Abstract
Background
Approximately half of patients with heart failure die suddenly as a result of ventricular arrhythmias. Although abnormal Ca2+ release from the sarcoplasmic reticulum (SR) through ryanodine receptors (RyR2) has been linked to arrhythmogenesis, the molecular mechanisms triggering release of arrhythmogenic Ca2+ remain unknown. We tested the hypothesis that increased RyR2 phosphorylation by Ca2+/calmodulin-dependent protein kinase II (CaMKII) is both necessary and sufficient to promote lethal ventricular arrhythmias.
Methods and Results
Mice in which the S2814 CaMKII site on RyR2 is constitutively activated (S2814D) develop pathological SR Ca2+ release events resulting in reduced SR Ca2+ load on confocal microscopy. These Ca2+ release events are associated with increased RyR2 open probability in lipid bilayer preparations. At baseline, young S2814D mice have structurally and functionally normal hearts without arrhythmias; however, they develop sustained ventricular tachycardia and sudden cardiac death upon catecholaminergic provocation by caffeine/epinephrine or programmed electrical stimulation. Young S2814D mice have a significant predisposition to sudden arrhythmogenic death after transverse aortic constriction (TAC) surgery. Finally, genetic ablation of the CaMKII site on RyR2 (S2814A) protects mutant mice from pacing-induced arrhythmias versus wild type mice after TAC surgery.
Conclusions
Our results suggest that CaMKII phosphorylation of RyR2 Ca2+ release channels at S2814 plays an important role in arrhythmogenesis and sudden cardiac death in mice with heart failure.
Keywords: Cardiac arrhythmias, Ca2+/calmodulin kinase II, heart failure, ryanodine receptor, sarcoplasmic reticulum
CLINICAL PERSPECTIVE.
Despite recent therapeutic advances including adrenergic blockers and implantable cardioverter-defibrillators (ICD) ventricular arrhythmias remain a prominent cause of death in patients with heart failure. Diastolic Ca2+ leak from the sarcoplasmic reticulum is believed to contribute to arrhythmia initiation in failing hearts, although the underlying mechanisms remain poorly understood. The expression and activity of the enzyme Ca2+/calmodulin-dependent protein kinase II (CaMKII) is upregulated in heart failure. Although increased CaMKII activity has been implicated in arrhythmogenesis, the specific CaMKII targets contributing to arrhythmia susceptibility have remained elusive. Our data revealed that mice in which the CaMKII phosphorylation site on the cardiac ryanodine receptor (RyR2) is constitutively activated exhibit an increased likelihood of intracellular Ca2+ releases. This diastolic Ca2+ ‘leak’ leads to an increased susceptibility to ventricular tachycardia in mice. Moreover, constitutive CaMKII phosphorylation of RyR2 caused an increase in arrhythmogenic sudden cardiac deaths following induction of experimental heart failure. Conversely, mice with genetic ablation of the CaMKII site on RyR2 exhibited protection from induced ventricular arrhythmias due to heart failure. Taken together, our studies suggest that CaMKII phosphorylation of RyR2 is an important downstream target of CaMKII that could be exploited therapeutically to minimize arrhythmia susceptibility in heart failure. Future studies utilizing pharmacological inhibition of this signaling event, once tested, may be a new avenue for reducing risk of sudden death in patients with heart failure.
INTRODUCTION
Congestive heart failure (HF) is a leading cause of mortality and morbidity worldwide. Approximately 50% of HF patients die of sudden cardiac death (SCD) attributed to ventricular arrhythmias (>300,000 in the U.S. annually) 1, 2. A large fraction of these arrhythmias are thought to be initiated by focal triggered mechanisms, such as spontaneous diastolic calcium (Ca2+) release from cardiac myocyte ryanodine receptors (RyR2) on the sarcoplasmic reticulum (SR), which activates an arrhythmogenic depolarizing inward Na+/Ca2+ exchange current 3, 4. Indeed, in HF there is enhanced diastolic SR Ca2+ release, and other changes in electrophysiological substrate that greatly enhance the propensity for triggered arrhythmias. Likewise, patients with inherited RyR2 point mutations exhibit catecholaminergic polymorphic ventricular tachycardia (CPVT), a known cause of SCD with sensitivity to adrenergic conditions such as exercise or stress 5, 6. HF is a chronic hyperadrenergic state, and a prominent theory suggested that β-adrenergic activation of protein kinase A (PKA) destabilized RyR2 through the loss of binding by FKBP12.6 7, 8, contributing to SR Ca2+ leak and consequent systolic dysfunction and arrhythmogenesis. However, subsequent work showed that acute inhibition of Ca2+/calmodulin-dependent protein kinase II (CaMKII) rather than PKA was sufficient to reverse the arrhythmogenic SR Ca2+ leak in HF 9, and that CaMKII mediates the β-adrenergic-induced increase in SR Ca2+ leak even in normal myocytes 10. Thus, in heart failure, CaMKII phosphorylation of RyR2 may be more important for arrhythmogenic events leading to sudden cardiac death.
CaMKII, which is upregulated and more active in HF 4, can phosphorylate and modulate numerous Ca2+ transport and ion channel proteins in cardiac myocytes, including RyR2 and voltage-gated Ca2+, Na+ and K+ channels, all of which could contribute to arrhythmogenesis 11. Moreover, transgenic overexpression of CaMKIV or CaMKIIδ (the predominant myocyte isoform) induces HF and cardiac arrhythmias 12–15, while inhibition and CaMKIIδ knockout limit the progression of HF and arrhythmias 16, 17. One weakness of currently available genetic models in which CaMKII activity is inhibited by gene deletion or transgenic expression of inhibitory peptides is they do not permit selective evaluation of specific downstream phosphorylation targets affected by CaMKII upregulation or inhibition. Given the role of CaMKII phosphorylation of RyR2 in both heart failure and diastolic SR Ca2+ release, we sought to define the role of CaMKII phosphorylation of RyR2 specifically in cardiac arrhythmogenesis. Therefore, we generated and studied knock-in mouse models in which the CaMKII phosphorylation site on RyR2 was either genetically inhibited or constitutively activated. Here we tested whether: a) the phosphomimetic S2814D mutant RyR2 alone increased the susceptibility to ventricular arrhythmias, and b) whether the non-phosphorylatable S2814A mutant prevents arrhythmias associated with cardiac hypertrophy and failure.
METHODS
Animals
Generation of RyR2-S2814A knock-in and AC3I transgenic mice was described previously 5, 17. RyR2-S2814D knock-in mice were created using a similar approach described for RyR2-S2814A knock-in mice 5 (see online supplement). All animal studies were performed according to protocols approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine conforming the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Co-Immunoprecipitation Assay
RyR2 was immunoprecipitated from heart lysates using an anti-RyR2 antibody (Thermo Scientific, Rockland, IL) incubated with Protein A-Sepharose beads (Rockland, Gilbertsville, PA) at room temperature for 1 h. For co-immunoprecipitation, antibody- attached beads were incubated with heart lysate aliquots containing 1000 μg total protein at 4°C overnight. Post-incubation, beads were washed with detergent-free Co-IP buffer, and resuspended in 2× LDS buffer (Invitrogen, Carlsbad, CA) containing β-mercaptoethanol. Samples were heated at 50°C for 15 min, and were resolved on 4–20% Criterion SDS-PAGE gels (Bio- Rad) for detection of RyR2 and FKBP12.6.
RyR2 phosphorylation assay
RyR2 was immunoprecipitated, and beads were washed with phosphorylation-assay buffer containing 50 mM Tris-Cl (pH 7.5), 10 mM MgCl2, 2 mM DTT, 0.1 mM Na2EDTA, 5 mM NaF, 1 mM Na3VO4, 1x protease inhibitors and 1x phosphatase inhibitors and resuspended in phosphorylation-assay buffer supplemented with 100 μM cold ATP and 1.5 μCi [γ-32P] ATP (Perkin Elmer, Waltham, MA). The phosphorylation reaction was initiated by adding CaMKII (250 U/reaction), which was pre-activated as per manufacturer’s (New England Biolabs, Ipswich, MA) instructions in the absence or presence of the CaMKII inhibitor KN-93 (10 μM) (Calbiochem, San Diego, CA). Reaction mixtures were incubated at 30°C for 20 minutes and stopped by adding 2× LDS buffer (Invitrogen, Carlsbad, CA) containing β-mercaptoethanol. Samples were heated at 50°C for 10 min and resolved on 5% SDS-PAGE gels. The protein gels were dried, and exposed to KODAK BioMax MR films.
Western blot analyses
Heart lysates were prepared from flash-frozen mouse hearts as described previously 18. Lysates were taken from mice at rest (Fig. 1), post-TAC or post-sham surgery (Fig. 7 and S5), immediately after pacing (Fig. 5 and S4), and with/without exposure to pacing (Fig. 7). For experimental details, please refer to the online supplement.
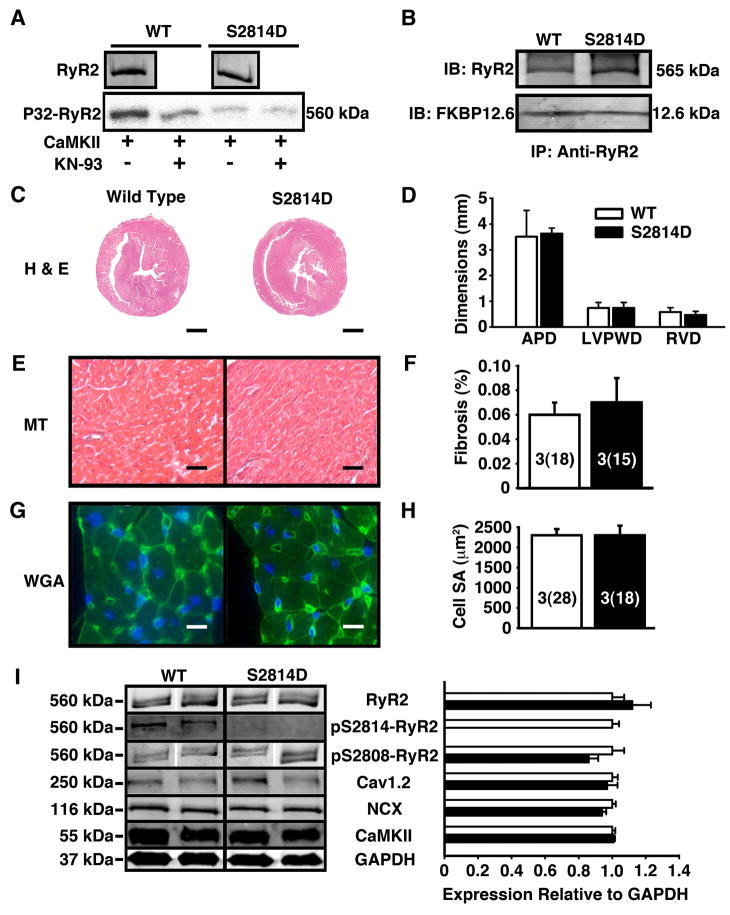
Figure 1. Baseline molecular and structural characteristics of S2814D mice.
(A) The S2814D mutation prevents CaMKII mediated phosphorylation of RyR2. Equal amounts of RyR2 protein were immunoprecipitated from WT and S2814D hearts and phosphorylated with CaMKII in the presence or absence of KN93. Shown is a representative example of an experiment repeated twice. (B) Co-immunoprecipitation showing that the binding of FKBP12.6 to RyR2 was not altered in S2814D mice. (C) Representative histological sections of 3 month-old mouse hearts stained with hematoxylin and eosin (H&E). Scale bar lines represent 1 mm (D) Cardiac wall dimensions assessed from H&E histological sections. Left-ventricular (LV) anterior-posterior diameter (APD), LV posterior wall diameter (LVPWD) and right ventricular (RV) wall diameter (RVD) were similar among WT and S2814D hearts. (E) Masson’s Trichrome for fibrosis of histological sections of 3 month-old mouse hearts. Scale bar lines represent 30 μm. (F) Percent fibrosis quantified from Masson’s Trichrome stain. (G) Representative wheat germ agglutinin fluorescence stains (WGA) of mouse cardiomyocytes. Scale bars represent 10 μm. (H) Cardiomyocyte surface area calculated from WGA stains. (I) Western blot and phosphorylation assays showing baseline protein expression and phosphorylation of RyR2 at baseline. On Western blot, expression of the cardiac ryanodine receptor (RyR2), the L-type Ca2+ channel (Cav1.2), Na+/Ca2+-exchanger (NCX), and Ca2+/calmodulin kinase II (CaMKII) were not statistically different between WT and S2814D mouse hearts at rest. GAPDH was used as a loading control for Western Blots. Number of animals (number of cells) is indicated in bar graphs.
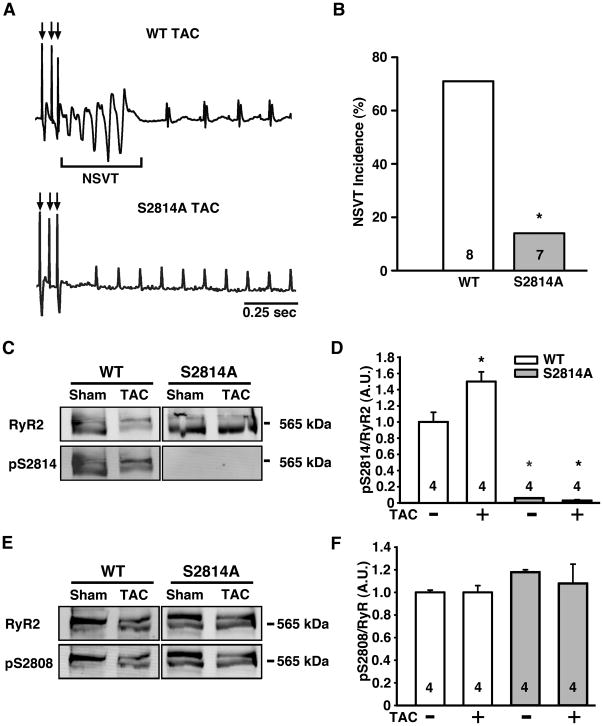
Figure 7. Genetic blockade of CaMKII phosphorylation of RyR2 reduces risk for pacing-induced ventricular ectopy.
(A) Representative ECG tracing of pacing-induced non-sustained VT (NSVT) in WT mice 8 weeks after transverse aortic constriction (TAC; top), whereas the S2814A TAC mice typically showed sinus rhythm (bottom). (B) Bar graph showing incidence of pacing-induced NSVT in WT and S2814A mice at 8 weeks post-TAC. (C) Representative Western blots showing total RyR2 and CaMKII-phosphorylated RyR2 at S2814 in WT and S2814A mice at 8 weeks after transverse aortic constriction (TAC). (D) Bar graphs showing averaged ratio between phosphorylated RyR2-pS2814 and total RyR2. (E) Representative Western blots showing total RyR2 and PKA-phosphorylated RyR2 at S2808 in WT and S2814A mice at 8 weeks after TAC. (F) Bar graphs showing averaged ratio between phosphorylated RyR2-pS2808 and total RyR2. N is shown in bar graphs. Fisher’s Exact test was used to compare NSVT incidence; Student’s t-test was used to compare Western blot data. *P<0.05 versus WT surgically matched control.
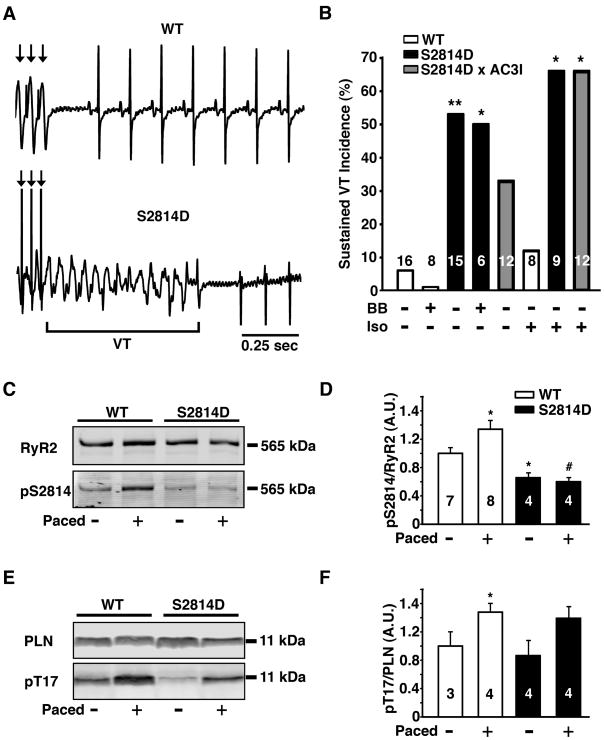
Figure 5. CaMKII phosphorylation of RyR2 is critical for development of ventricular arrhythmias in normal and failing hearts.
(A) Representative ECG traces after injection of 0.5 mg/kg isoproterenol and intracardiac overdrive pacing. Wild type (WT) mice exhibited normal sinus rhythm after intracardiac pacing, while S2814D mice were predisposed to development of ventricular tachycardia (VT). (B) Percent incidence of overdrive pacing-induced VT in WT, S2814D, and S2814D:AC3I mice with (+) and without (−) agonist isoproterenol (Iso, 0.5 mg/kg i.p.) and propranolol beta blocker (BB, 3 mg/kg i.p). (C) Representative Western blot of the RyR2 CaMKII site (S2814) in WT and S2814D mice before and after intracardiac pacing. (D) WT mice have a significant increase in phosphorylation at the CaMKII site S2814, whereas S2814D mice cannot be phosphorylated at the site. (E) Representative Western blot of the phospholamban (PLN) CaMKII site (T17) before and after intracardiac pacing. (F) There is a significant increase in phosphorylation of PLN by CaMKII in WT mice. N is indicated in bar graphs. Fisher’s Exact test was used to compare VT incidence data; an unpaired Student’s t-test was used to compare phosphorylation data. *P<0.05, **P<0.01 vs. WT treatment-matched control; # P<0.05 vs. S2814D non-paced control.
Histology
A transverse section of the heart was fixed in 10% buffered formalin for 48 hours. After paraffin embedding and sectioning, 5 μm sections were stained with hematoxylin-eosin (H&E) for cell morphology and Masson’s Trichrome for interstitial fibrosis as described 18.
Confocal imaging
Ca2+ sparks were recorded in saponin-permeabilized (50 μg/ml) or intact ventricular cardiomyocytes using a Zeiss LSM510 confocal microscopy. For experimental details, please refer to the online supplement.
Single channel recordings
Single-channel recordings of WT or S2814D RyR2 were performed and analyzed under voltage-clamp conditions as described previously 19 (see also online supplement).
Transthoracic echocardiography
Mice were anesthetized using 1.5% isoflurane in 95% O2. Body temperature was maintained at 36–37°C on a heated platform, and electrocardiograms and temperature were continuously monitored. Cardiac function was assessed using a VisualSonics VeVo 770 Imaging System (VisualSonics, Toronto, Canada) equipped with high-frequency 30 MHz probe, as described 20, 21. Data analysis was performed using VisualSonics software (VisualSonics, Toronto, Canada).
ECG Telemetry
Twenty-three animals (eleven S2814D and twelve WT) were studied with ECG telemetry according to published methods 22 (see online supplement).
Programmed electrical stimulation
Atrial and ventricular intracardiac electrograms were recorded using a 1.1F octapolar electrode catheter (EPR-800, Millar Instruments, Houston, Texas) inserted into the right ventricle via the right jugular vein, as described in the online supplement 23.
Transverse aortic constriction
Transverse aortic constriction (TAC) was performed as previously described in detail (see also online supplement) 21, 24, 25.
Statistical analysis
Continuous variables were expressed as mean ± SEM; whenever the distribution was skewed, medians with the first and third quartiles were expressed. Continuous variables were evaluated using an unpaired Student’s t-test or ANOVA. The Mann-Whitney test was used to compare continuous variables with a skewed distribution. Categorical data were expressed as percentages, and were compared using the Fisher’s Exact test. The Kaplan-Meier survival curve was evaluated by the log-rank test. P < 0.05 was considered statistically significant.
RESULTS
We generated a knock-in mouse model in which aspartic acid replaces serine at RyR2-2814 (S2814D) to mimic constitutive phosphorylation of RyR2 by CaMKII (Fig. S1). CaMKII can phosphorylate RyR2 immunoprecipitated from wild type (WT) hearts but not from S2814D mice (Fig. 1A). Furthermore, CaMKII inhibition by KN-93 prevents RyR2 phosphorylation by CaMKII in WT hearts, but has no effect in S2814D hearts, indicating that S2814 is the major CaMKII target site of RyR2 (Fig. 1A). FKBP12.6 binding to RyR2 can alter RyR2 function, but we found that the S2814D mutation did not alter FKBP12.6 binding to RyR2 in cardiac homogenates (Figure 1B), as in an earlier report 19. At baseline, cardiac structure and function are similar in young (3-month-old) WT and S2814D mice as determined by echocardiography (Table 1; Supplemental Table 1). Transverse H&E sections from WT and S2814D hearts showed there were no significant differences in right ventricular (RV) wall thickness (WT: 0.58 ± 0.17 mm; S2814D: 0.47 ± 0.14 mm, P = 0.10), left ventricular (LV) posterior wall thickness (WT: 0.74 ± 0.21 mm; S2814D: 0.74 ± 0.22 mm, P = 0.91), or LV anteroposterior diameter (WT: 3.51 ± 1.02 mm; S2814D: 3.63 ± 1.05 mm, P = 0.41) (Figure 1C-D). Quantitative analysis of Masson Trichrome stainings revealed no differences in the amount of interstitial fibrosis comparing WT (0.06 ± 0.02 % of total surface area) and S2814D mouse hearts (0.07 ± 0.02 %, P = 0.61) (Figure 1E-F). Wheat germ agglutinin (WGA) staining and quantification of myocardial cell size revealed no differences in myocyte surface areas in WT (2311 ± 166 μm2) and S8214D (2315 ± 256 μm2) mouse hearts (P = 0.99) (Figure 1G-H). On Western blots, expression of WT and S2814D heart lysates using antibodies against cardiac ryanodine receptor (RyR2), L-type Ca2+ channel (Cav1.2), Na+/Ca2+-exchanger (NCX), and Ca2+/calmodulin-dependent protein kinase II (CaMKII) was not statistically different (Cav1.2, P = 0.66; NCX, P = 0.17; CaMKII, P = 0.61) (Figure 1I).
Table 1.
Echocardiographic parameters of WT and S2814D mice at 3 and 12 months of age.
| Age | 3 months | 12 months | P Values | |||
|---|---|---|---|---|---|---|
| WT (n=14) | S2814D (n=14) | WT (n=14) | S2814D (n=14) | 3 months | 12 months | |
| HR (bpm) | 448.1±12.2 | 455.5±11.0 | 484.6±12.4 | 459.6±10.6 | 0.68 | 0.13 |
| EF (%) | 59.4±0.7 | 59.1±0.7 | 56.9±0.7 | 52.4±1.8 | 0.87 | 0.02 |
| FS (%) | 31.2±1.1 | 31.0±0.7 | 30.3±0.7 | 27.4±0.9 | 0.85 | 0.02 |
| ESD (mm) | 2.68±0.10 | 2.76±0.07 | 2.85±0.06 | 3.13±0.09 | 0.53 | 0.02 |
| EDD (mm) | 3.89±0.09 | 3.99±0.07 | 4.09±0.06 | 4.30±0.08 | 0.39 | 0.05 |
| IVSs (mm) | 0.90±0.03 | 0.89±0.03 | 0.94±0.02 | 0.95±0.03 | 0.82 | 0.58 |
| IVSd (mm) | 0.67±0.03 | 0.67±0.03 | 0.72±0.03 | 0.77±0.03 | 0.93 | 0.23 |
| LVPWs(mm) | 0.99±0.05 | 0.95±0.03 | 1.06±0.03 | 1.13±0.05 | 0.84 | 0.20 |
| LVPWd(mm) | 0.68±0.05 | 0.66±0.03 | 0.73±0.03 | 0.84±0.04 | 0.64 | 0.05 |
Data are expressed as mean ± SEM. HR = heart rate; bpm = beats per minute; EF = ejection fraction; FS = left ventricular fractional shortening; ESD = end-systolic diameter; EDD = end-diastolic diameter; IVSs/IVSd = intraventricular septal wall thickness in systole/diastole; LVPWs/ LVPWd = left ventricular posterior wall thickness in systole/diastole. Student’s t-test was used to compare intra-group differences; P values between WT and S2814D mice at 3 and at 12 months are displayed to the right.
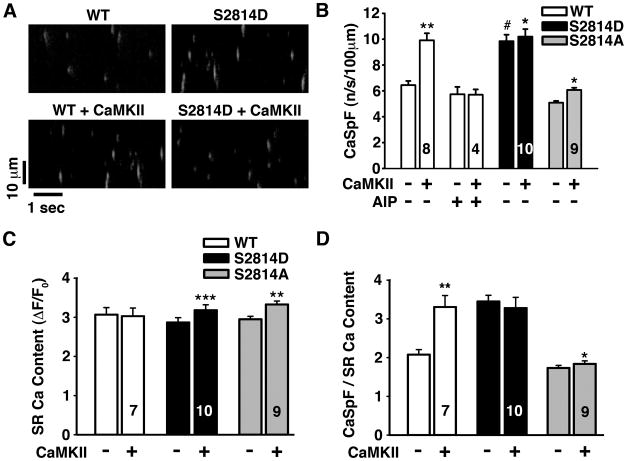
At the myocyte level, HF and CaMKII overexpression (and activation) enhance SR Ca2+ leak (manifested as increased Ca2+ sparks or waves mediated by RyR2) and can thus serve as the molecular trigger for arrhythmia 26. To determine how SR Ca2+ leak is altered in young S2814D and S2814A mice, we used confocal microscopy to image local SR Ca2+ release events, or Ca2+ sparks, in permeabilized isolated cardiomyocytes (Fig. 2A). At baseline, Ca2+ spark frequency (CaSpF) was significantly increased in S2814D (9.8 ± 0.5 /s/100μm) vs. WT mice (6.4 ± 0.3 /s/100μm; P < 0.001; Fig. 2A-B). Activation of endogenous CaMKII significantly increased CaSpF in WT myocytes (9.9 ± 0.5 /s/100μm), but had no additional effect in S2814D cells (which were already at this higher level, 10.2 ± 0.6 /s/100μm). In S2814A myocytes, CaSpF was comparable to WT at baseline both before (5.1 ± 0.1 /s/100μm) and after activation of CaMKII (6.1±0.2/s/100μm). The small rise with CaMKII was entirely attributable to enhanced SR Ca2+ content (Fig. 2C-D), and did not significantly alter Ca2+ spark amplitude, full duration at half-maximum (FDHM), full width at half-maximum (FWHM), or maximum Ca2+ release (Fig. S2). Inclusion of the specific CaMKII inhibitory peptide AIP (1 μM) prevented CaMKII-dependent activation of CaSpF in WT cells, but did not alter CaSpF in S2814D myocytes (Fig. 2B). Combined, these results show that constitutive RyR2-S2814 pseudo-phosphorylation mimics maximal CaMKII activation of WT RyR2 in ventricular myocytes, and that S2814 is the only functionally important CaMKII site with respect to these measurements.
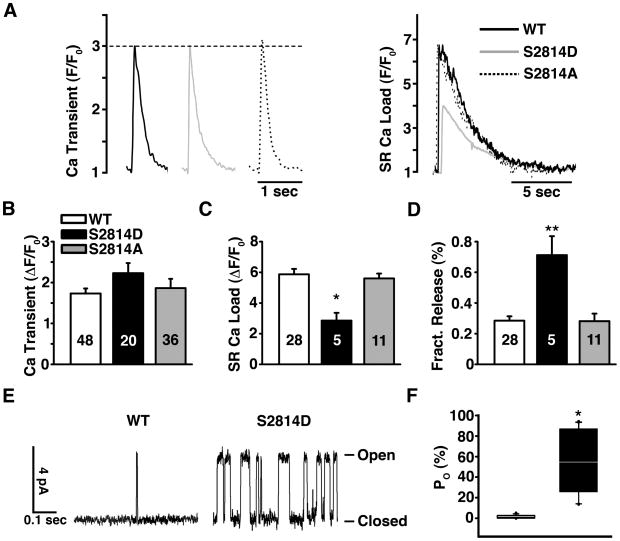
Figure 2. CaMKII phosphorylation induces RyR2 mediated SR calcium leak in permeabilized myocytes.
(A) Respresentative confocal line-scan images obtained in permeabilized cardiomyocytes from WT and S2814D mice before and after activation of endogenous CaMKII. (B) Quantification of Ca2+ spark frequency (CaSpF) in cardiomyocytes isolated from WT, S2814D and S2814A mice in the absence (−) or presence (+) of CaMKII and CaMKII inhibitor AIP. (C) SR calcium content (ΔF/F0) in permeabilized myocytes in the absence (−) or presence (+) of CaMKII. (D) Ratio of calcium spark frequency (CaSpF) to SR calcium content. WT myocytes had a significantly increased CaSpF/SR content ratio in the presence of CaMKII. N is indicated in bar graphs. 2-way repeated measures ANOVA was used to compare groups. *P<0.05, **P<0.01, *** P<0.001 vs. same genotype without CaMKII; # P<0.05 vs. WT.
We also measured Ca2+ transients, Ca2+ sparks and SR Ca2+ load in intact ventricular myocytes. Twitch Ca2+ transient amplitude during electrical field stimulation (1 Hz) was similar in WT, S2814D and S2814A cardiomyocytes (P=0.43; Fig. 3A-B). However, the time constant of twitch [Ca2+]i decline was significantly lower in S2814D (403.8±18 ms, P < 0.05) compared to WT (274.8±13 ms) and S2814A (238±18 ms). Moreover, SR Ca2+ load (assessed by rapid application of 10 mM caffeine) was 50% lower in S2814D compared to WT or S2814A myocytes (P = 0.02 Fig. 3A and C). This is presumably due to the slightly reduced SERCA2a function and higher diastolic SR Ca2+ leak in S2814D myocytes (see above), and also evidenced by significantly higher CaSpF in intact S2814D myocytes vs. WT or S2814A myocytes (Fig. S3A-B). However, other parameters such as Ca2+ spark amplitude, FDHM, FWHM, and rate of rise were unchanged among the groups (Fig. S3C-F). Note that CaMKIIδC transgenic mouse myocytes and rabbit HF myocytes also exhibit enhanced leak and reduced SR Ca2+ load 9, 12. There was no statistical difference in NCX function - measured as the time constant of [Ca2+]i decline during a caffeine-induced Ca2+ transient - among the three mouse groups: WT (τ=1.9±0.2 s), S2814D (τ=2.2±0.6 s) and S2814A (τ=1.7±0.2 s) (P = 0.54). However, the S2814D mice did exhibit enhanced fractional SR Ca2+ release (ratio of twitch:caffeine-induced Ca2+ transient) compared to WT or S2814A mice (P<0.001; Fig. 3D). Thus, S2814D mice maintain normal Ca2+ transients (and cardiac function) with a smaller SR Ca2+ load, but a larger fractional release at each contraction. This is consistent with prior work suggesting that CaMKII-dependent RyR2 phosphorylation sensitizes RyR2 to a given Ca2+ current trigger at a given SR Ca2+ load 27. Thus RyR2 phosphorylation at S2814 activates both diastolic and systolic RyR2 activation.
Figure 3. CaMKII phosphorylation induces RyR2 mediated SR calcium leak and reduced SR calcium load in intact myocytes.
(A) Representative Ca2+ transients (left) and superimposed SR load traces (right) expressed as F/F0. (B) Average twitch Ca2+ transient amplitude (DF/F0) in WT, S2814D, and S2814A cardiomyocytes. (C) Averaged peak fluorescence ratio (DF/F0) obtained by 10 mM caffeine application in WT, S2814D, and S2814A cardiomyocytes. (D) Fractional Ca2+ release expressed as ratio for twitch Ca2+ transient/caffeine transient in WT, S2814D, and S2814A cardiomyocytes. (E and F) CaMKII phosphorylation activates RyR2. RyR2 open probablity (Po) is increased in S2814D mice (see box plot in F) as calculated from RyR2 single channel recordings (n=14 channels for each group) (E). N is indicated in bar graphs. 2-way repeated measures ANOVA was used to compare Ca2+ imaging data. The Mann-Whitney U-test was used to compare Po. *P<0.05, **P<0.01, ***P<0.001 versus WT.
To directly assess single RyR2 channel opening we extracted microsomes containing RyR2 from young WT and S2814D mouse hearts and reconstituted them in lipid bilayers. RyR2-S2814D channels exhibited much higher open probability (Po 54.3%, [interquartile range, 25.8% to 86.5%]) compared to RyR2 from WT mice (1.0 % [interquartile range, 0.9% to 2.5%], P<0.001; Fig. 3E-F). It is likely, however, that the relative increase in open probability of S2814D mutant channels will be more modest in vivo, as the frequency of Ca2+ sparks in S2814D myocytes was only increased two-fold. The single channel and whole cell experiments demonstrate that pseudo-phosphorylation of RyR2 at the S2814 CaMKII site increases the open probability of RyR2, resulting in diastolic Ca2+ leak.
We then used young (3–4 month old) S2814D knock-in mice to evaluate the effects of CaMKII mediated RyR2 phosphorylation on arrhythmogenesis in structurally normal hearts. ECG telemeters were implanted in both WT and S2814D mice to allow recording of ambulatory ECG waveforms. S2814D mice had normal heart rhythm at rest, with unaltered electrophysiological parameters such as heart rate (HR), depolarization intervals (PQ, QRS), and repolarization intervals (QTc) (Table S2). Moreover, the ventricular effective refractory period (VERP) was also unaffected in S2814D mice (Table S3). However, when challenged with the β-adrenergic agonist isoproterenol (0.5 mg/kg i.p.), S2814D mice exhibited a significantly higher increase in premature ventricular complexes (PVCs) vs. WT (10.0 [interquartile range, 6.0 to 14.0] vs. 3.8 [interquartile range, 1.0 to 4.8]; P = 0.002), which is indicative of ventricular ectopic arrhythmic activity (Fig. 4A-B).
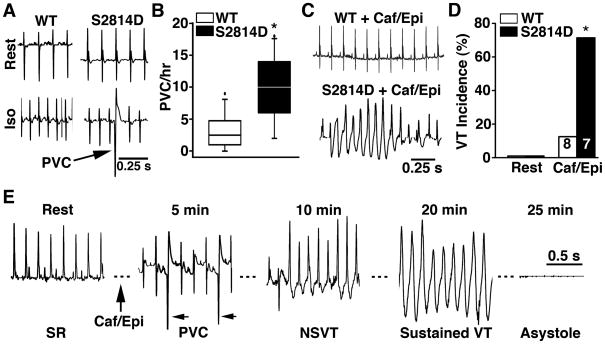
Figure 4. Constitutive CaMKII phosphorylation of RyR2 leads to ventricular arrhythmias and sudden cardiac death.
(A) Representative ECG telemetry tracings in wild type (WT) and S2814D knock-in mice at rest and following injection of isoproterenol (Iso; 0.5 mg/kg i.p.). Arrow indicates pro-arrhythmic premature ventricular complex (PVC). (B) Box plot showing incidence of PVCs in WT and S2814D mice before and after Iso challenge. (C) Representative telemetric ECG tracings from WT and S2814D mice following injection of caffeine and epinephrine (Caf/Epi; 120 mg/kg and 2 mg/kg i.p., respectively). (D) Ventricular tachycardia (VT) was observed in the majority of S2814D mice following Caf/Epi. (E) ECG waveform showing baseline ECG tracing of a S2814D mouse in sinus rhythm (heart rate 431 beats per minute, bpm). Upon the injection of caffeine and epinephrine (caf/epi), heart rate increased to 480 bpm, and premature ventricular contractions (PVC) occurred starting at 5 minutes post caf/epi. At 10 minutes, episodes of non-sustained ventricular tachycardia (NSVT) started occurring (570 bpm), eventually deteriorating into an episode of sustained VT starting at 20 minutes (ventricular rate varying between 588–667 bpm). Immediately following this VT episode, the mouse developed progressive heart block followed by asystole and death at 25 min. N is indicated in bar graphs. The Mann-Whitney U test was used to compare absolute PVC incidence; Fisher’s exact test was used to evaluate VT incidence. *P<0.05, **P<0.01 versus WT.
Given that hearts of S2814D mice were structurally and electrically normal at rest, but exhibited ventricular ectopy upon β-adrenergic stimulation, we considered that the model phenotype might resemble catecholaminergic polymorphic ventricular tachycardia (CVPT). To further test S2814D mice for predisposition to ventricular arrhythmias under more stringent catecholaminergic conditions 5, 28, we injected caffeine and epinephrine (120 mg/kg and 2 mg/kg i.p., respectively). There was a significantly increased incidence of sustained ventricular tachycardia in S2814D mice (71%) compared to WT mice (13%, P = 0.04) (Fig. 4C-D). This is consistent with the presence of a pro-arrhythmogenic substrate caused by CaMKII pseudo-phosphorylation of RyR2. One S2814D mouse exhibited persistent ventricular tachycardia following caffeine and epinephrine that deteriorated into bradycardia and then asystole (Fig. 4E). Thus, CaMKII-mediated RyR2 phosphorylation promotes in vivo ventricular arrhythmias, and increases the risk of sudden cardiac death.
In order to further differentiate the role of CaMKII activation of RyR2 from the effects of PKA activation, we sought to examine the role of elevated heart rate on arrhythmogenesis by performing in vivo intracardiac electrophysiology studies. Programmed electrical stimulation using ventricular burst pacing was performed to compare cardiac susceptibility to ventricular ectopic activity in WT and S2814D mice. Burst pacing evoked sustained ventricular tachycardia in 53% of S2814D mice compared with 6% of WT mice (P = 0.006; Fig. 5A-B). Consistent with previous studies 19, rapid pacing induced CaMKII but not PKA phosphorylation of RyR2; whereas, the S2814D mutation inhibited CaMKII phosphorylation of RyR2 under paced and non-paced conditions (Fig. 5, S4A-B). In contrast, pacing increased phospholamban (PLN) phosphorylation at the CaMKII site T17 (Fig. 5E-F), but not the PKA site S16 (Fig. S4C-D) in both S2814D and WT mice, suggesting that enhanced SR Ca2+ loading due to SERCA2a stimulation facilitates VT induction in S2814D mice.
Finally, treatment with the β-adrenergic receptor blocker propranolol (3 mg/kg) did not significantly alter the incidence of ventricular arrhythmia induction (S2814D: 50%; WT: 0%) (Fig. 5B). Because sudden changes in heart rate may induce a reflex sympathetic response and change in blood pressure, we also performed control experiments in which the arterial blood pressure was continuously monitored while the right ventricle was paced from 500 to 800 bpm at 100 bpm intervals. The blood pressure at a pacing rate of 800 bpm was not significantly higher (3.1 ± 0.1%; P = 0.88) than at 500 bpm, thus excluding that a reflex sympathetic response due to blood pressure changes is evoked in mice receiving EP studies.
To test whether CaMKII targets other than RyR2 may contribute to the observed arrhythmogenesis, we crossed S2814D mice with AC3I transgenic mice, in which the CaMKII-inhibitory peptide AC3I reduces CaMKII activity in the heart 17. S2814D:AC3I double mutant mice experienced a slight decline in arrhythmia incidence (33% versus 53% in S2814D), suggesting that CaMKII effects on other targets, such as L-type Ca2+ current 29 or PLN might promote SR Ca2+ loading and arrhythmogenesis. However, when these experiments were repeated after injection of β-adrenergic receptor agonist isoproterenol (0.5 mg/kg, i.p.), the incidence of sustained VT was not reduced in S2814D-AC3I mice (66%) (Fig. 5B). These results suggest that PKA activation and phosphorylation of Ca2+ handling proteins (e.g. PLN, L-type Ca2+ channel and RyR2) - even in the absence of CaMKII activation - can also enhance SR Ca2+ loading and promote arrhythmias in S2814D mice.
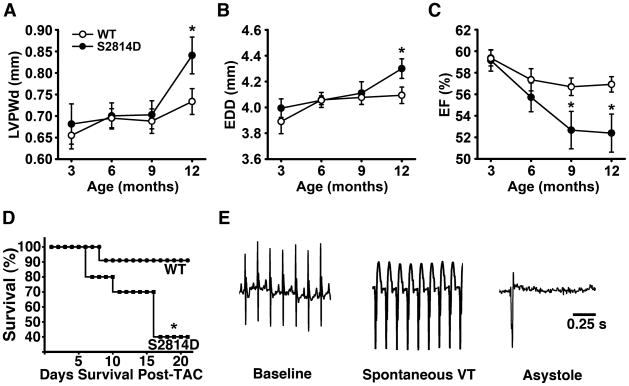
Previously, other groups have demonstrated that transgenic overexpression of CaMKII-δc induces heart failure in mice 12–15, and so we sought to define the specific role of CaMKII phosphorylation of RyR2 in progression to heart failure. As mentioned above, S2814D mice had no significant echocardiographic differences compared to WT littermates at 3 months of age (Table 1). However, at 12 months of age, S2814D mice demonstrated a significant increase in LV posterior wall diameter and end-diastolic diameter, and a small but significant decrease in ejection fraction (52.4 ± 1.8%) compared to WT mice (56.9 ± 0.7%; P = 0.005) (Fig. 6A-C, Table 1, P<0.05).
Figure 6. CaMKII phosphorylation of RyR2 causes cardiac dilation, loss of contractility, and early death from arrhythmias.
(A) Echocardiographic measurements of left ventricular posterior wall diameter (LVPWD), (B) end diastolic diameter (EDD), and (C) ejection fraction (EF) in S2814D and WT mice from 3 to 12 months. S2814D mice have significantly increased diastolic dimensions and reduction in EF versus WT mice (n = 14, both groups). (D) Kaplan-Meier survival curve in S2814D (n=10) and WT (n=11) mice 3 weeks post-TAC. (E) Representative ECG tracing of an arrhythmogenic death in a S2814D mouse that died post-TAC surgery day 21. Sudden death following ventricular tachycardia was only observed in S2814D mice, and in none of the WT mice after TAC. *P<0.05 vs. WT. Student’s t-test was used to compare echocardiographic data. A log-rank test was used to evaluate survival analysis.
Additionally, we performed transverse aortic constriction (TAC) in young (3–4 month old) S2814D and WT mice to evaluate the effects of constitutive CaMKII phosphorylation of RyR2 on the development of heart failure and arrhythmias. At 4 weeks post-TAC, CaMKII phosphorylation of RyR2 was not significantly elevated in WT mice (Fig. S5A-B). Moreover, phosphorylation of S2808 on RyR2 was not altered in WT and S2814D mice after TAC (Fig. S5C-D). However, survival was significantly lower for S2814D mice (40%) compared to WT mice (91%; P = 0.02) 3 weeks following TAC surgery (Fig. 6D). To determine whether the difference in survival was caused by arrhythmias, we repeated TAC studies in 5 WT and 5 S2814D mice in which a telemetric ECG transmitter was implanted 1 week prior to TAC. These studies revealed that the 2 S2814D mice in this group that died following TAC experienced episodes of ventricular arrhythmias immediately preceding death, whereas none of the WT mice died within 3 weeks after TAC (Fig. 6E). These results implicate CaMKII phosphorylation of RyR2 as an important factor contributing to arrhythmogenesis and sudden death in heart failure.
Next, we assessed whether prevention of CaMKII-mediated phosphorylation of RyR2 at S2814 could ameliorate ventricular arrhythmogenesis in mice with HF. To test this hypothesis, we used knock-in mice in which S2814 of RyR2 is replaced by alanine (S2814A) to genetically inhibit CaMKII phosphorylation of RyR2 5. Surgical TAC was performed in young (3–4 month old) WT and S2814A mice to induce HF. Following echocardiography at 8 weeks post-TAC, WT and S2814A mice were matched such that on average, both groups exhibited equal levels of cardiac dysfunction (see Table S4). Similar to patients with heart failure 30, WT mice subjected to TAC developed an increased propensity toward ventricular arrhythmias. Programmed electrical stimulation revealed that 75% (6 of 8) of WT mice developed non-sustained ventricular tachycardia following overdrive pacing at 8 weeks after TAC (Fig. 7A-B). In contrast, only 14% (1 of 7) of S2814A mice developed non-sustained VT (P = 0.04). Western blots using a phospho-epitope specific antibody revealed increased CaMKII phosphorylation of RyR2 in WT mice following TAC, whereas the S2814 phosphorylation site could not be phosphorylated in S2814A mice as expected (Fig. 7C-D). In contrast, phosphorylation of the PKA site S2808 was not altered in WT and S2814A mice compared to sham-operated controls (Fig. 7E-F). These results suggest that CaMKII phosphorylation of S2814 on RyR2 is an essential signaling event that promotes ventricular arrhythmias in TAC-induced heart failure. Taken together, our data in S2814D and S2814A mice demonstrate that CaMKII phosphorylation of RyR2 at this site is critical for the development of cardiac arrhythmia.
DISCUSSION
Previous work has demonstrated that increased CaMKII activity in failing hearts may contribute to abnormal Ca2+ handling, contractile failure, and arrhythmogenesis 9, 15. The novel knock-in mice, which express only the CaMKII phosphomimetic S2814D-mutant RyR2 or only non-phosphorylatable S2814A at this RyR2 site, have allowed unique tests concerning the importance of CaMKII phosphorylation of RyR2 at the cardiomyocyte and whole animal level. First, both biochemical and functional data (Fig. 1A-B) indicate that RyR2 phosphorylation at S2814 is the principal and possibly only site mediating CaMKII phosphorylation of RyR2 in mouse myocytes under physiological conditions. There may be other CaMKII target sites on RyR2 31, but they are neither obvious in our CaMKII-dependent 32P incorporation into S2814D mutant RyR2, nor at the functional level, as evidenced by Ca2+ release events. Additionally, CaMKII activation in WT myocytes did not produce stronger increase in the Ca2+ spark frequency than the S2814D myocytes exhibit at baseline. Also, in the S2814A myocytes the very small CaMKII-induced increase in CaSpF was explained by an enhanced SR Ca2+ load (presumably secondary to SR Ca2+-ATPase stimulation via CaMKII-dependent phospholamban phosphorylation). Note that a primary enhancement of RyR2 activity would decrease, rather than increase SR Ca2+ load.
A second major conclusion is that constitutive activation of the RyR2 CaMKII site increases the risk for ventricular arrhythmias in vivo, even in the absence of structural heart disease in young mice. Quantitative analysis of cardiac dimensions, fibrosis, and cardiac myocyte size revealed that structural remodeling is unlikely to be a significant cause of arrhythmogenesis in young S2814D mice. The elevated arrhythmia risk is also independent of altered binding of FKBP12.6, and can occur in the absence of increased PKA activity upon elevated HR. Interestingly, even though S2814D mice exhibit an increased incidence of Ca2+ sparks, ectopic activity was not observed under resting conditions. At baseline the myocytes may be effectively compensated by the combination of reduced SR Ca2+ load and increased fractional SR Ca2+ release during E-C coupling, such that the diastolic SR Ca2+ leak is insufficient to produce triggered arrhythmias unless SR Ca2+ load is driven toward WT levels, for example following phosphorylation of PLN.
Our findings have potentially important clinical implications with respect to arrhythmias in heart failure, because CaMKII expression, activity and phosphorylation of RyR2 at S2814, with resultant diastolic SR Ca2+ leak, are all enhanced in patients and animals with HF 9, 16. The level of S2814 phosphorylation is increased by about 50–100% in failing hearts as compared to non-failing control hearts 9, 16, 32, 33. Most of our studies were performed in homozygous S2814D to determine the physiological consequences of RyR2 phosphorylation. Whereas it is unlikely that S2814 on RyR2 will be maximally phosphorylated for prolonged amounts of time in patients or animals with heart failure, this model did enable us to elucidate the specific effects of RyR2 phosphorylation by CaMKII. Preliminary studies in heterozygous S2814D mice revealed arrhythmias incidences similar to homozygous S2814D (2 of 3 developed pacing-induced following isoproterenol administration), suggesting that our data may also be relevant as a model of RyR2 hyperphosphorylation seen in failing hearts.
Indeed, our findings suggest that CaMKII-dependent RyR2 phosphorylation may be a critical mediator of the high incidence of arrhythmias in human HF. Moreover, our data suggests that among the several CaMKII targets that have the potential to be pro-arrhythmic (Na+, Ca2+ and K+ channels), the RyR2 effects may predominate. The activating effect of CaMKII on RyR2 resembles the gain-of-function phenotype seen in inherited RyR2 mutations that are associated with CPVT. On the other hand, our data show that ventricular arrhythmias can be triggered in S2814D mice in the absence of increased PKA activation and PKA phosphorylation of RyR2 and PLN. Conversely, beta-adrenergic stimulation did increase the likelihood of arrhythmogenesis, possibly due to enhanced Ca2+ entry with increased SR Ca2+ loading via SERCA2a/PLN. Based on prior studies 34, enhanced SR Ca2+ release via RyR2 is likely sufficient to trigger Ca2+ waves and depolarizing transient inward current via NCX, although this remains to be confirmed in S2814D mice in future studies.
A third conclusion is that when RyR2 S2814 cannot be phosphorylated (S2814A), arrhythmias associated with the development of HF are prevented. This extends the above conclusion in an important way. That is, in addition to being pro-arrhythmogenic in normal and failing hearts, CaMKII-dependent phosphorylation of RyR2-S2814 may be an essential component for the triggered arrhythmias in HF, at least in the context of pressure overload- induced HF as examined here. This may extend to recent observations that CaMKIIδ knockout mice exhibit reduced transition to HF (and reduced RyR2-S2814 phosphorylation) during similar pressure overload-induced HF 16. The dramatic increase in diastolic SR Ca2+ leak (and Ca2+ spark frequency) in CaMKIIδ transgenic mice reduces SR Ca2+ load severely enough to depress systolic dysfunction, despite enhanced fractional SR Ca2+ release 12. Thus, CaMKII-dependent phosphorylation of RyR2-S2814 may be critical in both arrhythmogenesis and systolic dysfunction in HF.
Prior reports suggested that PKA-dependent hyperphosphorylation of RyR2 at S2808 and consequent FKBP12.6 dissociation are causative in the enhanced SR Ca2+ leak and arrhythmias in HF 35, 36, although key aspects of these observations remain highly controversial 37. Our results here do not resolve this controversy, although in our TAC model RyR2 phosphorylation at the CaMKII site and not the PKA site was increased. Moreover, it is clear that CaMKII-dependent phosphorylation of RyR2 (or pseudo-phosphorylation) at S2814 which occurs in HF can strongly activate RyR2 in myocytes and increase the propensity for arrhythmias and may even accelerate the transition from hypertrophy to HF 21.
It has been shown that inhibition of calmodulin (CaM)-binding to RyR2 by selective mutations in the binding site leads to a severe phenotype characterized by early-onset cardiomyopathy and postnatal death between days 9 and 16 38. Because CaM is thought to inhibit RyR2, disrupting the CaM binding site on RyR2 may lead to increased Ca2+ release. However, the functional effects of disrupting CaM binding appear to be more severe compared to increasing Ca2+ release by constitutive CaMKII phosphorylation (RyR2-ADA mice are lethal before weaning, RyR2-S2814D mice appear to have a normal lifespan). Thus, SR Ca2+ leak through RyR2-S2814D mice was sufficient to promote ventricular arrhythmias but not severe enough to cause early-onset cardiomyopathy due to activation of Ca2+-dependent hypertrophic signaling pathways.
In conclusion, our data suggest that increased CaMKII phosphorylation of RyR2 Ca2+ release channels at S2814 promotes the development of ventricular arrhythmias in mice even in the absence of structural heart disease. Our finding may have clinical implications with respect to arrhythmias in patients with congestive heart failure, as the activity of CaMKII is chronically enhanced in failing hearts. We have also demonstrated that inhibition of CaMKII phosphorylation of RyR2 can prevent ventricular arrhythmias in mice with heart failure. Thus, the effects of CaMKII on RyR2 may be a critical factor in the generation of arrhythmias in patients with heart failure, although this remains to be studied in humans. Our findings might lead to the development of more specific therapies that could modify the level of CaMKII phosphorylation of RyR2, thus reduce diastolic Ca2+ leak, and ultimately death from arrhythmias. Such novel anti-arrhythmic agents, once tested in randomized trials, may ultimately provide new avenues for treating ventricular arrhythmias and preventing sudden cardiac death in patients with heart failure.
Supplementary Material
Acknowledgments
The authors would like to thank Pumin Zhang, Ph.D., and Priyanka Desai for their assistance with generation of the knock-in mouse models, Satyam Sarma, M.D. for technical assistance, and Sameer Ather, M.D. for statistical advice.
FUNDING SOURCES
X.H.T.W. is a W.M. Keck Foundation Distinguished Young Scholar in Medical Research, and is supported by NIH/NHLBI grants R01-HL089598 and R01-HL091947, and Muscular Dystrophy Association grant #69238. M.D.M. is supported by NIH Training Grant 5T32HL066991-07. R.J.v.O. was the recipient of the 2008–2010 American Physiological Society Postdoctoral Fellowship in Physiological Genomics. D.M.B. is supported by NIH grant P01-HL80101. MEA is supported by R01 HL079031, R01 HL070250, and R01 HL096652. This project was supported by the Fondation Leducq Award to the ‘Alliance for Calmodulin Kinase Signaling in Heart Disease’ (M.E.A., D.M.B. and X.H.T.W.).
Footnotes
CONFLICT OF INTEREST DISCLOSURES: M.E.A. is a named inventor on several patents that claim to treat arrhythmias by CaMKII inhibition.
References
- 1.Farr MA, Basson CT. Sparking the failing heart. N Engl J Med. 2004;351:185–187. doi: 10.1056/NEJMcibr041466. [DOI] [PubMed] [Google Scholar]
- 2.Noseworthy PA, Newton-Cheh C. Genetic determinants of sudden cardiac death. Circulation. 2008;118:1854–1863. doi: 10.1161/CIRCULATIONAHA.108.783654. [DOI] [PubMed] [Google Scholar]
- 3.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 4.Pogwizd SM, Bers DM. Cellular basis of triggered arrhythmias in heart failure. Trends Cardiovasc Med. 2004;14:61–66. doi: 10.1016/j.tcm.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, Li N, Santonastasi M, Muller FU, Schmitz W, Schotten U, Anderson ME, Valderrabano M, Dobrev D, Wehrens XH. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest. 2009;119:1940–1951. doi: 10.1172/JCI37059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kannankeril PJ, Mitchell BM, Goonasekera SA, Chelu MG, Zhang W, Sood S, Kearney DL, Danila CI, De Biasi M, Wehrens XH, Pautler RG, Roden DM, Taffet GE, Dirksen RT, Anderson ME, Hamilton SL. Mice with the R176Q cardiac ryanodine receptor mutation exhibit catecholamine-induced ventricular tachycardia and cardiomyopathy. Proc Natl Acad Sci U S A. 2006;103:12179–12184. doi: 10.1073/pnas.0600268103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): Defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 8.Wehrens XH, Lehnart SE, Huang F, Vest JA, Reiken SR, Mohler PJ, Sun J, Guatimosim S, Song LS, Rosemblit N, D’Armiento JM, Napolitano C, Memmi M, Priori SG, Lederer WJ, Marks AR. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell. 2003;113:829–840. doi: 10.1016/s0092-8674(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 9.Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum ca2+ leak in heart failure. Circ Res. 2005;97:1314–1322. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- 10.Curran J, Hinton MJ, Rios E, Bers DM, Shannon TR. Beta-adrenergic enhancement of sarcoplasmic reticulum calcium leak in cardiac myocytes is mediated by calcium/calmodulin-dependent protein kinase. Circ Res. 2007;100:391–398. doi: 10.1161/01.RES.0000258172.74570.e6. [DOI] [PubMed] [Google Scholar]
- 11.Bers DM, Grandi E. Calcium/calmodulin-dependent kinase II regulation of cardiac ion channels. J Cardiovasc Pharmacol. 2009;54:180–187. doi: 10.1097/FJC.0b013e3181a25078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maier LS, Zhang T, Chen L, DeSantiago J, Brown JH, Bers DM. Transgenic CaMKII-delta C overexpression uniquely alters cardiac myocyte Ca2+ handling: Reduced SR Ca2+ load and activated SR Ca2+ release. Circ Res. 2003;92:904–911. doi: 10.1161/01.RES.0000069685.20258.F1. [DOI] [PubMed] [Google Scholar]
- 13.Wagner S, Dybkova N, Rasenack EC, Jacobshagen C, Fabritz L, Kirchhof P, Maier SK, Zhang T, Hasenfuss G, Brown JH, Bers DM, Maier LS. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J Clin Invest. 2006;116:3127–3138. doi: 10.1172/JCI26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Y, Temple J, Zhang R, Dzhura I, Zhang W, Trimble R, Roden DM, Passier R, Olson EN, Colbran RJ, Anderson ME. Calmodulin kinase II and arrhythmias in a mouse model of cardiac hypertrophy. Circulation. 2002;106:1288–1293. doi: 10.1161/01.cir.0000027583.73268.e7. [DOI] [PubMed] [Google Scholar]
- 15.Zhang T, Maier LS, Dalton ND, Miyamoto S, Ross J, Jr, Bers DM, Brown JH. The delta C isoform of CaMKII is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure. Circ Res. 2003;92:912–919. doi: 10.1161/01.RES.0000069686.31472.C5. [DOI] [PubMed] [Google Scholar]
- 16.Ling H, Zhang T, Pereira L, Means CK, Cheng H, Gu Y, Dalton ND, Peterson KL, Chen J, Bers D, Heller Brown J. Requirement for Ca2+/calmodulin-dependent kinase II in the transition from pressure overload-induced cardiac hypertrophy to heart failure in mice. J Clin Invest. 2009;119:1230–1240. doi: 10.1172/JCI38022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang R, Khoo MS, Wu Y, Yang Y, Grueter CE, Ni G, Price EE, Jr, Thiel W, Guatimosim S, Song LS, Madu EC, Shah AN, Vishnivetskaya TA, Atkinson JB, Gurevich VV, Salama G, Lederer WJ, Colbran RJ, Anderson ME. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med. 2005;11:409–417. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]
- 18.Sood S, Chelu MG, van Oort RJ, Skapura D, Santonastasi M, Dobrev D, Wehrens XH. Intracellular calcium leak due to FKBP12.6 deficiency in mice facilitates the inducibility of atrial fibrillation. Heart Rhythm. 2008;5:1047–1054. doi: 10.1016/j.hrthm.2008.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wehrens XH, Lehnart SE, Reiken SR, Marks AR. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ Res. 2004;94:e61–70. doi: 10.1161/01.RES.0000125626.33738.E2. [DOI] [PubMed] [Google Scholar]
- 20.Respress JL, Wehrens XH. Transthoracic echocardiography in mice. J Vis Exp. 2010;28:1738. doi: 10.3791/1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Oort RJ, Respress JL, Li N, Reynolds C, De Almeida AC, Skapura DG, De Windt LJ, Wehrens XH. Accelerated development of pressure overload-induced cardiac hypertrophy and dysfunction in an RyR2-R176Q knockin mouse model. Hypertension. 2010;55:932–938. doi: 10.1161/HYPERTENSIONAHA.109.146449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCauley MD, Wehrens XH. Ambulatory ECG recording in mice. J Vis Exp. 2010;39:1739. doi: 10.3791/1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li N, Wehrens XH. Programmed electrical stimulation in mice. J Vis Exp. 2010;39:1730. doi: 10.3791/1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.deAlmeida AC, van Oort RJ, Wehrens XH. Transverse aortic constriction in mice. J Vis Exp. 2010;38:1729. doi: 10.3791/1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.el Azzouzi H, van Oort RJ, van der Nagel R, Sluiter W, Bergmann MW, De Windt LJ. Mef2 transcriptional activity maintains mitochondrial adaptation in cardiac pressure overload. Eur J Heart Fail. 2010;12:4–12. doi: 10.1093/eurjhf/hfp165. [DOI] [PubMed] [Google Scholar]
- 26.Wehrens XH, Lehnart SE, Marks AR. Ryanodine receptor-targeted anti-arrhythmic therapy. Ann N Y Acad Sci. 2005;1047:366–375. doi: 10.1196/annals.1341.032. [DOI] [PubMed] [Google Scholar]
- 27.Li L, Satoh H, Ginsburg KS, Bers DM. The effect of Ca2+-calmodulin-dependent protein kinase II on cardiac excitation-contraction coupling in ferret ventricular myocytes. J Physiol. 1997;501:17–31. doi: 10.1111/j.1469-7793.1997.017bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cerrone M, Colombi B, Santoro M, di Barletta MR, Scelsi M, Villani L, Napolitano C, Priori SG. Bidirectional ventricular tachycardia and fibrillation elicited in a knock-in mouse model carrier of a mutation in the cardiac ryanodine receptor. Circ Res. 2005;96:e77–82. doi: 10.1161/01.RES.0000169067.51055.72. [DOI] [PubMed] [Google Scholar]
- 29.Adebanjo OA, Anandatheerthavarada HK, Koval AP, Moonga BS, Biswas G, Sun L, Sodam BR, Bevis PJ, Huang CL, Epstein S, Lai FA, Avadhani NG, Zaidi M. A new function for CD38/ADP-Ribosyl cyclase in nuclear Ca2+ homeostasis. Nat Cell Biol. 1999;1:409–414. doi: 10.1038/15640. [DOI] [PubMed] [Google Scholar]
- 30.Cleland JG, Chattopadhyay S, Khand A, Houghton T, Kaye GC. Prevalence and incidence of arrhythmias and sudden death in heart failure. Heart Fail Rev. 2002;7:229–242. doi: 10.1023/a:1020024122726. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez P, Bhogal MS, Colyer J. Stoichiometric phosphorylation of cardiac ryanodine receptor on serine 2809 by calmodulin-dependent kinase II and protein kinase A. J Biol Chem. 2003;278:38593–38600. doi: 10.1074/jbc.C301180200. [DOI] [PubMed] [Google Scholar]
- 32.Sossalla S, Fluschnik N, Schotola H, Ort KR, Neef S, Schulte T, Wittkopper K, Renner A, Schmitto JD, Gummert J, El-Armouche A, Hasenfuss G, Maier LS. Inhibition of elevated Ca2+/calmodulin-dependent protein kinase II improves contractility in human failing myocardium. Circ Res. 2010 doi: 10.1161/CIRCRESAHA.110.220418. In press. [DOI] [PubMed] [Google Scholar]
- 33.Chelu MG, Wehrens XH. Sarcoplasmic reticulum calcium leak and cardiac arrhythmias. Biochem Soc Trans. 2007;35:952–956. doi: 10.1042/BST0350952. [DOI] [PubMed] [Google Scholar]
- 34.Lehnart SE, Terrenoire C, Reiken S, Wehrens XH, Song LS, Tillman EJ, Mancarella S, Coromilas J, Lederer WJ, Kass RS, Marks AR. Stabilization of cardiac ryanodine receptor prevents intracellular calcium leak and arrhythmias. Proc Natl Acad Sci U S A. 2006;103:7906–7910. doi: 10.1073/pnas.0602133103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wehrens XH, Marks AR. Altered function and regulation of cardiac ryanodine receptors in cardiac disease. Trends Biochem Sci. 2003;28:671–678. doi: 10.1016/j.tibs.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Wehrens XH, Lehnart SE, Reiken SR, Deng SX, Vest JA, Cervantes D, Coromilas J, Landry DW, Marks AR. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science. 2004;304:292–296. doi: 10.1126/science.1094301. [DOI] [PubMed] [Google Scholar]
- 37.Guo T, Zhang T, Mestril R, Bers DM. Ca2+/calmodulin-dependent protein kinase II phosphorylation of ryanodine receptor does affect calcium sparks in mouse ventricular myocytes. Circ Res. 2006;99:398–406. doi: 10.1161/01.RES.0000236756.06252.13. [DOI] [PubMed] [Google Scholar]
- 38.Yamaguchi N, Takahashi N, Xu L, Smithies O, Meissner G. Early cardiac hypertrophy in mice with impaired calmodulin regulation of cardiac muscle ca release channel. J Clin Invest. 2007;117:1344–1353. doi: 10.1172/JCI29515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.