Abstract
There is a growing consensus that the balance between Angiotensin Type 1 (AT1R) and Angiotensin Type 2 (AT2R) signaling in many tissues may determine the magnitude and, in some cases the direction, of the biological response. Sympatho-excitation in cardiovascular diseases is mediated by a variety of factors and is, in part, dependent on Angiotensin II signaling in the central nervous system. Recent data have provided evidence that the AT2R can modulate sympatho-excitation in animals with hypertension and heart failure. The evidence for this concept is reviewed and a model is put forward to support the rationale that therapeutic targeting of the central AT2R may be beneficial in the setting of chronic heart failure.
Introduction
The renin angiotensin system (RAS) impacts a variety of important biological functions that are critical to circulatory and fluid volume homeostasis. It has been long accepted that the octapeptide Angiotensin II (Ang II) possesses potent effects in the central nervous system that regulate and modulate thirst, salt appetite, vasopressin release and sympathetic nerve activity. In this way strong activation of the central RAS can contribute to the pathogenesis of hypertension and other sympatho-excitatory states such as heart failure. There have been many excellent reviews on this topic [1**–5] and we will not attempt to summarize all the evidence for the central sympatho-excitatory effects of Ang II. Rather, this review will focus on an emerging area of central Ang II signaling through the Angiotensin Type 2 receptor (AT2R).
Angiotensin II Receptor Subtypes in the CNS
The central nervous system is well endowed with the two main receptor subtypes, AT1R and AT2R. These receptors are ubiquitously distributed in the brain and spinal cord [6–8] and located on neural, glial and vascular elements [9;10]. While Angiotensin II receptors are expressed throughout the brain, there appears to be a high density in those areas of the hypothalamus and medulla that regulate sympathetic outflow, arterial baroreflex function and thereby blood pressure [6;11;12]. This is especially relevant in areas that have no blood brain barrier and send projections to nuclei in the hypothalamus and medulla; the so called circumventricular organs [13]. In addition, there is evidence that an AT3R exists [6;14] and a small amount of evidence suggesting the possibility of a non AT1, AT2 or AT3 receptor signaling pathway [6;15].
In the central nervous system the downstream signaling pathways for Ang II are much the same as they are in other tissues. Both AT1 and AT2 receptors are G-protein coupled and signal through Gq and Gi, respectively [16]. Because the AT2R increases nitric oxide (NO) release [17;18] and facilitates neuronal potassium current [19], activation of this receptor should evoke sympatho-inhibition. This notion has been difficult to confirm, especially in disease states, because of the relative predominance of the AT1R and its sympatho-excitatory effects.
The prevailing dogma is that the AT2R subtype in the brain is predominant in the fetus, while the AT1R subtype is predominant in adults. This is based primarily on studies using autoradiography [20;21*], quantitative autoradiography [22], and in situ hybridization [23] techniques. Unfortunately, there are no data at the protein level to confirm or refute this idea.
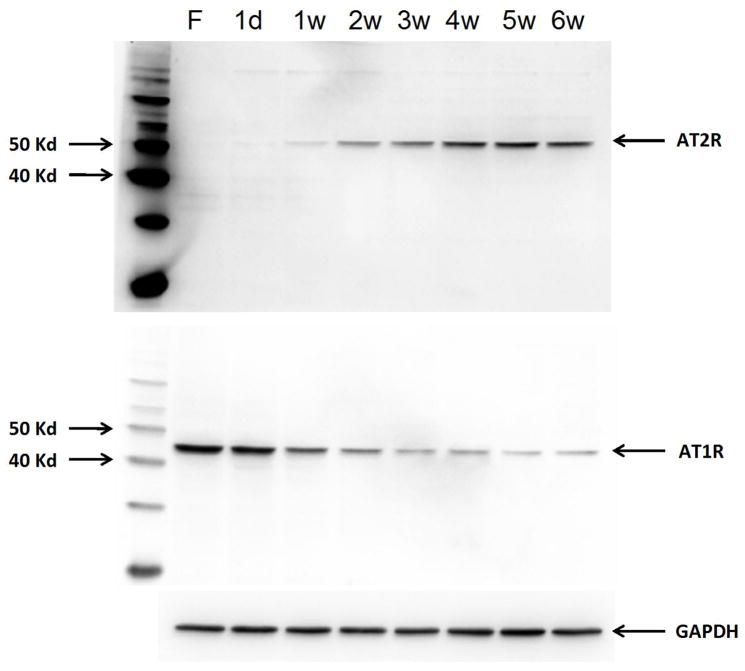
A recent study from our laboratory revealed a different Angiotensin receptor profile in both rats and mice during development which contrasts that currently based on the above studies. Using Western Blot analysis, we clearly demonstrated that, in brainstem, liver, and kidney, adult rats exhibit significantly higher AT2R, but significantly lower AT1R, protein expression compared to fetal or neonatal rats [24**]. Moreover, in the developmental mice, we got the same results as in rats. Figure 1 shows the time course of AT2R and AT1R protein expression in the brainstem from fetus to 6 week old mice. This figure clearly shows a gradual increase in AT2R expression in the brainstem during progression from fetal to adult life. On the other hand, expression of the AT1R appears to gradually decrease during maturation. It is not clear at which point in time this reversal in receptor expression occurs. However, 4 week old mice exhibit the same AT2R expression as do older mice, suggesting developmental changes in AT2R expression are complete in the mice at around one month. For the AT1R, stable expression appears at the 3 week time period, one week earlier than AT2R.
Figure 1.
Developmental changes in AT2R and AT1R protein expression in brainstem of mice. F: Fetus, d: day, w: week(s)
Based on our rodent data, it is our belief that the AT1R is always the dominant receptor subtype at all developmental stages, with higher expression in the fetus and neonate and lower expression in adulthood. On the other hand, the AT2R is expressed in a lower degree at first with an expression pattern which increases following birth into adulthood. The ratio of AT1R to AT2R protein therefore, is higher in early development compared to adulthood.
The reason for the differences in ATR protein expression versus the previous autoradiographical data is not completely clear. The autoradiography is a classical pharmacological method to detect receptor-ligand binding, which is a highly sensitive technique but its validity largely depends on the specificity of agonist and antagonist employed. In the previous autoradiographical study [20;21] whole animal binding was examined rather than select brain regions. Furthermore, these results were based primarily on changes in binding in response to AT1R or AT2R antagonists whereas we used specific antibodies to evaluate protein expression in various brain areas. Nevertheless additional work needs to be done in order to determine the mechanism for these differences.
If our biochemistry data are translated into functional significance, they suggest that activation of the AT2R may be a therapeutic strategy to limit the effects of AT1R stimulation in the adult in several diseases that are characterized by sympatho-excitation, such as chronic heart failure, hypertension, and diabetes by virtue of the fact that AT2R stimulation opposes activation of the AT1R in sympathetic regulatory areas of the central nervous system. Recent data from this laboratory have shown the functional influence of central AT2R activation on sympathetic outflow in adult rats using the AT2R agonist CGP123319 [25*] and by AT2R over expression using gene transfer [26], which is summarized in the following section.
Central AT2R and Sympathetic Regulation
Data from mice with AT2R gene deletions suggest the contribution of this receptor to sympatho-inhibition [27;28]. While the AT1R in the central nervous system has been solidly linked to sympatho-excitation, AT2R activation exhibits opposite influences on sympathetic tone. Siragy et al. [29] reported that AT2-null mice had slightly elevated systolic blood pressure compared with that of wild-type control mice. Infusion of a subpressor dose of Ang II in wild-type mice resulted in a pressor response in AT2R gene knock-out mice. Moreover, Li et al. [30] found that intracerebroventricular (icv) injection of Ang II evoked a larger increase in blood pressure in AT2R gene knock-out mice compared to wild type mice. In wild type mice central injection of Ang II plus PD123319 (an AT2R antagonist) initiated a greater pressor response than that induced by Ang II alone. These results strongly imply a potential inhibitory effect of stimulating central AT2Rs on blood pressure, which is likely mediated by sympatho-inhibition.
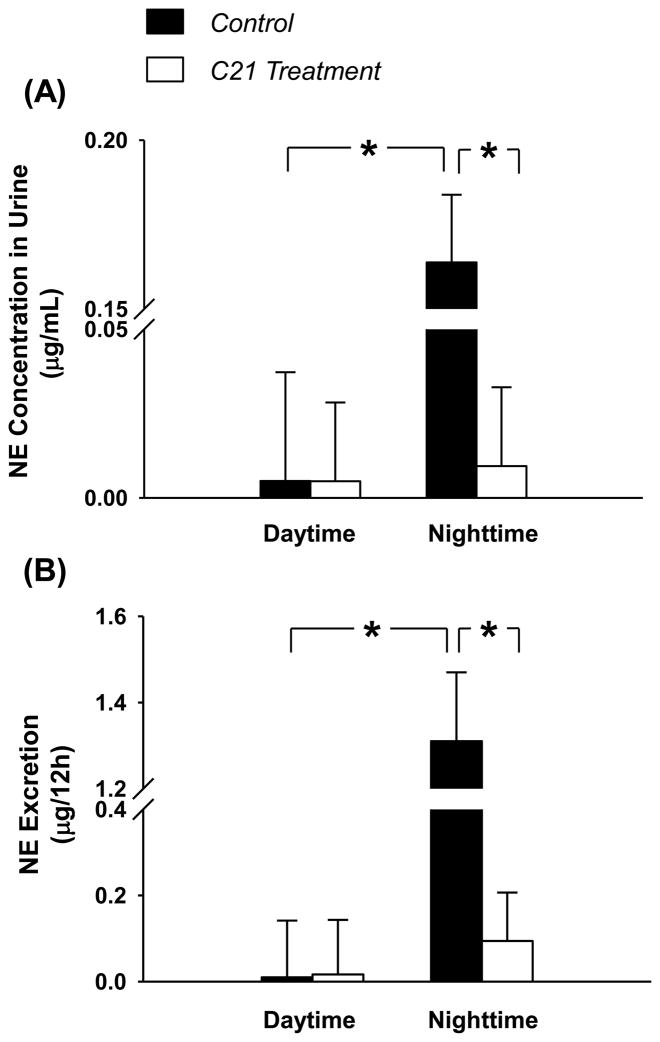
In a recent study we demonstrated that adenoviral gene transfer of the AT2R gene induced overexpression of AT2R protein in the rostral ventrolateral medulla (RVLM; a primary brainstem nucleus related to the control of sympathetic outflow) suppressed norepinephrine excretion and reduced arterial blood pressure in normal rats [26]. Similar effects were recently described by Li et al. [31]. We further found that in rats with chronic heart failure AT2R expression in the RVLM was significantly lower than in normal rats. This downregulation of AT2R expression contributed to the sympatho-excitation in this syndrome[25]. The regulation of cardiovascular function by AT2Rs in the RVLM was also recently documented by Tedesco and Ally who focused on the exercise pressor reflex [32]. These authors found that bilateral microdialysis of PD123319, the selective AT2R antagonist, into the RVLM augmented the pressor and tachycardia responses to static muscle contraction in anesthetized rats. They further demonstrated that the amplification by PD123319 contributed to the increased glutamate and decreased GABA levels within the RVLM. These data suggest that AT2Rs in the RVLM exhibit their influence on sympathetic outflow via regulating release of classical neurotransmitters locally. In addition, our recent experiments performed in normal rats demonstrated that chronic icv infusion of Compound 21 (C21), a newly created non-peptide AT2R agonist, decreased nocturnal norepinephrine (NE) excretion and blood pressure via a nNOS/NO signaling pathway within PVN and RVLM. Figure 2 shows NE concentration (ug/mL, panel A) and NE excretion (ug/12h, panel B) from day time and night time urine after C21 treatment for 7 days. C21 treated rats exhibited a significantly lower nocturnal NE concentration and excretion compared with control rats, suggesting a central inhibitory influence of C21 on sympathetic outflow.
Figure 2.
NE concentration (panel A) and NE excretion (panel B) in day time and night time urine after icv infusion of C21 for 7 days. *P < 0.05, n = 8 for the control group and 10 for the C21 treated group.
While it has been demonstrated that overexpression of AT2R in the periphery contributes to hypotensive responses following losartan administration [33], little information is available concerning the role of AT2Rs in the central nervous system on blood pressure or sympathetic regulation. AT2Rs appear to oppose enhanced renal sympathetic neurotransmission in response to stimulation of the AT1R [34]. On the other hand, Nap et al. [35] provide in vitro evidence to the contrary. Toney and Porter [36] showed that the AT2R was involved in the central vasopressin response to Ang II in young (4 week old) rats. This notion has recently been substantiated by electron micrographic-double labeling experiments showing the localization of AT2R and vasopressin on neurons and glia in the PVN [37]. While some data from anesthetized rats suggest that AT1R stimulation of RVLM neurons are inhibitory [38], most suggest that this stimulation is excitatory [25;39;40]. New data from our laboratory using a specific agonist for the AT2R suggests that activation of this pathway may, indeed, modulate excitatory Ang II responses in the RVLM [25].
AT2R and Neuronal Electrophysiology
Data from patch-clamp and extracellular single unit discharge recordings provide evidence for the above-mentioned inhibitory influence of central AT2R activation on sympathetic drive. Kang et al. [19] demonstrated that stimulation of AT2Rs significantly increased neuronal voltage-gated potassium channel current (Ikv) in cultured neurons from newborn rat hypothalamus and brainstem. They further indicated that the third intracellular loop of the AT2R is a key component in the stimulation of neuronal Ikv elicited by activation of this receptor [41]. Martens et al. [42] reported that activation of AT2R by CGP42112 modulates rat hypothalamic and brainstem neuronal whole-cell K+ current by increasing their open probability. Moreover, further study has demonstrated that, in single neurons, superfusion with Ang II (stimulation of AT1R and AT2R) induced a decrease in Ikv while superfusion of Ang II plus Losartan (stimulation of AT2R) induced an increase in Ikv, and superfusion of Ang II plus losartan and PD123319 resulted in no alterations of Ikv [43]. Consistent with the effects of AT2R stimulation on neuronal potassium current, Matsuura et al. [44] recently demonstrated a AT2R-mediated hyperpolarization and decrease in firing rate in bulbospinal RVLM neurons. Employing intracellular recording using the whole-cell patch-clamp technique and the brain stem-spinal cord preparation from AT1R knockout mice, they found that the resting membrane potential tended to be more negative, and the firing rate tended to be slower in bulbospinal RVLM neurons. They further found that superfusion with Ang II in AT1R KO mice hyperpolarized RVLM bulbospinal neurons and decreased their firing rate. On the other hand, using intracellular recordings, iontophoretic, micropressure and bath perfusion methods, Xiong and Marshall [45;46] demonstrated that Ang II, via stimulating the AT2R, depresses glutamate depolarizations and excitatory postsynaptic potentials in Locus Coeruleus of a brain slice preparation. Interestingly, the AT2R induced neuronal activation of delayed rectifier potassium channels has also been demonstrated to have a neuroprotective effect which is mediated through the AT2R [47]. These data strongly imply that AT2Rs supress neuronal activity via facilitating potassium channel current and decreasing firing rate.
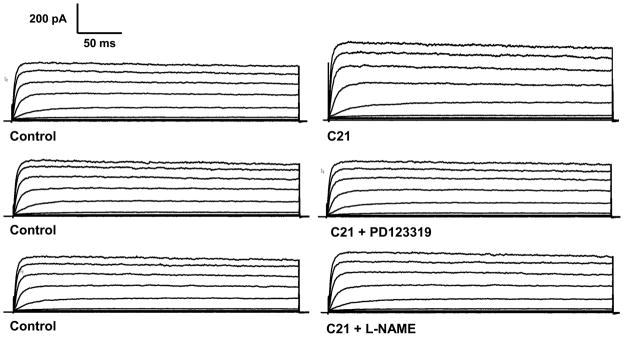
The majority of the neuronal AT2R intracellular signaling pathways are mediated via inhibitory G proteins (Gi) [41;48], even though evidence also exists showing that AT2Rs can couple via a G-protein-independent mechanism [49]. It has been demonstrated in neurons cultured from neonatal rat hypothalamus and brainstem, that inhibitory G proteins, PLA2, and protein phosphatase 2A (PP2A) are involved in the AT2R-dependent increase in K+ current [50–52]. AT2R activation stimulates PP2A activity [53] and PP2A may directly participate in a dephosphorylation-mediated activation of the K+ channel. Zu et al. [54] explored the involvement of a series of arachidonic acid (AA) metabolites in the AT2R-evoked increase in the K+ current in cultured neurons. They demonstrated that the PLA2/AA/12-LO pathway is responsible for the modulation of K+ currents by AT2R. This activation of the delayed rectifier K+ current (an outward K+ current in neuronal cells) will result in hyperpolarization of cellular membranes and suppression of neuronal activity. In preliminary experiments using CATH.a neurons, we demonstrated that C21 treatment significantly increased neuronal IKv, which was completely abolished by the AT2R antagonist, PD123319, and the NOS inhibitor, L-NAME (figure 3).
Figure 3.
Current tracings of IKv in CATH.a cells (a neuronal cell line) measured by whole cell patch clamp. C21: AT2R agonist; PD123319: AT2R antagonist; L-NAME: NOS blocker.
Central AT2R and Therapeutic Strategy
Many cardiovascular diseases, including chronic heart failure, essential hypertension, and diabetes, are characterized by heightened sympathetic tone. This excessive sympathetic outflow contributes to a variety of deleterious effects on the cardiovascular system, such as increased myocardial oxygen consumption and decreased blood flow to peripheral organs. Sympatho-excitation, therefore is an important therapeutic target and suppression of sympathetic outflow has become mainstream therapy for these diseases. Given the down regulated central AT2R expression in both chronic heart failure [25] and hypertension [55] and its role in the sympatho-excitation of these diseases, up regulation of AT2R expression in sympathetic regulatory areas of the brain by gene transfer or other methods, may have therapeutic potential in these diseases. As we have previously shown [26] viral vector-induced overexpression of the AT2R in the RVLM is associated with a reduction in blood pressure and urinary norepinephrine excretion. It will be relevant to determine the effects of AT2R overexpression in the heart failure state. In addition, C21 may be a valuable sympatho-inhibitory substance capable of activating central AT2Rs. Even though most reports have demonstrated its beneficial influence in spontaneously hypertensive rats [15] and rats with myocardial infarctions [56], our preliminary data suggest the inhibitory effects of C21 on sympathetic outflow is mediated by central mechanisms.
Conclusion and perspective
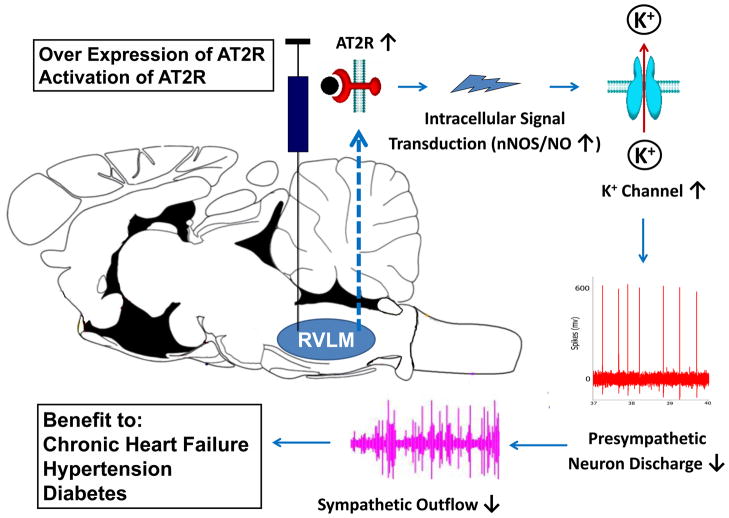
In this review we have concentrated on central AT2Rs and their potential involvement in sympathetic regulation. Although expression and functional significance of AT2Rs is still controversial new data have demonstrated a higher protein expression level of this receptor in mature rodent compared to that in the fetus and early neonate. Consistent with the Western blot data, we also documented an inhibitory influence of central AT2Rs on sympathetic tone in normal adult rats. In addition, the down regulated AT2R expression and signaling in the RVLM have been shown to contribute to the sympatho-excitation in chronic heart failure. These findings raise the possibility of a potential therapeutic strategy in diseases characterized by sympatho-excitation. The newly created non-peptide AT2R agonist, Compound 21 may potentially provide therapeutic efficacy. Figure 4 summarizes a possible therapeutic strategy that targets sympatho-excitation through stimulation of central AT2Rs or by overexpression of AT2R protein.
Figure 4.
Outline of therapeutic strategy by which activation or over expression of central AT2Rs may reduce sympatho-excitation in cardiovascular diseases such as heart failure and hypertension. RVLM: rostral ventrolateral medulla. (Modified from Zimmerman MC and Davisson RL [57]).
Acknowledgments
Some of the work described in this review was supported by PO-1 HL62222 (Dr. Irving H. Zucker), RO-1 HL093028 (Dr. Lie Gao), and a grant from the American Heart Association SDG 0635007N (Dr. Lie Gao).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1**.De Gasparao M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. PharmacolRev. 2000;52:415–472. This is a comprehensive review of the angiotensin receptors. [PubMed] [Google Scholar]
- 2.Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev. 2010;90:513–557. doi: 10.1152/physrev.00007.2009. [DOI] [PubMed] [Google Scholar]
- 3.Phillips MI, de Oliveira EM. Brain renin angiotensin in disease. J Mol Med. 2008;86:715–722. doi: 10.1007/s00109-008-0331-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zucker IH. Novel mechanisms of sympathetic regulation in chronic heart failure. Hypertension. 2006;48:1005–1011. doi: 10.1161/01.HYP.0000246614.47231.25. [DOI] [PubMed] [Google Scholar]
- 5.Zucker IH, Schultz HD, Patel KP, Wang W, Gao L. Regulation of central angiotensin type 1 receptors and sympathetic outflow in heart failure. Am J Physiol Heart Circ Physiol. 2009;297:H1557–H1566. doi: 10.1152/ajpheart.00073.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourassa EA, Fang X, Li X, Sved AF, Speth RC. AT(1) angiotensin II receptor and novel non-AT(1), non-AT(2) angiotensin II/III binding site in brainstem cardiovascular regulatory centers of the spontaneously hypertensive rat. Brain Res. 2010;1359:98–106. doi: 10.1016/j.brainres.2010.08.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oldfield BJ, Allen AM, Hards DK, McKinley MJ, Schlawe I, Mendelsohn FA. Distribution of angiotensin II receptor binding in the spinal cord of the sheep. Brain Res. 1994;650:40–48. doi: 10.1016/0006-8993(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 8.von Bohlen und HO, Albrecht D. Mapping of angiotensin AT1 receptors in the rat limbic system. Regul Pept. 1998;78:51–56. doi: 10.1016/s0167-0115(98)00109-8. [DOI] [PubMed] [Google Scholar]
- 9.Saavedra JM. Emerging features of brain angiotensin receptors. Regul Pept. 1999;85:31–45. doi: 10.1016/s0167-0115(99)00081-6. [DOI] [PubMed] [Google Scholar]
- 10.Downie LE, Vessey K, Miller A, Ward MM, Pianta MJ, Vingrys AJ, Wilkinson-Berka JL, Fletcher EL. Neuronal and glial cell expression of angiotensin II type 1 (AT1) and type 2 (AT2) receptors in the rat retina. Neuroscience. 2009;161:195–213. doi: 10.1016/j.neuroscience.2009.02.084. [DOI] [PubMed] [Google Scholar]
- 11.Lindley TE, Infanger DW, Rishniw M, Zhou Y, Doobay MF, Sharma RV, Davisson RL. Scavenging superoxide selectively in mouse forebrain is associated with improved cardiac function and survival following myocardial infarction. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1–R8. doi: 10.1152/ajpregu.00078.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferguson AV, Li Z. Whole cell patch recordings from forebrain slices demonstrate angiotensin II inhibits potassium currents in subfornical organ neurons. Regul Pept. 1996;66:55–58. doi: 10.1016/0167-0115(96)00049-3. [DOI] [PubMed] [Google Scholar]
- 13.Aars H, Akre S. Effect of angiotensin on sympathetic nerve activity. Acta Physiol Scand. 1968;74:134–141. doi: 10.1111/j.1748-1716.1968.tb04222.x. [DOI] [PubMed] [Google Scholar]
- 14.Sandberg K, Ji H, Clark AJ, Shapira H, Catt KJ. Cloning and expression of a novel angiotensin II receptor subtype. J Biol Chem. 1992;267:9455–9458. [PubMed] [Google Scholar]
- 15.Bosnyak S, Welungoda IK, Hallberg A, Alterman M, Widdop RE, Jones ES. Stimulation of angiotensin AT2 receptors by the non-peptide agonist, Compound 21, evokes vasodepressor effects in conscious spontaneously hypertensive rats. Br J Pharmacol. 2010;159:709–716. doi: 10.1111/j.1476-5381.2009.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaschina E, Unger T. Angiotensin AT1/AT2 receptors: regulation, signalling and function. Blood Press. 2003;12:70–88. doi: 10.1080/08037050310001057. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez-Alfonso MS, Gonzalez C. Nitric oxide and the renin-angiotensin system. Is there a physiological interplay between the systems? J Hypertens. 1999;17:1355–1361. doi: 10.1097/00004872-199917100-00001. [DOI] [PubMed] [Google Scholar]
- 18.Lee JH, Xia S, Ragolia L. Upregulation of AT2 receptor and iNOS impairs angiotensin II-induced contraction without endothelium influence in young normotensive diabetic rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R144–R154. doi: 10.1152/ajpregu.00191.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang J, Sumners C, Posner P. Angiotensin II type 2 receptor-modulated changes in potassium currents in cultured neurons. Am J Physiol. 1993;265:C607–C616. doi: 10.1152/ajpcell.1993.265.3.C607. [DOI] [PubMed] [Google Scholar]
- 20.Millan MA, Jacobowitz DM, Aguilera G, Catt KJ. Differential distribution of AT1 and AT2 angiotensin II receptor subtypes in the rat brain during development. Proc Natl Acad Sci U S A. 1991;88:11440–11444. doi: 10.1073/pnas.88.24.11440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21*.Cook VI, Grove KL, McMenamin KM, Carter MR, Harding JW, Speth RC. The AT2 angiotensin receptor subtype predominates in the 18 day gestation fetal rat brain. Brain Res. 1991;560:334–336. doi: 10.1016/0006-8993(91)91254-x. This is the first autographic data showing that AT2R is the predominant Angiotensin receptor subtype in rat fetal brain. [DOI] [PubMed] [Google Scholar]
- 22.Tsutsumi K, Seltzer A, Saavedra JM. Angiotensin II receptor subtypes and angiotensin-converting enzyme in the fetal rat brain. Brain Res. 1993;631:212–220. doi: 10.1016/0006-8993(93)91537-3. [DOI] [PubMed] [Google Scholar]
- 23.Nuyt AM, Lenkei Z, Palkovits M, Corvol P, Llorens-Cortes C. Ontogeny of angiotensin II type 2 receptor mRNA expression in fetal and neonatal rat brain. J Comp Neurol. 1999;407:193–206. [PubMed] [Google Scholar]
- 24**.Yu L, Zheng M, Wang W, Rozanski GJ, Zucker IH, Gao L. Developmental changes in AT1 and AT2 receptor-protein expression in rats. J Renin Angiotensin Aldosterone Syst. 2010 doi: 10.1177/1470320310379065. This is the first direct measurement of AT1R and AT2R protein expression in developing rat tissues. This paper reports the opposite expression pattern of the two Angiotensin receptors during animal development to the current opinion derived from autoradiography and in situ hybridization data. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25*.Gao L, Wang WZ, Wang W, Zucker IH. Imbalance of angiotensin type 1 receptor and angiotensin II type 2 receptor in the rostral ventrolateral medulla: potential mechanism for sympathetic overactivity in heart failure. Hypertension. 2008;52:708–714. doi: 10.1161/HYPERTENSIONAHA.108.116228. This is the first evidence showing the involvement of central AT2R in the regulation of sympathetic outflow and cardiovascular activity in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao L, Wang W, Wang W, Li H, Sumners C, Zucker IH. Effects of angiotensin type 2 receptor overexpression in the rostral ventrolateral medulla on blood pressure and urine excretion in normal rats. Hypertension. 2008;51:521–527. doi: 10.1161/HYPERTENSIONAHA.107.101717. [DOI] [PubMed] [Google Scholar]
- 27.Hein L, Barsh GS, Pratt RE, Dzau VJ, Kobilka BK. Behavioural and cardiovascular effects of disrupting the angiotensin II type-2 receptor in mice. Nature. 1995;377:744–747. doi: 10.1038/377744a0. [DOI] [PubMed] [Google Scholar]
- 28.Ichiki T, Labosky PA, Shiota C, Okuyama S, Imagawa Y, Fogo A, Niimura F, Ichikawa I, Hogan BL, Inagami T. Effects on blood pressure and exploratory behaviour of mice lacking angiotensin II type-2 receptor. Nature. 1995;377:748–750. doi: 10.1038/377748a0. [DOI] [PubMed] [Google Scholar]
- 29.Siragy HM, Inagami T, Ichiki T, Carey RM. Sustained hypersensitivity to angiotensin II and its mechanism in mice lacking the subtype-2 (AT2) angiotensin receptor. Proc Natl Acad Sci U S A. 1999;96:6506–6510. doi: 10.1073/pnas.96.11.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Z, Iwai M, Wu L, Shiuchi T, Jinno T, Cui TX, Horiuchi M. Role of AT2 receptor in the brain in regulation of blood pressure and water intake. Am J Physiol Heart Circ Physiol. 2003;284:H116–H121. doi: 10.1152/ajpheart.00515.2002. [DOI] [PubMed] [Google Scholar]
- 31.Li H, Gao H, Bourassa E, Speth R, Sumners C. Over expression of the angiotensin type 2 receptor in the rostral ventrolateral medulla (RVLM) decreases basal blood pressure in normotensive rats. [abstract] Molecular Therapy. 2007;12(Suppl 1) [Google Scholar]
- 32.Tedesco A, Ally A. Angiotensin II type-2 (AT2) receptor antagonism alters cardiovascular responses to static exercise and simultaneously changes glutamate/GABA levels within the ventrolateral medulla. Neurosci Res. 2009;64:372–379. doi: 10.1016/j.neures.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 33.Duke LM, Evans RG, Widdop RE. AT2 receptors contribute to acute blood pressure-lowering and vasodilator effects of AT1 receptor antagonism in conscious normotensive but not hypertensive rats. Am J Physiol Heart Circ Physiol. 2005;288:H2289–H2297. doi: 10.1152/ajpheart.01096.2004. [DOI] [PubMed] [Google Scholar]
- 34.Stegbauer J, Vonend O, Habbel S, Quack I, Sellin L, Gross V, Rump LC. Angiotensin II modulates renal sympathetic neurotransmission through nitric oxide in AT2 receptor knockout mice. J Hypertens. 2005;23:1691–1698. doi: 10.1097/01.hjh.0000179763.02583.8e. [DOI] [PubMed] [Google Scholar]
- 35.Nap A, Balt JC, Pfaffendorf M, Zwieten PA. No involvement of the AT2-receptor in angiotensin II-enhanced sympathetic transmission in vitro. J Renin Angiotensin Aldosterone Syst. 2003;4:100–105. doi: 10.3317/jraas.2003.009. [DOI] [PubMed] [Google Scholar]
- 36.Toney GM, Porter JP. Functional roles of brain AT1 and AT2 receptors in the central angiotensin II pressor response in conscious young spontaneously hypertensive rats. Brain Res Dev Brain Res. 1993;71:193–199. doi: 10.1016/0165-3806(93)90171-6. [DOI] [PubMed] [Google Scholar]
- 37.Coleman CG, Anrather J, Iadecola C, Pickel VM. Angiotensin II type 2 receptors have a major somatodendritic distribution in vasopressin-containing neurons in the mouse hypothalamic paraventricular nucleus. Neuroscience. 2009;163:129–142. doi: 10.1016/j.neuroscience.2009.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bertram D, Coote JH. Inhibitory effects of angiotensin II on barosensitive rostral ventrolateral medulla neurons of the rat. Clin Exp Pharmacol Physiol. 2001;28:1112–1114. doi: 10.1046/j.1440-1681.2001.03583.x. [DOI] [PubMed] [Google Scholar]
- 39.Head GA, Saigusa T, Mayorov DN. Angiotensin and baroreflex control of the circulation. Braz J Med Biol Res. 2002;35:1047–1059. doi: 10.1590/s0100-879x2002000900005. [DOI] [PubMed] [Google Scholar]
- 40.Chan SH, Hsu KS, Huang CC, Wang LL, Ou CC, Chan JY. NADPH oxidase-derived superoxide anion mediates angiotensin II-induced pressor effect via activation of p38 mitogen-activated protein kinase in the rostral ventrolateral medulla. Circ Res. 2005;97:772–780. doi: 10.1161/01.RES.0000185804.79157.C0. [DOI] [PubMed] [Google Scholar]
- 41.Kang J, Richards EM, Posner P, Sumners C. Modulation of the delayed rectifier K+ current in neurons by an angiotensin II type 2 receptor fragment. Am J Physiol. 1995;268:C278–C282. doi: 10.1152/ajpcell.1995.268.1.C278. [DOI] [PubMed] [Google Scholar]
- 42.Martens JR, Wang D, Sumners C, Posner P, Gelband CH. Angiotensin II type 2 receptor-mediated regulation of rat neuronal K+ channels. Circ Res. 1996;79:302–309. doi: 10.1161/01.res.79.2.302. [DOI] [PubMed] [Google Scholar]
- 43.Gelband CH, Zhu M, Lu D, Reagan LP, Fluharty SJ, Posner P, Raizada MK, Sumners C. Functional interactions between neuronal AT1 and AT2 receptors. Endocrinology. 1997;138:2195–2198. doi: 10.1210/endo.138.5.5236. [DOI] [PubMed] [Google Scholar]
- 44.Matsuura T, Kumagai H, Onimaru H, Kawai A, Iigaya K, Onami T, Sakata K, Oshima N, Sugaya T, Saruta T. Electrophysiological properties of rostral ventrolateral medulla neurons in angiotensin II 1a receptor knockout mice. Hypertension. 2005;46:349–354. doi: 10.1161/01.HYP.0000173421.97463.ac. [DOI] [PubMed] [Google Scholar]
- 45.Xiong H, Marshall KC. Angiotensin II depresses glutamate depolarizations and excitatory postsynaptic potentials in locus coeruleus through angiotensin II subtype 2 receptors. Neuroscience. 1994;62:163–175. doi: 10.1016/0306-4522(94)90322-0. [DOI] [PubMed] [Google Scholar]
- 46.Xiong HG, Marshall KC. Angiotensin II modulation of glutamate excitation of locus coeruleus neurons. Neurosci Lett. 1990;118:261–264. doi: 10.1016/0304-3940(90)90642-m. [DOI] [PubMed] [Google Scholar]
- 47.Grammatopoulos TN, Johnson V, Moore SA, Andres R, Weyhenmeyer JA. Angiotensin type 2 receptor neuroprotection against chemical hypoxia is dependent on the delayed rectifier K+ channel, Na+/Ca2+ exchanger and Na+/K+ ATPase in primary cortical cultures. Neurosci Res. 2004;50:299–306. doi: 10.1016/j.neures.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 48.Gelband CH, Sumners C, Lu D, Raizada MK. Angiotensin receptors and norepinephrine neuromodulation: implications of functional coupling. Regul Pept. 1998;73:141–147. doi: 10.1016/s0167-0115(97)11050-3. [DOI] [PubMed] [Google Scholar]
- 49.Gallinat S, Busche S, Raizada MK, Sumners C. The angiotensin II type 2 receptor: an enigma with multiple variations. Am J Physiol Endocrinol Metab. 2000;278:E357–E374. doi: 10.1152/ajpendo.2000.278.3.E357. [DOI] [PubMed] [Google Scholar]
- 50.Kang J, Posner P, Sumners C. Angiotensin II type 2 receptor stimulation of neuronal K+ currents involves an inhibitory GTP binding protein. Am J Physiol. 1994;267:C1389–C1397. doi: 10.1152/ajpcell.1994.267.5.C1389. [DOI] [PubMed] [Google Scholar]
- 51.Zhu M, Gelband CH, Moore JM, Posner P, Sumners C. Angiotensin II type 2 receptor stimulation of neuronal delayed-rectifier potassium current involves phospholipase A2 and arachidonic acid. J Neurosci. 1998;18:679–686. doi: 10.1523/JNEUROSCI.18-02-00679.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu M, Sumners C, Gelband CH, Posner P. Chronotropic effect of angiotensin II via type 2 receptors in rat brain neurons. J Neurophysiol. 2001;85:2177–2183. doi: 10.1152/jn.2001.85.5.2177. [DOI] [PubMed] [Google Scholar]
- 53.Huang XC, Sumners C, Richards EM. Angiotensin II stimulates protein phosphatase 2A activity in cultured neuronal cells via type 2 receptors in a pertussis toxin sensitive fashion. Adv Exp Med Biol. 1996;396:209–215. doi: 10.1007/978-1-4899-1376-0_22. [DOI] [PubMed] [Google Scholar]
- 54.Zhu M, Natarajan R, Nadler JL, Moore JM, Gelband CH, Sumners C. Angiotensin II increases neuronal delayed rectifier K(+) current: role of 12-lipoxygenase metabolites of arachidonic acid. J Neurophysiol. 2000;84:2494–2501. doi: 10.1152/jn.2000.84.5.2494. [DOI] [PubMed] [Google Scholar]
- 55.Peng JF, Phillips MI. Opposite regulation of brain angiotensin type 1 and type 2 receptors in cold-induced hypertension. Regul Pept. 2001;97:91–102. doi: 10.1016/s0167-0115(00)00218-4. [DOI] [PubMed] [Google Scholar]
- 56.Kaschina E, Grzesiak A, Li J, Foryst-Ludwig A, Timm M, Rompe F, Sommerfeld M, Kemnitz UR, Curato C, Namsolleck P, Tschope C, Hallberg A, Alterman M, Hucko T, Paetsch I, Dietrich T, Schnackenburg B, Graf K, Dahlof B, Kintscher U, Unger T, Steckelings UM. Angiotensin II type 2 receptor stimulation: a novel option of therapeutic interference with the renin-angiotensin system in myocardial infarction? Circulation. 2008;118:2523–2532. doi: 10.1161/CIRCULATIONAHA.108.784868. [DOI] [PubMed] [Google Scholar]
- 57.Zimmerman MC, Davisson RL. Redox signaling in central neural regulation of cardiovascular function. Prog Biophys Mol Biol. 2004;84:125–149. doi: 10.1016/j.pbiomolbio.2003.11.009. [DOI] [PubMed] [Google Scholar]