Abstract
The adherens junction (AJ) and tight junction (TJ) are key regulators of epithelial polarity and barrier function. Loss of epithelial phenotype is accompanied by endocytosis of AJs and TJs via unknown mechanisms. Using a model of calcium depletion, we defined the pathway of internalization of AJ and TJ proteins (E-cadherin, p120 and β-catenins, occludin, JAM-1, claudins 1 and 4, and ZO-1) in T84 epithelial cells. Proteinase protection assay and immunocytochemistry revealed orchestrated internalization of AJs and TJs into a subapical cytoplasmic compartment. Disruption of caveolae/lipid rafts did not prevent endocytosis, nor did caveolin-1 colocalize with internalized junctional proteins. Furthermore, AJ and TJ proteins did not colocalize with the macropinocytosis marker dextran. Inhibitors of clathrin-mediated endocytosis blocked internalization of AJs and TJs, and junctional proteins colocalized with clathrin and α-adaptin. AJ and TJ proteins were observed to enter early endosomes followed by movement to organelles that stained with syntaxin-4 but not with markers of late and recycling endosomes, lysosomes, or Golgi. These results indicate that endocytosis of junctional proteins is a clathrin-mediated process leading into a unique storage compartment. Such mechanisms may mediate the disruption of intercellular contacts during normal tissue remodeling and in pathology.
INTRODUCTION
Polarized epithelial cells create a highly efficient and selective barrier between different tissue compartments (Madara, 1998). Two junctional complexes constitute this barrier; the most apically located tight junction (TJ) and closely adjacent adherens junction (AJ; Farquhar and Palade, 1963). TJs and AJs play an important role in regulating paracellular permeability, cell differentiation, and proliferation (Gumbiner, 1996; Vleminckx and Kemler, 1999; Yeaman et al., 1999; Matter and Balda, 2003). Both junctions represent multiprotein complexes composed of transmembrane proteins and cytosolic plaque proteins (Yap et al., 1997; Tsukita et al., 2001; Gonzáles-Mariscal et al., 2003). The former proteins mediate cell-cell adhesion, whereas the latter link TJs and AJs to the cytoskeleton and participate in intracellular signaling. Transmembrane proteins in TJs include occludin, claudins, and junctional adhesion molecule (JAM), whereas its cytoplasmic plaque proteins consist of a number of scaffolding and signaling molecules such as the zonula occludens (ZO) protein family (Tsukita et al., 2001; Gonzáles-Mariscal et al., 2003). A transmembrane AJ protein, E-cadherin, is vital for initiating and maintaining cell-cell contacts (Gumbiner, 1996; Vleminckx and Kemler, 1999) and has unique calcium-binding properties (Koch et al., 1999). The cytoplasmic platform of AJs is comprised of several members of the catenin protein family interacting with E-cadherin (Yap et al., 1997).
Despite complex organization, neither AJs nor TJs are static structures, and they can be rapidly disassembled and reorganized in response to various extracellular stimuli (Gumbiner, 1996; Nusrat et al., 2000). Internalization of AJs and TJs appears to be a common mechanism to rapidly downregulate cell-cell adhesion and allow remodeling of intercellular junctions. Indeed, constitutive endocytosis of AJ and TJ components has been documented in several epithelial cell lines (Polak-Charcon and Ben-Shaul, 1979; Risinger and Larsen, 1981; Le et al., 1999) as well as in rat intestinal and retinal epithelia (Staehelin, 1973; Caldwell et al., 1984; Madara, 1990) and in the human fetal hindgut (Polak-Charcon et al., 1980). Internalization is also induced by various pathophysiologic stimuli including bacterial products (Nusrat et al., 2001; Scott et al., 2002; Hopkins et al., 2003), proinflammatory cytokines (Han et al., 2003), and oxidative stress (Basuroy et al., 2003). Despite abundance in different tissues and a putative role in downregulation of intercellular contacts under normal and diseased conditions, the mechanism by which AJs and TJs are endocytosed remains poorly understood. Studies examining internalization pathways of E-cadherin in several cell lines suggest a role of either clathrin-mediated endocytosis (Le et al., 1999; Palacios et al., 2001), caveolar-mediated pathway (Akhtar and Hotchin, 2001), or macropinocytosis (Paterson et al., 2003). The caveolar-mediated pathway has also been implicated in internalization of TJ proteins in T84 intestinal epithelial cells treated with Clostridium botulinum and Escherichia coli toxins (Nusrat et al., 2001; Hopkins et al., 2003). However, detailed analyses of internalization mechanisms of the apical junctions have not been performed.
Epithelial cells lose intercellular junctions after decrease in extracellular calcium concentration to the micromolar range (Cereijido et al., 1978) and these events are associated with rapid internalization of junctional proteins (Siliciano and Googenough, 1988; Kartenbeck et al., 1991; Kamei et al., 1999). The calcium depletion model allows short-term pharmacological modulation of AJ and TJ endocytosis without compromising cell viability, and it resembles endocytic processes documented in cancer cell lines and in a variety of carcinomas in situ (Polak-Charcon and Ben-Shaul, 1979; Risinger and Larsen, 1981; Remy, 1986; Vega-Salas et al., 1993).
The present study was designed to gain insight into mechanisms of internalization of AJ and TJ proteins in polarized T84 intestinal epithelial cells using the calcium depletion model. We report orchestrated endocytosis of major transmembrane and cytosolic AJ and TJ proteins that is clathrin-mediated and results in delivery of junctional proteins into a unique intracellular storage compartment.
MATERIALS AND METHODS
Antibodies and Other Reagents
The following primary polyclonal antibodies (pAb) and monoclonal antibodies (mAb) were used to detect junctional proteins and organelle markers by immunoflurescence labeling and Western blotting: anti-occludin, ZO-1, claudin-1, JAM-1, and Rab11 pAbs (Zymed Laboratories, San Francisco, CA); anti-claudin-4 mAb (Zymed) anti–JAM-1 mAb (Liu et al., 2000); anti–E-cadherin mAbs (HECD-1 hybridoma supernatant, gift of Dr. Alpha Yap, University of Queensland, Australia and DECMA-1, Sigma Chemical Co., St. Louis, MO); anti–β-catenin pAb (Sigma), anti–β-catenin, p120-catenin, caveolin-1, Rab5, syntaxin-4, LAMP-1 mAbs (BD PharMingen, San Diego, CA); anticlathrin heavy chain, α-adaptin, Rab9, trans-Golgi network (TGN) 38, and calnexin mAbs (Affinity Bioreagents, Golden, CO); anti–Rab4 and Rab5 pAbs (StressGen Biotechnology Corp., Victoria, BC, Canada); anti-Na+/K+ ATPase α-1 (Upstate Biotechnology, Lake Placid, NY). Anti-early endosomal antigen (EEA) 1, and Rab7 and antisyntaxin-3 pAbs were generously provided, respectively, by Dr. Marino Zerial (European Molecular Biology Laboratory, Heidelberg, Germany) and Dr. Thomas Weimbs (Lerner Research Institute, Cleveland, OH). Anti-transferrin receptor, and Golgi marker (GM) 130 mAbs as well as donkey anti-rabbit and goat anti-mouse secondary antibodies conjugated to fluorescent red or green Alexa dyes were obtained from Molecular Probes (Eugene, OR); horseradish peroxidase–conjugated goat antirabbit and anti-mouse secondary antibodies were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA).
Amiloride, 5-(N-ethyl-N-isopropyl)-amiloride (EIPA), phenylarsine oxide (POA), methyl-β-cyclodextrin (MβCD) trypsin, chymotrypsin, were obtained from Sigma; wortmannin, LY 294002, and brefeldin A were purchased from Biomol Research Laboratories (Plymouth Meeting, PA); cholesterol oxidase (Streptomyces sp.) were obtained from Calbiochem (La Jolla, CA); lysinefixable, tetramethylrhodamine-conjugated dextran (Mr = 10,000), rhodaminephalloidin and Alexa Fluor 488–conjugated cholera toxin subunit B were purchased from Molecular Probes. Other reagents were of the highest analytical grade and were obtained from Sigma.
Cell Culture
T84 intestinal epithelial cells (American Type Culture Collection, Manassas, VA) were cultured in a 1:1 mixture of DMEM and Ham's F-12 medium supplemented with 10 mM HEPES, 14 mM NaHCO3, 40 μg/ml penicillin, 90 μg/ml streptomycin, 5% newborn calf serum and adjusted to pH 7.4 (designated further as a complete T84 media). For all experiments, cells were grown for 6–8 d on collagen-coated, permeable polycarbonate filters, 0.4-μm pore size (Costar, Cambridge, MA). Filters with a surface area of 0.33 and 5 cm2 were used for immunocytochemical and biochemical experiments, respectively.
Calcium Depletion and Pharmacological Inhibition of Endocytosis
To deplete extracellular Ca2+, confluent T84 monolayers were washed twice with calcium-free Eagle's minimum essential medium for suspension culture (S-MEM; Sigma) supplemented with 2 mM EGTA, 10 mM HEPES, 14 mM NaHCO3, 40 μg/ml penicillin, 90 μg/ml streptomycin, and 5% dialyzed newborn calf serum and incubated in S-MEM for indicated times at 37°C. For pharmacological inhibition of endocytosis, cells were preincubated for 60 min with inhibitors in complete T84 media followed by a 60- or 120-min incubation in S-MEM containing the same concentration of inhibitor. Experiments utilizing cholesterol depletion were conducted in a serum-free medium supplemented with 0.5% bovine serum albumin. Stock solutions of water-insoluble inhibitors were prepared in DMSO and diluted in cell culture media immediately before each experiment. The final concentration of DMSO was 0.1% and was included in appropriate vehicle controls. Experiments with hypertonic media were conducted using complete T84 media and S-MEM containing 0.4 M sucrose. Experiments with acid media were conducted with complete T84 media and S-MEM without sodium bicarbonate, but containing 20 mM MES and 20 mM succinic acid, pH 5.5 (Shurety et al., 1996).
Immunofluorescence Labeling
Calcium-depleted T84 monolayers were rinsed twice with ice-cold calcium- and magnesium-free HBSS containing 10 mM HEPES (HBSS–), whereas control monolayers were rinsed with HBSS containing calcium and magnesium (HBSS+). Cells were fixed and permeabilized in absolute methanol for 20 min at –20°C followed by blocking in HBSS+ containing 1% bovine serum albumin (blocking buffer) for 1 h at room temperature and incubation for 60 min with primary antibodies in blocking buffer. Cell monolayers were then washed, incubated for 60 min with Alexa dye–conjugated secondary antibodies followed by rinsing and mounting on slides with ProLong Antifade medium (Molecular Probes). For double labeling of junctional proteins with F-actin, monolayers were fixed with 3.7% paraformaldehyde, permeabilized with 0.5% Triton X-100, and sequentially stained with primary and green Alexa dye–conjugated secondary antibodies, whereas F-actin was labeled with rhodamine-phalloidin. Stained monolayers were scanned using a Zeiss LSM510 laser scanning confocal microscope (Zeiss Microimaging Inc., Thornwood, NY) coupled to a Zeiss 100M axiovert and 63× or 100× Pan-Apochromat oil lenses. Fluorescent dyes were imaged sequentially in frame-interlace mode to eliminate crosstalk between channels. Images shown are representative of at least three experiments, with multiple images taken per slide.
Internalization of Cholera Toxin and Fluorescent Dextran
Internalization of cholera toxin was performed as previously described (McIntosh and Schnitzer, 1999) with minor modifications. Briefly, T84 monolayers were washed with ice-cold, serum-free T84 complete medium with 0.5% BSA and fluorescently labeled cholera toxin (2 μg/ml in the serum-free medium) was added to the apical and basolateral compartments. To allow for toxin binding, monolayers were incubated at 4°C for 30 min, washed three times with cold HBSS+, and transferred for 60 min at 37°C to induce endocytosis. Cells were then fixed with cold methanol for 20 min, counterstained for ZO-1, and processed for immunofluorescence as described above. To visualize internalized cholera toxin, all images were taken in the same x-y section below the tight junctions.
To evaluate internalization of the macropinocytosis marker, rhodamine-labeled dextran was dissolved in ice-cold S-MEM at 1 mg/ml and added to the apical and basolateral sides of T84 monolayers. Monolayers were then incubated at 4°C for 30 min to promote the marker diffusion into intercellular space and then incubated for 60 min at 37°C to induce endocytosis. Cells were fixed with 3.7% paraformaldehyde and permeabilized with 0.5% Triton X-100. For colocalization of fluorescent dextran with AJ/TJ proteins, cells were sequentially stained with primary and green Alexa dye–conjugated secondary antibodies.
Proteinase Protection Assay and Immunoblotting
Confluent T84 monolayers were incubated for 120 min in either complete T84 medium or in S-MEM, washed two times with HBSS– on ice, and incubated for 10 min at 37°C with trypsin (0.05%) or chymotrypsin (0.5%) dissolved in HBSS– with 2 mM EGTA. Proteinase solutions were then aspirated and residual proteolytic activity neutralized by incubation for 10 min with either 0.5% solution of soybean trypsin inhibitor or 100 μM solution of chymostatin in HBSS+ at 4°C. Thereafter, cells were homogenized in lysis buffer (20 mM Tris, 50 mM NaCl, 2 mM EDTA, 2 mM EGTA, 1% sodium deoxycholate, 1% Triton X-100, and 0.1% SDS, pH 7.4), containing a proteinase inhibitor cocktail (1:100, Sigma). Lysates were then cleared by centrifugation (30 min at 14, 0000 × g) and immediately boiled in SDS sample buffer. Gel electrophoresis and immunoblotting were conducted by standard methods with 10–20 μg protein per lane. As an internal control to ensure equal protein loading, immunoblots were probed for α-actin. The results shown are representative immunoblots of at least three independent experiments.
Transepithelial Resistance Measurement
The effect of calcium depletion on transepithelial electrical resistance (TEER) was measured using an epithelial voltammeter (EVOM)/End Ohm (World Precision Instruments, Sarasota, FL). The resistance of cell-free collagen-coated filters was subtracted from each experimental point.
RESULTS
Calcium Depletion Results in Orchestrated Internalization of Adherens and Tight Junction Proteins
Because disassembly of AJs and TJs may occur by either redistribution of junctional proteins within the plasma membrane (Revel et al., 1973; Troyanovsky et al., 1999; Fukuhara et al., 2002) or by endocytosis (Risinger and Larsen, 1981; Kartenbeck et al., 1991; Kamei et al., 1999), experiments were performed to determine whether calcium depletion-induced junctional disassembly in T84 cells was associated with internalization of AJ and TJ proteins. Both biochemical and morphological approaches were taken to answer this question.
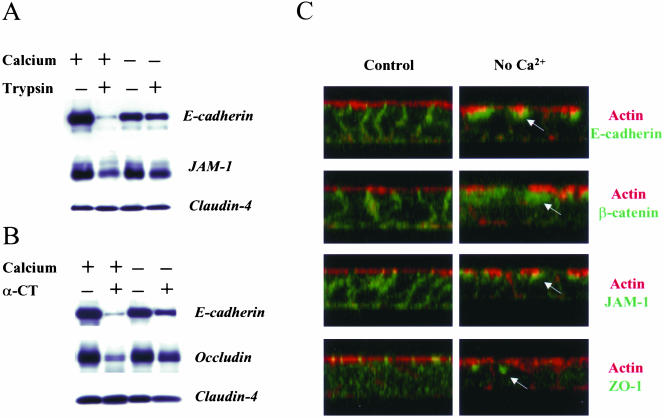
To biochemically assess internalization of AJ and TJ proteins, we used a proteinase protection assay (Le et al., 1999). As shown in Figure 1, A and B, brief (10–15 min) treatment of control T84 monolayers with either trypsin or α-chymotrypsin resulted in almost complete degradation of E-cadherin. In addition, trypsin cleaved a substantial amount of JAM-1. Occludin appeared to be insensitive to trypsin proteolysis, but was effectively degraded by α-chymotrypsin (Figure 1B). Claudin-4, on the other hand, was resistant to both proteinases. Depletion of calcium for 120 min significantly decreased amount of trypsin- and chymotrypsincleavable E-cadherin, trypsin-sensitive JAM-1, and chymotrypsin-cleavable occludin (Figure 1, A and B), suggesting the appearance of an intracellular proteinase-resistant pool of these proteins.
Figure 1.
Depletion of extracellular calcium decreases proteolytic sensitivity of AJ and TJ proteins by inducing their translocation into a subapical intracellular compartment. Confluent T84 monolayers were incubated in either complete T84 medium or in calcium-free S-MEM for 120 min followed by brief exposure to (A) trypsin (0.05%) or (B) α-chymotrypsin (α-CT; 0.5%) solutions as described in MATERIALS AND METHODS. Thereafter, cells were lysed and amounts of junctional proteins in total cell lysates determined by Western blotting. In Panel C, another subset of control and calcium-depleted T84 monolayers was fixed, double-labeled with actin and E-cadherin, β-catenin, JAM-1, or ZO-1, and analyzed by confocal microscopy (x-z images). Calcium depletion decreases the sensitivity of E-cadherin, JAM-1, and occludin to proteolysis indicating internalization of these proteins. Reconstructed confocal images in the x-z plane reveal internalization of E-cadherin, β-catenin, JAM-1, and ZO-1 into a subapical cytosolic compartment located under the apical F-actin (arrows).
As a complementary approach, localization of junctional proteins after calcium depletion was assessed by immunofluorescence labeling and confocal microscopy. As shown in Figure 1C, reconstructed confocal images in the x-z plane of control T84 monolayers revealed localization of ZO-1 in TJs at the level of apical perijunctional F-actin rings. E-cadherin, β-catenin, and JAM-1 were localized along the lateral membrane. Calcium depletion resulted in redistribution of these proteins into a subapical intracellular compartment, where condensed patches of E-cadherin, β-catenin, JAM-1, and ZO-1 were detected underneath the apical F-actin (Figure 1C, arrows).
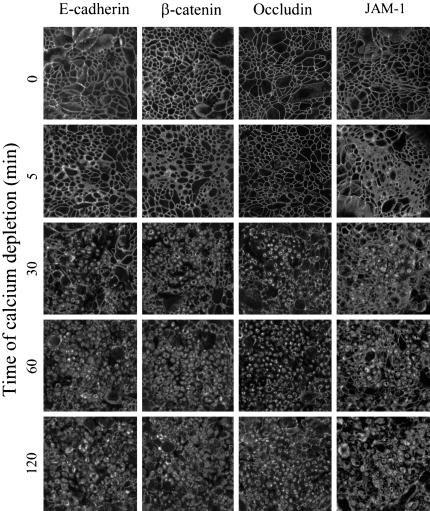
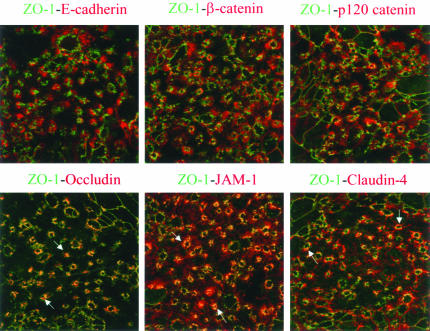
Immunofluorescence analysis of calcium-depleted T84 monolayers revealed similar internalization dynamics of AJ and TJ proteins (Figure 2). Indeed, staining for E-cadherin, β-catenin, occludin, and JAM-1 had a characteristic junctional localization in control T84 monolayers. At time points as early as 5 min after calcium depletion, a concerted movement of these proteins into cytosol was observed. The internalization of junctional proteins was accompanied by a dramatic decrease in transepithelial resistance, which fell from values averaging 2284 to 24 Ω cm2 during the first 5 min of calcium removal. After 30–120 min of the depletion, E-cadherin, β-catenin, occludin, and JAM-1 were found in large ring-like structures positioned centrally within the cell (Figure 2). x-z sections revealed their localization in a subapical position (Figure 1C). Similar findings were observed for other AJ (p120 catenin, afadin) and TJ (ZO-1, claudin-1, claudin-4) proteins (our unpublished results). Double-labeling experiments with E-cadherin-occludin, ZO-1-p120 catenin, and claudin-1-β-catenin pairs showed significant colocalization of AJ and TJ proteins in the cytosolic rings at early times (5–30 min) of calcium depletion (our unpublished results). Similar to intact apical junctions, these junctional protein-containing rings were resistant to extraction with ice-cold 1% solution of Triton-X-100 (our unpublished results), suggesting that calcium depletion leads to endocytosis of the entire apical junctional complex. However at later times (60–120 min) of internalization, AJ and TJ proteins became segregated into different endosomal populations. This is shown in the top panels in Figure 3, where double-immunolabeled calcium-depleted (120 min) monolayers demonstrate little colocalization of ZO-1 with AJ proteins E-cadherin, β-catenin, and p120 catenin. However, as shown in the bottom panels, there was significant colocalization of ZO-1 with TJ proteins occludin, JAM-1 and claudin-4 (Figure 3, arrows). Similar results were obtained when occludin, JAM-1, or claudin-1 were double-immunolabeled with other junctional proteins (our unpublished results).
Figure 2.
Calcium depletion induces rapid orchestrated endocytosis of AJ and TJ proteins. Confluent T84 monolayers were incubated in S-MEM for indicated times and intracellular localization of AJ proteins E-cadherin, β-catenin, and TJ proteins occludin, JAM-1 was determined by immunofluorescence labeling and confocal microscopy. In control T84 cells, all junctional proteins are localized at intercellular contacts revealing a characteristic “chicken wire” staining pattern. Depletion of extracellular calcium leads to a rapid orchestrated translocation of all junctional proteins from the cell border into centrally located ring-like structures.
Figure 3.
Internalized AJ and TJ proteins occupy different parts of a subapical endosomal compartment. T84 cells were incubated for 60 min in S-MEM followed by double immunolabeling for ZO-1 (green) with other junctional proteins (red). In the ring-like structures, ZO-1 colocalizes (yellow) with TJ proteins occludin, JAM-1, and claudin-4 (arrows), but not with AJ proteins E-cadherin, β-catenin, and p120 catenin.
Internalization of Adherens and Tight Junction Proteins after Calcium Depletion Is Not Mediated by Caveolae/Lipid Rafts or Macropinocytosis
Next, we sought to dissect endocytic pathways mediating internalization of junctional proteins after calcium depletion. Three major pathways involving caveolae/lipid rafts, macropinocytosis, and clathrin-coated pits were tested using pharmacological inhibition and immunofluorescence colocalization analysis with specific markers of each pathway. Effects of pharmacological inhibition and immunofluorescence colocalization data are shown in Figures 4, 5, 6, 7, 8, 9 for E-cadherin and occludin. Similar results, that are not reported here, were also obtained for ZO-1, JAM-1, and β-catenin.
Figure 4.
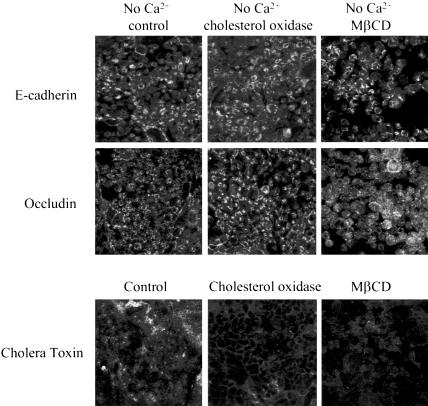
Inhibition of caveolar/lipid raft-mediated endocytosis does not block internalization of junctional proteins in calciumdepleted cells. T84 cells were preincubated for 60 min in serum-free T84 medium with either cholesterol oxidase (2 units/ml) or MβCD (10 mM) followed by a 120-min incubation in serum-free S-MEM containing the same concentrations of inhibitors. Control monolayers were incubated for the indicated times in serum-free T84 medium and S-MEM. Localization of E-cadherin and occludin was determined by immunofluorescence labeling and confocal microscopy. The bottom panel is a positive control, an effect of the inhibitors on cholera toxin uptake. As can be seen, both inhibitors attenuate cholera toxin uptake but fail to prevent internalization of junctional proteins.
Figure 5.
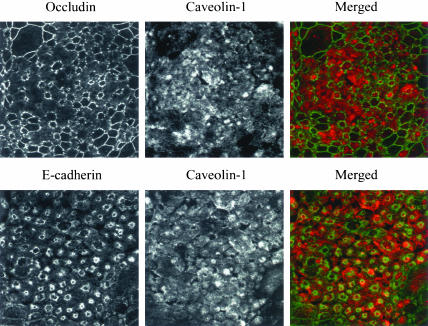
Internalized AJ and TJ proteins do not colocalize with the caveolar marker caveolin-1. T84 cells were depleted of calcium for 15 min and double-immunolabeled for caveolin-1 (red) and E-cadherin or occludin (green). As can be seen, caveolin-1 labeling does not show characteristic ring-like structures and does not colocalize with E-cadherin or occludin. Occasional yellow color observed in the lower merged image is due to focal oversaturation of the caveolin-1 signal.
Figure 6.
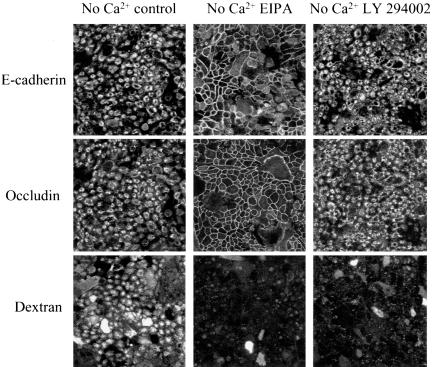
Differential effects of macropinocytosis inhibitors on internalization of junctional proteins in calcium-depleted cells. T84 cells were preincubated for 60 min in regular T84 medium with either 100 μM of inhibitor of the Na+/H+ exchanger, EIPA or 50 μM of the PI 3-kinase inhibitor LY 294002 followed by a 120-min incubation in S-MEM containing the same concentrations of inhibitors. Control monolayers were incubated in T84 medium and S-MEM containing vehicle (0.1% DMSO). Localization of E-cadherin and occludin was determined by immunofluorescence labeling and confocal microscopy. The effect of these inhibitors on internalization of fluorescently labeled dextran, was used as a positive control (bottom panel). Both types of macropinocytosis inhibitors prevent dextran uptake, however one of them (EIPA) significantly attenuates the internalization of junctional proteins, whereas another (LY 294002) is ineffective.
Figure 7.
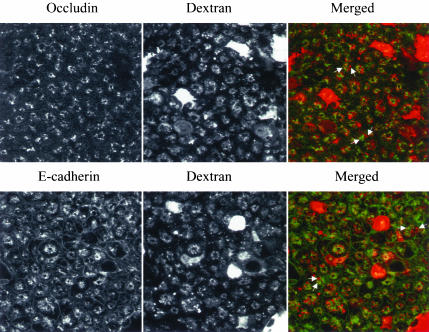
Internalized AJ and TJ proteins do not colocalize with the macropinocytosis marker, dextran. T84 cells were incubated for 60 min in S-MEM containing a 1 mg/ml fluorescently labeled dextran (red) followed by immunostaining for E-cadherin or occludin (green). Although internalized dextran localizes in an intracellular compartment resembling that of E-cadherin and occludin, it does not colocalize (arrows), suggesting a distinct endosomal population.
Figure 8.
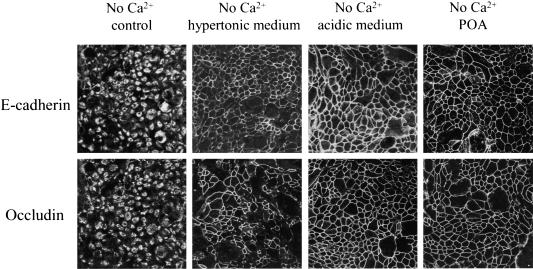
Inhibition of clathrin-mediated endocytosis blocks internalization of junctional proteins in calcium-depleted cells. T84 cells were preincubated for 60 min in either hypertonic (0.4 M sucrose) or acidic (pH 5.5) T84 media or with POA (20 μM) followed by a 120-min incubation in hypertonic or acidic or POA-containing S-MEM. Control monolayers were incubated in complete T84 medium and S-MEM. Localization of E-cadherin and occludin was determined by immunofluorescence labeling and confocal microscopy. All three inhibitors, which are known to prevent the formation of clathrin-coated pits, effectively block internalization of junctional proteins.
Figure 9.
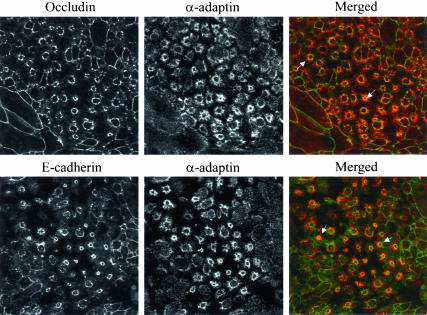
Internalized AJ and TJ proteins colocalize with a marker of clathrin-mediated endocytosis. T84 cells were depleted of calcium for 15 min and double-immunolabeled for occludin and E-cadherin (green) with α-adaptin (red). As can be seen, in the ring-like structures (arrows) occludin and Ecadherin clearly colocalize with the marker of clathrin-mediated endocytosis.
Caveolar-mediated pathway has been shown to be selectively blocked by depletion of cholesterol in plasma membrane (Simons and Toomre, 2000; Smart and Anderson, 2002). To deplete cholesterol, we used two different approaches, one of which involves its enzymatic modification by cholesterol oxidase (Smart et al., 1994; Coconnier et al., 2000; Shigematsu et al., 2003) and another that involves cholesterol extraction from plasma membrane with the cyclic heptasaccaride MβCD (Pike and Casey, 2002; Shigematsu et al., 2003). Incubation of T84 cells with either cholesterol oxidase (2 units/ml) or MβCD (10 mM) for 120 min attenuated intracellular accumulation of cholera toxin (Figure 4), a glycosphingolipid ligand that is dependent on caveolae/lipid rafts internalization pathways (Orlandi and Fishman, 1998). However, inclusion of these inhibitors during 120 min of calcium depletion failed to prevent endocytosis of E-cadherin and occludin (Figure 4). Interestingly, MβCD treatment disrupted cytosolic ring-like structures formed by junctional proteins (Figure 4), suggesting that cholesterol-dependent events occur later during endocytosis of AJ/TJ proteins. Double immunolabeling of occludin and E-cadherin with caveolin-1, a major protein component of caveolae (Razani and Lisanti, 2001), did not detect significant colocalization of internalized junctional proteins with the caveolae marker at different times (5–20 min) of calcium depletion (results of the 15-min time point are presented in Figure 5). Taken together, these data are inconsistent with a role for caveolae in internalization of AJ and TJ proteins in calcium-depleted T84 cells.
To examine the role of macropinocytosis, two standard pharmacological approaches were taken. One approach involved inhibition of the Na+/H+ exchanger using amiloride and its derivatives (West et al., 1989; Meier et al., 2002), and the other involved inhibition of phosphoinositide (PI) 3-kinase (Araki et al., 1996; Amyere et al., 2002). In our study, 5-(N-ethyl-N-isopropyl) amiloride (EIPA, 100 μM), a specific inhibitor of Na+/H+ exchanger, almost completely prevented disassembly of AJs and TJs at 120 min of calcium depletion (Figure 6). Similar results were obtained with amiloride (1 mM), a less potent inhibitor of the Na+/H+ exchanger (our unpublished results). In contrast, two different inhibitors of PI 3-kinase, namely LY 294002 (50 μM; Figure 6) and wortmannin (500 nM; our unpublished results) did not affect endocytosis of E-cadherin and occludin. Interestingly, inhibitors of both Na+/H+ exchanger and PI 3-kinase dramatically blocked internalization of the macropinocytosis marker dextran (Figure 6). To resolve these seemingly disparate results, we compared localization of internalized AJ and TJ proteins with dextran. A 60-min incubation of dextran with calcium-depleted T84 cells resulted in abundant intracellular accumulation of the marker, and the pattern of its intracellular localization was similar to those observed for internalized junctional proteins (Figure 7). However, colocalization analyses demonstrated that endosomes occupied by internalized dextran are clearly distinct from endosomes containing either occludin or E-cadherin (Figure 7, arrows). To verify that the differences in endosomal localization were not the result of late sorting events, we performed double-immunolabeling experiments with occludin, E-cadherin, and fluorescent dextran at earlier time points after calcium depletion (5, 15, and 30 min). There was no colocalization of junctional proteins with dextran at any of these earlier time points (our unpublished results). This lack of colocalization and PI 3-kinase inhibition results suggest that macropinocytosis is unlikely to be involved in the internalization of AJ and TJ proteins after calcium depletion.
Endocytosis of Adherens and Tight Junction Proteins Occurs via a Clathrin-mediated Pathway
To test for a role of clathrin-mediated endocytosis in the internalization of AJ and TJ proteins, we used a panel of inhibitors including hypertonic sucrose, cytosolic acidification, and POA, which are known to prevent assembly of clathrin-coated pits or their pinching off the plasma membrane (Sandvig et al., 1987; Heuser and Anderson, 1989; Moss and Ward, 1991; Shurety et al., 1996). Hyperosmotic media, containing 0.4 M sucrose, cytosolic acidification to pH 5.5, or treatment with POA (20 μM) effectively blocked internalization of E-cadherin and occludin after 120 min of calcium depletion (Figure 8).
Results of pharmacological inhibition were confirmed by immunofluorescence labeling of AJ and TJ proteins with clathrin, which is a major structural component of clathrin-coated pits and α-adaptin, a major component of the so-called AP-2 adaptor complex that mediates recruitment of cargo into clathrin-coated vesicles (Schmid, 1997). Indeed, at 15 min of calcium depletion, there was significant colocalization of both α-adaptin (Figure 9, arrows) and clathrin (our unpublished results) within ring-like structures containing internalized E-cadherin and occludin. In a time course experiment, there was marked colocalization of E-cadherin and occludin with clathrin and α-adaptin at early time points (5–30 min) but not at later time points (60 and 120 min) after chelation of Ca2+ (our unpublished results).
At Early Stages of Endocytosis, Junctional Proteins Are Associated with an Early Endosomal Compartment
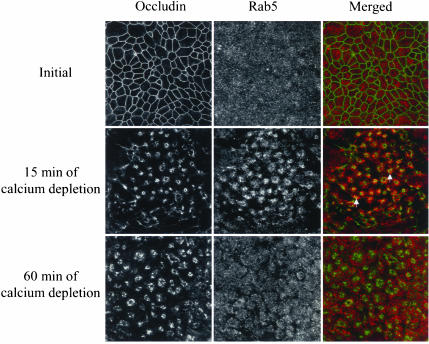
Because it is known that clathrin-coated pits deliver contents to early endosomes where they are sorted and distributed between different intracellular compartments (Mukherjee et al., 1997; Clague, 1998), we compared labeling patterns of internalized AJ and TJ proteins with those of early endosomes. Double immunolabeling experiments utilizing established markers of early endosomes, a small GTPase Rab5, and its effector protein EEA1 were performed. Representative confocal images showing occludin-Rab-5 colocalization are presented in Figure 10. Similar results were obtained in colocalization analysis of occludin with EEA1 as well as for double immunolabeling of E-cadherin, β-catenin, and JAM-1 with both early endosomal markers (Table 1). In T84 cells cultured under normal conditions, Rab5-positive endosomes were randomly distributed throughout the cell (Figure 10). After 15 min of calcium depletion, Rab5 significantly colocalized with ring-like structures containing occludin. This colocalization was evident at 5–30 min but drastically diminished after 60 min of calcium depletion (Figure 10).
Figure 10.
Internalized AJ and TJ proteins transiently localize in early endosomes. Intact T84 cell monolayers or monolayers depleted of calcium for 15 and 60 min were double-immunolabeled for occludin (green) and early endosomal marker, Rab5 (red). As can be seen, internalized occludin colocalizes with the early endosomal markers at early (5 min; arrows) but not late (60 min) times of endocytosis.
Table 1.
Colocalization of internalized apical junctional proteins with intracellular organelle markers
| Organelle marker | E-cadherin | β-catenin | Occludin | JAM-1 |
|---|---|---|---|---|
| Early endosomes | ||||
| Rab5 | +a | + | + | + |
| EEA1 | + | + | + | + |
| Recycling endosomes | ||||
| Rab4 | - | - | - | - |
| Rab11 | - | - | - | - |
| Late endosomes/lysosomes | ||||
| Rab7 | - | - | - | - |
| Rab9 | - | ND | - | ND |
| LAMP-1 | - | - | - | - |
| Golgi | ||||
| GM130 | - | ND | - | - |
| TGN38 | - | ND | - | - |
| Endoplasmic reticulum | ||||
| Calnexin | - | - | - | - |
| Vacuolar apical | ||||
| compartment | ||||
| Syntaxin-3 | - | - | - | - |
| Villin | - | - | - | - |
| Basolateral compartment | ||||
| Syntaxin-4 | + | + | + | + |
| Na+/K+ ATPase | + | + | - | - |
+, significant colocalization; -, no colocalization; ND, not determined.
At Late Stages of Endocytosis, Junctional Proteins Are Associated with a Unique Brefeldin A–resistant, Syntaxin-4–positive Endosomal Compartment
At later stages (60–120 min) of calcium depletion, we observed a loss of colocalization of AJ and TJ proteins with early endosomes (Figure 10). Experiments were performed to characterize this postearly endosomal compartment. As shown in Figure 7, after 60 min of calcium depletion, endosomes containing junctional proteins do not colocalize with fluorescent dextran-positive macropinosomes. Likewise, we observed no colocalization with early/recycling endosomes positive for the transferrin receptor (our unpublished results). Furthermore, the morphology of endosomes containing E-cadherin, β-catenin, JAM-1, and occludin did not change after a 60-min incubation with the fungal toxin, brefeldin A (35 μM), which contrasts with clustering effects of the toxin observed on transferrin receptor-containing endosomes in T84 cells (our unpublished results) and other cell lines (Lippincott-Schwartz et al., 1991).
We next investigated whether internalized AJ and TJ proteins are directed into well-characterized intracellular compartments participating in protein trafficking and processing. Extensive double immunolabeling of E-cadherin, β-catenin, JAM-1, and occludin with markers of different intracellular organelles after 60 and 120 min of calcium depletion was performed. The results summarized in Table 1 demonstrate a lack of colocalization of internalized junctional proteins with markers of recycling endosomes (Rab4 and Rab11), late endosomes (Rab7 and Rab9), lysosomes (LAMP-1), Golgi (GM130 and TGN38), and endoplasmic reticulum (calnexin).
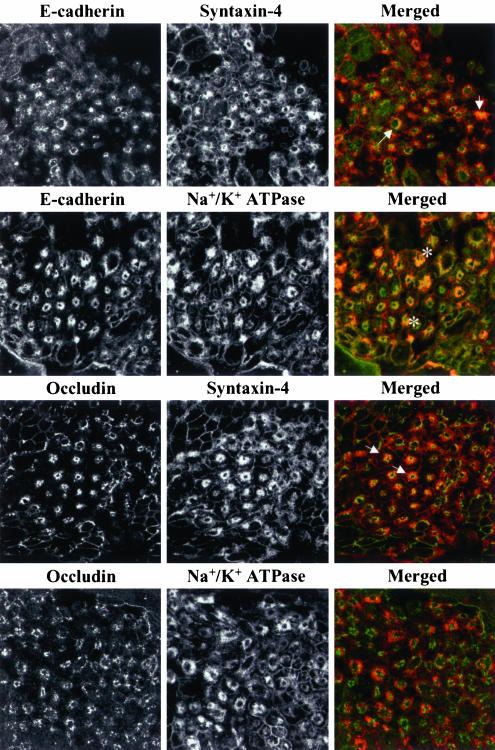
In addition to “classical” organelles involved in protein trafficking and processing, two unusual storage compartments have been described in epithelial cells that have lost polarity. The first is a vacuolar apical compartment containing proteins normally directed to apical plasma membranes (Vega-Salas et al., 1987), and the second is a storage compartment accumulating basolateral plasma membrane proteins (Low et al., 2000). We examined whether internalized junctional proteins localized to either of these compartments by double immunolabeling with markers of apical or basolateral plasma membrane (Table 1). Calcium depletion of T84 cells induced endocytosis of apical plasma membrane markers syntaxin-3 and villin into a cytosolic compartment appearing as condense endosomal clusters (our unpublished results) rather then vacuolae described in other cell lines (Vega-Salas et al., 1987). We did not observe a colocalization of internalized junctional proteins with these markers (Table 1). In contrast, after 60 min of calcium depletion there was marked colocalization of E-cadherin, occludin (Figure 11, arrows), β-catenin, and JAM-1 (Table 1) with syntaxin-4, indicating their accumulation into the storage compartment for basolateral membrane proteins. Surprisingly, another marker for this compartment, Na+/K+ ATPase colocalized only with AJ proteins E-cadherin, and β-catenin, but not with TJ proteins occludin and JAM-1 (Figure 11 and Table 1). These data provide further support to our observation that internalized AJ and TJ proteins are segregated into different endosomal populations at later times of calcium depletion (Figure 3). Syntaxin-4 was observed to colocalize with junctional proteins only at later times (60–120 min) of calcium depletion, when AJ and TJ proteins are segregated into different endosomal populations. To gain insight into the function of this syntaxin-4–containing compartment, we performed experiments to determine whether internalized junctional proteins could be recycled back to plasma membranes. T84 cell monolayers were incubated without calcium for 120 min in the presence of an inhibitor of protein synthesis, cycloheximide (20 μM) and then transferred into cycloheximide-containing medium with a normal calcium concentration. AJ and TJ proteins were then visualized by immunolabeling after 120 min of calcium depletion and after 60 and 180 min of incubation at normal calcium level. We observed that cytosolic ring-like structures containing internalized E-cadherin, β-catenin, occludin, and JAM-1 dispersed after 60 min of calcium repletion. This disruption was accompanied by targeting of the junctional proteins to sites of cell-cell contacts (our unpublished results). This observation suggests that internalized AJ and TJ proteins can recycle back to the apical region of the lateral membrane.
Figure 11.
Internalized AJ and TJ proteins are delivered into different endosomal populations within a storage compartment for basolateral proteins. T84 cells were depleted of calcium for 60 min and double-immunolabeled for E-cadherin and occludin with markers of the storage compartment for basolateral proteins, syntaxin-4 and Na+/K+ ATPase. As can be seen, both internalized E-cadherin and occludin colocalize with syntaxin-4 (arrows) but only E-cadherin colocalize with Na+/K+ ATPase (asterisks).
DISCUSSION
Calcium-dependent Internalizations of Junctional Proteins: General Features
Calcium-induced disassembly/reassembly of adhesive intercellular contacts is the oldest (Cereijido et al., 1978) and most widely used model to study structure and regulation of AJs and TJs. However, many of the mechanisms involved in this process are poorly understood. One important question that has not been resolved is whether intercellular junction are internalized (Siliciano and Goodenough, 1988, Kartenbeck et al., 1991; Kamei et al., 1999) or simply redistributed within the plasma membrane (Revel et al., 1973; Troyanovsky et al., 1999; Fukuhara et al., 2002) after calcium depletion. Our results indicate that endocytosis is the likely mechanism because calcium depletion markedly decreases the sensitivity of E-cadherin, JAM-1, and occludin to degradation by extracellular proteases (Figure 1, A and B). This observation is supported by our immunofluorescence analyses demonstrated translocation of junctional proteins from the lateral cell membrane into cytoplasmic ring-like structures directly underneath apically localized F-actin (Figure 1C).
Endocytosis of AJ and TJ proteins in T84 cells after Ca2+ chelation appears to be a highly synchronized event. Immunofluorescence labeling revealed virtually identical dynamics of internalization for E-cadherin, β-catenin, p120 catenin, ZO-1, JAM-1, occludin, and claudin-1 (Figure 2). This is not specific to T84 cells as we have also observed synchronized endocytosis of AJs and TJs in Caco-2 colonic epithelial cells (Ivanov, Nusrat, and Parkos, unpublished observation) and others have reported orchestrated internalization of AJ and TJ proteins in nontransformed Madin Darby canine kidney (MDCK) cells (Troxell et al., 1999; Rothen-Rutishauser et al., 2002). Furthermore, our double-immunolabeling experiments revealed significant colocalization of AJ and TJ proteins at early time points during endocytosis (Ivanov, Nusrat, Parkos, unpublished observation). These data suggest orchestrated internalization of the entire apical junctional complex in calcium-depleted cells. However, at later stages of endocytosis, we observed AJ and TJ proteins to segregated into two distinct but adjacent endosomal populations in the subapical cytoplasmic domain of T84 cells (Figure 3), which is consistent with separate localization of internalized AJ and TJ proteins in calcium-depleted MDCK cells (Rothen-Rutishauser et al., 2002).
Characterization of the Endocytic Pathway Involved in Internalization of Junctional Proteins
Epithelial cells internalize their plasma membrane proteins using specialized multiprotein endocytotic machineries that remove targets from the cell surface and determine their intracellular destinations (Mukherjee et al., 1997; Clague, 1998). Major endocytotic pathways involving clathrin-coated pits, caveolae, and macropinocytosis have been extensively characterized. Our data suggest that internalization of AJ and TJ proteins in calcium-depleted T84 cells occurs via a clathrin-mediated pathway.
Several lines of evidence support this conclusion. First, chemical agents preventing the assembly of clathrin-coated pits (hypertonic sucrose, cytosolic acidification, and POA) significantly attenuated endocytosis (Figure 8). Second, both clathrin and its auxiliary protein α-adaptin (Figure 9) colocalized with junctional proteins at early time points during internalization. Third, AJ and TJ proteins contain clatrin targeting sorting signals (Table 2). Indeed, protein targeting into clathrin-coated vesicles is generally determined by specific sorting signals that include tyrosine- and dileucine-based motifs usually represented by an YXXØ sequence, (Ø is a bulky hydrophobic residue, and X is any amino acid) and an EXXXLL sequences, with a negatively charged or neutral amino acids in the fourth position upstream of a leucine doublet, respectively (Bonifacino and Traub, 2003). As shown in Table 2, the sequences of the AJ and TJ proteins studied in this report except for JAM-1 contain either the tyrosine or the dileucine putative sorting signals. Such motifs may thus play a role in the clathrin-mediated endocytotic events reported here.
Table 2.
Sorting signals that may be involved in clathrin-mediated endocytosis of adherens and tight junction proteinsa
| Protein (PubMed accession number) | Sequence position | YXXØ- type sorting signals | EXXXLL- type sorting signals |
|---|---|---|---|
| E-cadherin (NP-004351) | 737-743 | VVKEPLL | |
| 827-830 | YDSL | ||
| 859-862 | YDYL | ||
| β-catenin (NP-001895) | 155-160 | ELTKLL | |
| 604-607 | YSPI | ||
| 716-719 | YRSF | ||
| p120 catenin (AAC39808) | 190-193 | YDDL | |
| 354-359 | ALVRLL | ||
| 731-734 | YKEL | ||
| Occludin (AAH29886) | 172-175 | YLSV | |
| 481-484 | YNRL | ||
| ZO-1 (Q07157) | 252-257 | ERATLL | |
| 495-498 | YRRI | ||
| 1512-1515 | YNRF | ||
| Claudin-4 (AAH00671) | 92-97 | ALGVLL | |
| 165-168 | YVGW |
Key residues are indicated in bold type.
Conversely, our results do not support a role for two alternative endocytic pathways for junctional proteins internalization after calcium depletion. Involvement of caveolar/lipid raft-mediated endocytosis is unlikely because cholesterol sequestration failed to prevent internalization of AJs and TJs, and no colocalization of internalized junctional proteins with caveolin-1 was found (Figures 4 and 5). Similarly, a role of macropinocytosis is not supported because there was no colocalization of internalized junctional proteins with markers of macropinocytosis, and there was no prevention of endocytosis after inhibition of an obligate mediator of macropinocytosis, PI 3-kinase (Figures 6 and 7). We did observe attenuation of junctional disassembly by amiloride and its derivatives. However, these substances have been shown to also block receptor-mediated endocytosis in kidney cells (Gekle et al., 2001) and prevent myosin phosphorylation in colonic epithelial cells (Turner et al., 2000). Therefore, the observed inhibitory effects of EIPA and amiloride may be explained by their nonselective blockage of endocytosis via modulation of the actin cytoskeleton. Although our results are in agreement with previous reports suggesting clathrin-mediated endocytosis of E-cadherin in MDCK cells (Le et al., 1999; Palacios et al., 2001), it appears that clathrin may not mediate junctional endocytosis in other types of epithelia. In particular, inhibition of this pathway does not affect internalization of E-cadherin in calcium-depleted keratinocytes where endocytosed E-cadherin colocalizes with caveolin-1 (Akhtar and Hotchin, 2001). These observations suggest that clathrin-coated pits and caveolae participate in the internalization of junctional proteins of nonstratified and stratified epithelia, respectively.
Characterization of Endosomal Compartment Targeted by Internalized Junctional Proteins
We found that internalized AJ and TJ proteins are delivered to a cytosolic compartment localized between the nucleus and the apical plasma membrane. Although this region also contains endosomes enriched with fluorescent dextran and the transferrin receptor, internalized junctional proteins did not colocalize with these markers. Such segregation is consistent with recent observations of distinct populations of subapical endosomes in polarized epithelial cells (Sheff et al., 1999; Brown et al., 2000). Our data suggest delivery of junctional proteins into early endosomes because internalized AJ and TJ proteins colocalize with two early endosomal markers, Rab5 and EEA1 (Figure 10; Table 1). The observed association of internalized junctional proteins with early endosomes was transient and disappeared at later stages (60–120 min) of calcium depletion. However at these times, endosomes containing junctional proteins did not acquire markers of any classic organelles involved in protein trafficking viz., recycling endosomes, late endosomes/lysosomes, trans-Golgi network, or the endoplasmic reticulum (Table 1). Another distinguishing feature of these AJ- and TJ-containing endosomes is their resistance to disruption by brefeldin A treatment. Taken together, these data suggest that at later stages of endocytosis, junctional proteins are delivered into an unusual endosomal compartment distinct from well-characterized intracellular organelles.
We observed that the late compartment containing internalized junctional proteins is enriched in syntaxin-4 (Figure 11) and therefore resembles a syntaxin-4–containing storage compartment for basolateral membrane proteins previously described in nonpolarized MDCK cells (Low et al., 2000). However, within this compartment AJ and TJ proteins are sorted into two different endosomal populations. AJ proteins-containing endosomes are enriched in another basolateral plasma membrane marker, Na+/K+ ATPase, whereas TJ protein-containing endosomes lack this marker (Figure 11). At present it is unknown whether AJ and TJ proteins occupy different parts of the basolateral storage compartment or TJ proteins are targeted to a previously unrecognized syntaxin-4–containing endosomal compartment. Such segregation implies different mechanism of intracellular trafficing AJ and TJ components. Consistent with this notion, a recent report demonstrated that in transfected fibroblasts, transport of the TJ protein claudin-1 and basolateral plasma membrane proteins was regulated by two different members of Rab family, Rab13 and Rab3B, respectively (Yamamoto et al., 2003). Late recruitment of syntaxin-4 to junctional protein-containing endosomes is coincident with segregation of internalized AJ and TJ proteins, suggesting a role of syntaxin-4 in intracellular sorting of junctional proteins. We also report that internalized AJ and TJ proteins readily recycle back to plasma membrane after restoration of normal concentration of calcium even if de novo protein synthesis is inhibited (Ivanov, Nusrat, and Parkos, unpublished observation). These findings suggest that the syntaxin-4–labeling intracellular compartment serves as a temporal depot for junctional proteins involved in rapid remodeling of adhesive intercellular contacts.
Internalizations of Junctional Proteins: Physiological Implications
A number of studies indicate that endocytosis is a fairly common mechanism regulating the biogenesis of AJs and TJs. For example, constitutive endocytosis of E-cadherin and TJ strands takes place in MDCK cells (Le et al., 1999), keratinocytes (Akhtar and Hotchin, 2001), and adenocarcinoma cells (Risinger and Larsen, 1981). Likewise, stimulation of epithelial cells with hormones and growth factors induce internalization of AJ (E-cadherin and β-catenin) and TJ (ZO-1, occludin, and claudin-1) proteins (Kamei et al., 1999; Harhaj et al., 2002; Hollande et al., 2003). Tissue remodeling and morphogenesis in vivo also involve endocytosis of intercellular junctions. For example, internalization of E-cadherin accompanies sea urchin gastrulation (Miller and McClay, 1997), whereas endocytosis of TJ strands has been observed in rat intestinal epithelia (Staehelin, 1974; Madara, 1990), rat retina (Caldwell et al., 1984), and human fetal hindgut (Polak-Charcon et al., 1980). Furthermore, internalization of several AJ and/or TJ proteins may mediate disruption of epithelial barriers by bacterial toxins (Nusrat et al., 2001; Scott et al., 2002; Hopkins et al., 2003), proinflammatory cytokines (Han et al., 2003), or oxidative agents (Basuroy et al., 2003).
Although there is a paucity of data to definitively answer whether internalization of junctional proteins in calcium-depleted cells occurs by the same mechanisms as those occurring under physiological or pathophysiological conditions, there are several lines of evidence suggesting similarities. In particular, orchestrated internalization of TJ proteins in calcium-depleted cells resembles endocytosis of intact TJ strands found in adenocarcinoma cells in vitro (Risinger and Larsen, 1981) and in animal tissues in vivo (Staehelin, 1974; Caldwell et al., 1984; Madara, 1990). In addition, both accelerated endocytosis of E-cadherin in calcium-depleted cells and constitutive processes under normal conditions (Le et al., 1999) appear to be mediated by clathrin. Furthermore, endosomal storage compartments in calcium-depleted cells resemble those reported in normal subconfluent MDCK cells (Low et al., 2000), in a variety of carcinoma cells (Vega-Salas et al., 1993), and in the duodenal mucosa of patients with microvillus inclusion disease (Ameen and Salas, 2000).
In conclusion, the present study demonstrates that depletion of extracellular calcium disrupts barrier function in polarized intestinal epithelial cells by causing orchestrated endocytosis of the entire apical junctional complex. AJ and TJ proteins are internalized via a clathrin-mediated pathway into a syntaxin-4–containing storage compartment from which these proteins may be recycled back to the plasma membrane. We propose that similar endocytosis may occur during normal tissue remodeling and during disruption of the epithelial barrier during pathological states.
Acknowledgments
We greatly appreciate generous donation of antibodies from Drs. M. Zerial, T. Weimbs, and A. Yap. This work was supported by National Institute of Health Grants DK 61379 (to C.A.P.), DK 55679, and DK 59888 (to A.N.); a Digestive Diseases Minicenter Grant DK 64399 (to A.N. and C.A.P.); a Senior Investigator Award from the Crohns and Colitis Foundation (to A.N.); and a Biomedical Sciences grant from the Arthritis Foundation (to A.N.).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–05–0319. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-05-0319.
Abbreviations used: AJ, adherens junction; EEA1, early endosomal antigen 1; EIPA, 5-(N-ethyl-N-isopropyl)-amiloride; JAM, junctional adhesion molecule, MβCD, methyl-β-cyclodextrin, PI, phosphatidylinositol; POA, phenylarsine oxide; TEER, transepithelial electrical resistance; TJ, tight junction; ZO-1, zonula occludens 1.
References
- Akhtar, N., and Hotchin, N.A. (2001). RAC1 regulates adherens junctions through endocytosis of E-cadherin. Mol. Biol. Cell 12, 847–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameen, N.A., and Salas, P.J.I. (2000). Microvillus inclusion disease: a genetic defect affecting apical membrane protein traffic in intestinal epithelium. Traffic 1, 76–83. [DOI] [PubMed] [Google Scholar]
- Amyere, M., Mettlen, M., Van Der Smissen, P., Platek, A., Payrastre, B., Veithen, A., and Courtoy, P.J. (2002). Origin, originality, functions, subversions and molecular signaling of macropinocytosis. Int. J. Med. Microbiol. 291, 487–494. [DOI] [PubMed] [Google Scholar]
- Araki, N., Johnson, M.T., and Swanson, J.A. (1996). A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J. Cell Biol. 135, 1249–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basuroy, S., Sheth, P., Kuppuswamy, D., Balasubramanian, S., Ray, R.M., and Ray, R.K. (2003). Expression of kinase-inactive s-Src delays oxidative stressinduced disassembly and accelerates calcium-mediated reassembly of tight junctions in the Caco-2 cell monolayer. J. Biol. Chem. 278, 11916–11924. [DOI] [PubMed] [Google Scholar]
- Bonifacino, J.S., and Traub, L.M. (2003). Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 72, 395–447. [DOI] [PubMed] [Google Scholar]
- Brown, P.S., Wang, E., Aroeti, B., Chapin, S.J., Mostov, K.E., and Dunn, K.W. (2000). Definition of distinct compartments in polarized Madin-Darby canine kidney (MDCK) cells for membrane-volume sorting, polarized sorting and apical recycling. Traffic 1, 124–140. [DOI] [PubMed] [Google Scholar]
- Caldwell, R.B., Wade, L.A., and McLaughlin, B.J. (1984). A quantitative study of intramembrane changes during cell junctional breakdown in the dystrophic rat retinal pigment epithelium. Exp. Cell Res. 150, 104–117. [DOI] [PubMed] [Google Scholar]
- Cereijido, M., Robbins, E.S., Dolan, W.J., Rotunno, C.A., and Sabatini, D.D. (1978). Polarized monolayers formed by epithelial cells on a permeable and translucent support. J. Cell Biol. 77, 853–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clague, M.L. (1998). Molecular aspects of the endocytic pathways. Biochem. J. 336, 271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coconnier, M.H., Lorrot, M., Barbat, A., Laboisse, C., and Servin, A.L. (2000). Listeriolysin O-induced stimulation of mucin exocytosis in polarized intestinal mucin-secreted cells: evidence for toxin recognition of membrane-associated lipids and subsequent toxin internalization through caveolae. Cell. Microbiol. 2, 487–504. [DOI] [PubMed] [Google Scholar]
- Farquhar, M.G., and Palade, G.E. (1963). Junctional complexes in various epithelia. J. Cell Biol. 17, 375–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara, A., Irie, K., Yamada, A., Katata, T., Honda, T., Shimizu, K., Nakanishi, H., and Takai, Y. (2002). Role of nectin in organization of tight junctions in epithelial cells. Genes Cells 7, 1059–1072. [DOI] [PubMed] [Google Scholar]
- Gekle, M., Freudinger, R., and Mildenberger, S. (2001). Inhibition of Na+-H+ exchanger-3 interferes with apical receptor-mediated endocytosis via vesicle fusion. J. Physiol. 531, 619–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzáles-Mariscal, L., Betanzos, A., Nava, P., and Jaramillo, B.E. (2003). Tight junction proteins. Prog. Biophys. Mol. Biol. 81, 1–44. [DOI] [PubMed] [Google Scholar]
- Gumbiner, B.M. (1996). Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell 84, 345–357. [DOI] [PubMed] [Google Scholar]
- Han, X., Fink, M.P., and Delude, R.L. (2003). Proinflammatory cytokines cause NO-dependent and independent changes in expression and localization of tight junction proteins in intestinal epithelial cells. Shock 19, 229–237. [DOI] [PubMed] [Google Scholar]
- Harhaj, N.S., Barber, A.J., and Antonetti, D.A. (2002). Platelet-derived growth factor mediates tight junction redistribution and increases permeability in MDCK cells. J. Cell. Physiol. 193, 349–364. [DOI] [PubMed] [Google Scholar]
- Heuser, J.E., and Anderson, R.G. (1989). Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J. Cell Biol. 108, 389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollande, F., Lee, D.J., Choquet, A., Roche, S., and Baldwin, G.S. (2003). Adherens junctions and tight junctions are regulated via different pathways by progastrin in epithelial cells. J. Cell Sci. 116, 1187–1197. [DOI] [PubMed] [Google Scholar]
- Hopkins, A.M., Walsh, S.V., Verkade, P., Boquet, P., and Nusrat, A. (2003). Constitutive activation of Rho proteins by CNF-1 influences tight junction structure and epithelial barrier function. J. Cell Sci. 116, 725–742. [DOI] [PubMed] [Google Scholar]
- Kamei, T., Matozaki, T., Sakisaka, T., Kodama, A., Yokoyama, S., Peng, Y.F., Nakano, K., Takaishi, K., and Takai, Y. (1999). Coendocytosis of cadherin and c-Met coupled to disruption of cell-cell adhesion in MDCK cells—regulation by Rho, Rac and Rab small G proteins. Oncogene 18, 6776–6784. [DOI] [PubMed] [Google Scholar]
- Kartenbeck, J., Schmelz, M., Franke, W.W., and Geiger, B. (1991). Endocytosis of junctional cadherins in bovine kidney epithelial (MDBK) cells cultured in low Ca2+ ion medium. J. Cell Biol. 113, 881–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch, A.W., Bozic, D., Pertz, O., and Engel, J. (1999). Homophilic adhesion by cadherins. Curr. Opin. Struct. Biol. 9, 275–281. [DOI] [PubMed] [Google Scholar]
- Le, T.L., Yap, A.S., and Stow, J.L. (1999). Recycling of E-cadherin: a potential mechanism for regulating cadherin dynamics. J. Cell Biol. 146, 219–232. [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz, J., Yuan, L., Tipper, C., Amherdt, M., Orci, L., and Klausner, R.D. (1991). Brefeldin A's effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell 67, 601–616. [DOI] [PubMed] [Google Scholar]
- Liu, Y., Nusrat, A., Schnell, F.J., Reaves, T.A., Walsh, S., Pochet, M., and Parkos, C.A. (2000). Human junctional adhesion molecules regulate tight junction resealing in epithelia. J. Cell Sci. 113, 2363–2374. [DOI] [PubMed] [Google Scholar]
- Low, S.H., Miura, M., Roche, P.A., Valdez, A.C., Mostov, K.E., and Weimbs, T. (2000). Intracellular redirection of plasma membrane trafficking after loss of epithelial cell polarity. Mol. Biol. Cell 11, 3045–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madara, J.L. (1990). Maintenance of the macromolecular barrier at cell extrusion sites in intestinal epithelium: physiological rearrangement of tight junctions. J. Membr. Biol. 116, 177–184. [DOI] [PubMed] [Google Scholar]
- Madara, J.L. (1998). Regulation of the movement of solutes across tight junctions. Annu. Rev. Physiol. 60, 143–159. [DOI] [PubMed] [Google Scholar]
- Matter, K., and Balda, M.S. (2003). Signaling to and from tight junctions. Nat. Rev. Mol. Cell. Biol. 4, 225–236. [DOI] [PubMed] [Google Scholar]
- McIntosh, D.P., and Schnitzer, J.E. (1999). Caveolae require intact VAMP for targeted transport in vascular endothelium. Am. J. Physiol. Heart Circ. Physiol. 277, H2222–H2232. [DOI] [PubMed] [Google Scholar]
- Meier, O., Boucke, K., Hammer, S.V., Keller, S., Stidwill, R.P., Hemmi, S., and Greber, U.F. (2002). Adenovirus triggers macropinocytosis and endosomal leakage together with its clathrin-mediated uptake. J. Cell Biol. 158, 1119–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, J.R., and McClay, D.R. (1997). Characterization of the role of cadherin in regulating cell adhesion during sea urchin development. Dev. Biol. 192, 323–339. [DOI] [PubMed] [Google Scholar]
- Moss, A., and Ward, W.F. (1991). Multiple pathways for ligand internalization in rat hepatocytes. Effects of anoxia, phenylarsine oxide and monensin. J. Cell. Physiol. 149, 313–318. [DOI] [PubMed] [Google Scholar]
- Mukherjee, S., Ghosh, R.N., and Maxfield, F.R. (1997). Endocytosis Physiol. Rev. 77, 759–803. [DOI] [PubMed] [Google Scholar]
- Nusrat, A., Turner, J.R., and Madara, J.L. (2000). Molecular physiology and pathophysiology of tight junctions IV. Regulation of tight junctions by extracellular stimuli: nutrients, cytokines, and immune cells. Am. J. Physiol. Gastrointest. Liver Physiol. 279, G851–G857. [DOI] [PubMed] [Google Scholar]
- Nusrat, A., von Eichel-Streiber, C., Turner, J. R., Verkade, P., Madara, J. L., and Parkos, C. A. (2001). Clostridium difficile toxins disrupt epithelial barrier function by altering membrane microdomain localization of tight junction proteins. Infect. Immun. 69, 1329–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandi, P.A., and Fishman, P.H. (1998). Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolaelike domains. J. Cell Biol. 141, 905–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios, F., Price, L., Schweitzer, J., Collard, J.G., and D'Souza-Schorey, C. (2001). An essential role for ARF6-regulated membrane traffic in adherens junction turnover and epithelial cell migration. EMBO J. 20, 4973–4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson, A.D., Parton, R.G., Ferguson, C., Stow, J.L., and Yap, A.S. (2003). Characterization of E-cadherin endocytosis in isolated MCF-7 and Chinese hamster ovary cells. The initial fate of unbound E-cadherin. J. Biol. Chem. 278, 21050–21057. [DOI] [PubMed] [Google Scholar]
- Pike, L.J., and Casey, L. (2002). Cholesterol levels modulate EGF receptormediated signaling by altering receptor function and trafficking. Biochemistry 41, 10315–10322. [DOI] [PubMed] [Google Scholar]
- Polak-Charcon, S., and Ben-Shaul, Y. (1979). Degradation of tight junctions in HT29, a human colon adenocarcinoma cell line. J. Cell Sci. 35, 393–402. [DOI] [PubMed] [Google Scholar]
- Polak-Charcon, S., Shoham, J., and Ben-Shaul, Y. (1980). Tight junctions in epithelial cells of human fetal hindgut, normal colon, and colon adenocarcinoma. J. Natl. Cancer Inst. 65, 53–62. [PubMed] [Google Scholar]
- Razani, B., and Lisanti, M.P. (2001). Caveolins and caveolae: molecular and functional relationships. Exp. Cell Res. 271, 36–44. [DOI] [PubMed] [Google Scholar]
- Remy, L. (1986). The intracellular lumen: origin, role and implications of a cytoplasmic neostructure. Biol. Cell 56, 97–105. [DOI] [PubMed] [Google Scholar]
- Revel, J.P., Yip, P., and Chang, L.L. (1973). Cell junctions in the early chick embryo—a freeze etch study. Dev. Biol. 35, 302–317. [DOI] [PubMed] [Google Scholar]
- Risinger, M.A., and Larsen, W.J. (1981). Endocytosis of cell-cell junctions and spontaneous cell disaggregation in a cultured human ovarian adenocarcinoma (COLO 316). Tissue Cell 13, 413–430. [DOI] [PubMed] [Google Scholar]
- Rothen-Rutishauser, B., Riesen, F.K., Braun, A., Gunthert, M., and Wunderli-Allenspach, H. (2002). Dynamics of tight and adherens junctions under EGTA treatment. J. Membr. Biol. 188, 151–162. [DOI] [PubMed] [Google Scholar]
- Sandvig, K., Olsnes, S., Petersen, O.W., and Van Deurs, B. (1987). Acidification of the cytosol inhibits endocytosis from coated pits. J. Cell Biol. 105, 679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid, S.L. (1997). Clathrin-coated vesicle formation and protein sorting: an integrated process. Annu. Rev. Biochem. 66, 511–548. [DOI] [PubMed] [Google Scholar]
- Scott, K.G.E., Meddings, J.B., Kirk, D.R., Lees-Miller, S.P., and Buret, A.G. (2002). Intestinal infection with Giardia spp. reduces epithelial barrier function in a myosin light chain kinase-dependent fashion. Gastroenterology 123, 1179–1190. [DOI] [PubMed] [Google Scholar]
- Sheff, D.R., Daro, E., Hull, M., and Mellman, I. (1999). The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions. J. Cell Biol. 145, 123–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigematsu, S., Watson, R.T., Khan, A.H., and Pessin, J.E. (2003). The adipocyte plasma membrane caveolin functional/structural organization is necessary for the efficient endocytosis of GLUT4. J. Biol. Chem. 278, 10683–10690. [DOI] [PubMed] [Google Scholar]
- Shurety, W., Bright, N.A., and Luzio, J.P. (1996). The effect of cytochalasin D and phorbol myristate acetate on the apical endocytosis of ricin in polarized Caco-2 cells. J. Cell Sci. 109, 2927–2935. [DOI] [PubMed] [Google Scholar]
- Siliciano, J.D., and Goodenough, D.A. (1988). Localization of the tight junction protein, ZO-1, is modulated by extracellular calcium and cell-cell contacts in Madin-Darby canine kidney epithelial cells. J. Cell Biol. 107, 2389–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons, K., and Toomre, D. (2000). Lipid rafts and signal transduction. Nat. Rev. Mol. Cell. Biol. 1, 31–41. [DOI] [PubMed] [Google Scholar]
- Smart, E.J., and Anderson, R.G.W. (2002). Alterations in membrane cholesterol that affect structure and function of caveolae. Methods Enzymol. 353, 131–139. [DOI] [PubMed] [Google Scholar]
- Smart, E.J., Ying, Y.S., Conrad, P.A., and Anderson, R.G.W. (1994). Caveolin moves from caveolae to the Golgi apparatus in response to cholesterol oxidation. J. Cell Biol. 127, 1185–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin, L.A. (1973). Further observations on the fine structure of freezecleaved tight junctions. J. Cell Sci. 13, 763–786. [DOI] [PubMed] [Google Scholar]
- Troxell, M.L., Chen, Y.T., Cobb, N., Nelson, W.J., and Marrs, J.A. (1999). Cadherin function in junctional complex rearrangement and posttranslational control of cadherin expression. Am. J. Physiol. Cell Physiol. 276, C404–C418. [DOI] [PubMed] [Google Scholar]
- Troyanovsky, R.B., Klingelhofer, J., and Troyanovsky, S. (1999). Removal of calcium ions triggers a novel type of intercadherin interaction. J. Cell Sci. 112, 4379–4387. [DOI] [PubMed] [Google Scholar]
- Tsukita, S., Furuse, M., and Itoh, M. (2001). Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell. Biol. 2, 285–293. [DOI] [PubMed] [Google Scholar]
- Turner, J.R., Black, E.D., Ward, J., Tse, C.M., Uchwat, F.A., Alli, H.A., Donowitz, M., Madara, J.L., and Angle, J.M. (2000). Transepithelial resistance can be regulated by the intestinal brush-border Na+/H+ exchanger NHE3. Am. J. Physiol. Cell Physiol. 279, C1918–C1924. [DOI] [PubMed] [Google Scholar]
- Vega-Salas, D.E., Salas, P.J.I., and Rodriguez-Boulan, E. (1987). Modulation of the expression of an apical plasma membrane protein of Madin-Darby canine kidney epithelial cells: cell-cell interactions control the appearance of a novel intracellular storage compartment. J. Cell Biol. 104, 1249–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega-Salas, D.E., San Martino, J.A., Salas, P.J., and Baldi, A. (1993). Vacuolar apical compartment (VAC) in breast carcinoma cell lines (MCF-7 and T47D): failure of the cell-cell regulated exocytosis mechanism of apical membrane. Differentiation 54, 131–141. [DOI] [PubMed] [Google Scholar]
- Vleminckx, K., and Kemler, R. (1999). Cadherins and tissue formation: integrating adhesion and signaling. BioEssays 21, 211–220. [DOI] [PubMed] [Google Scholar]
- West, M.A., Bretscher, M.S., and Watts, C. (1989). Distinct endocytic pathways in epidermal growth factor-stimulated human carcinoma A431 cells. J. Cell Biol. 109, 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, Y., Nishimura, N., Morimoto, S., Kitamura, H., Manabe, S., Kanayama, H., Kagawa, S., and Sasaki, T. (2003). Distinct roles of Rab3B and Rab13 in the polarized transport of apical, basolateral, and tight junctional membrane proteins to the plasma membrane. Biochem. Biophys. Res. Commun. 308, 270–275. [DOI] [PubMed] [Google Scholar]
- Yap, A.S., Brieher, W.M., and Gumbiner, B.M. (1997). Molecular and functional analysis of cadherin-based adherens junctions. Annu. Rev. Cell Dev. Biol. 13, 119–146. [DOI] [PubMed] [Google Scholar]
- Yeaman, C., Grindstaff, K.K., and Nelson, W.J. (1999). New perspectives on mechanisms involved in generating epithelial cell polarity. Physiol. Rev. 79, 73–98. [DOI] [PubMed] [Google Scholar]