Abstract
Introduction
These studies investigated the action of RAP-031, a soluble activin receptor type IIB (ActRIIB) comprised of a form of the ActRIIB extracellular domain linked to a murine Fc, and the NF-κB inhibitor ursodeoxycholic acid (UDCA) on the whole body strength of mdx mice.
Methods
The Whole Body Tension (WBT) method of assessing the forward pulling tension (FPT) exerted by dystrophic (mdx) mice was used.
Results and Discussion
RAP-031 produced a 41% increase in body mass and a 42.5% increase in FPT without altering the FPT normalized for body mass (WBT). Co-administration of RAP-031 with ursodeoxycholic acid (UDCA) produced increases in FPT that were associated with an increase in WBT. These results indicate that myostatin inhibition increases muscle mass without altering the fundamental weakness characteristic of dystrophic muscle and that co-treatment with an NF-κB inhibitor potentiates the effects of myostatin inhibition in improving FPT in mdx mice.
Keywords: Duchenne muscular dystrophy, mdx mouse, activin receptor type IIB, whole body tension, ursodeoxycholic acid
INTRODUCTION
The primary objective of these studies was to assess the efficacy of the soluble activin type IIB receptor, RAP-031, in enhancing muscle function using a reliable noninvasive procedure that rapidly evaluates the deficit in limb tension development in the mdx and other mouse models of Duchenne muscular dystrophy1–10. As described on the TREAT-NMD website11, the Whole Body Tension (WBT) procedure measures several variables related to the tension generated during a forward pulling maneuver. The variables include the forward pulling tension (FPT, gms) averaged over the top 5 or top 10 values (FPT5, FPT10, respectively); the top 5 and top 10 FPTs normalized to the body mass (WBT5, WBT10, respectively); the ratio WBT10/WBT5, which is termed the Functional Reserve (FR); and the proportional decline per pull (PDP), which is the negative slope of the proportional decline in FPT over the top 10 ordered FPTs9,11. A recent evaluation of the reliability of this technique indicates that the WBT5 and WBT10 values exhibit an inter-trial reliability of approximately 0.8 (Pearson correlation coefficient) in comparison to a value of 0.6 for the 4 limb wire grid hang test10. Previous experiments also indicate that the WBT measure in nondystrophic and mdx mice correlates with measurements of gastrocnemius tension development1, and that two distinct and effective inhibitors of the NF-κB pathway in mdx muscle produce a 12 to 21 % increase9 in WBT5 and WBT10 corresponding to a maximum recovery of approximately 25%. These results indicate that the WBT procedure provides a relatively rapid method for screening potential therapeutic compounds and combination therapies using the mdx mouse model for Duchenne muscular dystrophy.
In this study, the WBT method was used to assess the utility of a treatment which increases muscle mass by inhibiting ActRIIB activation (RAP-031). Previous studies using other means of inhibiting myostatin, one of the ligands which signals via ActRIIB to negatively regulate muscle mass, have indicated substantial increases in dystrophic muscle mass and tension development12,13. The primary purpose of this study was to utilize the normalized FPT measures (WBT5, WBT10) to more clearly determine whether RAP-031-induced inhibition of the ActRIIB pathway produces general improvement in the fundamental weakness characteristic of dystrophic limb muscle1.
Previous studies showed that a variety of inhibitors of the NF-κB pathway are useful in improving the structure and function of dystrophic muscle without altering muscle mass in the mdx mouse9, 14–18. Some of these inhibitors have been shown to increase FPT normalized to body mass, suggesting that the fundamental weakness characteristic of dystrophic muscle may be diminished by treatment with NF-κB inhibitors that do not increase body mass9. These results suggested that co-treatment with RAP-031 and an effective inhibitor of the NF-κB pathway may be particularly useful at both increasing muscle mass and reducing limb muscle weakness. To investigate this possibility, parallel studies were conducted to determine the combined efficacy of RAP-031 with ursodeoxycholic acid (UDCA), an NF-κB inhibitor that increases limb tension without increasing dystrophic muscle mass9.
The results indicate that treatment of 30 day old mdx mice with RAP-031 for up to 90 days substantially increased body mass and FPT but did not significantly influence the FPT normalized to body mass (WBT5, WBT10). The 90 day treatment did, however, lead to a small but significant increase in FR (WBT10/WBT5) associated with a corresponding reduction in PDP, thus indicating that prolonged RAP-031 treatment may reduce the characteristic weakening that occurs following a forward pulling maneuver9. In addition to increasing FPT and body mass, co-treatment with RAP-031 and the NF-κB inhibitor UDCA for 30 days produced a significant increase in WBT10. These results indicate that treatment with the ActRIIB inhibitor RAP-031 produces roughly proportional increases in muscle mass and tension generation without altering the fundamental weakness characteristic of dystrophic muscle. Co-treatment with the NF-κB inhibitor UDCA potentiated the beneficial effects of RAP-031 by producing further increases in limb tension that were associated with relatively small additional increases in body mass9.
METHODS
Drug Administration
Mdx mice, aged 30 days, were used for all of the RAP-031 and UDCA studies. RAP-031 mdx mice were treated twice weekly with subcutaneous (sc) RAP-031 (10 mg/kg) or an appropriate volume of vehicle (10 mM Tris buffered saline, pH 7.2). Mdx mice treated with both RAP-031 and UDCA were exposed to 10 mg/kg twice weekly of RAP-031 along with daily intraperitoneal (ip) administration of 40 mg/kg UDCA as described in Siegel et al.9. The vehicle for the UDCA (Sigma #5127) studies was a 1.02% NaCl solution (pH 8.4). In the combined treatment studies, mdx mice received either both experimental injections (RAP-031 and UDCA) or both vehicle injections.
Determination of Whole Body Tension
WBT determinations were obtained using the techniques described in Siegel et al.9 and at the TREAT NMD website11. All FPTs were obtained by a single investigator who was blinded to the identity of the mice being examined. The determinations were made at the same time period, between 1 and 4 PM, with the mice maintained on a 12 hour light-dark cycle with darkness at 7 PM. The mice were housed individually in cages for the duration of the experiment and were not exposed to an exercise regimen. Vehicle and drug-treated groups were age- and gender matched (equal numbers of male and female mdx mice in vehicle and drug-treated groups). The vehicle and experimental mdx mice were tested at 30, 60, and 90 days of treatment. The data was analyzed to determine the effect of drug treatment on the following variables:
FPT5, which represents the mean tension (gm) of the top 5 ordered FPTs.
FPT10, the mean tension of the top 10 FPTs.
WBT5 and WBT10, which are the FPT5 and FPT10, respectively, divided by the body mass.
FR, which is the WBT10/WBT5.
PDP, which is the proportional decline in FPT over the top 10 ordered FPTs.
Percent recovery was obtained by dividing the effect associated with drug treatment (i.e., drug treated mdx – vehicle treated mdx) by the initial deficit of the variable in the vehicle treated mdx mice (untreated nondystrophic – vehicle treated mdx) as described in Gillis6,19.
Statistics
Sigmaplot v 9.0 and Sigmaplot v 3.1 were used. Means and standard errors are shown and were analyzed using t tests or Mann-Whitney Rank Sums tests as appropriate. Significance was defined at the 0.05 level.
RESULTS
RAP-031 produces proportional increases in body mass and FPT without altering the WBT
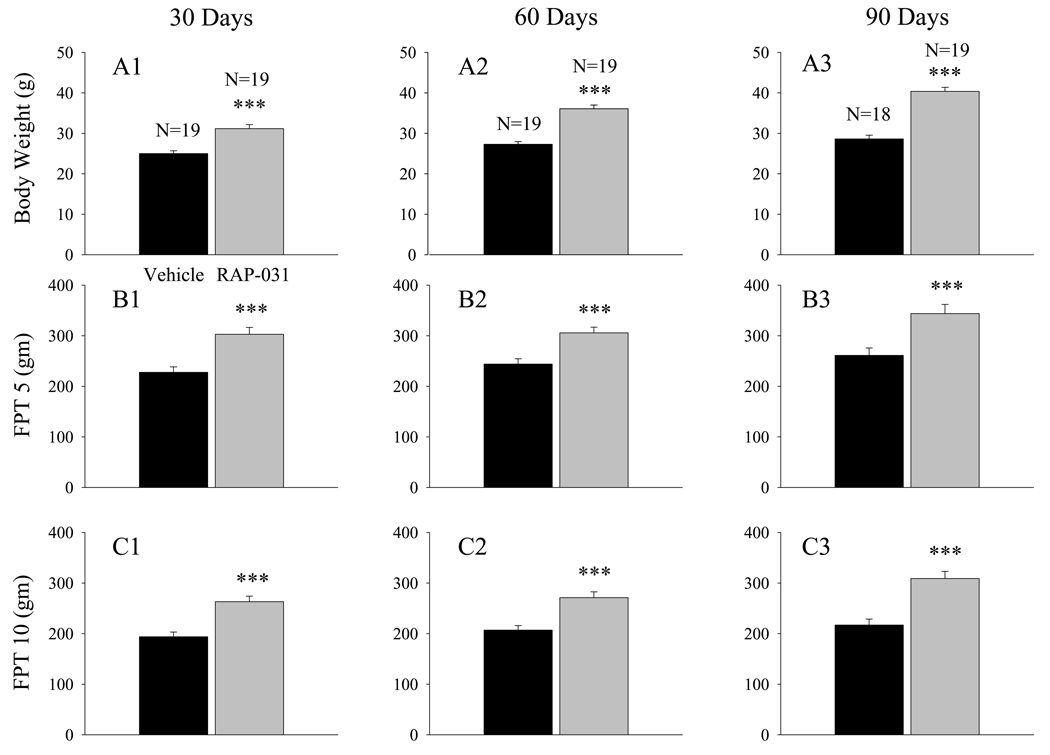
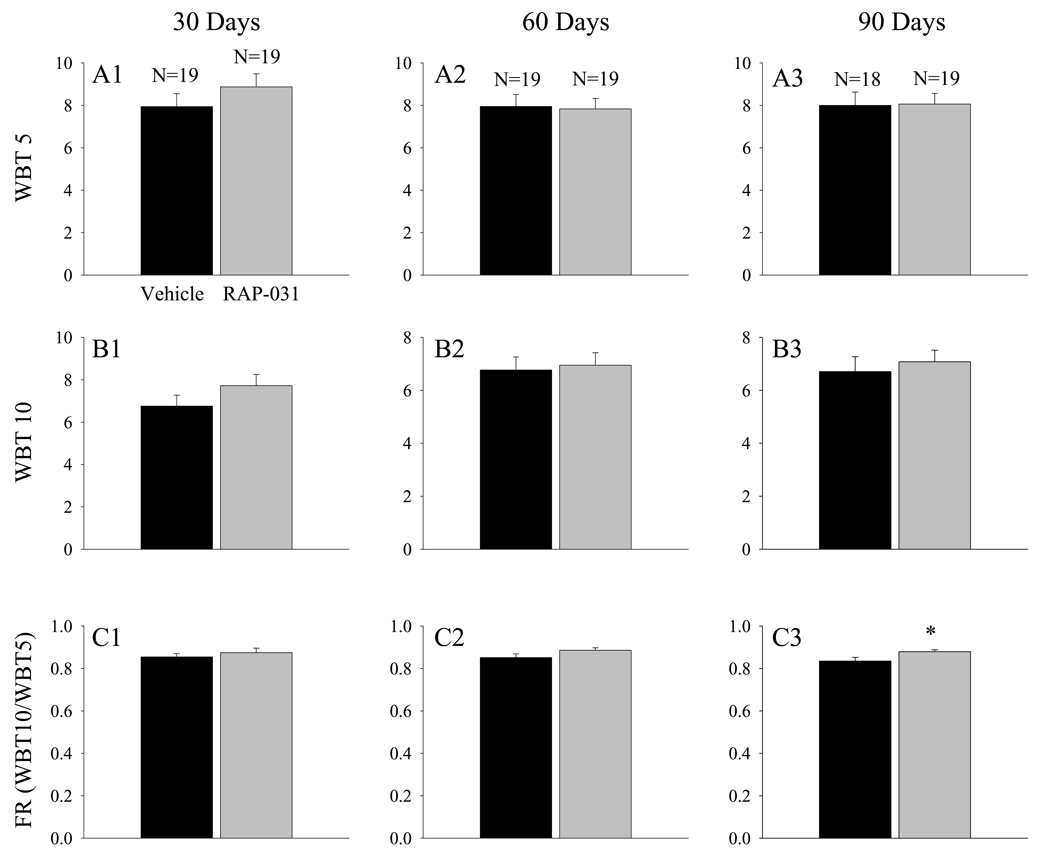
Body mass and WBT determinations were obtained at 30 day intervals in a group of mdx mice that were treated for 90 days with RAP-031, beginning at 30 days of age. After 30 days of treatment, the body mass of the RAP-031 treated mice was approximately 24% larger than the mass of the vehicle-treated mice (Fig. 1A1; p< 0.001), and the FPT5 (Fig. 1B1) and FPT10 (Fig. 1C1) had significantly (p<0.001) increased by 33 and 36 %, respectively. Since nondystrophic mice at 2 to 4 months of age exhibit FPT10 values that are approximately 15.5 times their body weight of 30 gms9, the FPT10 values in the RAP-031 treated mdx mice after 30 days of treatment (Fig. 1C1) represent a recovery6,19 in forward pulling strength of approximately 25%. The WBT5 (Fig. 2A1) and WBT10 (Fig 2B1) values of the RAP-031 treated mice at this age, however, were not significantly increased above the values seen in the corresponding vehicle-treated mdx mice. After 60 days of treatment, body mass had significantly (p<0.001) increased by 32% (Fig. 1A2), FPT5 by 25% (Fig 1B2), and FPT10 by 31.5% (Fig 1C2). No significant increases in either WBT5 or WBT10 were observed (Fig. 2A2, B2). Following 90 days of treatment, body mass was significantly (p<0.001) increased by 41% (Fig. 1A3). At this point, FPT5 (Fig. 1B3) and FPT10 (Fig. 1C3) were significantly (p<0.001) increased by 31.5 and 42.5%, respectively. Neither the WBT5 (Fig 2A3) nor the WBT10 (Fig. 2B3) were significantly elevated after 90 days of RAP-031 treatment, even though the recovery in FPT10 after 90 days reached approximately 37% (Fig. 1C3).
Figure 1.
RAP-031 treatment produces roughly proportional increases in body mass (A), FPT5 (B), and FPT10 (C) in young adult (30 day) mdx mice treated for 30 (A1, B1, C1), 60 (A2, B2, C2), or 90 days (A3, B3, C3). Black histobars – vehicle treated; grey histobars – RAP-031 treated. N represents the number of mdx mice in each group. *** - p< 0.001 in comparison to vehicle treated mdx mice.
Figure 2.
RAP-031 treatment does not influence FPT normalized to body mass. (A1 to A3) WBT5 values at 30, 60, and 90 days, respectively. (B1 to B3) WBT10 values at 30, 60, and 90 days. (C1 to C3) FR values at 30, 60, and 90 days. Black histobars – vehicle treated; grey histobars – RAP-031 treated. N represents the number of mdx mice in each group. * - p< 0.05 in comparison to vehicle treated mdx mice. Note that the FR is significantly increased in the RAP-031 treated mice after 90 days of treatment.
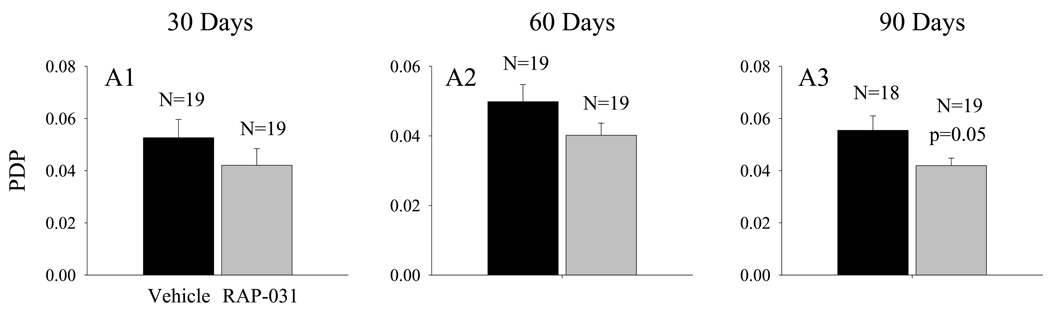
The proportional increases in FPT10 produced by RAP-031 after 30 to 90 days of treatment (Fig. 1C) were consistently slightly larger than the corresponding increases in FPT5 (Fig. 1B). This apparent trend was further evaluated by examining the FR, which is equal to WBT10/WBT5 (Fig. 2C1–C3). The results indicate that the FR was significantly (p<0.05) increased after 90 days of RAP-031 treatment (Fig. 2C3). The PDP, which represents the normalized decline in FPT over the top 10 ordered values, was consistently lower in the RAP-031 treated mdx mice and, at 90 days, the decrease just failed to reach statistical significance (Fig. 3A3; p=0.05).
Figure 3.
The effect of RAP-031 treatment on the PDP at 30 (A1), 60 (A2), and 90 (A3) days. Black histobars – vehicle treated; grey histobars – RAP-031 treated. N represents the number of mdx mice in each group.
In summary, these results indicate that RAP-031 treatment produced proportional increases in body mass and FPT and did not significantly influence either the WBT5 or WBT10. However, the treatment produced slightly larger proportional increases in FPT10 that resulted in a significant increase in FR (Fig. 2C3) associated with a reduction in PDP after 90 days of treatment (Fig. 3A3).
Treatment with RAP-031 and UDCA increases WBT10
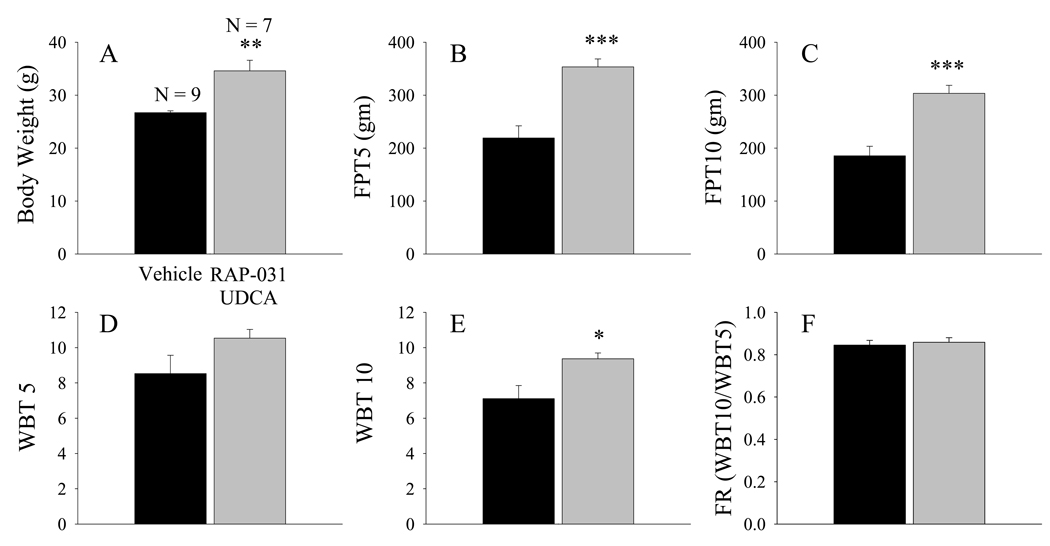
Previous experiments in this laboratory indicated that a 30 day treatment of 30-day-old mdx mice with UDCA produced a 21% increase in both WBT5 and WBT10 without corresponding increases in body mass and did not influence the FR or PDP9. In the present study, a group of 30 day old mdx mice were co-treated with RAP-031 and UDCA, and the results were compared to a control group treated with the appropriate vehicle solutions. Co-treatment with these agents for 30 days produced substantial increases in body mass (by 30%) and FPT, and a significant (p<0.05) 32% increase in WBT10 (Fig. 4) which corresponded to a recovery 6,19 of approximately 27%. Neither the FR (Fig 4F) nor the PDP were influenced by the 30 day treatment with both RAP-031 and UDCA.
Figure 4.
Combined RAP-031 and UDCA treatment for 30 days produced substantial increases in FPT and significant increases in WBT10. (A) Body weight, (B) FPT5, (C) FPT10, (D) WBT5, (E) WBT10, and (F) FR. Black histobars – vehicle treated, gray histobars – RAP-031 and UDCA treated. N is the number of mdx mice in each group. *, **, and *** indicates p< 0.05, p< 0.01, and p<0.001, respectively, in comparison to vehicle treated mice.
DISCUSSION
The WBT method for assessing the forward pulling tension of mice measures the combined tension generated by all of the limbs along with that generated by the abdominal and thoracic musculature and the distal limbs as the mice produce a forward pulling maneuver while continually gripping a tubular wire mesh1–10. An advantage of the WBT procedure over the similar method of assessing fore- or hind-limb grip strength is that the WBT force produced by all of the muscles involved in generating a forward pulling maneuver is more clearly related to body mass than the grip force, which is primarily generated by the distal limb musculature. Previous investigations of grip strength in a mouse model of amyotrophic lateral sclerosis, for example, showed that RAP-031 produced increases in grip strength that were roughly proportional to corresponding increases in body mass20. The results of our study are consistent with these observations. They indicate that treatment of mdx mice with RAP-031 produced substantial muscle hypertrophy and increases in absolute forward pulling tension, but they did not alter the forward pulling tension normalized to body mass (i.e., WBT5, WBT10; Fig. 2A, B).
These results are also consistent with the effect of long term treatment of mdx mice with myostatin antibodies that enhance absolute tension development but do not influence specific tension (tension normalized to cross sectional diameter) in isolated muscle experiments12. Similar results were obtained by Qiao et al13 who used an adenovirus delivery system to treat mdx mice with the myostatin inhibitor, myostatin propeptide, and produced an increase in the absolute grip strength and muscle tension, with no effect on specific twitch or tetanic tension in the tibialis anterior muscle. The WBT results that showed no effect of RAP 031 on WBT (Figure 2) suggest that this measure is the whole body equivalent of the total specific tension generated by the limb, abdominal and thoracic musculature during a forward pulling maneuver. Consistent with results obtained using other modes of inhibiting myostatin12,13, our results indicate that myostatin inhibition increases whole body strength through increases in muscle mass but does not alter the fundamental weakness that is characteristic of dystrophic fibers.
Previous results in this laboratory indicated that two distinct inhibitors of the NF-κB pathway, pyrrolidine dithiocarbamate (PDTC) and UDCA, produced moderate increases in WBT that were not associated with increases in body mass9. These effects are clearly distinct from those produced by ActRIIB inhibition, which increases muscle mass and tension without altering WBT. This supports the idea that the magnitude of the tension effect is directly proportional to the amount of lean mass amassed. The mechanism of action for the functional benefits of NF-κB inhibitors is not known, but it may involve increases in the resting membrane potential14,21, which would be expected to increase the quantity of sarcoplasmic Ca2+ released during an action potential. In our study, treatment with the NF-κB inhibitor UDCA in conjunction with RAP-031 produced increases in body mass that were associated with increases in WBT10 (Figure 4) similar to those produced by UDCA alone9. These results suggest that combined treatment with an effective NF-κB inhibitor and RAP-031 may increase muscle mass and FPT and additionally increase limb strength normalized to body mass (WBT10). Similar studies using the WBT procedure to assess various combination therapies will hopefully prove to be useful in the development and design of clinical trials to treat Duchenne and related muscular dystrophies.
Acknowledgements
This work was supported by grants awarded to CGC from the Association Francaise contre les Myopathies (AFM, 13980) and the NIH (R15AR055360). The authors also gratefully acknowledge the help of Ms. Laura McCarthy who contributed to the early stages of this project.
ABBREVIATIONS
- ActRIIB
activin receptor type IIb
- FPT
Forward pulling tension
- FPT5
mean of top 5 forward pulling tensions
- FPT10
mean of top 10 forward pulling tensions
- FR
functional reserve
- ip
intraperitoneal
- NF-κB
nuclear factor kappa B
- PDP
proportional decline per pull
- RAP-031
soluble ActRIIb – Fc fusion protein
- sc
subcutaneous
- UDCA
ursodeoxycholic acid
- WBT
Whole Body Tension
- WBT5
mean of top 5 forward pulling tensions divided by body mass
- WBT10
mean of top 10 forward pulling tensions divided by body mass
REFERENCES
- 1.Carlson CG, Makiejus RV. A non-invasive procedure to detect muscle weakness in the mdx mouse. Muscle and Nerve. 1990;3:480–484. doi: 10.1002/mus.880130603. [DOI] [PubMed] [Google Scholar]
- 2.Makiejus RV, Patel VK, Krishna G, Dierdorf SF, Bonsett C. Effect of adenylosuccinate on the strength of dystrophin lacking muscles of mdx mice. Biochem. Arch. 1991;7:95–103. [Google Scholar]
- 3.Hudecki MS, Pollina CM, Granchelli JA, Daly MK, Byrnes T, Wang JC, et al. Strength and endurance in the therapeutic evaluation of prednisolone-treated mdx mice. Res. Commun. Chem. Pathol. Pharmacol. 1993;79:45–60. [PubMed] [Google Scholar]
- 4.Deconinck N, Tinsley J, De Backer F, Fisher R, Kahn D, Phelps S, et al. Expression of truncated utrophin leads to major functional improvements in dystrophin deficient muscles of mice. Nat. Med. 1997;11:1216–1221. doi: 10.1038/nm1197-1216. [DOI] [PubMed] [Google Scholar]
- 5.Fougerousse F, Gonin P, Durand M, Richard I, Raymackers JM. Force impairment in calpain 3-deficient mice is not correlated with mechanical disruption. Muscle Nerve. 2003;27(5):616–623. doi: 10.1002/mus.10368. [DOI] [PubMed] [Google Scholar]
- 6.Gillis JP. Multivariate evaluation of the functional recovery obtained by the overexpression of utrophin in skeletal muscles of the mdx mouse. Neuromuscular Disorders. 2002;12:S90–S94. doi: 10.1016/s0960-8966(02)00088-3. [DOI] [PubMed] [Google Scholar]
- 7.Squire S, Raymackers JM, Vandebrouk C, Potter A, Tinsley J, Fisher R, et al. Prevention of pathology in mdx mice by expression of utrophin: analysis using an inducible transgenic expression system. Hum Mol Genet. 2002;11(26):3333–3344. doi: 10.1093/hmg/11.26.3333. [DOI] [PubMed] [Google Scholar]
- 8.Raymackers JM, Debaix H, Colson-Van Schoor M, De Backer F, Tajeddine N, Schwaller B, et al. Consequence of parvalbumin deficiency in the mdx mouse: histological, biochemical and mechanical phenotype of a new double mutant. Neuromuscular Disorders. 2003;13(5):376–387. doi: 10.1016/s0960-8966(03)00031-2. [DOI] [PubMed] [Google Scholar]
- 9.Siegel AL, Bledsoe C, Lavin J, Gatti F, Berge J, Millman G, et al. Treatment with inhibitors of the NF-kappaB pathway improves whole body tension development in the mdx mouse. Neuromuscular Disorders. 2009;19:131–139. doi: 10.1016/j.nmd.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Carlson CG, Rutter J, Bledsoe C, Singh R, Hoff H, Bruemmer K, et al. A simple protocol for assessing inter-trial and inter-examiner reliability for two noninvasive measures of limb muscle strength. J. Neurosci. Methods. 2010;186:226–230. doi: 10.1016/j.jneumeth.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Carlson CG. Whole body tension measurements. 2009 SOP number M.2.2_006, http://www.treat-nmd.eu/research/preclinical/SOPs/
- 12.Bogdanovich S, Krag TOB, Barton ER, Morris LD, Whittemore L-A, Ahima RS, et al. Functional improvement of dystrophic muscle by myostatin blockade. Nature. 2002;420:418–421. doi: 10.1038/nature01154. [DOI] [PubMed] [Google Scholar]
- 13.Qiao C, Jianbin L, Jiang J, Zhu X, Wang B, Li J, et al. Myostatin propeptide gene delivery by adeno-associated virus serotype 8 vectors enhances muscle growth and ameliorates dystrophic phenotypes in mdx mice. Human Gene Therapy. 2008;19:241–253. doi: 10.1089/hum.2007.159. [DOI] [PubMed] [Google Scholar]
- 14.Carlson CG, Samadi A, Siegel A. Chronic treatment with agents that stabilize cytosolic IκB-α enhances survival and improves resting membrane potential in mdx muscle fibers subjected to chronic passive stretch. Neurobiology of Disease. 2005;20:719–730. doi: 10.1016/j.nbd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Messina S, Bitto A, Aguennouz M, Minutoli L, Monici MC, Altavilla D, et al. Nuclear factor kappa-B blockade reduces skeletal muscle degeneration and enhances muscle function in Mdx mice. Experimental Neurology. 2006;198:234–241. doi: 10.1016/j.expneurol.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 16.Acharyya S, Villalta A, Bakkar N, Bupha-Intr T, Janssen PML, Carathers M, et al. Interplay of IKK/NF-κB signaling in macrophages and myofibers promotes muscle degeneration in Duchenne muscular dystrophy. J Clin Invest. 2007;117:889–901. doi: 10.1172/JCI30556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hnia V, Hugon G, Rivier V, Masmoudi V, Mercier V, Mornet V. Modulation of p38 mitogen-activated protein kinase cascade and metalloproteinase activity in diaphragm muscle in response to free radical scavenger administration in dystrophin-deficient mdx mice. Am J Pathol. 2007;170(2):633–643. doi: 10.2353/ajpath.2007.060344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitehead NP, Pham C, Gervasio OL, Allen DG. N-Acetylcysteine ameliorates skeletal muscle pathophysiology in mdx mice. J Physiol (Lond) 2008;586(7):2003–2014. doi: 10.1113/jphysiol.2007.148338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gillis JP. The recovery score to evaluate therapy efficiency in NMD: a common, quantitative, and comparative scoring system. 2008 SOP number M.1.1_001, http://www.treat-nmd.eu/research/preclinical/SOPs/
- 20.Morrison BM, Lachey JL, Warsing LC, Ting BL, Pullen AE, Underwood KW, et al. A soluble activin type IIB receptor improves function in a mouse model of amyotrophic lateral sclerosis. Exper. Neurol. 2009;217:258–268. doi: 10.1016/j.expneurol.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 21.Miles MT, Carlson CG. Acute inhibition of NFκB with pyrrolidine dithiocarbamate (PDTC) improves the resting membrane potential of the triangularis sterni (TS) muscle in the dystrophic (mdx) mouse. Neuroscience Abstracts. 2007:167.5. [Google Scholar]