Abstract
The most common microdeletion in humans involves the 22q11 region. Congenital anomalies associated with 22q11 loss include cardiac and facial defects. Less frequent is the co-presentation of malignant rhabdoid tumors that are highly aggressive childhood malignancies typically found in renal or extra-renal soft tissues and central nervous system. A newborn patient presented with multiple congenital anomalies consistent with 22q11 deletion syndrome including cleft lip and palate, ear tags and ventricular septal defects co-presenting with an axillary rhabdoid tumor. Comparative genomic hybridization revealed a 2.8 Mb germline deletion in the 22q11.2 region containing genes required for normal fetal development and the SMARCB1 tumor suppressor gene. Analysis of tumor DNA revealed a somatic deletion of exon 7 in the second allele of SMARCB1. Expression of SMARCB1 was absent, while tumor markers including MYC, GFAP and CLAUDIN-6 were upregulated. The presence of tandem oriented BCRL modules located within interspersed low copy repeat elements throughout the 22q11 distal region may predispose this area for microdeletions through nonalleleic homologous recombination.
Introduction
Genomic deletions within the 22q11.2 region are among the most common microdeletion in the human population (estimated at ∼1/4000 live births) and the second most frequent deletion associated with congenital cardiac defects (Goodship et al., 1998; Scambler, 2000; Kobrynski and Sullivan, 2007). Patients with 22q11 deletions manifest malformation phenotypes similar to animal models of neural crest disruption (Scambler, 2000). The 22q11.2 locus is a hotspot for de novo microdeletion, likely due to the presence of a series of 8 low copy repeat (LCR) elements interspersed throughout a 9Mb region that may allow for nonalleleic homologous recombination (Shaikh et al., 2007; Stankiewicz and Lupski, 2002). The most common of these microdeletions is a characteristic 3Mb deletion of a locus termed the “Typically Deleted Region,” or TDR (Shaikh et al., 2000). Deletion of the TDR that spans the proximal LCRs 2-4 (also referred to as LCR B-D) is associated with a broad spectrum of congenital anomalies ranging from cardiac defects, cleft lip and palate, thymic hypoplasia, parathyroid dysfunction, conotruncal anomalies and facial defects (Shprintzen et al., 1978), as well as psychotic features (Kook et al., 2010) and immunodeficiencies (Zemble et al., 2010). These phenotypes have been clinically referred to as DiGeorge syndrome (DGS; MIM 188400) or velo-cardio-facial syndrome (VCFS; MIM 192430). Both syndromes arise from similar deletions in 22q11, suggesting a similar etiology (Scambler et al., 1992). Deletion of the TBX1 gene has been proposed to account for many of the phenotypic features of DGS (Lindsay et al., 2001; Merscher et al., 2001). While TBX1 is a transcription factor that is required throughout all of development for the normal formation of the structures derived from the branchial arches (Xu et al., 2005), there are likely other genetic modifiers that contribute to the DGS phenotype (Aggarwal and Morrow, 2008). Distal deletions encompassing LCRs 4-6 (LCR D-F) have also been reported to be associated with developmental anomalies similar to DGS/VCFS (Mikhail et al., 2007), and have been referred to as 22q11.2 distal deletion syndrome (22q11.2DDS; MIM 611867) (Ben-Shachar et al., 2008; Lafay-Cousin et al., 2009). Germline deletions within this region predispose patients to a variable range of anomalies including cardiac defects and facial abnormalities (Ben-Shachar et al., 2008; Fernandez et al., 2009) that display limited phenotypic overlap with both DGS/VCFS and Goldenhar Syndrome/Oculo-Auriculo-Vertebral Spectrum (OAVS) (MIM 164210) (Derbent et al., 2003; Lafay-Cousin et al., 2009), suggesting the likelihood of many genetic modifiers of these phenotypes associated with defects in branchial arch development. 22q11 distal deletions removing genes residing between LCR6 and LCR7 (F-G) are associated with familial schwannomatosis (MIM 162091) (Hulsebos et al., 2007; Boyd et al., 2008) as well as rhabdoid predisposition syndrome (RPS; MIM 601607) (Sevenet et al., 1999; Roberts and Biegel, 2009) due to the loss of the SMARCB1 (INI1/SNF5) tumor suppressor gene.
A rhabdoid tumor is a highly aggressive and metastatic form of pediatric cancer that typically arises during infancy or early childhood, with time of initial diagnosis being made between 6 months and 5 years of age (median onset of 20 months) (Roberts and Biegel, 2009). Rhabdoid tumors are typically located within the kidneys or the CNS, but less commonly found in bone marrow and bladder. In the CNS, including the brain and spinal cord, they are classified as atypical teratoid/rhabdoid tumors (AT/RTs) (Rorke et al., 1995; Rorke et al., 1996; Pfister et al., 2010); whereas, in soft tissues including the kidney, liver and spleen they are designated malignant rhabdoid tumors (MRTs). Clinical presentation of the tumor depends in large part on the location of the tumor, but often includes a palpable mass, back/neck pain, loss of appetite, nausea/vomiting, and/or hematuria (Madigan et al., 2007). At the time of diagnosis, the majority of these tumors are already metastatic, rendering most therapies ineffective with overall survival of 23% and average survival of 16 months from the time of diagnosis (Tomlinson et al., 2005). These tumors arise from biallelic inactivation of SMARCB1 (INI1/hSNF5) that maps to 22q11.2 between LCRs 6-7 (F-G) and encodes a crucial subunit of the highly conserved SWI/SNF chromatin remodeling complex. The majority of rhabdoid tumors arise from somatic loss of both copies of SMARCB1, with 15-30% associated with germline mutation or deletion (Biegel et al., 1999; Biegel et al., 2002; Biegel, 2006). Many of the germline mutations are acquired de novo and individuals with these mutations appear to have a worse prognosis, often associated with earlier presentation, widespread and therapy-resistant metastases (Savla et al., 2000; Roberts and Biegel, 2009). There are only three published reports of four patients with congenital anomalies consistent with 22q11.2 deletion and co-presentation with aggressive rhabdoid tumors (Wieser et al., 2005; Jackson et al., 2007; Lafay-Cousin et al., 2009). In each of these reported cases, the age of rhabdoid tumor diagnosis ranged from 6 months to 5 years old. Our patient presented with phenotypic features consistent with either DGS/VCFS Syndrome or 22q11DDS with subsequent determination of a rhabdoid tumor at birth, suggesting a possible common germline deletion mediated by chromosome features that are a hallmark of the 22q11.2 region.
Materials and Methods
Immunohistochemistry
Tumor tissues were fixed in 10% buffered formalin and embedded in paraffin. Tissue sections (5-micron) were mounted and immunohistochemistry (IHC) was performed as described (Tang et al., 2008). Citric acid buffer (10mmol/L citrate buffer, pH 6.0) or Tris-EDTA Buffer (pH 9.0) was used for antigen retrieval. Antibodies and working concentrations for IHC detection included SMARCB1/INI1 (mab clone 3E10, 1:1000; Sigma-Aldrich, St. Louis, MO), C-MYC (sc-788, 1:500; Santa Cruz Biotech, Santa Cruz, CA), p53 (sc-4246, 1:500; Santa Cruz Biotech), GFAP (AB5804, 1:500; Millipore Inc., Billerica, MA) and CLAUDIN-6/CLDN6 (01-8865, 1:66; American Research Products, Belmont, MA). Detection was performed using the LSAB + System-HRP kit (K0679, Dako Corp., Carpinteria, CA) according to manufacturer protocols followed by light hematoxylin counterstaining.
Comparative Genome Hybridization (CGH) Array
A peripheral blood sample was used to carry out genomic microarray and FISH analyses by ARUP laboratories (Salt Lake City, UT). Hybridization was performed using the Human Genome CGH Microarray 44K (hg18 assembly) from Agilent Technologies (Santa Clara, CA, USA) with a custom design previously described (Baldwin et al., 2008). The data was analyzed using DNA Analytics 4.0.76software from Agilent Technologies with the ADM-1 algorithm. FISH analysis (probe RP11-8007) was performed to confirm to array results.
DNA Isolation and PCR Analyses
Tumor DNA was obtained from de-paraffinized 10-micron tissue sections using an Arcturus XT laser capture microdissection instrument (Arcturus Biosciences, MountainView, CA) as described (Yadav et al., 2010). The DNA was prepared using the Arcturus PicoPure® DNA extraction kit (Applied Biosystems, Carlsbad, CA) following the manufacturer's instructions. Standard PCR reactions were carried out using GoTaq® Green polymerase (Promega, Madison, WI) and specific primers (see supplementary materials).
Results and Discussion
Clinical Presentation
Our patient was a full term male infant born at 37 weeks via cesarean section to a healthy 26 year old G4 P2 mother. The pregnancy was complicated by gestational diabetes and mild polyhydramnios. Prenatal ultrasound revealed bilateral cleft lip and palate which prompted an amniocentesis that indicated a normal male karyotype (46XY). There was no extended family history of congenital anomalies or malignancies. At birth, physical examination revealed multiple congenital anomalies including bilateral cleft lip and palate, a 2 cm well-demarcated vascular-appearing growth on his left postero-lateral shoulder, bilateral ear tags, a 1cm poorly defined violaceous nodule just medial to the left anterior axillary line and bilateral clubbed feet. An echocardiogram revealed 2 small mid/apical VSDs (Table 1). Shortly after birth, a head CT and MRI revealed cystic encephalomalacia and possible migration disorder of the left frontal lobe, but not other lesions.
Table 1. Clinical and molecular features of patients diagnosed with co-presenting MRT and/or 22q11.2 distal deletion syndrome.
| Clinical feature | Patient 1a | Patient 2b | Patient 3c | Patient 4d | Patient 5d | Patient 6d | 22q11 distale |
|---|---|---|---|---|---|---|---|
| Deletion (LCR) | 2.8Mb (4/7) | 2.8Mb (4/7) | 2.8Mb (4/7) | 2.8Mb (4/7) | 2.8Mb (4/7) | 1.5MB (5/7+) | 1.4-2.1Mb (4/6) |
| Malignant rhabdoid tumor, AT/RT | + | + | + | + | + | + | 0/6 |
| Location of RT | axilla/MRT | MRT | AT/RT | MRT | AT/RT | axilla/MRT | NA |
| Age at RT diagnosis | birth | 6mos | 1yr | 5yr | 1yr | 5weeks | NA |
| Fetal bilateral cleft lip and/or palate | + | - | - | + | + | NR | 2/6 |
| Ear tags | + | + | + | - | - | NR | NR |
| Simple/folded ears | - | + | + | + | - | NR | NR |
| Skeletal defects (clubbed feet, spine deformity) | + | - | NR | NR | NR | NR | 3/6 |
| Cardiac defects (ASD, VSD) | + | + | NR | + | + | NR | 2/6 |
| Mongolian spots | + | - | NR | NR | + | NR | NR |
| Upslanting palpebral fissures | - | + | + | + | + | NR | NR |
| Smooth philtrum | - | + | + | NR | NR | NR | 6/6 |
| Developmental delay | NA | + | + | + | NR | NA | 4/6 |
| Facial dysmorphia/asymmetry | - | + | + | + | + | NR | NR |
| Downturned mouth | - | + | + | - | + | NR | NR |
| Gastroesophageal reflux | NR | NR | + | + | + | NR | NR |
| Hearing loss | NR | + | NR | + | NR | NR | NR |
This study;
NA, not applicable; NR, not reported; MRT, malignant rhabdoid tumor; AT/RT, atypical teratoid/rhabdoid tumor; LCR, low copy repeat
At 3 weeks of age, the vascular-appearing growth on the shoulder began to ulcerate and bleed (Fig. 1A). Over the following two weeks the mass significantly increased in size and the arm became edematous. An ultrasound of the left chest revealed the presence of 2 separate lobular, septated masses—a posterior mass measuring 3.0 × 2.5 × 1.8cm and anterior mass of 2.7 × 1.5 × 3.1cm. Two subsequent abdominal ultrasounds performed 1 week apart revealed an increasing number of hypoechoic lesions in the liver, demonstrating rapid progression of the tumor to metastatic growth. At 4 weeks of age, the infant developed a pleural effusion in which analysis of the pleural effusion fluid revealed many mitotic figures consistent with a large cell epitheloid malignant neoplasm. A chest/abdomen/pelvis CT scan revealed a heterogenous, multiloculated cystic lesion with rim enhancement in the left neck, shoulder, axilla, and thoracic chest wall with extension to the superior mediastinum with multiple hypodense nodules in the liver, subcutaneous tissue and retroperitoneum. MRI of the axilla done at the same time revealed the same large left-sided mass with associated axillary and supraclavicular lymphadenopathy (Fig. 1B). Following surgical debulking of the tumor the patient developed sepsis with vancomycin-resistant Enterococcus, his condition worsened, and the parents made the decision to withdraw care 8 days post-operatively at 7 weeks of age. At autopsy, the infant was found to have bilateral axillary malignant rhabdoid tumors (5.5 × 3.5 × 3cm on the right and 6 × 4 × 4.5cm on the left) with extensive invasion of the thoracic, upper abdominal and cervical organs including pericardium, diaphragm, thymus, thyroid, pleura, paraspinal region, retroperitoneum, mesentery, esophagus, liver, pancreas, and cervical/celiac/retroperitoneal lymph nodes.
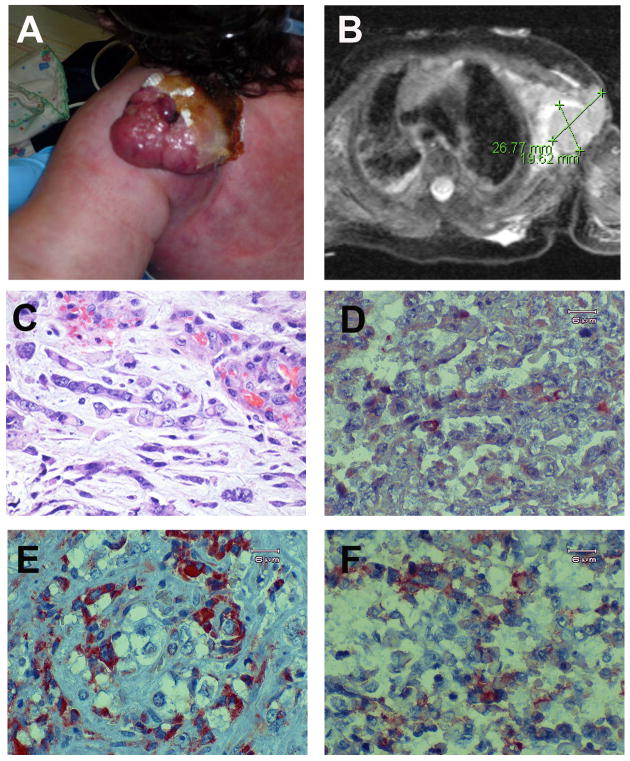
Figure 1. Rhabdoid tumor analysis.
(A) Photograph showing the vascular mass on the posterior aspect of the infant's left shoulder, shortly before excision. The mass had continued to grow in size and has some Vaseline gauze in place over the medial surface due to ulceration and bleeding. (B) T2-weighted MRI image showing a 2.7cm by 1.9cm septated subcutaneous mass in the left axilla. The associated high-signal of the surrounding tissues is consistent with edema. (C-F) Paraffin-embedded tumor sections were used for histologic examination of cellular morphology and immunohistochemical analyses. (C) Tumor section stained with hematoxylin and eosin (H&E). Note the characteristic malignant rhabdoid tumor phenotype, including the large off-center eccentric basophilic nucleus, eosinophilic cytoplasm and prominent nucleolus. (D-F) Immunohistochemical staining of rhabdoid tumor tissue sections. (D) SMARCB1/INI1. Note the absence of staining with the exception of a small population of infiltrating cells. (E) C-MYC and (F) CLDN6/CLAUDIN-6 were upregulated in the tumor cells compared to the non-rhabdoid surrounding cells.
Immunohistochemical and Molecular Analyses
Paraffin-embedded tumor tissue removed during surgery was cut and examined by staining with H&E to reveal the presence of cells of rhabdoid morphology (Fig. 1C), with large sections of the tumor displaying large polygonal cells with distinct borders, prominent nucleoli, eccentric basophilic nuclei, eosinophilic inclusions, and an abundant cytoplasm. There were also areas of the tumor that appeared to be highly necrotic (data not shown). As the axillary vascular growth was initially diagnosed as a possible hemangioma, we sought to verify that the tumor was an MRT by testing for the presence of several MRT-associated markers and confirmed that the tumor cells were positive for GFAP (glial fibrillary acid protein), vimentin, pan-keratin (AE1/AE3), EMA (epithelial membrane antigen), CD99 and smooth muscle actin (Fig. S1).
The overwhelming majority of malignant rhabdoid tumors result from inactivation or deletion of the SMARCB1/INI1 gene. Therefore, we performed an immunohistochemical (IHC) analysis for SMARCB1 and found the protein to be missing in cells displaying rhabdoid morphology (Fig. 2C). Some SMARCB1 positive staining cells were observed within the tumor tissue, likely due to infiltrating non-tumor epithelial and vascular cells.
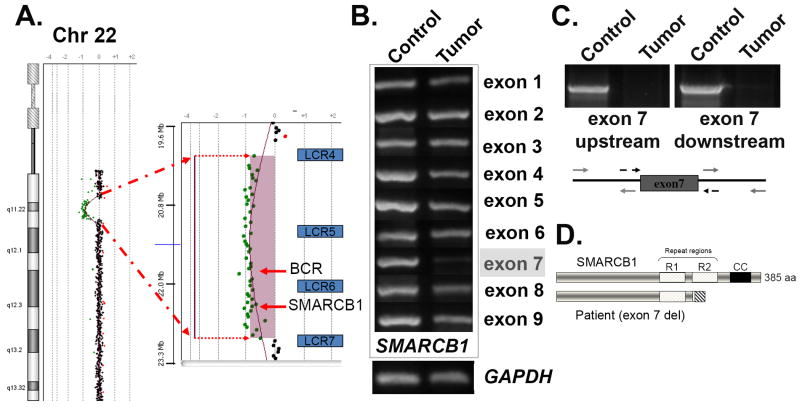
Figure 2. Germline mutation detection and molecular analysis of the rhabdoid tumor.
(A) A peripheral blood sample was used for comparative genomic hybridization (CGH) using the hg18 build on a U-array Cyto6000 platform. A ∼2.8Mb deletion resulted in partial monosomy (-1) in the chromosome 22q11.2 region, indicated by the green dots. A magnified view of the region shown on the right indicates loss of genetic material between the low copy repeat regions 4 and 7 (blu BCR and SMARCB1/hSNF5. (B) PCR analysis of the tumor sample. Chromosomal DNA was isolated following laser capture microdissection of rhabdoid tumor cells and used for PCR analysis. Each of the nine exons of SMARCB1 was individually amplified using both control and tumor cell DNA as templates. The GAPDH gene was used as a control for DNA concentration. Note the strongly reduced PCR product for exon 7. (C) PCR amplification of an extended region around exon 7 was used to verify that the entire exon was deleted in the tumor. The regions indicated by the grey arrows were used for amplification detection. (D) Schematic view of the deletion product that results in a frameshift and premature termination following exon 6. The SMARCB1 protein is shown (top), including the imperfect repeat regions (R1 and R2), as well as the C-terminal coiled-coil (CC). The deletion of exon 7 (lower diagram) results in the complete removal of residues within the R2 and CC regions are required for heterotypic protein interactions and assembly of SMARCB1 into SWI/SNF complexes.
Given the unusually early presentation of the MRT at birth and the extremely aggressive nature, we next sought to determine whether the rhabdoid tumor was positive for several tumor markers, including MYC and CLAUDIN-6/CLDN6. SMARCB1/INI1, a component of SWI/SNF chromatin remodeling complexes, interacts directly with the MYC promoter to decrease its levels in normal cells (Nagl et al., 2006). Recent analyses of rhabdoid tumor gene expression profiles associated with loss of SMARCB1 revealed that MYC transcription was indeed upregulated more than 2-fold (Gadd et al., 2010). Our MYC staining results revealed that the protein was strongly expressed in the rhabdoid morphology cells (Fig. 2E). MYC oncoproteins are a collection of transcription factors with a massive number of target genes (Lee and Dang, 2006; Keller et al., 2010) including those essential for coordinating angiogenic and anti-angiogenic factors in cancer and development. The aberrant regulation of angiogenic activity due to the large upregulation of MYC might contribute to the rapid tumor formation and metastasis of RTs (Albihn et al., 2010). MYC may also contribute to cancer formation through its role in proliferation control as repression of MYC is necessary for cells to differentiate (Eilers and Eisenman, 2008).
CLAUDIN-6 (CLDN6) is a component of tight junctions in renal podocytes where it is involved in the regulation of paracellular NaCl transport (Abuazza et al., 2006). CLDN6 displays elevated levels in early development with drastically reduced levels in adult mouse kidneys (Zhao et al., 2008). CLDN6 has been shown to be upregulated in the cell membranes of brain and spinal cord atypical teratoid/rhabdoid tumors (AT/RT) associated with loss of SMARCB1 (Birks et al., 2010). Although CLDN6 was recently found to be a marker for both rhabdoid glioblastomas (Kleinschmidt-DeMasters et al., 2010) and atypical teratoid/rhabdoid tumors (Birks et al., 2010), its explicit role in rhabdoid tumor pathology is currently unknown. We found strong but variable upregulation of CLDN6 in the membranes of the MRT cells (Fig. 2F), and to our knowledge, this is the first time that CLDN6 upregulation has been demonstrated in MRT. Rhabdoid tumor cells are similar to neural crest cells because each cell type possesses the ability to show divergent differentiation, can migrate over considerable distances, and can be present in both CNS and extra-CNS tissue (Gadd et al., 2010). RT cells may therefore arise from cells destined to become neural crest cells and thus retain the characteristics that the RT cells share with the neural crest cells. Elevated CLDN6 expression in rhabdoid cells may therefore be indicative of a developmentally primitive tumor and correlate with malignancy.
Germline deletion of SMARCB1 followed by a somatic mutation in the second allele is often associated with early onset MRT (<1 yr), aggressive behavior, poor prognosis and is found in ∼15-30% of patients with rhabdoid tumors (Roberts and Biegel, 2009). The co-presentation of congenital abnormalities consistent with 22q11 deletion syndromes (preauricular ear tags, cleft lip and palate, cardiac defects) with the MRT suggested that our patient harbored a large germline deletion of chromosome 22q in a region containing genes required for normal fetal development along with the SMARCB1 gene. Comparative genomic hybridization (CGH) was performed using Agilent arrays (version hg18) along with FISH analyses to determine if the patient harbored a deletion in the 22q11 region (Rickert and Paulus, 2004). These data revealed a 2.8Mb germline deletion (hg18 build; base pairs 20,138,750 – 22,973,264) that resulted in partial monosomy for the 22q11.2 region (Fig. 2A). As expected, the deleted material included the region implicated in 22q11 distal deletion syndrome and the SMARCB1 gene, as well as BCR, MAPK1 and other clinically important genes that reside between the low copy repeat LCR4 and LCR7 elements. The 22q11.2 proximal region implicated in DGS/VCFS was not deleted. In order to confirm the biallelic inactivation of SMARCB1, we isolated genomic DNA from the rhabdoid tumor cells using laser capture microdissection (Yadav et al., 2010). PCR amplification of all nine exons (Fig. 2B) revealed that 8/9 exons appeared to be present in equal amounts; however, the amplification product for exon 7 appeared to be greatly reduced compared to control, suggesting that exon 7 was deleted in the tumor. PCR amplification using additional flanking primer sets (Fig. 2C) confirmed that the exon was completely deleted in the tumor. The extent of the deletion removing exon 7 is not known as both exons 6 and 8 appear to be intact and the distance between these exons is approximately 16.6 kilobases. Predictions of protein coding potential with exon 7 removed indicate a frameshift would follow translation of exon 6 with a subsequent in-frame termination codon, effectively truncating SMARCB1 (Fig. 2D). Since there was no SMARCB1 IHC signal detected in the rhabdoid cells, we conclude that the tumor is null for SMARCB1 protein, most likely due to nonsense mediated decay of the mRNA.
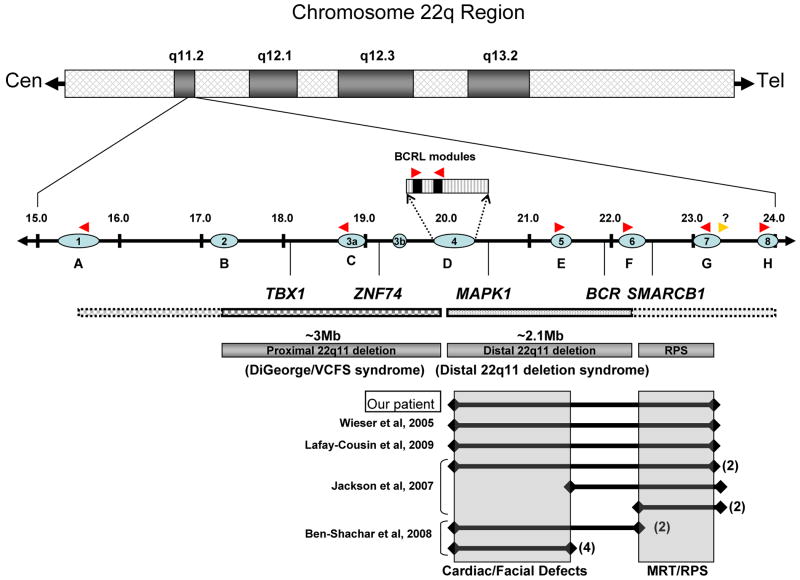
The similarity in clinical phenotype between our patient and others (Wieser et al., 2005; Jackson et al., 2007; Lafay-Cousin et al., 2009) with nearly identical deletions and/or deletion endpoints suggests that the distal 22q11 region may be a hotspot for nonallelic homologous recombination, likely due to the presence of tandem BCRL modules within the LCRs (Fig. 3). These LCRs can become improperly aligned fairly easily during meiotic recombination and result in the deletion of all genetic material between the two recombination sites (Edelmann et al., 1999a; Edelmann et al., 1999b; Shaikh et al., 2001; Shaikh et al., 2007; Ben-Shachar et al., 2008). Deleting the genetic material between LCR1/A and LCR4/D has been implicated in DGS/VCFS while SMARCB1 lies between LCR6/F and LCR7/G (Jackson et al., 2007). An examination of these published case reports reveals three interesting observations (Table 1 and Fig. 3). First, the appearance of a rhabdoid tumor at birth in our patient indicates that the somatic loss of the second SMARCB1 allele also occurred in utero during fetal development and that the presence of a large deletion did not influence the time of tumor onset as has been suggested (Jackson et al., 2007; Lafay-Cousin et al., 2009). Second, the presence of tandem BCRL modules may predispose patients for specific microdeletions, such that the distal oriented BCRL module embedded within LCR4 may be juxtaposed with the similar module on LCR7, while the proximal oriented BCRL module in LCR4 may juxtapose with the modules in LCR5 and LCR6. The presence of reported deletions between LCR5 or LCR6 and a site distal to LCR7 in three reported cases suggests that a cryptic distal-oriented BCRL module may be present between LCR7 and LCR8. Third, the cardio-facial phenotypes of the patients with similar 22q11.2 distal deletions (LCR4/7) are likely due to the removal of genes important for normal cardiac and facial development between LCR4 and LCR6. These genes are non-redundant with those associated with DGS/VCFS proximal deletions that are phenotypically similar. This similar phenotypic presentation has led to some confusion regarding diagnoses, with resolution dependent on molecular characterization.
Figure 3. Germline deletions in 22q11.2 predispose to a variety of developmental defects and cancer.
A schematic of the chromosome 22q11.2 region reveals the presence of a ∼9Mb segment implicated in several congenital birth defect and cancer predisposition syndromes. Interspersed within this region are 8 copies of low copy repeat sequences (LCR, blue ovals) that contain BCRL modules (red arrowheads, direction is related to orientation) that are likely the sites of non-allelic homologous recombination (NAHR). A potential undiscovered BCRL module may reside distal to LCR7 (gold arrow). Recombination between these BCRL modules may result in deletions of intervening chromosomal DNA. Frequent deletion of the region between LCR2 and LCR4 is associated with DGS/VCFS syndrome; while deletion of DNA between LCR4 and LCR6 is associated with 22q distal deletion syndrome. Clinical features of patients with deletions of either region are generally overlapping with several distinguishing features. Select genes located within the deletion syndrome regions are indicated. Molecularly defined germline deletion data obtained from several patients with 22q11 distal deletion syndrome reported in this study or others are indicated. Similar deletions from more than one patient in a given report are indicated by parentheses. Facial defects are frequently linked to loss of genes within the LCR4/6 region, while malignant rhabdoid tumors/rhabdoid predisposition syndrome (MRT/RPS) is associated with loss of genetic material between LCR6/7 (shaded boxes).
Summary
Patients with confirmed MRT have a grave prognosis in which survival is inversely related to age at diagnosis. Infants diagnosed at less than 6 months old have a median survival of approximately 2-4 months from the time of disease progression (Tomlinson et al., 2005). Germline deletions predispose patients for somatic mutations at any earlier age, possibly leading to more aggressive disease (Roberts and Biegel, 2009). Due to the presence of widespread metastases and the extensive invasion of neighboring vessels and organs, the ability to fully resect these tumors is greatly impaired (Madigan et al., 2007). While aggressive therapy (both surgical debulking and chemotherapy) has not been significantly effective in the treatment of rhabdoid tumors, there is some indication that early correct diagnosis may provide some opportunity to initiate therapy sooner and possibly extend survival of RT patients (Katsumi et al., 2008; Nicolaides et al., 2010). Infants diagnosed with congenital anomalies consistent with deletions in 22q11 may benefit from additional tests to determine whether the SMARCB1 gene had been inactivated. Moreover, patients with distal 22q11.2 deletions that extend beyond the SMARCB1 gene may benefit from continual monitoring by ultrasound or periodic head CT for signs of tumor development.
Supplementary Material
Paraffin-embedded tumor sections were used for histologic examination of cellular morphology and verification of MRT. (A) Control H&E stain to verify rhabdoid morphology within the sections used for IHC. (B-H) Immunohistochemical analyses of the rhabdoid tumor. Positive staining is indicated by the reddish/brown color. (B) GFAP immunostain shows elevated expression in the rhabdoid cells. (C) p53 protein is modestly elevated in tumor tissue, but not in surrounding non-tumor cells. Several studies have linked loss of SMARCB1 to upregulation of p53 in rhabdoid tumor cell lines and SMARCB1-deficient murine embryonic fibroblasts (MEF), where it is postulated to serve in oncogenic protection by upregulating genes involved in apoptosis (Isakoff et al., 2005; Kato et al., 2007). (D) Epithelial membrane antigen (EMA); (E) CD99; (F) Vimentin; (G) Smooth muscle actin; (H) Pan-keratin (AE1/AE3). All photographs were taken at 60× magnification.
Acknowledgments
We would like to thank Drs. Manuel Diaz and Nancy Zeleznik-Le for helpful comments and suggestions, Drs. Mitch Denning and Vipin Yadav for help with the laser capture microdissection, the SSOM Department of Pathology histology laboratory for tissue sectioning and Drs. Ricarchito Manera, Gladell Paner and Razvan Lapadat for advice and guidance. We acknowledge the assistance of Dr. Sarah South (ARUP Laboratories; Salt Lake City, UT) for help with the CGH analyses.
References
- Abuazza G, Becker A, Williams SS, Chakravarty S, Truong HT, Lin F, Baum M. Claudins 6, 9, and 13 are developmentally expressed renal tight junction proteins. Am J Physiol Renal Physiol. 2006;291:F1132–1141. doi: 10.1152/ajprenal.00063.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal VS, Morrow BE. Genetic modifiers of the physical malformations in velo-cardio-facial syndrome/DiGeorge syndrome. Dev Disabil Res Rev. 2008;14:19–25. doi: 10.1002/ddrr.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albihn A, Johnsen JI, Henriksson MA. MYC in oncogenesis and as a target for cancer therapies. Adv Cancer Res. 2010;107:163–224. doi: 10.1016/S0065-230X(10)07006-5. [DOI] [PubMed] [Google Scholar]
- Baldwin EL, Lee JY, Blake DM, Bunke BP, Alexander CR, Kogan AL, Ledbetter DH, Martin CL. Enhanced detection of clinically relevant genomic imbalances using a targeted plus whole genome oligonucleotide microarray. Genet Med. 2008;10:415–429. doi: 10.1097/GIM.0b013e318177015c. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar S, Ou Z, Shaw CA, Belmont JW, Patel MS, Hummel M, Amato S, Tartaglia N, Berg J, Sutton VR, Lalani SR, Chinault AC, Cheung SW, Lupski JR, Patel A. 22q11.2 distal deletion: a recurrent genomic disorder distinct from DiGeorge syndrome and velocardiofacial syndrome. Am J Hum Genet. 2008;82:214–221. doi: 10.1016/j.ajhg.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biegel JA. Molecular genetics of atypical teratoid/rhabdoid tumor. Neurosurg Focus. 2006;20:E11. doi: 10.3171/foc.2006.20.1.12. [DOI] [PubMed] [Google Scholar]
- Biegel JA, Tan L, Zhang F, Wainwright L, Russo P, Rorke LB. Alterations of the hSNF5/INI1 gene in central nervous system atypical teratoid/rhabdoid tumors and renal and extrarenal rhabdoid tumors. Clin Cancer Res. 2002;8:3461–3467. [PubMed] [Google Scholar]
- Biegel JA, Zhou JY, Rorke LB, Stenstrom C, Wainwright LM, Fogelgren B. Germ-line and acquired mutations of INI1 in atypical teratoid and rhabdoid tumors. Cancer Research. 1999;59:74–79. [PubMed] [Google Scholar]
- Birks DK, Kleinschmidt-DeMasters BK, Donson AM, Barton VN, McNatt SA, Foreman NK, Handler MH. Claudin 6 is a positive marker for atypical teratoid/rhabdoid tumors. Brain Pathol. 2010;20:140–150. doi: 10.1111/j.1750-3639.2008.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd C, Smith MJ, Kluwe L, Balogh A, Maccollin M, Plotkin SR. Alterations in the SMARCB1 (INI1) tumor suppressor gene in familial schwannomatosis. Clin Genet. 2008;74:358–366. doi: 10.1111/j.1399-0004.2008.01060.x. [DOI] [PubMed] [Google Scholar]
- Derbent M, Yilmaz Z, Baltaci V, Saygili A, Varan B, Tokel K. Chromosome 22q11.2 deletion and phenotypic features in 30 patients with conotruncal heart defects. Am J Med Genet A. 2003;116A:129–135. doi: 10.1002/ajmg.a.10832. [DOI] [PubMed] [Google Scholar]
- Edelmann L, Pandita RK, Morrow BE. Low-copy repeats mediate the common 3-Mb deletion in patients with velo-cardio-facial syndrome. Am J Hum Genet. 1999a;64:1076–1086. doi: 10.1086/302343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmann L, Spiteri E, McCain N, Goldberg R, Pandita RK, Duong S, Fox J, Blumenthal D, Lalani SR, Shaffer LG, Morrow BE. A common breakpoint on 11q23 in carriers of the constitutional t(11;22) translocation. Am J Hum Genet. 1999b;65:1608–1616. doi: 10.1086/302689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilers M, Eisenman RN. Myc's broad reach. Genes Dev. 2008;22:2755–2766. doi: 10.1101/gad.1712408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez L, Nevado J, Santos F, Heine-Suner D, Martinez-Glez V, Garcia-Minaur S, Palomo R, Delicado A, Pajares IL, Palomares M, Garcia-Guereta L, Valverde E, Hawkins F, Lapunzina P. A deletion and a duplication in distal 22q11.2 deletion syndrome region. Clinical implications and review. BMC Med Genet. 2009;10:48. doi: 10.1186/1471-2350-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadd S, Sredni ST, Huang CC, Perlman EJ. Rhabdoid tumor: gene expression clues to pathogenesis and potential therapeutic targets. Lab Invest. 2010;90:724–738. doi: 10.1038/labinvest.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodship J, Cross I, LiLing J, Wren C. A population study of chromosome 22q11 deletions in infancy. Arch Dis Child. 1998;79:348–351. doi: 10.1136/adc.79.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsebos TJ, Plomp AS, Wolterman RA, Robanus-Maandag EC, Baas F, Wesseling P. Germline mutation of INI1/SMARCB1 in familial schwannomatosis. Am J Hum Genet. 2007;80:805–810. doi: 10.1086/513207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isakoff MS, Sansam CG, Tamayo P, Subramanian A, Evans JA, Fillmore CM, Wang X, Biegel JA, Pomeroy SL, Mesirov JP, Roberts CW. Inactivation of the Snf5 tumor suppressor stimulates cell cycle progression and cooperates with p53 loss in oncogenic transformation. Proc Natl Acad Sci U S A. 2005;102:17745–17750. doi: 10.1073/pnas.0509014102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson EM, Shaikh TH, Gururangan S, Jones MC, Malkin D, Nikkel SM, Zuppan CW, Wainwright LM, Zhang F, Biegel JA. High-density single nucleotide polymorphism array analysis in patients with germline deletions of 22q11.2 and malignant rhabdoid tumor. Hum Genet. 2007;122:117–127. doi: 10.1007/s00439-007-0386-3. [DOI] [PubMed] [Google Scholar]
- Kato H, Honma R, Sanda T, Fujiwara T, Ito E, Yanagisawa Y, Imai J, Okamoto T, Watanabe S. Knock down of hSNF5/Ini1 causes cell cycle arrest and apoptosis in a p53-dependent manner. Biochem Biophys Res Commun. 2007;361:580–585. doi: 10.1016/j.bbrc.2007.07.035. [DOI] [PubMed] [Google Scholar]
- Katsumi Y, Kuwahara Y, Tamura S, Kikuchi K, Otabe O, Tsuchiya K, Iehara T, Kuroda H, Hosoi H, Sugimoto T. Trastuzumab activates allogeneic or autologous antibody-dependent cellular cytotoxicity against malignant rhabdoid tumor cells and interleukin-2 augments the cytotoxicity. Clin Cancer Res. 2008;14:1192–1199. doi: 10.1158/1078-0432.CCR-07-1661. [DOI] [PubMed] [Google Scholar]
- Keller U, Huber J, Nilsson JA, Fallahi M, Hall MA, Peschel C, Cleveland JL. Myc suppression of Nfkb2 accelerates lymphomagenesis. BMC Cancer. 2010;10:348. doi: 10.1186/1471-2407-10-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt-DeMasters BK, Alassiri AH, Birks DK, Newell KL, Moore W, Lillehei KO. Epithelioid versus rhabdoid glioblastomas are distinguished by monosomy 22 and immunohistochemical expression of INI-1 but not claudin 6. Am J Surg Pathol. 2010;34:341–354. doi: 10.1097/PAS.0b013e3181ce107b. [DOI] [PubMed] [Google Scholar]
- Kobrynski LJ, Sullivan KE. Velocardiofacial syndrome, DiGeorge syndrome: the chromosome 22q11.2 deletion syndromes. Lancet. 2007;370:1443–1452. doi: 10.1016/S0140-6736(07)61601-8. [DOI] [PubMed] [Google Scholar]
- Kook SD, An SK, Kim KR, Kim WJ, Lee E, Namkoong K. Psychotic features as the first manifestation of 22q11.2 deletion syndrome. Psychiatry Investig. 2010;7:72–74. doi: 10.4306/pi.2010.7.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafay-Cousin L, Payne E, Strother D, Chernos J, Chan M, Bernier FP. Goldenhar phenotype in a child with distal 22q11.2 deletion and intracranial atypical teratoid rhabdoid tumor. Am J Med Genet A. 2009;149A:2855–2859. doi: 10.1002/ajmg.a.33119. [DOI] [PubMed] [Google Scholar]
- Lee LA, Dang CV. Myc target transcriptomes. Curr Top Microbiol Immunol. 2006;302:145–167. doi: 10.1007/3-540-32952-8_6. [DOI] [PubMed] [Google Scholar]
- Lindsay EA, Vitelli F, Su H, Morishima M, Huynh T, Pramparo T, Jurecic V, Ogunrinu G, Sutherland HF, Scambler PJ, Bradley A, Baldini A. Tbx1 haploinsufficieny in the DiGeorge syndrome region causes aortic arch defects in mice. Nature. 2001;410:97–101. doi: 10.1038/35065105. [DOI] [PubMed] [Google Scholar]
- Madigan CE, Armenian SH, Malogolowkin MH, Mascarenhas L. Extracranial malignant rhabdoid tumors in childhood: the Childrens Hospital Los Angeles experience. Cancer. 2007;110:2061–2066. doi: 10.1002/cncr.23020. [DOI] [PubMed] [Google Scholar]
- Merscher S, Funke B, Epstein JA, Heyer J, Puech A, Lu MM, Xavier RJ, Demay MB, Russell RG, Factor S, Tokooya K, Jore BS, Lopez M, Pandita RK, Lia M, Carrion D, Xu H, Schorle H, Kobler JB, Scambler P, Wynshaw-Boris A, Skoultchi AI, Morrow BE, Kucherlapati R. TBX1 is responsible for cardiovascular defects in velo-cardio-facial/DiGeorge syndrome. Cell. 2001;104:619–629. doi: 10.1016/s0092-8674(01)00247-1. [DOI] [PubMed] [Google Scholar]
- Mikhail FM, Descartes M, Piotrowski A, Andersson R, Diaz de Stahl T, Komorowski J, Bruder CE, Dumanski JP, Carroll AJ. A previously unrecognized microdeletion syndrome on chromosome 22 band q11.2 encompassing the BCR gene. Am J Med Genet A. 2007;143A:2178–2184. doi: 10.1002/ajmg.a.31882. [DOI] [PubMed] [Google Scholar]
- Nagl NG, Jr, Zweitzig DR, Thimmapaya B, Beck GR, Jr, Moran E. The c-myc gene is a direct target of mammalian SWI/SNF-related complexes during differentiation-associated cell cycle arrest. Cancer Res. 2006;66:1289–1293. doi: 10.1158/0008-5472.CAN-05-3427. [DOI] [PubMed] [Google Scholar]
- Nicolaides T, Tihan T, Horn B, Biegel J, Prados M, Banerjee A. High-dose chemotherapy and autologous stem cell rescue for atypical teratoid/rhabdoid tumor of the central nervous system. J Neurooncol. 2010;98:117–123. doi: 10.1007/s11060-009-0071-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister SM, Korshunov A, Kool M, Hasselblatt M, Eberhart C, Taylor MD. Molecular diagnostics of CNS embryonal tumors. Acta Neuropathol. 2010;120:553–566. doi: 10.1007/s00401-010-0751-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickert CH, Paulus W. Chromosomal imbalances detected by comparative genomic hybridisation in atypical teratoid/rhabdoid tumours. Childs Nerv Syst. 2004;20:221–224. doi: 10.1007/s00381-003-0909-8. [DOI] [PubMed] [Google Scholar]
- Roberts CW, Biegel JA. The role of SMARCB1/INI1 in development of rhabdoid tumor. Cancer Biol Ther. 2009;8:412–416. doi: 10.4161/cbt.8.5.8019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorke LB, Packer R, Biegel J. Central nervous system atypical teratoid/rhabdoid tumors of infancy and childhood. J Neurooncol. 1995;24:21–28. doi: 10.1007/BF01052653. [DOI] [PubMed] [Google Scholar]
- Rorke LB, Packer RJ, Biegel JA. Central nervous system atypical teratoid/rhabdoid tumors of infancy and childhood: definition of an entity. J Neurosurg. 1996;85:56–65. doi: 10.3171/jns.1996.85.1.0056. [DOI] [PubMed] [Google Scholar]
- Savla J, Chen TT, Schneider NR, Timmons CF, Delattre O, Tomlinson GE. Mutations of the hSNF5/INI1 gene in renal rhabdoid tumors with second primary brain tumors. Journal of the National Cancer Institute. 2000;92:648–650. doi: 10.1093/jnci/92.8.648. [DOI] [PubMed] [Google Scholar]
- Scambler PJ. The 22q11 deletion syndromes. Hum Mol Genet. 2000;9:2421–2426. doi: 10.1093/hmg/9.16.2421. [DOI] [PubMed] [Google Scholar]
- Scambler PJ, Kelly D, Lindsay E, Williamson R, Goldberg R, Shprintzen R, Wilson DI, Goodship JA, Cross IE, Burn J. Velo-cardio-facial syndrome associated with chromosome 22 deletions encompassing the DiGeorge locus. Lancet. 1992;339:1138–1139. doi: 10.1016/0140-6736(92)90734-k. [DOI] [PubMed] [Google Scholar]
- Sevenet N, Sheridan E, Amram D, Schneider P, Handgretinger R, Delattre O. Constitutional mutations of the hSNF5/INI1 gene predispose to a variety of cancers. Am J Hum Genet. 1999;65:1342–1348. doi: 10.1086/302639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh TH, Kurahashi H, Emanuel BS. Evolutionarily conserved low copy repeats (LCRs) in 22q11 mediate deletions, duplications, translocations, and genomic instability: an update and literature review. Genet Med. 2001;3:6–13. doi: 10.1097/00125817-200101000-00003. [DOI] [PubMed] [Google Scholar]
- Shaikh TH, Kurahashi H, Saitta SC, O'Hare AM, Hu P, Roe BA, Driscoll DA, McDonald-McGinn DM, Zackai EH, Budarf ML, Emanuel BS. Chromosome 22-specific low copy repeats and the 22q11.2 deletion syndrome: genomic organization and deletion endpoint analysis. Hum Mol Genet. 2000;9:489–501. doi: 10.1093/hmg/9.4.489. [DOI] [PubMed] [Google Scholar]
- Shaikh TH, O'Connor RJ, Pierpont ME, McGrath J, Hacker AM, Nimmakayalu M, Geiger E, Emanuel BS, Saitta SC. Low copy repeats mediate distal chromosome 22q11.2 deletions: sequence analysis predicts breakpoint mechanisms. Genome Res. 2007;17:482–491. doi: 10.1101/gr.5986507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shprintzen RJ, Goldberg RB, Lewin ML, Sidoti EJ, Berkman MD, Argamaso RV, Young D. A new syndrome involving cleft palate, cardiac anomalies, typical facies, and learning disabilities: velo-cardio-facial syndrome. Cleft Palate J. 1978;15:56–62. [PubMed] [Google Scholar]
- Stankiewicz P, Lupski JR. Genome architecture, rearrangements and genomic disorders. Trends Genet. 2002;18:74–82. doi: 10.1016/s0168-9525(02)02592-1. [DOI] [PubMed] [Google Scholar]
- Tang M, Wei X, Guo Y, Breslin P, Zhang S, Wei W, Xia Z, Diaz M, Akira S, Zhang J. TAK1 is required for the survival of hematopoietic cells and hepatocytes in mice. J Exp Med. 2008;205:1611–1619. doi: 10.1084/jem.20080297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson GE, Breslow NE, Dome J, Guthrie KA, Norkool P, Li S, Thomas PR, Perlman E, Beckwith JB, D'Angio GJ, Green DM. Rhabdoid tumor of the kidney in the National Wilms' Tumor Study: age at diagnosis as a prognostic factor. J Clin Oncol. 2005;23:7641–7645. doi: 10.1200/JCO.2004.00.8110. [DOI] [PubMed] [Google Scholar]
- Wieser R, Fritz B, Ullmann R, Muller I, Galhuber M, Storlazzi CT, Ramaswamy A, Christiansen H, Shimizu N, Rehder H. Novel rearrangement of chromosome band 22q11.2 causing 22q11 microdeletion syndrome-like phenotype and rhabdoid tumor of the kidney. Hum Mutat. 2005;26:78–83. doi: 10.1002/humu.20195. [DOI] [PubMed] [Google Scholar]
- Xu H, Cerrato F, Baldini A. Timed mutation and cell-fate mapping reveal reiterated roles of Tbx1 during embryogenesis, and a crucial function during segmentation of the pharyngeal system via regulation of endoderm expansion. Development. 2005;132:4387–4395. doi: 10.1242/dev.02018. [DOI] [PubMed] [Google Scholar]
- Yadav V, Yanez NC, Fenton SE, Denning MF. Loss of protein kinase C delta gene expression in human squamous cell carcinomas: a laser capture microdissection study. Am J Pathol. 2010;176:1091–1096. doi: 10.2353/ajpath.2010.090816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemble R, Luning Prak E, McDonald K, McDonald-McGinn D, Zackai E, Sullivan K. Secondary immunologic consequences in chromosome 22q11.2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome) Clin Immunol. 2010;136:409–418. doi: 10.1016/j.clim.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Yaoita E, Nameta M, Zhang Y, Cuellar LM, Fujinaka H, Xu B, Yoshida Y, Hatakeyama K, Yamamoto T. Claudin-6 localized in tight junctions of rat podocytes. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1856–1862. doi: 10.1152/ajpregu.00862.2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Paraffin-embedded tumor sections were used for histologic examination of cellular morphology and verification of MRT. (A) Control H&E stain to verify rhabdoid morphology within the sections used for IHC. (B-H) Immunohistochemical analyses of the rhabdoid tumor. Positive staining is indicated by the reddish/brown color. (B) GFAP immunostain shows elevated expression in the rhabdoid cells. (C) p53 protein is modestly elevated in tumor tissue, but not in surrounding non-tumor cells. Several studies have linked loss of SMARCB1 to upregulation of p53 in rhabdoid tumor cell lines and SMARCB1-deficient murine embryonic fibroblasts (MEF), where it is postulated to serve in oncogenic protection by upregulating genes involved in apoptosis (Isakoff et al., 2005; Kato et al., 2007). (D) Epithelial membrane antigen (EMA); (E) CD99; (F) Vimentin; (G) Smooth muscle actin; (H) Pan-keratin (AE1/AE3). All photographs were taken at 60× magnification.