Abstract
Growing data suggest that antiretrovirals can be used as an effective means of HIV prevention. This paper reviews the current status and future clinical prospects of utilizing antiretroviral chemoprophylaxis before and after high-risk HIV exposure to prevent HIV transmission. The discussion about using antiretrovirals as a means of primary HIV prevention has moved to the forefront of public health discourse because of a growing evidence base, the increased tolerability of the medications, the decreased cost, the ever expanding formulary, and the limitations of other approaches.
Keywords: HIV, AIDS, primary prevention, ART, preexposure prophylaxis, postexposure prophylaxis, topical microbicides
WHY ANTIRETROVIRALS FOR PRIMARY HIV PREVENTION?
With more than 2.5 million new HIV infections annually1, HIV prevention is at a crucial juncture, since most biomedical interventions have failed to effectively decrease HIV acquisition,2 an effective HIV preventive vaccine remains years away,3 and many behavioral strategies have not led to durable reductions in the number of new HIV infections.4 Growing observational and modeling data suggest that antiretrovirals (ARVs) can be used as an effective means of HIV prevention.5 Antiretroviral therapy (ART) for prevention includes not only prompt initiation of HIV-infected individuals on treatment to decrease the risk of transmission6,7 (see El-Sadr in this Supplement) but also ART prophylaxis for at-risk uninfected individuals.8 Numerous animal model studies have demonstrated that ART administered parenterally, orally, or topically before or just after retroviral challenge was protective against HIV acquisition.9,10 Historically, treatment as prevention, or chemoprophylaxis, has been routinely used for other infectious diseases, including malaria, tuberculosis, and sexually transmitted infections (STIs),11,12 but the duration of administration is generally days to months rather than many years, as may be the case with HIV medications.
Antiretroviral postexposure prophylaxis (PEP) to prevent HIV acquisition was first recommended for use in occupational settings more than a decade ago.13 Subsequent studies have led to recommendations for its use in nonoccupational settings, and research is underway to examine whether ART preexposure prophylaxis (PrEP) could be an effective method of primary prevention for individuals who have ongoing risks for becoming HIV-infected.9,14. This paper reviews the current status and future clinical prospects of utilizing antiretroviral chemoprophylaxis before and after high-risk HIV exposure to prevent HIV transmission.
POSTEXPOSURE PROPHYLAXIS
The best proof of concept for the relationship between ARVs and HIV transmission comes from studies of the prevention of mother-to-child transmission (PMTCT)15,16 and a case-control study of postexposure prophylaxis (PEP) following needle stick injury in health care settings.17 The Centers for Disease Control and Prevention (CDC) registry documented that health care workers who took zidovudine (AZT) monotherapy following occupational exposure were one fifth as likely to become HIV-infected as those who did not take treatment.13,17 The rhesus macaque SIV challenge model has suggested that 28 days of ART is needed for optimally effective PEP18 and has been the basis for the recommendation of a 4-week course for humans exposed to HIV. In the past, a major impediment to wider implementation of PEP utilization was the relative intolerability of first-line agents, such as azidothymidine and protease inhibitors (PIs).19 A study examining PEP uptake in several European emergency departments found that almost half of the individuals did not complete the full 28-day PEP course because of decreased tolerability of regimens, including zidovudine/lamivudine and either ritonavir-boosted lopinavir or atazanavir.20 A subsequent case-control study of nonoccupational PEP found that men who took tenofovir–emtricitabine were more likely to complete a 28-day course of PEP than historical controls taking 2-drug AZT-based regimens.21 Other newer drugs may also offer opportunities for novel PEP strategies due to improved tolerability and novel mechanisms of action (eg, raltegravir)22 and/or high genital tract concentrations (eg, maraviroc).23 It remains to be fully elucidated whether 2 would be preferable to 3 for PEP. A 2-drug regimen would decrease regimen complexity (eg, 1 to 3 coformulated pills a day), increasing tolerability and completion rates, but alternatively, if the individual has been exposed to a treatment-experienced HIV-infected source, more drugs could provide extra protection against the selection for drug resistant virus.24
Though PEP has not been widely utilized outside occupational healthcare settings, concerns have been raised by some that wider utilization may be counterproductive among individuals at increased risk for HIV by decreasing their protective behaviors.. A study of MSM in Brazil did not demonstrate increases in risk-taking behaviors after PEP, and HIV incidence was significantly lower in PEP users.25 Because this was not a randomized controlled trial, unintended biases could have been present (eg, PEP users being generally less risky, etc). Other studies have included prevention counseling to increase the intervention’s long-term efficacy, to optimize the educable moment when at-risk individuals are seeking to protect themselves from infection.25,26 Despite efforts to use the PEP clinical encounter as a means to help people decrease risk-taking behavior, the San Francisco Health Department STD Clinic found that 1.3% of PEP users became HIV-infected within 6 months after completing their medication course, underscoring the need for sustained behavioral interventions for those at increased risk in addition to the provision of drugs.27
PREEXPOSURE PROPHYLAXIS
In situations where the likelihood of HIV exposure can be anticipated ahead of time, ARVs delivered in either oral or topical formulations could be a logical mode of primary prevention. The groundwork for current clinical PrEP research has been provided by animal studies over the past decade.10 The most widely studied antiretroviral for PrEP, tenofovir, has many features that are desirable in a chemoprophylactic agent, including long intracellular half-life, activity in monocyte/macrophages and other cells that may transmit HIV in genital secretions, and high concentrations in genital tissues.28 Preclinical nonhuman primate studies that evaluated the efficacy of tenofovir-based PreP found that the drug could protect against HIV acquisition when used topically or systemically.29 Subsequent macaque studies suggested possibility of intermittent dosing, given the high rates of protection found as long as animals received at least one dose preexposure, and another about 2 hours after the viral challenge.30,31
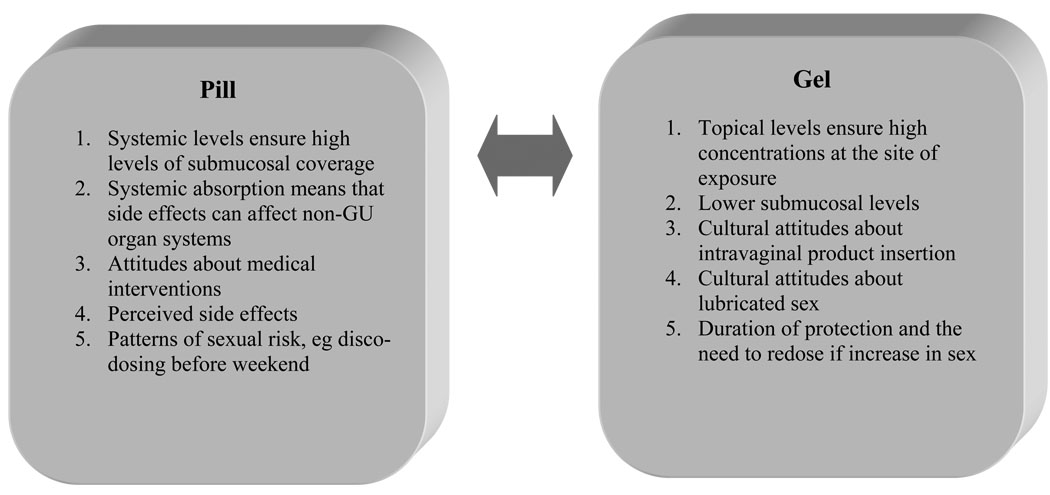
The ideal PrEP agent would have a long half-life, achieve high concentrations in target tissues, have a high barrier to the development of drug resistance, and be safe and inexpensive (Figure 1). Although all current clinical trials of PrEP involve tenofovir plus or minus emtricitabine, concerns have been raised about reliance on these drugs for chemoprophylaxis because they are mainstays of treatment. In the worst case scenario, individuals might use chemoprophylaxis intermittently, and if they did not undergo frequent HIV screening, they could select for, and then transmit, drug-resistant strains. Thus, other ARVs have been suggested as promising candidates for a multidrug PrEP regimen. Lamivudine and emtricitabine may be optimal for use as PrEP because of their great tolerability and long safety record, and strains that become resistant to these drugs almost invariably develop the M184V mutation, conferring decreased viral fitness,32 minimizing the risk of onward transmission to sex partners. Other well-studied antiretroviral agents may not be optimal for PrEP because they do not penetrate genital tissues in adequate concentrations,33 which is a function of relative protein binding and other pharmacodynamic properties.34 Protease inhibitors are highly protein-bound, achieving lower concentrations in the genital tract, compared to nucleoside analogues and nonnucleoside reverse transcriptase inhibitors.35,36 The oral CCR5 coreceptor antagonist maraviroc achieves high genital tract and rectal concentrations37 and could be effective as PrEP.37 If the initial tenofovir-based PrEP trials demonstrate efficacy, future studies may focus on the evaluation of other drugs with novel mechanisms of action and/or different pathways of resistance.
Figure 1.
PrEP delivered as a pill vs gel.
CURRENT STATUS OF PrEP CLINICAL TRIALS
Multiple clinical PrEP trials are underway among different communities globally to examine the impact of oral and/or vaginal tenofovir or emtricitabine/tenofovir on HIV acquisition (Table 1). These trials will enroll more than 20,000 HIV-uninfected men and women to address a variety of questions, including proof of concept, and if effective, then whether intermittent PrEP can be used, whether topical or oral PrEP is more effective, and whether drug resistance emerges as a clinical problem because of extensive use of these medications for chemoprophylaxis. All of these studies are examining the influence of PrEP on risk-taking behaviors.38 These studies are occurring among MSM in the Americas, Thailand, and South Africa; among at-risk women in sub-Saharan Africa; among HIV-discordant couples in Africa; and among Thai injection drug users. Most of these trials are evaluating daily dosing; a few will also include intermittent dosing strategies.39 A community-based organization, the AIDS Vaccine Advocacy Coalition (www.avac.org), has developed a PrEPWatch feature that provides continuously updated information about the status of clinical trials currently underway.
Table 1.
Current Clinical Trials of PrEP
| Trial Name | Population | Location | Drug | Means of Administration |
Sample Size |
Expected Results |
|---|---|---|---|---|---|---|
| US Extended Safety Trial (CDC* 4323) | Gay men; MSM | United States | TDF | Daily oral | 400 | TDF is safe and well-tolerated as PrEP for MSM |
| iPrEX | Gay men; MSM | Brazil, Ecuador, Peru, South Africa, Thailand, United States | TDF/FTC | Daily oral | 2500 | Fully enrolled 2009; results probable by late 2010 |
| Bangkok Tenofovir Study (CDC 4370) | Injecting drug users | Thailand | TDF | Daily oral | 2400 | Completed enrollment 2010; possible results 2010/early 2011 |
| CAPRISA 004 | Heterosexual women | South Africa | TDF | Coitally dependent topical vaginal gel | 1000 | First demonstration of the efficacy of tenofovir gel |
| TDF2 (CDC 4940) | Heterosexual men and women | Botswana | TDF/FTC | Daily oral | 1200 | Enrollment stopped 2009; safety data likely 2010 |
| Partners PrEP | Serodiscordant heterosexual couples | Kenya, Uganda | TDF and TDF/FTC | Daily oral | 3900 | Enrolling; data expected 2012 |
| FEM-PrEP | Heterosexual women | Kenya, Malawi, South Africa, Tanzania, Zambia | TDF/FTC | Daily oral | 3900 | Enrolling; data expected 2013 |
| VOICE (MTN 003) | Heterosexual women | South Africa, Uganda, Zambia, Zimbabwe; additional sites to be determined | TDF; TDF/FTC | Daily oral (TDF, TDF/FTC) Daily topical vaginal gel (TDF) |
5000 | Enrolling; data expected 2013 |
| ATN 082 | High-risk young MSM | United States | TDF/FTC | Daily dosing with or without a behavioral intervention | 100 | Enrolling; results in 2011 or 2012 |
| Studies Involving Intermittent PrEP (i-PrEP) | ||||||
| IAVI E001 and E002 | Serodiscordant couples and at-risk men and women | Kenya, Uganda | TDF/FTC | Daily oral; intermittent oral (twice weekly + coital dosing) | 150 | Full enrollment expected 2010 |
| HPTN 066 | Low-risk men and women | United States | TDF/FTC | Different dosing strategies planned | 48 | Intense pharmacokinetic study; full enrollment expected 2010 |
| HPTN 067 | High-risk women and MSM | Thailand, South Africa | TDF/FTC | Fixed interval vs coitally dependent | 360 | In planning stages |
CDC denotes Centers for Disease Control and Prevention; FTC, emtricitabine; MSM, men who have sex with men; TDF, tenofovir; IAVI, the International AIDS Vaccine Initiative; and HPTN, the HIV Prevention Trials Network.
The results of the first human PrEP study, a phase II, randomized double-blinded placebo controlled trial, was completed in 2007 and demonstrated the safety of daily oral tenofovir compared to a placebo for HIV prevention among high-risk West African women who received HIV testing, counseling, and condoms.40 Women in the intervention arm had fewer seroconversions than women in the control arm (8 vs 2), but this difference was not statistically significant.40,41 Importantly, both groups of women reduced their behavioral risk during the course of the study. Initial data suggests that efficient recruitment and implementation of large-scale phase III PrEP trials is possible with motivated participants and community engagement.42 One earlier planned PrEP study among Cambodian female sex workers never began enrollment because of community concerns, which led to extensive discussions among researchers, public officials, and other key stakeholders in order to anticipate concerns that might be raised in different communities—for example, regarding access to medication after trial completion.43 Over the next few years, data will become available to address whether oral tenofovir by itself, oral tenofovir coformulated with emtricitabine, or topical tenofovir gel (ie, a microbicide) will be more effective relative to placebo.44
An area of concern has been the development of drug resistance through the continued use of PrEP if participants unknowingly become HIV-infected, either by exposure to a drug-resistant virus, suboptimal adherence, or failure of the regimen (eg, inadequate tissue penetration). Individuals who had 2 weeks of tenofovir monotherapy (as a run-in to a study of a 3-drug-regimen) did not develop tenofovir resistance.45 In monkeys who were challenged with tenofovir-resistant SHIV, the use of tenofovir as PEP prevented HIV infection.46 Mathematical models of HIV prophylaxis have suggested that less than 1% of the predicted serconversions would develop a tenofovir-resistant strain.47 Due to the increasing use of tenofovir as part of first-line treatment regimens, continued monitoring to assess the effects of PrEP is warranted.
Data from oral and topical PrEP studies were presented at the International AIDS Society meetings in Vienna in July 2010. A study of 400 at-risk MSM, recruited in 3 US cities, compared the use of oral tenofovir to a placebo and found no evidence of increased clinical toxicities (particularly no increased renal insufficiency, bone demineralization, or other side effects) or increased sexual risk taking among the men assigned to tenofovir compared to those who received the placebo.48 Although this safety study was not powered to demonstrate drug efficacy, no new HIV infections were detected among the men who received the tenofovir. Another very important study, CAPRISA 004, studied tenofovir gel in high-risk women in KwaZulu-Natal, South Africa, and found that the women assigned to the tenofovir gel were significantly less likely to become HIV infected than those who used placebo gel49). Although the route of administration is different than oral chemoprophylaxis, the finding of a significant level of protection by a topical antiretroviral medication is a key observation, raising the hope that oral PrEP studies will also demonstrate a protective effect. Ultimately, it will be important to determine which route of administration is most effective for each specific population, since local anatomy (eg, vaginal versus rectal mucosa) and tissue pharmacology may determine the relative advantages of one approach over another. The VOICE trial being conducted by the NIH-funded Microbicide Trials Network (www.mtnstophiv.org) will be the first to evaluate an antiretroviral gel compared to oral medication, and may be a harbinger of future studies to determine the optimal chemoprophylactic strategies.
The success of the CAPRISA 004 study is important to prevention science, as the first demonstration of the ability of chemoprophylaxis to decrease HIV incidence and as the first proof of the protective effects of a topical microbicide. Future microbicide trials will evaluate other topical antiretroviral drugs, including dapivirine, a nonnucleoside reverse transcriptase agent, as well as other targets of the HIV life cycle, such as entry and integrase inhibitors. Future human studies will also assess whether other delivery mechanisms, ranging from vaginal rings to injectable compounds, might enhance product efficacy by decreasing the challenges of daily or coitally dependent adherence. More current information can be found on the Microbicide Trials Network website (www.mtnstopshiv.org) and the AIDS Vaccine Advocacy Coalition website (www.avac.org).
IMPLEMENTATION OF ANTIRETROVIRAL CHEMOPROPHYLAXIS
If PrEP is found to be an effective mode for HIV prevention, concerns about wider utilization include risk compensation, cost (ie, who will pay?), acquisition of resistant virus, adherence, and drug-related toxicities. The CDC and the World Health Organization have begun to meet regularly to anticipate community responses and to work with clinicians, national governments, and representatives of at-risk communities, as efficacy data becomes available.50 Providing ART to uninfected individuals will also need to be balanced with the imperative to provide prompt treatment access to HIV-infected individuals meeting guidelines for treatment initiation. However, it is possible that PrEP could be a cost-effective means of HIV prevention for younger and high-risk populations in the United States,51 given the high cost of treating new HIV infections over the life course.
If PrEP is found to be effective, questions will arise as to who should prescribe these drugs and how best to train providers. ART as PrEP will require researchers and clinicians to optimally utilize these agents in a focused manner to reduce the number of new HIV infections. Identifying individuals at high risk for HIV infection and then initiating them on PrEP could be a major challenge, given continued stigma associated with behaviors that put individuals at increased risk of acquiring HIV. Major areas that will require further clinical research include monitoring long-term safety, development of adherence strategies, assessing methods of optimal drug delivery and dosing, and minimizing selection of resistant viruses. Addressing these questions will require expanded safety and effectiveness trials as well as the development of public surveillance systems to monitor HIV incidence, risk compensation, and resistant virus evolution. Patients in clinical settings will require ongoing and regular HIV testing to avoid substandard therapy in the case of becoming HIV infected. Individuals with tenofovir-resistant virus could compromise their own treatment options and could spread resistant virus to their sex partners or offspring.
Potential risk compensation among individuals using PrEP may also compromise its effectiveness.52 It is possible that individuals could obtain off-label access to drugs in advance of efficacy data becoming available. Reports of ART being sold at clubs and of self administration prior to high-risk sexual activity have emerged,53,54 but studies from urban US centers have found minimal off-label PrEP use to date.55,56 However, the potential for widespread and unregulated use persists, and the demand could quickly change, based on when data becomes available to suggest a beneficial effect of PrEP.53 In order to ensure that PrEP does not lead to risk compensation and to provide PrEP as part of a risk reduction strategy, optimal prevention counseling packages will need to be developed.57
CONCLUSIONS: FUTURE OF ART FOR PRIMARY PREVENTION
The promise of ART chemoprophylaxis for uninfected individuals is great, but the field is still in an early stage, and major questions remain.. The discussion about using ART as a means of primary HIV prevention has moved to the forefront of public health discourse because of the increased tolerability of the medications, the decreased cost, the ever expanding formulary, and the limitations of other behavioral and biomedical approaches. Optimizing the use of antiretroviral agents for HIV prevention will require careful consideration of adherence, sustained access, behavioral risk reduction, and STI diagnosis and treatment. The ability to implement ART for primary prevention in the United States will depend on effectively engaging at risk individuals and educating their providers, as well as decreasing the economic impediments to optimal use.
It is conceivable that in the future certain drugs may be reserved for specific preventive and therapeutic interventions. Further research in pharmacology, virology, behavioral science, and health care service delivery will be needed to better understand the intended and unintended consequences of widely using ART for prevention. PEP and PrEP will likely be part of a larger HIV prevention toolbox that will be used to reduce the number of new infections, a toolbox that would also include behavioral risk reduction strategies, expanded HIV testing, male circumcision, prevention of mother-to-child transmission, and possibly STI treatment.4, 58 The search for an effective HIV vaccine must continue as well. But in the mean time, the use of multiple partially effective interventions could significantly slow the epidemic.
Acknowledgments
Funding: This research has been facilitated by the infrastructure and resources provided by the Lifespan/Tufts/Brown Center for AIDS Research (grant# P30 AI42853; PI, Charles Carpenter) and the Brown/Tufts/Miriam Fogarty AIDS International Training and Research Program (grant# D43TW000237; PI, Kenneth Mayer). KK Venkatesh is supported by NIMH Ruth Kirschstein National Research Service Award (NRSA; grant # F30 MH079738-01A2).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: K Mayer conceived the study. K Mayer and K Venkatesh synthesized analyses and wrote the manuscript.
Conflict of interest: The authors have no conflicts of interest.
REFERENCES
- 1.United Nations. Joint United Nations Programme on AIDS. [Accessed August 10, 2010];Geneva, Switzerland: Joint United Nations Programme on AIDS; Report on the Global AIDS Epidemic. 2008 Available at: http://www.unaids.org/en/KnowledgeCentre/HIVData/GlobalReport/2008/2008_Global_report.asp.
- 2.Padian NS, Buvé A, Balkus J, et al. Biomedical interventions to prevent HIV infection: evidence, challenges, and way forward. Lancet. 2008;372:585–599. doi: 10.1016/S0140-6736(08)60885-5. doi:10.1016/S0140-6736(08)60885-5. [DOI] [PubMed] [Google Scholar]
- 3.Johnston M, Fauci AS. An HIV vaccine--evolving concepts. N Engl J Med. 2007;356:2073–2081. doi: 10.1056/NEJMra066267. [DOI] [PubMed] [Google Scholar]
- 4.Coates T, Richter L, Caceres C. Behavioural strategies to reduce HIV transmission: how to make them work better. Lancet. 2008;372(9639):669–684. doi: 10.1016/S0140-6736(08)60886-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen MS, Gay CL. Treatment to prevent transmission of HIV-1. Clin Infect Dis. 2010;50 suppl 3:S85–S95. doi: 10.1086/651478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Granich R, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373:48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 7.Granich R, Crowley S, Vitoria M, et al. Highly active antiretroviral treatment for the prevention of HIV transmission. J Int AIDS Soc. 2010;13:1. doi: 10.1186/1758-2652-13-1. PMCID: PMC2822750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayer K. Antiretrovirals for HIV prevention: panacea or Pandora’s box? [paper #63]. Presented at: 17th Conference on Retroviruses and Opportunistic Infections; February 16–19, 2010; Francisco, CA. [Google Scholar]
- 9.Mayer K, Venkatesh KK. Antiretroviral therapy for HIV prevention: current status and future prospects. Am J Public Health. doi: 10.2105/AJPH.2009.184796. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen M, Kashuba AD. Antiretroviral therapy for prevention of HIV infection: new clues from an animal model. PLoS Medicine. 2006;5:e30. doi: 10.1371/journal.pmed.0050030. doi:10.1371/journal.pmed.0050030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Thoracic Society, Centers for Disease Control, and Infectious Diseases Society of America. Treatment of tuberculosis [erratum in: MMWR Recomm Rep. 2005;53:1203] MMWR Recomm Rep. 2003;52(RR11):1–77. [Google Scholar]
- 12.Division of STD Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control. Sexually transmitted diseases treatment guidelines, 2006 [erratum: MMWR Recomm Rep. 2006;55:997] MMWR Recomm Rep. 2006;55(RR11):1–94. [Google Scholar]
- 13.Centers for Disease Control and Prevention. Updated U.S. Public Health Service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep. 2001;50(RR11):1–52. [PubMed] [Google Scholar]
- 14.Cohen MS, Gay C, Kashuba AD, et al. Narrative review: antiretroviral therapy to prevent the sexual transmission of HIV-1. Ann Intern Med. 2007;146:591–601. doi: 10.7326/0003-4819-146-8-200704170-00010. [DOI] [PubMed] [Google Scholar]
- 15.Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal–infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331:1173–1180. doi: 10.1056/NEJM199411033311801. [DOI] [PubMed] [Google Scholar]
- 16.De Cock K, Fowler MG, Mercier E, et al. Prevention of mother-to-child HIV transmission in resource-poor countries: translating research into policy and practice. JAMA. 2000;283:1175–1182. doi: 10.1001/jama.283.9.1175. [DOI] [PubMed] [Google Scholar]
- 17.Cardo DM, Culver DH, Ciesielski CA, et al. A case-control study of HIV seroconversion in health care workers after percutaneous exposure. Centers for Disease Control and Prevention Needlestick Surveillance Group. N Engl J Med. 1997;337:1485–1490. doi: 10.1056/NEJM199711203372101. [DOI] [PubMed] [Google Scholar]
- 18.Tsai C, Emau P, Follis KE, Beck TW, et al. Effectiveness of postinoculation (R)-9-(2-phosphonylmethoxypropyl) adenine treatment for prevention of persistent simian immunodeficiency virus SIVmne infection depends critically on timing of initiation and duration of treatment. J Virol. 1998;72:4265–4273. doi: 10.1128/jvi.72.5.4265-4273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith DK, Grohskopf LA, Black RJ, et al. Antiretroviral postexposure prophylaxis after sexual, injection-drug use, or other nonoccupational exposure to HIV in the United States: recommendations from the U.S. Department of Health and Human Services. [Accessed September 1, 2008];MMWR Recomm Rep. 2005 54(RR21):1–20. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5402a1.htm. [PubMed] [Google Scholar]
- 20.Diaz-Brito V, León A, Knobel H, et al. the DATEMPEP Study Group. An open randomized multicenter clinical trial comparing zidovudine/lamivudine (ZDV/3TC) plus lopinavir/ritonavir (LP/r) or plus atazanavir (ATV) used as postexposure prophylaxis (PEP) for HIV infection [paper #956]. Presented at: 17th Conference on Retroviruses and Opportunistic Infections; February 16–19, 2010; San Francisco, CA. [Google Scholar]
- 21.Mayer KH, Mimiaga MJ, Cohen D, et al. Tenofovir DF plus lamivudine or emtricitabine for nonoccupational postexposure prophylaxis (NPEP) in a Boston Community Health Center. J Acquir Immune Defic Syndr. 2008;47:494–499. doi: 10.1097/QAI.0b013e318162afcb. [DOI] [PubMed] [Google Scholar]
- 22.Mayer K, Mimiaga M, Gelman M, et al. Tenofovir DF/emtricitabine/raltegravir (TDF/FTD/RAL) appears safe and well-tolerated for non-occupational post-exposure prophylaxis (NPEP) [abstract WEAC104]. Presented at: 5th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention; July 19–22, 2009; Cape Town, South Africa. [Google Scholar]
- 23.Dumond J. Maraviroc (MRV) genital tract (GT) fluid and tissue pharmacokinetics (PK) in healthy female volunteers: implications for pre- or post-exposure prophylaxis (PrEP or PEP) [paper #135LB]. Presented at: 15th Conference on Retroviruses and Opportunistic Infections; February 3–6, 2008; Boston, MA. [Google Scholar]
- 24.Bassett I, Freedberg KA, Walensky RP. Two drugs or three? Balancing efficacy, toxicity, and resistance in postexposure prophylaxis for occupational exposure to HIV. Clin Infect Dis. 2004;39:395–401. doi: 10.1086/422459. [DOI] [PubMed] [Google Scholar]
- 25.Schechter M, do Lago RF, Mendelsohn AB, et al. Behavioral impact, acceptability, and HIV incidence among homosexual men with access to postexposure chemoprophylaxis for HIV. J Acquir Immune Defic Syndr. 2004;35:519–525. doi: 10.1097/00126334-200404150-00010. [DOI] [PubMed] [Google Scholar]
- 26.Roland ME, Neilands TB, Krone MR, et al. Seroconversion following nonoccupational postexposure prophylaxis against HIV. Clin Infect Dis. 2005;41:1507–1513. doi: 10.1086/497268. [DOI] [PubMed] [Google Scholar]
- 27.Adamson P, Marcus J, Pipkin S, et al. Characteristics of and HIV seroconversion among patients receiving non-occupational HIV post-exposure prophylaxis in a sexually transmitted disease clinic: San Francisco, 2007 to 2009 [paper #958]. Presented at: 17th Conference on Retroviruses and Opportunistic Infections; February 16–19, 2010; San Francisco, CA. [Google Scholar]
- 28.Vourvahis M, Tappouni HL, Patterson KB, et al. The pharmacokinetics and viral activity of tenofovir in the male genital tract. J Acquir Immune Defic Syndr. 2008;47:329–333. doi: 10.1097/QAI.0b013e3181632cc3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dobard C, Parikh U, Sharma S, et al. Complete protection against repeated vaginal simian HIV exposures in macaques by a topical gel containing tenofovir alone or with emtricitabine [paper #46]. Presented at: 16th Conference on Retroviruses and Opportunistic Infections; February 8–11, 2009; Montreal, Quebec, Canada. [Google Scholar]
- 30.Garcia-Lerma G, Cong ME, Zheng Q, et al. Efficacy of intermittent prophylaxis with tenofovir and emtricitabine against rectal SHIV transmission in macaques and relationship to systemic and mucosal drug levels [paper #83]. Presented at: 17th Conference on Retroviruses and Opportunistic Infections; February 16–19, 2010; San Francisco, CA. [Google Scholar]
- 31.Garcia-Lerma G, Cong ME, Mitchell J, et al. Prevention of rectal simian HIV transmission in macaques by intermittent pre-exposure prophylaxis with oral truvada [paper #47]. Presented at: 16th Conference on Retroviruses and Opportunistic Infections; February 8–11, 2009; Montreal, Quebec, Canada. [Google Scholar]
- 32.Van Rompay KK, Matthews TB, Higgins J, et al. Virulence and reduced fitness of simian immunodeficiency virus with the M184V mutation in reverse transcriptase. J Virol. 2002;76:6083–6092. doi: 10.1128/JVI.76.12.6083-6092.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eron J, Vernazza PL, Johnston DM, et al. Resistance of HIV-1 to antiretroviral agents in blood and seminal plasma: implications for transmission. AIDS. 1998;12:F181–F191. doi: 10.1097/00002030-199815000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Taylor S, Pereira AS. Antiretroviral drug concentrations in semen of HIV-1 infected men. Sex Transm Infect. 2001;77:4–11. doi: 10.1136/sti.77.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghosn J, Chaix ML, Peytavin G, et al. Penetration of enfuvirtide, tenofovir, efavirenz, and protease inhibitors in the genital tract of HIV-1-infected men. AIDS. 2004;18:1958–1961. doi: 10.1097/00002030-200409240-00014. [DOI] [PubMed] [Google Scholar]
- 36.Pereira A, Kashuba AD, Fiscus SA, et al. Nucleoside analogues achieve high concentrations in seminal plasma: relationship between drug concentration and virus burden. J Infect Dis. 1999;180:2039–2043. doi: 10.1086/315149. [DOI] [PubMed] [Google Scholar]
- 37.Veazey R, Ketas T, Dufour J, et al. Protection of rhesus macaques from vaginal infection by maraviroc, an inhibitor of HIV-1 entry via the CCR5 co-receptor [paper #84LB]. Presented at: 17th Conference on Retroviruses and Opportunistic Infections; February 16–19, 2010; San Francisco, CA. [Google Scholar]
- 38.Buchbinder S. HIV prevention [paper #6]. Presented at: 16th Conference on Retroviruses and Opportunistic Infections; February 8–11, 2009; Montreal, Quebec, Canada. [Accessed August 16, 2010]. Available at: http://app2.capitalreach.com/esp1204/servlet/tc?c=10164&cn=retro&e=10642&m=1&s=20415&&espmt=2&mp3file=10642&m4bfile=10642&br=80&audio=false [Web cast]. [Google Scholar]
- 39.HIV Prevention Trials Network. [Accessed June 3, 2010];Ongoing studies and studies in development [Web page] Available at: www.htpn.org/research_studies.asp.
- 40.Peterson L, Taylor D, Roddy R, et al. Tenofovir disoproxil fumarate for prevention of HIV infection in women: a phase 2, double-blind, randomized, placebo-controlled trial. PloS Clinical Trials. 2007;2:e27. doi: 10.1371/journal.pctr.0020027. PMCID: PMC1876601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peterson L, Taylor D, Clarke EE, et al. Findings from a double-blind, randomized, placebo-controlled trial of tenofovir disoproxil fumarate (TDF) for prevention of HIV infection in women [abstract THLB0104]. Presented at: XVI International AIDS Conference; August 13–18, 2006; Toronto, Ontario, Canada. [Google Scholar]
- 42.Baeten J, Ndase P, Mugo N, et al. the Partners PrEP Study Team. Demographic, behavioral, and clinical characteristics of HIV-1 serodiscordant couples enrolled into an efficacy trial of pre-exposure prophylaxis [paper #959]. Presented at: 17th Conference on Retroviruses and Opportunistic Infections; February 16–19, 2010; San Francisco, CA. [Google Scholar]
- 43.HIV prevention trials require sophisticated community preparation. Controversial PrEP trials serve as cautionary tale. 2009;24:67–69. [PubMed] [Google Scholar]
- 44.Abdool Karim S, Coletti A, Richardson B, et al. Safety and effectiveness of vaginal microbicides Buffer Gel and 0.5% PRO 2000/5 gel for the prevention of HIV infection in women: results of the HPTN 035 Trial [paper #48LB]. Presented at: 16th Conference on Retroviruses and Opportunistic Infections; February 8–11, 2009; Montreal, Quebec, Canada. [Google Scholar]
- 45.Barditch-Crovo P, Deeks SG, Collier A, et al. Phase i/ii trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob Agents Chemother. 2001;45:2733–2739. doi: 10.1128/AAC.45.10.2733-2739.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Rompay KK, Johnson JA, Blackwood EJ, et al. Sequential emergence and clinical implications of viral mutants with K70E and K65R mutation in reverse transcriptase during prolonged tenofovir monotherapy in rhesus macaques with chronic RT-SHIV infection. Retrovirology. 2007;6:25. doi: 10.1186/1742-4690-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith D. Antiretroviral resistance is not an important risk of the oral tenofovir prophylaxis trial in Botswana: a simple mathematical modelling approach. Presented at: XVI International AIDS Conference; August 13–18, 2006; Toronto, Ontario, Canada. [Google Scholar]
- 48.Grohskopf L, Gvetadze R, Pathak S, et al. Preliminary analysis of biomedical data from the phase II clinical safety trial of tenofovir disoproxil fumarate (TDF) for HIV-1 pre-exposure prophylaxis (PrEP) among U.S. men who have sex with men (MSM). Presented at: XVIII International AIDS Conference; July 18–23, 2010; Vienna, Austria. [Google Scholar]
- 49.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. the CAPRISA 004 Trial Group. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women [published online ahead of print July 19, 2010] Science. doi: 10.1126/science.1193748. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. [Accessed December 26, 2009];New York, NY: AVAC; Part of the Solution: Setting Expectations for WHO and UNAIDS. Available at: http://www.avac.org/ht/d/sp/a/GetDocumentAction/i/2275. AVAC Report 2009.
- 51.Paltiel A, Freedberg KA, Scott CA, et al. HIV preexposure prophylaxis in the United States: impact on lifetime infection risk, clinical outcomes, and cost-effectiveness. Clin Infect Dis. 2009;48:806–815. doi: 10.1086/597095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abbas U, Anderson RM, Mellors JW. Potential impact of antiretroviral chemoprophylaxis on HIV-1 transmission in resource-limited settings. PLos ONE. 2007;2(9):e875. doi: 10.1371/journal.pone.0000875. doi:10.1371/journal.pone.0000875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mansergh G, Colfax G, McKirnan D, et al. the Project MIX Study Group. Use and sharing of antiretroviral medications for pre- and post-exposure prophylaxis to prevent sexual transmission of HIV among high-risk, substance-using men who have sex with men in 4 US cities [paper #957]. Presented at: 17th Conference on Retroviruses and Opportunistic Infections; February 16–19, 2010; San Francisco, CA. [Google Scholar]
- 54.Kellerman SE, Hutchinson AB, Begley EB, et al. Knowledge and use of HIV pre-exposure prophylaxis among attendees of minority gay pride events, 2004. J Acquir Immune Defic Syndr. 2006;43:376–377. doi: 10.1097/01.qai.0000234085.18914.d5. [DOI] [PubMed] [Google Scholar]
- 55.Mimiaga MJ, Case P, Johnson CV, et al. Preexposure antiretroviral prophylaxis attitudes in high-risk Boston area men who report having sex with men: limited knowledge and experience but potential for increased utilization after education. J Acquir Immune Defic Syndr. 2009;50:77–83. doi: 10.1097/QAI.0b013e31818d5a27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu AY, Kittredge PV, Vittinghoff E, et al. Limited knowledge and use of HIV post- and pre-exposure prophylaxis among gay and bisexual men. J Acquir Immune Defic Syndr. 2008;47:241–247. [PubMed] [Google Scholar]
- 57.Underhill K, Operario D, Skeer M, et al. Packaging PrEP to prevent HIV: an integrated framework to plan for pre-exposure prophylaxis implementation in clinical practice [published online ahead of print August 13, 2010] J Acquir Immune Defic Syndr. doi: 10.1097/qai.0b013e3181e8efe4. doi: 10.1097/QAI.0b013e3181e8efe4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burns D, Dieffenbach CW, Vermund SH. Rethinking HIV-1 prevention of HIV type 1 infection published online ahead of print August 13, 2010] Clin Infect Dis. 2010;51 doi: 10.1086/655889. doi: 10.1086/655889. [DOI] [PMC free article] [PubMed] [Google Scholar]