Abstract
Angiotensin converting enzyme (ACE) can cleave angiotensin I, bradykinin, neurotensin and many other peptide substrates in vitro. In part, this is due to the structure of ACE, a protein composed of two independent catalytic domains. Until very recently, little was known regarding the specific in vivo role of each ACE domain, and they were commonly regarded as equivalent. This is not true, as shown by mouse models with a genetic inactivation of either the ACE N- or C-domains. In vivo, most angiotensin II is produced by the ACE C-domain. Some peptides, such as the anti-fibrotic peptide AcSDKP, are substrates only of the ACE N-domain. Knowing the in vivo role of each ACE domain has great significance for developing ACE domain-specific inhibitors and for understanding the full effects of the anti-ACE pharmaceuticals in widespread clinical use.
Introduction
While renin and angiotensin-converting enzyme (ACE) work coordinately to produce angiotensin II, these enzymes are very different. Renin is extremely limited in its tissue expression, while ACE is expressed by many tissues, including vascular endothelium, proximal tubular epithelium of the kidney, absorptive epithelia of the duodenum, inflammatory cells and even developing male germ cells [1]. Renin is an aspartyl endoprotease; ACE is a zinc dependant dipeptidyl carboxypeptidase, meaning that ACE cleaves two amino acids from the carboxyl end of peptides. But the single most striking difference between renin and ACE is that renin is a highly specific enzyme, while ACE is promiscuous, capable of cleaving many different peptide substrates. In vitro studies have shown that ACE can hydrolyze angiotensin I, bradykinin, neurotensin, the tetrapeptide N-acetyl-seryl-aspartyl-lysyl-proline (AcSDKP), enkephalins and others. Even some substrates with modified C termini (for example substance P) can be hydrolyzed by ACE. This has significant physiologic consequences in that renin only contributes to the generation of angiotensin II, angiotensin 1-7 and similar angiotensin peptides. In contrast, the far larger number of bioactive peptides hydrolyzed by ACE implies wider physiologic effects, and in particular, effects apart from blood pressure control. As only one example, ACE inhibitor use in humans prevents the generation of angiotensin II, but it also leads to an accumulation of bradykinin, a vasodilator and potent proinflammatory molecule that can induce cough [2].
Structure of ACE
In part, the wide substrate specificity of ACE is because the ACE protein is composed of two catalytic domains. These domains, often called the N- and C- terminal domains (Figure 1), contain the consensus amino acid sequence HEMGH. This motif binds zinc and is crucial for enzymatic activity. Each domain is catalytically independent [3]. Overall amino acid homology between the two ACE domains is approximately 60%, but in the portions involved in catalysis, homology reaches 89% [1,4**]. Analysis of genomic DNA exon sizes and exon-intron boundaries, indicates that the modern ACE gene is the result of an ancient gene duplication [5]. This form of ACE is termed somatic ACE, as it is the ACE isozyme present in plasma and made by endothelium, the kidney and other somatic tissues. A second ACE isozyme, called testis ACE, is only expressed by developing male germ cells and is a smaller protein, composed of only the C-terminal domain. Nonetheless, testis ACE is catalytic and male mice lacking testis ACE produce far fewer offspring than wild-type mice [8-10**]. Some authors have suggested that testis ACE may be the primordial form of ACE [4**]. The two ACE isozymes result from two separate promoter regions in the ACE gene [6,7]. Unless otherwise specified, we will refer to somatic ACE as ACE.
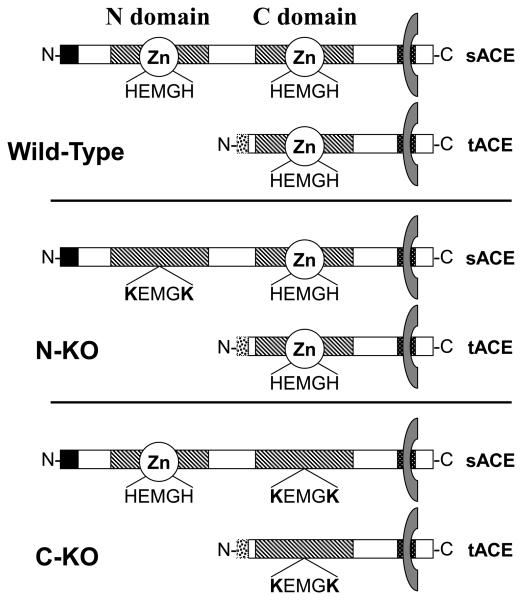
Figure 1.
Schematic representation of the WT, N-KO and C-KO ACE enzyme. The somatic isoform of ACE (sACE) is composed of two homologous regions termed N- and C- domains (hatched regions). Each domain contains the zinc-binding catalytic motif HEMGH. These domains are anchored to the plasma membrane by a hydrophobic region located at the carboxy-terminus extremity of the protein. In the N-KO strain the catalytic site of the N-domain was mutated to KEMGK abolishing its ability to bind zinc and rendering it inactive. In the C-KO strain, similar mutations inactivated the C-domain. The testis isoform (tACE), exclusively expressed in male germ cells, lacks the N-domain of sACE and has only one catalytic domain.
ACE or an ACE-like enzyme has been found in mammals, fish, worms, insects, crabs and even ticks [11,12]. The analysis of ACE in D. melanogaster is of particular interest [13-16]. Here, two ACE homologs termed Ance and Acer have been studied in detail. Each of these enzymes contains only one catalytic domain. Analysis of the gene structure and enzymatic properties of each of these proteins showed that Ance is more similar to the C-domain of somatic ACE while Acer appears more similar to the N-domain. The conclusion of these and other studies is that the gene duplication that resulted in the ACE present in vertebrates occurred approximately 330-350 million years ago [13]. That both catalytic domains of ACE have been conserved over such a long period of time indicates that each ACE domain must have an important and different physiologic role.
Even in vitro, the two catalytic domains of ACE do show some important biological differences. For example, optimal catalytic activity of the C-domain requires substantially higher concentrations of chloride than observed for optimal catalytic activity of the N-domain [3]. But perhaps the most significant difference between the two domains is a difference in enzymatic efficiency for individual peptide substrates. Some peptides, such as bradykinin, are hydrolyzed at approximately the same rate by both catalytic domains. In contrast, angiotensin I is hydrolyzed approximately three times more efficiently by the C-domain than the N-domain [3]. Finally, the peptides AcSDKP, angiotensin 1-7, and amyloid β-protein 1–42 are nearly exclusively cleaved by the N-domain [17-19*]. These conclusions are based on in vitro data, since, until recently, it was not possible to analyze the in vivo role of each ACE domain.
In vivo roles of ACE
There are two conceptual approaches to studying the in vivo function of the individual ACE domains. The first one is pharmacologic. All the commercially available ACE inhibitors used in clinical practice are insufficiently selective in their domain specificity to allow study of individual domain function. However, potent and specific inhibitors of each of the ACE domains have now been synthesized. For example, RXP 407, a peptide in which a phosphinic acid bond is used in place of a peptide bond, has a dissociation constant three orders of magnitude lower for the ACE N-domain than for the C-terminal domain [20]. This compound is reported as stable in vivo and, when used in a mouse, increases the plasma level of AcSDKP as much as 6-fold [21]. Another phosphinic peptide, RXPA 380, is reported as being a C-terminal specific inhibitor, with a dissociation constant more than 3 orders of magnitude lower for the ACE C-domain than the N-domain [22]. Again, this compound appears effective in mice. Finally, Sturrock and colleagues have prepared a different chemical class of C-terminal specific ACE inhibitors, termed ketomethylene inhibitors [23,24*]. Again, there is a 3 order of magnitude difference in dissociation constant, with very little effect on the N-terminal of ACE. Since mice genetically modified to express ACE with only one active catalytic site have a normal blood pressure (see below), we would predict that such site-specific ACE inhibitors would have only a limited effect reducing long term blood pressure. However, we believe they will be clinically useful for treating models of tissue injury, as discussed below.
The second major approach for studying ACE domain function is genetic, by using targeted homologous recombination in embryonic stem cells. The first targeted genetic models of ACE were null for all ACE expression [8,26]. However, targeting in ES cells has the capacity to create virtually any genetic change that can be envisioned [27]. Genes can be duplicated, promoters can be altered, and even individual nucleotide changes can be inserted into the genome. It was this last approach that was used to create mouse models expressing ACE with only one functional ACE domain. In other words, instead of truncating the ACE protein, our strategy was to selectively mutate two amino acids with the predicted result that the resulting protein would be full length but have only one active ACE catalytic domain (Figure 1). This work was based on elegant in vitro studies showing that mutations converting the two histidines responsible for binding zinc into lysine residues was sufficient to catalytically inactivate an ACE domain [3].
For the N-domain, the critical histidines are found at positions 395 and 399 within the 8th exon of the ACE gene; for the C-domain, it is histidines 993 and 997 in the 20th exon [28**,29**]. In both instances, targeting constructs were created in which His (codon CAT or CAC) were converted to Lys (codon AAG or AAA). To assist in positive selection, the targeting constructs incorporated a neomycin gene within either the 7th or the 19th intron. Finally, we used strategies to ultimately delete the neomycin cassette, such that the final mice would only possess a single LOX site within an intron plus mutation of HEMGH to KEMGK. In the original publications, mice with inactivation of either the N- or C-domain were referred to as ACE 7/7 and ACE 13/13 [28**,29**]. Now, we use the more logical nomenclature N-KO and C-KO.
To characterize these new models, we used Western blot analysis to study tissue expression of ACE in these new strains. These data establish that the introduction of point mutations into N-KO and C-KO mice had no effect on the 1) tissue distribution, 2) the levels of ACE expression, or 3) the molecular size of the ACE protein. Also, to verify the in vivo biochemical phenotype of ACE produced by the N-KO and C-KO mice, the plasma catalytic activity was assayed using the ACE substrates AcSDAcKP (cleaved exclusively by the N-terminal) and Hip-His-Leu (HHL, cleaved much more efficiently by the C-terminal domain). We also took advantage of three additional tools: the N-specific inhibitor RXP407, the difference in chloride sensitivity of the two ACE domains, and the fact that testis ACE only contains the C-domain. All of these studies were consistent: N-KO mice produce an ACE protein lacking N-domain catalytic activity while C-KO mice lack ACE C-domain activity. However, equally important, in each of these models the non-targeted domain of ACE was fully catalytic.
One of the misconceptions concerning ACE is that the enzyme is present in great physiologic excess. This is not true. For example, a mouse heterozygous for one WT ACE allele and for one ACE null allele produces about 60% normal plasma ACE activity [26]. Such a mouse has a normal blood pressure, but only because up-regulation of renin, and angiotensin I maintains angiotensin II levels. In fact, some mouse models made in our laboratory express ACE only in aberrant tissue locations, such as by liver hepatocytes [30]. In these and in other experimental models of ACE manipulation, the regulation of renin can compensate and maintain a normal blood pressure over wide changes in ACE location and expression levels [31,32]. Thus, it was expected that N-KO and C-KO mice would have normal blood pressures, and in fact, this is what was found. Further, while we originally found a small elevation of plasma angiotensin II levels in the N-KO mice, additional data and statistical analysis shows no significant differences between N-KO, C-KO and WT for plasma angiotensin II levels. Thus, any physiologic differences in these models cannot reasonably be attributed to differences of blood pressure or plasma angiotensin II levels.
Role of the N- and C-domain of ACE in the control of blood pressure
While the N-KO and C-KO mice have normal blood pressures, how they achieve homeostasis is very different. This is readily apparent from examining blood levels of angiotensin I. N-KO mice have plasma levels of angiotensin I and renin that were identical to WT mice [28**]. N-KO mice have a fully active C-terminal catalytic domain and the normal values of angiotensin I and renin indicate that, under steady state in vivo conditions, it is the C-terminal domain that is responsible for the vast majority of angiotensin II production. Because of this, no compensation of renin or angiotensin I is necessary to maintain blood pressure in the N-KO model. Different results were seen in the C-KO mice, which retain an active N-domain [29**]. These mice have a normal blood pressure but this is achieved with plasma angiotensin I levels that are greater than 7-fold those of WT and plasma renin activity 2.6-fold those of WT. These data reflect the relatively inefficient ability of the N-domain to produce angiotensin II, resulting in homeostatic up-regulation of renin, subsequent elevation of angiotensin I and, through mass action, reestablishment of normal angiotensin II levels.
The relative in vivo efficacy of the ACE N- and C-domains is bolstered by experiments in which N-KO, C-KO or WT mice were infused intravenously with either angiotensin I or angiotensin II peptide. For these experiments, the mice were anesthetized and prepared with an arterial catheter to monitor blood pressure. A venous catheter was used for peptide infusion. These studies showed that there was no difference in the magnitude of the blood pressure rise, the time response of the rise, or the shape of the pressure curves between WT and N-KO mice. These data were very different from those observed with the C-KO mice. These animals responded to angiotensin I infusion with a rise in blood pressure that was reduced by 48% as compared to WT mice. In contrast, when we measured the fall in blood pressure elicited by IV infusion of bradykinin, C-KO mice showed a response equivalent to WT, consistent with the finding of that bradykinin (1-9), and bradykinin (1-7) plasma levels were equivalent in N-KO, C-KO and WT mice. Thus, both the basal levels of angiotensin I and the response to infused angiotensin I peptide indicate that, in vivo, the vast majority of angiotensin II is produced by the conversion of angiotensin I by the C-terminal domain of ACE. Given this conclusion, it is difficult to argue that evolutionary maintenance of the ACE N-domain was due to genetic pressures related to the production of angiotensin II. What then is the physiologic role of the ACE N-domain?
N-domain of ACE controls AcSDKP degradation and lung fibrosis
We have noted that there are ACE peptide substrates that are exclusively cleaved by the N-domain. One such peptide is AcSDKP. This peptide is produced from the precursor protein thymosin β4 by the enzyme prolyl oligopeptidase (POP) and apparently is only degraded by the N-domain of ACE. A 7-fold increase in the plasma concentration of AcSDKP after the acute administration of an ACE inhibitor to normal volunteers shows the in vivo importance of ACE in the regulation of this peptide [33,34]. Plasma AcSDKP in N-KO mice is 7.3-fold that measured in WT mice. AcSDKP was first described as a natural regulator of hematopoietic stem cell proliferation [35]. Elegant recent work by Carretero and colleagues has demonstrated that AcSDKP also prevents the proliferation of fibroblasts in the myocardium, aorta and the kidney in models of injury [36*-38].
To study inflammation and fibrosis in the N-KO and C-KO strains, we used the model of lung injury induced by administration of the anti-cancer drug bleomycin [39*]. This is a well accepted model of lung injury and fibrosis which has direct clinical relevance, since the anti-neoplastic use of bleomycin is limited by its lung toxicity [40]. To induce injury in mice, we placed saline containing bleomycin directly into the trachea and evaluated the mice 14 days later. As anticipated, WT mice developed significant lung injury consisting of focal, typically subpleural pulmonary inflammation and fibrosis. Lung injury in C-KO mice was similar to WT. In contrast, inflammation and fibrosis were greatly reduced in N-KO mice, as indicated by the retention of a near normal histologic appearance [39*].
To objectively quantitate lung injury, we measure the amount of hydroxyproline in the whole lung of WT, N-KO and C-KO mice treated with intra-tracheal bleomycin. Since both the N-KO and CKO strains were on a mixed 129-C57BL/6 genetic background, separate littermate WT animals from each strain were used as controls. When treated with saline, the collagen content of WT, N-KO and CKO lungs were equivalent. However, two weeks after intra-tracheal bleomycin, WT and C-KO mice significantly increased the hydroxyproline content of their lungs. In contrast, N-KO mice did not show a significant increase of pulmonary collagen levels (Figure 2). Susceptibility to bleomycin can be influenced by the level of expression of bleomycin hydrolase in the lung [41]. However, Western blot analysis showed no significant differences in lung expression of this enzyme in N-KO, C-KO or WT mice.
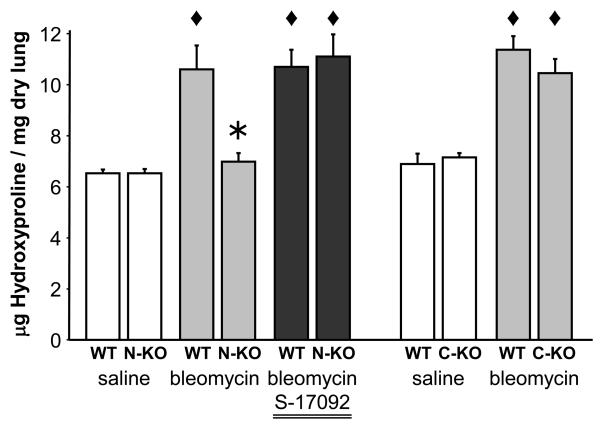
Figure 2.
Measurement of fibrosis. The determination of hydroxyproline content was used to objectively measure the degree of collagen deposition in the lung of WT, N-KO and C-KO mice 14 days after bleomycin or saline administration. The administration of bleomycin significantly increased the collagen deposition in lung of WT and C-KO mice when compared to saline treated animals. The inactivation of the N-terminal site of ACE prevented bleomycin-induced collagen deposition and fibrosis in the lung. * indicates P < 0.01 when compared to WT with the same treatment and ◆ indicates P < 0.01 when compared to saline-treated mice of the same genotype.
As discussed, AcSDKP is released from its precursor protein by the serine peptidase POP. To examine the role of AcSDKP in the N-KO model, we inhibited the generation of this peptide with the pharmaceutical S-17092, a POP inhibitor. S-17092 is a highly specific inhibitor of POP and does not result in a modification in concentration of other peptides including angiotensin II, substance P and Arg8-vasopressin in the rat heart, kidney and brain [42-44]. We administered S-17092 by daily intraperitoneal injections starting one day before the intra-tracheal instillation of bleomycin and continuing until the sacrifice of the mice. This protocol successfully decreased AcSDKP urine concentrations in the N-KO mice. When these mice were tested for susceptibility to bleomycin-induced lung injury, they showed a level of injury, as indicated by hydroxyproline levels that were now equivalent to those above WT (Figure 2). Please remember, that N-KO mice have normal blood pressure and plasma levels of angiotensin II. Thus, these experiments clearly implicate some other function of ACE that affects the response to bleomycin-induced lung injury. We believe this factor is AcSDKP.
If this hypothesis is true, then the delivery of exogenous AcSDKP should have a protective effect, even in WT mice. To test this, AcSDKP was administered to WT mice by osmotic mini pump. Four days after mini pump placement, bleomycin was administered, and two weeks later, lungs were collected and assayed for hydroxyproline. Treatment of WT mice with AcSDKP substantially reduced lung hydroxyproline content, as compared to mice treated with a saline-filled mini pump. These experiments and the other studies discussed above strongly argue that AcSDKP has important biochemical effects and that these could be modulated either through the use of a standard ACE inhibitor or possibly a pharmaceutical that inhibited only the ACE N-domain.
C-domain of ACE controls male fertility
One feature of ACE knockout mice (ie mice null for all ACE expression) is the marked reduction of fertility of the homozygous males [8,26,45]. The role of ACE in fertility is also true for other organisms like insects and mollusks [4**]. As discussed, a unique ACE isozyme is made in testes by developing sperm. Dr. Ramaj et al. showed that restoration of testis ACE expression in the sperm cells of ACE knockout mice was sufficient to rescue the fertility defect, without improving the other ACE knockout characteristics, such as low blood pressure and kidney abnormalities [46]. As already discussed, testis ACE is composed of only one catalytic domain, the C-domain of somatic ACE [47]. Therefore, C-KO mice express an inactivated testis ACE. We have tested these males and demonstrated that the enzymatic activity of this enzyme was absolutely crucial to male fertility [10**]. Until now, the detailed consequences of the inactivation of testis ACE remains unclear. C-KO males produce sperm cell with a normal abundance and mobility, but these cells are unable to bind to unfertilized ovocytes. It is therefore reasonable to postulate that the specific inactivation of the testis ACE isoform could constitute an approach to develop a male contraceptive.
Summary
In conclusion, there is little doubt that both catalytic domains of ACE play important physiologic roles. The conversion of angiotensin I to angiotensin II is the ken of the C-domain. In contrast, the N-domain efficiently hydrolyzes other peptides, including AcSDKP (Figure 3). We believe there are important reasons to develop specific inhibitors of either the N- or C-domains of ACE that would be soluble and applicable to animal models. However, it seems unlikely that such agents will chronically influence blood pressure. This is because inhibition of the C-domain, the locus for angiotensin II production, will result in compensatory elevation of renin and maintenance of blood pressure. In contrast, inhibitors of the N-domain would be expected to raise AcSDKP concentrations in the absence of the typical side effects associated with total ACE inhibition. While we do not yet understand all the physiologic consequences of elevated levels of AcSDKP, what we know so far indicates that this will be a fruitful area of investigation for processes as diverse as cardiac response to myocardial infarction and fibrotic lung injury.
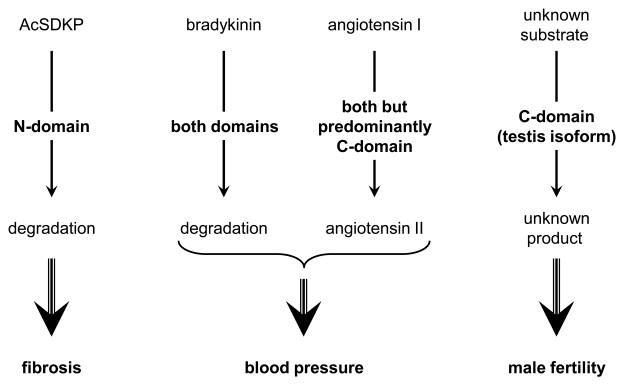
Figure 3.
Summary of the specific functions of the catalytic sites of ACE. Bradykinin and angiotensin I are substrates for both catalytic site of ACE, with a more efficient generation of angiotensin II by the C-terminal site. AcSDKP is a substrate for only the N-terminal site of ACE and affects inflammation and organ fibrosis. The C-terminal site is necessary for male fertility.
Acknowledgements
The authors want to acknowledge the contribution of John Adams and Ellen Bernstein for the genotyping and maintenance of the mouse colony. We also what to thank Dr. Mario Capecchi for the generation of the N-KO and C-KO strains, Dr. Pierre Corvol, Dr Duncan J. Campbell and Dr Hong D. Xiao for their technical help and scientific discussion during the investigation of these animals.
Support
National Institutes of Health grants RO1-DK039777, K99-HL088000, K99-DK083455 and the American Heart Association Beginning Grant in Aid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest:
The authors have no conflict of interest
References
- 1.Corvol P, Eyries M, Soubrier F. Peptidyl-dipeptidase A / Angiotensin 1-converting enzyme. In: Barrett AJ, Rawling ND, Woessner JF, editors. Handbook of Proteolytic Enzymes. 2nd ed. Vol. 1. Academic Press; San Diego: 2004. pp. 332–346. [Google Scholar]
- 2.Vegter S, de Jong-van den Berg LT. Misdiagnosis and mistreatment of a common side-effect--angiotensin-converting enzyme inhibitor-induced cough. Br J Clin Pharmacol. 2010;69:200–203. doi: 10.1111/j.1365-2125.2009.03571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei L, Alhenc-Gelas F, Corvol P, Clauser E. The two homologous domains of human angiotensin I-converting enzyme are both catalytically active. J Biol Chem. 1991;266:9002–9008. [PubMed] [Google Scholar]
- 4**.Riviere G. [Angiotensin-converting enzyme: a protein conserved during evolution] J Soc Biol. 2009;203:281–293. doi: 10.1051/jbio/2009032. This is an excellent review of the evolution of ACE. The ancestral form and function of ACE is discussed. [DOI] [PubMed] [Google Scholar]
- 5.Hubert C, Houot AM, Corvol P, Soubrier F. Structure of the angiotensin I-converting enzyme gene. Two alternate promoters correspond to evolutionary steps of a duplicated gene. J Biol Chem. 1991;266:15377–15383. [PubMed] [Google Scholar]
- 6.Howard TE, Shai SY, Langford KG, Martin BM, Bernstein KE. Transcription of testicular angiotensin-converting enzyme (ACE) is initiated within the 12th intron of the somatic ACE gene. Mol Cell Biol. 1990;10:4294–4302. doi: 10.1128/mcb.10.8.4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langford KG, Shai SY, Howard TE, Kovac MJ, Overbeek PA, Bernstein KE. Transgenic mice demonstrate a testis-specific promoter for angiotensin-converting enzyme. J Biol Chem. 1991;266:15559–15562. [PubMed] [Google Scholar]
- 8.Krege JH, John SW, Langenbach LL, Hodgin JB, Hagaman JR, Bachman ES, Jennette JC, O'Brien DA, Smithies O. Male-female differences in fertility and blood pressure in ACE-deficient mice. Nature. 1995;375:146–148. doi: 10.1038/375146a0. [DOI] [PubMed] [Google Scholar]
- 9.Esther CR, Marino EM, Howard TE, Michaud A, Corvol P, Capecchi MR, Bernstein KE. The critical role of tissue angiotensin-converting enzyme as revealed by gene targeting in mice. J Clin Invest. 1997;99:2375–2385. doi: 10.1172/JCI119419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10**.Fuchs S, Frenzel K, Hubert C, Lyng R, Muller L, Michaud A, Xiao HD, Adams JW, Capecchi MR, Corvol P, et al. Male fertility is dependent on dipeptidase activity of testis ACE. Nat Med. 2005;11:1140–1142. doi: 10.1038/nm1105-1140. This manuscript demonstrates the crucial importance of the catalytic activity of the testis isoform of ACE in male fertility. It also links sperm-egg binding with ACE enzymatic activity. [DOI] [PubMed] [Google Scholar]
- 11.Macours N, Hens K. Zinc-metalloproteases in insects: ACE and ECE. Insect Biochem Mol Biol. 2004;34:501–510. doi: 10.1016/j.ibmb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Coates D, Isaac RE, Cotton J, Siviter R, Williams TA, Shirras A, Corvol P, Dive V. Functional conservation of the active sites of human and Drosophila angiotensin I-converting enzyme. Biochemistry. 2000;39:8963–8969. doi: 10.1021/bi000593q. [DOI] [PubMed] [Google Scholar]
- 13.Cornell MJ, Williams TA, Lamango NS, Coates D, Corvol P, Soubrier F, Hoheisel J, Lehrach H, Isaac RE. Cloning and expression of an evolutionary conserved single-domain angiotensin converting enzyme from Drosophila melanogaster. J Biol Chem. 1995;270:13613–13619. doi: 10.1074/jbc.270.23.13613. [DOI] [PubMed] [Google Scholar]
- 14.Houard X, Williams TA, Michaud A, Dani P, Isaac RE, Shirras AD, Coates D, Corvol P. The Drosophila melanogaster-related angiotensin-I-converting enzymes Acer and Ance--distinct enzymic characteristics and alternative expression during pupal development. Eur J Biochem. 1998;257:599–606. doi: 10.1046/j.1432-1327.1998.2570599.x. [DOI] [PubMed] [Google Scholar]
- 15.Bingham RJ, Dive V, Phillips SE, Shirras AD, Isaac RE. Structural diversity of angiotensin-converting enzyme. Febs J. 2006;273:362–373. doi: 10.1111/j.1742-4658.2005.05069.x. [DOI] [PubMed] [Google Scholar]
- 16.Akif M, Ntai I, Sturrock ED, Isaac RE, Bachmann BO, Acharya KR. Crystal structure of a phosphonotripeptide K-26 in complex with angiotensin converting enzyme homologue (AnCE) from Drosophila melanogaster. Biochem Biophys Res Commun. 2010;398:532–536. doi: 10.1016/j.bbrc.2010.06.113. [DOI] [PubMed] [Google Scholar]
- 17.Rousseau A, Michaud A, Chauvet MT, Lenfant M, Corvol P. The hemoregulatory peptide N-acetyl-Ser-Asp-Lys-Pro is a natural and specific substrate of the N-terminal active site of human angiotensin-converting enzyme. J Biol Chem. 1995;270:3656–3661. doi: 10.1074/jbc.270.8.3656. [DOI] [PubMed] [Google Scholar]
- 18.Deddish PA, Marcic B, Jackman HL, Wang HZ, Skidgel RA, Erdos EG. N-domain-specific substrate and C-domain inhibitors of angiotensin-converting enzyme: angiotensin-(1-7) and keto-ACE. Hypertension. 1998;31:912–917. doi: 10.1161/01.hyp.31.4.912. [DOI] [PubMed] [Google Scholar]
- 19*.Zou K, Maeda T, Watanabe A, Liu J, Liu S, Oba R, Satoh Y, Komano H, Michikawa M. Abeta42-to-Abeta40- and angiotensin-converting activities in different domains of angiotensin-converting enzyme. J Biol Chem. 2009;284:31914–31920. doi: 10.1074/jbc.M109.011437. This study shows that the amyloid- ß protein 1-42 is a substrate of the N-terminal site of ACE, suggesting that ACE inhibitors may influence the development of Alzheimer's disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dive V, Cotton J, Yiotakis A, Michaud A, Vassiliou S, Jiracek J, Vazeux G, Chauvet MT, Cuniasse P, Corvol P. RXP 407, a phosphinic peptide, is a potent inhibitor of angiotensin I converting enzyme able to differentiate between its two active sites. Proc Natl Acad Sci U S A. 1999;96:4330–4335. doi: 10.1073/pnas.96.8.4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Junot C, Gonzales MF, Ezan E, Cotton J, Vazeux G, Michaud A, Azizi M, Vassiliou S, Yiotakis A, Corvol P, et al. RXP 407, a selective inhibitor of the N-domain of angiotensin I-converting enzyme, blocks in vivo the degradation of hemoregulatory peptide acetyl-Ser-Asp-Lys-Pro with no effect on angiotensin I hydrolysis. J Pharmacol Exp Ther. 2001;297:606–611. [PubMed] [Google Scholar]
- 22.Georgiadis D, Beau F, Czarny B, Cotton J, Yiotakis A, Dive V. Roles of the two active sites of somatic angiotensin-converting enzyme in the cleavage of angiotensin I and bradykinin: insights from selective inhibitors. Circ Res. 2003;93:148–154. doi: 10.1161/01.RES.0000081593.33848.FC. [DOI] [PubMed] [Google Scholar]
- 23.Watermeyer JM, Kroger WL, O'Neill HG, Sewell BT, Sturrock ED. Probing the basis of domain-dependent inhibition using novel ketone inhibitors of Angiotensin-converting enzyme. Biochemistry. 2008;47:5942–5950. doi: 10.1021/bi8002605. [DOI] [PubMed] [Google Scholar]
- 24*.Watermeyer JM, Kroger WL, O'Neill HG, Sewell BT, Sturrock ED. Characterization of domain-selective inhibitor binding in angiotensin-converting enzyme using a novel derivative of lisinopril. Biochem J. 2010;428:67–74. doi: 10.1042/BJ20100056. Based on X-ray crystallography analyses, the authors show that a chemical modification of the non-specific ACE inhibitor lisinopril can transform it into a highly C-terminal-specific ACE inhibitor. [DOI] [PubMed] [Google Scholar]
- 25.Capecchi MR. Targeted gene replacement. Sci Am. 1994;270:52–59. doi: 10.1038/scientificamerican0394-52. [DOI] [PubMed] [Google Scholar]
- 26.Esther CR, Jr., Howard TE, Marino EM, Goddard JM, Capecchi MR, Bernstein KE. Mice lacking angiotensin-converting enzyme have low blood pressure, renal pathology, and reduced male fertility. Lab Invest. 1996;74:953–965. [PubMed] [Google Scholar]
- 27.Deng C, Thomas KR, Capecchi MR. Location of crossovers during gene targeting with insertion and replacement vectors. Mol Cell Biol. 1993;13:2134–2140. doi: 10.1128/mcb.13.4.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28**.Fuchs S, Xiao HD, Cole JM, Adams JW, Frenzel K, Michaud A, Zhao H, Keshelava G, Capecchi MR, Corvol P, et al. Role of the N-terminal catalytic domain of angiotensin-converting enzyme investigated by targeted inactivation in mice. J Biol Chem. 2004;279:15946–15953. doi: 10.1074/jbc.M400149200. This paper shows that the selective inactivation of the N-terminal site of ACE in vivo has no effect on blood pressure; the presence of the C-terminal ACE catalytic domain is sufficient to maintain a functional renin-angiotensin system. It also strongly suggests that the anemia present in ACE null mice is not due to the accumulation of the tetrapeptide AcSDKP. [DOI] [PubMed] [Google Scholar]
- 29**.Fuchs S, Xiao HD, Hubert C, Michaud A, Campbell DJ, Adams JW, Capecchi MR, Corvol P, Bernstein KE. Angiotensin-converting enzyme C-terminal catalytic domain is the main site of angiotensin I cleavage in vivo. Hypertension. 2008;51:267–274. doi: 10.1161/HYPERTENSIONAHA.107.097865. This study shows that the C-domain of ACE is the predominant site of angiotensin I cleavage in vivo. [DOI] [PubMed] [Google Scholar]
- 30.Cole J, Quach du L, Sundaram K, Corvol P, Capecchi MR, Bernstein KE. Mice lacking endothelial angiotensin-converting enzyme have a normal blood pressure. Circ Res. 2002;90:87–92. doi: 10.1161/hh0102.102360. [DOI] [PubMed] [Google Scholar]
- 31.Krege JH, Kim HS, Moyer JS, Jennette JC, Peng L, Hiller SK, Smithies O. Angiotensin-converting enzyme gene mutations, blood pressures, and cardiovascular homeostasis. Hypertension. 1997;29:150–157. doi: 10.1161/01.hyp.29.1.150. [DOI] [PubMed] [Google Scholar]
- 32.Smithies O. Theodore Cooper Memorial Lecture. A mouse view of hypertension. Hypertension. 1997;30:1318–1324. doi: 10.1161/01.hyp.30.6.1318. [DOI] [PubMed] [Google Scholar]
- 33.Azizi M, Ezan E, Nicolet L, Grognet JM, Menard J. High plasma level of N-acetyl-serylaspartyl-lysyl-proline: a new marker of chronic angiotensin-converting enzyme inhibition. Hypertension. 1997;30:1015–1019. doi: 10.1161/01.hyp.30.5.1015. [DOI] [PubMed] [Google Scholar]
- 34.Azizi M, Rousseau A, Ezan E, Guyene TT, Michelet S, Grognet JM, Lenfant M, Corvol P, Menard J. Acute angiotensin-converting enzyme inhibition increases the plasma level of the natural stem cell regulator N-acetyl-seryl-aspartyl-lysyl-proline. J Clin Invest. 1996;97:839–844. doi: 10.1172/JCI118484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonnet D, Lemoine FM, Khoury E, Pradelles P, Najman A, Guigon M. Reversible inhibitory effects and absence of toxicity of the tetrapeptide acetyl-N-Ser-AspLys-Pro (AcSDKP) in human long-term bone marrow culture. Exp Hematol. 1992;20:1165–1169. [PubMed] [Google Scholar]
- 36*.Peng H, Carretero OA, Brigstock DR, Oja-Tebbe N, Rhaleb NE. Ac-SDKP reverses cardiac fibrosis in rats with renovascular hypertension. Hypertension. 2003;42:1164–1170. doi: 10.1161/01.HYP.0000100423.24330.96. The authors show that in hypertension, Ac-SDKP reverses cardiac fibrosis, due in part to a decrease in TGF-ß and CTGF in the heart. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37**.Lin CX, Rhaleb NE, Yang XP, Liao TD, D'Ambrosio MA, Carretero OA. Prevention of aortic fibrosis by N-acetyl-seryl-aspartyl-lysyl-proline in angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol. 2008;295:H1253–H1261. doi: 10.1152/ajpheart.00481.2008. This manuscript shows that, in angiotensin II-induced hypertension, Ac-SDKP prevents the development of aortic fibrosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liao TD, Yang XP, D'Ambrosio M, Zhang Y, Rhaleb NE, Carretero OA. N-acetyl-serylaspartyl-lysyl-proline attenuates renal injury and dysfunction in hypertensive rats with reduced renal mass: council for high blood pressure research. Hypertension. 2010;55:459–467. doi: 10.1161/HYPERTENSIONAHA.109.144568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39*.Li P, Xiao HD, Xu J, Ong FS, Kwon M, Roman J, Gal A, Bernstein KE, Fuchs S. Angiotensin-Converting Enzyme N-Terminal Inactivation Alleviates Bleomycin-Induced Lung Injury. Am J Pathol. 2010;177 doi: 10.2353/ajpath.2010.081127. This study shows that the inactivation of the N-terminal catalytic site of ACE significantly reduced bleomycin-induced lung fibrosis and implicates AcSDKP in the mechanism of protection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moeller A, Ask K, Warburton D, Gauldie J, Kolb M. The bleomycin animal model: a useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int J Biochem Cell Biol. 2008;40:362–382. doi: 10.1016/j.biocel.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haston CK, Wang M, Dejournett RE, Zhou X, Ni D, Gu X, King TM, Weil MM, Newman RA, Amos CI, et al. Bleomycin hydrolase and a genetic locus within the MHC affect risk for pulmonary fibrosis in mice. Hum Mol Genet. 2002;11:1855–1863. doi: 10.1093/hmg/11.16.1855. [DOI] [PubMed] [Google Scholar]
- 42.Barelli H, Petit A, Hirsch E, Wilk S, De Nanteuil G, Morain P, Checler F. S 17092-1, a highly potent, specific and cell permeant inhibitor of human proline endopeptidase. Biochem Biophys Res Commun. 1999;257:657–661. doi: 10.1006/bbrc.1999.0366. [DOI] [PubMed] [Google Scholar]
- 43.Szeltner Z, Polgar L. Structure, function and biological relevance of prolyl oligopeptidase. Curr Protein Pept Sci. 2008;9:96–107. doi: 10.2174/138920308783565723. [DOI] [PubMed] [Google Scholar]
- 44.Cavasin MA, Liao TD, Yang XP, Yang JJ, Carretero OA. Decreased endogenous levels of Ac-SDKP promote organ fibrosis. Hypertension. 2007;50:130–136. doi: 10.1161/HYPERTENSIONAHA.106.084103. [DOI] [PubMed] [Google Scholar]
- 45.Hagaman JR, Moyer JS, Bachman ES, Sibony M, Magyar PL, Welch JE, Smithies O, Krege JH, O'Brien DA. Angiotensin-converting enzyme and male fertility. Proc Natl Acad Sci U S A. 1998;95:2552–2557. doi: 10.1073/pnas.95.5.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramaraj P, Kessler SP, Colmenares C, Sen GC. Selective restoration of male fertility in mice lacking angiotensin-converting enzymes by sperm-specific expression of the testicular isozyme. J Clin Invest. 1998;102:371–378. doi: 10.1172/JCI3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ehlers MR, Fox EA, Strydom DJ, Riordan JF. Molecular cloning of human testicular angiotensin-converting enzyme: the testis isozyme is identical to the C-terminal half of endothelial angiotensin-converting enzyme. Proc Natl Acad Sci U S A. 1989;86:7741–7745. doi: 10.1073/pnas.86.20.7741. [DOI] [PMC free article] [PubMed] [Google Scholar]