Abstract
The GSK-3 family of serine/threonine kinases, which is comprised of two isoforms (α and β), was initially identified as a negative regulator of glycogen synthase, the rate limiting enzyme of glycogen synthesis [1, 2]. In the 30 years since its initial discovery, the family has been reported to regulate a host of additional cellular processes and, consequently, disease states such as bipolar disorders, diabetes, inflammatory diseases, cancer, and neurodegenerative diseases including Alzheimer’s Disease and Parkinson’s Disease [3, 4]. As a result, there has been intense interest on the part of the pharmaceutical industry in developing small molecule antagonists of GSK-3. Herein, we will review the roles played by GSK-3s in the heart, focusing primarily on recent studies that have employed global and tissue-specific gene deletion. We will highlight roles in various pathologic processes, including pressure overload and ischemic injury, focusing on some striking isoform-specific effects of the family. Due to space limitations and/or the relatively limited data in gene-targeted mice, we will not be addressing the family’s roles in ischemic pre-conditioning or its many interactions with various pro- and anti-apoptotic factors.
Introduction
The two isoforms of GSK-3 are encoded by distinct genes and are ubiquitously expressed. Unlike most protein kinases, the GSK-3s exhibit considerable activity in un-stimulated cells. In response to various stimuli, most notably growth factors, GSK-3s are phosphorylated (at serine 21 of GSK-3α and serine 9 of GSK-3β, herein referred to as S21 and S9 respectively) by a number of protein kinases including PKB/AKT, and this modification inhibits the GSK-3s by promoting pseudo-substrate interaction of the phosphorylated residue with a substrate docking motif. Since phosphorylation of substrates by GSK-3s very typically suppresses the substrates’ function, growth factors lead to activation of factors downstream of the GSK-3s via inhibiting the inhibitory effect of these kinases. GSK-3s can also be regulated by canonical Wnt signaling and GSK-3β (but not α) can be inhibited by p38-MAPK phosphorylation, though the physiological significance of the latter is not yet fully clear [5, 6].
The first studies implicating GSK-3 in regulating pathologic processes in the heart were published a decade ago and identified GSK-3β as a negative regulator of the hypertrophic response in cardiomyocytes in culture [7, 8]. Since then, numerous studies utilizing a variety of models have supported that conclusion, and have suggested additional roles for GSK-3 in the heart, probably most notably ischemic pre-conditioning and ischemic injury [7–20]. One caveat to all of the above referenced studies is that, until recently, all utilized transgenesis, knock-ins of constitutively-active mutants (serine to alanine mutations at Ser9 and/or Ser21), or non-isoform-selective small molecule inhibitors, and none employed true loss-of-function strategies. Herein, we will review findings from recent studies that have employed loss-of-function to, in some cases, confirm findings reached in prior studies and, in other cases, dispute those conclusions [21–23]. We will examine isoform-specific effects (with obvious implications for drug discovery), and will discuss novel roles recently identified for the family, most importantly the role of GSK-3α, but not -3β, in regulating β-adrenergic responsiveness and the role of GSK-3β in regulating post-MI remodeling and cardiomyocyte proliferation (summarized in Table 1). Figure 1 shows some of the more important upstream inputs into GSK-3 and downstream targets that have been validated in the heart as well as mechanisms of regulation of activity.
Table 1.
Evidence from mouse models suggesting roles of GSK-3s in the heart
| Model | Cardiac Phenotype |
|---|---|
| Cardiac-specific Tg-GSK-3β-S9A | Suppressed or reversed cardiac hypertrophy in response to TAC, calcineurin activation, or adrenergic stimulation [10, 15] |
| Cardiac-specific Tg-GSK-3β-DN | Induction of compensatory hypertrophy, inhibition of apoptosis and fibrosis, and increased cardiac contractility after PO [12] |
| Cardiac-specific Tg-GSK-3β | Impaired post-natal cardiomyocyte growth and led to contractile dysfunction [14] |
| Cardiac-specific Tg-GSK-3α | Inhibited cardiac growth and PO-induced cardiac hypertrophy yet increased fibrosis and apoptosis [17] |
| GSK-3β S9A KI | Attenuated hypertrophy and HF under PO [13] |
| GSK-3αS21A KI | Increased hypertrophy and HF under PO [13] |
| GSK-3β germline KO | Died in mid gestation from inability to recruit pro-survival NF-κB signaling in setting of increased TNFβ production in response to pathogens [24]; In absence of pathogens, died in late gestation or immediately after birth due to near obliteration of the ventricular cavities by proliferating cardiomyocytes, DORV and VSD also seen [21] |
| GSK-3α KO | Born without apparent cardiac abnormalities [21, 25]; Development of cardiac hypertrophy and contractile dysfunction after 2-months of age; PO-induced increased hypertrophy and HF; impaired beta-adrenergic responsiveness [23] |
| Inducible cardiac-specific GSK-3β KO | Normal PO-induced hypertrophy; less LV dilatation and better-preserved LV function post MI; increased cardiomyocyte proliferation following PO and MI [22] |
| GSK-3β-S9A/GSK-3α S21A double KI | Normal post MI hypertrophy [26]; attenuated pathological hypertrophy induced by chronic adrenergic stimulation [27] |
Abbreviations: TG, transgenic; S9A, serine-9 mutated to alanine; S21A, serine-21 mutated to alanine; DN, dominant negative; KI, knock-in; KO, knockout; HF, heart failure; PO, pressure overload; DORV, double outlet right ventricle; VSD, ventricular septal defect; MI, myocardial infarction; LV, left ventricle. Please see text for additional abbreviations.
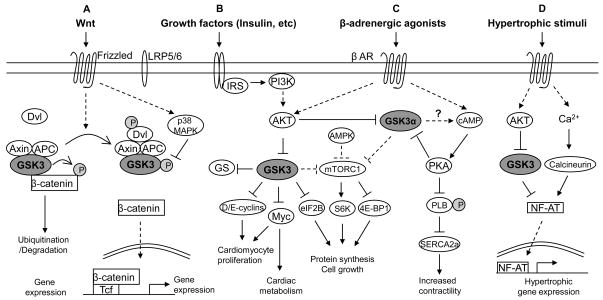
Figure 1. Pathways regulated by GSK-3.
A. Canonical Wnt signaling. In the absence of a Wnt signal (left side), the multiprotein complex assembled on axin and APC (the adenomatous polyposis coli gene product) includes active GSK-3 and β-catenin. GSK-3 phosphorylates β-catenin (the transcriptional co-activator that, together with the Tcf family of transcription factors regulates Wnt-dependent gene expression). Phosphorylation of β-catenin by GSK-3 leads to the ubiquitination and degradation of β-catenin by the proteasome, preventing gene expression. In the presence of a Wnt signal (right side), GSK-3 is re-directed to the LRP5/6 coreceptor via a somewhat unclear mechanism involving disheveled (Dvl). β-catenin is stabilized, and then translocates to the nucleus where it displaces transcriptional repressors (Groucho family) from Tcf/Lef, leading to gene expression. Wnt-dependent genes regulate a host of processes from carcinogenesis to cardiac hypertrophy [28]. An alternative mechanism to inhibit GSK-3 in this setting is mediated by p38 [5, 6].
B. Growth factor signaling: Insulin as an example. Following growth factor binding to cognate receptors, the PI3K/Akt pathway is activated, leading to inhibition of GSK-3. GSK-3 negatively regulates a host of factors downstream of growth factor receptors, so the consequences of GSK-3 inhibition are activation of these factors including: 1) glycogen synthase, leading to increased glycogen storage, 2) D- and E-type cyclins that promote cell cycle progression, 3) Myc, which also promotes cell cycle progression as well as regulating metabolic status of the cardiomyocyte, 4) mTORC1 which regulates protein synthesis and, secondarily, cell growth via interactions with a number of factors (2 are shown). In the heart, GSK-3α appears to be the dominant negative regulator of mTOR activity. Also shown is AMPK, the master energy sensor in the cell, that, in the presence of reduced energy stores, phosphorylates mTOR, priming it for further inhibitory phosphorylation by GSK-3.
C. β-adrenergic responsiveness. In the setting of stress, the β-adrenergic receptor (β-AR) is activated leading to increased cAMP and activation of PKA, which increases contractility in part by phosphorylating and inhibiting phospholamban, thereby activating SERCA2a. GSK-3α, via unclear mechanisms, amplifies the response by enhancing cAMP production. The enhanced PKA signaling eventually provides a negative feedback to inhibit GSK-3α, terminating the β-AR signal.
D. Hypertrophic signaling. Numerous hypertrophic stimuli (e.g. angiotensin II, α–adrenergic agonists, cell stretch, etc.) work through increasing Ca2+ and, in some cases, activating Akt to lead to inhibition of GSK-3. Increased Ca2+ activates calcineurin which de-phosphorylates NF-AT family members, allowing NF-ATs to translocate to the nucleus and activate hypertrophic gene expression. GSK-3 antagonizes hypertrophic signaling by phosphorylating NF-ATs, leading to their exclusion from the nucleus. CaMKII (not shown) promotes hypertrophy via a variety of effectors, but it is unclear at this point whether there is cross-talk between CaMKII and GSK-3.
GSK-3 promotes differentiation of embryonic stem (ES) cells
Defining the ground state of ES cell self-renewal has been an area of intense interest over the past several years. However, this has typically involved empiric formulations of media, and the signaling pathways that were able to maintain pluripotency in the absence of exogenous factors remained unclear. Sato et al. reported that the non-selective small molecule inhibitor BIO maintained pluripotency of mouse and human ES cells, and concluded that this was mediated via Wnt/β-catenin-dependent mechanisms [29]. More recently, Ying et al. utilized small molecule inhibitors of FGF receptors (SU5402), the ERK pathway (PD184352) and GSK-3s (CHIR99021) to maintain the ground state [30]. The obvious corollary to that was that the activation state of one or both GSK-3 isoforms (isoform-selective small molecule inhibitors do not exist) might regulate the decision of ES cells to decide whether to proliferate or differentiate. Therefore, we examined the role of GSK-3s in regulating differentiation of ES cells into a cardiomyocyte lineage in the embryoid body. We found that deletion of GSK-3β, but to a much lesser extent, GSK-3α, profoundly inhibited differentiation of ES cells into the cardiomyocyte lineage [21, 25]. However, the mechanism of this did not involve activation of Wnt signaling since deletion of both alleles of GSK-3β was insufficient to lead to stabilization of β-catenin and therefore to activation of canonical Wnt signaling [31].
The effects of deletion of GSK-3β on differentiation were most pronounced in the “late early” stages of differentiation, as reflected in comparable expression of the early lineage markers (GATA4 and Nkx2.5) but much less (in some cases > 6-fold less) expression of later markers of differentiation (α- and β-MHC, SERCA2, and BNP) [21]. The corollary to this was that proliferation was markedly increased in the GSK-3β KO embryoid bodies and a substantial component of that appeared to be due to cardiomyocyte proliferation as determined by double staining for GATA4 and Histone H3 phosphorylated on Ser 10, a marker of M phase progression. These studies suggest that GSK-3β plays a key role in the mid to later stages of cardiomyocyte differentiation, and in its absence, proliferation is enhanced.
GSK-3β, but not GSK-3α is a critical brake on cardiomyocyte proliferation during development
Based on the findings in the EBs, we turned to the developing heart to ask whether there might be a corollary in vivo. The GSK-3β germline KO is lethal early in development due to massive hepatic apoptosis that was traced to the failure to recruit cytoprotective NF-κB signaling in response to TNFα in the KO [24]. However, this phenotype requires that the mother be exposed to pathogens in the vivarium (in order to activate TNFα signaling). In the absence of these pathogens, GSK-3β KO embryos survive in the expected numbers to very late gestation or immediately post-birth and then die. In contrast, the GSK-3α KOs survive and exhibit no obvious cardiac abnormalities until later in life (see below). Pathological examination of the GSK-3β null newborns revealed double outlet right ventricle and VSD, but the dominant phenotype was that the ventricular cavities were virtually obliterated with cardiomycytes [21]. Cardiomyocytes positive for phospho-histone were >3-fold increased at E13.5 and were >2-fold increased at E15.5. A number of known GSK-3β targets were dysregulated, including the cell cycle regulators, the D- and E-type cyclins, as well as c-Myc. The latter is of particular interest since c-Myc had early on been proposed to regulate various metabolic pathways [30] and recently, MacLellan and coworkers confirmed and importantly extended these studies to the heart [32].
Thus, in this in vivo setting, deletion of GSK-3β leads to hyperproliferation of cardiomyocytes, consistent with the studies in the EBs. As will be discussed below, recent data confirm that this increased cardiomyocyte proliferation appears to be maintained in the adult cardiomyocyte-specific conditional GSK-3β KO [22]. Therefore, in all scenarios examined, selective deletion of GSK-3β leads to enhanced cardiomyocyte proliferation. The findings also raise the hypothesis that correctly timed inhibition of GSK-3β, such as by a small molecule inhibitor, could drive cardiomyocyte proliferation, and that properly-timed re-instatement of GSK-3β signaling via withdrawal of drug could drive immature cardiomyocytes toward differentiation (i.e. cardiac regeneration in situ).
GSK-3β promotes post-MI, but not post TAC, remodeling
GSK-3β has been, until very recently, the near-exclusive focus of most of the studies on GSK-3 regardless of the disease state being examined. This focus on GSK-3β may have arisen from two reports that suggested that GSK-3β was more effective than GSK-3α in rescuing Wnt/Wingless pathway defects due to the Zeste-white-3 (GSK-3) mutation in Drosophila [33, 34]. However, in those studies the level of expression of the two mammalian isoforms in the flies was not equalized. More recently, the two isoforms have been found to be entirely redundant in regulating Wnt/β-catenin signaling in ES cells [31]. As we will discuss below, however, such equivalency is not the case for several of the pathologic processes regulated by this family.
As noted above, Morisco et al. and Haq et al. reported in 2000 that over-expression of GSK-3β in NRVMs reduced hypertrophic responses to β-adrenergic stimulation, α-adrenergic stimulation and endothelin-1 [7, 8]. This appeared to be due, at least in part, to enhanced recruitment of calcineurin and, subsequently NF-AT family members, as well as CaMKII. Following these initial reports, several groups created transgenic mice over-expressing a GSK-3β mutant that cannot be inhibited (the regulatory S9 residue, that when phosphorylated inactivates the kinase, was mutated to alanine) and they consistently found that these mice did not develop as much hypertrophy as their WT littermates. This has been relatively consistent across the literature ([10, 13–17] and reviewed in [4]), and the converse (increased hypertrophy with over-expression of a dominant inhibitory mutant) was also found [12].
Interesting twists in the story began to appear when investigators used knock-ins (KI) of the inhibition-resistant form of GSK-3β. These studies confirmed that expression of GSK-3β (S9A) led to less hypertrophy and less heart failure. In contrast, expression of GSK-3α (S21A) led to increased hypertrophy and heart failure, suggesting very divergent roles for the two isoforms [13]. Thus, S9 phosphorylation and inhibition of GSK-3β promotes pathologic hypertrophy but S21 phosphorylation and inhibition of GSK-3α plays a beneficial compensatory role during pressure overload. We will return to these fairly startling differences in function of these two closely related kinases below.
To address the role of GSK-3β without employing over-expression, constitutive activation, or additional problems in interpretation of data arising from pan-expression, we recently employed conditional deletion of GSK-3β exclusively in the cardiomyocyte (via the Mer-Cre-Mer system) [22]. To our surprise, we found that deletion of GSK-3β had no effect on the hypertrophic response to TAC. This was not due to inadequate deletion of the gene and was irrespective of the magnitude of the gradient. In addition, no effects were found on LV function. We were particularly surprised that TAC-induced hypertrophy was not increased since we had previously reported that deletion of β-catenin, a key target of GSK-3, enhanced TAC-induced hypertrophy [28]. However, examination of cytosolic β-catenin levels (i.e. that which is able to translocate to the nucleus and activate gene expression), revealed no increase in the GSK-3β KO, supporting the concept that GSK-3α is equally competent to regulate β-catenin and the canonical Wnt pathway in the heart as it is in ES cells. The findings in the GSK-3β KO will be contrasted to the fairly profound effects of deletion of GSK-3α on the hypertrophic response to TAC (see below).
We next explored the possible role of GSK-3β in post-MI remodeling and, given the findings in the TAC model, were surprised to find that adverse LV remodeling was significantly reduced in the KO in that LV function was better preserved and LV dilatation was reduced [22] However, hypertrophy in the remote (non-infarct) zone was significantly increased, as one would expect from the transgenic studies. Of note, these findings were not due to cardioprotection from deletion of GSK-3β in this permanent occlusion model since we did not delete the gene until > 5d post-MI, at which point the infarct would have been completed. The findings were particularly surprising in that the role played by GSK-3β appears to depend on the stimulus to hypertrophy, with deletion having no impact on TAC-induced hypertrophy but promoting post-MI hypertrophy.
Recently Marber and colleagues reported that the double KI mouse with constitutive activation of both GSK-3α and GSK-3β had a similar degree of hypertrophy as WT and concluded that remodeling post-MI is independent of GSK-3 activation state [26]. These results are difficult to reconcile with the findings in the GSK-3β KO. However, an alternative explanation that derives from studies by Matsuda et al [13], which showed that the KI of GSK-3β attenuated hypertrophy whereas KI of GSK-3α increased hypertrophy, would be that the phenotypes of the two KIs counter-balanced and attenuated one another in the double KI. Other confounders impairing interpretation of KO vs. KI data are the caveats that were mentioned above: 1) phosphorylation of S9 is only one mechanism of inhibition of GSK-3β and the S to A mutation does not prevent Wnt-mediated or p38-mediated regulation of the kinase; 2) the KI is expressed in all cells. These seemingly contradictory findings and others will be discussed further (below).
GSK-3α protects against TAC-induced hypertrophy and enhances β-adrenergic responsiveness
We next asked what specific roles GSK-3α might play in the heart. Since GSK-3β appeared to play little role in TAC-induced hypertrophy, we asked whether GSK-3α might. To our surprise, given that the GSK-3α S21A KI developed increased hypertrophy and heart failure [13], we observed a similar effect upon knocking out GSK-3α, even in the absence of a stressor [23]. These results, to say the least, are also difficult to reconcile.
Perhaps not surprisingly, given that the GSK-3α KO was a global tissue KO, the hypertrophy was present in both cardiac and skeletal muscle, though was more apparent in the heart. Along with the hypertrophy was a modest reduction in LV function. On examination of signaling pathways regulated by GSK-3s, there was a striking dysregulation of mTORC1 as evidenced by significantly increased phosphorylation of the mTORC1 targets, 4E-BP1 and ribosomal S6 kinase [23]. These data support the contention that GSK-3α is a central negative regulator of mTORC1, and with the loss of that control, hypertrophy develops even in the absence of a stress.
Most strikingly, when younger GSK-3α KO mice, which had preserved LV function at baseline, were subjected to TAC, the mice were simply unable to generate a systolic pressure greater than 110 mmHg whereas WT mice generated SBPs of > 180 mmHg. Despite the lower pressure achieved by the KO, the hypertrophic response in the KO was markedly exaggerated. This was associated with increased expression of the fetal gene program, with marked replacement fibrosis, likely consistent with cardiomyocyte loss, and with heart failure [23].
In searching for underlying mechanisms, we found a significantly blunted response of the KO mice to β-adrenergic stimulation, even in the absence of TAC. Since β-adrenergic responsiveness is critical in the heart’s response to stressors, it is probably not surprising that the KO hearts rapidly failed when exposed to pressure overload. The mechanism of this effect is under investigation, but it is clear that GSK-3α interacts with the β-adrenergic system at a very proximal point in the cascade, above the level of adenylyl cyclase and probably at the receptor/GRK2/5, β-arrestin complex [23]. These findings are consistent with Webb et al. who employed an alternate strategy (isoproteronol infusion in the A9/A21 constitutively active double KI mouse) to show that persistent activation of GSK-3 prevented adverse isoproterenol-induced remodeling [27]. Taken together, these studies suggest GSK-3α is indeed a critical regulator of the β-adrenergic signaling pathway.
One hypothesis following on these findings is the possibility that moderate inhibition of GSK-3α, such as one would achieve with pharmacologic inhibition, could provide an alternative strategy to achieve β-blockade. A benefit of this strategy could come from the positive effects of GSK-3s in improving insulin sensitivity/glucose tolerance (see below), an effect that most traditional β-blockers do not share; indeed, they typically worsen glucose tolerance [35].
GSK-3β deletion promotes cardiomyocyte proliferation in the adult heart
As noted above, GSK-3 regulates a number of factors that regulate cell cycle progression, but the first suggestion that this might apply to cardiomyocytes was from Keating and co-workers who reported that the small molecule GSK-3 inhibitor, BIO, promoted proliferation of NRVMs [36]. Although BIO is not selective for GSK-3 isoforms, and thus cannot differentiate isoform-specific effects, based on the significant increase in cardiomyocyte proliferation we observed in both embryoid bodies and the developing heart, we asked whether deletion of GSK-3β could induce cell cycle progression in adult cardiomyocytes in situ. We found that selective deletion of GSK-3β indeed resulted in a significant increase in cardiomyocyte proliferation that was further increased in the setting of stress induced by either TAC or MI. Thus, in all scenarios tested, inhibition/deletion of GSK-3β promotes proliferation. Although molecular mechanisms have not been clearly identified, we believe that it is likely via effects on cell cycle regulators (cyclins, Myc, etc [21].) as well as effects on metabolism mediated, at least in part, by Myc [32]. The physiological consequences of this proliferation, if any, remain to be determined.
GSK-3 deletion/inhibition improves insulin sensitivity/glucose tolerance: a role for GSK-3 inhibitors in diabetes?
The function of GSK-3 in insulin signaling and glucose metabolism is one of the best characterized of the family’s multiple functions and has major potential therapeutic implications in type 2 diabetes (T2DM) and related disorders. Inactivation of GSK-3 isoforms promotes dephosphorylation and activation of glycogen synthase (GS), and the resulting increase in glycogen synthesis leads to improved glucose tolerance. But GSK-3s have multiple mechanisms by which their inhibition can improve insulin resistance, the forerunner of T2DM. Insulin resistance is characterized by normal insulin secretion by pancreatic β cells but impaired signaling in insulin-responsive tissues. Numerous studies have implicated GSK-3s in the pathogenesis of insulin resistance and T2DM. One central mechanism involves phosphorylation of IRS-1 on S332, thereby impairing the ability of the insulin receptor (IR) to phosphorylate IRS1 on Tyr residues, and this attenuates insulin action [37–40]. Furthermore, two recent studies revealed a novel mechanism involving proteasomal degradation of IRS1/2 phosphorylated at S332, and this contributes to high glucose-induced insulin resistance [41] and limits islet beta cell growth and function [42].
Until recently, evidence for a role of GSK-3 in glucose homeostasis was largely based on studies employing pharmacological inhibitors. Several ATP-competitive inhibitors and synthetic peptides have been used in a variety of model systems to stimulate glycogen synthesis and/or glucose uptake in insulin-sensitive cells such as hepatocytes, skeletal myocytes, and adipocytes, either in culture or in diabetic animal models [43]. For example, chronic inhibition of GSK-3 in diabetic Zucker rats with CT118637 [44] or aminopyrimidine derivatives (e.g. CHIR98014 and CHIR99021) [45] improved oral glucose tolerance and whole body insulin sensitivity, with an amelioration of dyslipidemia and an improvement in IRS-1-dependent insulin signaling in skeletal muscle. Treatment with L803-mts, a highly selective peptide inhibitor, improved insulin resistance and glucose homeostasis in ob/ob mice [46] and in high fat-fed C57BL/6J mice [47].
Isoform-specific GSK-3 inhibitors do not exist as yet, but studies with transgenic or knockout mice have revealed tissue-specific functions of GSK-3α and -3β in glucose homeostasis. Mice over-expressing human GSK-3β in skeletal muscle developed glucose intolerance with reduced GS activity [48] Furthermore, mice lacking GSK-3α displayed improved glucose tolerance and insulin sensitivity, and reduced fat mass.[49] In these mice, increased glycogen content was only observed in liver, where insulin signaling, due in part to a marked increase in IRS-1 expression, was enhanced, suggesting a tissue-specific role of GSK-3α. Skeletal-muscle-specific deletion of GSK-3β resulted in improved glucose tolerance and insulin sensitivity, while liver-specific deletion of GSK-3β did not, suggesting a predominant role for GSK-3β in skeletal muscle [50].
Knockdown or inhibition of GSK-3 also promotes replication and survival of pancreatic β cells [51, 52]. Consistent with these findings, β-cell-specific overexpression of GSK-3β reduced β cell mass and proliferation, providing direct evidence that overactivation of GSK3β is associated with β cell failure in diabetic mouse models [53]. Of note, the stability of two β-cell-specific transcriptional factors that play crucial roles in glucose-stimulated insulin expression, MafA [54] and PDX-1 [42, 53], were both modulated by GSK-3. Finally, and most definitively, Tanabe et al. showed that β-cell specific ablation of GSK-3β in two diabetic mouse models (KOs of the insulin receptor or Irs2) reversed the reduction in β cell mass and corrected diabetes [55] All of these finding support the possibility that specific inhibitors of GSK-3 may have practical applications in β cell regenerative therapies for the treatment of advanced T2DM and possibly even T1DM.
In the heart, studies employing the constitutively active GSK3α/3β KI mice demonstrated that both GSK-3 isoforms participated in the regulation of heart GS by insulin, with GSK-3β playing a more prominent role [56]. Moreover, inactivation of GSK-3β either by a non-selective small molecule inhibitor (SB216763) or cardiac-specific over-expression of metallothionein (which inhibits GSK-3s via an unknown mechanism) prevented diabetes-induced cardiomyopathy in mice [57], suggesting a therapeutic potential for GSK-3β inhibition in the treatment of diabetic cardiomyopathy. In support of this notion, SB216763 enhanced the recovery of post ischemic LV function in isolated working rat hearts, and this was associated with accelerated glycogen synthesis, reduced glycolysis and H+ production, and, subsequently, reduced Ca2+ overload [58].
Roles in the human heart
In light of the large amount of data generated on the regulatory roles of GSK-3s in various mouse models, it is surprising that very little is known about the role of these kinases in the human heart. We have previously reported that Akt was activated and GSK-3β was markedly inhibited in failing human hearts but not in hypertrophied hearts [59]. GSK-3β has also been reported to be activated in failing hearts after left ventricular assist device support [60]; however, this finding is still controversial since it was not confirmed in other similar studies [61–63]. Thus, the significance of changes in GSK-3 activity in the setting of human heart failure is unclear.
Inhibition of GSK-3s with the small molecule BIO enhanced the growth and survival of cardiac stem cells isolated from patients that had undergone cardiac surgery [64]. This is consistent with the findings in mouse and human ES cells that GSK-3 promotes differentiation and inhibits proliferation [21, 25, 29, 30].
Translational potential and challenges for future research
The two isoforms of GSK-3 share virtually identical catalytic domains, a similar mode of N-terminal domain regulation by serine phosphorylation and are functionally redundant with respect to Wnt signaling. Furthermore, their expression levels do not appear to differ between tissues, and there are no validated examples of substrates that are selective for one isoform over the other. Yet, as exemplified dramatically in the heart as well as in several other tissues, the two isoforms can clearly play distinct roles in regulation of biological processes. For example, while GSK-3α is the primary regulator of glycogen synthase in liver, GSK-3β is dominant in skeletal muscle. Little is known of the underlying mechanisms that allow such distinctive functions but they likely include differential subcellular localization or sequestration, as suggested in a study by Matsuda et al. which reported that GSK-3α was nuclear-localized whereas GSK-3β was not [13].
We believe that the non-catalytic domains flanking the kinase core, which are highly divergent, likely mediate differential affinities for various binding partners, including substrates and scaffolding proteins that determine sub-cellular localization. This may in part explain the strikingly divergent roles of GSK-3s as predicted by the phenotypes of the KOs vs. KIs. For example, from the GSK-3β KO, which did not modulate hypertrophy, one would predict that GSK-3β either plays no role in hypertrophy following TAC (unlikely) or that GSK-3α compensates for loss of GSK-3β. From the GSK-3β S9A KI, one would predict, as virtually all prior transgenic data have shown, that GSK-3β is a negative regulator of TAC-induced hypertrophy. We suggest that the scaffolding functions and protein-protein interactions, which would be maintained in the KI as opposed to the KO, account in part for the different conclusions. The significant increase in total GSK-3 activity of the S9A or S21A KIs is also not physiologic, and may lead to erroneous conclusions as to function. Kinase-inactive KIs of each isoform may be necessary to fully address this issue, but as we go forward toward the clinic, we believe it will be the loss-of-function KO-derived data that will be most informative.
Given the number of disease states purported to be affected by dysregulation of GSK-3s, one might imagine that there would be intense interest on the part of pharmaceutical companies in developing inhibitors. While occasional review articles do appear that tout the strategy and several potent and relatively selective inhibitors have become available, there seems to be little clinical development in this arena. Indeed in searching Clinicaltrials.gov, we could find no trials in cardiovascular disease and only two trials employing an agent other than the traditional inhibitors of GSK-3 (lithium and valproic acid) that inhibit total GSK-3 activity in situ by no more than 10–20%. The agent is NP031112 (Noscira SA) and is in two Phase I/II clinical trials, one in Alzhemier Disease and the other in progressive supranuclear palsy. Trials with lithium or valproic acid include patients with bipolar disorder (already approved for this indication) and spino-cerebellar ataxia type 3. To our knowledge, no trials have reported any adverse consequences in the heart, suggesting indications for use could expand, even to the heart. That said, based on the data discussed above, showing the widely divergent consequences of inhibiting one or the other isoforms by gene deletion, one suspects that the inability to make isoform-specific inhibitors, and the variety of basic and essential processes regulated by GSK-3s, has caused hesitation even among the intrepid. Furthermore, our findings in the developing heart raise concerns about the long-term use of more potent GSK-3 inhibitors in women of child-baring potential (these subjects were excluded from the Noscira trials). That said, drug therapy virtually never achieves the degree of inhibition that one sees in a KO animal, and it will be of great interest to see the toxicity profile of NP031112.
The greatest promise for drug development may lie in the derivation of isoform-selective strategies such as RNAi, or a strategy recently reported in which peptides or small molecules are designed to target the divergent C-terminal domains of both GSK-3α and -3β [65]. This strategy is predicted to lead to kinase inhibition by destabilizing GSK-3 structure, and may be able to do so in an isoform-specific manner. This may be key to extracting therapeutic value. We believe that such precision drugs, while much harder to develop, will likely be essential for the widespread chronic use of GSK-3 inhibitors in disorders other than those in which there are no other viable options.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Woodgett JR. Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J. 1990;9:2431–8. doi: 10.1002/j.1460-2075.1990.tb07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woodgett JR, Cohen P. Multisite phosphorylation of glycogen synthase. Molecular basis for the substrate specificity of glycogen synthase kinase-3 and casein kinase-II (glycogen synthase kinase-5) Biochim Biophys Acta. 1984;788:339–47. doi: 10.1016/0167-4838(84)90047-5. [DOI] [PubMed] [Google Scholar]
- 3.Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116:1175–86. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sugden PH, Fuller SJ, Weiss SC, Clerk A. Glycogen synthase kinase 3 (GSK3) in the heart: a point of integration in hypertrophic signalling and a therapeutic target? A critical analysis. Br J Pharmacol. 2008 Mar;153(Suppl 1):S137–53. doi: 10.1038/sj.bjp.0707659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bikkavilli RK, Feigin ME, Malbon CC. p38 mitogen-activated protein kinase regulates canonical Wnt-beta-catenin signaling by inactivation of GSK3beta. J Cell Sci. 2008 Nov 1;121(Pt 21):3598–607. doi: 10.1242/jcs.032854. [DOI] [PubMed] [Google Scholar]
- 6.Thornton TM, Pedraza-Alva G, Deng B, Wood CD, Aronshtam A, Clements JL, et al. Phosphorylation by p38 MAPK as an alternative pathway for GSK3beta inactivation. Science. 2008 May 2;320(5876):667–70. doi: 10.1126/science.1156037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haq S, Choukroun G, Kang ZB, Lee K-H, Ranu H, Matsui T, et al. Glycogen synthase kinase-3β is a negative regulator of cardiomyocyte hypertrophy. J Cell Biol. 2000;151:117–29. doi: 10.1083/jcb.151.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morisco C, Zebrowski D, Condorelli G, Tsichlis P, Vatner SF, Sadoshima J. The Akt-glycogen synthase kinase 3β pathway regulates transcription of atrial natriuretic factor induced by β-adrenergic receptor stimulation in cardiac myocytes. J Biol Chem. 2000;275:14466–75. doi: 10.1074/jbc.275.19.14466. [DOI] [PubMed] [Google Scholar]
- 9.Morisco C, Seta K, Hardt SE, Lee Y, Vatner SF, Sadoshima J. Glycogen synthase kinase 3β regulates GATA4 in cardiac myocytes. J Biol Chem. 2001;276:28586–97. doi: 10.1074/jbc.M103166200. [DOI] [PubMed] [Google Scholar]
- 10.Antos CL, McKinsey TA, Frey N, Kutschke W, McAnally J, Shelton JM, et al. Activated glycogen synthase kinase-3β suppresses cardiac hypertrophy in vivo. Proc Nat Acad Sci. 2002;99:907–12. doi: 10.1073/pnas.231619298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haq S, Michael A, Andreucci M, Bhattacharya K, Dotto P, Walters B, et al. Stabilization of beta-catenin by a Wnt-independent mechanism regulates cardiomyocyte growth. Proc Natl Acad Sci U S A. 2003 Apr 15;100(8):4610–5. doi: 10.1073/pnas.0835895100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirotani S, Zhai P, Tomita H, Galeotti J, Marquez JP, Gao S, et al. Inhibition of glycogen synthase kinase 3beta during heart failure is protective. Circ Res. 2007 Nov 26;101(11):1164–74. doi: 10.1161/CIRCRESAHA.107.160614. [DOI] [PubMed] [Google Scholar]
- 13.Matsuda T, Zhai P, Maejima Y, Hong C, Gao S, Tian B, et al. Distinct roles of GSK-3alpha and GSK-3beta phosphorylation in the heart under pressure overload. Proc Natl Acad Sci U S A. 2008 Dec 30;105(52):20900–5. doi: 10.1073/pnas.0808315106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michael A, Haq S, Chen X, Hsich E, Cui L, Walters B, et al. Glycogen synthase kinase-3beta regulates growth, calcium homeostasis, and diastolic function in the heart. J Biol Chem. 2004 May 14;279(20):21383–93. doi: 10.1074/jbc.M401413200. [DOI] [PubMed] [Google Scholar]
- 15.Sanbe A, Gulick J, Hanks MC, Liang Q, Osinska H, Robbins J. Reengineering inducible cardiac-specific transgenesis with an attenuated myosin heavy chain promoter. Circ Res. 2003;92:609–16. doi: 10.1161/01.RES.0000065442.64694.9F. [DOI] [PubMed] [Google Scholar]
- 16.Trivedi CM, Luo Y, Yin Z, Zhang M, Zhu W, Wang T, et al. Hdac2 regulates the cardiac hypertrophic response by modulating Gsk3beta activity. Nat Med. 2007;13:324–31. doi: 10.1038/nm1552. [DOI] [PubMed] [Google Scholar]
- 17.Zhai P, Gao S, Holle E, Yu X, Yatani A, Wagner T, et al. Glycogen synthase kinase-3alpha reduces cardiac growth and pressure overload-induced cardiac hypertrophy by inhibition of extracellular signal-regulated kinases. J Biol Chem. 2007 Nov 9;282(45):33181–91. doi: 10.1074/jbc.M705133200. [DOI] [PubMed] [Google Scholar]
- 18.Murphy E, Steenbergen C. Does inhibition of glycogen synthase kinase protect in mice? Circ Res. 2008 Aug 1;103(3):226–8. doi: 10.1161/CIRCRESAHA.108.181602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008 Apr;88(2):581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Juhaszova M, Zorov DB, Yaniv Y, Nuss HB, Wang S, Sollott SJ. Role of glycogen synthase kinase-3beta in cardioprotection. Circ Res. 2009 Jun 5;104(11):1240–52. doi: 10.1161/CIRCRESAHA.109.197996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerkela R, Kockeritz L, Macaulay K, Zhou J, Doble BW, Beahm C, et al. Deletion of GSK-3beta in mice leads to hypertrophic cardiomyopathy secondary to cardiomyoblast hyperproliferation. J Clin Invest. 2008 Nov;118(11):3609–18. doi: 10.1172/JCI36245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woulfe KC, Gao E, Lal H, Harris D, Fan Q, Vagnozzi R, et al. Glycogen synthase kinase-3beta regulates post-myocardial infarction remodeling and stress-induced cardiomyocyte proliferation in vivo. Circ Res. 2010 May 28;106(10):1635–45. doi: 10.1161/CIRCRESAHA.109.211482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou J, Lal H, Chen X, Shang X, Song J, Li Y, et al. GSK-3alpha directly regulates beta-adrenergic signaling and the response of the heart to hemodynamic stress in mice. J Clin Invest. 2010 Jul 1;120(7):2280–91. doi: 10.1172/JCI41407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature. 2000 Jul 6;406(6791):86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- 25.Force T, Woodgett JR. Unique and overlapping functions of GSK-3 isoforms in cell differentiation and proliferation and cardiovascular development. J Biol Chem. 2009 Apr 10;284(15):9643–7. doi: 10.1074/jbc.R800077200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Webb IG, Sicard P, Clark JE, Redwood S, Marber MS. Myocardial stress remodelling after regional infarction is independent of glycogen synthase kinase-3 inactivation. J Mol Cell Cardiol. 2010 Nov;49(5):897–900. doi: 10.1016/j.yjmcc.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Webb IG, Nishino Y, Clark JE, Murdoch C, Walker SJ, Makowski MR, et al. Constitutive glycogen synthase kinase-3alpha/beta activity protects against chronic beta-adrenergic remodelling of the heart. Cardiovasc Res. 2010 Aug 1;87(3):494–503. doi: 10.1093/cvr/cvq061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen X, Shevtsov S, Hsich E, Cui L, Haq S, Aronovitz MJ, et al. The β-catenin/T-cell factor/Lymphocyte enhancer factor signaling pathway is required for normal and stress-induced cardiac hypertrophy. Mol Cell Biol. 2006;26:4462–73. doi: 10.1128/MCB.02157-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004 Jan;10(1):55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 30.Ying QL, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, et al. The ground state of embryonic stem cell self-renewal. Nature. 2008 May 22;453(7194):519–23. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doble BW, Patel S, Wood GA, Kockeritz LK, Woodgett JR. Functional redundancy of GSK-3alpha and GSK-3beta in Wnt/beta-catenin signaling shown by using an allelic series of embryonic stem cell lines. Dev Cell. 2007 Jun;12(6):957–71. doi: 10.1016/j.devcel.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahuja P, Zhao P, Angelis E, Ruan H, Korge P, Olson A, et al. Myc controls transcriptional regulation of cardiac metabolism and mitochondrial biogenesis in response to pathological stress in mice. J Clin Invest. 2010 May 3;120(5):1494–505. doi: 10.1172/JCI38331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruel L, Bourouis M, Heitzler P, Pantesco V, Simpson P. Drosophila shaggy kinase and rat glycogen synthase kinase-3 have conserved activities and act downstream of Notch. Nature. 1993 Apr 8;362(6420):557–60. doi: 10.1038/362557a0. [DOI] [PubMed] [Google Scholar]
- 34.Siegfried E, Chou TB, Perrimon N. wingless signaling acts through zeste-white 3, the Drosophila homolog of glycogen synthase kinase-3, to regulate engrailed and establish cell fate. Cell. 1992 Dec 24;71(7):1167–79. doi: 10.1016/s0092-8674(05)80065-0. [DOI] [PubMed] [Google Scholar]
- 35.Klapholz M. Beta-blocker use for the stages of heart failure. Mayo Clin Proc. 2009 Aug;84(8):718–29. doi: 10.4065/84.8.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tseng AS, Engel FB, Keating MT. The GSK-3 inhibitor BIO promotes proliferation in mammalian cardiomyocytes. Chem Biol. 2006;13:957–63. doi: 10.1016/j.chembiol.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 37.Eldar-Finkelman H, Krebs EG. Phosphorylation of insulin receptor substrate 1 by glycogen synthase kinase 3 impairs insulin action. Proc Nat Acad Sci. 1997;94:9660–4. doi: 10.1073/pnas.94.18.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liberman Z, Eldar-Finkelman H. Serine 332 phosphorylation of insulin receptor substrate-1 by glycogen synthase kinase-3 attenuates insulin signaling. J Biol Chem. 2005 Feb 11;280(6):4422–8. doi: 10.1074/jbc.M410610200. [DOI] [PubMed] [Google Scholar]
- 39.Dokken BB, Sloniger JA, Henriksen EJ. Acute selective glycogen synthase kinase-3 inhibition enhances insulin signaling in prediabetic insulin-resistant rat skeletal muscle. Am J Physiol Endocrinol Metab. 2005 Jun;288(6):E1188–94. doi: 10.1152/ajpendo.00547.2004. [DOI] [PubMed] [Google Scholar]
- 40.Henriksen EJ, Kinnick TR, Teachey MK, O’Keefe MP, Ring D, Johnson KW, et al. Modulation of muscle insulin resistance by selective inhibition of GSK-3 in Zucker diabetic fatty rats. Am J Physiol Endocrinol Metab. 2003 May;284(5):E892–900. doi: 10.1152/ajpendo.00346.2002. [DOI] [PubMed] [Google Scholar]
- 41.Leng S, Zhang W, Zheng Y, Liberman Z, Rhodes CJ, Eldar-Finkelman H, et al. Glycogen synthase kinase 3 beta mediates high glucose-induced ubiquitination and proteasome degradation of insulin receptor substrate 1. J Endocrinol. 2010 Aug;206(2):171–81. doi: 10.1677/JOE-09-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y, Tanabe K, Baronnier D, Patel S, Woodgett J, Cras-Meneur C, et al. Conditional ablation of Gsk-3beta in islet beta cells results in expanded mass and resistance to fat feeding-induced diabetes in mice. Diabetologia. 2010 Sep 7; doi: 10.1007/s00125-010-1882-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rayasam GV, Tulasi VK, Sodhi R, Davis JA, Ray A. Glycogen synthase kinase 3: more than a namesake. Br J Pharmacol. 2009 Mar;156(6):885–98. doi: 10.1111/j.1476-5381.2008.00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dokken BB, Henriksen EJ. Chronic selective glycogen synthase kinase-3 inhibition enhances glucose disposal and muscle insulin action in prediabetic obese Zucker rats. Am J Physiol Endocrinol Metab. 2006 Aug;291(2):E207–13. doi: 10.1152/ajpendo.00628.2005. [DOI] [PubMed] [Google Scholar]
- 45.Ring DB, Johnson KW, Henriksen EJ, Nuss JM, Goff D, Kinnick TR, et al. Selective glycogen synthase kinase 3 inhibitors potentiate insulin activation of glucose transport and utilization in vitro and in vivo. Diabetes. 2003 Mar;52(3):588–95. doi: 10.2337/diabetes.52.3.588. [DOI] [PubMed] [Google Scholar]
- 46.Kaidanovich-Beilin O, Eldar-Finkelman H. Long-term treatment with novel glycogen synthase kinase-3 inhibitor improves glucose homeostasis in ob/ob mice: molecular characterization in liver and muscle. J Pharmacol Exp Ther. 2006 Jan;316(1):17–24. doi: 10.1124/jpet.105.090266. [DOI] [PubMed] [Google Scholar]
- 47.Rao R, Hao CM, Redha R, Wasserman DH, McGuinness OP, Breyer MD. Glycogen synthase kinase 3 inhibition improves insulin-stimulated glucose metabolism but not hypertension in high-fat-fed C57BL/6J mice. Diabetologia. 2007 Feb;50(2):452–60. doi: 10.1007/s00125-006-0552-5. [DOI] [PubMed] [Google Scholar]
- 48.Pearce NJ, Arch JR, Clapham JC, Coghlan MP, Corcoran SL, Lister CA, et al. Development of glucose intolerance in male transgenic mice overexpressing human glycogen synthase kinase-3beta on a muscle-specific promoter. Metabolism. 2004 Oct;53(10):1322–30. doi: 10.1016/j.metabol.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 49.MacAulay K, Doble BW, Patel S, Hansotia T, Sinclair EM, Drucker DJ, et al. Glycogen synthase kinase 3alpha-specific regulation of murine hepatic glycogen metabolism. Cell Metab. 2007 Oct;6(4):329–37. doi: 10.1016/j.cmet.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 50.Patel S, Doble BW, MacAulay K, Sinclair EM, Drucker DJ, Woodgett JR. Tissue-specific role of glycogen synthase kinase 3beta in glucose homeostasis and insulin action. Mol Cell Biol. 2008 Oct;28(20):6314–28. doi: 10.1128/MCB.00763-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mussmann R, Geese M, Harder F, Kegel S, Andag U, Lomow A, et al. Inhibition of GSK3 promotes replication and survival of pancreatic beta cells. J Biol Chem. 2007 Apr 20;282(16):12030–7. doi: 10.1074/jbc.M609637200. [DOI] [PubMed] [Google Scholar]
- 52.Stukenbrock H, Mussmann R, Geese M, Ferandin Y, Lozach O, Lemcke T, et al. 9-cyano-1-azapaullone (cazpaullone), a glycogen synthase kinase-3 (GSK-3) inhibitor activating pancreatic beta cell protection and replication. J Med Chem. 2008 Apr 10;51(7):2196–207. doi: 10.1021/jm701582f. [DOI] [PubMed] [Google Scholar]
- 53.Liu Z, Tanabe K, Bernal-Mizrachi E, Permutt MA. Mice with beta cell overexpression of glycogen synthase kinase-3beta have reduced beta cell mass and proliferation. Diabetologia. 2008 Apr;51(4):623–31. doi: 10.1007/s00125-007-0914-7. [DOI] [PubMed] [Google Scholar]
- 54.Han SI, Aramata S, Yasuda K, Kataoka K. MafA stability in pancreatic beta cells is regulated by glucose and is dependent on its constitutive phosphorylation at multiple sites by glycogen synthase kinase 3. Mol Cell Biol. 2007 Oct;27(19):6593–605. doi: 10.1128/MCB.01573-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tanabe K, Liu Z, Patel S, Doble BW, Li L, Cras-Meneur C, et al. Genetic deficiency of glycogen synthase kinase-3beta corrects diabetes in mouse models of insulin resistance. PLoS Biol. 2008 Feb;6(2):e37. doi: 10.1371/journal.pbio.0060037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mora A, Sakamoto K, McManus EJ, Alessi DR. Role of the PDK1-PKB-GSK3 pathway in regulating glycogen synthase and glucose uptake in the heart. FEBS Lett. 2005 Jul 4;579(17):3632–8. doi: 10.1016/j.febslet.2005.05.040. [DOI] [PubMed] [Google Scholar]
- 57.Wang Y, Feng W, Xue W, Tan Y, Hein DW, Li XK, et al. Inactivation of GSK-3beta by metallothionein prevents diabetes-related changes in cardiac energy metabolism, inflammation, nitrosative damage, and remodeling. Diabetes. 2009 Jun;58(6):1391–402. doi: 10.2337/db08-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Omar MA, Wang L, Clanachan AS. Cardioprotection by GSK-3 inhibition: role of enhanced glycogen synthesis and attenuation of calcium overload. Cardiovasc Res. 2010 Jun 1;86(3):478–86. doi: 10.1093/cvr/cvp421. [DOI] [PubMed] [Google Scholar]
- 59.Haq S, Choukroun G, Lim HW, Tymitz KM, del Monte F, Gwathmey J, et al. Differential activation of signal transduction pathways in human hearts with hypertrophy versus advanced heart failure. Circulation. 2001;103:670–7. doi: 10.1161/01.cir.103.5.670. [DOI] [PubMed] [Google Scholar]
- 60.Baba HA, Stypmann J, Grabellus F, Kirchhof P, Sokoll A, Schafers M, et al. Dynamic regulation of MEK/Erks and Akt/GSK-3beta in human endstage heart failure after left ventricular mechanical support: myocardial mechanotransduction-sensitivity as a possible molecular mechanism. Cardiovasc Res. 2003;59:390–9. doi: 10.1016/s0008-6363(03)00393-6. [DOI] [PubMed] [Google Scholar]
- 61.Razeghi P, Bruckner BA, Sharma S, Youker KA, Frazier OH, Taegtmeyer H. Mechanical unloading of the failing human heart fails to activate the protein kinase B/Akt/glycogen synthase kinase-3beta survival pathway. Cardiology. 2003;100:17–22. doi: 10.1159/000072387. [DOI] [PubMed] [Google Scholar]
- 62.Razeghi P, Taegtmeyer H. Activity of the Akt/GSK-3beta pathway in the failing human heart before and after left ventricular assist device support. Cardiovasc Res. 2004;61:196–7. doi: 10.1016/j.cardiores.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 63.Chen Y, Park S, Li Y, Missov E, Hou M, Han X, et al. Alterations of gene expression in failing myocardium following left ventricular assist device support. Physiol Genomics. 2003 Aug 15;14(3):251–60. doi: 10.1152/physiolgenomics.00022.2003. [DOI] [PubMed] [Google Scholar]
- 64.Tateishi K, Ashihara E, Honsho S, Takehara N, Nomura T, Takahashi T, et al. Human cardiac stem cells exhibit mesenchymal features and are maintained through Akt/GSK-3beta signaling. Biochem Biophys Res Commun. 2007 Jan 19;352(3):635–41. doi: 10.1016/j.bbrc.2006.11.096. [DOI] [PubMed] [Google Scholar]
- 65.Buescher JL, Phiel CJ. A noncatalytic domain of glycogen synthase kinase-3 (GSK-3) is essential for activity. J Biol Chem. 2010 Mar 12;285(11):7957–63. doi: 10.1074/jbc.M109.091603. [DOI] [PMC free article] [PubMed] [Google Scholar]