Abstract
Efforts to move from malaria control to eradication will require new approaches to block malaria transmission, such as the development of anti-malarial drugs with gametocytocidal activity. Here fluorescent oxidoreduction indicator alamarBlue is used to develop a screen for gametocyte viability. The fluorescent signal increases linearly with gametocyte number (R2=0.99) and determination of the IC50 of epoxomicin demonstrated the assay was reproducible and sensitive (IC50 2.16±0.57 nM, Z’-factor 0.81±0.01). Six anti-malarials were also tested and at 10 µM only primaquine and dihydroartemisinin (DHA) had gametocytocidal activity. This new assay provides an important tool to efficiently screen compounds for gametocytocidal activity.
Keywords: alamarBlue, drug screening, gametocyte, Plasmodium falciparum
The resurgence of Plasmodium falciparum mortality seen in the wake of the spread of chloroquine-resistant parasites in the 1990s was a grim reminder of the importance of effective anti-malaria therapy [1]. After the introduction of artemisinin combination therapy (ACT) as first line treatment in 2004 mortality rates again decreased. However, reports of recrudescence and delayed clearance following ACT treatment are a concern and are being carefully monitored [2–3]. There has also been increased support for the discovery and development of new anti-malarials. A number of small compound and natural product libraries have been screened by monitoring DNA or parasite replication in intraerythrocytic parasites and several novel compounds have been identified for further development [4–7]. The assays used in the screens to date identify compounds that block asexual parasite growth, but do not evaluate the effect of the compounds on other stages of the life cycle, including intraerythrocytic parasites undergoing sexual differentiation into gametocytes. During gametocyte development DNA replication is blocked and only a single gametocyte is produced following red blood cell (RBC) invasion. Therefore, in contrast to asexual parasites this stage is not easily monitored using DNA or cell proliferation markers.
Once an intraerythrocytic parasite begins sexual differentiation it takes 10–12 day for the production of a mature gametocyte capable of infecting a mosquito and spreading malaria through the population. In addition to not undergoing DNA replication, gametocytes are less sensitive to all the commonly used anti-malarial drugs, except primaquine, than asexual parasites [8]. In fact, it has been reported that treatment with the anti-malarial pyrimethamine stimulates sexual differentiation [9]. As a consequence of this difference in drug sensitivity and the long time course of gametocytogenesis, a patient can remain infectious to mosquitoes for more than 2 weeks after the clearance of asexual parasites. These factors complicate elimination of the parasite and facilitate the propagation of drug-resistant parasites. In contrast to pyrimethamine and other anti-malarials that block DNA replication, artemisinin derivatives are effective against early gametocytes [8, 10]. This activity may contribute to the marked reduction in malaria seen after the introduction of ACT. In a recent study by Smithuis et al. adding a single dose of primaquine to ACT has been shown to decrease the number of patients with gametocyte-positive thick blood smears from ~30% to ~4 % in 7 days, while in the absence of primaquine ~27% of the patients remained gametocytemic [11]. Such findings have lead to suggestion that primaquine be used as a method to reduce and possibly eliminate malaria hypoendemic areas. However, hemolysis can be a side effect of primaquine and this can be severe in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency, which is common in malaria endemic areas. This side effect has raised concerns about mass administration of primaquine without a rapid assay for G6PD deficiency that can be used in remote field settings [12].
The identification of new compounds with gametocytocidal activity could facilitate control and eradication efforts. However, screening compound libraries for activity against mature gametocytes has been limited by several factors. In in vitro culture the yield of P. falciparum gametocytes is low in comparison to asexual parasites and the method used to analyze development, manual quantitation of Giemsa-stained blood smears, is slow and labor intensive.
The present study describes a method to screen compounds for gametocytocidal activity using oxidoreduction indicator, alamarBlue, as a general measure of metabolic activity. Only a single fluorescent reduced form is monitored which simplifies quantification and the signal can be enhanced by increasing the incubation time because the fluorescence of reduced alamarBlue is stable at 37°C for several days. AlamarBlue has already been used to evaluate viability of a number of cell lines, including Trypanosoma and Leishmania [6, 13]. Another benefit of this assay is that it can be used to directly evaluate any parasite line or natural isolate, since it does not require the introduction of a reporter gene by transformation.
Assay Optimization
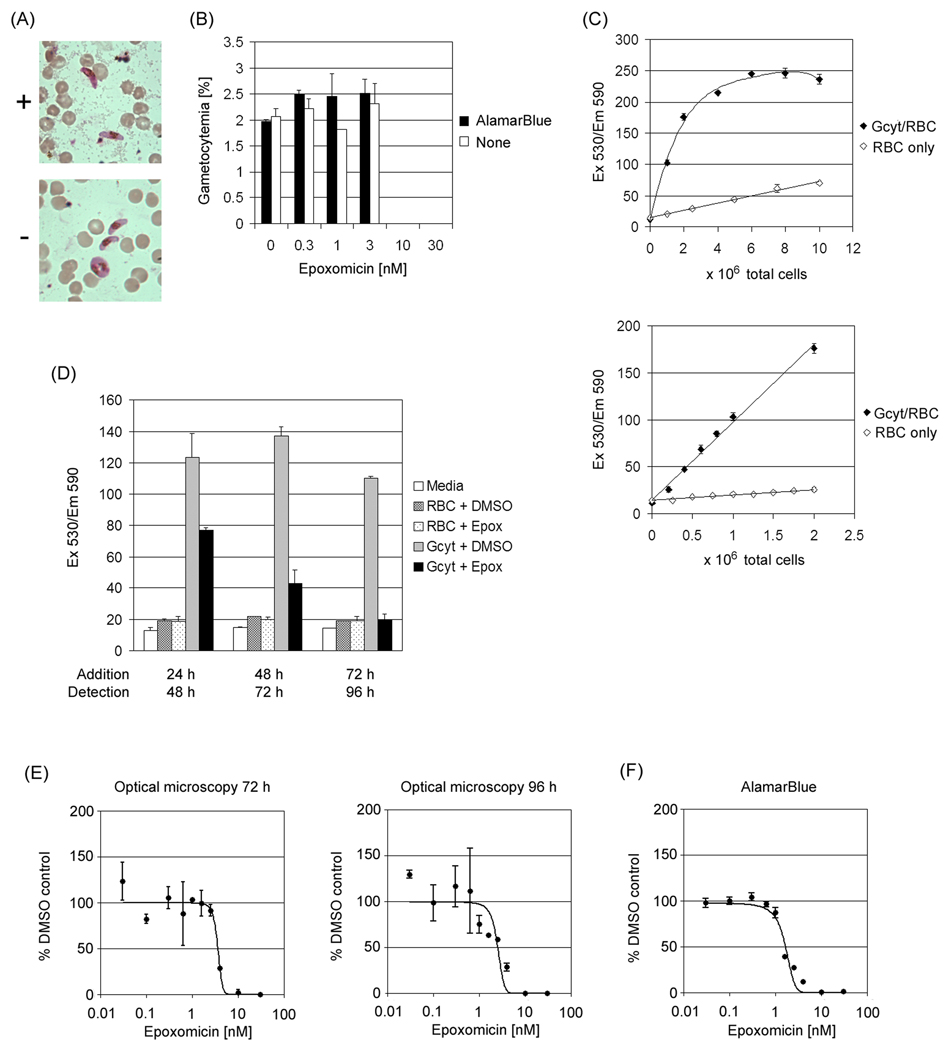
AlamarBlue was first screened to ensure that it did not interfere with gametocyte development or the gametocytocidal activity of proteasome inhibitor, epoxomicin [14]. Gametocyte (3D7 strain) cultures were set up in complete media (RPMI 1640 supplemented with L-Glutamine, 25mM HEPES, 50 µg ml−1 hypoxanthine (KD Biomedical), 10µg ml−1 gentamicin, 0.25% NaHCO3, and 10% human serum) as described [15] and the parasites were treated with N-acetyl glucosamine (NAG) from day 9 to day 11 to eliminate asexual parasites [16]. On day 13, 100 µl of the gametocyte (stage III-V) culture were incubated with epoxomicin (0.3 – 30 nM) or carrier alone, dimethyl sulfoxide (DMSO), in a 96-well plate for 72 h followed by the addition of 10 µl of alamarBlue (Invitrogen, Carlsbad, CA). After 24 h incubation with alamarBlue, a Giemsa-stained smear of each well was prepared (day 17). Following DMSO or epoxomicin treatment neither gametocytemia, the ability to respond to a gametogenesis signal, nor gametocyte morphology were affected by alamarBlue suggesting that it is not toxic and does not alter epoxomicin activity (Fig. 1A, B).
Fig. 1.
AlamarBlue assay development. (A) Giemsa-stained smear of DMSO control cultures with (+) and without (−) alamarBlue. (B) Gametocytemia of cultures in the presence of epoxomicin (0.3 – 30 nM) or DMSO as the carrier control (0 nM) with (■) and without (□) alamarBlue. NAG-treated gametocyte cultures were incubated with DMSO or epoxomicin for 72 h prior to the addition of alamarBlue. Giemsa-stained smears were prepared 24 h after alamarBlue addition. (C) Gametocyte-dependent alamarBlue fluorescence. NAG-Percoll-treated ametocyte cultures, 0 – 1 × 107 total cells (45.6 % gametocytemia, stage III-V) (Gcyt/RBC) (♦) or 0 – 1 × 107 RBCs (RBC only) (◊) were incubated in 96-well plates with alamarBlue. Fluorescence was measured 24 h after alamarBlue addition and the average signal and standard deviation from duplicate wells in a representative experiment are shown. The data from 0 and 2 to 10 × 106 total cells is shown in the upper graph and the data from 0 to 2 × 106 total cells is shown in the lower graph. (D) Timing of alamarBlue addition. NAG-Percoll-treated gametocyte cultures (4 × 105 gametocytes, stage III-V/ and 6 × 105 RBCs) or 6 × 105 RBCs were incubated in 96-well plates with DMSO or 100 nM epoxomicin. Medium alone without cells was included as a negative control. AlamarBlue was added at 24, 48 or 72 h after epoxomicin addition, and fluorescence was measured 24 h later. Medium alone, open bar; RBC with DMSO, cross-hatched bar; RBC with epoxomicin, dotted bar; gametocyte culture with DMSO, grey bar; gametocyte culture with epoxomicin, black bar (E) Inhibition curves of epoxomicin by optical microscopy. NAG-treated gametocyte cultures (stage III-V) were incubated in a 24-well plate with DMSO or epoxomicin. Giemsa-stained smears were prepared at 72 and 96 h after epoxomicin addition. (F) Epoxomicin inhibition curves using the alamarBlue assay. NAG-Percoll-treated gametocyte cultures (stage III-V) were incubated in a 96-well plate with DMSO or epoxomicin. AlamarBlue was added at 72 h after epoxomicin addition and fluorescence was measured 24 h later. Statistical analysis was done with GraphPad Prism 5 software (GraphPad Software, Inc.).
To determine the detection limit of the assay, gametocytes (stage III-V) cultured as described above were enriched by Percoll (65 % (v/v)) density gradient centrifugation and a range of cell concentrations (0 – 1 × 107 cells (45.6 % gametocytemia, stages III-V) /100 µl) were distributed into a 96-well plate containing 10 µl of alamarBlue. Wells containing an equivalent number of uninfected RBCs were also tested. After overnight incubation, the fluorescence of each well was detected at 590/35 nm following excitation at 530/25 nm with a Synergy TM HT Multi-Detection Microplate Reader (BIO-TEC). The fluorescence signal (Ex 530/Em 590) increased linearly with gametocyte number to 176 Arbitrary Units (AU), before saturating above 200 AU (Fig. 1C). The data is represented by two graphs, one to evaluate saturation above 0.9 × 106 gametocytes/2 × 106 total cells (Fig. 1C, upper panel) and the other to evaluate linearity from 0.23 to 0.9 × 106 gametocytes/0.5 to 2 × 106 total cells (Fig. 1C, lower graph). The linear correlation coefficients in two independent assays of 0 – 2 × 106 cells (45.6 % and 50 % gametocytemia) with duplicate wells were both 0.99 which was significant (p<0.0001) by linear regression analysis. After correcting for the number of gametocytes in each well the fluorescent signal from uninfected RBCs was more 20 times lower than the signal from the same number of gametocytes. Based on these results the cell concentration range selected for use in subsequent assays was 0.4 – 1 × 106 gametocytes/well.
Previous work using optical microscopy indicated that the maximal effect of epoxomicin treatment, even at 100 nM concentration, was observed several days after treatment [14]. To determine whether this would also be the optimal time point for the alamarBlue assay, alamarBlue was added to NAG-treated, Percoll-purified gametocyte cultures (4 × 105 stage III-V gametocytes and 6 × 105 RBCs)/100 µl) or RBCs alone (6 × 105 RBCs/100 µl) at 24, 48 or 72 h after 1 µl of DMSO alone or in combination with 10 µM epoxomicin was added and fluorescence was measured 24 h later at 48, 72 and 96 h, respectively. The fluorescent signals from the DMSO-treated cultures remained constant; at 48, 72 and 96 h they were 124 ± 14.9 AU, 137 ± 5.67 AU and 110 ± 1.41 AU, respectively. In contrast, the signals from the epoxomicin treated cultures declined from 77 ± 1.41 AU at 48 h to the level of the uninfected RBCs at 96 h, 19.5 ± 3.54 AU. This indicated that, as seen previously with the optical assay, all the gametocytes died within 96 hours of treatment with 100 nM epoxomicin and this was selected as the time point to use in subsequent assays (Fig. 1D) [14]. Moreover the RBC signal was not altered by epoxomicin, indicating that the decreased fluorescence observed in the epoxomicin treated-gametocyte cultures was due to gametocyte mortality not a direct affect of epoxomicin on alamarBlue fluorescence. As an independent marker of viability gametocytes treated 72 hr with DMSO or epoxomicin were tested for the ability to respond to a gametogenesis stimulus by rounding up and exflagellating. The DMSO-treated gametocytes rounded up and the males successfully exflagellated, while there was no exflagellation or other change in the morphology of the few residual gametocyte fragments found in the epoxomicin-treated cultures in response to the stimulus. These findings are consistent with prior viability studies demonstrating that treatment with ≤ 1 % DMSO does not affect gametogenesis or oocyst production [14].
Final Assay Conditions
Once the basic conditions were established for the alamarBlue assay it was directly compared with the current standard assay, optical microscopy of Giemsa-stained smears. The epoxomicin dose response was used for comparison. For the optical assay, 10 µl of DMSO alone or in combination with the indicated concentration of epoxomicin were added to NAG-treated gametocyte cultures (1.45 % gametocytemia (stage III-V)/ 3 % hematocrit on day 13). Giemsa-stained smears were prepared at 72 and 96 h after incubation with epoxomicin and the gametocytes counted manually.
The alamarBlue assay was carried out as is described here in detail. Gametocytes were produced from asexual P. falciparum (3D7 strain) by setting up a culture in a 75 cm2 flask at 0.1 % parasitemia and 6 % hematocrit in 12.5 ml on day 1. The culture was maintained under standard culture conditions (37°C in 90% Nitrogen, 5% CO2 and 5% O2), fed 12.5 ml medium on day 3, and then fed daily with 25 ml medium from day 4 to 11. On days 9–11 50 mM NAG was included in the medium to eliminate asexual parasites. On day 12, infected RBCs were enriched by Percoll density gradient centrifugation. Briefly, gametocytes and uninfected RBCs were collected by centrifugation at 800 × g for 5 min, and adjusted to 10–20% hematocrit in RPMI medium without serum (incomplete). Two-2.5 ml were layered on top of 9 ml of 65 % (v/v) Percoll/PBS in a 15 ml tube. The tube was centrifuged at 1,870 × g for 10 min in a swinging bucket rotor and the gametocyte layer at the medium/Percoll interface was collected. The gametocytes were washed twice in incomplete medium and cultivated in complete medium overnight. The following day (day 13) the medium was changed and the gametocytemia (stage III-V)) determined by Giemsa stain. The culture volume was adjusted to 5 × 105 gametocytes and 0.7 – 4.2 × 106 RBC (40 – 10.5 % gametocytemia)/100 µl and 100 µl were added to each well of 96-well-flat-bottom plate. Reproducibility was found to be optimal if the gametocytemia was between 10 – 20% as it resulted in more uniform distribution to the wells. One microliter epoxomicin or DMSO was added to each well and the gametocytes were cultivated under standard conditions without changing medium. On day 16, 10 µl of alamarBlue were added to each well and the plate was returned to standard culture conditions for 24 h. On day 17 the plate was centrifuged at 1,870 × g for 5 min, 80 µl of supernatant was collected in a new 96-well plate, and the fluorescence of reduced alamarBlue in the supernatant was measured at 590/35 nm following excitation at 530/25 nm.
In both the optical and alamarBlue assays the gametocytes were killed completely with 10 and 30 nM epoxomicin and no mortality was observed at less than 0.3 nM epoxomicin. By optical microscopy the epoxomicin IC50 at 72 and 96 h after treatment were 3.42 and 2.87 nM and in the corresponding alamarBlue assay the IC50 measured 96 h after treatment was 1.44 nM (Fig. 1E, F). To test reproducibility, the almarBlue assay was repeated 6 times and the average epoxomicin IC50 determined to be 2.16 ± 0.57 nM. In two independent optical assays, the average IC50 was 3.60 ± 0.26 and 3.47 ± 0.85 nM at 72 and 96 h, respectively. The IC50 values are similar in both assays, however, in contrast to the 8–12 hours required to count Giemsa slides from 96 wells, it took less than 1 minute to measure the alamarBlue assay plate and the standard deviation was lower. For further evaluation of this method as a drug screen, the Z’-factor was calculated as described [17]. This factor evaluates both the dynamic range and variability of the assay and a value over 0.7 is considered acceptable for compound library analysis. The well-to-well and plate-to-plate Z’-factor values for the alamarBlue assay were 0.87 ± 0.08 and 0.81 ± 0.01 for 7 and 4 independent alamarBlue assays, respectively, indicating it should provide a reliable tool to screen compounds for gametocytocidal activity.
Gametocytocidal activity of common anti-malarial drugs
As a further test, the alamarBlue assay was used to screen the gametocytocidal activity of a panel of current anti-malarial drugs, pyrimethamine, chroloquine, quinine, mefloquine, dihydroartemisinine (DHA) and primaquine. Consistent with previous reports pyrimethamine, chloroquine, quinine or mefloquine did not show gametocytocidal activity, while primaquine showed a reduction of 69.4 ± 17.2% activity at 10 µM. DHA, the active metabolite of artemisinin was the most effective against late stage gametocytes inducing a 92.8 ± 1.3% reduction at 10 µM and a 70.4 ± 2.5% reduction at 1 µM in two independent experiments. Artesunate has been reported to have an IC50 of 108 nM for gametocytes (stage IV-V) when assayed by Giemsa smear [8], but the effect of DHA against gametocytes has not been published previously. Although DHA is more efficacious than the other drugs tested, the effective dose against asexual parasites is much lower than that required to kill gametocytes and this is reflected in the ability of gametocytes to remain infectious to mosquitoes for a week after artemisinin treatment [10, 18–19]. Together these results demonstrate that alamarBlue can be used to efficiently screen compounds for gametocytocidal activity. The assay is much faster and less variable than counting gametocytes in Giemsa-stained smears. The Z’-factor of 0.81 (range 1 to −1) calculated from the epoxomicin and anti-malarial drug data indicates that the assay is sensitive and reproducible. Specific-gametocyte stages can be tested and the kinetics of gametocyte mortality can be monitored by adding alamarBlue at different times after compound addition. Importantly, unlike assays developed using parasites lines transformed with reporter genes, such as GFP or luciferase, any parasite line including field isolates can be assayed directly using alamarBlue. This feature is particularly valuable when testing multiple clinical isolates for drug sensitivity. Additionally, although parasites transformed with gametocyte-specific reporter constructs are useful for gametocyte induction assays [9], the GFP signal can persist in dying and dead gametocytes making viability measurements difficult. As mentioned before gametocytes do not replicate, therefore markers for cell replication, such as cell-permeant and impermeant DNA and RNA stains [20] are not very sensitive and can cross-react with the residual hemozoin bodies that accumulate in the culture during the 14–16 days of gametocyte development. In summary the alamarBlue gametocytocidal assay developed here provides a simple, reproducible and economical screen to identify new compounds with the potential to be future malaria transmission-blocking drugs.
Fig. 2.
Anti-malarial gametocytocidal activity. AlamarBlue was added to NAG-Percoll-treated gametocyte cultures (stage III-V) 72 h after compound addition and fluorescence was measured 24 h later, Pyr, pyrimethamine; Qn, quinine; MQ, mefloquine; CQ, chloroquine; DHA, dihydroartemisinine; PriQ, primaquine. 10 µM, black bar; 1 µM, grey bar; 0.1 µM, open bar.
Acknowledgements
This research was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. We thank Dr. S. Desai for use of the fluorescent plate reader and Dr. B. Morahan for critical reading of the manuscript.
Abbreviations
- ACT
artemisinin combination therapy
- AU
arbitrary units
- CQ
chloroquine
- DHA
dihydroartemisinin
- DMSO
dimethyl sulfoxide
- DHA
dihydroartemisinine
- MQ
mefloquine
- PriQ
primaquine
- Pyr
pyrimethamine
- Qn
quinine
- RBC
red blood cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Trape JF. The public health impact of chloroquine resistance in Africa. Am J Trop Med Hyg. 2001;64:12–17. doi: 10.4269/ajtmh.2001.64.12. [DOI] [PubMed] [Google Scholar]
- 2.Carrara VI, Zwang J, Ashley EA, Price RN, Stepniewska K, Barends M, et al. Changes in the treatment responses to artesunate-mefloquine on the northwestern border of Thailand during 13 years of continuous deployment. PLoS One. 2009;4:e4551. doi: 10.1371/journal.pone.0004551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Witkowski B, Iriart X, Soh PN, Menard S, Alvarez M, Naneix-Laroche V, et al. pfmdr1 amplification associated with clinical resistance to mefloquine in West Africa: implications for efficacy of artemisinin combination therapies. J Clin Microbiol. 2010;48:3797–3799. doi: 10.1128/JCM.01057-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kato N, Sakata T, Breton G, Le Roch KG, Nagle A, Andersen C, et al. Gene expression signatures and small-molecule compounds link a protein kinase to Plasmodium falciparum motility. Nature Chem Biol. 2008;4:347–356. doi: 10.1038/nchembio.87. [DOI] [PubMed] [Google Scholar]
- 5.Plouffe D, Brinker A, McNamara C, Henson K, Kato N, Kuhen K, et al. In silico activity profiling reveals the mechanism of action of antimalarials discovered in a high-throughput screen. Proc Natl Acad Sci U S A. 2008;105:9059–9064. doi: 10.1073/pnas.0802982105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guiguemde WA, Shelat AA, Bouck D, Duffy S, Crowther GJ, Davis PH, et al. Chemical genetics of Plasmodium falciparum. Nature. 2010;465:311–315. doi: 10.1038/nature09099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gamo FJ, Sanz LM, Vidal J, de Cozar C, Alvarez E, Lavandera JL, et al. Thousands of chemical starting points for antimalarial lead identification. Nature. 2010;465:305–310. doi: 10.1038/nature09107. [DOI] [PubMed] [Google Scholar]
- 8.Benoit-Vical F, Lelievre J, Berry A, Deymier C, Dechy-Cabaret O, Cazelles J, et al. Trioxaquines are new antimalarial agents active on all erythrocytic forms, including gametocytes. Antimicrob Agents Chemother. 2007;51:1463–1472. doi: 10.1128/AAC.00967-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peatey CL, Skinner-Adams TS, Dixon MW, McCarthy JS, Gardiner DL, Trenholme KR. Effect of antimalarial drugs on Plasmodium falciparum gametocytes. J Infect Dis. 2009;200:1518–1521. doi: 10.1086/644645. [DOI] [PubMed] [Google Scholar]
- 10.Chen PQ, Li GQ, Guo XB, He KR, Fu YX, Fu LC, et al. The infectivity of gametocytes of Plasmodium falciparum from patients treated with artemisinin. Chin Med J. 1994;107:709–711. [PubMed] [Google Scholar]
- 11.Smithuis F, Kyaw MK, Phe O, Win T, Aung PP, Oo APP, et al. Effectiveness of five artemisinin combination regimens with or without primaquine in uncomplicated falciparum malaria: an open-label randomised trial. Lancet Infect Dis. 2010;10:673–681. doi: 10.1016/S1473-3099(10)70187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baird JK, Surjadjaja C. Consideration of ethics in primaquine therapy against malaria transmission. Trends Parasitol. 2010 doi: 10.1016/j.pt.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Sykes ML, Avery VM. Development of an Alamar Blue viability assay in 384-well format for high throughput whole cell screening of Trypanosoma brucei brucei bloodstream form strain 427. Am J Trop Med Hyg. 2009;81:665–674. doi: 10.4269/ajtmh.2009.09-0015. [DOI] [PubMed] [Google Scholar]
- 14.Czesny B, Goshu S, Cook JL, Williamson KC. The proteasome inhibitor epoxomicin has potent Plasmodium falciparum gametocytocidal activity. Antimicrob Agents Chemother. 2009;53:4080–4085. doi: 10.1128/AAC.00088-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ifediba T, Vanderberg JP. Complete in vitro maturation of Plasmodium falciparum gametocytes. Nature. 1981;294:364–366. doi: 10.1038/294364a0. [DOI] [PubMed] [Google Scholar]
- 16.Ponnudurai T, Lensen AH, Meis JF, Meuwissen JH. Synchronization of Plasmodium falciparum gametocytes using an automated suspension culture system. Parasitol. 1986;93(Pt 2):263–274. doi: 10.1017/s003118200005143x. [DOI] [PubMed] [Google Scholar]
- 17.Zhang JH, Chung TDY, Oldenburg KR. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomolec Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 18.Karl S, Wong RPM, St Pierre TG, Davis TME. A comparative study of a flow-cytometry-based assessment of in vitro Plasmodium falciparum drug sensitivity. Malar J. 2009;8 doi: 10.1186/1475-2875-8-294. 294- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okell LC, Drakeley CJ, Ghani AC, Bousema T, Sutherland CJ. Reduction of transmission from malaria patients by artemisinin combination therapies: a pooled analysis of six randomized trials. Malar J. 2008;7:125. doi: 10.1186/1475-2875-7-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chevalley S, Coste A, Lopez A, Pipy B, Valentin A. Flow cytometry for the evaluation of anti-plasmodial activity of drugs on Plasmodium falciparum gametocytes. Malar J. 2010;9:49. doi: 10.1186/1475-2875-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]