Abstract
Flagella and cilia are structurally polarized organelles whose lengths are precisely defined, and alterations in length are related to several human disorders [1, 2]. Intraflagellar transport (IFT) and protein signaling molecules are implicated in specifying flagellar/ciliary length [3–6], but evidence has been lacking for a flagellum/cilium length sensor that could participate in active length control or establishment of structural polarity. Previously, we showed that the phosphorylation state of the aurora-like protein kinase CALK in Chlamydomonas is a marker of the absence of flagella. Here, we show that CALK phosphorylation state also is a marker for flagellar length. CALK is phosphorylated in cells without flagella, and during flagellar assembly it becomes desphosphorylated. Dephosphorylation is not simply a consequence of initiation of flagellar assembly or of time after experimentally-induced flagellar loss, but requires flagellar assembly to a threshold length. Analysis of cells with flagella of varying lengths shows that the threshold length for CALK dephosphorylation is ~6 µm (half-length). Studies with short and long flagellar mutants indicate that cells detect absolute rather than relative flagellar length. Our results demonstrate that cells possess a mechanism for translating flagellar length into a posttranslational modification of a known flagellar regulatory protein.
Results and Discussion
Dephosphorylation of CALK during flagellar assembly
Cells possess exquisite and cell type-specific mechanisms for specifying the structure and length of cilia and flagella. For example, flagella of wild type strains of the biflagellate green alga, Chlamydomonas reinhardtii, are 11.6 µm +/− 1.1 (derived from [4]). In addition to specifying steady-state length, Chlamydomonas cells also detect and respond to assembling flagella of ~half-length. Levels of flagellar protein transcripts fall abruptly when newly assembling flagella reach ~ 6 µm [7, 8]. Moreover, the axoneme is structurally polarized. Some inner dynein arms are localized either in the proximal or distal half of the flagella [9, 10]; polyglutamylated tubulin exhibits proximal-distal polarity [11]; and, beak-like structures within microtubule doublets of the axoneme are confined to the proximal half [12]. Although cell biologists can use optical methods to measure ciliary/flagellar length, cells must use molecules, and to date no molecular marker for flagellar or ciliary length has been reported.
Here, we have examined Chlamydomonas aurora-like protein kinase (CALK), a member of the aurora protein kinase family, as a possible molecular marker of flagellar length. Aurora protein kinases are known to regulate cilia in vertebrate cells and in Chlamydomonas [13, 14]. In both systems, the aurora proteins are in the desphosphorylated state in cells with organelles at steady-state length. When flagellar disassembly is triggered, however, the aurora proteins in both undergo changes in their phosphorylation state, and when the levels of the auroras are experimentally reduced, disassembly is inhibited. In our previous studies, we showed that phosphorylated CALK was a marker for the absence of flagella. The phosphorylated state of CALK occurred independently of the path to the deflagellated condition. Thus, CALK was phosphorylated on cells that were flagella-less because of their culture conditions (growth at an air-agar interface), due to experimentally-induced flagellar detachment, to environmentally- or developmentally-triggered flagellar shortening, or to basal body mutations incompatible with flagellar assembly [13]. Because CALK is phosphorylated in cells lacking flagella and non-phosphorylated in cells with flagella at steady-state length, we have examined CALK phosphorylation during the flagellar assembly that occurs upon deflagellation. Specifically, we tested whether CALK phosphorylation state might be a marker of flagellar length.
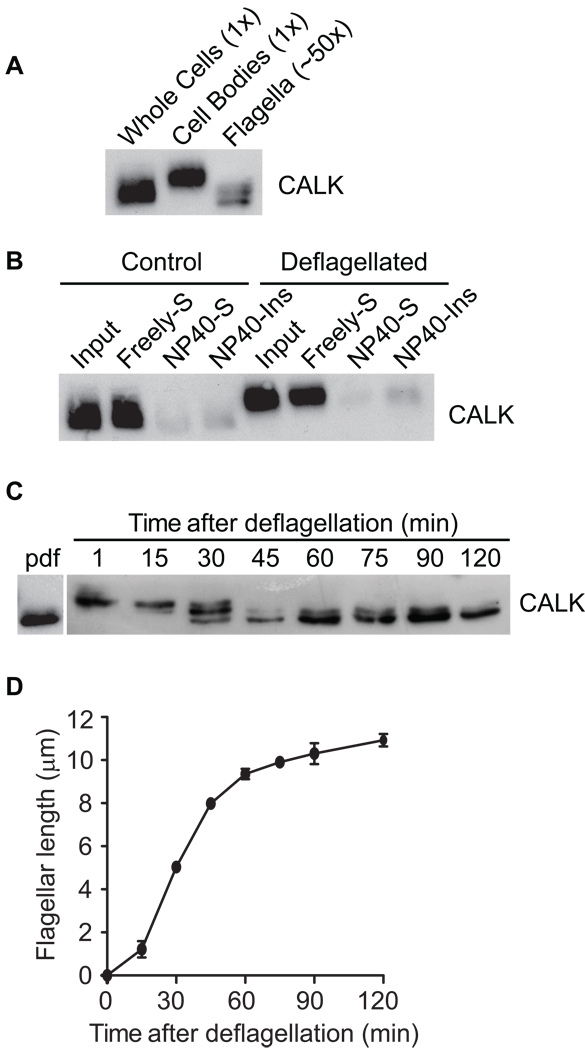
We used SDS-PAGE and immunoblot analysis with an anti-CALK antibody to determine the phosphorylation state of CALK. The non-phosphorylated form migrates at a lower position on SDS-PAGE than the fully phosphorylated form [13]. In resting cells with flagella at steady-state length, CALK was non-phosphorylated (Figure 1A (left lane). And, when cells were deflagellated by pH shock, immunoblotting showed that CALK, most of which was in the cell body, was phosphorylated (Figure 1A, middle panel). Only a small portion of total CALK was in the flagella (Figure 1A, right panel) [13]. Cell fractionation showed that both the non-phosphorylated and the phosphorylated forms of CALK were released by freezing and thawing into the freely soluble, cytoplasmic fraction of cells (Figure 1B).
Figure 1. CALK is Localized to the Cell Body and Undergoes Dephosphorylation during Flagellar Assembly.
(A) CALK is phosphorylated upon pH shock-induced deflagellation and present predominantly in the cell body. Equal amounts of protein from whole cells, cell bodies and flagella were extracted in lysis buffer and analyzed by SDS-PAGE and immunoblotting. Because flagella represent about 2% of total cellular protein, the flagellar sample represents ~50 times as many cell equivalents as the cell body sample.
(B) CALK in control and deflagellated cells is present in the freely soluble, cytoplasmic fraction of cells. Control or pH-shocked cells were disrupted by freezing and thawing and subjected to centrifugation to yield the freely soluble fractions (Freely-S). The insoluble fractions were resuspended in buffer containing the detergent NP40, and centrifuged again to yield the soluble, membrane fractions (NP40-S) and the insoluble fractions (NP-40-Ins). Equal portions of each fraction were analyzed by immunoblot.
(C) Dephosphorylation of CALK begins when regenerating flagella reach about half-length. Cell samples prior to deflagellation (pdf) and at the indicated times after deflagellation were analyzed by SDS-PAGE and immunoblotting. The CALK seen in some experiments migrating between the upper and lower bands indicates that CALK can be phosphorylated at more than one site.
(D) Kinetics of flagellar regeneration after flagellar detachment by pH shock. Error bars indicate the standard error of the mean (SEM).
To assess the phosphorylation states of CALK during flagellar biogenesis, cells were harvested at the indicated times after flagellar assembly was induced by deflagellation (Figure 1C and D) for determination of flagellar lengths and immunoblotting with CALK antibody. As shown in Figure 1C, CALK was in the fully phosphorylated state in deflagellated cells. To our surprise, when the newly assembling flagella reached about half-length, desphosphorylated CALK appeared; and at 45 min and thereafter, as the flagella regenerated to full length (Figure 1D) [15], the fully phosphorylated form had become undetectable (Figure 1C).
CALK dephosphorylation requires flagellar assembly
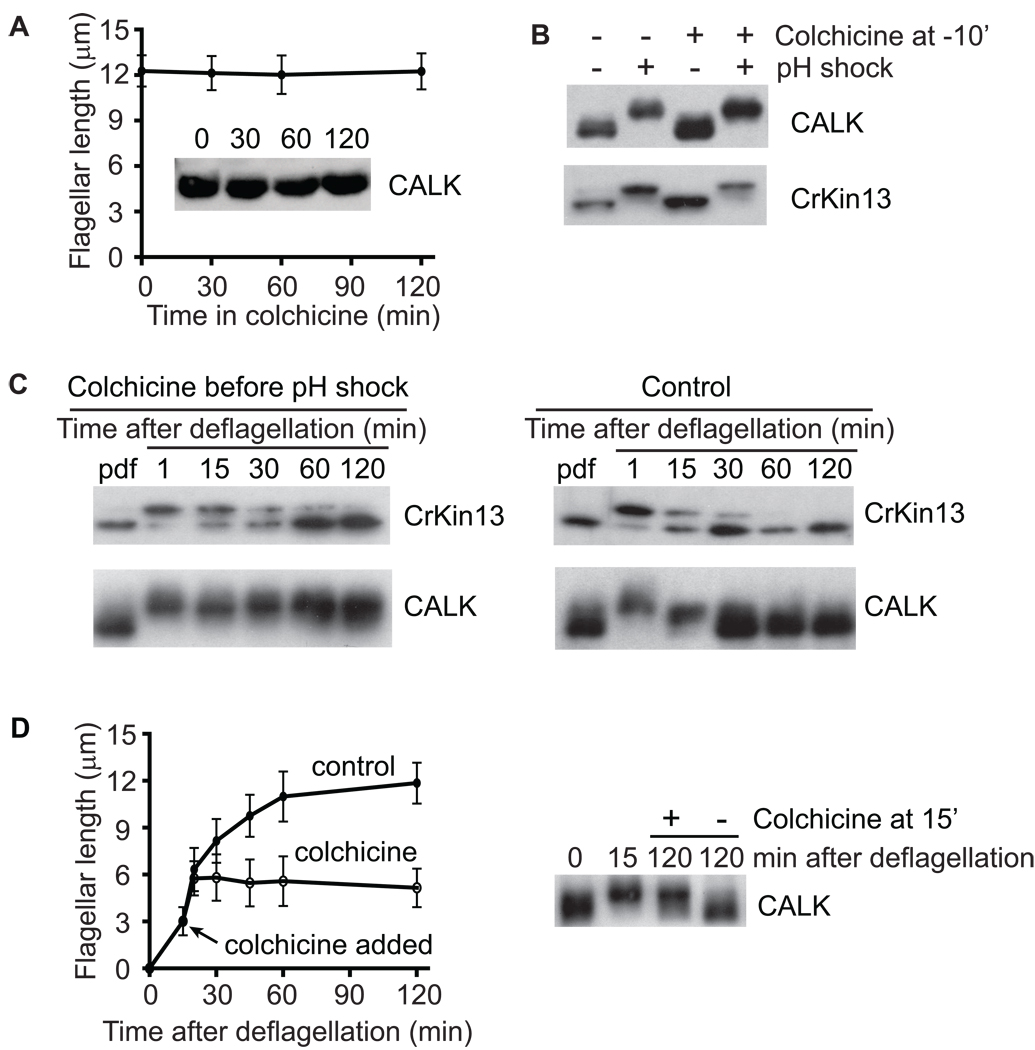
Previously, we showed that Chlamydomonas depolymerizing kinesin, CrKinesin13, which also became phosphorylated upon deflagellation, gradually became dephosphorylated as the flagella assembled, CrKinesin13 [16]. To determine whether dephosphorylation of either protein was related to flagellar biogenesis, we prevented deflagellated cells from initiating flagellar assembly by adding the flagellar assembly inhibitor colchicine just before deflagellation [5, 15]. Control, non-deflagellated cells incubated with colchicine retained full length flagella for 2 hr and possessed only the lower, non-phosphorylated forms of CALK (Figure 2A) and CrKinesin13 (data not shown). Furthermore, colchicine did not block pH-shock induced deflagellation (not shown), nor did it block the concomitant phosphorylation of CALK or CrKinesin13 (Figure 2B). As expected [15], the cells deflagellated in colchicine failed to assemble flagella (data not shown). Furthermore, even though assembly was blocked, the CrKinesin13 in the colchicine-treated cells became desphosphorylated (Figure 2C, left panel) similarly to that in control cells without colchicine (Figure 2C, right panel). In contrast, however, the CALK in the cells that failed to initiate flagellar biogenesis remained phosphorylated for the duration of the experiment (Figure 2C, left panel), unlike the CALK in control cells, which underwent the typical dephosphorylation during assembly (Figure 2C, right panel). Thus, dephosphorylation of CrKinesin13 after flagellar loss was unrelated to flagellar assembly, whereas dephosphorylation of CALK was linked to some aspect of flagellar biogenesis.
Figure 2. Flagellar Assembly Is Essential to Trigger Dephosphorylation of CALK but not CrKinesin-13.
(A) Incubation of cells in an agent, colchicine, that blocks flagellar assembly does not induce phosphorylation of CALK or changes in flagellar length in cells with steady-state length flagella. Error bars in this and subsequent figures indicate standard deviation (SD).
(B) Colchicine does not block deflagellation or the concomitant phosphorylation of CALK and CrKinesin13. Control cells and cells to which colchicine was added were subjected to pH shock and then CALK and CrKinesin-13 were analyzed by SDS-PAGE and immunoblotting.
(C) Dephosphorylation of CALK but not CrKinesin-13 requires flagellar assembly. Cells deflagellated by pH shock were incubated in the presence or absence of colchicine and at the indicated times the phosphorylation states of CALK and Crkinesin13 were analyzed by SDS-PAGE and immunoblotting.
(D) Initiation of flagellar assembly alone fails to trigger dephosphorylation of CALK. Flagellar lengths and CALK phosphorylation states were assessed at the indicated times in control, deflagellated cells and in deflagellated cells to which colchicine was added at 15 min (arrow) to block further flagellar assembly.
To determine whether CALK dephosphorylation was linked simply to initiation of flagellar assembly, we allowed deflagellated cells to begin flagellar assembly, blocked further assembly by adding colchicine, and then assessed the phosphorylation state of CALK. As shown in Figure 2D, cells that were allowed to initiate flagellar assembly for 15 minutes before assembly was blocked by colchicine (left panel) failed to dephosphorylate CALK when examined 2 hr after deflagellation (right panel). In contrast, CALK became fully dephosphorylated in the control (Figure 2D, right panel) cells that assembled full-length flagella (left panel). Thus, intiation of flagellar assembly per se failed to trigger CALK dephosphorylation. Taken together, our results indicated that dephosphorylation of CALK was not related simply to time after deflagellation or to initiation of flagellar assembly. Rather, the results suggested that dephosphorylation was related to flagellar length.
The phosphorylation state of CALK is linked to flagellar length
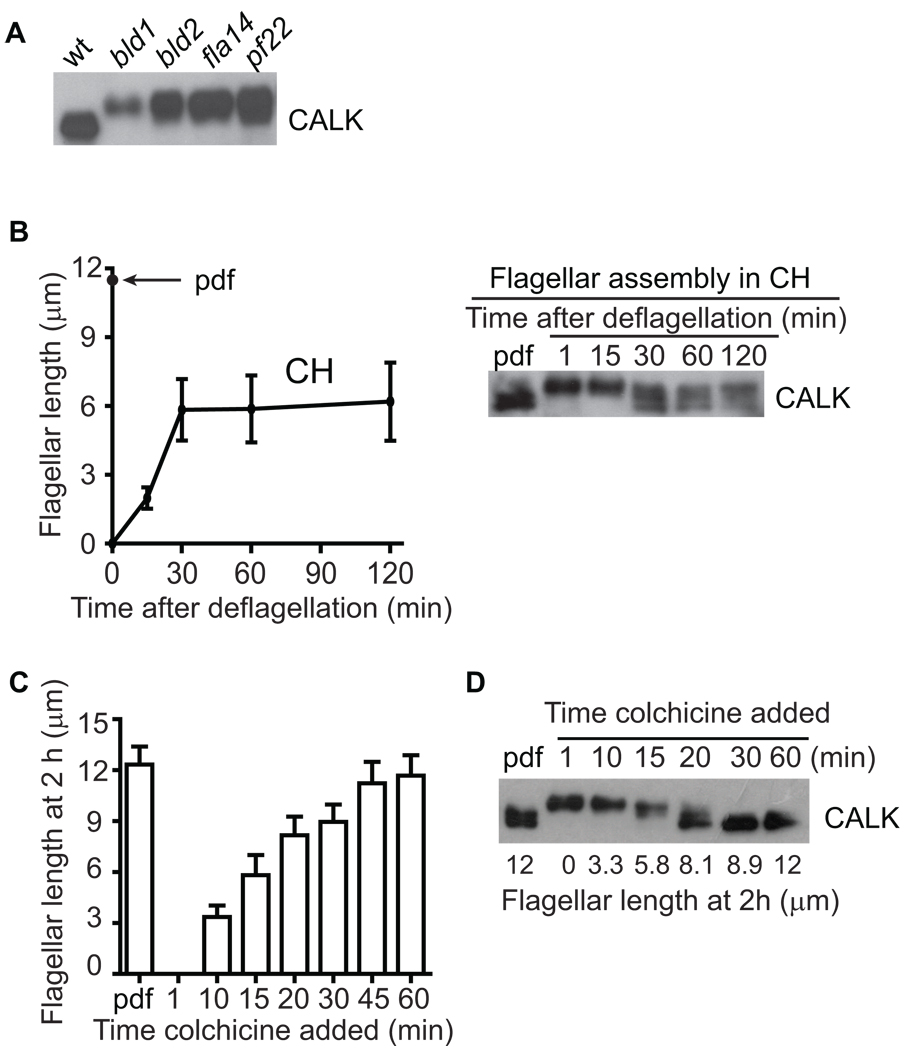
To test for a direct relationship between the phosphorylation state of CALK and flagellar length, we studied CALK in several mutants with flagella of varying lengths, and in wild type cells experimentally treated to generate flagella of varying lengths. Previously, we showed that CALK was constitutively phosphorylated in several strains of aflagellate cells, including wild type cells growing on agar plates, and IFT mutant cells dhc1b, ift88, and fla10 null [13, 17]. CALK was similarly constitutively phosphorylated in the flagella-less mutants bld1 (an IFT52 mutant) and bld2 (an epsilon tubulin mutant) [18, 19]; and in cells with short flagella, fla14 (a retrograde IFT motor mutant) [20] and pf22 (a flagellar dynein arm mutant) [9] (Figure 3A). Thus, independently of the circumstances that led to the diminished length or absence of flagella, cells with flagella of less than half-length contained only the phosphorylated form of CALK.
Figure 3. CALK Phosphorylation State is Responsive to Flagellar Length.
(A) CALK is constitutively phosphorylated in the aflagellate mutants bld1 and bld2 and in IFT mutants with short flagella, fla14 and pf22.
(B) CALK dephosphorylation begins but fails to be completed in cells that assemble half-length flagella in the absence of protein synthesis. Flagellar lengths (left panel) and CALK phosphorylation states (right panel) were determined at the indicated times in cells deflagellated and maintained in cycloheximide (CH).
(C) Pharmacologically-regulated half-length is a flagellar threshold for CALK dephosphorylation. Colchicine was added to deflagellated cells at the indicated times. At 2 h after deflagellation, flagellar lengths (left panel) and CALK phosphorylation states (right panel) were determined.
To examine CALK in cells whose flagella were around half-length (5 – 7 µm), we took advantage of the observation that cells deflagellated in a protein synthesis inhibitor draw upon a pool of flagellar precursors during regeneration that is sufficient to generate ~half-length flagella [15]. As shown in Figure 3B, within 30 min after deflagellation in the protein synthesis inhibitor cycloheximide, cells had assembled half-length flagella, and then growth had ceased. As expected, CALK was phosphorylated upon deflagellation in the presence of cycloheximide. Furthermore, at 30 min after deflagellation, CALK dephosphorylation had begun to occur, but then had ceased. At 120 min, the flagella remained at half-length and the phosphorylation state of CALK remained at an intermediate level (Figure 3B). These results suggested that half-length represented a threshold length during flagellar biogenesis to which cells responded by triggering initiation of dephosphorylation of CALK. Thus, in these circumstances, a posttranslational modification of CALK was a molecular marker of ~half-length flagella.
To test whether the phosphorylation state of CALK was a more general marker of ~half-length, we generated cells with flagella of varying lengths using colchicine [15] and assessed CALK phosphorylation. For this experiment, colchicine was added at the times indicated after deflagellation to block further assembly, and at 2 hr we determined flagellar length (Figure 3C, left panel) and assessed the phosphorylation state of CALK (right panel). CALK phosphorylation state indeed was a remarkably accurate indicator of flagellar length. In cells with flagella less than half-length (5 – 7 µm), CALK was phosphorylated, and in cells with flagella greater than ~half-length, CALK was dephosphorylated.
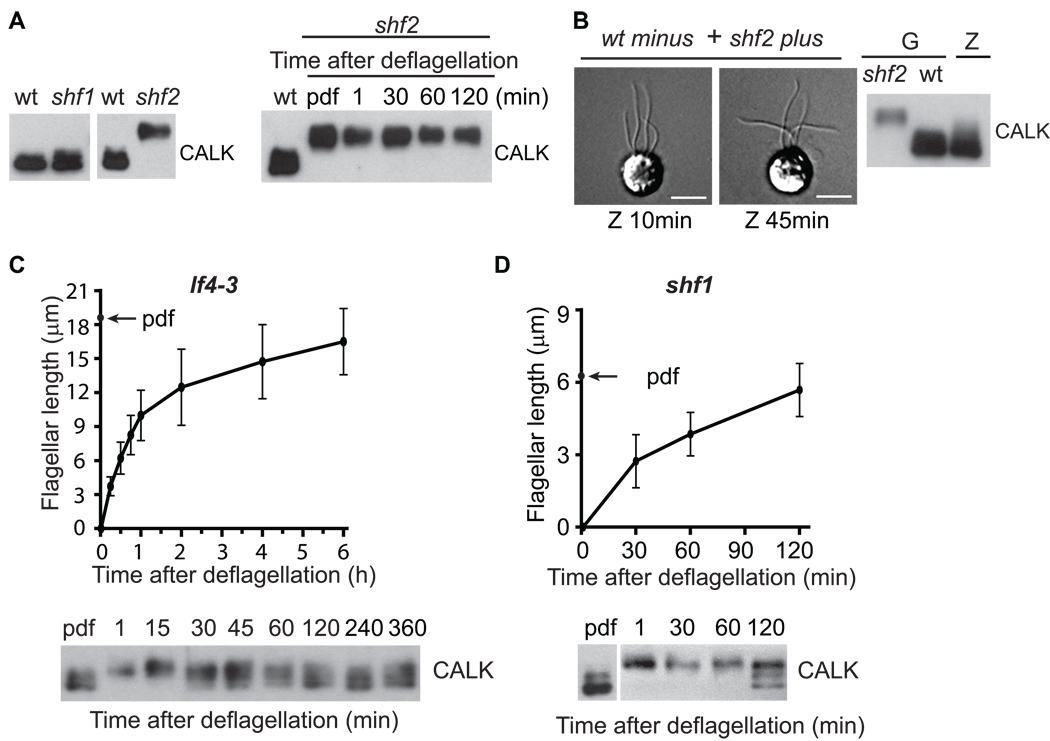
The differing states of CALK phosphorylation in two short flagella mutants also indicate that half-length is a threshold to which CALK is finely tuned
Because we had found that half-length was a threshold for the change in phosphorylation state of CALK in cells with pharmacologically generated half-length flagella, we anticipated that we would find similar evidence for a half-length threshold in mutants whose flagella were about half-length. Among 3 reported short (half-length) flagella mutants, shf1, shf2 and shf3, only shf1 and shf2 retained the half-length phenotype and were studied further. The CALK in shf1 cells, whose flagella were between 6 – 8 µm in average length [21] was mostly non-phosphorylated (Figure 4A). On the other hand, the CALK in shf2 mutants, whose flagella were also around half-length, was mostly phosphorylated. Occasionally, intermediate states of CALK phosphorylation were observed in shf1 cells (data not shown). As expected, the CALK in shf1 cells was capable of becoming phosphorylated upon deflagellation (see below).
Figure 4. Genetically-Specified Half-Length Is A Flagellar Threshold For Triggering CALK Dephosphorylation, And Cells Detect Absolute, Not Relative Flagellar Length.
(A) Mutants shf1 and shf2 with ~half-length flagella exhibit different, all-or-none states of CALK phosphorylation. CALK is in the dephosphorylated state in wt and shf1 cells and in the phosphorylated state in shf2 cells (left panel). Flagellar assembly after deflagellation in shf2 cells fails to induce CALK dephosphorylation (right panel).
(B) CALK in shf2 cells is capable of being desphosphorylated. The flagella of shf2 cells assembled to wild type length after wild type minus and shf2 plus gametes (G) fused to form zygotes (Z) (left panels), and CALK became dephosphorylated (right panel). Scale bar represents 5 µm.
(C) CALK dephosphorylation in the long flagellar mutant lf4–3. CALK dephosphorylation begins when assembling flagella are ~6 µm long. Flagellar lengths (upper panels) and CALK phosphorylation states (lower panels) were determined at the indicated times during flagellar assembly after deflagellation.
(D) CALK dephosphorylation also begins when assembling flagella reach ~6 µm long in the short flagellar mutant shf1.
Because it was possible that the shf2 mutation rendered CALK incapable of becoming dephosphoryated, we generated shf2/wild type dikaryons and tested for CALK dephosphorylation. We mixed wild type minus (6145C) gametes with shf2 plus gametes and allowed them to fuse to form zygotes whose cytoplasm, therefore, represented an amalgam of the 2 parental cytoplasms. Confirming earlier results [21], the short flagella began to elongate soon after fusion (Figure 4B, left panel). And, strikingly, all of the CALK in the zygotes became dephosphorylated (Figure 4B, right panel). Thus, shf2 CALK was fully capable of becoming dephosphorylated. Taken together with the results above, these results that CALK exhibits different, all-or-none phosphorylation states in 2 short flagella mutants whose flagella were ~ half-length, indicate that half-length is a very finely monitored threshold during flagellar assembly.
CALK phosphorylation state is responsive to absolute flagellar length
We next tested whether the phosphorylation state of CALK was responsive to the absolute or relative length of flagella by use of long (lf) and short flagella mutants. Of the four lf mutants available [22], lf1, lf2, and lf3 cells have flagella of varying lengths, are defective in flagellar regeneration, and thus were not suitable for our assay [3, 22, 23]. In contrast, lf4–3 null mutants have flagella of relatively uniform length of around 20 µm, and are able to regenerate flagella after deflagellation [4, 22] (Figure 4C, upper panel). As expected, CALK was phosphophorylated during flagellar loss and underwent dephosphorylation during flagllar regeneration. Of particular importance, dephosphorylation of CALK became detectable around 30 min after flagellar detachment when the flagella were about 6 µm long (Figure 4C, lower panel). Although CALK never became fully dephosphorylated in this experiment, the result that this long flagellar mutant initiated dephosphorylation of CALK when its flagella became ~6 µm long (i. e., half-the length of a wild type flagellum) indicated that cells are responsive to absolute and not relative flagellar length. Experiments with shf1 cells led to a similar conclusion. CALK in shf1 cells became phosphorylated upon deflagellation; and, even though flagellar assembly took much longer than in the lf4–3 cells (Figure 4D, upper panel), only when the flagella of the shf1 cells reached about 6 µm in length, was CALK dephosphorylation initiated (Figure 4D, lower panel). Thus, independently of the time required to assemble flagella or of ultimate flagellar length, cells detect absolute not relative flagellar length.
Translating flagellar length into a posttranslational modification of a flagellar regulatory protein
Our results that the phosphorylation state of the protein kinase CALK is a marker of flagellar length demonstrate for the first time that cells possess a mechanism for translating flagellar length into a posttranslational modification. The simplest models to account for such a sensing mechanism must include molecular events that occur only when cells begin to extend their flagella beyond a half-length threshold. For example, a cell body regulator of CALK dephosphorylation could transit through flagella by IFT and become activated only when it interacts with flagellar components uniquely present in the distal half of the flagellum. Or, in a time-of-flight model, the regulator might be activated only if its transit time in flagella is increased as a consequence of increased flagellar length. In the cilium-based sonic hedgehog signaling pathway, pathway activation depends on transit of cytoplasmic components into cilia [24].
In a variant of these models, CALK itself could be modified by cycling through flagella. In any of these models, CALK dephosphorylation would fail in shf2 cells if the SHF2 protein were essential for assembly of unique components of the distal portion of flagella, or if SHF2 were a sensor essential for detecting half-length flagella and triggering formation of unique distal components.
Chlamydomonas cells could exploit the changes in CALK phosphorylation state for at least 3 interconnected, length-related features of flagella: control of flagellar length, specification during assembly of transport of proteins unique to distal versus proximal portions of flagella, and regulation of flagellar protein transcripts during flagellar assembly.
Length regulation
Two of the most prominent models for length regulation (see review ([1]) are the feedback model that posits that assembly or disassembly of flagella occurs upon deviations from the genetically set length [25], and the balanced point model that posits that length is determined by a passive mechanism that depends on length-dependent assembly and length-independent disassembly of flagellar components [5]. A fundamental difference between the two models is that the feedback model depends on a sensor or a mechanism that detects flagellar length, whereas the balance point model does not.
The discoveries that several signaling molecules are essential for flagellar length control including cAMP [26], calcium [6], and protein kinases [3, 4, 27] are consistent with a feedback model [28]. And, our results here that CALK phosphorylation state is responsive to flagellar length provide the first molecular evidence for a length-sensing system. Whether a half-length sensor could be used to specify full length flagella remains unknown. Future studies on cells whose flagella are of different lengths - - uni-flagella mutants [29] and “long-zero flagella” cells generated by mechanical shearing [15] - - should provide important insights into CALK phosphorylation and length regulation.
Structural polarity
Previously, we temporally linked CALK phosphorylation to a nearly 4-fold increase in flagellar trafficking of IFT subcomplexes and to regulation of IFT cargo loading during regulated flagellar disassembly [17]. Thus, it is possible that CALK phosphorylation state during flagella regeneration is used by cells to regulate loading of distal versus proximal cargo onto IFT particles to create flagellar polarity.
Gene transcription
It is also possible cells exploit the phosphorylation state of CALK to regulate transcription of flagellar genes. Immediately after detachment of flagella, when CALK is phosphorylated, transcription of flagellar genes is activated. And, when flagella are ~half-length, CALK is dephosphorylated and transcription of flagellar genes is terminated [8]. Although evidence is lacking in Chlamydomonas that transcription of flagellar genes regulates length, ciliary length in zebrafish Kupffer’s vesicles is regulated by FGF-dependent gene transcription [30].
In summary, our results demonstrate for the first time in any system the existence of a mechanism to translate flagellar length into a protein posttranslational modification. Our work lays the basis for a deeper understanding of control of flagellar assembly.
Supplementary Material
Acknowledgments
We thank Drs. Pete Lefebvre and Nedra Wilson for providing the lf4 strains. We are grateful to Drs. Joel Rosenbaum, George Witman and Pete Lefebvre for helpful discussions. This work was supported by NIH Grant GM25661 (to W. J. S.) and National Natural Science Foundation of China (Grants 30830057, 30988004 and 30771084), 973 program (Grant 2007CB914401), and SRFDP (to J. P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Data
Supplemental information, including Experimental Procedures, is available at http://www.current�biology.com/content/supplemental.
References
- 1.Wemmer KA, Marshall WF. Flagellar length control in chlamydomonas--paradigm for organelle size regulation. Int. Rev. Cytol. 2007;260:175–212. doi: 10.1016/S0074-7696(06)60004-1. [DOI] [PubMed] [Google Scholar]
- 2.Gerdes JM, Davis EE, Katsanis N. The vertebrate primary cilium in development, homeostasis, and disease. Cell. 2009;137:32–45. doi: 10.1016/j.cell.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tam LW, Wilson NF, Lefebvre PA. A CDK-related kinase regulates the length and assembly of flagella in Chlamydomonas. J. Cell Biol. 2007;176:819–829. doi: 10.1083/jcb.200610022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berman SA, Wilson NF, Haas NA, Lefebvre PA. A novel MAP kinase regulates flagellar length in Chlamydomonas. Curr. Biol. 2003;13:1145–1149. doi: 10.1016/s0960-9822(03)00415-9. [DOI] [PubMed] [Google Scholar]
- 5.Marshall WF, Rosenbaum JL. Intraflagellar transport balances continuous turnover of outer doublet microtubules: implications for flagellar length control. J. Cell Biol. 2001;155:405–414. doi: 10.1083/jcb.200106141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tuxhorn J, Daise T, Dentler WL. Regulation of flagellar length in Chlamydomonas. Cell Motil. Cytoskeleton. 1998;40:133–146. doi: 10.1002/(SICI)1097-0169(1998)40:2<133::AID-CM3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 7.Stolc V, Samanta MP, Tongprasit W, Marshall WF. Genome-wide transcriptional analysis of flagellar regeneration in Chlamydomonas reinhardtii identifies orthologs of ciliary disease genes. Proc. Natl. Acad. Sci. USA. 2005;102:3703–3707. doi: 10.1073/pnas.0408358102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker EJ, Schloss JA, Rosenbaum JL. Rapid changes in tubulin RNA synthesis and stability induced by deflagellation in Chlamydomonas. J. Cell Biol. 1984;99:2074–2081. doi: 10.1083/jcb.99.6.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piperno G, Ramanis Z. The proximal portion of Chlamydomonas flagella contains a distinct set of inner dynein arms. J. Cell Biol. 1991;112:701–709. doi: 10.1083/jcb.112.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yagi T, Uematsu K, Liu Z, Kamiya R. Identification of dyneins that localize exclusively to the proximal portion of Chlamydomonas flagella. J. Cell Sci. 2009;122:1306–1314. doi: 10.1242/jcs.045096. [DOI] [PubMed] [Google Scholar]
- 11.Kann ML, Soues S, Levilliers N, Fouquet JP. Glutamylated tubulin: diversity of expression and distribution of isoforms. Cell Motil. Cytoskeleton. 2003;55:14–25. doi: 10.1002/cm.10107. [DOI] [PubMed] [Google Scholar]
- 12.Hoops HJ, Witman GB. Outer doublet heterogeneity reveals structural polarity related to beat direction in Chlamydomonas flagella. J. Cell Sci. 1983;97:902–908. doi: 10.1083/jcb.97.3.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan J, Wang Q, Snell WJ. An aurora kinase is essential for flagellar disassembly in Chlamydomonas. Dev. Cell. 2004;6:445–451. doi: 10.1016/s1534-5807(04)00064-4. [DOI] [PubMed] [Google Scholar]
- 14.Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis EA. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell. 2007;129:1351–1363. doi: 10.1016/j.cell.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenbaum JL, Moulder JE, Ringo DL. Flagellar elongation and shortening in Chlamydomonas. The use of cycloheximide and colchicine to study the synthesis and assembly of flagellar proteins. J. Cell Biol. 1969;41:600–619. doi: 10.1083/jcb.41.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piao T, Luo M, Wang L, Guo Y, Li D, Li P, Snell WJ, Pan J. A microtubule depolymerizing kinesin functions during both flagellar disassembly and flagellar assembly in Chlamydomonas. Proc. Natl. Acad. Sci. USA. 2009;106:4713–4718. doi: 10.1073/pnas.0808671106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan J, Snell WJ. Chlamydomonas shortens its flagella by activating axonemal disassembly, stimulating IFT particle trafficking, and blocking anterograde cargo loading. Dev. Cell. 2005;9:431–438. doi: 10.1016/j.devcel.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Dutcher SK, Morrissette NS, Preble AM, Rackley C, Stanga J. Epsilon-tubulin is an essential component of the centriole. Mol. Biol. Cell. 2002;13:3859–3869. doi: 10.1091/mbc.E02-04-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brazelton WJ, Amundsen CD, Silflow CD, Lefebvre PA. The bld1 mutation identifies the Chlamydomonas osm-6 homolog as a gene required for flagellar assembly. Curr. Biol. 2001;11:1591–1594. doi: 10.1016/s0960-9822(01)00485-7. [DOI] [PubMed] [Google Scholar]
- 20.Pazour GJ, Wilkerson CG, Witman GB. A dynein light chain is essential for the retrograde particle movement of intraflagellar transport (IFT) J. Cell Biol. 1998;141:979–992. doi: 10.1083/jcb.141.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuchka MR, Jarvik JW. Short-Flagella Mutants of Chlamydomonas reinhardtii. Genetics. 1987;115:685–691. doi: 10.1093/genetics/115.4.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barsel SE, Wexler DE, Lefebvre PA. Genetic analysis of long-flagella mutants of Chlamydomonas reinhardtii. Genetics. 1988;118:637–648. doi: 10.1093/genetics/118.4.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tam LW, Dentler WL, Lefebvre PA. Defective flagellar assembly and length regulation in LF3 null mutants in Chlamydomonas. J. Cell Biol. 2003;163:597–607. doi: 10.1083/jcb.200307143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tukachinsky H, Lopez LV, Salic A. A mechanism for vertebrate Hedgehog signaling: recruitment to cilia and dissociation of SuFu-Gli protein complexes. J. Cell Biol. 2010;191:415–428. doi: 10.1083/jcb.201004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silflow CD, Lefebvre PA. Assembly and motility of eukaryotic cilia and flagella. Lessons from Chlamydomonas reinhardtii. Plant Physiol. 2001;127:1500–1507. [PMC free article] [PubMed] [Google Scholar]
- 26.Besschetnova TY, Kolpakova-Hart E, Guan Y, Zhou J, Olsen BR, Shah JV. Identification of signaling pathways regulating primary cilium length and flow-mediated adaptation. Curr. Biol. 2010;20:182–187. doi: 10.1016/j.cub.2009.11.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson NF, Lefebvre PA. Regulation of flagellar assembly by glycogen synthase kinase 3 in Chlamydomonas reinhardtii. Eukaryot. Cell. 2004;3:1307–1319. doi: 10.1128/EC.3.5.1307-1319.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenbaum J. Organelle size regulation: length matters. Curr. Biol. 2003;13:R506–R507. doi: 10.1016/s0960-9822(03)00440-8. [DOI] [PubMed] [Google Scholar]
- 29.Huang B, Ramanis Z, Dutcher SK, Luck DJ. Uniflagellar mutants of Chlamydomonas: evidence for the role of basal bodies in transmission of positional information. Cell. 1982;29:745–753. doi: 10.1016/0092-8674(82)90436-6. [DOI] [PubMed] [Google Scholar]
- 30.Neugebauer JM, Amack JD, Peterson AG, Bisgrove BW, Yost HJ. FGF signalling during embryo development regulates cilia length in diverse epithelia. Nature. 2009;458:651–654. doi: 10.1038/nature07753. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.