Abstract
Objective
To understand disorder-unique and common pathophysiology, studies in multiple patient groups with overlapping symptoms are needed. Deficits in emotion processing and hyperarousal symptoms are prominent features of bipolar disorder, attention deficit hyperactivity disorder (ADHD), and severe mood dysregulation. The authors compared amygdala response during emotional and nonemotional ratings of neutral faces in youths with these disorders as well as a group of healthy comparison youths.
Method
Blood-oxygen-level-dependent (BOLD) signal in the amygdala was examined in children with bipolar disorder (N=43), ADHD (N=18), and severe mood dysregulation (N=29) and healthy comparison subjects (N=37). During functional magnetic resonance imaging (fMRI), participants attended to emotional and nonemotional aspects of neutral faces.
Results
While rating subjective fear of neutral faces, youths with ADHD demonstrated left amygdala hyperactivity relative to the other three groups, whereas youths with severe mood dysregulation demonstrated hypoactivity.
Conclusions
These findings support the role of unique neural correlates in face-emotion processing among youths with bipolar disorder, ADHD, and severe mood dysregulation.
Pathophysiological studies in psychiatry typically compare one small, clinically homogeneous patient sample with healthy peers. However, comparisons of patient groups with overlapping symptoms allow investigators to map unique and common perturbations. In the present study, we compared amygdala activation during face-emotion processing in youths with attention deficit hyperactivity disorder (ADHD), bipolar disorder, and severe nonepisodic irritability (operationalized using Leibenluft et al.'s criteria for severe mood dysregulation [1]) as well as in healthy comparison subjects.
Several studies have examined the neural circuitry mediating face-emotion processing in bipolar disorder (2–6) and childhood psychopathology (7–13). For both pediatric and adult bipolar disorder, amygdala dysfunction is perhaps the most commonly reported finding in functional magnetic resonance imaging (fMRI) studies of the illness, with amygdala hyperactivity being reported in a variety of paradigms involving face emotions (2, 3, 6, 14). Of particular note, Pavuluri et al. (10, 12) found increased amygdala activation during face-emotion processing in pediatric bipolar disorder. In the present study, we focused on neutral faces because we previously found that 1) children with bipolar disorder rate neutral faces as more hostile and fear producing than comparison subjects, and 2) these differences are associated with amygdala hyperactivity (11). When viewing neutral faces that transform gradually into an emotional expression, patients with bipolar disorder and severe mood dysregulation require more intense emotional information than comparison subjects in order to identify the expression (15). Although patients with bipolar disorder or severe mood dysregulation both have impairments in face-emotion labeling, they differ in clinical presentation (16), outcome (17–20), family history (21), and psychophysiological correlates of frustration (22). Therefore, one important question is the extent to which neural circuitry mediating face-emotion processing of neutral faces differs between bipolar disorder and severe mood dysregulation.
To our knowledge, no previous fMRI study has included youths with severe mood dysregulation, despite considerable interest in whether these youths have a developmental presentation of bipolar disorder (1, 23). Epidemiological studies (17–20) suggest that severe mood dysregulation may be a risk factor for major depressive disorder. Identifying similarities and differences between severe mood dysregulation and other psychopathology is essential, and determining the neural circuitry engaged in processing neutral faces may assist in the differential diagnosis of disorders with overlapping clinical features.
Given the high rates of ADHD in children with bipolar disorder and severe mood dysregulation (16), nonirritable children with ADHD are an important comparison group. Emotion regulation and face-emotion labeling deficits emerge in some (24–28) but not all (29) studies of ADHD. Studies in pediatric disruptive behavior disorders have found biased threat appraisal, manifested by subjects rating neutral or ambiguous social situations as affectively negative (30, 31). Structural and functional imaging studies report amygdala dysfunction in ADHD (32, 33) (e.g., hyperactivity during reward processing in adults with ADHD [34]).
Employing a face-emotion processing task, we compared amygdala perturbations among children with bipolar disorder, ADHD, and severe mood dysregulation as well as healthy comparison subjects. Specifically, subjects made emotional or nonemotional ratings of neutral faces. The contrasts of interest compared neural activity during fear ratings with that of nose-width ratings and neural activity during hostility ratings with that of nose-width ratings (11). Based on previous work using these contrasts (11), we hypothesized that relative to healthy comparison subjects, children with bipolar disorder would demonstrate amygdala hyperactivity during emotional (fear or hostility) as opposed to nonemotional (nose-width) ratings of neutral faces. Children with severe mood dysregulation also have deficits in face-emotion labeling (29), including on a behavioral paradigm that involves neutral faces (15). Data suggest that this form of mood dysregulation is a risk factor for depressive disorders (17–20), and two studies suggest that youths with major depressive disorder exhibit amygdala hypoactivation when viewing faces in some contexts (9, 13). Accordingly, we hypothesized that children with severe mood dysregulation would show decreased amygdala activation while rating emotional or nonemotional aspects of neutral faces. No prior studies, to our knowledge, have examined amygdala response to emotional and nonemotional ratings of faces in ADHD patients, and behavioral results in face-emotion labeling studies are inconsistent (24–29). Therefore, while we expected functional amygdala perturbation in patients with ADHD, data to generate specific hypotheses are insufficient.
Method
Subjects
Usable fMRI data were acquired from subjects with bipolar disorder (N=43), ADHD (N=18), and severe mood dysregulation (N=29) as well as healthy comparison subjects (N=37). Participants, ages 8 to 17 years, were enrolled in an Institutional Review Board-approved study at the National Institute of Mental Health. Parents and youths gave written informed consent/assent. Patients were recruited through advertisements to mental health support groups and mental healthcare professionals. Healthy comparison subjects were recruited by advertisement and had no lifetime psychiatric diagnoses and no first-degree relatives with a mood disorder.
Subjects were assessed using the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL) (35). Interviewers were master's- and doctoral-level clinicians, with excellent interrater reliability (κ>0.9 for all diagnoses, including differentiating bipolar disorder from severe mood dysregulation). Diagnoses were based on best-estimate procedures generated in a consensus conference led by two psychiatrists. Youths with bipolar disorder met “narrow phenotype” criteria, with at least one DSM-IV full-duration hypomanic/manic episode characterized by abnormally elevated mood and at least three B mania symptoms (1). Youths with severe mood dysregulation had nonepisodic irritability, overreactivity to negative emotional stimuli ≥3 times per week, and hyperarousal (i.e., at least three of the following symptoms: insomnia, distractibility, psychomotor agitation, racing thoughts/flight of ideas, pressured speech, intrusiveness). Symptoms began before age 12; were present for at least 1 year, with no symptom-free periods exceeding 2 months; and caused severe impairment in at least one setting (i.e., home, school, peer) and mild impairment in another. Euphoric mood or distinct episodes lasting more than 1 day were exclusionary (1). Youths with ADHD met DSM-IV criteria for ADHD but not for severe mood dysregulation or any mood disorder. In the ADHD group, anxiety disorders were exclusionary, except for separation anxiety and social phobia.
The Wechsler Abbreviated Scale of Intelligence was administered to determine IQ. To evaluate mood in patients with bipolar disorder or severe mood dysregulation, clinicians with interrater reliability (κ>0.9) administered the Children's Depression Rating Scale and the Young Mania Rating Scale to the parent and child within 48 hours of scanning. Elevated Young Mania Rating Scale scores in patients with severe mood dysregulation reflect hyperarousal symptoms because, by definition (1), patients with this type of mood dysregulation cannot meet criteria for hypomania, mania, or a mixed episode.
Exclusion criteria for all subjects were an IQ <70, a history of head trauma, a neurological disorder, a pervasive developmental disorder, an unstable medical illness, or substance abuse/dependence. Patients with ADHD taking short-acting stimulants were included but were medication-free for ≥48 hours before scanning. Thus, both healthy comparison subjects and ADHD patients were medication-free at testing. Patients receiving medication for bipolar disorder or severe mood dysregulation were included. For ethical reasons, only those patients who were not responding to current psychotropic medication were withdrawn from treatment.
One hundred eighty-six subjects were scanned, yielding 127 (68.3%) usable scans. Groups differed in the proportion of excluded scans (p=0.02) but not in reasons for exclusion. Relative to patients with bipolar disorder, more severe mood dysregulation patients (p<0.01) and healthy comparison subjects (p=0.02) had unusable scans. Of the 59 excluded scans, 25 were excluded for poor behavioral data (no response ≥7 times), 22 for a >3.5-mm movement in any plane, and 12 for technical malfunction. Data from 20 bipolar disorder patients and 12 healthy comparison subjects have been published previously (11). Thus, among the 127 participants studied, data from 95 have not been presented previously.
Behavioral Paradigm
Subjects viewed 32 gray-scale adult faces (eight happy, eight angry, eight fearful, eight neutral [36]), drawn from three stimulus sets (37–39), as described by Rich et al. (11). The experiment consisted of the following four blocks: 1) passive viewing; 2) rating the perceived threat (“How hostile is this face?”); 3) rating subjective fear (“How afraid are you of this face?”); and 4) rating nose width (“How wide is the nose?”). Blocks were randomly ordered across subjects. Prior to each condition, an instruction screen was presented for 3,000 msec. Each randomly ordered stimulus event (eight faces, two fixation trials) was displayed for 4,000 msec. Using a five-key button box (MRI Devices, Waukesha, Wisc.), ratings (1 [not at all] to 5 [very]) were recorded while subjects viewed the face. Randomization controlled for the potential influence of facial features on activation. Each individual was exposed to a randomly selected set of 32 faces drawn from the three stimulus sets. This insured that potential confounds (e.g., facial feature, gender, or race of the stimulus) were controlled across each group. Stimuli were presented during one 14.2-minute 160-trial run.
Scanning Acquisition and Preprocessing
Whole brain blood-oxygen-level-dependent (BOLD) T2-weighted fMRI data were acquired on a General Electric Signa 3T scanner (Milwaukee) using Avotec Silent Vision Glasses (Stuart, Fla.). Images were acquired using an echo planar single-shot gradient echo pulse sequence (matrix size=64×64, repetition time=2,000 msec, echo time=40 msec, field of view=240 mm, voxels=3.75×3.75×5 mm). Images were acquired in 23 contiguous slices covering the entire brain, positioned parallel to the anterior commissure-posterior commissure plane. A high-resolution T1-weighted anatomical image was acquired for spatial normalization (180 1-mm sagittal slices; field of view=256 mm; number of excitations=1; repetition time=11.4 msec; echo time=4.4 msec; matrix size=256×256; time to inversion=300 msec; bandwidth=130 Hz/pixel). Given our focus on the amygdala and for consistency with prior approaches (9), we relied on unsmoothed data.
fMRI Data
Amygdala boundaries were defined using standard anatomical criteria (40) on a single Montreal Neurological Institute template and applied to all normalized brain images at the group level. Using Statistical Parametric Mapping 1999 (SPM99) software (Wellcome Trust Centre for Neuroimaging, University College of London), BOLD signal changes were averaged across all voxels in each amygdala structure, providing a single average amygdala value for each event type for every subject. Subjects' results were then entered into multivariate group-level models implemented in SPSS.
During preprocessing, we corrected functional data for slice timing and motion, coregistered functional and anatomical data, and spatially normalized the data to the Montreal Neurological Institute T1-weighted template image in SPM99. Event-related response amplitudes for each event type (e.g., rating fear or nose width of the neutral facial expression) were estimated using the general linear model. The waveform in the general linear model was a rectangular pulse (4-second duration) convolved with the hemodynamic response function. Contrast images were created for each subject using pairwise comparisons of the event-related BOLD response amplitudes across conditions. Each contrast image was then divided by the subject-specific voxel time series mean, yielding the percent of fMRI signal change (41).
For all group-level analyses, a random effects model was employed to permit population-level inferences (42). Using SPSS, analyses of covariance (ANCOVAs), with age as a covariate and including all four groups (bipolar disorder, ADHD, severe mood dysregulation, and healthy comparison), were performed in the left and right amygdala for the two a priori contrasts of interest while subjects viewed neutral faces. We examined subjects' neural activity 1) during ratings of fear relative to ratings of nose width and 2) during ratings of hostility relative to ratings of nose width. We also examined nose-width ratings relative to fixation trials. Given our a priori hypotheses and the use of a single mean value for each subject's amygdala activation, a statistical threshold of p<0.05 was used for both primary and post hoc analyses. In the left amygdala, the omnibus four-group ANCOVA was significant for the fear versus nose-width contrast (p<0.05). A priori-planned two-way contrasts were performed in order to understand the overall between-group differences observed. In particular, these planned contrasts tested the hypotheses that bipolar disorder patients would show amygdala hyperactivity when viewing neutral faces, while severe mood dysregulation patients would exhibit amygdala hypoactivity when viewing neutral faces. Exploratory post hoc analyses included adding reaction time and ratings as covariates as a result of between-group behavioral differences, comparing bipolar disorder patients with and without ADHD and medicated and unmedicated patients. We also performed an analysis excluding the three ADHD youths with comorbid anxiety disorder. Finally, we used at test to compare bipolar disorder patients with and without euthymia and Pearson correlations to examine associations between amygdala activation, number of medications (for the bipolar disorder and severe mood dysregulation groups), and behavioral performance (ratings and reaction time).
Behavioral Data
Behavioral ratings and reaction time were analyzed using separate ANCOVAs covaried for age. Post hoc analyses employed t tests. We performed Pearson correlations to assess associations between mood and performance. A statistical threshold of p<0.05 was used.
Results
Demographic Characteristics
Groups differed in age (F=3.43, df=3, 123, p=0.02), with the severe mood dysregulation group being younger than the bipolar disorder group (p<0.01) (Table 1). Subsequent analyses covaried for age. There was no significant difference in IQ among the groups. Fifty-six percent of bipolar disorder patients (N=24/43) and 96% of severe mood dysregulation patients (N=26/27; data missing for two subjects) were euthymic at the time of imaging.
TABLE 1. Demographic and Clinical Characteristics of Bipolar Disorder, Severe Mood Dysregulation, ADHD, and Healthy Comparison Subjects.
| Characteristic | Bipolar Disorder Subjects (N=43) | Severe Mood Dysregulation Subjects (N=29) | ADHD Subjects (N=18) | Healthy Comparison Subjects (N=37) | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age (years)a | 14.81 | 2.66 | 12.94 | 1.91 | 13.87 | 2.31 | 13.73 | 2.72 |
| Wechsler Abbreviated Scale of Intelligence full-scale IQ scoreb | 107.65 | 12.05 | 107.22 | 15.23 | 113.11 | 14.78 | 108.20 | 12.72 |
| Young Mania Rating Scale scorec, d | 9.56 | 6.48 | 12.64 | 4.92 | ||||
| Children's Depression Rating Scale scoree | 28.37 | 9.13 | 28.44 | 8.02 | ||||
| Number of medicationse | 2.35 | 1.70 | 2.11 | 1.55 | 0 | 0 | 0 | 0 |
| N | % | N | % | N | % | N | % | |
| Male | 17 | 40 | 17 | 59 | 13 | 72 | 21 | 57 |
| Euthymiae, f | 24 | 56 | 26 | 96 | ||||
| Bipolar I disorder | 40 | 93 | ||||||
| Bipolar II disorder | 3 | 7 | ||||||
| Comorbid disorders | ||||||||
| ADHD | 20 | 47 | 24 | 83 | ||||
| Anxiety disorder | 20 | 47 | 15 | 52 | 3 | 17 | ||
| Oppositional defiant disorder or conduct disorder | 12 | 28 | 17 | 59 | ||||
| Medicatione | ||||||||
| No medication | 11 | 26 | 6 | 22 | 18 | 100 | 37 | 100 |
| Atypical antipsychotic | 19 | 44 | 10 | 37 | ||||
| Lithium | 15 | 35 | 4 | 15 | ||||
| Antiepileptic | 22 | 51 | 12 | 44 | ||||
| Antidepressant | 13 | 30 | 8 | 30 | ||||
| Stimulant | 10 | 23 | 10 | 37 | ||||
| Other | 5 | 12 | 5 | 19 | ||||
Significant differences among groups (p=0.02).
Data missing for two severe mood dysregulation patients and two healthy comparison subjects.
Data missing for one severe mood dysregulation patient.
Significant between-group difference for bipolar disorder and severe mood dysregulation patients (p=0.04).
Data missing for two severe mood dysregulation patients.
Significant between-group difference for bipolar disorder and severe mood dysregulation patients (p<0.01).
Behavioral Data
Ratings
ANCOVA revealed a significant group effect on fear ratings of neutral faces (F=3.12, df=3, 122, p=0.03) (Table 2). Patients with bipolar disorder (p<0.01) and severe mood dysregulation (p=0.02) were more afraid of neutral faces than healthy comparison subjects. ADHD patients did not differ significantly from the other three groups. Groups did not differ on hostility or nose-width ratings. There was no relationship between ratings and Young Mania Rating Scale or Children's Depression Rating Scale scores.
TABLE 2. Emotional and Nonemotional Ratings of Neutral Faces Among Bipolar Disorder, Severe Mood Dysregulation, ADHD, and Healthy Comparison Subjects.
| Characteristic | Bipolar Disorder Subjects (N=43) | Severe Mood Dysregulation Subjects (N=29) | ADHD Subjects (N=18) | Healthy Comparison Subjects (N=37) | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Behavioral ratings | ||||||||
| Hostility | 2.04 | 0.71 | 1.91 | 0.73 | 1.84 | 0.80 | 1.77 | 0.58 |
| Feara | 1.94 | 0.78 | 1.86 | 0.82 | 1.70 | 0.80 | 1.45 | 0.52 |
| Nose width | 2.25 | 0.71 | 2.26 | 0.66 | 2.08 | 0.52 | 2.19 | 0.53 |
| Overall | 2.07 | 0.56 | 2.01 | 0.60 | 1.87 | 0.60 | 1.80 | 0.42 |
| Reaction time (msec) | ||||||||
| Hostilityb | 1,969.84 | 400.05 | 1,800.52 | 447.40 | 1,790.12 | 416.29 | 1,753.79 | 438.51 |
| Fear | 1,802.61 | 444.39 | 1,540.45 | 529.76 | 1,739.49 | 409.48 | 1,642.47 | 464.95 |
| Nose width | 1,880.34 | 395.94 | 1,699.51 | 371.63 | 1,803.12 | 354.53 | 1,852.50 | 390.99 |
| Overall | 1,884.26 | 331.22 | 1,680.16 | 389.53 | 1,777.58 | 342.90 | 1,749.59 | 385.60 |
Significant between-group differences (p=0.03); patients with bipolar disorder (p<0.01) and severe mood dysregulation (p=0.02) rated higher levels of fear of neutral faces relative to healthy comparison subjects.
Between-group differences (p=0.06), with bipolar disorder patients slower than healthy comparison subjects (p=0.01) and severe mood dysregulation patients (p=0.05).
Reaction time
ANCOVA revealed nearly significant reaction time differences in ratings for hostility of neutral faces (F=2.53, df=3, 122, p=0.06), with bipolar disorder patients reacting slower than healthy comparison subjects (p=0.01) and severe mood dysregulation patients (p=0.05) (Table 2). Groups did not differ significantly on reaction time when rating fear or nose width. Higher Young Mania Rating Scale scores were associated with faster reaction times for fear ratings (r=–0.26, p=0.03). Children's Depression Rating Scale scores were not related to reaction time.
fMRI Data
Fear and nose-width ratings contrast
Data revealed disorder-specific perturbations in left amygdala activation (F=4.53, df=3, 122, p>0.01) (Figure 1). Relative to healthy comparison subjects, ADHD patients manifested hyperactivation in this brain region (p=0.05), whereas severe mood dysregulation patients demonstrated hypoactivation (p=0.04). Bipolar disorder patients did not differ significantly from healthy comparison subjects. Disorder-specific perturbations extended beyond comparisons with healthy subjects. ADHD patients manifested hyperactivity when compared with bipolar disorder patients (p=0.05) and severe mood dysregulation patients (p<0.01). Severe mood dysregulation patients showed hypoactivity relative to bipolar disorder patients (p=0.04) and ADHD patients (p<0.01). There were no significant between-group differences in the right amygdala.
FIGURE 1. Left Amygdala Activation During Ratings of Fear and Nose Width.
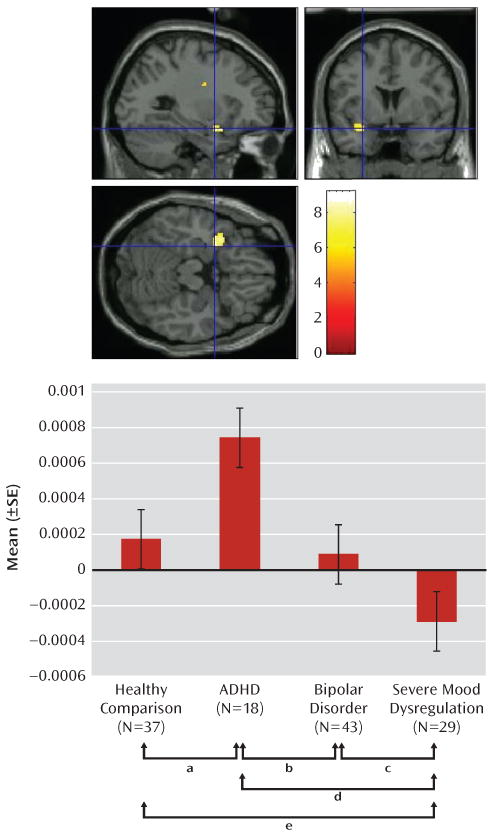
a Amygdala activation in ADHD patients was greater than that for healthy comparison subjects (p=0.05).
b Amygdala activation in ADHD patients was greater than that for bipolar disorder patients (p=0.05).
c Amygdala activation in severe mood dysregulation patients was less than that for bipolar disorder patients (p=0.04).
d Amygdala activation in severe mood dysregulation patients was less than that for ADHD patients (p<0.01).
e Amygdala activation in severe mood dysregulation patients was less than that for healthy comparison subjects (p=0.04).
Hostility and nose-width ratings contrast
There were no significant between-group differences in the left or right amygdala.
Nose-width ratings and fixation trials
There was a significant group effect in the left amygdala (F=3.28, df=3, 122, p=0.02), with severe mood dysregulation patients showing hyperactivation relative to the other three groups (all p values <0.01). Nearly significant differences emerged in the right amygdala (F=2.25, df=3, 122, p=0.09): severe mood dysregulation patients showed hyperactivation relative to healthy comparison subjects (p=0.02), and healthy comparison subjects showed hypoactivation relative to bipolar disorder (p=0.08) and ADHD patients (p=0.06).
Post Hoc Analyses of Fear and Nose-Width Ratings Contrast in the Left Amygdala
Covarying behavioral ratings and reaction time
When ratings and reaction times were covaried, results remained the same as the aforementioned, except the difference between severe mood dysregulation and bipolar disorder patients fell short of significance (p=0.09). Thus, behavioral differences did not account for the neural differences.
Comorbidities, mood state, medications, and behavioral performance
There were no significant differences in left amygdala activation between bipolar disorder patients with (N=20) and without (N=23) ADHD. In an ANCOVA excluding the three ADHD patients with anxiety disorders, the ADHD group still showed hyperactivation relative to the other three groups (all p values <0.03). Among bipolar disorder patients, those with (N=24) and without (N=19) euthymia did not differ significantly in amygdala activation.
There were no significant differences between medicated (N=32) and unmedicated (N=11) bipolar disorder patients or between medicated (N=21) and unmedicated (N=6) severe mood dysregulation patients (data missing for two subjects). There was no relationship between the number of medications and amygdala activations in the bipolar disorder or severe mood dysregulation group. There also was no relationship between the presence of any specific medication class (i.e., antipsychotic, lithium, antiepileptic, antidepressant, or stimulant) and left amygdala activation.
Ratings and reaction times were related to amygdala activation in healthy comparison subjects and patients with severe mood dysregulation and ADHD but not bipolar disorder. Increased amygdala activation was associated with 1) slower reaction time for hostility ratings among patients with severe mood dysregulation (r=0.38, p=0.04), 2) slower reaction time during nose-width ratings among ADHD patients (r=0.50, p=0.03), and 3) higher fear ratings among healthy comparison subjects (r=0.40, p=0.02).
Discussion
It is important to examine phenotypic entities with overlapping clinical features to determine similarities and differences in neural perturbations. Face-emotion processing is a salient feature of social cognition and is deficient in several childhood pathologies, including bipolar disorder, severe mood dysregulation, and, possibly, ADHD (25–29). We compared amygdala activation during a face-emotion processing task in youths with ADHD, bipolar disorder, or severe mood dysregulation as well as healthy comparison subjects (i.e., subjects with no axis I diagnosis). This is the first study, to our knowledge, to compare amygdala activation in these clinically overlapping groups using a face-emotion processing paradigm and the first fMRI study to examine face processing in severe mood dysregulation.
We found an imaging-based double dissociation in amygdala activation in patients with ADHD and severe mood dysregulation. Nonirritable ADHD youths showed amygdala hyperactivity when completing subjective fear ratings of neutral faces relative to healthy youths and those with bipolar disorder or severe mood dysregulation. In contrast, children with chronic irritability (operationalized using Leibenluft et al.'s criteria for severe mood dysregulation [1]) showed amygdala hypoactivity relative to healthy youths and those with bipolar disorder or ADHD. Contrary to our hypothesis, patients with bipolar disorder did not differ significantly from healthy comparison subjects. These findings suggest that there may be functional differences among ADHD, bipolar disorder, and severe mood dysregulation patients, despite the presence of overlapping behavioral deficits and clinical symptoms.
Severe mood dysregulation is characterized by severe, nonepisodic irritability and hyperarousal. Although youths with severe mood dysregulation typically meet criteria for ADHD (16) and are often assigned the diagnosis of bipolar disorder, they may be seen as clinically “in between” these two groups. Unlike ADHD, severe mood dysregulation is characterized by a distinct and highly impairing mood component. However, in contrast to bipolar disorder, it does not involve discrete hypomanic or manic episodes. Several studies indicate that similar to youths with bipolar disorder, youths with severe mood dysregulation are deficient in the ability to identify and label facial emotions (15, 29), which is consistent with the behavioral and neural findings in the present study. When performing emotional ratings (i.e., subjective fear) of neutral faces, severe mood dysregulation patients exhibited reduced activity in the amygdala relative to healthy comparison subjects and patients with bipolar disorder or ADHD. The amygdala mediates emotional processing and valence (43) and is involved in the processing of facial affect (44). In severe mood dysregulation, the deficit in amygdala engagement while processing emotional aspects of facial expressions could contribute to interpersonal difficulties and mood problems.
Our finding in severe mood dysregulation resembles data reported for youths with major depressive disorder. Using this same task, viewing both fearful and neutral faces, Beesdo et al. (9) found amygdala hypoactivation in children with major depressive disorder. Similarities between fMRI findings in severe mood dysregulation and major depressive disorder are interesting because longitudinal epidemiological research suggests that severe mood dysregulation, and chronic irritability in general, are associated with subsequent depressive disorders (17–20, 45). Thus, both longitudinal and neuroimaging data suggest associations between severe mood dysregulation and major depressive disorder. Pathophysiological similarities between these two disorders merit further investigation, with a particular emphasis on whether amygdala dysfunction in severe mood dysregulation predicts later major depressive disorder. In addition, the psychological and physiological underpinnings of amygdala hypoactivation in severe mood dysregulation during fear versus nose-width ratings of neutral faces warrant further exploration. For example, our data suggest that this finding could, in part, reflect relative hyperactivation in youths with severe mood dysregulation, compared with other youths, while rating nose width, perhaps because youths with this disorder have difficulty attending away from face emotions. Alternatively, it could reflect perturbations in other baseline conditions, given questions regarding the appropriate baseline in fMRI (46).
Given the high rate of ADHD in youths with severe mood dysregulation (16), it is interesting that the neural correlates of face-emotion processing differ markedly between these two patient groups. Although both severe mood dysregulation and ADHD patients have symptoms of distractibility and increased motor activity, they differ in that patients with severe mood dysregulation, but not ADHD, have significant irritability. Using the K-SADS-PL, with an additional severe mood dysregulation module, the ADHD subjects in the present sample were evaluated carefully to ensure that they did not have significant irritability or other mood symptoms. Thus, it is somewhat surprising that the ADHD children demonstrated amygdala hyperactivity during a face processing task. However, studies demonstrate structural (32) and dopaminergic (33) abnormalities in the amygdala in ADHD patients, suggesting that amygdala abnormalities in ADHD are not without precedent. In the only previous fMRI study of face processing in ADHD, Marsh et al. (47), employing a partially overlapping ADHD group, found that amygdala activity did not differ between ADHD patients and comparison subjects. The task used by Marsh et al. and the one used in the present study differ significantly in attentional demands (i.e., implicit versus explicit processing), possibly accounting for the disparate results.
Using a subset of the present sample, we previously found increased activation in the left amygdala in bipolar disorder patients relative to healthy comparison subjects in the hostility and subjective fear conditions (11). In both our previous study (11) and the present study, bipolar disorder patients, relative to healthy comparison subjects, rated neutral faces as more fear producing and exhibited slower reaction times when rating face hostility. Our failure to extend this previous neuroimaging finding might be the result of a type II error, particularly since the current analysis includes four groups rather than two. Indeed, this face-viewing paradigm is particularly prone to type II error because it includes multiple face emotions and attention states, each sampled relatively sparsely (eight replicates per attentional condition). Such sparse sampling was necessary to maintain short task duration and increase tolerability for youths with severe psychopathology.
In addition, we were interested in a task that included neutral faces, given our prior behavioral and imaging results (11, 15). However, in examining responses to neutral faces, it is important to embed them in other face emotions, since other emotions can affect the interpretation of neutral faces (48). Thus, our task represents a compromise. We sampled activation when neutral faces were viewed in multiple attention states against a background of multiple emotions, but the number of trials for neutral faces in each attention state was relatively low.
Indeed, it is possible that the presence of other emotions may have influenced our findings of amygdala activity in response to neutral faces. Although all subjects were exposed to the same number of emotional facial expressions, one group may have been particularly affected by exposure to angry and/or fearful faces, and this, in turn, may have influenced the group's processing of neutral faces. Research in amygdala dysfunction in pediatric bipolar disorder could use more powerful paradigms, perhaps focused specifically on emotional ratings of neutral faces or on other face emotions, such as fear or anger (49).
Additional limitations complicate interpretations. First, most bipolar disorder and severe mood dysregulation youths were medicated. However, prior work suggests that medications do not typically cause type I errors and may even diminish between-group differences (50). Youths with ADHD but not bipolar disorder or severe mood dysregulation were withdrawn from stimulants before scanning. The influence of recent medication withdrawal on activation remains unknown. Second, some bipolar disorder and severe mood dysregulation patients were not experiencing a period of euthymia at the time of testing, and negative affect on the day of the scan was not assessed in the ADHD group. Third, age differed among the groups, although analyses controlled for this difference. Fourth, we used standard anatomical criteria to locate the amygdala boundaries. Since studies report abnormalities in amygdala structure in patients with bipolar disorder and ADHD (32, 51), standard anatomical criteria may not be the most accurate method of identifying this region of interest. Future research might trace the individual amygdala for each subject, although this may become infeasible as studies continue to increase in size.
The present study suggests unique neural mechanisms mediating face-emotion processing deficits in clinically overlapping groups. There was a double dissociation in amygdala activity in youths with ADHD and severe mood dysregulation when completing fear (emotional) versus nonemotional ratings of neutral faces. ADHD patients demonstrated hyperactivation, while patients with severe mood dysregulation demonstrated hypoactivation, relative to the other groups. Additional neuroimaging studies are needed to examine amygdala activity in response to other emotional facial expressions in these youths and further specify the neural perturbations associated with nosologically and phenotypically similar and distinct clinical entities.
Acknowledgments
Supported by the NIMH Intramural Research Program.
Footnotes
The authors report no financial relationships with commercial interests.
References
- 1.Leibenluft E, Charney DS, Towbin KE, Bhangoo RK, Pine DS. Defining clinical phenotypes of juvenile mania. Am J Psychiatry. 2003;160:430–437. doi: 10.1176/appi.ajp.160.3.430. [DOI] [PubMed] [Google Scholar]
- 2.Blumberg HP, Donegan NH, Sanislow CA, Collins S, Lacadie C, Skudlarski P, Gueorguieva R, Fulbright RK, McGlashan TH, Gore JC, Krystal JH. Preliminary evidence for medication effects on functional abnormalities in the amygdala and anterior cingulate in bipolar disorder. Psychopharmacology (Berl) 2005;183:308–313. doi: 10.1007/s00213-005-0156-7. [DOI] [PubMed] [Google Scholar]
- 3.Foland LC, Altshuler LL, Bookheimer SY, Eisenberger N, Townsend J, Thompson PM. Evidence for deficient modulation of amygdala response by prefrontal cortex in bipolar mania. Psychiatry Res. 2008;162:27–37. doi: 10.1016/j.pscychresns.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altshuler L, Bookheimer S, Townsend J, Proenza MA, Sabb F, Mintz J, Cohen MS. Regional brain changes in bipolar I depression: a functional magnetic resonance imaging study. Bipolar Disord. 2008;10:708–717. doi: 10.1111/j.1399-5618.2008.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, Frangou S, Ecker C, Phillips ML. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry. 2004;55:578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 6.Yurgelun-Todd DA, Gruber SA, Kanayama G, Killgore WD, Baird AA, Young AD. fMRI during affect discrimination in bipolar affective disorder. Bipolar Disord. 2000;2:237–248. doi: 10.1034/j.1399-5618.2000.20304.x. [DOI] [PubMed] [Google Scholar]
- 7.Killgore WD, Yurgelun-Todd DA. Ventromedial prefrontal activity correlates with depressed mood in adolescent children. Neuroreport. 2006;17:167–171. doi: 10.1097/01.wnr.0000198951.30939.73. [DOI] [PubMed] [Google Scholar]
- 8.Jones AP, Laurens KR, Herba CM, Barker GJ, Viding E. Amygdala hypoactivity to fearful faces in boys with conduct problems and callous-unemotional traits. Am J Psychiatry. 2009;166:95–102. doi: 10.1176/appi.ajp.2008.07071050. [DOI] [PubMed] [Google Scholar]
- 9.Beesdo K, Lau JY, Guyer AE, McClure-Tone EB, Monk CS, Nelson EE, Fromm SJ, Goldwin MA, Wittchen HU, Leibenluft E, Ernst M, Pine DS. Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Arch Gen Psychiatry. 2009;66:275–285. doi: 10.1001/archgenpsychiatry.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pavuluri MN, Passarotti AM, Harral EM, Sweeney JA. An fMRI study of the neural correlates of incidental versus directed emotion processing in pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2009;48:308–319. doi: 10.1097/CHI.0b013e3181948fc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rich BA, Vinton DT, Roberson-Nay R, Hommer RE, Berghorst LH, McClure EB, Fromm SJ, Pine DS, Leibenluft E. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci U S A. 2006;103:8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pavuluri MN, O'Connor MM, Harral E, Sweeney JA. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biol Psychiatry. 2007;62:158–167. doi: 10.1016/j.biopsych.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 13.Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, Axelson D, Whalen PJ, Casey BJ. Amygdala response to fearful faces in anxious and depressed children. Arch Gen Psychiatry. 2001;58:1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- 14.Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:829, 833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rich BA, Grimley ME, Schmajuk M, Blair KS, Blair RJ, Leibenluft E. Face emotion labeling deficits in children with bipolar disorder and severe mood dysregulation. Dev Psychopathol. 2008;20:529–546. doi: 10.1017/S0954579408000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dickstein DP, Rich BA, Binstock AB, Pradella AG, Towbin KE, Pine DS, Leibenluft E. Comorbid anxiety in phenotypes of pediatric bipolar disorder. J Child Adolesc Psychopharmacol. 2005;15:534–548. doi: 10.1089/cap.2005.15.534. [DOI] [PubMed] [Google Scholar]
- 17.Brotman MA, Schmajuk M, Rich BA, Dickstein DP, Guyer AE, Costello EJ, Egger HL, Angold A, Pine DS, Leibenluft E. Prevalence, clinical correlates, and longitudinal course of severe mood dysregulation in children. Biol Psychiatry. 2006;60:991–997. doi: 10.1016/j.biopsych.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 18.Leibenluft E, Cohen P, Gorrindo T, Brook JS, Pine DS. Chronic versus episodic irritability in youth: a community-based, longitudinal study of clinical and diagnostic associations. J Child Adolesc Psychopharmacol. 2006;16:456–466. doi: 10.1089/cap.2006.16.456. [DOI] [PubMed] [Google Scholar]
- 19.Stringaris A, Cohen P, Pine DS, Leibenluft E. Adult outcomes of youth irritability: a 20-year prospective community-based study. Am J Psychiatry. 2009;166:1048–1054. doi: 10.1176/appi.ajp.2009.08121849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stringaris A, Goodman R. Longitudinal outcome of youth oppositionality: irritable, headstrong, and hurtful behaviors have distinctive predictions. J Am Acad Child Adolesc Psychiatry. 2009;48:404–412. doi: 10.1097/CHI.0b013e3181984f30. [DOI] [PubMed] [Google Scholar]
- 21.Brotman MA, Kassem L, Reising MM, Guyer AE, Dickstein DP, Rich BA, Towbin KE, Pine DS, McMahon FJ, Leibenluft E. Parental diagnoses in youth with narrow phenotype bipolar disorder or severe mood dysregulation. Am J Psychiatry. 2007;164:1238–1241. doi: 10.1176/appi.ajp.2007.06101619. [DOI] [PubMed] [Google Scholar]
- 22.Rich BA, Schmajuk M, Perez-Edgar KE, Fox NA, Pine DS, Leibenluft E. Different psychophysiological and behavioral responses elicited by frustration in pediatric bipolar disorder and severe mood dysregulation. Am J Psychiatry. 2007;164:309–317. doi: 10.1176/ajp.2007.164.2.309. [DOI] [PubMed] [Google Scholar]
- 23.Moreno C, Laje G, Blanco C, Jiang H, Schmidt AB, Olfson M. National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Arch Gen Psychiatry. 2007;64:1032–1039. doi: 10.1001/archpsyc.64.9.1032. [DOI] [PubMed] [Google Scholar]
- 24.Singh SD, Ellis CR, Winton AS, Singh NN, Leung JP, Oswald DP. Recognition of facial expressions of emotion by children with attention-deficit hyperactivity disorder. Behav Modif. 1998;22:128–142. doi: 10.1177/01454455980222002. [DOI] [PubMed] [Google Scholar]
- 25.Cadesky EB, Mota VL, Schachar RJ. Beyond words: How do children with ADHD and/or conduct problems process nonverbal information about affect. J Am Acad Child Adolesc Psychiatry. 2000;39:1160–1167. doi: 10.1097/00004583-200009000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Corbett B, Glidden H. Processing affective stimuli in children with attention-deficit hyperactivity disorder. Child Neuropsychol. 2000;6:144–155. doi: 10.1076/chin.6.2.144.7056. [DOI] [PubMed] [Google Scholar]
- 27.Rapport LJ, Friedman SL, Tzelepis A, Van Voorhis A. Experienced emotion and affect recognition in adult attention-deficit hyperactivity disorder. Neuropsychology. 2002;16:102–110. doi: 10.1037//0894-4105.16.1.102. [DOI] [PubMed] [Google Scholar]
- 28.Pelc K, Kornreich C, Foisy ML, Dan B. Recognition of emotional facial expressions in attention-deficit hyperactivity disorder. Pediatric Neurol. 2006;35:93–97. doi: 10.1016/j.pediatrneurol.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 29.Guyer AE, McClure EB, Adler AD, Brotman MA, Rich BA, Kimes AS, Pine DS, Ernst M, Leibenluft E. Specificity of facial expression labeling deficits in childhood psychopathology. J Child Psychol Psychiatry. 2007;48:863–871. doi: 10.1111/j.1469-7610.2007.01758.x. [DOI] [PubMed] [Google Scholar]
- 30.Milich R, Dodge KA. Social information processing in child psychiatric populations. J Abnorm Child Psychol. 1984;12:471–489. doi: 10.1007/BF00910660. [DOI] [PubMed] [Google Scholar]
- 31.Dodge KA, Price JM, Bachorowski JA, Newman JP. Hostile attributional biases in severely aggressive adolescents. J Abnorm Psychol. 1990;99:385–392. doi: 10.1037//0021-843x.99.4.385. [DOI] [PubMed] [Google Scholar]
- 32.Plessen KJ, Bansal R, Zhu H, Whiteman R, Amat J, Quackenbush GA, Martin L, Durkin K, Blair C, Royal J, Hugdahl K, Peterson BS. Hippocampus and amygdala morphology in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2006;63:795–807. doi: 10.1001/archpsyc.63.7.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Volkow ND, Wang G, Newcorn J, Telang F, Solanto MV, Fowler JS, Logan J, Ma Y, Schulz K, Pradhan K, Wong C, Swanson JM. Depressed dopamine activity in caudate and preliminary evidence of limbic involvement in adults with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2007;64:932–940. doi: 10.1001/archpsyc.64.8.932. [DOI] [PubMed] [Google Scholar]
- 34.Plichta MM, Vasic N, Wolf RC, Lesch K, Brummer D, Jacob C, Fallgatter AJ, Gron G. Neural hyporesponsiveness and hyperresponsiveness during immediate and delayed reward processing adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2009;65:7–14. doi: 10.1016/j.biopsych.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 35.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 36.Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, Charney DS, Ernst M, Pine DS. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20:420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- 37.Gur RE, Skolnick BE, Gur RC, Caroff S, Rieger W, Obrist WD, Younkin D, Reivich M. Brain function in psychiatric disorders, I: regional cerebral blood flow in medicated schizophrenics. Arch Gen Psychiatry. 1983;40:1250–1254. doi: 10.1001/archpsyc.1983.01790100096013. [DOI] [PubMed] [Google Scholar]
- 38.Ekman P, Friesen WV. Pictures of Facial Affect. Palo Alto, Calif: Consulting Psychologists Press; 1976. [Google Scholar]
- 39.Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson C. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Szeszko PR, Robinson D, Alvir JM, Bilder RM, Lencz T, Ashtari M, Wu H, Bogerts B. Orbital frontal and amygdala volume reductions in obsessive-compulsive disorder. Arch Gen Psychiatry. 1999;56:913–919. doi: 10.1001/archpsyc.56.10.913. [DOI] [PubMed] [Google Scholar]
- 41.Zarahn E, Aguirre G, D'Esposito M. A trial-based experimental design for fMRI. Neuroimage. 1997;6:122–138. doi: 10.1006/nimg.1997.0279. [DOI] [PubMed] [Google Scholar]
- 42.Holmes AP, Friston KJ. Generalizability, random effects, and population inference. Neuroimage. 1998;7:S754. [Google Scholar]
- 43.LeDoux JE. Emotion circuits in the brain. Ann Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 44.Fitzgerald DA, Angstadt M, Jelsone LM, Nathan PJ, Phan KL. Beyond threat: amygdala reactivity across multiple expressions of facial affect. Neuroimage. 2006;30:1441–1448. doi: 10.1016/j.neuroimage.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Burke JD, Loeber R, Lahey BB, Rathouz PJ. Developmental transitions among affective and behavioral disorders in adolescent boys. J Child Psychol Psychiatry. 2005;46:1200–1210. doi: 10.1111/j.1469-7610.2005.00422.x. [DOI] [PubMed] [Google Scholar]
- 46.Stark CE, Squire LR. When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proc Natl Acad Sci U S A. 2001;98:12760–12766. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marsh AA, Finger EC, Mitchell DG, Reid ME, Sims C, Kosson DS, Towbin KE, Leibenluft E, Pine DS, Blair RJ. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. Am J Psychiatry. 2008;165:712–720. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- 48.Phillips ML, Medford N, Young AW, Williams L, Williams SC, Bullmore ET, Gray JA, Brammer MJ. Time courses of left and right amygdalar responses to fearful facial expressions. Hum Brain Mapp. 2001;12:193–202. doi: 10.1002/1097-0193(200104)12:4<193::AID-HBM1015>3.0.CO;2-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Killgore WD, Gruber SA, Yurgelun-Todd DA. Abnormal corticostriatal activity during fear perception in bipolar disorder. Neuroreport. 2008;19:1523–1527. doi: 10.1097/WNR.0b013e328310af58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Phillips ML, Travis MJ, Fagiolini A, Kupfer DJ. Medication effects in neuroimaging studies of bipolar disorder. Am J Psychiatry. 2008;165:313–320. doi: 10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pfeifer JC, Welge J, Strakowski SM, Adler CM, DelBello MP. Meta-analysis of amygdala volumes in children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2008;47:1289–1298. doi: 10.1097/CHI.0b013e318185d299. [DOI] [PubMed] [Google Scholar]


