Abstract
Importance of the field
Pain, dysphagia, respiratory problems, sexual and cardiovascular dysfunctions may occur in patients with multiple sclerosis (MS).
Areas covered in the field
In the present review we attempt to summarize the current knowledge on the impact that pain, dysphagia, respiratory problems, sexual and cardiovascular dysfunctions have in patients with MS.
What the reader will gain
The current understanding on pain, dysphagia, respiratory problems, sexual and cardiovascular dysfunctions and future research perspectives to expand the knowledge of this field.
Take home message
To effectively manage MS it is essential that these symptoms are recognised as early as possible and treated by a rehabilitative multidisciplinary approach, based on proven scientific evidence.
Keywords: multiple sclerosis, dysautonomia, pain, pharmacotherapy
1 Introduction
Multiple sclerosis (MS), a chronic disease of the central nervous system (CNS), is one of the leading causes of neurological disability of young adults [1]. The disease is sustained by a complex and not entirely defined inter-play between inflammation and neurodegeneration.
The diagnosis of MS is based on the (revised) McDonald criteria [2] and is multidisciplinary. Both clinical and paraclinical measures are included. The presence of at least two clinical attacks accompanied by clinical evidence of at least two lesions by magnetic resonance imaging (MRI) is indicative of MS. If clinical evidence of at least two clinical lesions cannot be found by neurological examination, dissemination in space may be provided by specific MRI criteria [3], and by the presence oligoclonal bands in the cerebrospinal fluid (CSF). The new criteria for the diagnosis of MS, revised in 2005, acknowledge the capability of making a definitive diagnosis of MS either after a monosymptomatic presentation or in the context of a primary progressive course. Revision of these diagnostic criteria, states that a) a new lesion, with respect to a previous MRI performed at least 30 days after the initial clinical event, can be used to demonstrate dissemination in time; b) spinal lesions may also play a role in defining specific abnormalities on MRI compatible with MS (dissemination in space); c) CSF positive for the presence of oligoclonal bands may be useful to diagnose, though in the presence of appropriate MRI changes, CSF abnormalities are not required for a definitive diagnosis.
From a clinical standpoint, lesion dissemination in space makes MS potentially manifest with a variety of neurological symptoms. Some symptoms are easily detectable by patients, caregivers and physicians. Those symptoms include motor, sensory and visual disturbances as well as, to some degree, cognitive impairment. Other symptoms are more difficult to recognize as MS-induced by the patients hence to be reported. Such symptoms include disturbances of the cardiovascular system, sexual impairment, dysphagia, respiratory problems and pain [4]. All and each of these symptoms can profoundly impact patient quality of life (QoL) of patients and caregivers and also be very disabling for patients. It is important to emphasize that numerous other symptoms beyond motor, visual and sensory disability and besides pain, dysphagia, respiratory problems, sexual and cardiovascular dysfunctions afflict patients with MS. These symptoms include bladder and bowel problems and, outside the dysautonomia area, cognitive and emotional dysfunctions. However, since the knowledge on possible diagnostic algorithms, therapeutic approaches and impact that pain, dysphagia, respiratory problems, sexual and cardiovascular dysfunctions may have in MS patients is limited with respect to the one about bladder and bowel problems or cognitive and emotional disability, in the present manuscript we focus on pain, dysphagia, respiratory problems, sexual and cardiovascular dysfunctions. For each of the discussed clinical entities, we will describe the prevalence and type, the hypothesized pathophyisiology in relation to the presence of MS, the imaging correlates and the pharmacological and non- pharmacological therapies currently available to overcome them.
Although pain, dysphagia, respiratory problems, sexual and cardiovascular dysfunctions are very common in MS patients and to some extent likely induced by the disease, they remain partially non-specific in the way they manifest clinically. Also, since large case-control studies focusing in these symptoms are lacking, much remains unknown regarding the relation between MS and pain, dysphagia, respiratory problems, sexual and cardiovascular dysfunctions.
2. METHODS
Electronic database (Medline, PubMed and collections of books available through the National Institutes of Health library) were selected for material in English, French, Spanish and Italian language without limiting the publication date. The search terms used were as follows: MS, MRI, cardiovascular dysautonomia, central neuropathic pain, dysphagia, erectile dysfunction, fatigue, orgasm, orthostatic intolerance, pain, respiratory deficits, sexual dysfunction. Due to the paucity in literature regarding therapeutics, no classification of the literature according to common criteria (level of evidence I–IV, e.g.) and level of recommendation was performed.
3. DYSPHAGIA
3.1 Prevalence, symptoms and physiopathology of dysphagia in MS
Dysphagia is a disabling, life-threatening symptom that can cause death through aspiration pneumonia. Prevalence of dysphagia ranges from 33% to 43% [5, 6]. Permanent dysphagia may be visible in mildly impaired patients, such as those with a score of 3.0–3.5 at the Expanded Disability Status Scale (EDSS) [7], and reach a prevalence of 65% in most severely disabled patients [8, 9] (EDSS 8.0–9.0). Dysphagia may also occur in as many as 17% patients with mild disability (EDSS <2.5) [10].
Despite complaints made by both patients and caregivers, this symptom is often underestimated; indeed, as it is not considered to be life-threatening, it is not always evaluated in clinical practice. Family and caregivers should be encouraged to pay careful attention to cough when patients are eating or drinking, to weight loss, to a rise in temperature related to respiratory symptoms and to dysarthrophonia. Such symptoms should be investigated further. A videofluorographic swallowing study (VFSS) and a fiberoptic endoscopic examination may be considered to be equally effective in detecting dysphagia and should be considered fundamental for the subsequent treatment of this symptom.
The most common symptoms in moderate dysphagia are choking episodes, food retention in the pharynx, leaking of oral content in the pharynx and larynx, obstructive apnoea and acute pneumonia due to aspiration of bolus into the bronchial tree [11].
Dysphagia in MS may be due to impairment of both the oral and the pharyngeal phases of swallowing. Abraham and co-workers found impairment of the upper oesophageal sphincter in 100% of a small cohort of 13 patients with MS and EDSS scores of 2.0–9.0 [10]. Calcagno and co-workers [12] classified 49 patients with primary progressive (PP) MS and secondary progressive (SP) MS in three patient groups on the basis of severe, moderate and slight dysphagia. Patients with severe dysphagia had impairment of both the oral and pharyngeal phases of swallowing; patients with moderate dysphagia had motor impairment of the tongue, lips, velum or larynx, while the group with mild dysphagia had deficiency either of the velum or of the glottic closure.
Quantity and consistency of food has the potential to worsen dysphagia as shown in a recent paper by Taniguchi and co-workers [13]. The authors demonstrated that the main effect of harder food consistency is a delay in oral ejection time, which markedly delays total swallowing time. Conversely, pharyngeal bolus transit is dependent on food viscosity. That is, the risk of failure during the pharyngeal phase of swallowing is often related to a weak pushing-back movement of the tongue; increased food viscosity can delay the bolus progression towards the oesophagus because the bolus adheres to the pharyngeal walls.
The role of MS related factors triggering and sustaining dysphagia has not been extensively investigated. A combination of various factors, such as the involvement of the corticobulbar tracts, cerebellar and brainstem dysfunctions, lower cranial nerve paresis, cognitive impairment, overall disability, depressed mood and low vital capacity are believed to sustain dysphagia in MS patients [12, 14]. The use of some drugs, especially anticholinergic agents to relief spasticity or modulate bladder problems (i.e., atropine, baclofen) has been reported to impair swallowing functions, though no specific studies on MS exist.
3.2 The development of compensatory mechanisms to overcome dysphagia
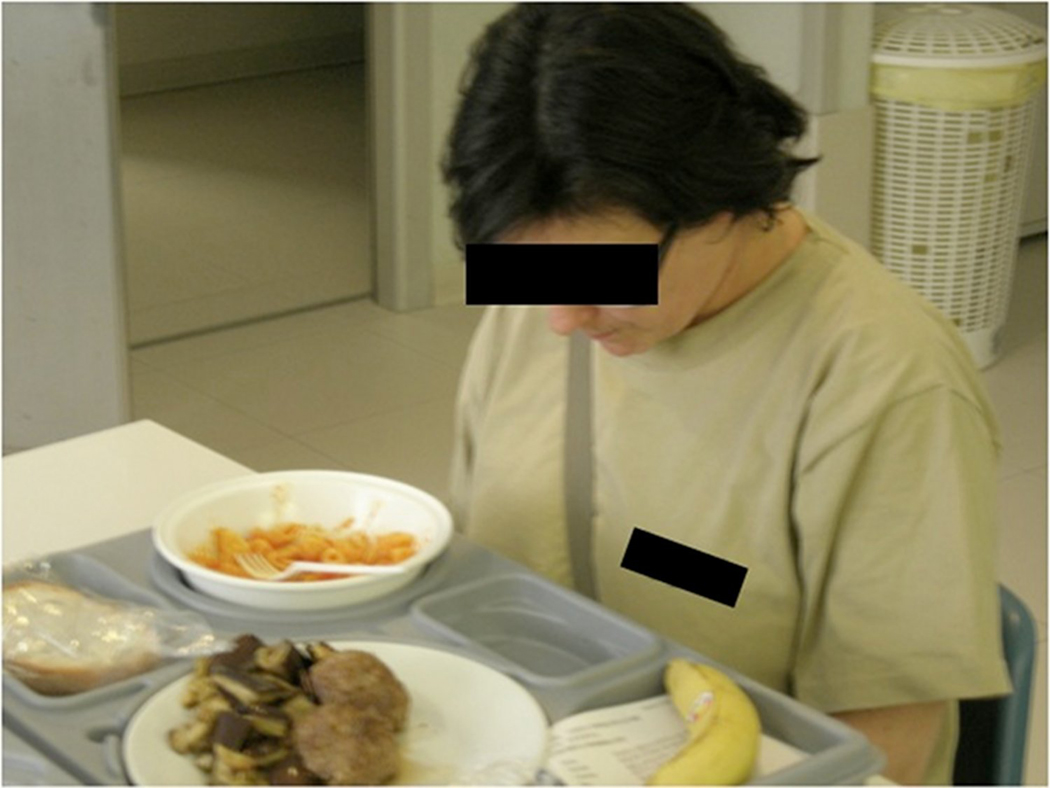
Currently, no drug therapies are available to treat the MS associated dysphagia. The only available therapeutic approaches are those based upon rehabilitative and coping strategies to reduce the fatal consequences of dysphagia. Different treatment strategies can be divided according to their aims, which may be restorative, compensatory or adaptive. The aim of therapeutic procedures, which consist of motor exercises and stimulations and can be defined as restorative, is to optimize swallowing. On the other hand, compensatory strategies, such as changes in food consistency, taste and temperature, or postural positions, do not have a direct effect on swallowing, though they do improve oral feeding conditions, thereby reducing the risk of aspiration. Adaptive equipment, such as feeding tubes, percutaneous endoscopic gastrostomy (PEG) and their related electrical devices, may be used to supplement oral intake by means of other nutritional methods in patients in whom swallowing is considered to be unsafe. The choice of treatment needs to be based on the degree of severity of the symptoms (Table 1 [15]). As of today, no pharmacological treatments are available to treat dysphagia. Only a recent study reported the effectiveness of botulinum toxin on dysphagia associated with upper sphincter hyperactivity in 14 MS patients, which points to new possible strategies in this field [16]. In mild dysphagia, the first step consists of informing the patient about the specific mechanisms and circumstances of eating and the events associated with swallowing so that he/she can try to voluntary control these actions. Patients are urged to focus their attention on modulation of cough, improving swallowing, and on particular head and neck positions while eating and swallowing (Figure 1). Before eating, patients must be correctly positioned. They should be sitting upright, with a comfortable support for the forearms and feet flat on the ground. The room where the meal is consumed must be quiet and well lit. The patient should eat slowly, respecting each individual bite volume recommended, and not introduce a second bite until the previous has been fully swallowed (beware of food residue in the mouth). The meal should not, however, last more than 45 minutes as fatigue and distractibility increase the risk of inhalation. During the meal, it is the caregiver's duty to ensure that the patient's level of attention remains high and to suspend the meal at the first signs of fatigue. The patient should not talk during the meal, watch television or be distracted in any way.
TABLE 1.
ASHA NOMS SWALLOWING SCALE (O’ Neil) AND RELATED STRATEGIES TO MANAGE DYSPHAGIA [15].
| Degree 0: Oral feeding – Normal Diet | |||
| Level 7 | Normal | Normal Diet | Normal swallowing function, safe and efficient for all consistencies. The patient is able to eat independently. No strategies are effectively needed. |
| Level 6 | Functionall y sufficient | Some diet restriction, compensatory and restorative strategies useful | The patient eats and drinks independently as swallowing is safe. May require minimal observation by therapist and/or care-givers, but can self-manage when difficulty occurs. May need to avoid specific food items (such as food with solid and liquid components mixed), or requires additional time due to dysphagia. |
| Degree 1: Oral feeding – Diet restrictions | |||
| Level 5 | Slight Dysphagia | Some diet restriction, therapist’s observation useful, compensatory and restorative strategies needed | Swallowing is safe, some minimal diet restrictions may occasionally be required. Therapist’s supervision to use compensatory strategies is also useful. All nutrition and hydration needs are met by mouth. |
| Level 4 | Medium- slight dysphagia | Diet restriction, therapist’s observation needed, compensatory and restorative strategies needed | Swallowing is possible, but usually requires compensatory strategies, and/or the patient has moderate diet restrictions and/or still requires tube feedings and/or oral supplements. |
| Level 3 | Moderate dysphagia | Diet restriction, therapist’s observation needed, compensatory, adaptive, and restorative strategies needed | Oral intakes provide less than 50% of nutrition and hydration by mouth, and/or swallowing is safe with use of compensatory strategies and/or requires maximum diet restrictions. Alternative method of feeding is also required |
| Degree 2 No oral feeding – Minimal or no oral intake | |||
| Level 2 | Quite severe dysphagia | Heavy Diet restriction, therapist needed, compensatory adaptive, and restorative strategies needed | Swallowing is not reliable, the patient may take some selected foods only under supervision of the therapist. Alternative method of feeding is required. |
| Level 1 | Severe dysphagia | No oral feeding, adaptive strategies needed | Food can’t be swallowed safely by mouth. All nutrition and hydration is provided through other ways, such as nasogastric tube, PEG. |
FIGURE 1.
Head and neck position which can help ensure safe swallowing and protect airways from inhalation of food. The figure shows that a forward flexed position of the head is the best way to protect the airways, while the tongue pushes the food during the pharyngeal phase of swallowing.
Work from our group [12] has demonstrated that compensatory strategies may develop and resolve dysphagia in as many as 94% of 49 the patients. Those compensatory strategies include changes in posture, in the amount and speed of food presentation and in food consistency eliminated aspiration. It is also important noting that these compensatory strategies help to redirect and improve the flow of food, thereby avoiding aspiration and reducing the risk of pneumonia, but do not eliminate dysphagia permanently [17, 18]. Direct rehabilitation with the aid of sensorial tactile or thermal stimulation of the mouth and faucial isthmus by speech therapists may increase the awareness of pharyngeal swallowing in the patient [19, 20, 21].
In severe cases, the use of intermittent feeding via a temporary naso-gastric tube may be useful to ensure an adequate calorie intake. If the dysphagia worsens and nutrition must be administered exclusively via an enteral tube, the best choice is to perform a PEG, which allows natural gastro-intestinal function and has a low complication rate [22]. The most common complications associated with upper endoscopic procedures include cardiopulmonary compromise, aspiration, haemorrhage and perforation. Mortality attributable to upper endoscopy is exceedingly low (0.005–0.01%). Severe haemorrhage is a rare complication of upper endoscopy (0.02% to 0.06% cases). Complications associated with PEG use and wound care are peristomal pain, abscess and wound infections, PEG dislodgement and site herniation, gastrointestinal bleeding and ulceration [23].
4. RESPIRATORY IMPAIRMENT IN MS
The prevalence of respiratory disturbance has been estimated to be 64% [24], with results ranging from 36% in ambulatory patients to 83% in non-ambulatory patients. A multidisciplinary approach, together with a pneumological screening, should thus be adopted in all phases of the disease. Generally, respiratory impairment is more common in the advanced stage of MS although it may occur earlier, during acute relapses [25]. In addition, alterations in expiratory muscle efficiency, ultimately impairing breathing and coughing may be detected by instrumental evaluations even in the absence of clinical symptoms [26]. Severity of MS, disease duration [27, 28, 29] and cerebellar impairment, as identified clinically and quantified by the EDSS score [24], are the main factors associated with the occurrence of respiratory deficit. Among all the respiratory problems, pneumonia is the most frequent underlying cause of death in MS [30, 31] and accounts for as many as 46% of MS-related deaths [32]. There are two typical patterns of respiratory dysfunction associated with MS. In the first pattern, acute respiratory failure develops as a consequence of demyelinating lesions of the cervical spinal cord or the respiratory nuclei of the medulla oblungata. In the second pattern, aspiration, pneumonia and respiratory failure may gradually develop and worsen as MS progresses due to a number of factors such as, but not only, spinal cord thoracic disease, long-standing wheelchair use, bed-confinement and scarce motility in general.
No specific therapies are available to treat MS-related respiratory problems. Adequate pulmonary management should support inspiratory and expiratory muscle function. Non-invasive positive-pressure ventilation via a nasal mask is often effective for the support of inspiratory muscle, though it may be contraindicated in some patients; other treatments include theophylline in selected patients [33]. Recent studies suggest the importance of training respiratory muscles for both strength and endurance in MS people [26, 29, 34]. However, up to now, it is recommended that on a patient-by-patient basis, the effectiveness of specific treatments is evaluated by means of multidisciplinary team.
5. CARDIOVASCULAR DYSFUNCTION (CD) IN MS
5.1 Prevalence, symptoms and physiopathology of CD in MS
CD occurs rarely in patients with MS although its clinical manifestations may be dangerous [35]. Proper differential diagnosis between MS-induced CD symptoms and CD disorders is mandatory as not to initiate inappropriate treatment strategies. Nevertheless, disentangling CD symptoms related to cardiovascular autonomic neuropathy in MS, induced by drugs or due to primary heart disease may represent a critical challenge in neurological disease management and mandates the involvement of a cardiologist, first.
Clinically relevant cardiovascular events seem to be more likely to occur in MS patients with more severe clinical disability [36] and/or during acute relapses [37]. However, abnormal responses to cardiovascular reflex tests may be virtually found at any stage of the disease [38, 39]. A large inter-study variability is reported on the co-morbidity MS-CD, depending upon the type of CD and the autonomic reflex tests used to detect CD. Orthostatic dizziness (OD) has been reported in variable percentage of both relapsing remitting (RR) and SPMS patients ranging from 24% to up to 50% [40, 41, 42]. Conversely, postural orthostatic tachycardia syndrome (POTS) has been rarely described [43], and even more so prior to the diagnosis of MS [43, 44]. CD due to reduced left ventricular ejection fraction and cardiac heart failure may also occur in approximately 12% and 0.4% of patients, respectively, whose MS is managed with mitoxantrone [45]. Hence, it is recommended that in these patients cardiac function is monitored monthly during therapy and yearly upon therapy discontinuation by means of electrocardiogram, echocardiograph or multiple gated acquisitions [45]. Treatment with glatiramer acetate – a first line agent for RRMS patients – may also be associated to transient post-injection dyspnea, palpitation and chest pain not related to myocardial ischemia [46].
Fatigue, palpitations (when moving to upright posture) and OD are the most common clinical findings in MS patients affected by CD [36, 39]. Syncope or near syncope is generally uncommon in MS although orthostatic intolerance, possibly leading to syncope, has been reported in nearly half of studied MS patients [35, 37, 39, 40, 42, 47]. Such high incidence of syncope reported in a few studies might be due to the small group of selected patients [43] in whom either an absolute heart rate (HR) ≥120 beats per minute (bpm) or an increase by ≥30 bpm and concomitant fall of blood pressure (BP) within the first 10 minutes of an upright tilt test were seen. This hemodynamic postural response led to the diagnosis of POTS by the authors.
In patients with MS, CD is thought to be related to impairment of the reflex pathways in the brainstem [48] with involvement of the central-autonomic connections [38]. Both sympathetic and parasympathetic branches of the autonomic nervous system may be involved. While impaired sympathetic functions are seen mainly in clinically active patients, cardiovagal parasympathetic impairment tends to appear during remitting phases of the disease [49]. Similarly, catecholamine levels tend to reduce in RRMS and to increase in chronic progressive MS [49]. These findings yield the hypothesis that while parasympathetic dysfunction may only be a sequel of the disease, sympathetic dysfunction may function as trigger of new relapses [49].
In line with the hypothesis of a reduced parasympathetic vagal neuroregulation directed to the heart in clinically stable patients, previous work from our group [38] has shown that, at rest, MS patients have decreased spectrum total power, very low frequency and low frequency oscillatory components as compared to healthy controls. For a better appreciation of these findings, we report in Figure 3 patterns of the above parameters in a healthy subject as an example. However, reciprocal variations from rest to standing of low frequency increase and high frequency reduction, were similar in the two populations. Additionally, the cross-spectrum and coherence analyses of respirogram and tachogram showed that the low frequency power of each subject, both in the resting and standing positions, was not influenced by respiration.
FIGURE 3.
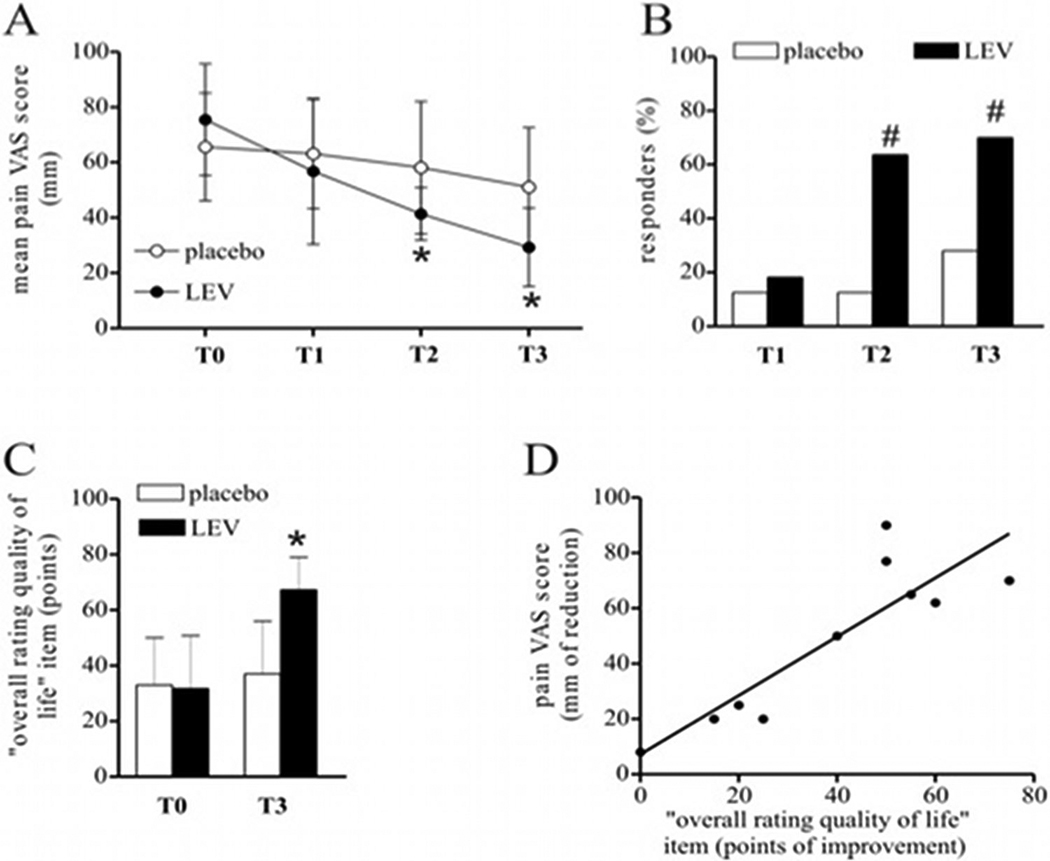
Effects of levetiracetam in MS chronic neuropathic pain, adapted from [111].
Figure 3A. Over a 3-month trial with 4 monthly measurements (i.e., T0 or baseline, T1, T2 and T3) levetiracetam (LEV, closed black circles) significantly reduced (p<0.05 at T2 and T3 vs T0 value, by ANOVA and follow-up t-test) the mean intensity pain at the VAS (100 mm visual analog scale for pain, ranging from “no pain” to “worst possible pain”) compared to placebo (closed white circles).
Figure 3B. The percent of responder patients, defined as patients with a clinically significant reduction of VAS score by >20 mm vs baseline, was larger (p<0.05 at T2 and T3 vs placebo, by ANOVA and follow-up t-test) in the levetiracetam-group (black columns) than in the placebo one (white columns).
Figure 3C. At the end of the study period, treated patients (black columns) had better quality of life (p<0.05 at T3 vs T0 value, by ANOVA and follow-up t-test) as measured by the MSQOL-54 scale, than non-treated ones (white columns).
Figure 3D. Correlation plot showing a significant association between pain reduction and quality of live improvement at the end of three-month trial (r=0.78, p<0.01, by Spearman correlation test).
5.2 MRI correlates of CD in MS
It remains uncertain whether CD is consequence of MS plaques strategically located in the brainstem, spinal cord, hypothalamus and cerebral cortex, or is rather either an epiphenomenon or an incidental co-morbidity. To this end, results of imaging studies investigating the relations between white matter lesion volume (WM-LV) in T2-weighted (T2-w) images by MRI and CD are controversial. While some early studies relying upon chronic lesion counting in relatively small number of patients have denied such an association [38, 40], larger studies utilizing automated procedures for measuring WM-LV, have disclosed an association between total intracranial WM-LV and CD [39], decreased diastolic BP, midbrain WM-LV and decreased BP responses immediately, at 2 and at 7 minutes after tilting, parietal WM-LV and decreased BP responses at 2 minutes after tilting [50]. Therefore, one cannot exclude the hypothesis that a centrally-mediated mechanism may be partially responsible for inducing damage in several critical pathways of autonomic nervous system, in their turn causing CD in MS patients.
5.C Pharmacological and non-pharmacological therapies to overcome CD in MS
The therapeutical management of orthostatic hypotension and orthostatic intolerance syndromes as POTS in patients with MS is similar to that of non-MS patients. The good clinical practice relies upon the recent European Society of Cardiology Guidelines for the diagnosis and management of syncope [51]. First line intervention is patient education about the nature of the CD in conjunction with lifestyle modifications, which can improve orthostatic symptoms. Raising the standing BP just enough to be within the autoregulatory zone can make a substantial functional difference. Expansion of extracellular volume is also an important goal. Gravitational venous pooling can be treated with abdominal binders or compression stockings. Counter-pressure maneuvers such as leg crossing and squatting should be encouraged in patients with warning symptoms able to perform them [51] Peripheral alpha1 (a1) adrenal-receptor agonists that induce venous and arterial vasoconstriction, such as midodrine hydrochloride, are useful additions to the first-line treatment in patients with chronic orthostatic intolerance [52]. Midodrine has been shown to be effective in increasing BP in both supine and upright posture. Adverse effects include paresthesia and itching (probably secondary to piloerection). This drug is not recommended for patients with disease of coronary or peripheral arteries [53].
Fludrocortisone, inducing renal sodium retention and fluid volume expansion, is presently recommended only in selected patients and is effective only if sodium intake is adequate [54]. This drug may also improve the peripheral vasoconstrictor response to sympathetic stimulation. Supine hypertension, heart failure and hypokalemia may occur [55]. Caffeine and nonsteroidal antiinflammatory drugs (NSAID) such as ibuprofen or indomethacin, may also be suggested. NSAIDs may inhibit prostaglandin-induced vasodilation, increasing peripheral vascular resistance [56]. However, NSAIDs may cause unwanted vasopressor reactions with concurrent use of sympathomimetic drugs. Propranolol may enhance the beneficial effects of volume expansion. β-Blockade leads to unopposed α-adrenergic peripheral vascular vasoconstriction, preventing the inappropiate orthostatic vasodilation [57]. In patients with orthostatic intolerance and reflex syncope paroxetine was shown to be effective in one placebo-controlled trial, which included highly symptomatic patients [58]. Paroxetine may reduce anxiety, which precipitates CD, hence its use is properly indicated only in patients with co-morbidity reflex syncope/psychiatric disease. No specific clinical trials have been conducted and specific evidence reported in MS patients on this topic and physicians dealing with this problem in MS need to refer to general practice in cardiology.
6. SEXUAL DYSFUNCTION (SD) IN MS
6.1 Prevalence, symptoms and putative physiopathology of SD in MS
SD is a common but often undiagnosed feature of MS. Reported rates range from 50% to 84% in men and from 45% to 85% in women [59, 60, 61]. SD occur significantly more frequently in MS patients than in patients with other chronic diseases or healthy subjects [62]. SD in MS is associated with a marked reduction in the QoL, also among patients with otherwise low disability, and it may compromise fertility [61, 63]. The nature of SD in MS patients is complex and multifactorial, and a multidisciplinary approach to diagnosis is required.
SD can arise at any stage of the disease. By the time of the disease diagnosis, a variable proportion of 10% to 34.9% of the newly diagnosed women may report SD [62, 64, 65]. Between 2 to 5 years after diagnosis [66], 50% of the men and 14% of the women may report dissatisfaction with their sexual functioning. However, the risk of developing SD and the extent or number of SD associated symptoms increase significantly as function of time [59]. The increased risk is independent from sex and from any other disease variables such as disease duration, age at disease onset and disability [67].
A conceptual model [65, 68] includes a primary, secondary and tertiary component. Primary SD refers to MS-related neurological changes that may directly impair libido, sexual response and orgasm. These changes are determined by a direct damage to the nervous system pathways and genitals. The most frequent symptoms are loss or decreased sexual desire, reduced vaginal lubrication, erectile dysfunction (ED), ejaculatory dysfunction, altered genital sensation, hyporgasmia. Secondary SD encompasses symptoms related to the physical disability of MS, such as fatigue, spasticity, spasms, physical limitations, poor coordination, tremors, burning sensations, cognitive changes, bladder and bowel dysfunction, side effects of MS medications. To this end, one should bear in mind that while several studies have reported a significant relation between MS induced physical disability and SD [60, 63, 69, 70], others failed in demonstrating such an association [64, 71, 72]. Fatigue [73, 74], pyramidal signs in the lower limbs [74], weakness of pelvic floor [69], age at onset of symptoms and PP disease [75] were all reported to be positively related to SD. Studies have shown an association also between SD and MS-induced bladder dysfunction [59, 64, 74, 75, 76, 77]. However urodynamic studies in MS patients, although rarely performed, found no correlation with objective urometric parameters [68, 75].
Tertiary SD results from psychological, emotional, social and cultural aspects. This includes depression, anxiety, low self-esteem and distorted body image, anger, fear of rejection and feeling or fear of abandonment. To this end, significant associations were seen between SD and depression [75, 76, 77], low educational level, physical disorders, cognitive symptoms [75]. A recent study from our group involving 122 consecutive fully ambulatory MS patients [77], reported a sex-specific correlation between SD and emotional disability. Specifically, in women depression was associated with reduction in orgasm, satisfaction and sexual desire. Conversely in men reduced orgasm and sexual desire were respectively associated with high post-void residual volume and slow urinary flow rate.
6.3 MRI correlates of SD in MS
Few studies have examined brain MRI in relation to SD in MS patients. An association was seen between SD and WM-LV in T2-w images. With respect to specific lesion topography, prominent areas appeared to be the brainstem and pyramidal tracts [71, 76]. Pontine atrophy and ventricular enlargement have also been associated with impaired QoL with respect to SD in MS [71]. The importance of atrophy over WM focal lesions in determining SD has also been reported by Zivadinov and co-workers. The authors found a significant association between pontine volume loss and ED in men or decreased vaginal lubrication in women [70]. The data strengthen the concept of a crucial role of the brainstem in the regulation of sexual functioning. However, the limited number of these studies in MS, the different utilised methodologies and the lack of spinal MRI investigations, make the correlation between MRI findings in MS patients and SD still inconclusive.
6.4 Pharmacological and non-pharmacological therapies to overcome SD in MS
Given the multifactorial nature of SD, a holistic approach is required for successful assessment and treatment. All the patients should be queried about sexual problems by an open communication, should be informed about the expected impact of the disease on their sexual function, and should be made aware of the available therapeutic options. A psychosexual counselling, including the participation of the partner is mostly recommended. Medications possibly impairing libido and erectile function, such as antidepressants and muscle-relaxants, ought to be discontinued.
Pharmacological treatment in men is mainly addressed to ED. Oral phosphodiesterase-5 (PDE-5) inhibitors, such as sildenafil, tadalafil, or vardenafil, are currently first-line treatments [78]. Sildenafil is generally safe and may improve ED in men with MS to a variable extent [79, 80]. Tadalafil, a long-acting PDE5 inhibitor, has also been proven to be safe and effective for males with MS suffering from ED [81]. In the general population most commonly reported adverse events with PDE5 inhibitors are usually transient and dose-related. Those include headeache, flushing, rhinitis and dyspepsia [78]. The incidence of serious cardiovascular events results higher in patients treated with Sildenafil (0,5%), then in Tadalafil (0,3%) Vardenafil or placebo (0,2%) recipients [78]. In refractory cased to oral agents, intracavernosal self-injections of vasoactive drugs are an effective option, the commonest of which is Prostaglandin E1; this treatment modality may be complicated in patients with advanced disability. Vacuum erection device is a non-invasive treatment, effective in neurogenic ED [82], Nevertheless premature loss of rigidity during intercourse is commonly reported.
As of today there are no specific treatments proven to be effective for female SD in MS. Topical estrogens creams may improve vaginal dryness, burning and dyspareunia. Although sildenafil treatment showed some benefits in post-menopausal and younger women with SD [83, 84, 85], in MS patients was not associated to significant improvement in lubrication, sensation or orgasm compared with placebo [83].
7. PAIN
7.1 Prevalence, symptoms and physiopathology of pain in MS
Pain is a frequent complaint of patients with MS. At any stage of the disease, as many as 30% to 85% MS patients may suffer from pain as chronic or acute symptom. The variable incidence of pain depends upon the definition of specific pain conditions and the different modalities of data collection [4, 86, 87, 88, 89]. Pain significantly interferes with the patients’ daily activities and has a dramatic impact on their QoL [90, 91(77)]. Various types of pain occur in MS, and frequently multiple pain problems may co-exist in the same patient. Those include: (1) dysesthetic extremity pain, (2) Lhermitte's sign, (3) pain associated with optic neuritis and trigeminal neuralgia, (4) pain associated with immunomodulatory treatment, (5) painful tonic spasms, (6) back pain, and (7) headache [4, 92, 93, 94, 95, 96]. Dysesthetic extremity pain, Lhermitte’s sign and trigeminal neuralgia fulfil the definition of continuous or intermittent central neuropathic pain, and should be classified as MS-related pain since typically following the formation of demyelinating lesions in specific brain areas. Painful tonic spasms, low back pain, and muscle spasms are classified as musculoskeletal pain, and are generally the result of spasticity and/or altered postures. Headache conditions are classified as mixed neuropathic and non-neuropathic pain, and may be MS-independent or treatment-related pain disturbances [87].
While dysesthetic pain, trigeminal neuralgia and painful tonic spasms may present with features typical of the classical MS demyelinating plaques, all the other pain conditions may present with similar features between MS and other neurological conditions. Therefore, for the purpose of the present review, we will focus only on dysesthetic pain, trigeminal neuralgia and painful tonic spasms.
Dysesthetic extremity pain is the most frequent pain condition in MS patients, occurring in approximately 23% of the patients at least once during the disease lifespan [89, 93, 95]. It is a continuous, bilateral and burning pain, usually worsening at night. In our limited experience, patients with dysesthetic extremity pain worsening at night may benefit of dopaminergic drugs such as those administered for the treatment of restless legs syndrome (Centonze D, preliminary results). This observation raises the question of a possible pathophysiological overlap between dysesthetic extremity pain and restless legs syndrome in MS patients.
The prevalence of trigeminal neuralgia in MS patients is estimated to be 20 times greater than in general population [97], and is classically associated with demyelinating plaques located in the brainstem [98]. Trigeminal neuralgia can affect one or both fifth cranial nerve at the same time and can be the presenting symptom in 10–15% of the MS cases [96, 97, 98]. Although MS-associated trigeminal neuralgia may be in some cases indistinguishable from the idiopathic form, few characteristics may help clinicians in discriminating the two types. In MS, trigeminal neuralgia has the tendency to appear in very young people, can be bilateral and has often a longer duration of pain attacks. Furthermore, MS patients are less likely to have the concomitant involvement of ophthalmic nerve [98].
Painful tonic spasms are the third most frequent type of pain associated with MS [99, 100]. Lesions associated with painful tonic spasms were seen in the basal ganglia, in the internal capsula, medulla oblongata and the cerebral peduncle [101]. However, it is commonly accepted that painful tonic spasms follows an ephaptic spread of discharges as one can see in demyelinated axons such as those of MS patients [100, 101].
7.2 Pharmacological and non-pharmacological therapies to combat MS-related pain
Previous authors have been extensively reviewed the topic of pharmacological strategies to defeat pain in MS [86, 87, 102]. As seen for non-MS related pain, in the daily clinical practice, treatment of MS pain syndromes remains largely empirical and symptomatic, and relies upon the use of older and newer antidepressants and anticonvulsants, alone or in various combinations. In fact, only few systematic clinical studies have been performed to evaluate the efficacy of different drugs to defeat pain in MS (Table 2). With some exceptions [102, 103, 104], the majority of these studies suffer the limitation of not being randomized, double-blind, placebo-controlled clinical studies. Furthermore, different pain syndromes tend to be not differentiated enough by the treating physicians, resulting into largely unsatisfactory pharmacological approaches to pain.
TABLE 2.
DRUGS TESTED TO CONTROL MS-INDUCED PAIN
| Study | Number of patients | Drug | Efficacy | Comments |
|---|---|---|---|---|
| Centonze et al (2009) [98] | 20 | Sativex | Not proven | N/A |
| Chitsaz et al (2009) [121] | 27 | Nortriptyline | Proven | Average dose 50 mg |
| Solaro et al (2009) [122] | 16 | Pregabalin | Proven | Effective only on painful paroxysmal symptoms |
| Rossi et al (2009) [97] | 20 | Levetiracetam | Proven | N/A |
| Breuer et al (2007) [90] | 12 | Lamotrigine | Not proven | N/A |
| Rog et al (2007) [89] | 66 | Sativex | Proven | Effective only on dysesthetic painful spasms |
Sativex: Oromucosal delta9-tetrahydrocannabinol/cannabidiol.
N/A=not applicable
In our group, on the notion that levetiracetam has analgesic properties in both animals and humans [105, 106, 107, 108, 109, 110], we investigated the effect of levetiracetam in reducing central neuropathic pain in MS patients in patients with intolerance to conventional medications (i.e., gabapentin, carbamazepine, pregabalin, amitriptyline, duloxetine, baclofen) or non-responder to them [111]. Levetiracetam was well tolerated, significantly reduced the pain intensity score (Figure 4A) and maintained a higher proportion of patients (Figure 4B) with significant pain reduction than placebo. Quality of life rating improved in treated patients (Figure 4C), as a function of pain reduction (Figure 4D). Pain tended to be more markedly decreased in patients suffering for higher pre-therapy level of pain.
Conversely, we failed in demonstrating the analgesic properties of a cannabis-based pharmaceutical preparation containing delta9-tetrahydrocannabinol and cannabidiol, approved in some countries as a second-line therapy in the treatment of chronic neuropathic pain in MS. Different patient inclusion criteria and the small number of patients in our study might contribute to explain the discrepancy between our results and those of others [112 and references therein].
Alternative to pharmacological strategies, non-invasive brain stimulation techniques such as anodal transcranial direct current stimulation (tDCS) and repetitive transcranial magnetic stimulation (rTMS) can be considered for the treatment of pain. tDCS applied over the sensory-motor cortex decreases pain sensation and increase pain threshold in healthy subjects, and repeated sessions of anodal tDCS may ease pain in patients with several non-MS conditions [113, 114, 115, 116, 117]. A double blind, randomized, placebo controlled study, addressing the effects of daily sessions of anodal tDCS repeated for five consecutive days was performed with 16 MS patients with central neuropathic pain in our group. We showed that tDCS may relieve pain sensation in MS patients [118]. In contrast, we did not observe analgesic effects of rTMS in MS patients (Mori F and Centonze D, unpublished observations). It is likely that tDCS and rTMS have slightly different neuromodulatory properties, tDCS being preferable to treat pain of MS patients. Conversely, rTMS is beneficial effect in reducing spasticity in MS patients [119, 120], suggesting that rTMS might be useful to treat painful spasms associated with spasticity in MS.
9. FUTURE PERSPECTIVE
Future research should have several aims.
First effort should be devoted to improve the diagnostic algorithm and to standardize diagnostic tests to disclose pain, dysphagia, respiratory problems, sexual and cardiovascular dysfunctions and pain in MS. Second, clinical scientists should endeavour to design clinical scales accurately heightening the impact that dysphagia, respiratory disturbances, SD and CD may have in the daily life and overall disability level of MS patients. Third, the use of non-conventional MRI techniques nowadays exploited for understanding the basis of other components of the MS disability should be applied towards a better understanding of pain, dysphagia, respiratory disturbances, SD and CD imaging correlates. Fourth, large randomized double blind placebo controlled or active comparators clinical trials are needed in order to test the effect of new drugs or rehabilitative strategies to prevent the occurrence of the symptoms in MS patients or modulate their severity. Last, effort should be devoted to render currently available treatment modalities more effective and tolerable.
10. EXPERT OPINION
Pain, dysphagia, respiratory problems, sexual and cardiovascular dysfunctions may significantly affect patients with MS, even in the early stage of the disease. The role of these symptoms in the context of MS, as primary disease sign or as a side effect of currently employed medications, remains unknown.
Pain, dysphagia, respiratory problems, sexual and cardiovascular dysfunctions do significantly impact patient daily life and may be a major source of disability. Hence, the assessment of these symptoms should be performed as early as possible after the diagnosis of MS and even in the absence of clinically evident problems.
Because of the insidious and often times delicate nature of pain, dysphagia, respiratory problems, sexual and cardiovascular dysfunctions, they may be often neglected by physicians and rarely reported by patients. Hence, it is extremely important that neurologists are fully aware of the entity and the complexity of dysphagia, respiratory problems CD, SD, pain in MS and sufficiently trained to discuss and manage those problems with their patients. Up to now, there is limited knowledge about these clinical problems and possible strategies to overcome them. High level studies to symptomatic MS therapy are rare and recommendations often refer to effects of treatment in other neurological diseases.
It remains mandatory that once diagnosed, a multidisciplinary approach is undertaken to promptly set up symptomatic therapies aimed to at least an improvement in patient QoL.
10.1 HIGHLIGHTS
Physicians, neurologists specifically, need to be aware of pain, dysphagia, respiratory problems, sexual and cardiovascular dysfunctions and ask about them because patients are often not forthcoming. However, if these symptoms are unrecognized can lead to many serious problems.
These symptoms need to be evaluated at any and every stage of the disease. On the basis of their degree of severity, planning and performing specific individualized approaches is required by a multidisciplinary team.
Their evalutation of SD in MS patients requires insight into neurological, physical, psychological and social aspects. A holistic and multidisciplinary approach is required for their successful management of SD.
For each of these symptoms, non-specific therapies are available and often. tend to be refractory to conventional treatments in MS.
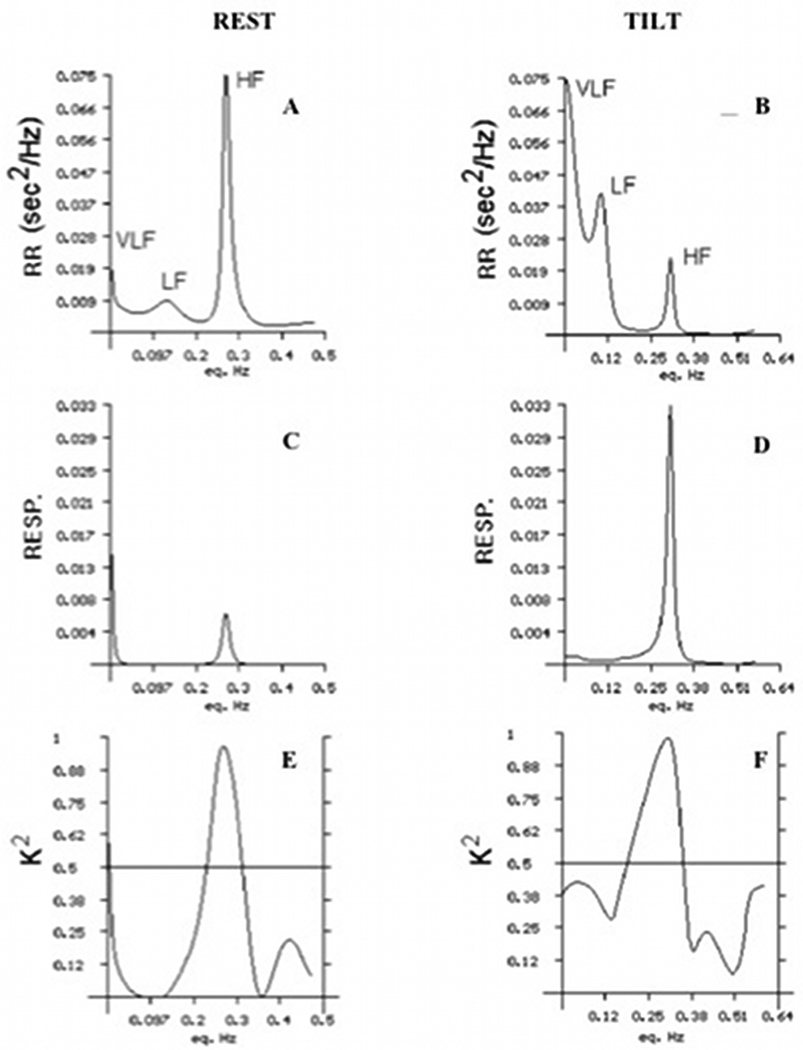
FIGURE 2.
Spectral Analysis of beat-to-beat heart rate oscillations, adapted from [38].
Figure 2A and 2B. Example of a normal subject at rest and after orthostatic activation induced by passive tilting. The very low frequency, low frequency (parasympathetic + sympathetic) and high frequency (parasympathetic) components are indicated.
Figure 2C and 2D. The respiratory signal (respirogram) of the same subject at rest and after orthostatic activation. The main respiratory frequency is always synchronous with the high frequency component.
Figure 2E and 2F. The coherence function resulting from the cross-spectral analysis of tachogram and respirogram. As one can see, the main respiratory frequency shows a high coherence with the high frequency. The coherence in the low frequency band is very low.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the work of A Cuccaro and V Ikonomidou in preparing the manuscript.
Footnotes
Declaration of interest
F Bagnato’s contribution was sustained by the Intramural Program of the NINDS, NIH, Bethesda, MD, USA. The authors state no conflict of interest and have received no payment in preparation of this manuscript
REFERENCES
- 1.Compston A, Coles A. Multiple Sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 2.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58:840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 3.Barkhof F, Filippi M, Miller DH, et al. Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain. 1997;120:2059–2069. doi: 10.1093/brain/120.11.2059. [DOI] [PubMed] [Google Scholar]
- 4.Solaro C, Messmer Uccelli M. Pharmacological management of pain in patients with multiple sclerosis. Drugs. 2010;70:1245–1254. doi: 10.2165/11537930-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Hartelius L, Svensson P. Speech and swallowing symptom associated with Parkinson’s disease and multiple sclerosis: a survey. Folia Phoniatr Logop. 1994;46:9–17. doi: 10.1159/000266286. [DOI] [PubMed] [Google Scholar]
- 6.Logemann JA. Dysphagia: evaluation and treatment. Folia Phoniatr Logop. 1995;47:140–164. doi: 10.1159/000266348. [DOI] [PubMed] [Google Scholar]
- 7.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 8.Tassorelli C, Bergamaschi R, Buscone S, et al. Dysphagia in multiple sclerosis: from pathogenesis to diagnosis. Neurol Sci. 2008;(29 Suppl 4):360–363. doi: 10.1007/s10072-008-1044-9. [DOI] [PubMed] [Google Scholar]
- 9.De Pauw A, Dejaeger E, D'hooghe B, et al. Dysphagia in multiple sclerosis. Clin Neurol Neurosurg. 2002;104:345–351. doi: 10.1016/s0303-8467(02)00053-7. [DOI] [PubMed] [Google Scholar]
- 10.Abraham SS, Yun PT. Laryngopharyngeal dysmotility in multiple sclerosis. Dysphagia. 2002;17:69–74. doi: 10.1007/s00455-001-0103-7. [DOI] [PubMed] [Google Scholar]
- 11.Hoppers P, Holm SE. The role of fiberoptic endoscopy in Dysphagia rehabilitation. J Head Trauma Rehabil. 1999;14:475–485. doi: 10.1097/00001199-199910000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Calcagno P, Ruoppolo G, Grasso MG, et al. Dysphagia in multiple sclerosis - Prevalence and prognostic factors. Acta Neurol Scand. 2002;105:40–43. doi: 10.1034/j.1600-0404.2002.10062.x. [DOI] [PubMed] [Google Scholar]
- 13.Taniguchi H, Tsukada T, Ootaki S, et al. Correspondence between food consistency and suprahyoid muscle activity, tongue pressure, and bolus transit times during the oropharyngeal phase of swallowing. J Appl Physiol. 2008;105:791–799. doi: 10.1152/japplphysiol.90485.2008. [DOI] [PubMed] [Google Scholar]
- 14.Thomas FJ, Wiles CM. Dysphagia and nutritional status in multiple sclerosis. J Neurol. 1999;246:677–682. doi: 10.1007/s004150050431. [DOI] [PubMed] [Google Scholar]
- 15.American Speech-Language Hearing Association. National Outcomes Measurements System (NOMS): Adult Speech-Language Pathology Training Manual. Rockville (Maryland): American Speech-Language Hearing Association; 1998. [Google Scholar]
- 16.Restivo DA, Marchese Ragona R, Patti F, Maimone D, Zappaà G, Pavone A. Botulinum toxin improves dysphagia associated with multiple sclerosis. Eur J Neurol. 2010 Aug; doi: 10.1111/j.1468-1331.2010.03189.x. [DOI] [PubMed] [Google Scholar]
- 17.DeAngelis T, Lublin F. Multiple sclerosis: new treatment trials and emerging therapeutic targets. Curr Opin Neurol. 2008;21:261–271. doi: 10.1097/WCO.0b013e328300c70d. [DOI] [PubMed] [Google Scholar]
- 18.Inagaki D, Miyaoka Y, Ashida I, et al. Influence of food properties and body position on swallowing related muscle activity amplitude. J Oral Rehabil. 2009;36:176–183. doi: 10.1111/j.1365-2842.2008.01927.x. [DOI] [PubMed] [Google Scholar]
- 19.Henze T. What is new in symptom management? Int MS J. 2007;14:22–27. [PubMed] [Google Scholar]
- 20.Payne A. Nutrition and diet in the clinical management of multiple sclerosis. J Hum Nutr Diet. 2001;14:349–357. doi: 10.1046/j.1365-277x.2001.00308.x. [DOI] [PubMed] [Google Scholar]
- 21.Prosiegel M, Schelling A, Wagner-Sonntag E. Dysphagia and multiple sclerosis. Int MS J. 2004;11:22–31. [PubMed] [Google Scholar]
- 22.Giusti A, Giambuzzi M. Management of Dysphagia in patients affected by multiple sclerosis: state of the art. Neurol Sci. 2008;(29 Suppl 4):364–366. doi: 10.1007/s10072-008-1045-8. [DOI] [PubMed] [Google Scholar]
- 23.Schrag SP, Sharma R, Jaik NJ. Complications Related to Percutaneous EndoscopicGastrostomy (PEG) Tubes. A Comprehensive Clinical ReviewJ Gastrointestin Liver Dis. 2007 December;4:407–418. [PubMed] [Google Scholar]
- 24.Grasso MG, Lubich S, Guidi L, et al. Cerebellar deficit and respiratory impairment: a strong association in multiple sclerosis? Acta Neurol Scand. 2000;101:98–103. doi: 10.1034/j.1600-0404.2000.101002098.x. [DOI] [PubMed] [Google Scholar]
- 25.Gosselink R, Kovacs L, Decramer M. Respiratory muscle involvement in multiple sclerosis. Eur Respir J. 1999;13:449–454. doi: 10.1183/09031936.99.13244999. [DOI] [PubMed] [Google Scholar]
- 26.Fry DK, Pfalzer LA, Chokshi AR, et al. Randomized control trial of effects of a 10-week inspiratory muscle training program on measures of pulmonary function in persons with multiple sclerosis. J Neurol Phys Ther. 2007;31:162–172. doi: 10.1097/NPT.0b013e31815ce136. [DOI] [PubMed] [Google Scholar]
- 27.Aiello M, Rampello A, Granella F, et al. Cough efficacy is related to the disability status in patients with multiple sclerosis. Respiration. 2008;76:311–316. doi: 10.1159/000119641. [DOI] [PubMed] [Google Scholar]
- 28.Mutluay FK, Gürses HN, Saip S. Effects of multiple sclerosis on respiratory functions. Clin Rehabil. 2005;19:426–432. doi: 10.1191/0269215505cr782oa. [DOI] [PubMed] [Google Scholar]
- 29.Gosselink R, Kovacs L, Ketelaer P, et al. Respiratory muscle weakness and respiratory muscle training in severely disabled multiple sclerosis patients. Arch Phys Med Rehabil. 2000;81:747–751. doi: 10.1016/s0003-9993(00)90105-9. [DOI] [PubMed] [Google Scholar]
- 30.Sadovnick AD, Eisen K, Ebers GC, et al. Cause of death in patients attending multiple sclerosis clinics. Neurology. 1991;41:1193–1196. doi: 10.1212/wnl.41.8.1193. [DOI] [PubMed] [Google Scholar]
- 31.Midgard R, Albrektsen G, Riise T, et al. Prognostic factors for survival in multiple sclerosis: a longitudinal, population based study in Møre and Romsdal, Norway. J Neurol Neurosurg Psychiatry. 1995;58:417–421. doi: 10.1136/jnnp.58.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirst C, Swingler R, Compston DA, et al. Survival and cause of death in multiple sclerosis: a prospective population-based study. J Neurol Neurosurg Psychiatry. 2008;79:1016–1021. doi: 10.1136/jnnp.2007.127332. [DOI] [PubMed] [Google Scholar]
- 33.Aboussouan LS. Respiratory disorders in neurologic disease. Cleve Clin J Med. 2005;72(6):511–520. doi: 10.3949/ccjm.72.6.511. [DOI] [PubMed] [Google Scholar]
- 34.Chiara T, Martin AD, Davenport PW, et al. Expiratory muscle strength training in persons with multiple sclerosis having mild to moderate disability: effect on maximal expiratory pressure, pulmonary function, and maximal voluntary cough. Arch Phys Med Rehabil. 2006;87:468–473. doi: 10.1016/j.apmr.2005.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olindo S, Guillon B, Helias J, Philibert B, et al. Decrease in heart ventricular ejection fraction during multiple sclerosis. Eur J Neurol. 2002;9:287–291. doi: 10.1046/j.1468-1331.2002.00400.x. [DOI] [PubMed] [Google Scholar]
- 36.Merkelbach S, Haensch CA, Hemmer B, et al. Multiple sclerosis and the autonomic nervous system. J Neurol. 2006;(253 Suppl 1):21–25. doi: 10.1007/s00415-006-1105-z. [DOI] [PubMed] [Google Scholar]
- 37.Scroth WS, Tenner SM, Rappaport BA, et al. Multiple sclerosis as a cause of atrial fibrillation and electro- cardiographic changes. Arch Neurol. 1992;49:422–424. doi: 10.1001/archneur.1992.00530280116034. [DOI] [PubMed] [Google Scholar]
- 38.Frontoni M, Fiorini M, Strano S, et al. Power spectrum analysis contribution to the detection of cardiovascular dysautonomia in multiple sclerosis. Acta Neurol Scand. 1996;93:241–245. doi: 10.1111/j.1600-0404.1996.tb00514.x. [DOI] [PubMed] [Google Scholar]
- 39.Linden D, Diehl RR, Berlit P. Subclinical autonomic disturbances in multiple sclerosis. J Neurol. 1995;242:374–378. doi: 10.1007/BF00868392. [DOI] [PubMed] [Google Scholar]
- 40.Vita G, Fazio MC, Milone S, et al. Cardiovascular autonomic dysfunction in multiple sclerosis is likely related to brainstem lesions. J Neurol Sci. 1993;120:82–86. doi: 10.1016/0022-510x(93)90029-x. [DOI] [PubMed] [Google Scholar]
- 41.Anema JR, Heijenbrok MW, Faes TJ, et al. Cardiovascular autonomic function in multiple sclerosis. J Neurol Sci. 1991;104:129. doi: 10.1016/0022-510x(91)90301-m. 134. [DOI] [PubMed] [Google Scholar]
- 42.Flachenecker P, Wolf A, Krauser M, et al. Cardiovascular autonomic dysfunction in multiple sclerosis: correlation with orthostatic intolerance. J Neurol. 1999;246:578–586. doi: 10.1007/s004150050407. [DOI] [PubMed] [Google Scholar]
- 43.Flachenecker P, Reiners K. Abnormal baroreflex responses in multiple sclerosis. Clin Auton Res. 2005;15:419. doi: 10.1007/s10286-005-0315-2. [DOI] [PubMed] [Google Scholar]
- 44.Kanjwal K, Karabin B, Kanjwal Y, Grubb BP. Autonomic Dysfunction Presenting as Postural Orthostatic Tachycardia Syndrome in Patients with Multiple Sclerosis. Int. J. Med. Sci. 2010;7:62–67. doi: 10.7150/ijms.7.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marriott JJ, Miyasaki JM, Gronseth G, et al. Evidence Report: The efficacy and safety of mitoxantrone (Novantrone) in the treatment of multiple sclerosis: Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2010;74:1463–1470. doi: 10.1212/WNL.0b013e3181dc1ae0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ziemssen T, Neuhaus O, Hohlfeld R. Risk-Benefit Assessment of Glatiramer Acetate in Multiple Sclerosis. Drug Safety. 2001;24(13):979–990. doi: 10.2165/00002018-200124130-00005. [DOI] [PubMed] [Google Scholar]
- 47.Linden D, Diehl RR, Berlit P. Subclinical autonomic disturbances in multiple sclerosis. J Neurol. 1995;242:374–378. doi: 10.1007/BF00868392. [DOI] [PubMed] [Google Scholar]
- 48.Acevedo AR, Nava C, Arriada N, et al. Cardiovascular dysfunction in multiple sclerosis. Acta Neurol Scand. 2000;10:85–88. doi: 10.1034/j.1600-0404.2000.101002085.x. [DOI] [PubMed] [Google Scholar]
- 49.Flachenecker P, Reiners K, Krauser M, et al. Autonomic dysfunction in multiple sclerosis is related to disease activity and progression disability. Mult Scler. 2001;7:327–334. doi: 10.1177/135245850100700509. [DOI] [PubMed] [Google Scholar]
- 50.Saari A, Tolonen U, Paakko E, et al. Cardiovascular autonomic dysfunction correlates with brain MRI lesion load in MS. Clin Neurophysiol. 2004;115:1473–1478. doi: 10.1016/j.clinph.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 51.Moya A, Sutton R, Ammirati F, et al. Guidelines for the diagnosis and management of syncope (version 2009): the task force for the diagnosis and management of syncope of the European Society of Cardiology (ESC) Eur Heart J. 2009;30:2631–2671. doi: 10.1093/eurheartj/ehp298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quaglia MG, Farina A, Palmery M, et al. Chiral investigation of midodrine, a long-acting alpha-adrenergic stimulating agent. Chirality. 2004;16:356–362. doi: 10.1002/chir.20041. [DOI] [PubMed] [Google Scholar]
- 53.Wright RA, Kaufman HC, Perera R, et al. A double-blind, dose - response study of midodrine in neurogenic orthostatic hypotension. Neurology. 1998;51:120–124. doi: 10.1212/wnl.51.1.120. [DOI] [PubMed] [Google Scholar]
- 54.Pathak A, Raoul V, Montastruc JL, Senard JM. Adverse drug reactions related to drugs used in orthostatic hypotension: a prospective and systematic pharmacovigilance study in France. Eur J Clin Pharmacol. 2005;61:471–474. doi: 10.1007/s00228-005-0941-6. [DOI] [PubMed] [Google Scholar]
- 55.van Lieshout JJ, ten Harkel AD, Wieling W. Fludrocortisone and sleeping in the head-up position limit the postural decrease in CO in autonomic failure. Clin Auton Res. 2000;10:35–42. doi: 10.1007/BF02291388. [DOI] [PubMed] [Google Scholar]
- 56.Bayorh MA, Eatman D, Wang M, et al. Indomethacin attenuates post-suspension hypotension in Sprague-Dawley rats. J Gravit Physiol. 2001;8:77–83. [PubMed] [Google Scholar]
- 57.Wyller VB, Thaulow E, Amlie JP. Treatment of chronic fatigue and orthostatic intolerance with propranolol. J Pediatr. 2007;150:654–655. doi: 10.1016/j.jpeds.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 58.Di Girolamo E, Di Iorio C, Sabatini P, et al. Effects of paroxetine hydrochloride, a selective serotonin reuptake inhibitor, on refractory vasovagal syncope: a randomized, double-blind, placebo-controlled study. J Am Coll Cardiol. 1999;33:1227–1230. doi: 10.1016/s0735-1097(98)00694-9. [DOI] [PubMed] [Google Scholar]
- 59.Zorzon M, Zivadinov R, Monti Bragadin L, et al. Sexual dysfunction in multiple sclerosis: a 2-year follow-up study. J Neurol Sci. 2001;187:1–5. doi: 10.1016/s0022-510x(01)00493-2. [DOI] [PubMed] [Google Scholar]
- 60.Demirkiran M, Sarica Y, Uguz S, et al. Multiple sclerosis patients with and without sexual dysfunction: are there any differences? Mult Scler. 2006;12:209–214. doi: 10.1191/135248506ms1253oa. [DOI] [PubMed] [Google Scholar]
- 61.Tepavcevic DK, Kostic J, Basuroski ID, et al. The impact of sexual dysfunction on the quality of life measured by MSQoL-54 in patients with multiple sclerosis. Mult Scler. 2008;14:1131–1136. doi: 10.1177/1352458508093619. [DOI] [PubMed] [Google Scholar]
- 62.Zorzon M, Zivadinov R, Bosco A, et al. Sexual dysfunction in multiple sclerosis: a case-control study. I. Frequency and comparison of groups. Mult Scler. 1999;5:418–427. doi: 10.1177/135245859900500i609. [DOI] [PubMed] [Google Scholar]
- 63.Nortvedt MW, Riise T, Myhr KM, et al. Reduced quality of life among multiple sclerosis patients with sexual disturbance and bladder dysfunction. Mult Scler. 2001;7:231–235. doi: 10.1177/135245850100700404. [DOI] [PubMed] [Google Scholar]
- 64.Tzorzis V, Skriapas K, Hadjigeorgiou G, et al. Sexual dysfunction in newly diagnosed multiple sclerosis women. Mult Scler. 2008;14:561–563. doi: 10.1177/13524585080140040901. [DOI] [PubMed] [Google Scholar]
- 65.Stenager E, Stenager EN, Jensen K. Sexual function in multiple sclerosis: a 5-year follow-up study. Ital J Neurol Sci. 1996;17:67–69. doi: 10.1007/BF01995711. [DOI] [PubMed] [Google Scholar]
- 66.Nortvedt MW, Riise T, Frugard, et al. Prevalence of bladder, bowel and sexual problems among multiple sclerosis patients two to five years after diagnosis. Mult Scler. 2007;13:106–112. doi: 10.1177/1352458506071210. [DOI] [PubMed] [Google Scholar]
- 67.Mattson D, Petrie M, Srivastava DK, et al. Multiple sclerosis. Sexual dysfunction and its response to medications. Arch Neurol. 1995;52:862–868. doi: 10.1001/archneur.1995.00540330040012. [DOI] [PubMed] [Google Scholar]
- 68.Fletcher SG, Castro-Borrero W, Remington G, et al. Sexual dysfunction in patients with multiple sclerosis: a multidisciplinary approach to evaluation and management. Nat Clin Pract Urol. 2009;6:96–107. doi: 10.1038/ncpuro1298. [DOI] [PubMed] [Google Scholar]
- 69.Hulter BM, Lundberg PO. Sexual function in women with advanced multiple sclerosis. J Neurol Neurosurg Psychiatry. 1995;59:83–86. doi: 10.1136/jnnp.59.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zivadinov R, Zorzon M, Locatelli L, et al. Sexual dysfunction in multiple sclerosis: a MRI, neuropsychological and urodynamic study. J Neurol Sci. 2003;210:73–76. doi: 10.1016/s0022-510x(03)00025-x. [DOI] [PubMed] [Google Scholar]
- 71.Janardhan V, Bakshi R. Quality of life and its relationship to brain lesions and atrophy on magnetic resonance images in 60 patients with multiple sclerosis. Arch Neurol. 2000;57:1485–1491. doi: 10.1001/archneur.57.10.1485. [DOI] [PubMed] [Google Scholar]
- 72.McCabe MP. Exacerbation of symptoms among people with multiple sclerosis patients: impact on sexuality and relationships over time. Arch Sex Behav. 2004;33:593–601. doi: 10.1023/B:ASEB.0000044743.41613.fc. [DOI] [PubMed] [Google Scholar]
- 73.Valleroy ML, Kraft GH. Sexual dysfunction in multiple sclerosis. Arch Phys Med Rehabil. 1984;65:125–128. [PubMed] [Google Scholar]
- 74.Betts CD, Jones SJ, Fowler CG, et al. Erectile dysfunction in multiple sclerosis. Associated neurological and neurophysiological deficits, and treatment of the condition. Brain. 1994;117:1303–1310. doi: 10.1093/brain/117.6.1303. [DOI] [PubMed] [Google Scholar]
- 75.Zivadinov R, Zorzon M, Bosco A, et al. Sexual dysfunction in multiple sclerosis: II. Correlation analysis. Mult Scler. 1999;5:428–431. doi: 10.1177/135245859900500i610. [DOI] [PubMed] [Google Scholar]
- 76.Barak Y, Achiron A, Elizur A, et al. Sexual dysfunction in relapsing-remitting multiple sclerosis: magnetic resonance imaging, clinical, and psychological correlates. J Psychiatry Neurosci. 1996;21:255–258. [PMC free article] [PubMed] [Google Scholar]
- 77.Haggiag S, Ricci G, Bolla G, et al. Gender differences in sexual dysfunction among a Multiple Sclerosis population. J Neurol. 2010;257:S75. [Google Scholar]
- 78.Tsertsvadze A, Fink S, Yazdi F, et al. Oral phosphodiesterase-5 inhibitors and hormonal treatments for erectile dysfunction: a systematic review and meta-analysis. Ann Intern Med. 2009;151(9):650–661. doi: 10.7326/0003-4819-151-9-200911030-00150. [DOI] [PubMed] [Google Scholar]
- 79.Fowler CJ, Miller JR, Sharief MK, et al. A double blind, randomised study of sildenafil citrate for erectile dysfunction in men with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2005;76:700–705. doi: 10.1136/jnnp.2004.038695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Safarinejad MR. Evaluation of the safety and efficacy of sildenafil citrate for erectile dysfunction in men with multiple sclerosis: a double-blind, placebo controlled, randomized study. J Urol. 2009;181:252–258. doi: 10.1016/j.juro.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 81.Lombardi G, Macchiarella A, Del Popolo G. Efficacy and safety of Tadalafil for erectile dysfunction in patients with multiple sclerosis. J Sex Med. 2010 April 1; doi: 10.1111/j.1743-6109.2010.01797.x. [DOI] [PubMed] [Google Scholar]
- 82.Denil J, Ohl DA, Smythe C. Vacuum erection device in spinal cord injured men: patients and partner satisfaction. Arch Phys Rehabilit. 1996;77:750–753. doi: 10.1016/s0003-9993(96)90252-x. [DOI] [PubMed] [Google Scholar]
- 83.Dasgupta R, Wiseman OJ, Kanabar G, et al. Efficacy of sildenafil in the treatment of female sexual dysfunction due to multiple sclerosis. J Urol. 2004;171:1189–1193. doi: 10.1097/01.ju.0000113145.43174.24. [DOI] [PubMed] [Google Scholar]
- 84.Kaplan SA, Reis RB, Kohn IJ, et al. Safety and efficacy of sildenafil in postmenopausal women with sexual dysfunction. Urology. 1999;53:481–486. doi: 10.1016/s0090-4295(98)00633-5. [DOI] [PubMed] [Google Scholar]
- 85.Caruso S, Intellisano G, Lupo L, et al. Premenopausal women affected by sexual arousal disorder treated with sildenafil: a double-blind, cross-over, placebo-controlled study. Br J Obstet Gynaecol. 2001;108:623–628. doi: 10.1111/j.1471-0528.2001.00143.x. [DOI] [PubMed] [Google Scholar]
- 86.Kenner M, Menon U, Elliott DG. Multiple sclerosis as a painful disease. Int Rev Neurobiol. 2007;79:303–321. doi: 10.1016/S0074-7742(07)79013-X. [DOI] [PubMed] [Google Scholar]
- 87.O'Connor AB, Schwid SR, Herrmann DN, et al. Pain associated with multiple sclerosis: systematic review and proposed classification. Pain. 2008;137:96–111. doi: 10.1016/j.pain.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 88.Nurmikko TJ, Gupta S, Maclver K. Multiple sclerosis-related central pain disorders. Curr Pain Headache Rep. 2010;14:189–195. doi: 10.1007/s11916-010-0108-8. [DOI] [PubMed] [Google Scholar]
- 89.Osterberg A, Boivie J. Central pain in multiple sclerosis - sensory abnormalities. Eur J Pain. 2010;14:104–110. doi: 10.1016/j.ejpain.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 90.Kalia LV, O’Connor PW. Severity of chronic pain and its relationship to quality of life in multiple sclerosis. Mult Scler. 2005;11:322–327. doi: 10.1191/1352458505ms1168oa. [DOI] [PubMed] [Google Scholar]
- 91.Svendsen KB, Jensen TS, Hansen HJ, Bach FW. Sensory function and quality of life in patients with multiple sclerosis and pain. Pain. 2005;114:473–481. doi: 10.1016/j.pain.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 92.Ehde DM, Gibbons LE, Chwastiak L, et al. Chronic pain in a large community sample of persons with multiple sclerosis. Mult Scler. 2003;9:605–611. doi: 10.1191/1352458503ms939oa. [DOI] [PubMed] [Google Scholar]
- 93.Osterberg A, Boivie J, Thuomas KA. Central pain in multiple sclerosis: prevalence and clinical characteristics. Eur J Pain. 2005;9:531–542. doi: 10.1016/j.ejpain.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 94.Cruccu G, Biasiotta A, Di Rezze S, et al. Trigeminal neuralgia and pain related to multiple sclerosis. Pain. 2009;143:186–191. doi: 10.1016/j.pain.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 95.Solaro C, Brichetto G, Amato MP, et al. The prevalence of pain in multiple sclerosis: a multicenter cross-sectional study. Neurology. 2004;63:919–921. doi: 10.1212/01.wnl.0000137047.85868.d6. [DOI] [PubMed] [Google Scholar]
- 96.Hooge JP, Redekop WK. Trigeminal neuralgia in multiple sclerosis. Neurology. 1995;45:1294–1296. doi: 10.1212/wnl.45.7.1294. [DOI] [PubMed] [Google Scholar]
- 97.Katusic S, Beard CM, Bergstralh E, Kurland LT. Incidence and clinical features of trigeminal neuralgia, Rochester, Minnesota, 1945–1984. Ann Neurol. 1990;27:89–95. doi: 10.1002/ana.410270114. [DOI] [PubMed] [Google Scholar]
- 98.De Simone R, Marano E, Brescia Morra V, et al. A clinical comparison of trigeminal neuralgic pain in patients with and without underlying multiple sclerosis. Neurol Sci. 2005;(26 Suppl 2):150–151. doi: 10.1007/s10072-005-0431-8. [DOI] [PubMed] [Google Scholar]
- 99.Matthews WB. Paroxysmal symptoms in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1975;38:617–623. doi: 10.1136/jnnp.38.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Spissu A, Cannas A, Ferrigno P, et al. Anatomic correlates of painful tonic spasms in multiple sclerosis. Mov Disord. 1999;14:331–335. doi: 10.1002/1531-8257(199903)14:2<331::aid-mds1020>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 101.Restivo DA, Tinazzi M, Patti F, et al. Botulinum toxin treatment of painful tonic spasms in multiple sclerosis. Neurology. 2003;61:719–720. doi: 10.1212/01.wnl.0000080081.74117.e4. [DOI] [PubMed] [Google Scholar]
- 102.Solaro C, Tanganelli P, Messmer Uccelli M. Pharmacological treatment of pain in multiple sclerosis. Expert Rev Neurother. 2007;7:1165–1174. doi: 10.1586/14737175.7.9.1165. [DOI] [PubMed] [Google Scholar]
- 103.Rog DJ, Nurmikko TJ, Friede T, Young CA. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology. 2005;65:812–819. doi: 10.1212/01.wnl.0000176753.45410.8b. [DOI] [PubMed] [Google Scholar]
- 104.Breuer B, Pappagallo M, Knotkova H, et al. A randomized, double-blind, placebo-controlled, two-period, crossover, pilot trial of lamotrigine in patients with central pain due to multiple sclerosis. Clin Ther. 2007;29:2022–2030. doi: 10.1016/j.clinthera.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 105.Ardid D, Lamberty Y, Alloui A, et al. Antihyperalgesic effect of levetiracetam in neuropathic pain models in rats. Eur J Pharmacol. 2003;473:27–33. doi: 10.1016/s0014-2999(03)01933-2. [DOI] [PubMed] [Google Scholar]
- 106.Guay DR. Oxcarbazepine, topiramate, zonisamide, and levetiracetam: potential use in neuropathic pain. Am J Geriatr Pharmacother. 2003;1:18–37. doi: 10.1016/s1543-5946(03)80013-2. [DOI] [PubMed] [Google Scholar]
- 107.Rowbotham MC, Manville NS, Ren J. Pilot tolerability and effectiveness study of levetiracetam for postherpetic neuralgia. Neurology. 2003;61:866–867. doi: 10.1212/01.wnl.0000079463.16377.07. [DOI] [PubMed] [Google Scholar]
- 108.Price MJ. Levetiracetam in the treatment of neuropathic pain: three case studies. Clin J Pain. 2004;20:33–36. doi: 10.1097/00002508-200401000-00007. [DOI] [PubMed] [Google Scholar]
- 109.Enggaard TP, Klitgaard NA, Sindrup SH. Specific effect of levetiracetam in experimental human pain models. Eur J Pain. 2006;10:193–198. doi: 10.1016/j.ejpain.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 110.Archer DP, Lamberty Y, Wang B, et al. Levetiracetam reduces anesthetic-induced hyperalgesia in rats. Anesth Analg. 2007;104:180–185. doi: 10.1213/01.ane.0000247788.57318.1f. [DOI] [PubMed] [Google Scholar]
- 111.Rossi S, Mataluni G, Codecà C, et al. Effects of levetiracetam on chronic pain in multiple sclerosis: results of a pilot, randomized, placebo-controlled study. Eur J Neurol. 2009;16:360–366. doi: 10.1111/j.1468-1331.2008.02496.x. [DOI] [PubMed] [Google Scholar]
- 112.Centonze D, Mori F, Koch G, et al. Lack of effect of cannabis-based treatment on clinical and laboratory measures in multiple sclerosis. Neurol Sci. 2009;30:531–534. doi: 10.1007/s10072-009-0136-5. [DOI] [PubMed] [Google Scholar]
- 113.Fregni F, Freedman S, Pascual-Leone A. Recent advances in the treatment of chronic pain with non-invasive brain stimulation techniques. Lancet Neurol. 2007;6:188–191. doi: 10.1016/S1474-4422(07)70032-7. [DOI] [PubMed] [Google Scholar]
- 114.Boggio PS, Zaghi S, Lopes M, Fregni F. Modulatory effects of anodal transcranial direct current stimulation on perception and pain thresholds in healthy volunteers. Eur J Neurol. 2008;15:1124–1130. doi: 10.1111/j.1468-1331.2008.02270.x. [DOI] [PubMed] [Google Scholar]
- 115.Fregni F, Gimenes R, Valle AC, et al. A randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis Rheum. 2006;54:3988–3998. doi: 10.1002/art.22195. [DOI] [PubMed] [Google Scholar]
- 116.Rosen AC, Ramkumar M, Nguyen T, Hoeft F. Noninvasive transcranial brain stimulation and pain. Curr Pain Headache Rep. 2009;13:12–17. doi: 10.1007/s11916-009-0004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Knotkova H, Cruciani RA. Non-invasive transcranial direct current stimulation for the study and treatment of neuropathic pain. Methods Mol Biol. 2010;617:505–515. doi: 10.1007/978-1-60327-323-7_37. [DOI] [PubMed] [Google Scholar]
- 118.Mori F, Codecà C, Kusayanagi H, et al. Effects of anodal transcranial direct current stimulation on chronic neuropathic pain in patients with multiple sclerosis. J Pain. 2010;11:436–442. doi: 10.1016/j.jpain.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 119.Centonze D, Koch G, Versace V, et al. Repetitive transcranial magnetic stimulation of the motor cortex ameliorates spasticity in multiple sclerosis. Neurology. 2007;68:1045–1050. doi: 10.1212/01.wnl.0000257818.16952.62. [DOI] [PubMed] [Google Scholar]
- 120.Mori F, Codecà C, Kusayanagi H, et al. Effects of intermittent theta burst stimulation on spasticity in patients with multiple sclerosis. Eur J Neurol. 2010;17:295–300. doi: 10.1111/j.1468-1331.2009.02806.x. [DOI] [PubMed] [Google Scholar]
- 121.Chitsaz A, Janghorbani M, Shaygannejad V, et al. Sensory complaints of the upper extremities in multiple sclerosis: relative efficacy of nortriptyline and transcutaneous electrical nerve stimulation. Clin J Pain. 2009;25:281–285. doi: 10.1097/AJP.0b013e318190862b. [DOI] [PubMed] [Google Scholar]
- 122.Solaro C, Boehmker M, Tanganelli P. Pregabalin for treating paroxysmal painful symptoms in multiple sclerosis: a pilot study. J Neurol. 2009;256 doi: 10.1007/s00415-009-5203-6. 1773-1734. [DOI] [PubMed] [Google Scholar]