Abstract
Phytosterol supplements lower low density lipoprotein (LDL) cholesterol, but accumulate in vascular lesions of patients and limit the anti-atherosclerotic effects of LDL lowering in apolipoprotein E deficient mice, suggesting that the cholesterol lowering benefit of phytosterol supplementation may not be fully realized. Individual phytosterols have cell-type specific effects that may either be beneficial or deleterious with respect to atherosclerosis, but little is known concerning their effects on macrophage function. The effects of phytosterols on ABCA1 and ABCG1 abundance, cholesterol efflux, and inflammatory cytokine secretion were determined in cultured macrophage foam cells. Among the commonly consumed phytosterols, stigmasterol increased expression of ABCA1 and ABCG1 and increased efflux of cholesterol to apolipoprotein (Apo) AI and high density lipoprotein (HDL). Campesterol and sitosterol had no effect on ABCA1 or ABCG1 levels. Sitosterol had no effect of cholesterol efflux to Apo AI or HDL, whereas campesterol had a modest, but significant reduction in cholesterol efflux to HDL in THP-1 macrophages. Whereas stigmasterol blunted aggregated LDL-induced increases in tumor necrosis factor (TNF)-α, interleukin (IL)-6 and IL-1β secretion, sitosterol exacerbated these effects. The presence of campesterol had no effect on agLDL-induced inflammatory cytokine secretion from THP-1 macrophages. In conclusion, the presence of stigmasterol in modified lipoproteins promoted cholesterol efflux and suppressed inflammatory cytokine secretion in response to lipid loading in macrophage foam cells. While campesterol was largely inert, the presence of sitosterol increased the proinflammatory cytokine secretion.
Keywords: phytosterol, ABC transporter, LXR, tumor necrosis factor α, interleukin-6, interleukin-1β
Introduction
Functional Foods is a food industry marketing term that describes products that naturally contain, or are supplemented with, compounds conferring health benefits. A number of functional foods contain added phytosterols, a mixture of non-cholesterol sterols found in the oils of the seeds, beans, and legumes of the plants from which they are extracted [1]. Commonly consumed dietary phytosterols (sitosterol, campesterol and stigmasterol) are structurally similar to cholesterol, differing only by methyl or ethyl substitution at C-24 alone or in combination with a double bond at C-22 [1]. When supplied at a dose of 2–4 g per day, phytosterol-esters and their fully hydrogenated stanol-ester derivatives reduce LDL cholesterol by approximately 10%, even when added to statin therapy [2–4]. The absorption of phytosterols is opposed by the ABCG5 ABCG8 (G5G8) sterol transporter [5, 6]. However, patients consuming phytosterols in the form of supplements and functional foods have increased phytosterols in plasma and tissues [1–4, 7]. It is not known if this increase in plasma phytosterols is required for their cholesterol lowering effect, nor is it known if this level of accumulation confers cardiovascular risk or benefit.
There is considerable controversy in the literature concerning the association between plasma levels of plant sterols and the incidence of cardiovascular disease [2, 4, 8]. As with the clinical data, studies in mouse models of atherosclerosis have generated mixed results. Phytosterol supplementation in mice lacking one copy of the LDL receptor resulted in a reduction in both plasma cholesterol and vascular lesion area [9]. However, a more recent study in ApoE deficient mice showed that phytosterol supplementation impaired endothelial function, increased lesion size following cerebral artery occlusion, and increased atherosclerotic lesion area compared to mice treated with the cholesterol absorption inhibitor, ezetimibe [7]. This study also addressed phytosterol consumption and accumulation in plasma and aortic valve cusps in patients undergoing valve replacement. Plasma and lesion concentrations of phytosterols were positively correlated. In addition, patients that reported regular use of phytosterol supplements had the highest phytosterol concentrations in both plasma and lesions. [7]. However, no conclusions can be made concerning the role of phytosterols in disease progression.
Studies addressing cardiovascular phenotypes in both humans and rodents have generally been limited to commercially available mixtures of phytosterols. However, it is clear from a variety of in vitro studies that individual phytosterols have distinct biological activities that include the modulation of signaling pathways and activation of cellular stress responses, growth arrest, and death mechanisms [10–13]. Many of these have implications for lipid metabolism, inflammation and the development of cardiovascular disease. Sitosterol, campesterol and stigmasterol have each been shown to reduce Apo B48 secretion from both intestinal and hepatic cell lines and to reduce cholesterol synthesis [14]. When supplied in atherogenic lipoproteins, sitosterol activates cellular stress response mechanisms and induces death of cultured macrophages [12]. Similar effects were reported in cancer cell lines where sitosterol has been suggested for use as a cytotoxic and chemotherapeutic-sensitizing agent [11, 13]. When fed to rats, stigmasterol reduced cholesterol absorption, decreased hepatic cholesterol content and suppressed expression of both HMG-CoA reductase (HMGCR) and Cholesterol 7-α-hydroxylase (CYP7A1) [15]. Stigmasterol and campesterol, but not sitosterol, interfere with SREBP processing and reduce the expression of genes in the cholesterol biosynthetic and uptake pathways in Y1 adrenal cells [16]. Independently of SREBP processing, stigmasterol and 22- and 24-unsaturated cholesterol biosynthetic intermediates were shown to be LXR ligands that promote the expression of ABCA1 and ABCG1, two transporters involved in the reverse cholesterol transport pathway that opposes cholesterol accumulation in tissues [16, 17]. Conversely, stigmasterol had no effect on LXR dependent gene expression, and antagonized farnesoid X-activated receptor (FXR) and pregnane X receptor (PXR) activity in hepatocytes [18]. Collectively, these observations indicate that the biological activity of phytosterols is both cell-type and sterol specific.
Although phytosterols accumulate in vascular lesions, the effects of phytosterols on macrophage function are poorly understood. We hypothesized that individual phytosterols would differentially influence macrophage ABC transporter abundance, cholesterol efflux and inflammatory cytokine secretion. Our results indicate that stigmasterol increases ABCA1 and ABCG1 expression as well as cholesterol efflux to HDL and Apo AI in cholesterol loaded macrophages, whereas campesterol and sitosterol had no effect or modestly reduced cholesterol efflux. In addition, stigmasterol decreased aggregated LDL-induced secretion of TNFα, IL-6 and IL-1β. Conversely, sitosterol exacerbated the proinflammatory effects of lipid loading. Our results indicate that among the commonly consumed phytosterols, stigmasterol has beneficial effects on in vitro correlates of macrophage function whereas sitosterol is proinflammatory.
Methods and Materials
Reagents and Buffers
Stigmasterol, 22(R)-dehydrocholesterol, and 5α-cholestane were purchased from Steraloids (Newport, RI). Cholesterol, β-sitosterol, campesterol, brassicasterol, Phorbol 12-myristate 13-acetate (PMA) and 1α, 2α[3H ]-cholesterol were purchased from Sigma (St. Louis, MO). Sterols were solubilized in 100% ethanol at a final concentration of 5 mg/ml. RPMI 1640 medium, Fetal Bovine Serum (FBS), and L-glutamine were purchased from Atlanta Biologicals (Lawrenceville, GA). Penicillin/Streptomycin was obtained from Invitrogen/Gibco (Carlsbad, CA). Human Apolipoprotein AI (Apo AI) was purchased from Biodesign International (Saco, ME). Anti-ABCG1 antibody was purchased from GeneTex (San Antonio, TX). Anti-ABCA1 antibody was kind gift from Mason Freeman (Harvard Medical School, Boston, MA). Anti-Calnexin antibody was purchased from Nventa (San Diego, CA). Horseradish peroxidase conjugated secondary antibodies and SuperSignal West Pico Chemiluminiscent Reagent were purchased from (Thermo/Pierce, Rockford, IL). Quantitative real-time PCR, the preparation of membrane proteins, SDS-PAGE, immunoblotting, and densitometry analysis were conducted as previously described [19].
Cell Culture
All animal procedures were conducted in accordance with the university animal care and use committee. C57BL6/J male mice (Jackson Laboratories, 8–10 weeks) were injected intraperitoneally with 2 ml of sterile 10% Brewer’s thioglycollate medium. Five days after injection, macrophages were collected by peritoneal lavage using sterile phosphate-buffered saline (PBS). Mouse peritoneal macrophages (MPMs) were washed with PBS, recovered by centrifugation at 500xg (10 min, 22°C), suspended in Medium A (RPMI 1640 containing 10 mM HEPES buffer, gentamicin (50 μg/ml), streptomycin (100 μg/ml), penicillin (100 IU/ml), sodium bicarbonate (2 g/L), and 7.5% FBS). Cells (9 × 106) were plated in 10 cm dishes for 4 hours. Cells were washed once, fed Medium A and cultured for 24 hr prior to initiation of experiments. For treatment with sterols, cells were incubated in Medium B (RPMI 1640 containing 10 mM HEPES buffer, gentamicin (50 μg/ml), streptomycin (100 μg/ml), penicillin (100 IU/ml), sodium bicarbonate (2 g/L), and 2 mg/ml fatty acid free BSA). Medium C consisted of Medium B supplemented with sodium compactin (5 μM) and mevalonate (50 μM).
Human monocyte/macrophages (THP-1) were obtained from the American Type Culture Collection (ATCC) and maintained in Medium D (RPMI 1640 containing 10 mM HEPES buffer, gentamicin (50 μg/ml), streptomycin (100 μg/ml), penicillin (100 IU/ml), sodium bicarbonate (2 g/L), and 5% FBS) according to the suppliers instructions. For studies of THP-1 macrophages, monocytes were seeded at a density of 1.5 × 106 cells per well in 6-well plates in Medium D containing 50 ng/ml PMA and allowed to differentiate into macrophages for 72 hrs. Following differentiation, the medium was removed, the cells were washed twice with Medium B, and treatments applied as in MPMs as indicated.
Lipoproteins
Low density lipoprotein (LDL; d=1.020–1.063 g/ml) and HDL (d=1.063–1.21 g/ml) were isolated as previously described and generously provided by Dr. Marcielle de Beer (Cardiovascular Research Center, University of Kentucky) [20]. Aggregated LDL (agLDL) was prepared as previously described [21]. Briefly, isolated LDL (1 mg/ml protein) was aggregated by vortexing for 1 min. To break large aggregates, the solution was sonicated for 10 min (70% duty cycle) on ice using a Branson Sonifier and passed through a 0.45 μm filter. Measurement of thiobarbituric acid-reactive substances (TBARS) was conducted to confirm the absence of oxidation during the aggregation procedure. For the incorporation of phytosterols into agLDL, aggregation was conducted in the presence of the indicated sterol. Partitioning of exogenously added sterols into agLDL was confirmed using [3H]-cholesterol and [3H]-sitosterol. Greater than 99% of labeled sterols were TCA precipitable under these conditions (not shown).
Cholesterol Loading and Analysis
To measure cholesterol loading, macrophages were incubated for 48 hr at 37°C in Medium B alone, in the presence of the indicated sterols delivered in ethanol, or in 100 μg protein/ml agLDL containing the indicated sterols and their concentrations. Following extensive washing, total cellular lipids were extracted twice with 2 ml of hexane:isopropanol (3:2), dried under nitrogen gas and suspended in 1 ml of 33% KOH (in ethanol) containing 5 μg of 5α-cholestane as an internal standard. Samples were saponified at 70°C for 2 h. Water (1 ml) and of petroleum ether (2 ml) were added to each sample. Samples were vigorously vortexed for 2 min, centrifuged (2000xg, 10min, 22°C), the organic phase was collected, and dried under nitrogen gas. Sterols were derivatized using N,O-Bis(trimethylsilyl)trifluoroacetamide:pyridine (1:1) (Sigma) and assayed by gas chromatography-mass spectroscopy (GC-MS) as previously described [22]. Cell proteins were solublized in 1N NaOH overnight and total protein determined by BCA assay (Pierce). Total cellular sterol content was expressed as μg sterol per mg total cell protein after normalization to the internal standard. The limit of detection for sterols by GC-MS is 50 ng/mg total cell protein.
Cholesterol Efflux
THP-1 and mouse peritoneal macrophages were loaded with agLDL (100 μg/ml protein) containing the indicated sterols and 1 μCi/ml [3H]-cholesterol for 24 hours in Medium D (RPMI 1640 containing 10 mM HEPES buffer, gentamicin (50 μg/ml), streptomycin (100 μg/ml), penicillin (100 IU/ml), and 2 g/L sodium bicarbonate, 1% FBS). Cells were washed and incubated in Medium E (RPMI 1640 + 10 mM HEPES buffer + 0.2 mg/ml fatty acid free BSA) for 1 hr. Cells were washed and cholesterol efflux was determined in the presence or absence of Apo AI (30 μg/ml) or human HDL (100 μg/mL) in Medium E.
Inflammatory Cytokine Measurement
Inflammatory cytokine production was measured in the supernatants of THP-1 macrophages cultured as described above. Cytometric Bead Array (CBA) Kits (BD Pharmingen, San Jose, CA) were utilized to simultaneously quantify the following cytokine concentrations: TNFα, interleukin (IL) -1β, IL-6, IL-8, IL-10 and IL-12 as previously described [23, 24]. These cytokines, with the exception of IL-10, are indicative of the inflammatory function of macrophages produced at high levels through classical activation. Bead populations with distinct fluorescence intensities coated with capture antibodies specific for each cytokine were incubated with fluorochrome-conjugated detection antibodies along with 50 μl of a two-fold dilution of each sample for 3 hours at room temperature. Fluorescence intensities were assayed by flow cytometry and compare to a standard curve generated for each cytokine to determine the concentration in each sample.
Statistical Analysis
Data were analyzed by one-way ANOVA to determine if there were differences among treatment groups. A post-hoc Dunnett’s multiple comparison test was conducted to compare each treatment to the control. Multiple comparisons among treatments were conducted with Bonferroni tests where indicated.
Results
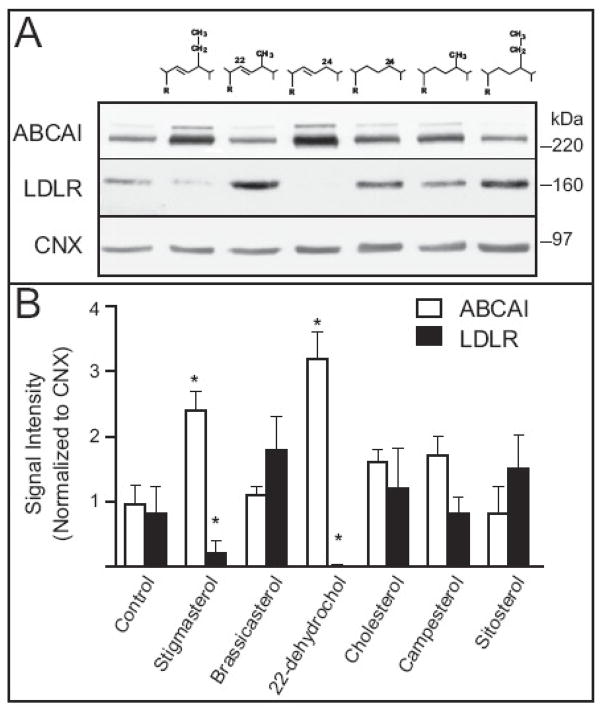
Previous reports indicate that the effects of phytosterols on LXR and SREBP-2 target genes are cell type dependent. To determine their effects in macrophages, we evaluated ABCA1 and LDLR protein abundance in elicited MPMs following treatment with individual non-cholesterol sterols differing at the 22 and 24 carbon positions within the cholesterol side chain (Figure 1). Cells were harvested, cultured for 24 hr and incubated in the presence of serum free medium (Control) supplemented with the indicated sterol for 48 hr. Consistent with previous reports in Y1 adrenal cells, stigmasterol and 22(R)-dehydrocholesterol increased ABCA1 expression and decreased LDLR abundance whereas the remaining sterols had no effect. One potential explanation for the differences in responses among the sterols is their entry and accumulation in macrophages. We extracted lipids from control and sterol treated cells and analyzed cholesterol and phytosterol content by GC-MS. Although there were substantial differences in the mass of sterols extracted from cultured macrophages, no correlation between cell-associated sterols and the expression of either ABCA1 or LDLR could be established (not shown).
Figure 1.
Effect of 22 and 24 substitution of the cholesterol side-chain on expression of ABCA1 and LDLR and sterol accumulation in MPMs. A) MPMs were isolated and cultured as described in Methods. On day 2, the medium was removed and replaced with medium containing carrier (Control, ethanol) or 50 μg/ml of the indicated sterols for 48h. Total cellular membranes were prepared and analyzed by immunoblotting for levels of ABCA1 and LDLR. Representative immunoblots from an experiment conducted two times are shown. B) Immunoblots were scanned and analyzed by densitometry. Sum signal intensities for ABCA1 and LDLR were normalized to Calnexin in each experiment. Bars represent the means ± standard deviations. Bars represent the means ± standard deviations. Asterisks denote significant differences from control treated cells (p < 0.01).
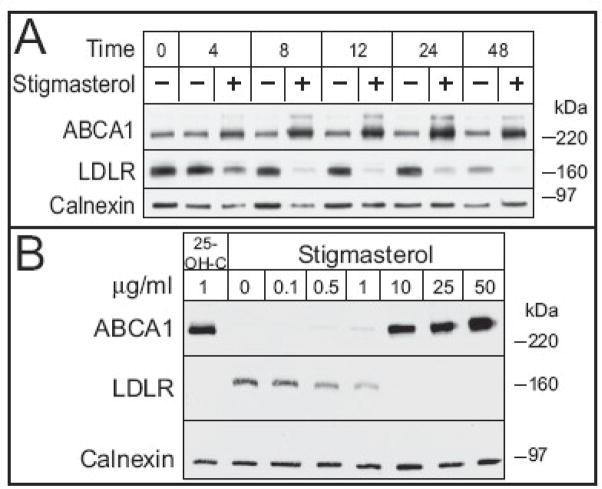
The concentrations of phytosterols used in this and previous studies are substantially greater than what are typically observed in plasma of individuals consuming phytosterol supplements. To determine if phytosterols affected the expression of ABCA1 and LDLR at concentrations that are observed in vivo (4–20 μg/ml [15]), we conducted a time-course experiment using 10 μg/ml phytosterol (Figure 2A). Among the commonly consumed phytosterols only stigmasterol increased expression of ABCA1 and decreased expression of LDLR. Neither campesterol nor sitosterol altered immunoreactive ABCA1 and LDLR during over the 48 hour period (not shown). Densitometric analysis indicated that the effects of stigmasterol on ABCA1 and LDLR were discernable by 4 hours and persisted up to 48 hours (Figure S1). However, LDLR expression decreased in control cells after 24 hours, suggesting that reductions over this period were unrelated to the presence of stigmasterol. This result also implies that the cellular content of cholesterol is dynamic over the 72 hr culture period and that the effects of stigmasterol may be dependent upon, or secondary to changes in endogenous cholesterol synthesis, a known source of LXR ligands [17]. To address this concern and to determine the minimal concentration of stigmasterol required to elicit changes in ABCA1 and LDLR abundance, a dose-response experiment was conducted in the presence of the HMG-CoA Reductase (HMGCR) inhibitor, compactin (Figure 2B). The presence of compactin suppresses ABCA1 and upregulates LDLR. Therefore, 25-hydroxycholesterol was used as a positive control since it is known to be both an LXR agonist and a suppressor of SREBP processing. Stigmasterol treatment resulted in an increase in ABCA1 that was detectible at 1 μg/ml, increased substantially at 10 μg/ml, and continued to increase, albeit to a lesser extent from 10 to 50 μg/ml (Figure S1). For LDLR, a modest suppression was observed at 0.5 μg/ml and further increases in stigmasterol resulted in complete suppression of immunoreactive LDL receptor. These results demonstrate that the effects of stigmasterol on LDLR and ABCA1 in macrophages are dose-dependent and not due to accumulation of cholesterol biosynthetic intermediates.
Figure 2.
Effect of time and concentration of stigmasterol on immunoreactive ABCA1 and LDLR in elicited MPMs. A) MPMs were isolated and cultured as in Figure 1. On day 2, the medium was removed and replaced with Medium B containing stigmasterol (10 μg/ml) for up to 48h. B) On day 2, the medium was removed and replaced with Medium C containing 25-OH-C (1 μg/ml) or stigmasterol (0 to 50 μg/ml) for 24 h. Total cellular membranes were prepared and analyzed by immunoblotting for levels of ABCA1 and LDLR. Calnexin was used as a control for equal loading of proteins. Representative immunoblots from experiments conducted three times are shown.
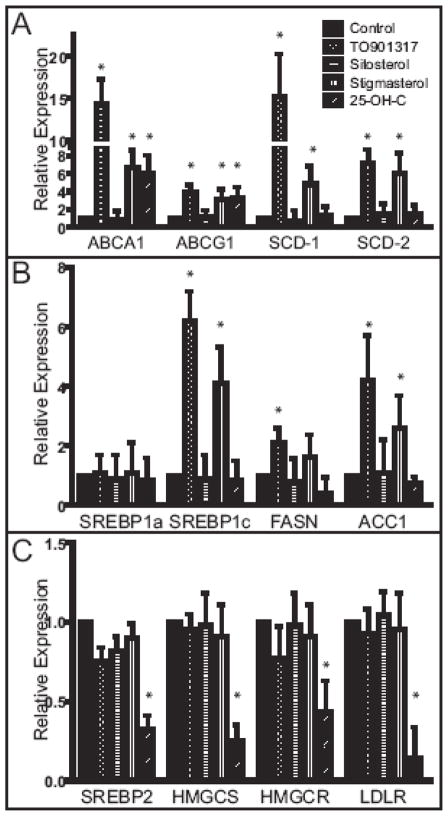
Next we determined if the effect of stigmasterol on ABCA1 abundance was associated with changes in mRNA levels for this and other LXR target genes (Figure 3). Each of the LXR target genes was increased by stigmasterol, but not sitosterol. Similarly, campesterol did not alter LXR target gene expression. We also evaluated expression of SREBPs and selected targets. Not surprisingly, SREBP-1c was also upregulated by stigmasterol as well as its downstream targets fatty acid synthase (FASN) and acetyl-CoA carboxylase (ACC1). However, stigmasterol had no effect on SREBP2 or its target genes, suggesting the mechanism by which stigmasterol suppresses LDLR protein is post-transcriptional, distinct from that of 25-OH-C, and independent of interference with SREBP processing.
Figure 3.
Effect of stigmasterol on the expression of LXR (A) and SREBP1 (B), SREBP2 (C) and selected target genes in mouse peritoneal macrophages. Macrophages were elicited and cultured as in Figure 1. On day 2, the medium was removed and replaced with Medium C (Control) or Medium C supplemented with TO901317 (1 μM), sitosterol (10 μg/ml), stigmasterol (10 μg/ml), or 25-OH-C (1 μg/ml) for 24 h. Total RNA was isolated and processed for quantitative RT-PCR. Asterisks denote significant differences from control treated cells (p < 0.01).
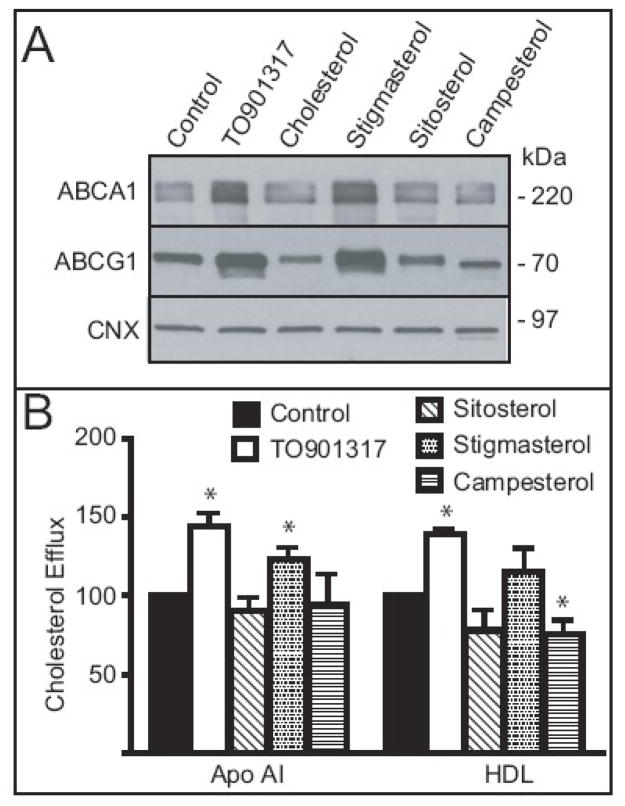
Although plant sterols may affect gene expression and cholesterol trafficking when added directly to the culture medium, macrophage foam cells acquire plant sterols from modified lipoproteins in vivo. Aggregation of LDL by vortexing substantially increased the incorporation of phytosterols into LDL particles compared to oxidation and acetlyation (not shown). However, LDL aggregates are poorly processed by mouse peritoneal macrophages [25]. Therefore we selected THP-1 cells since these cells are an established model of macrophage foam cells that readily internalize and process agLDL in lysosomes [21]. First, we confirmed that the effects of individual phytosterols on ABC transporter expression would persist in lipid loaded cells and that they were not unique to MPMs. Following differentiation, THP-1 macrophages were cultured with medium supplemented with agLDL (Control) or agLDL prepared in the presence of the indicated sterol. As a positive control, cells were incubated in medium containing both agLDL and an LXR agonist (TO901317). As an additional control, agLDL was prepared in the presence of cholesterol to maintain equality of total added sterols. Following 48 hr of treatment, membrane proteins were prepared and analyzed by SDS-PAGE and immunoblotting (Figure 4A). Incubation of THP-1 macrophages with agLDL in the absence of additional sterols increases ABCA1 and suppresses LDLR below the limits of detection (not shown). The addition of the LXR agonist further increased ABCA1 protein in agLDL loaded macrophages. ABCG1 was also increased in THP-1 loaded macrophages, the measurements of which proved difficult in mouse macrophages using commercially available antibodies. The incorporation of cholesterol, sitosterol and campesterol in agLDL had no effect on ABCA1 or ABCG1 abundance. Consistent with mRNA data in MPMs, the inclusion of stigmasterol in agLDL increased both transporters in human macrophages, whereas other phytosterols had no effect.
Figure 4.
Effect of major phytosterols on expression of ABCA1 and ABCG1 and cholesterol efflux to ApoAI and HDL in agLDL loaded THP-1 macrophages. Native human LDL (1 mg protein) was aggregated in the presence of carrier (Control), TO901317 (10 μM), cholesterol, stigmasterol, sitosterol, or campesterol at concentration of 10μg/ml, sonicated and filtered. Following differentiation, the cells were washed and incubated in medium supplemented with 100 μg/ml agLDL protein prepared in the presence of the indicated agonist or sterol for 48 hr A) Total cellular membranes were prepared and analyzed by immunoblotting for levels of ABCA1 and ABCG1. B) In a parallel experiment, [3H]-cholesterol (10 μCi/mg LDL protein) was added to LDL prior to aggregation. Following loading, cells were washed, allowed to equilibrate, and incubated in medium containing 100 μg/ml human HDL or 30 μg/ml recombinant human Apo AI for 4 hr. [3H]-cholesterol present in the medium and cells were determined by liquid scintillation counting, normalized to total cell protein and percent efflux calculated. Percent efflux from cells incubated in medium containing neither acceptor was subtracted as background. Data are the mean ± SD of three replicates. Asterisks denote significant differences (p < 0.05) compared to control cells. This experiment was conducted four times with similar results.
Cellular sterol content was determined before and after incubation with agLDL (Table 1). Lipids were extracted and analyzed by GC-MS. Incubation of THP-1 macrophages with agLDL resulted in a 5–6 fold increase in total cellular sterol content. The addition of phytosterols collectively and individually had no effect on the extent of cholesterol accumulation or total cellular sterol content when compared to the cholesterol control, indicating that changes in ABC transporter expression are not merely a function of total cholesterol or sterol content of THP-1 macrophages.
Table 1.
Cellular sterol content (μg/mg total cell protein) following incubation with agLDL prepared in the presence (+) of the indicated sterol. Values represent means ±standard deviations. Empty cells indicate that levels were below the limits of detection by GC-MS (50 ng/mg total cell protein).
| Sterol | agLDL + |
|||||
|---|---|---|---|---|---|---|
| Control | Carrier | Cholesterol | Stigmasterol | Sitosterol | Campesterol | |
| Cholesterol | 7.89± 0.74 | 42.96± 5.74 | 47.36± 6.52 | 49.00± 6.18 | 45.21± 10.18 | 44.68± 1.92 |
| Stigmasterol | 6.41± 2.43 | |||||
| Sitosterol | 5.28± 1.22 | |||||
| Campesterol | 14.16± 1.01 | |||||
| Total Sterols | 7.89± 1.04 | 42.96± 5.74 | 47.36± 6.52 | 55.41± 5.14 | 50.49± 3.65 | 58.84± 3.37 |
Next we determined if phytosterols altered efflux of cholesterol from agLDL loaded THP-1 macrophages to Apo AI and HDL (Figure 4B). THP-1 monocytes were differentiated into macrophages and incubated with agLDL prepared in the presence of [3H]-cholesterol and the indicated sterol for 48 hours (100 μg/ml LDL, 10 μg/ml sterol, 1 μCi/ml [3H]-cholesterol). Following the loading phase, the cells were washed and allowed to equilibrate in serum free medium for 2 hr. The equilibration medium was removed, the cells were washed and the medium replaced in Medium B containing Apo AI (30 μg/ml) or HDL (100 μg/ml) for 4hr. Relative to control cells, in which no additional sterols were added to the LDL aggregates, TO901317 enhanced efflux of [3H]-cholesterol to both Apo AI and HDL (p<0.05). Sitosterol had no effect on efflux to either acceptor, although there was a tendency for a decrease to HDL. The presence of stigmasterol increased efflux to Apo AI by 25% (p<0.05) and tended to increase efflux to HDL. Campesterol resulted in a modest, but significant decrease in efflux to HDL (p<0.05), but did not alter efflux to Apo AI. Similar stimulatory effects of stigmasterol were observed in MPMs (Figure S2). These results indicate that the effects of individual phytosterols on ABC transporter expression and cholesterol efflux are largely consistent among cultured macrophages of both human and mouse origin.
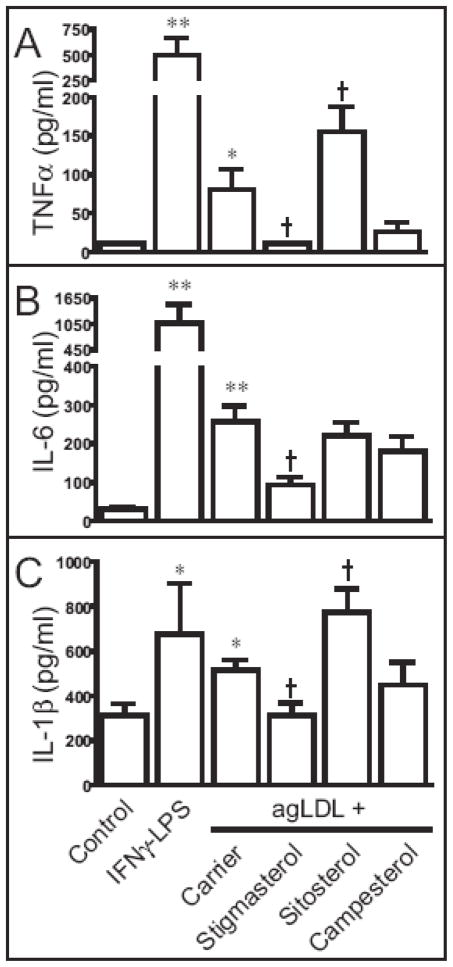
Beyond the accumulation of lipid, macrophages contribute to the inflammatory state of the atherosclerotic lesion. To determine if phytosterols alter the inflammatory response to agLDL loading, we evaluated the secretion of inflammatory cytokines in the culture medium using a commercially available cytometric bead assay (CBA) inflammation panel (Figure 5). First, we used pretreatment with interferon (IFN) -γ followed by lipopolysaccharide (LPS) as a control for classical activation of macrophages. Following pretreatment with IFN-γ, LPS dramatically increased the secretion of TNFα, IL-6 and IL-1β. Compared to untreated cells, incubation with agLDL increased the expression of each of these proinflammatory cytokines. The effect of phytosterols on the response to agLDL loading was assessed by comparing the levels of secreted cytokines to cells treated with agLDL prepared in the presence of the carrier (ethanol). The presence of stigmasterol decreased agLDL-induced secretion of TNFα, IL-6 and IL-1β. The presence of sitosterol increased the secretion of TNFα and IL-1β, but not IL-6. Campesterol had no effect on the inflammatory response to agLDL. Levels of IL-8 were unaffected by treatments. IL-10 and IL12p70 were below the limits of detection in our assay (not shown).
Figure 5.
Sterol loading of THP-1 cells induces the synthesis and secretion of TNFα, IL-6 and IL-1β. THP-1 macrophages were incubated in medium (control), agLDL (100 μg/ml) prepared in the presence of carrier or the indicated sterol (10 μg/ml) for 48 hours. As a positive control for activation of macrophages, cells were incubated for 24 hr in the presence of IFNγ (20 ng/ml) followed by LPS (100 ng/ml). The culture media were collected and centrifuged to remove non-adherent cells. The amount of TNFα (A), IL-6 (B) and IL-1β (C) released to the media were analyzed by CBA assay. Data are the mean ± SEM of six replicates. ** p < 0.01 vs. untreated control, * p < 0.05 vs. untreated control, † p < 0.05 vs. AgLDL + carrier. This experiment was conducted twice with similar results.
Discussion
We report for the first time that stigmasterol increases expression of ABCA1 and ABCG1, enhances cholesterol efflux, and decreases the inflammatory response to uptake of modified lipoproteins in multiple models of macrophage foam cells. Conversely, sitosterol exacerbated the inflammatory response of agLDL loading and tended to decrease cholesterol efflux. Although campesterol had no effect on the abundance of ABC transporters or secretion of cytokines, it had a modest inhibitory effect on cholesterol efflux from agLDL loaded macrophages to HDL.
The increase in efflux to Apo AI and HDL in the presence of stigmasterol is presumably mediated by the increase in ABCA1 and ABCG1 protein, respectively. However, a role for other sterol transporting proteins such as SR-BI, CD36 and ABCG4 cannot be excluded. Further complicating matters is the fact that LXR agonists have been shown to enhance efflux of cholesterol by promoting the transport of cholesterol to the cell surface in human macrophages [26]. Given that stigmasterol interacts with at least two independent sterol sensing mechanisms in other cell types, the precise mechanism(s) by which stigmasterol enhances cholesterol efflux to Apo AI and HDL in macrophages remains difficult to definitively establish. Similarly, the mechanism for suppression of inflammatory cytokine secretion remains unknown, but is likely related to activation of LXR signaling based on the increase in LXR target genes by this sterol and emerging role of this nuclear hormone receptor in the suppression of inflammation [27].
The suppression of LDLR by stigmasterol appears to be independent of disruptions in SREBP2 processing since target genes for this transcription factor are unaffected by this phytosterol. It is tempting to speculate that the mechanism is also LXR dependent based on recent reports of LXR-mediated LDLR degradation [28]. However, we did not pursue this effect of stigmasterol in macrophage foam cells since lipid loading in the absence of stigmasterol or the synthetic LXR ligand is sufficient to suppress LDLR levels below the limits of detection.
In general terms, the effects of sitosterol were opposite of stigmasterol. Sitosterol increased the inflammatory response of agLDL loaded macrophages and tended to reduce cholesterol efflux. However, sitosterol had no effect on immunoreactive levels of ABC transporters or mRNA levels of any of the transcripts examined. Previous reports in macrophages and other cell types indicate that sitosterol inhibits cell growth, activates components of the integrated stress response and at sufficient concentrations is toxic to cells [12, 13]. We did not observe cytotoxicity with sitosterol treatment in these studies, but it is important to note that did not quantify direct measures of cellular stress since these effects were beyond the scope of our study.
This study adds to a growing body of literature demonstrating that individual phytosterols affect a number of signaling, trafficking, and enzymatic mechanisms with implications in the development and progression of cardiovascular disease. The studies detailing interactions with between phytosterols, nuclear hormone receptors and cholesterol homeostasis have generally evaluated levels that are observed in patients with sitosterolemia or receiving parenteral nutrition in which the G5G8 transporter is effectively bypassed. The relative abundance of stigmasterol in commercially available phytosterol supplements and functional foods compounded with its limited absorption make it unlikely that the levels of this individual phytosterol accumulate in sufficient quantities to have a significant positive impact on the reverse cholesterol transport or inflammatory pathways within macrophages. However, supplying stigmasterol as the sole source of phytosterol in the diet increased its levels to 20 μg/ml in serum and reduced cholesterol absorption, plasma cholesterol and hepatic HMGCR activity, suggesting that the beneficial effects of this phytosterol are achievable [15].
A critical question with respect to the use of phytosterols as supplements and within functional foods is whether the benefits of cholesterol lowering are greater than potential risk associated with the accumulation of plant sterols in plasma and tissues. It is important to note that humans consume significant amounts of phytosterols depending on their diet and phytosterol supplements are generally regarded as safe. However, cholesterol lowering therapies persist for decades. Increasing phytosterol consumption to levels sufficient for cholesterol lowering, particularly in patients that harbor polymorphisms in ABCG5/ABCG8, may limit cardiovascular benefit depending on sterol composition. On the other hand, added benefit may be achievable through the use of supplements enriched in stigmasterol or other 22-dehydrosterols. Additional studies of individual phytosterols are required to determine if sterol composition can be optimized to achieve added cardiovascular benefit beyond cholesterol lowering.
Acknowledgments
Funding Sources: This work was sponsored by grants from the American Heart Association (Graf: 053025N) and the National Institute of General Medical Sciences (Li: 1R01GM085231-01).
The authors wish to thank Saloni Bhatnagar for technical assistance and Maria deBeer for preparation of lipoproteins.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moghadasian MH, Frohlich JJ. Effects of dietary phytosterols on cholesterol metabolism and atherosclerosis: Clinical and experimental evidence. Am J Med. 1999;107:588–94. doi: 10.1016/s0002-9343(99)00285-5. [DOI] [PubMed] [Google Scholar]
- 2.John S, Sorokin AV, Thompson PD. Phytosterols and vascular disease. Curr Opin Lipidol. 2007;18:35–40. doi: 10.1097/MOL.0b013e328011e9e3. [DOI] [PubMed] [Google Scholar]
- 3.Patel MD, Thompson PD. Phytosterols and vascular disease. Atherosclerosis. 2006;186:12–9. doi: 10.1016/j.atherosclerosis.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 4.Thompson GR, Grundy SM. History and development of plant sterol and stanol esters for cholesterol-lowering purposes. Am J Cardiol. 2005;96:3D–9D. doi: 10.1016/j.amjcard.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 5.Kidambi S, Patel SB. Sitosterolaemia: Pathophysiology, clinical presentation and laboratory diagnosis. Journal of clinical pathology. 2008;61:588–94. doi: 10.1136/jcp.2007.049775. [DOI] [PubMed] [Google Scholar]
- 6.Hui DY, Labonte ED, Howles PN. Development and physiological regulation of intestinal lipid absorption. Iii. Intestinal transporters and cholesterol absorption. American journal of physiology. 2008;294:G839–43. doi: 10.1152/ajpgi.00061.2008. [DOI] [PubMed] [Google Scholar]
- 7.Weingartner O, Lutjohann D, Ji S, Weisshoff N, List F, Sudhop T, von Bergmann K, Gertz K, Konig J, et al. Vascular effects of diet supplementation with plant sterols. Journal of the American College of Cardiology. 2008;51:1553–61. doi: 10.1016/j.jacc.2007.09.074. [DOI] [PubMed] [Google Scholar]
- 8.Windler E, Zyriax BC, Kuipers F, Linseisen J, Boeing H. Association of plasma phytosterol concentrations with incident coronary heart disease data from the cora study, a case-control study of coronary artery disease in women. Atherosclerosis. 2008 doi: 10.1016/j.atherosclerosis.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 9.Plat J, Beugels I, Gijbels MJ, de Winther MP, Mensink RP. Plant sterol or stanol esters retard lesion formation in ldl receptor-deficient mice independent of changes in serum plant sterols. Journal of lipid research. 2006;47:2762–71. doi: 10.1194/jlr.M600346-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.Awad AB, Begdache LA, Fink CS. Effect of sterols and fatty acids on growth and triglyceride accumulation in 3t3-l1 cells. The Journal of nutritional biochemistry. 2000;11:153–8. doi: 10.1016/s0955-2863(99)00087-x. [DOI] [PubMed] [Google Scholar]
- 11.Awad AB, Fink CS. Phytosterols as anticancer dietary components: Evidence and mechanism of action. The Journal of nutrition. 2000;130:2127–30. doi: 10.1093/jn/130.9.2127. [DOI] [PubMed] [Google Scholar]
- 12.Bao L, Li Y, Deng SX, Landry D. Tabas I Sitosterol-containing lipoproteins trigger free sterol-induced caspase-independent death in acat-competent macrophages. The Journal of biological chemistry. 2006;281:33635–49. doi: 10.1074/jbc.M606339200. [DOI] [PubMed] [Google Scholar]
- 13.Rubis B, Paszel A, Kaczmarek M, Rudzinska M, Jelen H, Rybczynska M. Beneficial or harmful influence of phytosterols on human cells? The British journal of nutrition. 2008:1–9. doi: 10.1017/S0007114508981423. [DOI] [PubMed] [Google Scholar]
- 14.Ho SS, Pal S. Margarine phytosterols decrease the secretion of atherogenic lipoproteins from hepg2 liver and caco2 intestinal cells. Atherosclerosis. 2005;182:29–36. doi: 10.1016/j.atherosclerosis.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 15.Batta AK, Xu G, Honda A, Miyazaki T, Salen G. Stigmasterol reduces plasma cholesterol levels and inhibits hepatic synthesis and intestinal absorption in the rat. Metabolism: clinical and experimental. 2006;55:292–9. doi: 10.1016/j.metabol.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 16.Yang C, Yu L, Li W, Xu F, Cohen JC, Hobbs HH. Disruption of cholesterol homeostasis by plant sterols. J Clin Invest. 2004;114:813–22. doi: 10.1172/JCI22186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang C, McDonald JG, Patel A, Zhang Y, Umetani M, Xu F, Westover EJ, Covey DF, Mangelsdorf DJ, et al. Sterol intermediates from cholesterol biosynthetic pathway as liver x receptor ligands. The Journal of biological chemistry. 2006;281:27816–26. doi: 10.1074/jbc.M603781200. [DOI] [PubMed] [Google Scholar]
- 18.Carter BA, Taylor OA, Prendergast DR, Zimmerman TL, Von Furstenberg R, Moore DD, Karpen SJ. Stigmasterol, a soy lipid-derived phytosterol, is an antagonist of the bile acid nuclear receptor fxr. Pediatr Res. 2007;62:301–6. doi: 10.1203/PDR.0b013e3181256492. [DOI] [PubMed] [Google Scholar]
- 19.Sabeva NS, Rouse EJ, Graf GA. Defects in the leptin axis reduce abundance of the abcg5-abcg8 sterol transporter in liver. The Journal of biological chemistry. 2007;282:22397–405. doi: 10.1074/jbc.M702236200. [DOI] [PubMed] [Google Scholar]
- 20.Coetzee GA, Strachan AF, van der Westhuyzen DR, Hoppe HC, Jeenah MS, de Beer FC. Serum amyloid a-containing human high density lipoprotein 3. Density, size, and apolipoprotein composition. The Journal of biological chemistry. 1986;261:9644–51. [PubMed] [Google Scholar]
- 21.Griffin EE, Ullery JC, Cox BE, Jerome WG. Aggregated ldl and lipid dispersions induce lysosomal cholesteryl ester accumulation in macrophage foam cells. Journal of lipid research. 2005;46:2052–60. doi: 10.1194/jlr.M500059-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Wilund KR, Yu L, Xu F, Vega GL, Grundy SM, Cohen JC, Hobbs HH. No association between plasma levels of plant sterols and atherosclerosis in mice and men. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:2326–32. doi: 10.1161/01.ATV.0000149140.00499.92. [DOI] [PubMed] [Google Scholar]
- 23.Cook EB, Stahl JL, Lowe L, Chen R, Morgan E, Wilson J, Varro R, Chan A, Graziano FM, Barney NP. Simultaneous measurement of six cytokines in a single sample of human tears using microparticle-based flow cytometry: Allergics vs. Non-allergics. Journal of immunological methods. 2001;254:109–18. doi: 10.1016/s0022-1759(01)00407-0. [DOI] [PubMed] [Google Scholar]
- 24.Chen R, Lowe L, Wilson JD, Crowther E, Tzeggai K, Bishop JE, Varro R. Simultaneous quantification of six human cytokines in a single sample using microparticle-based flow cytometric technology. Clinical chemistry. 1999;45:1693–4. [PubMed] [Google Scholar]
- 25.Hoff H, Zyromski N, Armstrong D, O’Neil J. Aggregation as well as chemical modification of ldl during oxidation is responsible for poor processing in macrophages. J Lipid Res. 1993;34:1919–29. [PubMed] [Google Scholar]
- 26.Rigamonti E, Helin L, Lestavel S, Mutka AL, Lepore M, Fontaine C, Bouhlel MA, Bultel S, Fruchart JC, et al. Liver x receptor activation controls intracellular cholesterol trafficking and esterification in human macrophages. Circulation research. 2005;97:682–9. doi: 10.1161/01.RES.0000184678.43488.9f. [DOI] [PubMed] [Google Scholar]
- 27.Rigamonti E, Chinetti-Gbaguidi G, Staels B. Regulation of macrophage functions by ppar-alpha, ppar-gamma, and lxrs in mice and men. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:1050–9. doi: 10.1161/ATVBAHA.107.158998. [DOI] [PubMed] [Google Scholar]
- 28.Zelcer N, Hong C, Boyadjian R, Tontonoz P. Lxr regulates cholesterol uptake through idol-dependent ubiquitination of the ldl receptor. Science. 2009;325:100–4. doi: 10.1126/science.1168974. [DOI] [PMC free article] [PubMed] [Google Scholar]