardiolipin complexes to accept electrons from complex III (Basova et al., 2007) – could be a significant switch utilized by the cell as an initiating event of apoptosis.
The possible role of Cytc phosphorylation as an independent regulator of the interplay between Cytc and p66shc is currently unknown. However, given its effect on downstream caspase activation (see section 8) one might speculate that Cytc phosphorylation provides an additional safeguard mechanism to regulate ROS production independent of Ser36 phosphorylation of p66shc (Fig. 4).
7.3. Testis-specific cytochrome c: an isoform with superior ROS scavenging properties
A unique isoform of Cytc was first identified in the testicular cells of rodents. This testis-specific isoform of Cytc (T-Cytc) is expressed in the germinal epithelial cells (Goldberg et al., 1977). Although the amino acid sequence of T-Cytc is 86% homologous to somatic Cytc (S-Cytc or simply Cytc; Fig. 3), its expression pattern and biological functions are distinct. S-Cytc is expressed in all cells including those of the testis, however its expression is gradually decreased in the testicular germ cells. As spermatogenesis proceeds, T-Cytc expression increases, becoming the predominant form in mature sperm (Goldberg et al., 1977; Hess et al., 1993; Liu et al., 2006). T-Cytc operates with a three-fold increased H2O2 reduction activity; however, at the same time its apoptotic activity is also significantly increased (about fourfold compared to S-Cytc) (Liu et al., 2006). The authors concluded that these effects help prevent radical damage of sperm and serve as a selective agent by initiating cellular death of dysfunctional or damaged sperm. These functions are of particular importance in testes where the integrity of sperm must be highly regulated in order to ensure efficient and complete DNA transmission.
Interestingly, a cell line derived from Cytc knockout mice is respiratory-competent due to induction and expression of the testes isoform (Vempati et al., 2007). Furthermore, the authors showed that T-Cytc was able to promote apoptosis through both the intrinsic and extrinsic pathway, suggesting that both death pathways converge at Cytc.
The testis isoform of Cytc was lost during primate evolution, and it is now a non-transcribed pseudogene in humans (Hüttemann et al., 2003; Zhang and Gerstein, 2003). Consequently, human Cytc has to exercise both testis- and non-testis-specific functions using the same basic molecule. It is therefore not surprising that Cytc underwent some evolutionary changes in the lineage leading to humans, which is also reflected in an altered Cytc-CcO binding epitope (Schmidt et al., 2005). Human Cytc shares more homology with mouse Cytc-S (91% identity) compared to Cytc-T (82% identity) (Fig. 3). Among the 20 sites that are variable in the alignment of the three sequences, in humans 11 of those correspond to the somatic and only 2 sites correspond to the testis isoform in mouse (Fig. 3).
8. Role of cytochrome c in apoptosis
8.1. Cytochrome c release is an essential step in the apoptotic cascade
The importance of Cytc during development and life is not restricted to its involvement in ATP production and as a radical scavenger, but extends to its essential role in apoptosis. The first report showing that Cytc plays a crucial role in the cell death pathway was published in 1996 using a cell free apoptotic system to which compounds can be added, such as Cytc and dATP, another factor required for induction of the program (Liu et al., 1996). Other early work suggested that molecular changes at the level of Cytc, but not its degradation, occurred during the course of apoptosis (Krippner et al., 1996). Since then thousands of studies have been published confirming and extending those initial findings. It is now generally accepted that a key step in the apoptotic cascade involves the release of Cytc into the cytosol where it binds with apoptotic protease-activating factor 1 (Apaf-1). Binding of Cytc results in an increased affinity of the complex for dATP whose binding is necessary for oligomerization and formation of the apoptosome (Wang, 2001). The apoptosome recruits multiple procaspase-9 molecules and promotes their cleavage to an active form, known as the initiators of apoptosis. Caspase-9 bound to the apoptosome acts as the cleavage factor of caspase-3, or what is considered the major enzyme in the committal to apoptosis (Green, 2000; Wang, 2001). Recently developed spectroscopic methods now allow the real-time measurements of Cytc distribution in the cell based on changes in its redox state, when it is released into the reducing cytosolic environment during apoptosis (Ripple et al., 2010).
Cell lines null for Cytc exhibit reduced caspase-3 activation when stimulated with apoptosis inducing agents (Li et al., 2000a), indicating that Cytc is essential for caspase activation. Therefore cells with Cytc deficiencies will not only have decreased metabolic rates, but also will be resistant to stress signals that would typically induce cell death.
Other recent studies have suggested that Cytc release initiates apoptosis by binding inositol trisphosphate (IP3) receptors, causing calcium release into the cytosol (Beresewicz et al., 2006) and subsequent calpain activation and apoptosis-inducing factor (AIF) release (Cao et al., 2007). Interestingly, it has long been known that isolated mitochondria can reversibly release and take up Cytc, while the latter restores their function (Jacobs and Sanadi, 1960). Cytc can be selectively released from the mitochondrial intermembrane space in a fashion that does not necessarily involve membrane rupture or mitochondrial permeability transition pore opening (Doran and Halestrap, 2000). Thus, Cytc release during apoptosis is not all-or-none; instead a certain threshold of released Cytc must be reached before a cell irreversibly commits to apoptosis (Clayton et al., 2005). The release of Cytc precedes permeability transition (Chalmers and Nicholls, 2003) and is accompanied by a sharp increase in ROS production that involves complex I (Kushnareva et al., 2002), thus elevated ROS could then serve as an initiator for permeability transition.
An interesting and medically important example for the reversibility of Cytc release to restore ETC activity was demonstrated in an animal model for sepsis. Sepsis is a systemic inflammatory condition and it can lead to multiple organ failure and death. In a mouse model for sepsis the authors found inhibition of oxidative phosphorylation at the level of Cytc and CcO in the heart (Piel et al., 2008; Piel et al., 2007). The intravenous injection of cow Cytc led to an uptake of Cytc into the cardiomyocytes and significantly improved mitochondrial function, and survival increased from 15% for the controls to about 50% in the septic mice that received the Cytc injections. At first sight these findings may seem puzzling, because Cytc not only has to be taken up by the mitochondria, but it also has to translocate across the cell membrane. Interestingly, it was recently shown that Cytc contains cell penetrating peptide (CPP) epitopes in the N- and C-terminal helices (Jones et al., 2010). Such CPPs can mediate the cellular uptake of proteins and may explain that intravenous injection of Cytc can restore ETC function in heart in vivo. However, yet another question needs attention: why does the treatment with exogenous Cytc, which will be taken up into the cytosol first, not amplify the apoptotic cascade but instead lead to increased survival of the septic mice? Although no definite answer can be given at this point one can speculate that the amino acid differences between cow Cytc, which was administered to the septic mice, cause ineffective binding to mouse Apaf-1, insufficient to trigger caspase activation. Small changes in Cytc are sufficient to render it incapable of triggering apoptosis. For example, nitration of Tyr74 still allows Cytc to bind to Apaf-1 but the complex is unable to activate downstream caspases (Garcia-Heredia et al. 2010). Alternatively, Cytc might become phosphorylated by a serum kinase before entering the cells.
8.2. Regulation of cytochrome c release and apoptosome formation via cytochrome c phosphorylation
Since the release of Cytc from the mitochondria and subsequent formation of the apoptosome is the key step that controls the fate of the cell, one would expect it to be tightly regulated, and we propose that this regulation involves phosphorylation of Cytc. We have recently shown that cow liver Cytc is phosphorylated on Tyr48 in vivo (Yu et al., 2008). Strikingly, mutant Cytc containing the negatively charged and thus phosphomimetic amino acid Glu48 instead of Tyr48 was completely incapable of inducing downstream caspase activation (Pecina et al., 2010), suggesting a switch for the regulation of apoptosis.
Critical protein-protein interactions involving Cytc and Apaf-1 occur in the cytosol, which may be affected by Cytc phosphorylation. This binding involves several surface residues of Cytc and results in Apaf-1 enveloping the molecule. A mutational analysis of Cytc has identified several amino acids that are required for apoptosome formation and Lys7 is one of them, without which binding affinity is greatly diminished (Yu et al., 2001). Lys7 is spatially located next to Tyr97 (Fig. 2), which was shown to be phosphorylated in vivo in cow heart (Lee et al., 2006). A phosphorylated Tyr97 might obstruct this binding via salt bridge formation with Lys7 and prevent formation of the apoptosome, thus providing a distinct means of regulating the apoptotic pathway in heart tissue where this phosphorylation occurs, a hypothesis that we will test in future work.
8.3. Cytochrome c-catalyzed cardiolipin oxidation precedes cytochrome c release
Normally, about 15-20% of Cytc is bound to cardiolipin, a mitochondria-specific inner membrane lipid (Schlame et al., 2000, Kagan et at., 2004). This fraction of hydrophobically membrane-bound Cytc cannot be removed by treatment with high ionic strength solutions (Belikova et al., 2006), and likely does not participate in electron transport due to a highly negative redox potential. During apoptosis, trans-membrane migration of cardiolipin facilitates its interactions with Cytc in the intermembrane space and the formation of Cytc/cardiolipin complexes (Kagan et al, 2005). The free soluble pool of Cytc, which is re-instated after cardiolipin peroxidation due to low affinity of Cytc for peroxidized cardiolipin (Iverson and Orrenius, 2004, Kagan et al., 2005).
Cytc is bound to cardiolipin via two sites termed the A-site and C-site (Rytomaa and Kinnunen, 1994) (Fig. 2). These two sites are distinct in that the A-site interaction is predominantly of a loose electrostatic nature that participates in the electron transfer and radical scavenging functions of Cytc. In contrast, the C-site utilizes hydrophobic interactions and hydrogen bonding for a tight conformation associated with the oxidation of cardiolipin, a Cytc-catalyzed reaction that occurs during the course of the apoptotic process as detailed below. Cardiolipin oxidation is required for and thus precedes the release of Cytc from the mitochondria (Kagan et al., 2009).
The binding of cardiolipin to the A-site of Cytc is reversible and easily displaced by ATP or by increasing ionic strength (Rytomaa and Kinnunen, 1994; Rytomaa et al., 1992). The C-site binding by Cytc is much more stable, and cardiolipin cannot be easily dissociated by nucleotides (Rytomaa et al., 1992). The spectral changes observed with C-site binding suggest the occurrence of conformational changes within the protein that affect the heme-environment of Cytc (Tuominen et al., 2002), whereas A-site binding resulted in no detectable changes in the absorption spectrum.
The C-site is formed by a hydrophobic cavity from the surface of Cytc to the embedded heme group (Dickerson et al., 1971). Cardiolipin interaction with the C-site results in a tightly bound configuration, in which one cardiolipin acyl chain is inserted into the hydrophobic groove while keeping the other chains embedded within the mitochondrial inner membrane (Tuominen et al., 2002). This interaction essentially tethers Cytc to the membrane and gives rise to the extended lipid anchorage hypothesis that is supported by mounting evidence of heme-iron spectral changes (Choi and Swanson, 1995; Spooner and Watts, 1991; Tuominen et al., 2002; Vincent et al., 1987; Vincent and Levin, 1988). An alternative hypothesis suggesting interactions of two fatty acyls of cardiolipin with Cytc has also been suggested (Sinibaldi et al., 2010). The binding results in both partial protein unfolding and conformational changes of the Met80-heme iron interaction, as well as spectral changes in the heme iron spin state (Tuominen et al., 2002). These changes could allow for H2O2 access to the heme environment of Cytc (Choi and Swanson, 1995; Tuominen et al., 2002) and may account for a peroxide-dependent initiation of Cytc-catalyzed cardiolipin peroxidation (Kagan et al., 2005).
Prior to the release of Cytc from the mitochondria during apoptosis, a redistribution of cardiolipin occurs (Garcia Fernandez et al., 2002). The majority of cardiolipin, almost 80%, is located in the inner mitochondrial membrane and constitutes about 20% of the membrane lipids found there. In apoptotic cells a membrane translocation occurs that results in almost 40% of the cardiolipin of the inner mitochondrial membrane relocating to the outer-membrane (Kagan et al., 2006). Here it participates in the formation of Cytc/cardiolipin peroxidase complexes and – after cardiolipin peroxidation – the formation of the mitochondrial permeability transition pore, which facilitates release of Cytc and other pro-apoptotic factors into the cytosol. In addition, there are changes in the molar ratio of cardiolipin distribution between the two monolayers of the inner-membrane (Kagan et al., 2006). This redistribution occurs prior to the release of pro-apoptotic factors and other indicators of apoptosis, but takes place after the generation and accumulation of ROS (Garcia Fernandez et al., 2002; Gonzalvez and Gottlieb, 2007; Kagan et al., 2005).
The oxidation activity that occurs during apoptosis is specific for cardiolipin and fully dependent upon Cytc (Kagan et al., 2005). The extent of cardiolipin oxidation and sensitivity to the apoptosis-inducing agent actinomycin D is directly proportional to the amount of Cytc present in the cell lines tested (Kagan et al., 2005). Once cardiolipin was oxidized, the affinity between cardiolipin and Cytc was greatly decreased (Kagan et al., 2005; Nakagawa, 2004) allowing for cardiolipin redistribution and likely association with Bcl-2 family proteins at the mitochondrial outer membrane. The decreased affinity between Cytc and peroxidized cardiolipin also provides a greater abundance of free Cytc within the intermembrane space. Free Cytc is needed for release into the cytosol followed by caspase activation. Cytc knockout cells showed no cardiolipin oxidation activity and lacked release of pro-apoptotic factors, such as Smac/Diablo, when treated with actinomycin D (Kagan et al., 2005). Interestingly, normal release of these factors can be achieved by addition of oxidized cardiolipin. Such treatment has also been shown to induce specific release of pro-apoptotic factors even in the absence of apoptotic stimuli (Kagan et al., 2005). These findings suggest that the oxidation of cardiolipin fully relies upon Cytc. Subsequently, permeabilization of the outer membrane increases, but passage through the membrane is restricted to apoptotic factors as opposed to non-specific damage of mitochondria.
The cardiolipin-Cytc interaction involved in the oxidation of cardiolipin occurs via the C-site. This hydrophobic binding pocket is bordered by Phe10 and Tyr97 (Dickerson et al., 1971). This also puts the binding in close proximity to the embedded heme group of Cytc which is believed to be involved in the oxidation process and necessary for the transfer of electrons (Dickerson et al., 1971; Kagan et al., 2005). Activation of peroxidases that function via a heme group, such as Cytc, typically requires the formation of a protein-bound radical; in the case of Cytc a tyrosine radical has been identified (Kagan et al., 2005; Svistunenko, 2005; Tyurina et al., 2006). Tyr97 is in close proximity to the cardiolipin binding site and can be covalently modified via phosphorylation, thus providing a possible means of regulating the Cytc-cardiolipin interaction and the formation of the peroxidase complex. In addition, recent evidence suggests that Tyr67, which is one of four conserved tyrosines present in Cytc and in closest proximity to the heme group is a likely candidate involved in the Cytc-driven oxidation of cardiolipin (Kapralov et al., submitted).
The peroxidase activity of Cytc is dependent on the physical interaction between Cytc and cardiolipin. Solubilized Cytc was shown to have weak peroxidase activity and a barely detectable electron paramagnetic resonance (EPR) signal for tyrosyl radical formation (Tyurina et al., 2006). When cardiolipin is bound to Cytc in the presence of H2O2, the heme group of Cytc reacts to form the highly reactive intermediates, compounds I and II (see equations 2 and 3 below). Compound I is a short-lived ferrylporphyrin radical formed by the H2O2 driven oxidation of the heme-iron center (Barr et al., 1996). The heme-iron is oxidized, with the reduction of H2O2 to water, from a ferric oxidation state to Fe(V), and compound II is formed via the abstraction of an electron from a tyrosine residue, resulting in reduction of Fe(V) to Fe(IV) and the formation of a tyrosyl radical (Dunford, 1987; Tyurina et al., 2006). In the presence of nonoxidizable cardiolipin this tyrosyl radical can dimerize with other tyrosyl radicals that have been formed (see equation 4 below). Alternatively, in the presence of oxidizable cardiolipin the tyrosyl radical catalyzes the oxidation of cardiolipin (Tyurina et al., 2006) resulting in decreased affinity between and dissociation of cardiolipin and Cytc (Nakagawa, 2004).
Proposed reaction intermediates for Cytc-catalyzed peroxidation of cardiolipin (CL) (Tyurina et al., 2006):
CL + Cytc-[Fe3+ + Tyr] → CL/Cytc-[Fe3+ + Tyr]
CL/Cytc-[Fe3+ + Tyr] + H2O2 → CL/Cytc-[Fe5+=O + Tyr] (Compound I) + H2O
CL/Cytc-[Fe5+=O + Tyr] → CL/Cytc-[Fe4+=O + Tyr•] (Compound II)
2x Compound II → Cytc-Tyr–Tyr-Cytc (Cytc dimers and oligomers)
CL/Cytc-[Fe4+=O + Tyr•]→ CL•/Cytc-[Fe4+=O + Tyr]
CL•/Cytc-[Fe4+=O + Tyr] + O2 →→ CL-OO• + Cytc-[Fe4+=O + Tyr]
The tyrosine radical formed in the activation of peroxidases like that of Cytc is readily detectable by EPR. Studies of Cytc associated with non-oxidizable 1,1‘,2,2‘-tetraoleoyl-cardiolipin (TOCL), and with readily oxidizable 1,1‘,2,2‘-teralinoleoyl- cardiolipin (TLCL), demonstrated that both resulted in the formation of tyrosine radicals when treated with H2O2 (Tyurina et al., 2006). In the absence of cardiolipin an EPR signal corresponding to a tyrosine radical of Cytc was barely detectable, emphasizing the Cytc peroxidation specificity for cardiolipin. The magnitude of the tyrosine radical EPR signal from the Cytc/TLCL complex was significantly less than that of Cytc/TOCL. This 30±7% decrease is thought to be due to quenching of the tyrosine radical by its involvement in the peroxidation of cardiolipin (Tyurina et al., 2006). Recently, by mutating each of the four Tyr residues in Cytc, Kapralov et al., (submitted) identified Tyr67 – the tyrosine residue closest to the heme moiety – as the major contributor to the peroxidase activity of Cytc-cardiolipin complex.
Since two out of the four conserved tyrosines in Cytc, Tyr48 and Tyr97, are phosphorylated in vivo, it is possible that they also exert an effect on its peroxidase activity. Although studies with in vivo phosphorylated Cytc remain to be performed, we have shown for one of the four tyrosine residues that the phosphomimetic substitution Tyr48Glu decreased Cytc-cardiolipin binding by about 30% compared to wild-type unphosphorylated Cytc, and that Cytc peroxidase activity of the Tyr48Glu mutant was cardiolipin-inducible only at high cardiolipin concentrations, unlike controls. It is possible that the more bulky phosphate group present in vivo may exert an even more pronounced effect on the peroxidase activity of Cytc. Since cardiolipin oxidation is required for Cytc release during apoptosis, the Cytc phosphorylation state may control this rather early step of the death program.
9. To the next level: a proposal for the regulation of mitochondrial energy and ROS production via phosphorylation of cytochrome c
The fact that Cytc is phosphorylated in vivo, and that four phosphorylation sites have already been mapped strongly suggests that the multiple functions of Cytc are tightly regulated and that this regulation is tissue-specific. For Tyr97- and Tyr48-phosphorylated Cytc as well as Tyr48Glu phosphomimetic mutant Cytc we have shown that the reaction with CcO is partially inhibited leading to ‘controlled respiration.’ We propose in what follows that this effect plays an essential role in the prevention of ROS under healthy conditions; in contrast, during cellular stress Cytc becomes dephosphorylated, ‘controlled respiration’ is lost, and Cytc can now also function in apoptosis (Fig. 4). Under these conditions of cellular stress, the mitochondrial membrane potential (ΔΨm) will be affected and needs to be taken into account in order to understand the consequences of changes in OxPhos activity. This review focuses on phosphorylation of Cytc but other OxPhos components may be affected in a similar fashion.
9.1. The mitochondrial membrane potential (ΔΨm) integrates key determinants of cellular fate and is regulated through phosphorylation of OxPhos components
For a better understanding of the role of ROS production in a physiological context the mitochondrial membrane potential (ΔΨm) has to be considered. Respiratory control, the traditional mechanism regulating the activity of the ETC complexes, is thought to operate via ΔΨm. At high ΔΨm further proton pumping is inhibited, whereas a decrease in ΔΨm through proton utilization by ATP synthase would in turn allow the ETC to pump protons. We recently proposed a model as a significant extension of the traditional concept, in which cell signaling pathways control the activity of the ETC complexes. This in turn controls ΔΨm, maintaining healthy, low ΔΨm levels between 80-140 mV (Hüttemann et al., 2008). Such regulation is crucial for higher organisms because ΔΨm is directly related to the production of ROS (Fig. 4): at ΔΨm>140 mV, ROS production increases exponentially, whereas mitochondria of resting cells with a low ΔΨm do not produce significant amounts of ROS (reviewed in Liu, 1999). Studies based on isolated mitochondria or reconstituted CcO vesicles showed high ΔΨm values of 180-220 mV (e.g., Cossarizza et al., 1996; Nicholls and Ferguson, 1992; Steverding and Kadenbach, 1991), but they may not represent physiological conditions because the phosphorylation state was not preserved during mitochondria isolation (discussed in Hüttemann et al., 2008). Other studies performed under more physiological conditions reported ΔΨm values from 80-140 mV in perfused rat hearts, intact cultured fibroblasts, neuroblastoma cells, lymphocytes, embryonic heart cells, and osteocarcoma cells (Backus et al., 1993; Brand and Felber, 1984; Porteous et al., 1998; Wan et al., 1993; Zhang et al., 2001). Thus, the maintenance of physiologically low ΔΨm values avoids excessive generation of ROS but provides the full capability to produce ATP because maximal rates of ATP synthesis by ATP synthase occur at physiological ΔΨm values of 100-120 mV (Kaim and Dimroth, 1999).
Under unstressed conditions the OxPhos system does not work at full capacity. However, since excess capacity is available it has to be good for something. Given the consequences of high ΔΨm values, i.e., excessive ROS production, one can conclude that a hyperpolarization of ΔΨm is a trigger for cell death. Likely, excessive calcium release is involved in stress signaling to the mitochondria. Calcium induces dephosphorylation of most mitochondrial proteins (Hopper et al., 2006) and is believed to be the most important signal for mitochondrial activation (Robb-Gaspers et al., 1998). According to our model, cellular stress results in excessive calcium release leading to dephosphorylation of Cytc allowing maximal electron transfer rates, followed by increased ΔΨm levels at which ROS production occurs, which triggers and amplifies apoptosis (Fig. 4). This idea is consistent with various recent reports showing that ΔΨm increases after induction of apoptosis, initiated by, e.g., calcium, Bax, ROS, and ceramides (reviewed in Kadenbach et al., 2004a). Our findings that phosphorylation of Cytc (and CcO) lead to controlled respiration provide a unifying mechanism for maintaining optimal mitochondrial membrane potentials under healthy conditions, avoiding ROS (Lee et al., 2005; Lee et al., 2009; Lee et al., 2006; Samavati et al., 2008; Yu et al., 2008, Pecina et al., 2010). In contrast, during apoptotic stress, Cytc dephosphorylation will contribute to the cell death decision in multiple ways: 1) increased respiration rates, followed by increased ΔΨm and ROS levels, 2) increased cardiolipin oxidation rates that allow Cytc to dissociate from the inner mitochondrial membrane and release into the cytosol, and 3) the dephosphorylated form of Cytc is required to form a functional apoptosome for downstream caspase activation (Fig. 4).
9.2. Neurodegenerative and cardiovascular diseases as possible examples of dysregulated Cytc function
Multiple neurodegenerative diseases are characterized by loss of specific neuron populations due to induction of apoptosis. Mitochondrial dysfunction, that leads to release of Cytc into the cytoplasm and apoptosis, has been demonstrated in acute neurologic trauma such as stroke (Fujimura et al., 1998; Rabuffetti et al., 2000; Sugawara et al., 1999) and traumatic brain and spinal cord injury (Li et al., 2000b) as well as chronic diseases such as Amyotrophic Lateral Sclerosis (ALS; Li et al., 2000c; Rowland and Shneider, 2001), Huntington's Disease (Martin, 1999; Wang et al., 2003b; Wang et al., 2008), and Parkinson Disease (Wu et al., 2002). If release of Cytc could be inhibited, apoptosis could be prevented, slowing the disease progression or limiting neurologic damage after trauma. Reperfusion of brain tissue following ischemia initiates a cell death cascade exhibiting hallmarks of our proposed Cytc-centered mechanism including altered mitochondrial Ca2+ homeostasis (Kobayashi et al., 2003; Starkov et al., 2004), Ca2+ dependent dephosphorylation of mitochondrial proteins (Schuh et al., 2005), excessive ROS generation (Wang et al., 2005; Yamato et al., 2003), and cardiolipin peroxidation and redistribution (Kowalczyk et al., 2009), all culminating in release of Cytc from mitochondria and induction of apoptotic cell death.
Multiple studies have demonstrated that therapeutic intervention at the level of Cytc release is an effective neuroprotective strategy (Endo et al., 2006; Sanderson et al., 2008), and that Cytc release is required for apoptosis to occur (Matapurkar and Lazebnik, 2006). For example, administration of insulin, a hormone we have shown to induce Cytc phosphorylation in ischemic brain (unpublished data), prevents Cytc release and neuronal death following brain ischemia (Sanderson et al., 2008). These data suggest a possible mechanism of neuroprotection where Cytc release can be prevented and apoptosis avoided by using substances that regulate the phosphorylation state of Cytc. Interestingly, another robust neuroprotective therapy, preconditioning, requires p66shc activation and translocation to the mitochondria to confer neuroprotection (Brown et al., 2010), suggesting that some ROS are required as a preconditioning signal. Similar patterns of injury progression have been demonstrated following traumatic injury to the brain (Sullivan et al., 2002) and spinal cord (Xu et al., 2005), demonstrating a central role of Cytc release in acute neurologic trauma. Familial ALS, a chronic neurodegenerative disease, is characterized by the presence of high levels of ROS due to mutations in the radical scavenger superoxide dismutase (SOD1) (Rosen et al., 1993). Increased ROS levels damage cells, eventually resulting in loss of specific motor neuron populations through apoptosis. Radical scavengers have been proven beneficial in ALS transgenic mice models (Gurney et al., 1996) as well as over-expression of antiapoptotic Bcl-2 protein (Kostic et al., 1997). Minocycline, a second generation tetracycline, confers neuroprotection in several models of neurodegeneration including delay of ALS progression in mice (Zhu et al., 2002). More recently methazolamide has been tested for efficacy in Huntington's disease. In transgenic mice it delayed the onset of disease and mortality (Wang et al., 2008). The authors concluded that the mutual neuroprotective effect may be linked to their shared inhibition of Cytc release from mitochondria. Promising results like these demonstrate the need for determining the specific function and signaling events that regulate Cytc.
Cardiomyopathy is a major contributor to congestive heart failure and is propagated by apoptosis of cardiomyocytes. Immunogold labeling and immunoblotting of cardiomyopic hearts with anti-Cytc and anti-caspase 3 antibodies revealed significant release of Cytc to the cytoplasm and activation of caspase 3 when compared to normal heart tissue (Narula et al., 1999). Cardiac ischemia/reperfusion injury follows a similar pathway with sudden reperfusion causing excessive ROS production that leads to Cytc release and ultimately apoptosis. Cytc release in cardiomyocytes has been attributed to many mechanisms including ROS generation, cardiolipin peroxidation, and Ca2+ overload in the mitochondria. Indeed, compounds that prevent cardiolipin peroxidation like melatonin protect mitochondria exposed to Ca2+ overload (Petrosillo et al., 2009b) and limit reperfusion injury in heart (Petrosillo et al., 2009a). Interestingly, hearts from p66shc knockout mice show decreased ROS generation during reperfusion and improved tissue protection (Carpi et al., 2009). These studies suggest a role for p66shc induced ROS production and cardiolipin peroxidation in release of Cytc from the mitochondria and apoptosis induction. Further studies, in particular the elucidation of the signaling pathways leading to reversible phosphorylation of Cytc, are necessary to determine the contribution of Cytc phosphorylation in the regulation of cell death. This knowledge may allow the development of therapies that target Cytc directly or through cell signaling to prevent stress stimuli from damaging otherwise functional cell populations. Furthermore, conditions in which increased apoptotic activity would be beneficial could be targeted, such as cancer. A general problem of cancer control are adaptive mechanisms that allow cancer cells to evade the apoptotic pathway, and Cytc (hyper-) phosphorylation or the inability to dephosphorylate Cytc may be such an underlying mechanism.
10. Conclusion
The involvement of Cytc in several processes crucial for cellular life and death, including electron transfer, redox-coupled protein import, cardiolipin oxidation, radical scavenging, and apoptosome formation (Fig. 1), make it a likely target of regulation by post-translational modifications. To date, of the four phosphorylation sites mapped on Cytc there is convincing evidence for the regulatory importance of two tyrosine phosphorylations identified in mammalian heart and liver. The potential role of these phosphorylations to control respiration rates and thus ΔΨm and the production of ROS, in addition to direct regulation of cell-death associated processes such as cardiolipin oxidation and apoptosome formation open up the exciting possibility for future manipulation of Cytc phosphorylation in pathologic conditions where decreased or, in contrast, increased apoptotic activity would be beneficial, such as neurodegenerative diseases and cancer, respectively.
ACKNOWLEDGEMENTS
This work was supported by grants from the National Institutes of Health (GM089900, MH; HL70755, HL094488, U19A1068021, and OH008282, VEK), the Center for Molecular Medicine and Genetics, and the Cardiovascular Research Institute, Wayne State University School of Medicine, Detroit.
Abbreviations
- Apaf-1
apoptotic protease-activating factor 1
- CcO
cytochrome c oxidase
- Cytc
cytochrome c
- ΔΨm
mitochondrial membrane potential
- EPR
electron paramagnetic resonance
- ETC
electron transport chain
- OxPhos
oxidative phoshorylation
- ROS
reactive oxygen species
- TIM
translocase of the inner membrane
- TOM
translocase of the outer membrane
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Acin-Perez R, Bayona-Bafaluy MP, Bueno M, Machicado C, Fernandez-Silva P, Perez-Martos A, Montoya J, Lopez-Perez MJ, Sancho J, Enriquez JA. An intragenic suppressor in the cytochrome c oxidase I gene of mouse mitochondrial DNA. Hum Mol Genet. 2003;12:329–339. doi: 10.1093/hmg/ddg021. [DOI] [PubMed] [Google Scholar]
- Allen S, Balabanidou V, Sideris DP, Lisowsky T, Tokatlidis K. Erv1 mediates the Mia40-dependent protein import pathway and provides a functional link to the respiratory chain by shuttling electrons to cytochrome c. J Mol Biol. 2005;353:937–944. doi: 10.1016/j.jmb.2005.08.049. [DOI] [PubMed] [Google Scholar]
- Aoki H, Kang PM, Hampe J, Yoshimura K, Noma T, Matsuzaki M, Izumo S. Direct activation of mitochondrial apoptosis machinery by c-Jun N-terminal kinase in adult cardiac myocytes. J Biol Chem. 2002;277:10244–10250. doi: 10.1074/jbc.M112355200. [DOI] [PubMed] [Google Scholar]
- Arnold S, Kadenbach B. The intramitochondrial ATP/ADP-ratio controls cytochrome c oxidase activity allosterically. FEBS Lett. 1999;443:105–108. doi: 10.1016/s0014-5793(98)01694-9. [DOI] [PubMed] [Google Scholar]
- Backus M, Piwnica-Worms D, Hockett D, Kronauge J, Lieberman M, Ingram P, LeFurgey A. Microprobe analysis of Tc-MIBI in heart cells: calculation of mitochondrial membrane potential. Am J Physiol. 1993;265:C178–187. doi: 10.1152/ajpcell.1993.265.1.C178. [DOI] [PubMed] [Google Scholar]
- Barr DP, Gunther MR, Deterding LJ, Tomer KB, Mason RP. ESR spin-trapping of a protein-derived tyrosyl radical from the reaction of cytochrome c with hydrogen peroxide. J Biol Chem. 1996;271:15498–15503. doi: 10.1074/jbc.271.26.15498. [DOI] [PubMed] [Google Scholar]
- Basova LV, Kurnikov IV, Wang L, Ritov VB, Belikova NA, Vlasova II, Pacheco AA, Winnica DE, Peterson J, Bayir H, Waldeck DH, Kagan VE. Cardiolipin switch in mitochondria: shutting off the reduction of cytochrome c and turning on the peroxidase activity. Biochemistry. 2007;46:3423–3434. doi: 10.1021/bi061854k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belikova NA, Vladimirov YA, Osipov AN, Kapralov AA, Tyurin VA, Potapovich MV, Basova LV, Peterson J, Kurnikov IV, Kagan VE. Peroxidase activity and structural transitions of cytochrome c bound to cardiolipin-containing membranes. Biochemistry. 2006;45:4998–5009. doi: 10.1021/bi0525573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beresewicz M, Kowalczyk JE, Zablocka B. Cytochrome c binds to inositol (1,4,5) trisphosphate and ryanodine receptors in vivo after transient brain ischemia in gerbils. Neurochem Int. 2006;48:568–571. doi: 10.1016/j.neuint.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Brand MD, Felber SM. Membrane potential of mitochondria in intact lymphocytes during early mitogenic stimulation. Biochem J. 1984;217:453–459. doi: 10.1042/bj2170453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JE, Zeiger SL, Hettinger JC, Brooks JD, Holt B, Morrow JD, Musiek ES, Milne G, McLaughlin B. Essential role of the redox-sensitive kinase p66shc in determining energetic and oxidative status and cell fate in neuronal preconditioning. J Neurosci. 2010;30:5242–5252. doi: 10.1523/JNEUROSCI.6366-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell GW, Louie GV, Brayer GD. High-resolution three-dimensional structure of horse heart cytochrome c. J Mol Biol. 1990;214:585–595. doi: 10.1016/0022-2836(90)90200-6. [DOI] [PubMed] [Google Scholar]
- Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- Cao G, Xing J, Xiao X, Liou AK, Gao Y, Yin XM, Clark RS, Graham SH, Chen J. Critical role of calpain I in mitochondrial release of apoptosis-inducing factor in ischemic neuronal injury. J Neurosci. 2007;27:9278–9293. doi: 10.1523/JNEUROSCI.2826-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpi A, Menabo R, Kaludercic N, Pelicci P, Di Lisa F, Giorgio M. The cardioprotective effects elicited by p66(Shc) ablation demonstrate the crucial role of mitochondrial ROS formation in ischemia/reperfusion injury. Biochim Biophys Acta. 2009;1787:774–780. doi: 10.1016/j.bbabio.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Chacinska A, Pfannschmidt S, Wiedemann N, Kozjak V, Sanjuan Szklarz LK, Schulze-Specking A, Truscott KN, Guiard B, Meisinger C, Pfanner N. Essential role of Mia40 in import and assembly of mitochondrial intermembrane space proteins. Embo J. 2004;23:3735–3746. doi: 10.1038/sj.emboj.7600389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers S, Nicholls DG. The relationship between free and total calcium concentrations in the matrix of liver and brain mitochondria. J Biol Chem. 2003;278:19062–19070. doi: 10.1074/jbc.M212661200. [DOI] [PubMed] [Google Scholar]
- Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125:1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Choi S, Swanson JM. Interaction of cytochrome c with cardiolipin: an infrared spectroscopic study. Biophys Chem. 1995;54:271–278. doi: 10.1016/0301-4622(94)00151-9. [DOI] [PubMed] [Google Scholar]
- Clayton R, Clark JB, Sharpe M. Cytochrome c release from rat brain mitochondria is proportional to the mitochondrial functional deficit: implications for apoptosis and neurodegenerative disease. J Neurochem. 2005;92:840–849. doi: 10.1111/j.1471-4159.2004.02918.x. [DOI] [PubMed] [Google Scholar]
- Cossarizza A, Ceccarelli D, Masini A. Functional heterogeneity of an isolated mitochondrial population revealed by cytofluorometric analysis at the single organelle level. Exp Cell Res. 1996;222:84–94. doi: 10.1006/excr.1996.0011. [DOI] [PubMed] [Google Scholar]
- Craig DB, Wallace CJ. Studies of 8-azido-ATP adducts reveal two mechanisms by which ATP binding to cytochrome c could inhibit respiration. Biochemistry. 1995;34:2686–2693. doi: 10.1021/bi00008a036. [DOI] [PubMed] [Google Scholar]
- Dalmonte ME, Forte E, Genova ML, Giuffre A, Sarti P, Lenaz G. Control of respiration by cytochrome c oxidase in intact cells: role of the membrane potential. J Biol Chem. 2009;284:32331–32335. doi: 10.1074/jbc.M109.050146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson R, Timkovich R. Cytochrome c. In: Boyer P, editor. The Enzymes. 3rd ed. Academic Press; New York: 1975. pp. 397–472. [Google Scholar]
- Dickerson RE, Kopka ML, Borders CL, Jr., Varnum J, Weinzier JE. A centrosymmetric projection at 4A of horse heart oxidized cytochrome c. J Mol Biol. 1967;29:77–95. doi: 10.1016/0022-2836(67)90182-9. [DOI] [PubMed] [Google Scholar]
- Dickerson RE, Takano T, Eisenberg D, Kallai OB, Samson L, Cooper A, Margoliash E. Ferricytochrome c. I. General features of the horse and bonito proteins at 2.8 A resolution. J Biol Chem. 1971;246:1511–1535. [PubMed] [Google Scholar]
- Diekert K, de Kroon AI, Ahting U, Niggemeyer B, Neupert W, de Kruijff B, Lill R. Apocytochrome c requires the TOM complex for translocation across the mitochondrial outer membrane. Embo J. 2001;20:5626–5635. doi: 10.1093/emboj/20.20.5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran E, Halestrap AP. Cytochrome c release from isolated rat liver mitochondria can occur independently of outer-membrane rupture: possible role of contact sites. Biochem J. 2000;348(Pt 2):343–350. [PMC free article] [PubMed] [Google Scholar]
- Dumont ME, Cardillo TS, Hayes MK, Sherman F. Role of cytochrome c heme lyase in mitochondrial import and accumulation of cytochrome c in Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:5487–5496. doi: 10.1128/mcb.11.11.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunford HB. Free radicals in iron-containing systems. Free Radic Biol Med. 1987;3:405–421. doi: 10.1016/0891-5849(87)90019-0. [DOI] [PubMed] [Google Scholar]
- Duvigneau JC, Piskernik C, Haindl S, Kloesch B, Hartl RT, Hüttemann M, Lee I, Ebel T, Moldzio R, Gemeiner M, Redl H, Kozlov AV. A novel endotoxin-induced pathway: upregulation of heme oxygenase 1, accumulation of free iron, and free iron-mediated mitochondrial dysfunction. Lab Invest. 2008;88:70–77. doi: 10.1038/labinvest.3700691. [DOI] [PubMed] [Google Scholar]
- Endo H, Saito A, Chan PH. Mitochondrial translocation of p53 underlies the selective death of hippocampal CA1 neurons after global cerebral ischaemia. Biochem Soc Trans. 2006;34:1283–1286. doi: 10.1042/BST0341283. [DOI] [PubMed] [Google Scholar]
- Ferguson-Miller S, Brautigan DL, Margoliash E. Correlation of the kinetics of electron transfer activity of various eukaryotic cytochromes c with binding to mitochondrial cytochrome c oxidase. J Biol Chem. 1976;251:1104–1115. [PubMed] [Google Scholar]
- Fujimura M, Morita-Fujimura Y, Murakami K, Kawase M, Chan PH. Cytosolic redistribution of cytochrome c after transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 1998;18:1239–1247. doi: 10.1097/00004647-199811000-00010. [DOI] [PubMed] [Google Scholar]
- Garcia Fernandez M, Troiano L, Moretti L, Nasi M, Pinti M, Salvioli S, Dobrucki J, Cossarizza A. Early changes in intramitochondrial cardiolipin distribution during apoptosis. Cell Growth Differ. 2002;13:449–455. [PubMed] [Google Scholar]
- Garcia-Heredia JM, Diaz-Moreno I, Nieto PM, Orzaez M, Kocanis S, Teixeira M, Perez-Paya E, Diaz-Quintana A, De la Rosa MA. Nitration of tyrosine 74 prevents human cytochrome c to play a key role in apoptosis signaling by blocking caspase-9 activation. Biochim Biophys Acta. 1797:981–993. doi: 10.1016/j.bbabio.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, Pelliccia G, Luzi L, Minucci S, Marcaccio M, Pinton P, Rizzuto R, Bernardi P, Paolucci F, Pelicci PG. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221–233. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Goldberg E, Sberna D, Wheat TE, Urbanski GJ, Margoliash E. Cytochrome c: immunofluorescent localization of the testis-specific form. Science. 1977;196:1010–1012. doi: 10.1126/science.193188. [DOI] [PubMed] [Google Scholar]
- Gonzalvez F, Gottlieb E. Cardiolipin: setting the beat of apoptosis. Apoptosis. 2007;12:877–885. doi: 10.1007/s10495-007-0718-8. [DOI] [PubMed] [Google Scholar]
- Green DR. Apoptotic pathways: paper wraps stone blunts scissors. Cell. 2000;102:1–4. doi: 10.1016/s0092-8674(00)00003-9. [DOI] [PubMed] [Google Scholar]
- Gurney ME, Cutting FB, Zhai P, Doble A, Taylor CP, Andrus PK, Hall ED. Benefit of vitamin E, riluzole, and gabapentin in a transgenic model of familial amyotrophic lateral sclerosis. Ann Neurol. 1996;39:147–157. doi: 10.1002/ana.410390203. [DOI] [PubMed] [Google Scholar]
- Han D, Canali R, Rettori D, Kaplowitz N. Effect of glutathione depletion on sites and topology of superoxide and hydrogen peroxide production in mitochondria. Mol Pharmacol. 2003;64:1136–1144. doi: 10.1124/mol.64.5.1136. [DOI] [PubMed] [Google Scholar]
- Han D, Williams E, Cadenas E. Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space. Biochem J. 2001;353:411–416. doi: 10.1042/0264-6021:3530411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess RA, Miller LA, Kirby JD, Margoliash E, Goldberg E. Immunoelectron microscopic localization of testicular and somatic cytochromes c in the seminiferous epithelium of the rat. Biol Reprod. 1993;48:1299–1308. doi: 10.1095/biolreprod48.6.1299. [DOI] [PubMed] [Google Scholar]
- Hinkle PC, Kumar MA, Resetar A, Harris DL. Mechanistic stoichiometry of mitochondrial oxidative phosphorylation. Biochemistry. 1991;30:3576–3582. doi: 10.1021/bi00228a031. [DOI] [PubMed] [Google Scholar]
- Hopper RK, Carroll S, Aponte AM, Johnson DT, French S, Shen RF, Witzmann FA, Harris RA, Balaban RS. Mitochondrial matrix phosphoproteome: effect of extra mitochondrial calcium. Biochemistry. 2006;45:2524–2536. doi: 10.1021/bi052475e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüttemann M, Jaradat S, Grossman LI. Cytochrome c oxidase of mammals contains a testes-specific isoform of subunit VIb--the counterpart to testes-specific cytochrome c? Mol Reprod Dev. 2003;66:8–16. doi: 10.1002/mrd.10327. [DOI] [PubMed] [Google Scholar]
- Hüttemann M, Lee I, Pecinova A, Pecina P, Przyklenk K, Doan JW. Regulation of oxidative phosphorylation, the mitochondrial membrane potential, and their role in human disease. J Bioenerg Biomembr. 2008;40:445–456. doi: 10.1007/s10863-008-9169-3. [DOI] [PubMed] [Google Scholar]
- Hüttemann M, Lee I, Samavati L, Yu H, Doan JW. Regulation of mitochondrial oxidative phosphorylation through cell signaling. Biochim Biophys Acta. 2007;1773:1701–1720. doi: 10.1016/j.bbamcr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Iverson SL, Orrenius S. The cardiolipin-cytochrome c interaction and the mitochondrial regulation of apoptosis. Arch Biochem Biophys. 2004;423:37–46. doi: 10.1016/j.abb.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Jacobs EE, Sanadi DR. The reversible removal of cytochrome c from mitochondria. J Biol Chem. 1960;235:531–534. [PubMed] [Google Scholar]
- Jezek P, Hlavata L. Mitochondria in homeostasis of reactive oxygen species in cell, tissues, and organism. Int J Biochem Cell Biol. 2005;37:2478–2503. doi: 10.1016/j.biocel.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Jones S, Holm T, Mager I, Langel U, Howl J. Characterization of bioactive cell penetrating peptides from human cytochrome c: protein mimicry and the development of a novel apoptogenic agent. Chem Biol. 2010;17:735–744. doi: 10.1016/j.chembiol.2010.05.018. [DOI] [PubMed] [Google Scholar]
- Kadenbach B, Arnold S, Lee I, Hüttemann M. The possible role of cytochrome c oxidase in stress-induced apoptosis and degenerative diseases. Biochim Biophys Acta. 2004a;1655:400–408. doi: 10.1016/j.bbabio.2003.06.005. [DOI] [PubMed] [Google Scholar]
- Kadenbach B, Arnold S, Lee I, Hüttemann M. The possible role of cytochrome c oxidase in stress-induced apoptosis and degenerative diseases. Biochim Biophys Acta. 2004b;1655:400–408. doi: 10.1016/j.bbabio.2003.06.005. [DOI] [PubMed] [Google Scholar]
- Kagan VE, Bayir HA, Belikova NA, Kapralov O, Tyurina YY, Tyurin VA, Jiang J, Stoyanovsky DA, Wipf P, Kochanek PM, Greenberger JS, Pitt B, Shvedova AA, Borisenko G. Cytochrome c/cardiolipin relations in mitochondria: a kiss of death. Free Radic Biol Med. 2009;46:1439–1453. doi: 10.1016/j.freeradbiomed.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan VE, Borisenko GG, Tyurina YY, Tyurin VA, Jiang J, Potapovich AI, Kini V, Amoscato AA, Fujii Y. Oxidative lipidomics of apoptosis: redox catalytic interactions of cytochrome c with cardiolipin and phosphatidylserine. Free Radic Biol Med. 2004;37:1963–1985. doi: 10.1016/j.freeradbiomed.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritow VB, Amoscato AA, Osipov AN, Belikova NA, Kapralov AA, Kini V, Vlasova II, Zhao Q, Zou M, Di P, Svistunenko DA, Kurnikov IV, Borisenko GG. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nature Chem Biol. 2005;1:223–232. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- Kagan VE, Tyurina YY, Bayir H, Chu CT, Kapralov AA, Vlasova II, Belikova NA, Tyurin VA, Amoscato A, Epperly M, Greenberger J, Dekosky S, Shvedova AA, Jiang J. The “pro-apoptotic genies” get out of mitochondria: oxidative lipidomics and redox activity of cytochrome c/cardiolipin complexes. Chem Biol Interact. 2006;163:15–28. doi: 10.1016/j.cbi.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Kaim G, Dimroth P. ATP synthesis by F-type ATP synthase is obligatorily dependent on the transmembrane voltage. Embo J. 1999;18:4118–4127. doi: 10.1093/emboj/18.15.4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapralov AA, Yanamala N, Tyurina YY, Castro L, Samhan-Arias AK, Vladimirov YA, Maeda A, Mylnikov D, Demicheli V, Tortora V, Klein-Seetharaman J, Radi R, Kagan VE. Topography of tyrosine residues and their involvement in peroxidase activity of cytochrome c/cardiolipin complex. submitted. [DOI] [PMC free article] [PubMed]
- Kobayashi T, Kuroda S, Tada M, Houkin K, Iwasaki Y, Abe H. Calcium-induced mitochondrial swelling and cytochrome c release in the brain: its biochemical characteristics and implication in ischemic neuronal injury. Brain Res. 2003;960:62–70. doi: 10.1016/s0006-8993(02)03767-8. [DOI] [PubMed] [Google Scholar]
- Korshunov SS, Krasnikov BF, Pereverzev MO, Skulachev VP. The antioxidant functions of cytochrome c. FEBS Lett. 1999;462:192–198. doi: 10.1016/s0014-5793(99)01525-2. [DOI] [PubMed] [Google Scholar]
- Kostic V, Jackson-Lewis V, de Bilbao F, Dubois-Dauphin M, Przedborski S. Bcl-2: prolonging life in a transgenic mouse model of familial amyotrophic lateral sclerosis. Science. 1997;277:559–562. doi: 10.1126/science.277.5325.559. [DOI] [PubMed] [Google Scholar]
- Kowalczyk JE, Beresewicz M, Gajkowska B, Zablocka B. Association of protein kinase C delta and phospholipid scramblase 3 in hippocampal mitochondria correlates with neuronal vulnerability to brain ischemia. Neurochem Int. 2009;55:157–163. doi: 10.1016/j.neuint.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Krippner A, Matsuno-Yagi A, Gottlieb RA, Babior BM. Loss of function of cytochrome c in Jurkat cells undergoing fas-mediated apoptosis. J Biol Chem. 1996;271:21629–21636. doi: 10.1074/jbc.271.35.21629. [DOI] [PubMed] [Google Scholar]
- Kushnareva Y, Murphy AN, Andreyev A. Complex I-mediated reactive oxygen species generation: modulation by cytochrome c and NAD(P)+ oxidation-reduction state. Biochem J. 2002;368:545–553. doi: 10.1042/BJ20021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange C, Hunte C. Crystal structure of the yeast cytochrome bc1 complex with its bound substrate cytochrome c. Proc Natl Acad Sci U S A. 2002;99:2800–2805. doi: 10.1073/pnas.052704699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebiedzinska M, Duszynski J, Rizzuto R, Pinton P, Wieckowski MR. Age-related changes in levels of p66Shc and serine 36-phosphorylated p66Shc in organs and mouse tissues. Arch Biochem Biophys. 2009;486:73–80. doi: 10.1016/j.abb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Lee AK, Tse A. Dominant role of mitochondria in calcium homeostasis of single rat pituitary corticotropes. Endocrinology. 2005;146:4985–4993. doi: 10.1210/en.2005-0358. [DOI] [PubMed] [Google Scholar]
- Lee I, Salomon AR, Ficarro S, Mathes I, Lottspeich F, Grossman LI, Hüttemann M. cAMP-dependent tyrosine phosphorylation of subunit I inhibits cytochrome c oxidase activity. J Biol Chem. 2005;280:6094–6100. doi: 10.1074/jbc.M411335200. [DOI] [PubMed] [Google Scholar]
- Lee I, Salomon AR, Samavati L, Pecina P, Pecinova A, Hüttemann M. Isolation of regulatory-competent, phosphorylated cytochrome c oxidase. Methods Enzymol. 2009;345 doi: 10.1016/S0076-6879(09)05011-3. in press. [DOI] [PubMed] [Google Scholar]
- Lee I, Salomon AR, Yu K, Doan JW, Grossman LI, Hüttemann M. New prospects for an old enzyme: mammalian cytochrome c is tyrosine-phosphorylated in vivo. Biochemistry. 2006;45:9121–9128. doi: 10.1021/bi060585v. [DOI] [PubMed] [Google Scholar]
- Li K, Li Y, Shelton JM, Richardson JA, Spencer E, Chen ZJ, Wang X, Williams RS. Cytochrome c deficiency causes embryonic lethality and attenuates stress-induced apoptosis. Cell. 2000a;101:389–399. doi: 10.1016/s0092-8674(00)80849-1. [DOI] [PubMed] [Google Scholar]
- Li M, Ona VO, Chen M, Kaul M, Tenneti L, Zhang X, Stieg PE, Lipton SA, Friedlander RM. Functional role and therapeutic implications of neuronal caspase-1 and -3 in a mouse model of traumatic spinal cord injury. Neuroscience. 2000b;99:333–342. doi: 10.1016/s0306-4522(00)00173-1. [DOI] [PubMed] [Google Scholar]
- Li M, Ona VO, Guegan C, Chen M, Jackson-Lewis V, Andrews LJ, Olszewski AJ, Stieg PE, Lee JP, Przedborski S, Friedlander RM. Functional role of caspase-1 and caspase-3 in an ALS transgenic mouse model. Science. 2000c;288:335–339. doi: 10.1126/science.288.5464.335. [DOI] [PubMed] [Google Scholar]
- Liu SS. Cooperation of a “reactive oxygen cycle” with the Q cycle and the proton cycle in the respiratory chain--superoxide generating and cycling mechanisms in mitochondria. J Bioenerg Biomembr. 1999;31:367–376. doi: 10.1023/a:1018650103259. [DOI] [PubMed] [Google Scholar]
- Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- Liu Z, Lin H, Ye S, Liu QY, Meng Z, Zhang CM, Xia Y, Margoliash E, Rao Z, Liu XJ. Remarkably high activities of testicular cytochrome c in destroying reactive oxygen species and in triggering apoptosis. Proc Natl Acad Sci U S A. 2006;103:8965–8970. doi: 10.1073/pnas.0603327103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammucari C, Rizzuto R. Signaling pathways in mitochondrial dysfunction and aging. Mech Ageing Dev. 2010;131:536–543. doi: 10.1016/j.mad.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JB. Molecular basis of the neurodegenerative disorders. N Engl J Med. 1999;340:1970–1980. doi: 10.1056/NEJM199906243402507. [DOI] [PubMed] [Google Scholar]
- Matapurkar A, Lazebnik Y. Requirement of cytochrome c for apoptosis in human cells. Cell Death Differ. 2006;13:2062–2067. doi: 10.1038/sj.cdd.4401968. [DOI] [PubMed] [Google Scholar]
- McIntosh DB, Parrish JC, Wallace CJ. Definition of a nucleotide binding site on cytochrome c by photoaffinity labeling. J Biol Chem. 1996;271:18379–18386. doi: 10.1074/jbc.271.31.18379. [DOI] [PubMed] [Google Scholar]
- Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–313. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- Morriss GM, New DA. Effect of oxygen concentration on morphogenesis of cranial neural folds and neural crest in cultured rat embryos. J Embryol Exp Morphol. 1979;54:17–35. [PubMed] [Google Scholar]
- Nakagawa Y. Initiation of apoptotic signal by the peroxidation of cardiolipin of mitochondria. Ann N Y Acad Sci. 2004;1011:177–184. doi: 10.1007/978-3-662-41088-2_18. [DOI] [PubMed] [Google Scholar]
- Napiwotzki J, Shinzawa-Itoh K, Yoshikawa S, Kadenbach B. ATP and ADP bind to cytochrome c oxidase and regulate its activity. Biol Chem. 1997;378:1013–1021. doi: 10.1515/bchm.1997.378.9.1013. [DOI] [PubMed] [Google Scholar]
- Narula J, Pandey P, Arbustini E, Haider N, Narula N, Kolodgie FD, Dal Bello B, Semigran MJ, Bielsa-Masdeu A, Dec GW, Israels S, Ballester M, Virmani R, Saxena S, Kharbanda S. Apoptosis in heart failure: release of cytochrome c from mitochondria and activation of caspase-3 in human cardiomyopathy. Proc Natl Acad Sci U S A. 1999;96:8144–8149. doi: 10.1073/pnas.96.14.8144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto S, Combs CA, French S, Ahn BH, Fergusson MM, Balaban RS, Finkel T. The mammalian longevity-associated gene product p66shc regulates mitochondrial metabolism. J Biol Chem. 2006;281:10555–10560. doi: 10.1074/jbc.M511626200. [DOI] [PubMed] [Google Scholar]
- Nemoto S, Finkel T. Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science. 2002;295:2450–2452. doi: 10.1126/science.1069004. [DOI] [PubMed] [Google Scholar]
- Nicholls DG, Ferguson SJ. Bioenergetics 2. Academic Press Limited; London, San Diego: 1992. [Google Scholar]
- Pecina P, Borisenko GG, Belikova NA, Tyurina YY, Pecinova A, Lee I, Samhan-Arias AK, Przyklenk K, Kagan VE, Hüttemann M. Phosphomimetic substitution of cytochrome C tyrosine 48 decreases respiration and binding to cardiolipin and abolishes ability to trigger downstream caspase activation. Biochemistry. 2010;49:6705–6714. doi: 10.1021/bi100486s. [DOI] [PubMed] [Google Scholar]
- Pereverzev MO, Vygodina TV, Konstantinov AA, Skulachev VP. Cytochrome c, an ideal antioxidant. Biochem Soc Trans. 2003;31:1312–1315. doi: 10.1042/bst0311312. [DOI] [PubMed] [Google Scholar]
- Petrosillo G, Colantuono G, Moro N, Ruggiero FM, Tiravanti E, Di Venosa N, Fiore T, Paradies G. Melatonin protects against heart ischemiareperfusion injury by inhibiting mitochondrial permeability transition pore opening. Am J Physiol Heart Circ Physiol. 2009a;297:H1487–1493. doi: 10.1152/ajpheart.00163.2009. [DOI] [PubMed] [Google Scholar]
- Petrosillo G, Moro N, Ruggiero FM, Paradies G. Melatonin inhibits cardiolipin peroxidation in mitochondria and prevents the mitochondrial permeability transition and cytochrome c release. Free Radic Biol Med. 2009b;47:969–974. doi: 10.1016/j.freeradbiomed.2009.06.032. [DOI] [PubMed] [Google Scholar]
- Piccoli C, Scrima R, Boffoli D, Capitanio N. Control by cytochrome c oxidase of the cellular oxidative phosphorylation system depends on the mitochondrial energy state. Biochem J. 2006;396:573–583. doi: 10.1042/BJ20060077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piel DA, Deutschman CS, Levy RJ. Exogenous cytochrome C restores myocardial cytochrome oxidase activity into the late phase of sepsis. Shock. 2008;29:612–616. doi: 10.1097/SHK.0b013e318157e962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piel DA, Gruber PJ, Weinheimer CJ, Courtois MR, Robertson CM, Coopersmith CM, Deutschman CS, Levy RJ. Mitochondrial resuscitation with exogenous cytochrome c in the septic heart. Crit Care Med. 2007;35:2120–2127. doi: 10.1097/01.ccm.0000278914.85340.fe. [DOI] [PubMed] [Google Scholar]
- Pinton P, Rimessi A, Marchi S, Orsini F, Migliaccio E, Giorgio M, Contursi C, Minucci S, Mantovani F, Wieckowski MR, Del Sal G, Pelicci PG, Rizzuto R. Protein kinase C beta and prolyl isomerase 1 regulate mitochondrial effects of the life-span determinant p66Shc. Science. 2007;315:659–663. doi: 10.1126/science.1135380. [DOI] [PubMed] [Google Scholar]
- Porteous WK, James AM, Sheard PW, Porteous CM, Packer MA, Hyslop SJ, Melton JV, Pang CY, Wei YH, Murphy MP. Bioenergetic consequences of accumulating the common 4977-bp mitochondrial DNA deletion. Eur J Biochem. 1998;257:192–201. doi: 10.1046/j.1432-1327.1998.2570192.x. [DOI] [PubMed] [Google Scholar]
- Rabuffetti M, Sciorati C, Tarozzo G, Clementi E, Manfredi AA, Beltramo M. Inhibition of caspase-1-like activity by Ac-Tyr-Val-Ala-Asp-chloromethyl ketone induces long-lasting neuroprotection in cerebral ischemia through apoptosis reduction and decrease of proinflammatory cytokines. J Neurosci. 2000;20:4398–4404. doi: 10.1523/JNEUROSCI.20-12-04398.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehling P, Wiedemann N, Pfanner N, Truscott KN. The mitochondrial import machinery for preproteins. Crit Rev Biochem Mol Biol. 2001;36:291–336. doi: 10.1080/20014091074200. [DOI] [PubMed] [Google Scholar]
- Richter C, Park JW, Ames BN. Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc Natl Acad Sci U S A. 1988;85:6465–6467. doi: 10.1073/pnas.85.17.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemer J, Fischer M, Herrmann JM. Oxidation-driven protein import into mitochondria: Insights and blind spots. Biochim Biophys Acta. 2010 doi: 10.1016/j.bbamem.2010.06.003. in press, doi: 10.1016/j.bbamem.2010.1006.1003. [DOI] [PubMed] [Google Scholar]
- Ripple MO, Abajian M, Springett R. Cytochrome c is rapidly reduced in the cytosol after mitochondrial outer membrane permeabilization. Apoptosis. 2010;15:563–573. doi: 10.1007/s10495-010-0455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb-Gaspers LD, Burnett P, Rutter GA, Denton RM, Rizzuto R, Thomas AP. Integrating cytosolic calcium signals into mitochondrial metabolic responses. Embo J. 1998;17:4987–5000. doi: 10.1093/emboj/17.17.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts VA, Pique ME. Definition of the interaction domain for cytochrome c on cytochrome c oxidase. III. Prediction of the docked complex by a complete, systematic search. J Biol Chem. 1999;274:38051–38060. doi: 10.1074/jbc.274.53.38051. [DOI] [PubMed] [Google Scholar]
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O'Regan JP, Deng HX, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med. 2001;344:1688–1700. doi: 10.1056/NEJM200105313442207. [DOI] [PubMed] [Google Scholar]
- Rytomaa M, Kinnunen PK. Evidence for two distinct acidic phospholipid-binding sites in cytochrome c. J Biol Chem. 1994;269:1770–1774. [PubMed] [Google Scholar]
- Rytomaa M, Mustonen P, Kinnunen PK. Reversible, nonionic, and pH-dependent association of cytochrome c with cardiolipin-phosphatidylcholine liposomes. J Biol Chem. 1992;267:22243–22248. [PubMed] [Google Scholar]
- Salemme FR. Structure and function of cytochromes c. Annu Rev Biochem. 1977;46:299–329. doi: 10.1146/annurev.bi.46.070177.001503. [DOI] [PubMed] [Google Scholar]
- Samavati L, Lee I, Mathes I, Lottspeich F, Hüttemann M. Tumor necrosis factor α inhibits oxidative phosphorylation through tyrosine phosphorylation at subunit I of cytochrome c oxidase. J Biol Chem. 2008;283:21134–21144. doi: 10.1074/jbc.M801954200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson TH, Kumar R, Sullivan JM, Krause GS. Insulin blocks cytochrome c release in the reperfused brain through PI3-K signaling and by promoting Bax/Bcl-XL binding. J Neurochem. 2008;106:1248–1258. doi: 10.1111/j.1471-4159.2008.05473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanishvili R, Volz KW, Westbrook EM, Margoliash E. The low ionic strength crystal structure of horse cytochrome c at 2.1 A resolution and comparison with its high ionic strength counterpart. Structure. 1995;3:707–716. doi: 10.1016/s0969-2126(01)00205-2. [DOI] [PubMed] [Google Scholar]
- Schlame M, Rua D, Greenberg ML. The biosynthesis and functional role of cardiolipin. Progress in lipid research. 2000;39:257–288. doi: 10.1016/s0163-7827(00)00005-9. [DOI] [PubMed] [Google Scholar]
- Schmidt TR, Wildman DE, Uddin M, Opazo JC, Goodman M, Grossman LI. Rapid electrostatic evolution at the binding site for cytochrome c on cytochrome c oxidase in anthropoid primates. Proc Natl Acad Sci U S A. 2005;102:6379–6384. doi: 10.1073/pnas.0409714102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuh RA, Kristian T, Fiskum G. Calcium-dependent dephosphorylation of brain mitochondrial calcium/cAMP response element binding protein (CREB). J Neurochem. 2005;92:388–394. doi: 10.1111/j.1471-4159.2004.02873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan JP, Swerdlow RH, Parker WD, Miller SW, Davis RE, Tuttle JB. Altered calcium homeostasis in cells transformed by mitochondria from individuals with Parkinson's disease. J Neurochem. 1997;68:1221–1233. doi: 10.1046/j.1471-4159.1997.68031221.x. [DOI] [PubMed] [Google Scholar]
- Sinibaldi F, Howes BD, Piro MC, Polticelli F, Bombelli C, Ferri T, Coletta M, Smulevich G, Santucci R. Extended cardiolipin anchorage to cytochrome c: a model for protein-mitochondrial membrane binding. J Biol Inorg Chem. 2010;15:689–700. doi: 10.1007/s00775-010-0636-z. [DOI] [PubMed] [Google Scholar]
- Spooner PJ, Watts A. Reversible unfolding of cytochrome c upon interaction with cardiolipin bilayers. 1. Evidence from deuterium NMR measurements. Biochemistry. 1991;30:3871–3879. doi: 10.1021/bi00230a010. [DOI] [PubMed] [Google Scholar]
- Starkov AA, Chinopoulos C, Fiskum G. Mitochondrial calcium and oxidative stress as mediators of ischemic brain injury. Cell Calcium. 2004;36:257–264. doi: 10.1016/j.ceca.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Steverding D, Kadenbach B. Influence of N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline modification on proton translocation and membrane potential of reconstituted cytochrome-c oxidase support “proton slippage”. J Biol Chem. 1991;266:8097–8101. [PubMed] [Google Scholar]
- Stock D, Leslie AG, Walker JE. Molecular architecture of the rotary motor in ATP synthase. Science. 1999;286:1700–1705. doi: 10.1126/science.286.5445.1700. [DOI] [PubMed] [Google Scholar]
- Sugawara T, Fujimura M, Morita-Fujimura Y, Kawase M, Chan PH. Mitochondrial release of cytochrome c corresponds to the selective vulnerability of hippocampal CA1 neurons in rats after transient global cerebral ischemia. J Neurosci. 1999;19:RC39. doi: 10.1523/JNEUROSCI.19-22-j0002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PG, Keller JN, Bussen WL, Scheff SW. Cytochrome c release and caspase activation after traumatic brain injury. Brain Res. 2002;949:88–96. doi: 10.1016/s0006-8993(02)02968-2. [DOI] [PubMed] [Google Scholar]
- Sun L, Xiao L, Nie J, Liu FY, Ling GH, Zhu XJ, Tang WB, Chen WC, Xia YC, Zhan M, Ma MM, Peng YM, Liu H, Liu YH, Kanwar YS. p66Shc mediates high glucose and angiotensin II induced oxidative stress renal tubular injury via mitochondrial dependent apoptotic pathway. Am J Physiol Renal Physiol. 2010 doi: 10.1152/ajprenal.00414.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svistunenko DA. Reaction of haem containing proteins and enzymes with hydroperoxides: the radical view. Biochim Biophys Acta. 2005;1707:127–155. doi: 10.1016/j.bbabio.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Tuominen EK, Wallace CJ, Kinnunen PK. Phospholipid-cytochrome c interaction: evidence for the extended lipid anchorage. J Biol Chem. 2002;277:8822–8826. doi: 10.1074/jbc.M200056200. [DOI] [PubMed] [Google Scholar]
- Tuominen EK, Zhu K, Wallace CJ, Clark-Lewis I, Craig DB, Rytomaa M, Kinnunen PK. ATP induces a conformational change in lipid-bound cytochrome c. J Biol Chem. 2001;276:19356–19362. doi: 10.1074/jbc.M100853200. [DOI] [PubMed] [Google Scholar]
- Turrens JF, Alexandre A, Lehninger AL. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch Biochem Biophys. 1985;237:408–414. doi: 10.1016/0003-9861(85)90293-0. [DOI] [PubMed] [Google Scholar]
- Tyurina YY, Kini V, Tyurin VA, Vlasova II, Jiang J, Kapralov AA, Belikova NA, Yalowich JC, Kurnikov IV, Kagan VE. Mechanisms of cardiolipin oxidation by cytochrome c: relevance to pro- and antiapoptotic functions of etoposide. Mol Pharmacol. 2006;70:706–717. doi: 10.1124/mol.106.022731. [DOI] [PubMed] [Google Scholar]
- Vempati UD, Diaz F, Barrientos A, Narisawa S, Mian AM, Millan JL, Boise LH, Moraes CT. Role of cytochrome C in apoptosis: increased sensitivity to tumor necrosis factor alpha is associated with respiratory defects but not with lack of cytochrome C release. Mol Cell Biol. 2007;27:1771–1783. doi: 10.1128/MCB.00287-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villani G, Attardi G. In vivo control of respiration by cytochrome c oxidase in wild-type and mitochondrial DNA mutation-carrying human cells. Proc Natl Acad Sci U S A. 1997;94:1166–1171. doi: 10.1073/pnas.94.4.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villani G, Greco M, Papa S, Attardi G. Low reserve of cytochrome c oxidase capacity in vivo in the respiratory chain of a variety of human cell types. J Biol Chem. 1998;273:31829–31836. doi: 10.1074/jbc.273.48.31829. [DOI] [PubMed] [Google Scholar]
- Vincent JS, Kon H, Levin IW. Low-temperature electron paramagnetic resonance study of the ferricytochrome c-cardiolipin complex. Biochemistry. 1987;26:2312–2314. doi: 10.1021/bi00382a036. [DOI] [PubMed] [Google Scholar]
- Vincent JS, Levin IW. Interaction of ferricytochrome c with zwitterionic phospholipid bilayers: a Raman spectroscopic study. Biochemistry. 1988;27:3438–3446. doi: 10.1021/bi00409a047. [DOI] [PubMed] [Google Scholar]
- Wan B, Doumen C, Duszynski J, Salama G, Vary TC, LaNoue KF. Effects of cardiac work on electrical potential gradient across mitochondrial membrane in perfused rat hearts. Am J Physiol. 1993;265:H453–460. doi: 10.1152/ajpheart.1993.265.2.H453. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Jackson JG, Thayer SA. Altered distribution of mitochondria impairs calcium homeostasis in rat hippocampal neurons in culture. J Neurochem. 2003a;87:85–94. doi: 10.1046/j.1471-4159.2003.01970.x. [DOI] [PubMed] [Google Scholar]
- Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–2933. [PubMed] [Google Scholar]
- Wang X, Pal R, Chen XW, Limpeanchob N, Kumar KN, Michaelis EK. High intrinsic oxidative stress may underlie selective vulnerability of the hippocampal CA1 region. Brain Res Mol Brain Res. 2005;140:120–126. doi: 10.1016/j.molbrainres.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhu S, Drozda M, Zhang W, Stavrovskaya IG, Cattaneo E, Ferrante RJ, Kristal BS, Friedlander RM. Minocycline inhibits caspase-independent and -dependent mitochondrial cell death pathways in models of Huntington's disease. Proc Natl Acad Sci U S A. 2003b;100:10483–10487. doi: 10.1073/pnas.1832501100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhu S, Pei Z, Drozda M, Stavrovskaya IG, Del Signore SJ, Cormier K, Shimony EM, Wang H, Ferrante RJ, Kristal BS, Friedlander RM. Inhibitors of cytochrome c release with therapeutic potential for Huntington's disease. J Neurosci. 2008;28:9473–9485. doi: 10.1523/JNEUROSCI.1867-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZB, Li M, Zhao Y, Xu JX. Cytochrome C is a hydrogen peroxide scavenger in mitochondria. Protein and peptide letters. 2003c;10:247–253. doi: 10.2174/0929866033479013. [DOI] [PubMed] [Google Scholar]
- Wu DC, Jackson-Lewis V, Vila M, Tieu K, Teismann P, Vadseth C, Choi DK, Ischiropoulos H, Przedborski S. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J Neurosci. 2002;22:1763–1771. doi: 10.1523/JNEUROSCI.22-05-01763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Chi L, Xu R, Ke Y, Luo C, Cai J, Qiu M, Gozal D, Liu R. Increased production of reactive oxygen species contributes to motor neuron death in a compression mouse model of spinal cord injury. Spinal Cord. 2005;43:204–213. doi: 10.1038/sj.sc.3101674. [DOI] [PubMed] [Google Scholar]
- Yamato M, Egashira T, Utsumi H. Application of in vivo ESR spectroscopy to measurement of cerebrovascular ROS generation in stroke. Free Radic Biol Med. 2003;35:1619–1631. doi: 10.1016/j.freeradbiomed.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Yu H, Lee I, Salomon AR, Yu K, Hüttemann M. Mammalian liver cytochrome c is tyrosine-48 phosphorylated in vivo, inhibiting mitochondrial respiration. Biochim Biophys Acta. 2008;1777:1066–1071. doi: 10.1016/j.bbabio.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Wang X, Purring-Koch C, Wei Y, McLendon GL. A mutational epitope for cytochrome C binding to the apoptosis protease activation factor-1. J Biol Chem. 2001;276:13034–13038. doi: 10.1074/jbc.M009773200. [DOI] [PubMed] [Google Scholar]
- Zhang H, Huang HM, Carson RC, Mahmood J, Thomas HM, Gibson GE. Assessment of membrane potentials of mitochondrial populations in living cells. Anal Biochem. 2001;298:170–180. doi: 10.1006/abio.2001.5348. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Gerstein M. The human genome has 49 cytochrome c pseudogenes, including a relic of a primordial gene that still functions in mouse. Gene. 2003;312:61–72. doi: 10.1016/s0378-1119(03)00579-1. [DOI] [PubMed] [Google Scholar]
- Zhao X, Leon IR, Bak S, Mogensen M, Wrzesinski K, Hojlund K, Jensen ON. Phosphoproteome analysis of functional mitochondria isolated from resting human muscle reveals extensive phosphorylation of inner membrane protein complexes and enzymes. Mol Cell Proteomics. 2010 doi: 10.1074/mcp.M110.000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Stavrovskaya IG, Drozda M, Kim BY, Ona V, Li M, Sarang S, Liu AS, Hartley DM, Wu DC, Gullans S, Ferrante RJ, Przedborski S, Kristal BS, Friedlander RM. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature. 2002;417:74–78. doi: 10.1038/417074a. [DOI] [PubMed] [Google Scholar]