Abstract
The capacity of stem cells to differentiate into specific cell types makes them very promising in tissue regeneration and repair. However, realizing this promise requires novel methods for guiding lineage-specific differentiation of stem cells. In this study, hepatocyte growth factor (HGF), an important morphogen in liver development, was co-printed with collagen I (Col) to create arrays of protein spots on glass. Human adipose stem cells (ASCs) were cultured on top of the HGF/Col spots for 2 weeks. The effects of surface-immobilized HGF on hepatic differentiation of ASCs were analyzed using RT-PCR, ELISA and immunocytochemistry. Stimulation of stem cells with HGF from the bottom-up caused an upregulation in synthesis of α-fetoprotein and albumin, as determined by immunocytochemistry and ELISA. RT-PCR results showed that the mRNA levels for albumin, α-fetoprotein and α1 antitrypsin were 10 to 20 fold higher in stem cells cultured on the HGF/Col arrays compared to stem cells on Col only spots. Our results show that surfaces containing HGF co-printed with ECM proteins may be used to differentiate mesenchymal stem cells such as ASCs into hepatocyte-like cells. These results underscore the utility of growth factor-containing culture surfaces for stem cell differentiation.
Keywords: Growth factor immobilization, Extracellular matrix, Hepatic differentiation, Mesenchymal stem cells, Protein microarrays
INTRODUCTION
Liver dysfunction is a major health problem in the world. Liver transplantation, while being the only established successful treatment of end-stage liver disease, is limited by the availability of donor livers, and as a result, many patients die each year while on a waiting list[1,2]. Hence, researchers have focused on hepatocyte transplantation which would offer a safer and readily available alternative to whole organ transplantation[3,4]. However, the major limitations of using human primary hepatocytes as the cell source for transplantations are the need for a large number of these cells and low proliferative capacity of primary cells[5]. Therefore, researchers are focusing on differentiation of human stem cells towards hepatocytes as an alternative cell source for liver-based cell therapies [6,7].
Stem cells are capable of self-renewal and have the capacity to differentiate into specific cell types. These properties make them very promising in treating metabolic disorders, such as liver disease [5]. Other advantages of using stem cells in tissue engineering and regenerative medicine include their ease of isolation, proliferative capacity, and the possibility of using autologous stem cells [8]
Several studies have investigated differentiation of stem cells toward hepatocytes. Human mesenchymal stem cells (hMSCs)[9], as well as human embryonic stem cells (hESC)[10], liver stem cells/oval cells [11], cord blood cells[12], bone marrow stem cells [13], and fetal hepatocytes [14] are stem cell types that display potential to develop into hepatocytes. Adipose tissue represents an abundant and accessible source of adult stem cells that can differentiate along several lineage pathways, including hepatic lineage [15,16,17,18].
While multiple factors can contribute to the induction of stem cell differentiation, growth factors (GFs) and extracellular matrix (ECM) proteins represent some of the most important inducers. GFs have been shown to drive hepatic differentiation of embryonic and adult stem cells[19] However, stem cell differentiation studies are typically confounded by the high cost of having to add expensive recombinant proteins (GFs) into the culture media and to frequently change the medium. Supplementing media with GFs also makes it difficult to screen the effects of multiple GF types and combinations on the stem cell phenotype. In contrast to in vitro experiments where GF molecules are dissolved in the medium (present in soluble phase), in vivo GFs interact noncovalently with ECM proteins and are presented to cells in solid-phase [20]. Binding to ECM proteins has been shown to enhance both the strength and the duration of GF signaling to cells in vitro[21]. Therefore, there is a growing interest in solid-phase presentation of GFs on culture surfaces for stimulation of cells [22,23,24,25]. Overall, tethering GF molecules to the surface or immobilizing these molecules in complex with ECM proteins may be a more cost-effective way of utilizing expensive GFs and may represent a more physiological way of providing GF signals to cells.
We have previously described solid-phase immobilization of GFs on ECM arrays for cultivation of primary rat hepatocytes[26,27] and mouse embryonic stem cells[28,29] In the present study, we explored GFs presentation on printed ECM arrays as a means to deliver liver-inductive stimuli to ASCs from the bottom-up. We demonstrate that HGF immobilized on the culture surface induces expression of hepatic phenotype in these mesenchymal stem cells. Our studies underscore the potential use of surface-immobilized GF molecules for guiding differentiation of adult stem cells.
MATERIALS AND METHODS
Chemicals and materials
Glass slides (75×25 mm2) were obtained from VWR (West Chester, PA). (3-Acryloxypropyl)trichlorosilane was purchased from Gelest, Inc. (Morrisville, PA). Sulfuric acid, hydrogen peroxide, ethanol, collagen from rat tail (type I), hepatocyte growth factor (HGF), were obtained from Sigma–Aldrich (St. Louis, MO). Concentrated phosphate-buffered saline (10× PBS) was purchased from Lonza (Walkersville, MD). Minimal essential medium (MEM), sodium pyruvate, non-essential amino acids, fetal bovine serum (FBS), Superscript III, RNase mini kit RNA extraction and cDNA synthesis kit, were purchased from Qiagen (Valencia, CA, USA), 384-well polypropylene microarray plates were obtained from Genetix (New Milton, Hampshire). Goat anti-human albumin antibody and goat anti-human α1-antitrypsin (AAT) antibody, reference serum and Albumin ELISA Quantitation Kit were obtained from Bethyl Laboratories (Montgomery, TX). Mouse anti- goat IgG Texas Red conjugate and rabbit anti-goat FITC-conjugated IgG were purchased from Santa Cruz Biotechnologies, Inc. Formalin was purchased from Fisher (Pittsburgh, PA). Slide-A-Lyzer Mini Dialysis Units were purchased from Pierce (Rockford, IL).
ASCs culture
Human (h)ASCs, were provided by Dr. Jan Nolta, Stem Cell Program, University of California, Davis. The isolated hASCs were characterized by flow cytometric analysis, and the methods for mesodermal differentiation, and their culture and maintenance conditions as described previously [30,31]. Briefly, MSCs were cultivated in MEM containing 10% fetal bovine serum and 2 mM L-glutamine, 100 U mL−1 penicillin, 100 μg mL−1 streptomycin in a polysterene plastic tissue-culture flask. Cultivation of hASCs was carried out at 37°C in a humidified atmosphere containing 95% air and 5% CO2 according to the supplier’s instructions. For all experiments hMSCs were cultivated up to the 4th passage until transfer onto protein arrays.
Preparation of glass substrates
Glass slides were cleaned by immersion in “piranha” solution consisting of 3:1 ratio of aqueous solutions of 50% v/v of sulfuric acid and 30% w/v of hydrogen peroxide for 30 min. The glass slides were thoroughly rinsed with deionized water, dried under nitrogen, and kept in Class 10000 air prior to use. For silane modification, the glass slides were exposed to oxygen plasma for 5 min at 300W (YES3, Yield Engineering Systems, Livermore, CA) and then placed for 10 min in a 2 mM solution of (3-acrylopropyl) trichlorosilane diluted in anhydrous toluene. The reaction was performed in a glove bag under a nitrogen blanket to avoid exposure to atmospheric moisture. After silanization, the slides were rinsed with fresh toluene, dried under nitrogen, and cured at 100° C for 4 h. The silane quality was assessed using contact angle goniometer (Rame-Hart, Netcong, NJ). The silane-modified glass slides were stored in a desiccator before use.
Printing HGF/Col spots
Printing solution consisted of 0.2 mg/mL collagen (I) (Col) and 0.005% (v/v) Tween 20 in 1× PBS. HGF was then added into this solution (500 ng/mL) and was allowed to interact with collagen for 30 min at room temperature prior to printing. Protein arrays were contact-printed under ambient conditions on 75×25 mm2 silane-modified glass slides using a MicroCaster hand-held microarrayer system (Schleicher & Schuell). The pins collected protein (0.2 mg/mL ECM+ 500 ng/mL HGF in 1× PBS with 0.005% Tween) from a 382-well plate, dispensing 20-70 nL of solution onto the glass slide and forming circular spots ~500 μm in diameter. A typical array contained 6 × 12 spots. Protein arrays were kept in a refrigerator before use and were functional for at least one week.
Cultivation of ASCs on collagen and HGF/collagen arrays
After four passages under culture conditions described above, ASCs were transferred into the hepatic induction medium containing IMDM supplemented with 15% FBS, 20ng mL−1 of HGF, 20ng mL−1 of Oncostatin M, 100 U mL−1 penicillin, 100 μg mL−1 streptomycin, 1 mM nonessential amino acids, 0.5 U mL −1, insulin, 14 ng mL−1 glucagon, and 100 nM dexamethasone. ASCs were cultivated in this medium for 7 days. In experiments testing the effects of soluble GFs, stem cells were cultured on Col spots in the above mentioned differentiation media that was supplemented with 20 ng/mL HGF.
For cell seeding experiments, the glass slides with printed protein spots were placed into wells of a conventional six-well plate. The samples were sterilized with 70% ethanol, and washed twice with 1× PBS. Cellular micropatterning was carried out by incubating glass slides ASC suspension in culture medium at a concentration of 1×106 cells/mL at 37° C. After incubation for 1 hour, the medium containing unattached cells was removed, surfaces were washed twice with 1× PBS and stem cell cultures were transferred into a differentiation medium consisting of IMDM supplemented with 15% FBS, 20 ng mL−1 of oncostatin M, 100 U mL−1 penicillin, 100 μg mL−1 streptomycin, 1 mM nonessential amino acids, 0.5 U mL −1, insulin, 14 ng mL−1 glucagon, and 100 nM dexamethasone. ASCs were cultured for 2 weeks in the differentiation medium on Col spots with or without immobilized HGF.
Fluorescent immunohistochemistry analysis
After 2 weeks of differentiation, ASCs cultured on protein spots were stained by immunohistochemistry. Stem cells were washed twice with PBS, fixed for 30 min in a 4% paraformaldehyde solution at RT and then permeabilized with 0.4% Triton X-100 for 20 min. Corresponding primary antibodies including the goat anti-human albumin (1:1000) and goat anti-human α-fetoprotein (AFP) (1:500) were then added to the washed cells and incubated overnight at 4°C. The cells were subsequently washed three times with PBS and incubated with FITC-labeled goat anti-mouse IgG at 37°C, for 1 hour in the dark. After washing with PBS, the cells were incubated with 4, 6-diamidino-2-phenylindole (DAPI) (1:1000) for the purpose of nuclear staining. Stained cells were visualized and imaged using a confocal microscope (Zeiss LSM Pascal)
ELISA analysis of hepatic function
Samples of the medium bathing ASCs on Col and HGF/Col spots were collected at day 0, 5, 10, and 15 and were analyzed for secreted albumin and AAT using ELISA. This analysis was carried out in accordance with manufacturer’s instructions (Bethyl Laboratories) using a microplate reader. Secretion levels of these proteins were normalized by the total cell number. The number of samples (glass surfaces) was n=4 for each condition.
Real-time RT-PCR reaction
Real-time quantitative RT-PCR (qRT-PCR) was used to determine the expression of albumin, AAT and AFP. Briefly, total cellular RNA was prepared using the RNeasy Mini Kit from Qiagen following the manufacturer’s instructions. Single-stranded cDNA was performed using the reverse transcription-PCR protocol of the First Strand cDNA Synthesis Kit (Qiagen) according to the manufacturer’s instructions. For quantitative analysis of samples, the first-strand cDNA was analyzed by TaqMan PCR. Concentrations of all primers were optimized before use and standard curves were plotted. Amplification efficiencies were calculated from the slope of the standard curves generated on parallel reactions. All PCR reactions were performed on three samples (n=3). GAPDH was used as a housekeeping gene in these experiments. Expression of liver specific genes was reported relative to GAPDH.
RESULTS AND DISCUSSION
This study explored the effects of solid-phase presented HGF on hepatic differentiation of ASCs. Stem cells cultured on top of protein spots containing HGF and Col were shown to express hepatic markers and to secrete serum protein associated with liver. Immobilizing GFs on cell culture surfaces represents a promising approach for stem cell differentiation because of the dramatic decrease in cost of culture, the possibility to screen cell-GF interactions in a multiplexed fashion and the enhancement of stimulatory effects of GFs.
Cultivation of ASCs on HGF/Col microarrays
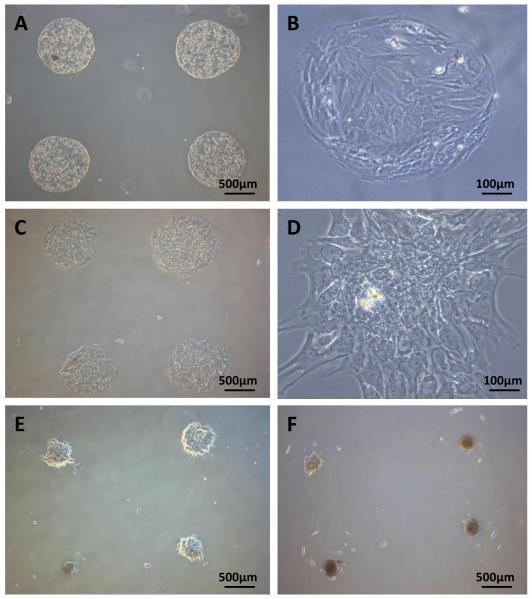
Prior to protein micropatterning, glass slides were modified with acrylated silane ((3-acryloxypropyl)trichlorosilane). The silanization step provided a surface coating that made glass substrates slightly hydrophobic (contact angle ~ 50°), thus, improving the quality of printed protein spots. As collagen type I (Col) is abundant in the liver and has been used as a supporting matrix for culturing primary hepatocytes and stem cells [32], it is used as the ECM protein in this study. To test the ability of surface immobilized HGF to promote hepatic differentiation of ASCs, HGF/Col spots were printed onto silane-modified glass substrates. Glass slides with Col spots without HGF were used as controls. We have previously verified that HGF is retained on the surface of printed protein spots over days in culture [26]. When seeded on these glass substrates, ASCs selectively attached on printed arrays forming ~500 μm diameter clusters of ~100 cells/spot (Fig 1A-D). Cell attachment was defined by the presence of Col in the spots and happened regardless of HGF content. Stem cell organization on protein arrays occurred with high fidelity and minimal non-specific attachment on silanized glass regions. ASCs clusters started to roll-up and formed three-dimensional spheroids after 10 days of culture on HGF/ECM spots (Fig 1E), and by day 15 approximately 90% of the clusters were in a spheroid formation (Fig 1F). In comparison, ASCs cultured on ECM arrays without HGF showed no changes in morphology. The organization of hepatocytes derived from ASCs into 3D spheroids may well be responsible for the enhanced hepatic function induced by the HGF microarrays as a number of recent studies suggested that hepatocytes cultured in 3D configurations such as a spheroids are more functional than standard monolayer cultures [33,34,35]. We have previously observed similar transition from monolayer to spheroid format in primary hepatocytes cultured on HGF spots[26]. We hypothesize that spheroid formation is connected to the high-local concentration of HGF in printed protein spots. It should also be noted that spheroid formation in mesenchymal stem cells did not happen when HGF was added in the media. The likely reason is that concentration of soluble HGF was not high enough to drive this cell re-organization process.
Figures 1. Colonies of ASCs forming on protein arrays.
Mesenchymal stem cells cultured on 500 μm diameter HGF/Col arrays. (A–B) Hepatocytes on Col spots without HGF at 5 days in culture. (C–D) ASCs on HGF/Col spots at day 5 in culture. (E) Start of the spheroid formation on HGF/Col spots after 10 days in culture. Note the edges of the stem cell monolayer are starting to roll up. (F) An array of stem cell spheroids formed on HGF/Col on day 15. No spheroid formation was observed in stem cells cultured on Col spots without HGF.
Evaluation of hepatic differentiation of ASCs by immunohistochemistry analysis
To determine the extent of hepatic differentiation of ASCs, intracellular albumin (liver specific protein) and AFP (a protein indicative of immature hepatocytes) were evaluated by immunohischemical analysis. As shown in Fig 2, a large fraction of ASCs cultured on HGF/Col spots expressed AFP and smaller fraction stained positive for albumin. These results point to an immature phenotype of cells differentiated from ASCs. It should be noted that no AFP and albumin signal was observed in ASCs cultured on Col only spots (data not shown). Given that stem cells residing on both types of surfaces were immersed in the same differentiation medium, HGF presentation on the culture surface appears to be the key determinant of hepatic differentiation in these experiments.
Figure 2. Immunofluorescent staining of albumin and α-fetoprotein.
(A) Staining for albumin (green) in mesenchymal stem cells after 15 day differentiation on HGF/Col spots. (B) Merged albumin and DAPI images show frequency of albumin-expressing stem cells within the field of view. DAPI (blue) stains cell nuclei. (C) Immunostaining for α-fetoprotein (red) after 15 days of differentiation on HGF/col spots. (D) Merged α-fetoprotein and DAPI images.
Evaluation of Hepatic Gene Expression by RT-PCR
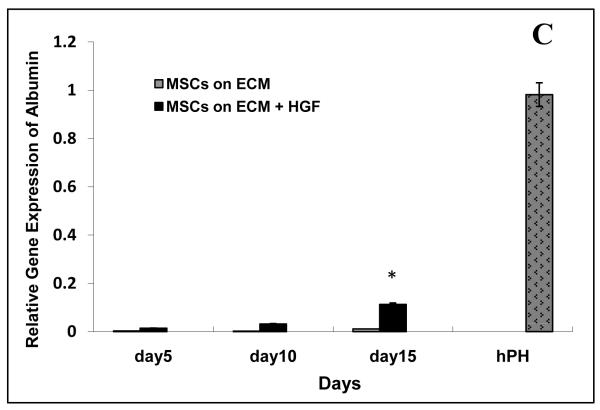
RT-PCR was used to analyze expression of liver specific genes (albumin, AAT, and AFP) in ASCs cultured on Col spots with and without HGF. This analysis revealed that the levels of albumin, AAT and AFP expression were significantly higher (10 to 20 fold) in mesenchymal stem cells cultured on HGF/Col spots then on Col only spots (Fig 3). By day 15, albumin and AAT expression levels in ASCs cultured on HGF were 15% of gene expression in adult hepatocytes. In addition, the mRNA levels for liver specific genes increased significantly over time in stem cells cultured on HGF/Col spots and only moderately in stem cells on Col spot. Overall, our data demonstrate that inclusion of HGF in the printed protein spots had a dramatic effect in upregulating liver-specific gene expression.
Figure 3. qRT-PCR analysis of liver specific gene expression in mesenchymal stem cells cultured on protein spots.
The mRNA expression of albumin, α-fetoprotein (αFP) and α1-antitrypsin (AAT) was determined by real time quantitative RT-PCR at day 5, 10, 15 after differentiation on Col spots with and without HGF. Gene expression is relative to a housekeeping gene GAPDH. (A) AFP gene expression. (B) AAT gene expression. (C) Albumin gene expression.
Hepatic Function of ASCs Cultured on HGF/Col Surfaces
Secretion of proteins into the blood stream is an important function of the liver. Therefore, production of liver proteins such as albumin and AAT is commonly used to assess phenotype of hepatocytes cultured in vitro. ASCs cultured on HGF/Col and Col only spots were analyzed for synthesis of albumin and AAT using ELISA. As shown in Fig 4 stem cells cultured on HGF-containing spots secreted 3 to 4 fold more serum proteins than ASCs residing on Col only spots. Looking at the dynamics of protein secretion over the course of 15 days, one notices only a gradual increase in stem cells on Col spots vs. a dynamic change in production of albumin and AAT in stem cells cultured on HGF/Col spots. This suggests a faster and more pronounced hepatic differentiation of ASCs cultured on HGF-containing surfaces.
Figure 4. ELISA analysis of albumin and α-1 antitrypsin secreted by mesenchymal stem cells during differentiation.
Secretion of liver proteins was analyzed for three cultivation conditions: stem cells on Col spots, stem cells on Col/HGF spots and stem cells on Col spots with HGF added in solution. The amount of secreted protein was normalized by the total number of cells present at the time point of collection. (A) ELISA analysis of AAT production. (B) ELISA analysis of albumin production.
In another set of experiments, protein synthesis in stem cells cultured on HGF/Col spots was compared to protein production in stem cells cultured on Col spots and supplement with HGF in culture media (20 ng/mL). As seen in Fig 4, soluble HGF induced slightly higher (~20 to 30%) levels of protein secretion in ASCs. However, this moderate gain in protein synthesis levels needs to be viewed in light of key differences in how differentiation experiments were performed. Soluble HGF was replenished during daily media changes over the course of 15 days. In contrast, HGF/Col spots were printed once at the beginning of the experiment and, while the medium was changed daily, no HGF supplementation occurred until experiment was stopped at day 15. Experiments with soluble HGF required 60 ng per day of this expensive reagent per well of a 6-well plate, whereas solid-phase presentation experiment required a one-time use of 5.4 ng per well. Therefore, surface immobilization allowed using 165 times less of HGF over the course of a 15 day experiment.
In the present study we have demonstrated that solid phase presentation of HGF was effective in driving differentiation of ASCs towards a hepatocyte lineage. Future directions include cultivating of stem cells on heparin-based hydrogels[36] in order to investigate the synergy of GF signaling and substrate mechanical properties in driving hepatic differentiation of stem cells. Overall, immobilization of signaling molecules on surfaces has broad implications for stem cell differentiation towards liver and other tissue types.
ACKNOWLEDGEMENTS
We thank Prof. Jan Nolta for providing stem cells used in our experiments. We gratefully acknowledge Drs. Yuyu Duan and Ji Youn Lee for technical assistance. This work was supported by an NIH grant (R01DK079977) awarded to AR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Murray KF, Carithers RL, Jr, AASLD AASLD practice guidelines: Evaluation of the patient for liver transplantation. Hepatology. 2005;41:1407–1432. doi: 10.1002/hep.20704. [DOI] [PubMed] [Google Scholar]
- [2].Mohamadnejad M, Alimoghaddam K, Mohyeddin-Bonab M, Bagheri M, Bashtar M, Ghanaati H, Baharvand H, Ghavamzadeh A, Malekzadeh R. Phase 1 Trial of Autologous Bone Marrow Mesenchymal Stem Cell Transplantation in Patients with Decompensated Liver Cirrhosis. Arch Iran Med. 2007;10(4):435–438. [PubMed] [Google Scholar]
- [3].Muraca M, Gerunda G, Neri D, Vilei MT, Granato A, Feltracco P, Meroni M, Giron G, Burlina AB. Hepatocyte transplantation as a treatment for glycogen storage disease type 1a. Lancet. 2002;359(9303):317–318. doi: 10.1016/S0140-6736(02)07529-3. [DOI] [PubMed] [Google Scholar]
- [4].Khan AA, Parveen N, Mahaboob VS, Rajendraprasad A, Ravindraprakash HR, Venkateswarlu J, P. G, Rao P, Narusu ML, Khaja MN, Pramila R, Habeeb A, Habibullah CM. Treatment of Crigler-Najjar Syndrome type 1 by hepatic progenitor cell transplantation: a simple procedure for management of hyperbilirubinemia. Transplant Proc. 2008;40(4):1148–1150. doi: 10.1016/j.transproceed.2008.03.022. [DOI] [PubMed] [Google Scholar]
- [5].Habibullah CM. stem cell in digestive diseases. Indian journal of Gastrology. 2007;26(1):s23–s24. [PubMed] [Google Scholar]
- [6].Lee KD, Kuo TK, Whang-Peng J, Chung YF, Lin CT, Chou SH, Chen JR, Chen YP, Lee OK. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 2004;40:1275–1284. doi: 10.1002/hep.20469. [DOI] [PubMed] [Google Scholar]
- [7].Ochiya T, Yamamoto Y, Banas A. Commitment of stem cells into functional hepatocytes. Differentiation. 2010;79(2):65–73. doi: 10.1016/j.diff.2009.10.002. [DOI] [PubMed] [Google Scholar]
- [8].Jorgensen C, Gordeladze J, Noel D. Tissue engineering through autologous mesenchymal stem cells. Curr Opin Biotechnol. 2004;15(5):406–410. doi: 10.1016/j.copbio.2004.08.003. [DOI] [PubMed] [Google Scholar]
- [9].Stock P, Brückner S, Ebensing S, Hempel M, Dollinger MM, Christ B. The generation of hepatocytes from mesenchymal stem cells and engraftment into murine liver. Nature Protocols. 2010;5:617–627. doi: 10.1038/nprot.2010.7. [DOI] [PubMed] [Google Scholar]
- [10].Duan Y, Catana A, Meng Y, Yamamoto N, He S, Gupta S, Gambhir SS, Zern MA. Differentiation and enrichment of hepatocyte-like cells from human embryonic stem cells in vitro and in vivo. Stem Cells. 2007;25(12):3058–3068. doi: 10.1634/stemcells.2007-0291. [DOI] [PubMed] [Google Scholar]
- [11].Gupta S, LaBrecque DR, Shafritz DA. Mitogenic effect of hepatic stimulator substance on cultured nonparenchymal liver epithelial cells. Hepatology. 1992;15:485–491. doi: 10.1002/hep.1840150322. [DOI] [PubMed] [Google Scholar]
- [12].Newsome PN, Johannessen I, Boyle S, Dalakas E, McAulay KA, Samuel K, Rae F, Forrester L, Turner ML, Hayes PC, Harrison DJ, Bickmore WA, Plevris JN. Human cord blood-derived cells can differentiate into hepatocytes in the mouse liver with no evidence of cellular fusion. Gastroenterology. 2003;124:1891. doi: 10.1016/s0016-5085(03)00401-3. [DOI] [PubMed] [Google Scholar]
- [13].Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T, Lenvik T, Johnson S, Hu WS, Verfaillie CM. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest. 2002;109:1291. doi: 10.1172/JCI15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Malhi H, Irani AN, Gagandeep S, Gupta S. Isolation of human progenitor liver epithelial cells with extensive replication capacity and differentiation into mature hepatocytes. J Cell Sci. 2002;115:2679–2688. doi: 10.1242/jcs.115.13.2679. [DOI] [PubMed] [Google Scholar]
- [15].Gimble JM, Guilak F, Bunnell BA. Clinical and preclinical translation of cell-based therapies using adipose tissue-derived cells. Stem Cell Res Ther. 2010;1(2):19–27. doi: 10.1186/scrt19. 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zuk PA. The Adipose-derived stem cell: looking back and looking ahead. Mol Biol Cell. 2010;21(11):1783–1787. doi: 10.1091/mbc.E09-07-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lue J, Lin G, Ning H, Xiong A, Lin CS, Glenn JS. Transdifferentiation of adipose-derived stem cells into hepatocytes: a new approach. Liver Int. 2010;30(6):913–922. doi: 10.1111/j.1478-3231.2010.02231.x. [DOI] [PubMed] [Google Scholar]
- [18].Coradeghini R, Guida C, Scanarotti C, Sanguineti R, Bassi AM, Parodi A, Santi PL, Raposio E. A comparative study of proliferation and hepatic differentiation of human adipose-derived stem cells. Cells Tissues Organs. 2010;191(6):466–477. doi: 10.1159/000273266. [DOI] [PubMed] [Google Scholar]
- [19].Zaret KS. Hepatocyte differentiation: from the endoderm and beyond. Curr Opin Genet Dev. 2001;11:568–574. doi: 10.1016/s0959-437x(00)00234-3. [DOI] [PubMed] [Google Scholar]
- [20].Schuppan D, Schmid M, Somasundaram R, Ackermann R, Ruehl M, Nakamura T. Collagens in the liver extracellular matrix bind hepatocyte growth factor. Gastroenterology. 1998;114(1):139–152. doi: 10.1016/s0016-5085(98)70642-0. [DOI] [PubMed] [Google Scholar]
- [21].Paralkar VM, Vukicevic S, Reddi AH. Transforming growth factor-beta binds collagen IV of basement membrane matrix: Implications for development. Dev. Biol. 1991;143:303–308. doi: 10.1016/0012-1606(91)90081-d. [DOI] [PubMed] [Google Scholar]
- [22].Kuhl PR, Griffith-Cima LG. Tethered epidermal growth factor as a paradigm for growth factor-induced stimulation from the solid phase. Nature Med. 1996;2:1022–1027. doi: 10.1038/nm0996-1022. [DOI] [PubMed] [Google Scholar]
- [23].Mann BK, Schmedlen RH, West JL. Tethered-TGF-beta increases extracellular matrix production of vascular smooth muscle cells. Biomaterials. 2001;22:439–444. doi: 10.1016/s0142-9612(00)00196-4. [DOI] [PubMed] [Google Scholar]
- [24].Fan VH, Au A, Tamama K, Littrell R, Richardson LB, Wright JW, Wells A, Griffith LG. Tethered epidermal growth factor provides a survival advantage to mesenchymal stem cells. Stem Cells. 2007;25:1241–1251. doi: 10.1634/stemcells.2006-0320. [DOI] [PubMed] [Google Scholar]
- [25].Alberti K, Davey RE, Onishi K, George S, Salchert K, Seib FP, Bornhauser M, Pompe T, Nagy A, Werner C, Zandstra PW. Functional immobilization of signaling proteins enables control of stem cell fate. Nature Methods. 2008;5:645–650. doi: 10.1038/nmeth.1222. [DOI] [PubMed] [Google Scholar]
- [26].Jones CN, Tuleuova N, Lee JY, Ramanculov E, Reddi AH, Zern MA, Revzin A. Cultivation of liver cells on printed arrays of hepatocyte growth factor. Biomaterials. 2009;30:3733–3741. doi: 10.1016/j.biomaterials.2009.03.039. [DOI] [PubMed] [Google Scholar]
- [27].Jones CN, Tuleuova N, Lee JY, Ramanculov E, Reddi AH, Zern MA, Revzin A. Cultivating hepatocytes on printed arrays of HGF and BMP7 to characterize protective effects of these growth factors during in vitro alcohol injury. Biomaterials. 2010;31:5936–5944. doi: 10.1016/j.biomaterials.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lee JY, Tuleuova N, Jones CN, Ramanculov E, Zern MA, Revzin A. Directing hepatic differentiation of embryonic stem cells with protein microarray-based co-cultures. Integrative Biology. 2009;1:460–468. doi: 10.1039/b905757a. [DOI] [PubMed] [Google Scholar]
- [29].Tuleuova N, Lee JY, Lee J, Ramanculov E, Zern MA, Revzin A. Using growth factor arrays and micropatterned co-cultures to induce hepatic differentiation of embryonic stem cells. Biomaterials. 2010;31:9221–9231. doi: 10.1016/j.biomaterials.2010.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Rosová I, Dao M, Capoccia B, Link D, Nolta JA. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008;26(8):2173–2182. doi: 10.1634/stemcells.2007-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Rosová I, Link D, Nolta JA. shRNA-Mediated Decreases in c-Met Levels Affect the Differentiation Potential of Human Mesenchymal Stem Cells and Reduce Their Capacity for Tissue Repair. Tissue Eng Part A. 2010 doi: 10.1089/ten.tea.2009.0363. should be formally published now. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ishii T, Fukumitsu K, Yasuchika K, Adachi K, Kawase E, Suemori H, Nakatsuji N, Ikai I, Uemoto S. Effects of extracellular matrixes and growth factors on the hepatic differentiation of human embryonic stem cells. Am J Physiol Gastrointest Liver Physiol. 2008;295(2):313–321. doi: 10.1152/ajpgi.00072.2008. [DOI] [PubMed] [Google Scholar]
- [33].Chang TT, Hughes-Fulford M. Monolayer and spheroid culture of human liver hepatocellular carcinoma cell line cells demonstrate distinct global gene expression patterns and functional phenotypes. Tissue Eng Part A. 2009;15(3):559–567. doi: 10.1089/ten.tea.2007.0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Curcio E, Salerno S, Barbieri G, De Bartolo L, Drioli E, Bader A. Mass transfer and metabolic reactions in hepatocyte spheroids cultured in rotating wall gaspermeable membrane system. Biomaterials. 2007;28(36):5487–5497. doi: 10.1016/j.biomaterials.2007.08.033. [DOI] [PubMed] [Google Scholar]
- [35].Nakazawa K, Lee SW, Fukuda J, Yang DH, K. T. Hepatocyte spheroid formation on a titanium dioxide gel surface and hepatocyte long-term culture. J Mater Sci Mater Med. 2006;17(4):359–364. doi: 10.1007/s10856-006-8237-7. [DOI] [PubMed] [Google Scholar]
- [36].Kim M, Lee J, Jones CN, Revzin A, Tae G. Heparin-based hydrogel as a matrix for encapsulation and cultivation of primary hepatocytes. Biomaterials. 2010;31:3596–3603. doi: 10.1016/j.biomaterials.2010.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]