Abstract
In recent years a major research effort has focused on the role of inflammation, and in particular adaptive immunity, in the genesis of hypertension. Hypertension stimulates the accumulation of inflammatory cells including macrophages and T lymphocytes in peripheral tissues important in blood pressure control, such as the kidney and vasculature. Angiotensin II modulates blood pressure via actions on the central nervous system (CNS) and the adaptive immune system. Recent work suggests that the central actions of angiotensin II via the circumventricular organs lead to activation of circulating T-cells and vascular inflammation. The neuroimmune system plays an essential role in the pathogenesis of hypertension and further understanding of this relationship could lead to the development of new treatment strategies.
Introduction
Despite extensive study, the etiology of most cases of human hypertension continues to be heavily debated. Blood pressure regulation is a complex process involving renal, vascular and central mechanisms. The brain is essential for processing and integrating neurohumoral signals from the periphery to maintain pressure and fluid homeostasis. Several distinct nuclei in the forebrain, hypothalamus and brainstem contribute to regulation of blood pressure and perturbations of these central sites contribute to the etiology of hypertension [1–3]. For example, human and experimental animal models of hypertension exhibit autonomic dysfunction, elevated sympathetic nerve activity (SNA) and altered baroreceptor sensitivity [4]. These neurogenic mechanisms are influenced by humoral factors such as angiotensin II and mineralcorticoids and by environmental factors such as stress and high salt intake. There is, however, emerging evidence that inflammation, and in particular adaptive immunity, contributes to hypertension. This raises the question of whether there is any link between the central regulation of blood pressure and adaptive immunity in the genesis of hypertension. Considering that the central nervous system (CNS) exerts powerful influences on the immune system and vice versa, it is plausible that CNS actions of factors such as angiotensin II, might enhance adaptive immune responses that lead to hypertension. This review provides a brief overview of the neuroimmune system, the central regulation of blood pressure and how the neuroimmune link contributes to the etiology of hypertension.
Interactions of the neuroimmune system and immunity
The bi-directional communication between the central nervous system (CNS) and the immune system are well known [5]. The sympathetic nervous system (SNS) and hypothalamic pituitary axis (HPA) are two major pathways that modulate this communication. The SNS innervates both primary (thymus, bone marrow) and secondary (spleen, lymph nodes, Peyer’s patches) lymphoid tissues while most immune cells express receptors for neurohormones (i.e. glucocorticoids and angiotensin II) and for catecholamines (i.e. norepinephrine)[6]. Depending on immune cell type and level of activation, factors released from the HPA and SNS can have immunosuppressive and immuno-enhancing effects. Immunosuppressive effects of glucocorticoids, the end product of HPA axis activation, are well documented [7]. On the other hand, norepinephrine released from sympathetic nerve terminals can both enhance and inhibit adaptive and innate immune cells [8]. Thus, the SNS and HPA can regulate the magnitude of innate or adaptive immune response in multiple ways. In reciprocal, the immune system also regulates the CNS [9, 10]. Pro-inflammatory cytokines produced in the periphery can feedback to the brain, passing through the blood brain barrier at leaky points such as the organum vasculosum lamina terminalis (OVLT) or median eminence [11]. Interestingly, these brain regions, referred to as circumventricular organs (CVOs), are also essential to blood pressure and fluid homeostasis. In addition, cytokines can be produced locally in the brain by glia and neurons and contribute to a neuroinflammatory response implicated in neurodegenerative diseases and more recently identified in hypertension [12–14]. As proposed by Paton and colleagues [15], increased circulating inflammatory cells and cytokines in the brain can impair central blood pressure regulation and promote hypertension. While CNS-immune interactions have been extensively studied in the setting of autoimmune and psychiatric disorders, the role of the neuroimmune system in hypertension is far less understood.
The Central control of blood pressure, the renin angiotensin system (RAS) and inflammation
The neurohumoral effects of the RAS significantly contribute to the central regulation of blood pressure. Components of the RAS, including renin, angiotensinogen and angiotensin type 1 (AT1a) receptors are present in various brain regions and cell types [16–18]. The importance of the RAS and locally derived angiotensin II in the brain is highlighted by numerous studies where brain manipulations, such as lesioning of specific regions [19] and over expression of various components of the RAS in the brain [20] produce long term changes in blood pressure [3, 21]. In addition to brain-derived angiotensin II, blood-borne angiotensin peptides can enter the brain and modulate blood pressure and fluid homeostasis. Circulating angiotensin II accesses the CNS via the CVOs, which are hypothalamic regions around the third and fourth ventricles that have a weak blood brain barrier and contain a high density of angiotensin type 1 (AT1a) receptors. These regions include the OVLT, the area postrema (AP) and subfornical organ (SFO)[22], and are essential to the regulation of blood pressure and fluid balance [23]. In particular, the anteroventral third ventricle (AV3V) region, which encompasses periventricular neural structures including the OVLT and portions of the preoptic nucleus [22], has been well documented to play a role in various forms of hypertension [24]. The AV3V region receives input from the SFO and sends efferent projections to key cardiovascular regulatory brainstem sites such as the nucleus of the solitary tract (NTS) and rostral ventrolateral medulla (RVLM). Electrolytic lesions that disrupt the AV3V region have been shown to virtually abolish all of the central actions of angiotensin II including drinking behavior, sympathetic outflow, vasopressin release as well as preventing and/or reversing several forms of experimental hypertension [22, 24].
Angiotensin II not only has important central effects in regulation of blood pressure, but also contributes to key events in inflammation [25]. For example, AT1a receptor blockade or anti-inflammatory treatment can lower blood pressure and reverse renal and vascular accumulation of inflammatory cells [26]. Moreover, angiotensin II dependent hypertension increases production of pro-inflammatory cytokines within specific brain regions involved in blood pressure control [2]. In addition, Zhang et al., recently reported that angiotensin II increases permeability of the blood brain barrier and cerebral microvasculature inflammation [27]. Interestingly, other forms of experimental hypertension also promote inflammation in the brainstem microvasculature [15]. Overall, these studies clearly demonstrate that inflammation in the blood vessels, kidneys and CNS can contribute to the development of hypertension. In an effort to better understand inflammation in hypertension, our laboratory has performed several recent studies examining the adaptive immune system in the pathophysiology of hypertension [28–33].
Adaptive immunity in hypertension
In 2007, Guzik et al., found that mice lacking T-lymphocytes are resistant to the development of both angiotensin II and DOCA-salt induced hypertension [28]. Adoptive transfer of T, but not B cells restored hypertension in these animals. More recently, Crowley et al., showed that SCID mice, which lack lymphocyte responses, have a blunted blood pressure response and reduced sodium retention during angiotensin II-dependent hypertension [34]. In addition, subsequent studies have extended these findings and provide further evidence of a role for T lymphocytes in salt-sensitive and genetic forms of hypertension [35–37]. Despite the growing evidence for the role of T cells in hypertension, the mechanisms underlying T cell activation and T cell infiltration into the vasculature and kidney and these effects on blood pressure remain unclear.
The relationship between angiotensin II and increased sympathetic nerve activity in hypertension are well documented [38, 39]. The SNS, as discussed above, can also impact the adaptive immune response in several ways. For example, T cell rich organs such as the spleen and lymph nodes are highly innervated with sympathetic nerves [40, 41]. Most immune cells possess adrenergic receptors, and T and B cells almost exclusively express the β2 subtype [42]. During increased sympathetic activation, norepinephrine released from the sympathetic nerve terminal has been shown to both inhibit and stimulate T cell activation and proliferation [43]. For example, naïve CD4+ lymphocytes cultured under Th1-promoting conditions produces 3 to 4-fold more IFN in norepinephrine stimulated versus unstimulated cells [44]. In keeping with this concept, Ganta et al., showed that administration of angiotensin II in the lateral cerebral ventricle increased mRNA expression of pro-inflammatory splenic cytokines such as IL-1 beta and IL-6 and splenic sympathetic denervation abrogated these responses [45]. Based on these studies we sought to further examine the role of central angiotensin II signaling and T cell mediated inflammation in hypertension.
Our initial studies were designed to increase the central effects of angiotensin II by locally increasing oxidative stress in the brain, specifically in the SFO [30]. As mentioned above, the SFO is a CVO region that contains an NADPH oxidase and has direct efferent projections to the AV3V region. Zimmerman et al., were the first to demonstrate that angiotensin II dependent hypertension is characterized by increased oxidative stress in the SFO [46]. We asked the question of whether increased oxidative stress in the SFO could contribute to T cell mediated hypertension. Oxidative stress was increased in the SFO by specifically deleting extracellular superoxide dismutase (SOD3) via ICV injection of an adenovirus encoding cre-recombinase. Interestingly, SOD3 deletion in the SFO increased sympathetic outflow and markedly enhanced the blood pressure response to a low dose infusion of angiotensin II (140ng/kg/min). Moreover, SOD3 deletion also markedly increased circulating T cells expressing the activation markers CD69 and CD44high, as well as increased vascular infiltration of inflammatory cells.
To further explore the link between the CNS, T cell activation and inflammation in hypertension, we examined the effect of blocking the central actions of angiotensin II using the well-known AV3V ablation method [32]. We then examined properties of circulating T cells and the vascular accumulation of these cells in response to chronic angiotensin II infusion. AV3V lesioning attenuated the rise in blood pressure in response to a slow pressor dose of angiotensin II and completely prevented the activation and vascular infiltration of T cells. This striking finding demonstrated that the central-mediated effects of angiotensin II, but not the peripheral effects, were responsible for the hypertension and inflammation. In light of these findings, T cell activation and vascular inflammation by angiotensin II may be dependent on increased sympathetic outflow and catecholamine release or other central signals that were blocked by AV3V ablation. Alternatively, increased T cell activation and vessel inflammation during angiotensin II maybe secondary to the rise in blood pressure, which can be blocked by AV3V ablation.
To differentiate between these scenarios, we chronically infused norepinephrine as a peripherally acting hypertensive stimulus, thus bypassing the effect of the central lesion and directly acting on peripheral adrenergic receptors in a fashion suggested by Ganta et al. [45]. We also administered hydralazine to prevent the elevation in blood pressure to either angiotensin II or norepinephrine. In contrast to the case with angiotensin II infusion, AV3V lesions did not prevent the hypertension, T cell activation or vascular inflammation caused by norepinephrine infusion. Importantly, prevention of blood pressure elevation with hydralazine completely abrogated T cell activaton and vascular inflammation caused by both angiotensin II and norepinephrine infusion. Overall, these recent studies clearly show that the central actions of angiotensin II on the CVOs contributes to increased systemic activation of T cells and vascular inflammation. Therefore, we have proposed a new paradigm to explain how hypertensive stimuli promote inflammation in hypertension.
Proposed mechanism for the role of central hypertensive stimuli and T cell action and inflammation in hypertension
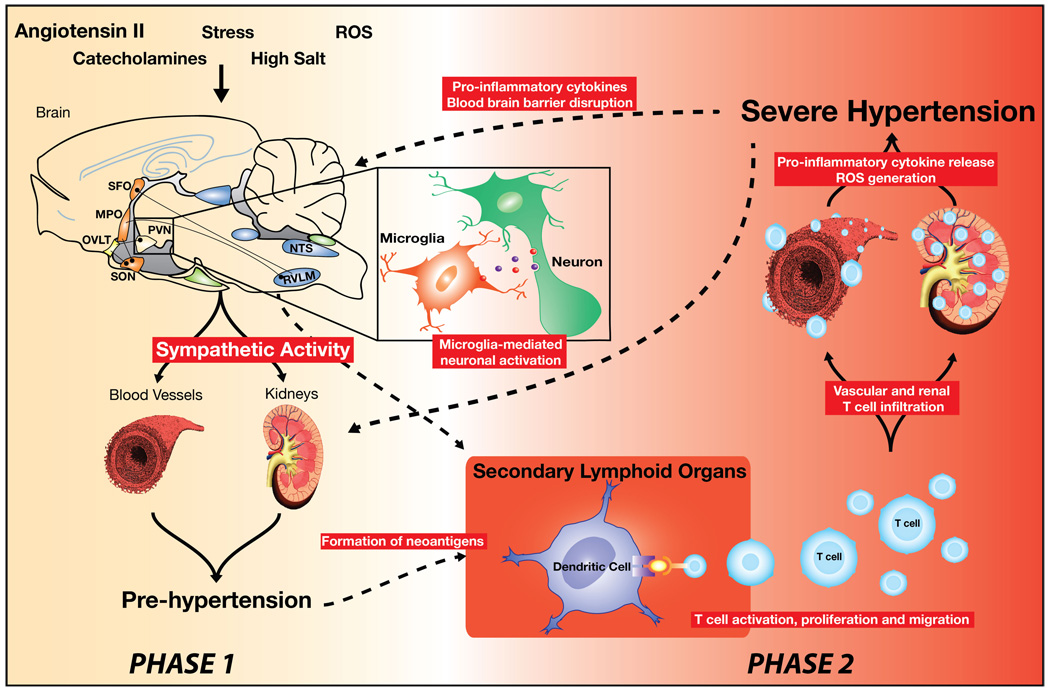
Our findings are compatible with a pathway in which central stimuli such as angiotensin II cause modest elevations of blood pressure, which leads to T cell activation, and ultimately severe hypertension. This proposed mechanism occurs in a two-phase feed forward fashion and is highly dependent on the actions of hypertensive stimuli such as angiotensin II on the CVOs (Figure 1). The SNS and increased sympathetic nerve activation, likely contributes to varying degrees at both phases in this mechanism. The first phase leads to a modest elevation in blood pressure (i.e. pre-hypertension), giving rise to an inflammatory response, possibly by generating “neoantigens” that activate T cells. Oxidative modification of proteins, lipids or DNA may be potential candidates for a neoantigen. This inflammatory response leads to entry of effector-like T cells into the perivascular fat and kidney as well as macrophage infiltration due to signals from T cells. Cytokines and other inflammatory mediators released by these cells work in concert with the direct effects of angiotensin II, catecholamines, and salt to cause vascular and renal dysfunction, promote vasoconstriction, vascular remodeling, a shift in the pressure-natriuresis curve and sodium retention, promoting a second phase of severe, sustained hypertension (Figure1).
Figure 1. Proposed role for the circumventricular organs (CVOs) in T cell mediated inflammation in hypertension.
Hypertensive stimuli such as angiotensin II, high salt or stress act in large part on the CVOs neurocircuitry causing a modest elevation in pressure (Phase 1- pre-hypertension). Local inflammation at the level of the CVOs involving microglia activation and increased cytokine production may also contribute to increased sympathetic outflow. We hypothesize that in a feed forward fashion this leads to neoantigen formation, promoting T cell activation. Activated T cells then enter the kidney and vasculature (Phase 2). T cell derived signals such as IL-17 promote entry of other inflammatory cells, such as macrophages. These inflammatory cells release cytokines that cause vasoconstriction and promote sodium and water absorption, ultimately causing severe hypertension (Phase 2).
Conclusion
The central and peripheral activation of T cells in hypertension and the extent to which the neuroimmune system impacts hypertension is far from being completely understood. The CVOs are known to be involved in blood pressure regulation and our recent studies describe a new role for these brain regions in contributing to peripheral T cell activation and vascular inflammation during hypertension. Given the emerging role of immunity in hypertension and the role of the CNS in blood pressure regulation, the data presented here highlight the importance of the neuroimmune interface in this disease process. Further understanding of the neuroimmune response in hypertension may provide additional insight and targeting for therapeutic intervention.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Paton JF, Raizada MK. Neurogenic hypertension. Exp Physiol. 2010;95(5):569–571. doi: 10.1113/expphysiol.2009.047282. [DOI] [PubMed] [Google Scholar]
- 2. Shi P, Diez-Freire C, Jun JY, Qi Y, Katovich MJ, Li Q, Sriramula S, Francis J, Sumners C, Raizada MK. Brain microglial cytokines in neurogenic hypertension. Hypertension. 2010;56(2):297–303. doi: 10.1161/HYPERTENSIONAHA.110.150409. Using novel gene transfer approaches these authors demonstrate that microglial cells in the paraventricular nucleus (PVN) produce pro-inflammatory cytokines that contribute to the alteration of blood pressure in the setting of angiotensin II induced hypertension.
- 3.Feng Y, Xia H, Cai Y, Halabi CM, Becker LK, Santos RA, Speth RC, Sigmund CD, Lazartigues E. Brain-selective overexpression of human Angiotensin-converting enzyme type 2 attenuates neurogenic hypertension. Circ Res. 2010;106(2):373–382. doi: 10.1161/CIRCRESAHA.109.208645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7(5):335–346. doi: 10.1038/nrn1902. Provides a detailed analysis and review for how the sympathetic nervous system contributes to the development of hypertension.
- 5.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52(4):595–638. [PubMed] [Google Scholar]
- 6.Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987–2007) Brain Behav Immun. 2007;21(6):736–745. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Webster JI, Tonelli L, Sternberg EM. Neuroendocrine regulation of immunity. Annu Rev Immunol. 2002;20:125–163. doi: 10.1146/annurev.immunol.20.082401.104914. [DOI] [PubMed] [Google Scholar]
- 8.Kohm AP, Sanders VM. Norepinephrine and beta 2-adrenergic receptor stimulation regulate CD4+ T and B lymphocyte function in vitro and in vivo. Pharmacol Rev. 2001;53(4):487–525. [PubMed] [Google Scholar]
- 9.Besedovsky HO, Del Rey A. Central and Peripheral Cytokines Mediate Immune-Brain Connectivity. Neurochem Res. 2010 doi: 10.1007/s11064-010-0252-x. [DOI] [PubMed] [Google Scholar]
- 10.Rivest S. How circulating cytokines trigger the neural circuits that control the hypothalamic-pituitary-adrenal axis. Psychoneuroendocrinology. 2001;26(8):761–788. doi: 10.1016/s0306-4530(01)00064-6. [DOI] [PubMed] [Google Scholar]
- 11.Banks WA, Kastin AJ, Broadwell RD. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation. 1995;2(4):241–248. doi: 10.1159/000097202. [DOI] [PubMed] [Google Scholar]
- 12.Agostinho P, Cunha RA, Oliveira C. Neuroinflammation, oxidative stress and the pathogenesis of Alzheimer's disease. Curr Pharm Des. 2010;16(25):2766–2778. doi: 10.2174/138161210793176572. [DOI] [PubMed] [Google Scholar]
- 13.Paton JF, Waki H, Abdala AP, Dickinson J, Kasparov S. Vascular-brain signaling in hypertension: role of angiotensin II and nitric oxide. Curr Hypertens Rep. 2007;9(3):242–247. doi: 10.1007/s11906-007-0043-1. [DOI] [PubMed] [Google Scholar]
- 14. Shi P, Raizada MK, Sumners C. Brain cytokines as neuromodulators in cardiovascular control. Clin Exp Pharmacol Physiol. 2010;37(2):e52–e57. doi: 10.1111/j.1440-1681.2009.05234.x. This paper presents current evidence for the neuromodulatory role of brain cytokines in the neural control blood pressure.
- 15. Paton JF, Waki H. Is neurogenic hypertension related to vascular inflammation of the brainstem? Neurosci Biobehav Rev. 2009;33(2):89–94. doi: 10.1016/j.neubiorev.2008.05.020. These authors present evidence and propose a hypothesis for the role of brainstem microvasculature inflammation in the pathogenesis of hypertension.
- 16.Cuadra AE, Shan Z, Sumners C, Raizada MK. A current view of brain renin-angiotensin system: Is the (pro)renin receptor the missing link? Pharmacol Ther. 2010;125(1):27–38. doi: 10.1016/j.pharmthera.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grobe JL, Dickson ME, Park S, Davis DR, Born EJ, Sigmund CD. Cardiovascular consequences of genetic variation at -6/235 in human angiotensinogen using "humanized" gene-targeted mice. Hypertension. 2010;56(5):981–987. doi: 10.1161/HYPERTENSIONAHA.110.157354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKinley MJ, Albiston AL, Allen AM, Mathai ML, May CN, McAllen RM, Oldfield BJ, Mendelsohn FA, Chai SY. The brain renin-angiotensin system: location and physiological roles. Int J Biochem Cell Biol. 2003;35(6):901–918. doi: 10.1016/s1357-2725(02)00306-0. [DOI] [PubMed] [Google Scholar]
- 19.Hendel MD, Collister JP. Contribution of the subfornical organ to angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol. 2005;288(2):H680–H685. doi: 10.1152/ajpheart.00823.2004. [DOI] [PubMed] [Google Scholar]
- 20.Shan Z, Shi P, Cuadra AE, Dong Y, Lamont GJ, Li Q, Seth DM, Navar LG, Katovich MJ, Sumners C, et al. Involvement of the brain (pro)renin receptor in cardiovascular homeostasis. Circ Res. 2010;107(7):934–938. doi: 10.1161/CIRCRESAHA.110.226977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morimoto S, Cassell MD, Sigmund CD. Glia- and neuron-specific expression of the rennin-angiotensin system in brain alters blood pressure, water intake, and salt preference. J Biol Chem. 2002;277(36):33235–33241. doi: 10.1074/jbc.M204309200. [DOI] [PubMed] [Google Scholar]
- 22.Brody MJ, Johnson AK. In: Role of the anteroventral third ventricle region in fluid and electrolyte balance, arterial pressure regulation, and hypertension. MLaG WF, editor. New York: Frontiers in Neuroendocrinology Raven Press; 1980. pp. 249–292. [Google Scholar]
- 23. McKinley MJ, McAllen RM, Davern P, Giles ME, Penschow J, Sunn N, Uschakov A, Oldfield BJ. The sensory circumventricular organs of the mammalian brain. Adv Anat Embryol Cell Biol. 2003;172:III–XII. doi: 10.1007/978-3-642-55532-9. 1-122, back cover. This book serves as an excellent reference for the neurocircuitry of the circumventricular organs.
- 24.Brody M, Fink G, Buggy J, Haywood J, Gordon F, Knuepfer M, Mow M, Mahoney L, Johnson A. Critical role of the AV3V region in development and maintenance of experimental hypertension. In: Schmitt H, Meyers P, editors. Perspectives in Nephrology and Hypertension. New York: Wiley and Flammarion; 1978. pp. 76–84. in Perspectives in Nephdogy and Hypertension. [Google Scholar]
- 25. Benigni A, Cassis P, Remuzzi G. Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med. 2010;2(7):247–257. doi: 10.1002/emmm.201000080. A recent review article on the role of angiotensin II in inflammation, tissue injury, autoimmunity, oxidative stress and aging.
- 26. Rodriguez-Iturbe B, Quiroz Y, Nava M, Bonet L, Chavez M, Herrera-Acosta J, Johnson RJ, Pons HA. Reduction of renal immune cell infiltration results in blood pressure control in genetically hypertensive rats. Am J Physiol Renal Physiol. 2002;282(2):F191–F201. doi: 10.1152/ajprenal.0197.2001. In the spontaneously hypertensive rat (SHR) these authors demonstrate that the hypertension, increased renal lymphocyte infiltration and oxidative stress could be normalized following administration of the immunosuppressive drug mycophenolate mofeltil.
- 27. Zhang M, Mao Y, Ramirez SH, Tuma RF, Chabrashvili T. Angiotensin II induced cerebral microvascular inflammation and increased blood-brain barrier permeability via oxidative stress. Neuroscience. 2010 doi: 10.1016/j.neuroscience.2010.09.029. Using C57bl/6 mice these authors show that a 2 week slow pressor dose (490ng/kg/min) model of angiotensin II dependent hypertension leads to increased permeability of the blood brain barrier and increased leukocyte infiltration into the cerebral microvasculature. Treatment with the anti-oxidant tempol could reverse these effects.
- 28. Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204(10):2449–2460. doi: 10.1084/jem.20070657. Using gentically modified mice these authors demonstrate an essential role for T cells in the genesis of angiotensin II dependent and DOCA-Salt hypertension.
- 29. Hoch NE, Guzik TJ, Chen W, Deans T, Maalouf SA, Gratze P, Weyand C, Harrison DG. Regulation of T-cell function by endogenously produced angiotensin II. Am J Physiol Regul Integr Comp Physiol. 2009;296(2):R208–R216. doi: 10.1152/ajpregu.90521.2008. These authors demonstrate that T cells contain an endogenous renin-angiotensin system that modulates T-cell function, NADPH oxidase activity, and production of superoxide and TNF-alpha.
- 30. Lob HE, Marvar PJ, Guzik TJ, Sharma S, McCann LA, Weyand C, Gordon FJ, Harrison DG. Induction of Hypertension and Peripheral Inflammation by Reduction of Extracellular Superoxide Dismutase in the Central Nervous System. Hypertension. 2009 doi: 10.1161/HYPERTENSIONAHA.109.142646. These authors induced oxidative stress in the subfornical organ (SFO) using a novel transgenic approach which promoted hypertension and peripheral T cell activation and vascular inflammation. This paper demonstrates an important link between central hypertensive stimuli and peripheral vascular inflammation.
- 31. Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 Promotes Angiotensin II-Induced Hypertension and Vascular Dysfunction. Hypertension. 2009 doi: 10.1161/HYPERTENSIONAHA.109.145094. Using IL17−/− mice these authors demonstrate an important role for increased circulating TH17 cells and IL17 production in angiotensin II dependent hypertension.
- 32. Marvar PJ, Thabet SR, Guzik TJ, Lob HE, McCann LA, Weyand C, Gordon FJ, Harrison DG. Central and peripheral mechanisms of T-lymphocyte activation and vascular inflammation produced by angiotensin II-induced hypertension. Circ Res. 2010;107(2):263–270. doi: 10.1161/CIRCRESAHA.110.217299. This paper identified an important role for the circumventricular organs in modulating peripheral vascular inflammation in hypertension.
- 33. Harrison DG, Vinh A, Lob H, Madhur MS. Role of the adaptive immune system in hypertension. Curr Opin Pharmacol. 2010;10(2):203–207. doi: 10.1016/j.coph.2010.01.006. A recent review article on the role of adaptive immunity in hypertension.
- 34. Crowley SD, Song YS, Lin EE, Griffiths R, Kim HS, Ruiz P. Lymphocyte responses exacerbate angiotensin II-dependent hypertension. Am J Physiol Regul Integr Comp Physiol. 2010;298(4):R1089–R1097. doi: 10.1152/ajpregu.00373.2009. An important paper that suggests lymphocytes play a role in sodium handling and pressure natriuresis in hypertension. The authors demonstrate that immunodeficient SCID mice have lower blood pressure and reduced renal injury and increased sodium excretion following 4 weeks of angiotensin II infusion.
- 35.De Miguel C, Das S, Lund H, Mattson DL. T lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol. 2010;298(4):R1136–R1142. doi: 10.1152/ajpregu.00298.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez-Iturbe B, Johnson RJ. The role of renal microvascular disease and interstitial inflammation in salt-sensitive hypertension. Hypertens Res. 2010;33(10):975–980. doi: 10.1038/hr.2010.148. [DOI] [PubMed] [Google Scholar]
- 37.Viel EC, Lemarie CA, Benkirane K, Paradis P, Schiffrin EL. Immune regulation and vascular inflammation in genetic hypertension. Am J Physiol Heart Circ Physiol. 2010;298(3):H938–H944. doi: 10.1152/ajpheart.00707.2009. [DOI] [PubMed] [Google Scholar]
- 38.Osborn JW, Fink GD. Region-specific changes in sympathetic nerve activity in angiotensin II-salt hypertension in the rat. Exp Physiol. 2010;95(1):61–68. doi: 10.1113/expphysiol.2008.046326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev. 2010;90(2):513–557. doi: 10.1152/physrev.00007.2009. [DOI] [PubMed] [Google Scholar]
- 40.Felten DL, Felten SY, Carlson SL, Olschowka JA, Livnat S. Noradrenergic and peptidergic innervation of lymphoid tissue. J Immunol. 1985;135(2 Suppl):755s–765s. [PubMed] [Google Scholar]
- 41.Felten DL, Livnat S, Felten SY, Carlson SL, Bellinger DL, Yeh P. Sympathetic innervation of lymph nodes in mice. Brain Res Bull. 1984;13(6):693–699. doi: 10.1016/0361-9230(84)90230-2. [DOI] [PubMed] [Google Scholar]
- 42.Kin NW, Sanders VM. CD86 stimulation on a B cell activates the phosphatidylinositol 3- kinase/Akt and phospholipase C gamma 2/protein kinase C alpha beta signaling pathways. J Immunol. 2006;176(11):6727–6735. doi: 10.4049/jimmunol.176.11.6727. [DOI] [PubMed] [Google Scholar]
- 43.Madden KS, Sanders VM, Felten DL. Catecholamine influences and sympathetic neural modulation of immune responsiveness. Annu Rev Pharmacol Toxicol. 1995;35:417–448. doi: 10.1146/annurev.pa.35.040195.002221. [DOI] [PubMed] [Google Scholar]
- 44.Swanson MA, Lee WT, Sanders VM. IFN-gamma production by Th1 cells generated from naive CD4+ T cells exposed to norepinephrine. J Immunol. 2001;166(1):232–240. doi: 10.4049/jimmunol.166.1.232. [DOI] [PubMed] [Google Scholar]
- 45. Ganta CK, Lu N, Helwig BG, Blecha F, Ganta RR, Zheng L, Ross CR, Musch TI, Fels RJ, Kenney MJ. Central angiotensin II-enhanced splenic cytokine gene expression is mediated by the sympathetic nervous system. Am J Physiol Heart Circ Physiol. 2005;289(4):H1683–H1691. doi: 10.1152/ajpheart.00125.2005. These authors demonstrate an important neuroimmunological effect of central angiotensin II. Intracerebralventricular (ICV) injection of angiotensin II leads to increase splenic nerve activity and splenic pro-inflammatory cytokine production.
- 46. Zimmerman MC, Lazartigues E, Lang JA, Sinnayah P, Ahmad IM, Spitz DR, Davisson RL. Superoxide mediates the actions of angiotensin II in the central nervous system. Circ Res. 2002;91(11):1038–1045. doi: 10.1161/01.res.0000043501.47934.fa. Using a novel adenoviral vector for superoxide dismutase (SOD), this is the first paper to demonstrate a role for angiotensin II dependent superoxide signaling in the subfornical organ (SFO) during hypertension.