Abstract
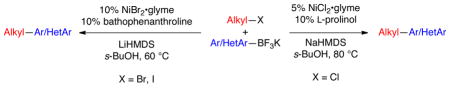
A method for the cross-coupling of alkyl electrophiles with various potassium aryl- and heteroaryltrifluoroborates has been developed. Nearly stoichiometric amounts of organoboron species could be employed to cross-couple a large variety of challenging heteroarylnucleophiles. Several functional groups were tolerated on both the electrophilic and the nucleophilic partners. Chemoselective reactivity of C(sp3)-Br bonds in the presence of C(sp2)-Br bonds was achieved.
Several recent studies have focused on the development of cross-coupling strategies to unite alkyl electrophiles with aryl nucleophilic partners.1 Among these, the Suzuki–Miyaura reaction has emerged as one of the most powerful methods because of the low toxicity, air- and water stability, functional group compatibility and commercial availability of the organoboron compounds.2,3 Nickel catalysts were reported to be among the most successful for C(sp2)–C(sp3) bond-formation via the Suzuki–Miyaura reaction.1 Nevertheless, limitations to the developed method remain:4–6 depending on the nucleophile, a significant excess of boronic acid is usually required, and ortho-substituted arylboronic acids only cross-couple to a limited extent. Perhaps most critically, only a few isolated examples have been reported to partner alkyl halides with heteroarylboron nucleophiles (e.g., indole-5-boronic acid, thiophene-3-boronic acid), and virtually all protocols explicitly have failed for other important heterocyclic systems.5
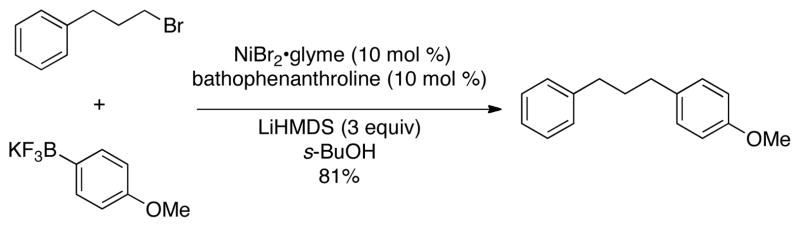
We envisioned that these limitations could be overcome through the use of the more robust potassium aryl- and heteroaryltrifluoroborates as nucleophilic coupling partners in the Ni-catalyzed cross-coupling with various alkyl electrophiles.7 Using the reaction between 1-bromo-3-phenylpropane and a stoichiometric amount of potassium (4-methoxyphenyl)-trifluoroborate (eq 1) as a template, a wide variety of catalyst/ligand combinations, solvents, bases and temperatures were screened.8 The best coupling conditions were determined to be 10 mol % of NiBr2·glyme, 10 mol % of 4,7-diphenyl-1,10-phenanthroline (bathophenanthroline) and 3 equivalents of LiHMDS in sec-butanol.8
With these results in hand, we investigated the cross-coupling of 2-(bromomethyl)tetrahydro-2H-pyran with different potassium aryltrifluoroborates (Table 1).
Table 1.
Cross-Coupling of 2-(Bromomethyl)tetrahydro-2H-pyran with Potassium Aryltrifluoroborates
 | |||||
|---|---|---|---|---|---|
| entry | R-BF3K | yield % | entry | R-BF3K | yield % |
| 1 |  |
R = H, 86 R = OMe, 72 |
6 |  |
68 |
| 2 | R = o-Me, 76 R = m-Me, 81(76)b R = p-Me, 70 |
7 |  |
81 | |
| 3 |  |
64 | |||
| 4 | 71 | 8a |  |
66 | |
| 5 | 53 | 9a |  |
68 | |
Reactions run at 80 °C.
Reaction performed on 5 mmol scale using 1 mol % catalyst loading.
Both electron rich and electron poor substituted trifluoroborates successfully underwent cross-coupling using nearly stoichiometric amounts of the nucleophile (1.02 equiv). Sterically hindered ortho-substituted trifluoroborates also underwent the reaction in good yields (Table 1, entries 2 and 9). Additionally, substrates containing a secondary alcohol (entry 7), a ketone (entries 8 and 9), as well as an alkene (entry 5) were tolerated. Importantly, the reactions could be performed on gram scale using only 1 mol % catalyst loading with little effect on the yield (entry 2).
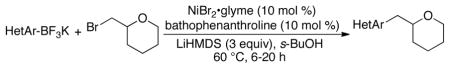
To expand the scope of this method, we examined the cross-coupling of the same alkyl bromide with a large variety of heteroaryltrifluoroborates including furans, benzo- and dibenzofuran, pyridines, pyrimidines, thiophenes, quinolines, indole and imidazole, again using virtually stoichiometric organotrifluoroborates (Table 2).
Table 2.
Cross-Coupling of 2-(Bromomethyl)tetrahydro-2H-pyran with Potassium Heteroaryltrifluoroborates
 | |||||
|---|---|---|---|---|---|
| entry | R-BF3K | yield % | entry | R-BF3K | yield % |
| 1 | X = O, 71 X = S, 63 |
7 |  |
78 | |
| 2 |  |
66 | 8 |  |
82 |
| 3 | 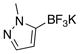 |
35 | 9 |  |
70 |
| 4 | X = O, 68 X = S, 81(74)a |
10 | 71 | ||
| 11 | 59 | ||||
| 5 |  |
X = O, 80 X = S, 58 |
12 |  |
51 |
| 6 |  |
R = H, 77 R = OMe, 71 |
13 | 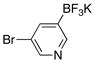 |
63 |
Reaction performed on 5 mmol scale using 1 mol % catalyst loading.
The corresponding cross-coupled products were obtained in moderate to good yields. Given the extraordinarily high propensity for heteroarylboron reagents to undergo protodeboronation,9 these transformations represent a significant advance in the formation of core substructures of greatest interest in the pharmaceutical, agrochemical, and materials science sectors. As in the case of the aryltrifluoroborates, a heteroaryl cross-coupling could be carried out on gram scale using only 1 mol % of the nickel catalyst (entry 4). Of additional interest, the C(sp2)–Br bond in 5-bromo-3-pyridyltrifluroborate remained intact during the cross-coupling conditions, maintaining the potential for further functionalization of the pyridine (entry 13).
To test the general applicability of our method to other electrophiles, potassium 2-benzofuranyl- and 4-pyridinyl-trifluoroborate were reacted with various functionalized alkyl halides (Table 3).
Table 3.
Cross-Coupling of Diverse Alkyl Halides with Potassium 2-Benzofuranyl- and 4-Pyridinyltrifluoroborates
 | |||||
|---|---|---|---|---|---|
| entry | Alkyl-X | yield % | entry | Alkyl-X | yield % |
| 1 |
1, 79 2, 81 |
7 |
1, 70 2, 61 |
||
| 2 | 2, 61 | 8 |
1, 84 2, 63 |
||
| 3 |
1, 80 2, 76 |
9 |
1, 60 2, 71 |
||
| 4 |
1, 76 2, 78 |
10 |
1, 67 2, 68 |
||
| 5 | 2, 72a | 11 |
1, 71 2, 69 |
||
| 6 |
1, 63 2, 68 |
12 |
1, 62 2, 63 |
||
Isolated as the s-Bu ester.
Electrophiles containing an acetal, a benzyl ether and a distal olefin were tolerated (Table 3). p-Bromo-(3-bromopropyl)-benzene smoothly underwent chemo-selective cross-coupling at the C(sp3)–Br bond, leaving the C(sp2)–Br bond available for further functionalization (entry 2). Another bidirectional functionalization opportunity arises from the selective reactivity of alkyl bromides and iodides over alkyl chlorides (entries 7 and 8). The same set of conditions could be applied to the successful cross-coupling of secondary bromides and iodides (entries 9–12). Although stereochemical studies have not been conducted, on the basis of previously reported investigations5 we would anticipate that these processes would transpire via radical intermediates, and thereby the stereochemical fidelity of enantioenriched starting materials would not be transferred to the products.
The conditions employed for the cross-coupling of alkyl iodides and bromides were not successful when applied to the more challenging alkyl chlorides. As previously reported in the literature,5 the use of L-prolinol as a ligand was required for the reaction of alkyl chlorides (Table 4). Although the ortho-substituted boronic acids have been problematic in the Ni-catalyzed cross-coupling with alkyl chlorides,5 use of the more stable potassium trifluoroborates led to the formation of the desired product in 60% yield (entry 5).10
Table 4.
Cross-Coupling of Various Alkyl Chlorides with Potassium Aryl- and Heteroaryltrifluoroborates
 | |||
|---|---|---|---|
| entry | Alkyl-Cl | Ar/HetAr | yield % |
| 1 | 60 | ||
| 2 | 54 | ||
| 3 |  |
47 | |
| 4 |  |
42 | |
| 5 |  |
60 | |
| 6 |  |
49a | |
Reaction using KHMDS (3 equiv)
In conclusion, we have developed an efficient method for the Ni-catalyzed cross-coupling of unactivated halides. Bathophenanthroline was used as a ligand for the Suzuki–Miyaura cross-coupling of alkyl bromides and iodides with aryl- and heteroaryl nucleophiles, while L-prolinol was required for the cross-coupling of alkyl chlorides. Several major advances have derived from these studies. 1) only 1 equiv of the organoboron reagent is necessary, as opposed to 1.2 to 2 equiv reported in previous studies; 2) both heteroaryl- and sterically hindered ortho-substituted organoborons, previously reported to be problematic substrates, can be coupled in good yields; and 3) on larger scale, low (1 mol %) catalyst loadings can be used to effect efficient cross-coupling. The use of organotrifluoroborates thus represents a significant advance in cross-coupling with alkyl halide electrophiles.
Supplementary Material
Scheme 1.
Optimization of the cross-coupling conditions
Acknowledgments
We thank Frontier Scientific for boronic acids. Deidre L. Sandrock (University of Pennsylvania) is acknowledged for performing HTE experiments. Financial support has been provided by the NIH General Medical Sciences (R01 GM035249) and the NSF GOALI program (CHE-0848460). IA thanks CURF (University of Pennsylvania) for a fellowship. Dr. Rakesh Kohli (University of Pennsylvania) is acknowledged for obtaining HRMS data.
Footnotes
Supporting Information Available: Experimental procedures and compound characterization data (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Rudolph A, Lautens M. Angew Chem Int Ed. 2009;48:2656–2670. doi: 10.1002/anie.200803611. and references cited therein; [DOI] [PubMed] [Google Scholar]; Ejiri S, Odo S, Takahashi H, Nishimura Y, Gotoh K, Nishihara Y, Takagi K. Org Lett. 2010;12:1692–1695. doi: 10.1021/ol100210u. [DOI] [PubMed] [Google Scholar]; Hatakeyama T, Hashimoto T, Kondo Y, Fujiwara Y, Seike H, Takaya H, Tamada Y, Ono T, Nakamura M. J Am Chem Soc. 2010;132:10674–10676. doi: 10.1021/ja103973a. [DOI] [PubMed] [Google Scholar]; Owson NA, Fu GC. J Am Chem Soc. 2010;132:11908–11909. doi: 10.1021/ja105924f. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lundin PM, Fu GC. J Am Chem Soc. 2010;132:11027–11029. doi: 10.1021/ja105148g. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lou S, Fu GC. J Am Chem Soc. 2010;132:1264–1266. doi: 10.1021/ja909689t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.For reviews see: Miyaura N, Suzuki A. Chem Rev. 1995;95:2457–2483.Little AF, Fu GC. Angew Chem, Int Ed. 2002;41:4177–4211.
- 3.For studies and examples of palladium-catalyzed cross-coupling reactions of alkyl electrophiles see: Ariafard A, Lin Z. Organometallics. 2006;25:4030–4033.Zhou J, Fu GC. J Am Chem Soc. 2003;125:12527–12530. doi: 10.1021/ja0363258.Kirchhoff JH, Netherton MR, Hills ID, Fu GC. J Am Chem Soc. 2002;124:13662–13663. doi: 10.1021/ja0283899.He A, Falck JR. J Am Chem Soc. 2010;132:2524–2525. doi: 10.1021/ja910582n.
- 4.Zhou J, Fu GC. J Am Chem Soc. 2004;126:1340–1341. doi: 10.1021/ja039889k. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez-Bobes F, Fu GC. J Am Chem Soc. 2006;128:5360–5361. doi: 10.1021/ja0613761. [DOI] [PubMed] [Google Scholar]
- 6.Duncton MAJ, Estiarte MA, Tan D, Kaub C, O’Mahony DJR, Johnson RJ, Cox M, Edwards WT, Wan M, Kincaid J, Kelly MG. Org Lett. 2008;10:3259–3262. doi: 10.1021/ol8011327. [DOI] [PubMed] [Google Scholar]
- 7.For reviews on potassium organotrifluoroborates in cross-coupling reactions see: Molander GA, Ellis NM. Acc Chem Res. 2007;40:275–286. doi: 10.1021/ar050199q.Darses S, Genet JP. Chem Rev. 2008;108:288–325. doi: 10.1021/cr0509758.Molander GA, Canturk B. Angew Chem, Int Ed. 2009;48:9240–9286. doi: 10.1002/anie.200904306.Stefani HA, Cella R, Vieira AS. Tetrahedron. 2007;63:3623–3658.
- 8.The screening was performed through microscale High Throughput Experimentation (HTE). Dreher SD, Dormer PG, Sandrock DL, Molander GA. J Am Chem Soc. 2008;130:9257–9259. doi: 10.1021/ja8031423.See Supporting Information.
- 9.(a) Molander GA, Canturk B, Kennedy LE. J Org Chem. 2009;74:973–980. doi: 10.1021/jo802590b. and references therein. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Knapp DM, Gillis EP, Burke MD. J Am Chem Soc. 2009;131:6961–6963. doi: 10.1021/ja901416p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Under these conditions, reactions of other heteroaryl-trifluoroborates (furans, thiophenes, pyrazoles) proceeded in relatively low yield (<40%, data not shown). The cross-coupling of alkyl chlorides with aryl and heteroaryltrifluoroborates is currently under study.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.