Abstract
As part of the insulin signaling pathway, AKT influences growth and metabolism. The AKT1 gene G205T (rs1130214) polymorphism has potential functional effects. Thus, we determined whether the G205T polymorphism influences metabolic variables and their responses to aerobic exercise training. Following dietary stabilization, healthy, sedentary, 50-75 yr old Caucasian men (n = 51) and women (n = 58) underwent 6 months of aerobic exercise training. Before and after completing the intervention, dual-energy x-ray absorptiometry measured percent body fat, computed tomography measured visceral and subcutaneous fat, and oral glucose tolerance testing measured glucose total area under the curve (AUC), insulin AUC, and insulin sensitivity. Taqman assay determined AKT1 G205T genotypes. At baseline, men with the GG genotype (n = 29) had lower VO2max values (p = 0.026), and higher percent body fat (p = 0.046), subcutaneous fat (p = 0.021), and insulin AUC (p = 0.003) values than T allele carriers (n = 22). Despite their rather disadvantageous starting values, men with the GG genotype seemed to respond to exercise training more robustly than men with the T allele, highlighted by significantly greater fold change improvements in insulin AUC (p = 0.012) and glucose AUC (p = 0.035). Although the GG group also significantly improved VO2max with training, the change in VO2max was not as great as that of the T allele carriers (p = 0.037). In contrast, after accounting for hormone replacement therapy use, none of the variables differed in the women at baseline. As a result of exercise training, women with the T allele (n = 20) had greater fold change improvements in fasting glucose (p = 0.011), glucose AUC (p = 0.017), and insulin sensitivity (p = 0.044) than GG genotype women (n = 38). Our results suggest that the AKT1 G205T polymorphism influences metabolic variables and their responses to aerobic exercise training in older previously sedentary individuals.
Keywords: polymorphism, exercise, glucose
Introduction
As a critical component in the insulin and insulin-like growth factor signaling cascades, AKT affects growth, differentiation, and metabolism. AKT [also known as protein kinase B (PKB)] is a serine and threonine kinase with three distinct mammalian genes: AKT1, AKT2, and AKT3. Of them, AKT1 is the most widely expressed (ubiquitously), and studies involving targeted disruption of the AKT1 gene in mice have indicated its involvement in organismal growth, with AKT1 knockout mice being smaller (∼20 % lower body weight) than wildtype controls (Chen et al., 2001;Cho et al., 2001). Furthermore, AKT1 has been implicated in muscle differentiation as knockdown of AKT1 in cell culture experiments inhibited MyoD activity and myoblast differentiation (Wilson & Rotwein, 2007). A role for AKT1 in adipocyte differentiation has also emerged from studies involving AKT1 knockdown mice embryonic fibroblasts (Baudry et al., 2006) and the downregulation of AKT1 expression via RNA interference (Xu & Liao, 2004) and, in both of these instances, adipocyte differentiation was impaired. Thus, with its connections to growth and adipogenesis, AKT1 would appear to be a likely candidate gene related to obesity-related metabolic variables.
Little data exist to our knowledge regarding AKT1 polymorphisms and metabolic phenotypes. However, the AKT1 gene region has been well studied in connection with schizophrenia, and despite result inconsistencies (Ide et al., 2006;Liu et al., 2006;Ohtsuki et al., 2004;Sanders et al., 2008;Turunen et al., 2007), several reports have implicated AKT1 haplotypes with disease risk (Bajestan et al., 2006;Emamian et al., 2004;Ikeda et al., 2004;Norton et al., 2007;Schwab et al., 2005;Thiselton et al., 2008;Xu et al., 2007). The AKT1 G205T (rs1130214) polymorphism acts as a tagging polymorphism for the haplotype (Emamian et al., 2004;Schwab et al., 2005), and because the G205T polymorphism may affect AKT1 expression by altering a putative transcription factor binding site (Thiselton et al., 2008), we investigated the polymorphism in relation to metabolic phenotypes in our cohort of generally healthy, older individuals. The response of many health and fitness parameters to aerobic exercise training tends to be highly variable and at least partly dependent on genetic factors (Bray et al., 2009). In fact, it has recently been reported that 23 % of the exercise training response variability in maximal oxygen consumption could be explained by 11 different DNA variants (Timmons et al., 2010). In keeping with these ideas, our secondary purpose was to determine if aerobic exercise training-induced changes in our participants' metabolic phenotypes were AKT1 G205T genotype-specific.
Methods
Ethical approval
This study was approved by the University of Maryland, College Park Institutional Review Board. All participants provided written informed consent.
Participant Selection and Screening
This was a retrospective cohort study of participants in the Gene Exercise Research Study (GERS) at the University of Maryland, College Park. The methods and design of the GERS have been described in detail previously (McKenzie et al., 2004;Obisesan et al., 2006). Data reported here are from 109 Caucasian participants (51 men, 58 women) who completed all baseline and final testing procedures as part of cohorts taking part in the GERS during the years 1998-2006. Previous reports from this group have included participants from earlier cohorts of the GERS (e.g. 1998-2004) (McKenzie et al., 2004;Obisesan et al., 2006;Obisesan et al., 2004). More recently, our group reported PLIN haplotype relationships involving this same subset of participants (Jenkins et al., 2010).
Briefly, participants had to be ≥ 50 yrs of age but ≤ 75 yrs of age, sedentary (< 20 minutes of physical activity ≤ twice per week), non-diabetic (fasting glucose concentration < 126 mg/dL and 2-hour OGTT glucose concentration < 200 mg/dL), non-smoking, have no history or characteristics of cardiovascular, lung, liver, or kidney disease, have a BMI ≤ 37 kg/m2, have a hematocrit > 35, and have no orthopedic conditions that would preclude exercise. In addition, participants had to have 1) resting blood pressure measurements > 120/80 mmHg but < 160/100 mmHg or 2) at least one National Cholesterol Education Program (NCEP) lipid abnormality and be normotensive or blood pressure controlled with non-lipid and non-glucose altering medication. Finally, women participating in the study had to be postmenopausal for at least 2 years and willing to maintain their hormone therapy status, either receiving or not receiving hormone replacement therapy (HRT), throughout the duration of the study.
Dietary Stabilization
After screening into the study, participants attended dietary stabilization classes twice a week for 6 weeks. A registered dietician provided instructional sessions on the principles of the American Heart Association Step 1 diet, similar to the American Heart Association Dietary Guidelines for the General Population, which emphasized the consumption of 55-60 % of dietary calories from carbohydrates, < 30 % of dietary calories from fat, and alcohol in moderation (American Heart Association, 1988). Participants maintained the diet throughout the study's duration, with periodic dietary recalls and food frequency checks to ensure adherence. Body weight was stabilized before baseline testing occurred and was maintained ± 5 % throughout the study.
Baseline Testing
Following the completion of the dietary classes, participants underwent baseline testing. Total percent body fat and fat free mass were measured via dual-energy x-ray absorptiometry (DPX-L or DPX-IQ, Lunar Corporation, Madison, WI), whereas visceral and subcutaneous adipose tissue depots were measured using single slice computed tomography (GE Hi-Light CT scanner). Standard procedures were used as previously described (Nicklas et al., 1996).
After a 12-hour overnight fast, a 2-hour OGTT began between the hours of 6:30 and 9:00 AM. A 20- or 22-gauge indwelling catheter was placed into a vein in the antecubital region, and blood sampling occurred before and every 30 minutes after the ingestion of a 75 gram D-glucose solution, for 2 hours. Blood samples were centrifuged, and plasma was separated and stored at -80 degrees Celsius until assayed for glucose and insulin levels. The glucose oxidase method was used to determine plasma glucose concentration via a glucose analyzer (YSI 2300 Stat Plus, YSI, Inc., Yellow Springs, OH), and a competitive radioimmunoassay (kit HI-14K, Linco Research, St. Charles, MO) was used to determine plasma insulin concentration. Glucose total area under the curve (AUC) was calculated via the trapezoidal method and an insulin sensitivity index (ISI) was calculated using the method of Matsuda and DeFronzo (Matsuda & DeFronzo, 1999).
Maximal oxygen consumption (VO2max) and heart rate were measured during a maximal graded treadmill exercise test, as previously described (Dengel et al., 1994). A physician presided over the test, and electrocardiogram and blood pressure measurements were recorded throughout. Standard criteria were used to ensure that VO2max was achieved (American College of Sports Medicine, 2010), and heart rates measured from the electrocardiogram recording were used in the exercise prescription.
Aerobic Exercise Training Intervention
Following the completion of baseline testing, participants began 24 weeks of supervised aerobic exercise training. An exercise training heart rate range was determined for each participant corresponding to the percent intensity ± 5 % using the Karvonen (heart rate reserve) formula. During the exercise training sessions, Polar heart rate monitors were used to assess heart rate. Training equipment available to the participants included stair climbers, stationary bicycles, recumbent bicycles, rowing ergometers, treadmills, elliptical machines, and cross country ski machines. To gradually acclimatize the sedentary participants to exercise, the first week of training involved 20 minutes of activity at 50 % intensity for 3 days per week. Exercise duration then progressed by 5 minutes weekly, until 40 minutes was achieved. Then, exercise intensity increased gradually, 5 % per week, until 70 % was attained. Thus, from week 9 through week 24, participants exercised for 40 minutes at an intensity of 70 %. Exercise heart rates were monitored throughout each training session by the exercise training staff to ensure training at the required intensity level, and average exercise heart rate for each participant for each exercise session was recorded in an exercise log. Finally, in weeks 10-24, participants added an additional day of exercise to their routine (an unsupervised home workout session). Exercise percent attendance rates were calculated as the number of sessions completed as prescribed (i.e. at the required exercise intensity and duration) divided by the total number of possible sessions.
Final Testing
Final testing occurred following the completion of 24 weeks of aerobic exercise training. Final testing procedures were similar to baseline testing procedures except that all final testing took place 24-36 hours after an exercise training bout. This time frame ensured measurement of training rather than acute exercise effects.
Genotyping
A 10 mL blood sample was collected during the screening process and DNA extracted and stored for genotyping. The AKT1 G205T polymorphism (rs1130214) was genotyped using a predesigned TaqMan allelic discrimination assay (C_26352825_10). All reactions were carried out using an Applied Biosystems 7300 Real Time PCR System and optical reaction plates. Fluorescence (via allele specific VIC and FAM dyes – probe sequence [vic/fam] GAGTCCAGAGCCCTCCAGCGCAAGC[A/C]CAAAAACCTCCTGGGAGAAACCCCA) was measured and genotypes were determined using the Applied Biosystems 7300 System Sequence Detection Software. Each plate contained four no template controls and four sequencing control samples of known genotype.
Statistical Analyses
All statistical analyses were performed using SPSS (version 17.0) software. Before any statistical analyses were conducted, the assumptions for each procedure were examined, and as a result, transformation using the common logarithm was required for intra-abdominal fat, insulin AUC, and ISI. Chi-square tests were used to assess Hardy-Weinberg equilibrium and to compare differences in categorical variables between groups. Analysis of covariance (ANCOVA) was used to compare differences in obesity-related variables between AKT1 G205T genotype groups at baseline and in response to aerobic exercise training. The fold change with training was calculated as the ratio of the final value and the baseline value. The fold decrease was reported for fold changes below one, and was calculated by dividing one by the fold change. When appropriate, covariates for the baseline analyses included age and BMI, and covariates for the after aerobic exercise training analyses included age, BMI, and the baseline value of the outcome variable. Due to a significant difference in the frequency of hormone replacement therapy (HRT) users by genotype group in the women participating in the study, HRT use was also accounted for in the baseline and after training analyses. Data from men and women were analyzed separately due to the potential for sex-specific effects (Ordovas, 2007). Due to the small number of non-Caucasian participants, the possibility of allele frequency differences by race, and the added strength resulting from a homogeneous population (Rodriguez-Murillo & Greenberg, 2008), only data from Caucasians were included in the analyses. Paired samples t-tests were used to determine significant changes within groups with aerobic exercise training. Statistical significance was set at p ≤ 0.05.
Results
AKT1 G205T allele and genotype frequencies (Table 1) for the 109 participants differed slightly from Hardy-Weinberg expectancies in the total group (χ2 = 5.63, p = 0.018) and in the women participating in the study (χ2 = 5.43, p = 0.020), but were in Hardy-Weinberg equilibrium for the men participating in the study (χ2 = 1.05, p = 0.305). Repeat genotyping to verify these results revealed consistent genotype results indicating a possible sampling bias but robust laboratory techniques. Due to the low frequency of individuals with the TT genotype and non-statistically different GT and TT group means for the major outcome variables (data not shown), the GT and TT genotype groups were combined for analyses.
Table 1. AKT1 G205T allele and genotype frequencies with sample sizes.
| Allele | Genotype | ||||
|---|---|---|---|---|---|
| G | T | GG | GT | TT | |
| Total | 0.76 (165) | 0.24 (53) | 0.62 (67) | 0.28 (31) | 0.10 (11) |
| Men | 0.74 (75) | 0.27 (27) | 0.57 (29) | 0.33 (17) | 0.10 (5) |
| Women | 0.78 (90) | 0.22 (26) | 0.66 (38) | 0.24 (14) | 0.10 (6) |
Data are frequencies (sample size in parentheses).
Men
As shown in Table 2, men participating in the study had normal fasting glucose measurements, but were overweight with below average VO2max values (19). Although weight, fat free mass, and BMI were not different between the GG and GT+TT genotype groups, significant differences were detected for percent body fat and subcutaneous body fat measurements, with the GG genotype group having higher values. Consistent with the body composition differences, the GG genotype group also had higher insulin AUC values than the GT+TT group. Furthermore, covarying for percent body fat or subcutaneous body fat in place of BMI did not significantly alter the insulin AUC results (p = 0.007 and p = 0.024, respectively). Lastly, VO2max values were higher in the GT+TT group than in the GG group.
Table 2. Baseline characteristics of men grouped by AKT1 G205T genotype.
| GG (n = 17-29) |
GT+TT (n = 13-22) |
P-value | |
|---|---|---|---|
| Age (yr) | 58 ± 1 | 61 ± 1 | 0.073 |
| VO2max (mLkg-1min-1) | 27.3 ± 0.7 | 30.0 ± 0.9 | 0.026* |
| Weight (kg) | 91.1 ± 2.7 | 86.7 ± 3.1 | 0.292 |
| BMI (kgm-2) | 28.7 ± 0.7 | 27.3 ± 0.8 | 0.205 |
| Fat free mass (kg) | 58.7 ± 1.3 | 57.6 ± 1.5 | 0.598 |
| IA fat (cm2) | 150 (129-175) | 131 (110-156) | 0.254 |
| SC fat (cm2) | 280 ± 16 | 220 ± 18 | 0.021* |
| Total fat (%) | 31.0 ± 1.1 | 27.3 ± 1.3 | 0.046* |
| Glucose (mmolL-1) | 5.2 ± 0.1 | 5.4 ± 0.1 | 0.130 |
| Insulin (pmolL-1) | 79 ± 5 | 91 ± 6 | 0.136 |
| ISI | 2.9 (2.5-3.3) | 3.3 (2.7-3.9) | 0.263 |
| Glucose AUC (mmolL-1 × min) | 960 ± 41 | 904 ± 46 | 0.370 |
| Insulin AUC (pmolL-1 × min) | 64863 (55208-76208) | 43351 (35892-52240) | 0.003* |
Data are expressed as adjusted means ± SE with the exception of intra-abdominal fat, ISI, and insulin AUC, which are presented as the back transformed mean of the log transform (95 % confidence interval). VO2max, maximal oxygen consumption; BMI, body mass index; IA; intra-abdominal; SC, subcutaneous; ISI, insulin sensitivity index; AUC, total area under the curve; n, sample size. Sample sizes are varied due to the inability to obtain all measurements on all participants. P-value is for the main effect of genotype.
indicates significant difference between genotype groups p ≤ 0.05.
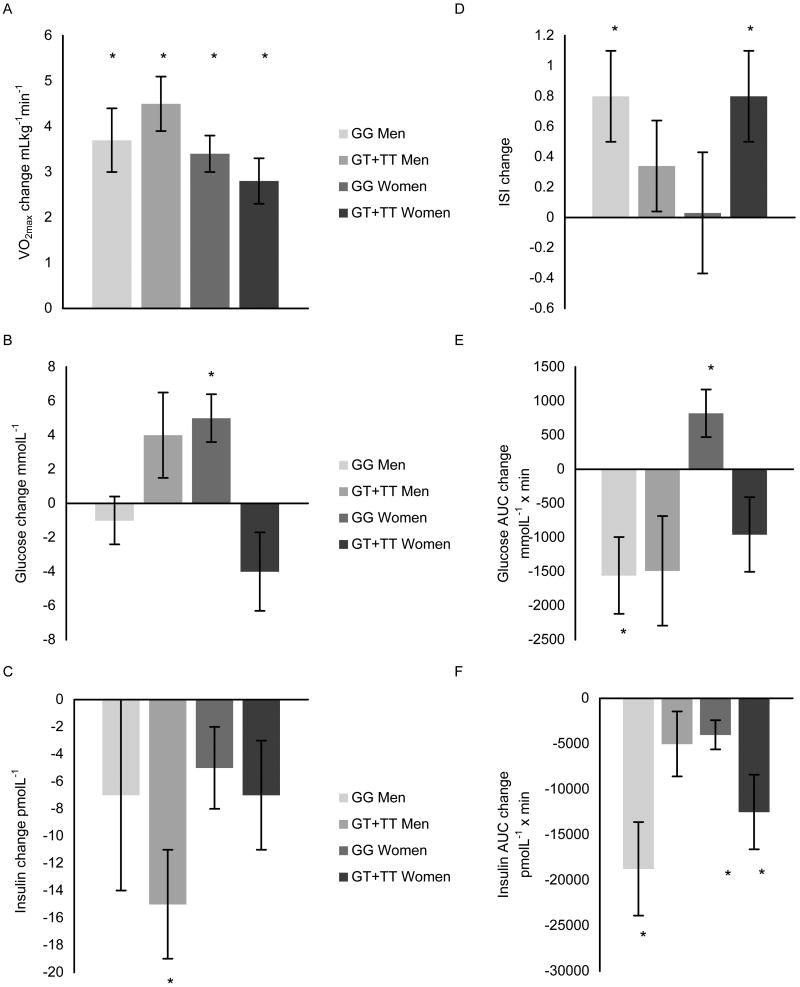
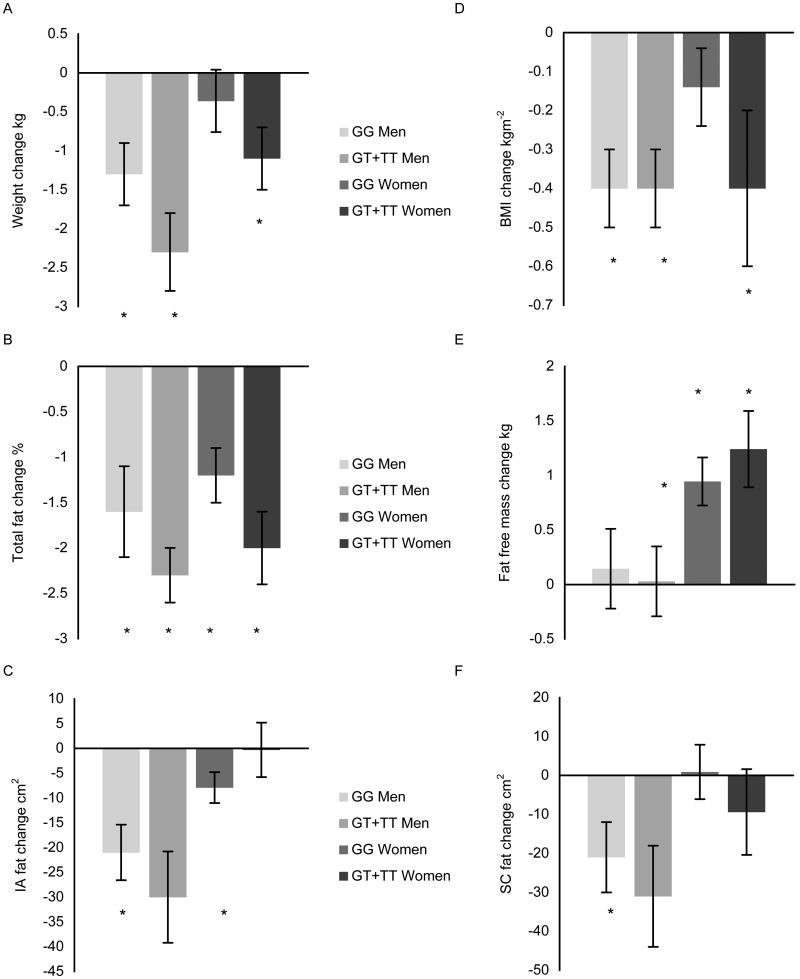
Percent attendance at the exercise sessions did not differ between the groups, with the GG group averaging 90.3 ± 1.3 % and the GT+TT group averaging 91.5 ± 1.5 %. As displayed in Figures 1 and 2, after 24 weeks of aerobic exercise training in the men, the GG group increased VO2max by ∼13 %, decreased subcutaneous fat by ∼8 %, and improved insulin sensitivity via decreases in insulin AUC and glucose AUC and an increase in the ISI. In addition, slight but statistically significant changes in weight, intra-abdominal fat, BMI, and percent body fat occurred in the GG men. Similarly, the GT+TT men had slight but statistically significant decreases in weight, percent body fat, and BMI. They also experienced a significant decrease in fasting insulin with aerobic exercise training, a slight increase in fat free mass, and a 19 % increase in VO2max.
Figure 1. Aerobic exercise training-induced changes in A) VO2max, B) fasting glucose, C) fasting insulin, D) ISI, E) glucose AUC, and F) insulin AUC by AKT1 G205T genotype and gender.
Data are expressed as unadjusted means ± SE. VO2max, maximal oxygen consumption; ISI, insulin sensitivity index; AUC, total area under the curve. * indicates significant difference within genotype group with training p ≤ 0.05.
Figure 2. Aerobic exercise training-induced changes in A) weight, B) total body fat, C) IA fat, D) BMI, E) fat free mass, and F) SC fat by AKT1 G205T genotype and gender.
Data are expressed as unadjusted means ± SE. BMI, body mass index; IA; intra-abdominal; SC, subcutaneous. * indicates significant difference within genotype group with training p ≤ 0.05.
Overall, the GG group responded more favorably to aerobic exercise training than the GT+TT group. When expressed as a fold change, the GG group improved insulin AUC and glucose AUC more than the GT+TT group (fold decreases of 1.4 ± 0.06 versus 1.0 ± 0.06, p = 0.012 and 1.1 ± 0.04 versus 1.0 ± 0.04, p = 0.035, respectively). Furthermore, although both genotype groups significantly improved VO2max with training, the fold change in VO2max was slightly greater in the GT+TT group than in the GG group (fold increases of 1.2 ± 0.02 versus 1.1 ± 0.02, p = 0.037).
Women
Since HRT users had a lower baseline weight (p = 0.028) and intra-abdominal fat content (p = 0.047) than HRT non-users, and the frequency of HRT users and HRT non-users differed between the genotype groups (χ2 = 4.08, p = 0.043), HRT use was accounted for in the analyses. After adjusting for HRT use among women participating in the study, none of the baseline glucose or obesity-related variables differed by genotype group (Table 3). As with the men, exercise adherence did not differ in the women, averaging 90.1 ± 1.2 % and 92.5 ± 1.7 % in the GG and GT+TT groups, respectively. Aerobic exercise training-induced changes in the major outcome variables are shown in Figures 1 and 2. Following the completion of aerobic exercise training, the GT+TT genotype group had more favorable changes than the GG genotype group in fasting glucose (fold increases of 1.0 ± 0.03 versus 1.1 ± 0.02, p = 0.011), glucose AUC (fold decrease of 1.1 ± 0.04 versus fold increase of 1.1 ± 0.03, p = 0.017), and ISI values (fold increases of 1.2 ± 0.08 versus 1.0 ± 0.06, p = 0.044), respectively. These differences resulted from the significant increase in ISI that occurred in the GT+TT group and significant, but disadvantageous, increases in glucose AUC and fasting glucose that were experienced by the GG group with training. Other changes that occurred with aerobic exercise training in the GG women included a 15 % increase in VO2max, a slight increase in fat free mass, and slight decreases in percent body fat, intra-abdominal fat, and insulin AUC. The GT+TT group also increased VO2max (∼12 %) and fat free mass, and slightly decreased weight, percent body fat, BMI, and insulin AUC with aerobic exercise training.
Table 3. Baseline characteristics of women grouped by AKT1 G205T genotype.
| GG (n = 20-38) |
GT+TT (n = 15-20) |
P-value | |
|---|---|---|---|
| Age (yr) | 57 ± 1 | 58 ± 1 | 0.684 |
| HRT use (yes/no) | 22/16 | 6/14 | ------ |
| VO2max (mLkg-1min-1) | 23.1 ± 0.5 | 23.3 ± 0.7 | 0.816 |
| Weight (kg) | 73.8 ± 2.0 | 76.0 ± 2.8 | 0.531 |
| BMI (kgm-2) | 27.7 ± 0.7 | 27.9 ± 1.0 | 0.868 |
| Fat free mass (kg) | 38.9 ± 0.9 | 40.1 ± 1.2 | 0.443 |
| IA fat (cm2) | 110 (100-121) | 111 (97-127) | 0.880 |
| SC fat (cm2) | 347 ± 19 | 374 ± 26 | 0.420 |
| Total fat (%) | 42.0 ± 1.1 | 42.7 ± 1.5 | 0.745 |
| Glucose (mmolL-1) | 4.8 ± 0.1 | 5.1 ± 0.1 | 0.079 |
| Insulin (pmolL-1) | 76 ± 5 | 77 ± 6 | 0.953 |
| ISI | 3.7 (3.1-4.4) | 3.3 (2.6-4.1) | 0.373 |
| Glucose AUC (mmolL-1 × min) | 857 ± 39 | 965 ± 49 | 0.100 |
| Insulin AUC (pmolL-1 × min) | 45499 (37844-54702) | 48753 (38726-61376) | 0.644 |
Data are expressed as adjusted means ± SE with the exception of intra-abdominal fat, ISI, and insulin AUC, which are presented as the back transformed mean of the log transform (95 % confidence interval). HRT, hormone replacement therapy; VO2max, maximal oxygen consumption; BMI, body mass index; IA; intra-abdominal; SC, subcutaneous; ISI, insulin sensitivity index; AUC, total area under the curve; n, sample size. Sample sizes are varied due to the inability to obtain all measurements on all participants. P-value is for the main effect of genotype.
indicates significant difference between genotype groups p ≤ 0.05.
Discussion
AKT1 has been implicated as a positive regulator of growth (Chen et al., 2001;Cho et al., 2001) and skeletal muscle differentiation (Wilson & Rotwein, 2007). Mouse models have also indicated the importance of AKT1 in adipocyte differentiation (Baudry et al., 2006;Xu & Liao, 2004), and although several polymorphisms in AKT1 were not associated with type 2 diabetes in an Ashkenazi Jewish population (Matsubara et al., 2001), no one to our knowledge thus far has published data on the AKT1 G205T polymorphism (rs1130214) in relation to metabolic variables or their responses to aerobic exercise training. Furthermore, the G205T polymorphism may have a functional effect (Thiselton et al., 2008). Recently, Harmon and colleagues (2010) reported that the T allele was part of an AKT1 haplotype that resulted in enhanced transcription in muscle and decreased transcription in fat. In keeping with these findings, we found that several obesity-related metabolic variables differed prior to exercise training by the AKT1 G205T genotype in generally healthy, older men. Furthermore, men with the T allele (GT or TT genotype) were found to have lower subcutaneous fat, percent body fat, and insulin AUC values as compared to men with the GG genotype. In addition, VO2max, expressed relative to body weight, was found to be higher in the T allele carriers. Thus, it seems that the T allele may be beneficial in sedentary men.
As insulin resistance and obesity are often related, it is possible that the genotype differences detected for insulin AUC reflect the significant body fat differences in the groups. However, when either percent body fat or subcutaneous body fat was used as a covariate in place of BMI, the insulin AUC results did not change substantially. Thus, the AKT1 G205T genotype differences detected for insulin AUC in the men appear to be independent of the genotype-related differences in body composition.
Although the GG genotype was associated with less favorable outcomes at baseline in men, it was advantageous in terms of responsiveness to aerobic exercise training as significantly greater improvements in insulin AUC and glucose AUC occurred in the GG genotype group as compared to the GT+TT genotype group. Furthermore, an examination of the 10 % most robust responders versus non-responders for each of the major outcome variables found similar patterns as 80 % of the top 10 % most responsive to aerobic exercise training in terms of change in subcutaneous body fat, change in percent body fat, change in fasting glucose, and change in insulin AUC were individuals with the GG genotype. Of the 10 % least responsive to aerobic exercise training, GT+TT individuals were found to comprise 100 % of the non-responders in glucose AUC change and 80 % of the non-responders in insulin AUC change. Interestingly, 100 % of the 10 % least responsive to VO2max change with training were individuals with the GG genotype. This may help to explain the slightly better change in VO2max with training in the GT+TT genotype group.
Interestingly, the genotype-dependent differences found in the men were not replicated in the women. Although more evidence is needed, research suggests that sex may modulate interactions between genetic factors, the environment, and health outcomes (Ordovas, 2007). Although speculative, sex-specific physiological differences in hormone levels or body composition may influence AKT1 genotype associations with obesity-related metabolic variables. This is an interesting avenue for further research since AKT1 variants appear to have tissue-specific effects (Harmon et al., 2010). On the other hand, in the current study, these sex differences may be due in part to a statistical power issue resulting from the inclusion of HRT usage in the analyses involving women. Among the women, we did however find genotype-related differences in the aerobic exercise training-induced changes in fasting glucose, ISI, and glucose AUC. In all three instances, the T allele carriers had more advantageous values after the aerobic exercise training intervention than the GG genotype group. A comparison of the 10 % most robust responders to non-responders for these variables found that 80 % of the most responsive to aerobic exercise training in terms of change in fasting glucose and 100 % of the most responsive to aerobic exercise training in terms of change in glucose AUC and change in ISI were women with the GT+TT genotype. Of the 10 % least responsive to aerobic exercise training, women with the GG genotype were found to comprise 100 % of the non-responders in fasting glucose change and 80 % of the non-responders in glucose AUC change.
Given the recent attention to personalized medicine, genetic screening for exercise prescription would enable the identification and subsequent targeting of individuals with a potentially adverse response to exercise (with regard to a given phenotype) for more individualized and/or more closely monitored programs (Roth, 2008). In our current study, the rather adverse glucose response to exercise seen in the female GG genotype group lends support to these ideas and reinforces the need for replication in additional gene-exercise training studies.
Besides the retrospective nature of our study, a limitation is that the genotype frequencies for the total study group and the female participants were not in Hardy-Weinberg equilibrium. Consequently, the results must be viewed with caution as they may reflect some unknown sampling bias in the selection of our study population. Another limitation involves the high selectivity of our participants. As cardiovascular disease is the leading cause of death in the United States and the effects of regular exercise training on cardiovascular risk have been well-characterized, we chose to study 50-75 year old individuals with at least 1 risk factor for cardiovascular disease. Furthermore, due to a relatively small sample size of non-Caucasian participants and the need for a homogeneous population (Rodriguez-Murillo & Greenberg, 2008) only data from Caucasians were included in the analyses. Thus, our results may not be applicable to individuals with different health and demographic characteristics. In addition, due to sample size constraints, we combined the GT and TT genotype groups for analysis. Thus, replication and/or larger sample sizes are needed to verify our results.
As the prevalence of modifiable metabolic diseases such as type 2 diabetes and obesity continue at epidemic proportions, increased importance is given to interventions which can help to delay, prevent, or lessen the disease burden. One such intervention is aerobic exercise training; however, not everyone responds similarly to exercise and physical activity. In fact, our results suggest that the AKT1 G205T polymorphism influences obesity-related variables and their aerobic exercise training-induced changes, with differences in the response of men and women. Although the results should be viewed with caution, they provide support for the influence of genetic variation on aerobic exercise training-induced health outcomes and provide a framework for future studies.
Acknowledgments
This research was supported by National Institutes of Health grants AG15389 (J.M.H), AG17474 (J.M.H.), and AG00268 (J.A.M, S.W., and A.T.L.).
Footnotes
Conflicts of Interest: None
References
- American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription. 8. Lippincott Williams and Wilkins; Philadelphia: 2010. [Google Scholar]
- American Heart Association. Dietary guidelines for healthy American adults. A statement for physicians and health professionals by the Nutrition Committee, American Heart Association. Circulation. 1988;77:721A–724A. [PubMed] [Google Scholar]
- Bajestan SN, Sabouri AH, Nakamura M, Takashima H, Keikhaee MR, Behdani F, Fayyazi MR, Sargolzaee MR, Bajestan MN, Sabouri Z, Khayami E, Haghighi S, Hashemi SB, Eiraku N, Tufani H, Najmabadi H, Arimura K, Sano A, Osame M. Association of AKT1 haplotype with the risk of schizophrenia in Iranian population. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:383–386. doi: 10.1002/ajmg.b.30291. [DOI] [PubMed] [Google Scholar]
- Baudry A, Yang ZZ, Hemmings BA. PKBalpha is required for adipose differentiation of mouse embryonic fibroblasts. J Cell Sci. 2006;119:889–897. doi: 10.1242/jcs.02792. [DOI] [PubMed] [Google Scholar]
- Bray MS, Hagberg JM, Perusse L, Rankinen T, Roth SM, Wolfarth B, Bouchard C. The human gene map for performance and health-related fitness phenotypes: the 2006-2007 update. Med Sci Sports Exerc. 2009;41:35–73. doi: 10.1249/mss.0b013e3181844179. [DOI] [PubMed] [Google Scholar]
- Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol K, Shiyanova T, Roninson I, Weng W, Suzuki R, Tobe K, Kadowaki T, Hay N. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 2001;15:2203–2208. doi: 10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem. 2001;276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- Dengel DR, Hagberg JM, Coon PJ, Drinkwater DT, Goldberg AP. Effects of weight loss by diet alone or combined with aerobic exercise on body composition in older obese men. Metabolism. 1994;43:867–871. doi: 10.1016/0026-0495(94)90268-2. [DOI] [PubMed] [Google Scholar]
- Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA. Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat Genet. 2004;36:131–137. doi: 10.1038/ng1296. [DOI] [PubMed] [Google Scholar]
- Harmon BT, Devaney SA, Gordish-Dressman H, Reeves EK, Zhao P, Devaney JM, Hoffman EP. Functional characterization of a haplotype in the AKT1 gene associated with glucose homeostasis and metabolic syndrome. Hum Genet. 2010 doi: 10.1007/s00439-010-0891-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide M, Ohnishi T, Murayama M, Matsumoto I, Yamada K, Iwayama Y, Dedova I, Toyota T, Asada T, Takashima A, Yoshikawa T. Failure to support a genetic contribution of AKT1 polymorphisms and altered AKT signaling in schizophrenia. J Neurochem. 2006;99:277–287. doi: 10.1111/j.1471-4159.2006.04033.x. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Iwata N, Suzuki T, Kitajima T, Yamanouchi Y, Kinoshita Y, Inada T, Ozaki N. Association of AKT1 with schizophrenia confirmed in a Japanese population. Biol Psychiatry. 2004;56:698–700. doi: 10.1016/j.biopsych.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Jenkins NT, McKenzie JA, Damcott CM, Witkowski S, Hagberg JM. Endurance exercise training effects on body fatness, VO2max, HDL-C subfractions, and glucose tolerance are influenced by a PLIN haplotype in older Caucasians. J Appl Physiol. 2010;108:498–506. doi: 10.1152/japplphysiol.01018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YL, Fann CS, Liu CM, Wu JY, Hung SI, Chan HY, Chen JJ, Pan CC, Liu SK, Hsieh MH, Hwang TJ, Ouyang WC, Chen CY, Lin JJ, Chou FH, Chueh CM, Liu WM, Tsuang MM, Faraone SV, Tsuang MT, Chen WJ, Hwu HG. Absence of significant associations between four AKT1 SNP markers and schizophrenia in the Taiwanese population. Psychiatr Genet. 2006;16:39–41. doi: 10.1097/01.ypg.0000180681.80546.f3. [DOI] [PubMed] [Google Scholar]
- Matsubara A, Wasson JC, Donelan SS, Welling CM, Glaser B, Permutt MA. Isolation and characterization of the human AKT1 gene, identification of 13 single nucleotide polymorphisms (SNPs), and their lack of association with Type II diabetes. Diabetologia. 2001;44:910–913. doi: 10.1007/s001250100577. [DOI] [PubMed] [Google Scholar]
- Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- McKenzie JA, Weiss EP, Ghiu IA, Kulaputana O, Phares DA, Ferrell RE, Hagberg JM. Influence of the interleukin-6 -174 G/C gene polymorphism on exercise training-induced changes in glucose tolerance indexes. J Appl Physiol. 2004;97:1338–1342. doi: 10.1152/japplphysiol.00199.2004. [DOI] [PubMed] [Google Scholar]
- Nicklas BJ, Rogus EM, Colman EG, Goldberg AP. Visceral adiposity, increased adipocyte lipolysis, and metabolic dysfunction in obese postmenopausal women. Am J Physiol. 1996;270:E72–E78. doi: 10.1152/ajpendo.1996.270.1.E72. [DOI] [PubMed] [Google Scholar]
- Norton N, Williams HJ, Dwyer S, Carroll L, Peirce T, Moskvina V, Segurado R, Nikolov I, Williams NM, Ikeda M, Iwata N, Owen MJ, O'Donovan MC. Association analysis of AKT1 and schizophrenia in a UK case control sample. Schizophr Res. 2007;93:58–65. doi: 10.1016/j.schres.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Obisesan TO, Leeuwenburgh C, Ferrell RE, Phares DA, McKenzie JA, Prior SJ, Hagberg JM. C-reactive protein genotype affects exercise training-induced changes in insulin sensitivity. Metabolism. 2006;55:453–460. doi: 10.1016/j.metabol.2005.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obisesan TO, Leeuwenburgh C, Phillips T, Ferrell RE, Phares DA, Prior SJ, Hagberg JM. C-reactive protein genotypes affect baseline, but not exercise training-induced changes, in C-reactive protein levels. Arterioscler Thromb Vasc Biol. 2004;24:1874–1879. doi: 10.1161/01.ATV.0000140060.13203.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuki T, Inada T, Arinami T. Failure to confirm association between AKT1 haplotype and schizophrenia in a Japanese case-control population. Mol Psychiatry. 2004;9:981–983. doi: 10.1038/sj.mp.4001559. [DOI] [PubMed] [Google Scholar]
- Ordovas JM. Gender, a significant factor in the cross talk between genes, environment, and health. Gend Med. 2007;4 B:S111–S122. doi: 10.1016/s1550-8579(07)80052-0. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Murillo L, Greenberg DA. Genetic association analysis: a primer on how it works, its strengths and its weaknesses. Int J Androl. 2008;31:546–556. doi: 10.1111/j.1365-2605.2008.00896.x. [DOI] [PubMed] [Google Scholar]
- Roth SM. Perspective on the future use of genomics in exercise prescription. J Appl Physiol. 2008;104:1243–1245. doi: 10.1152/japplphysiol.01000.2007. [DOI] [PubMed] [Google Scholar]
- Sanders AR, Duan J, Levinson DF, Shi J, He D, Hou C, Burrell GJ, Rice JP, Nertney DA, Olincy A, Rozic P, Vinogradov S, Buccola NG, Mowry BJ, Freedman R, Amin F, Black DW, Silverman JM, Byerley WF, Crowe RR, Cloninger CR, Martinez M, Gejman PV. No significant association of 14 candidate genes with schizophrenia in a large European ancestry sample: implications for psychiatric genetics. Am J Psychiatry. 2008;165:497–506. doi: 10.1176/appi.ajp.2007.07101573. [DOI] [PubMed] [Google Scholar]
- Schwab SG, Hoefgen B, Hanses C, Hassenbach MB, Albus M, Lerer B, Trixler M, Maier W, Wildenauer DB. Further evidence for association of variants in the AKT1 gene with schizophrenia in a sample of European sib-pair families. Biol Psychiatry. 2005;58:446–450. doi: 10.1016/j.biopsych.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Thiselton DL, Vladimirov VI, Kuo PH, McClay J, Wormley B, Fanous A, O'Neill FA, Walsh D, Van den Oord EJ, Kendler KS, Riley BP. AKT1 is associated with schizophrenia across multiple symptom dimensions in the Irish study of high density schizophrenia families. Biol Psychiatry. 2008;63:449–457. doi: 10.1016/j.biopsych.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons JA, Knudsen S, Rankinen T, Koch LG, Sarzynski M, Jensen T, Keller P, Scheele C, Vollaard NB, Nielsen S, Akerstrom T, MacDougald OA, Jansson E, Greenhaff PL, Tarnopolsky MA, van Loon LJ, Pedersen BK, Sundberg CJ, Wahlestedt C, Britton SL, Bouchard C. Using molecular classification to predict gains in maximal aerobic capacity following endurance exercise training in humans. J Appl Physiol. 2010;108:1487–1496. doi: 10.1152/japplphysiol.01295.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turunen JA, Peltonen JO, Pietilainen OP, Hennah W, Loukola A, Paunio T, Silander K, Ekelund J, Varilo T, Partonen T, Lonnqvist J, Peltonen L. The role of DTNBP1, NRG1, and AKT1 in the genetics of schizophrenia in Finland. Schizophr Res. 2007;91:27–36. doi: 10.1016/j.schres.2006.11.028. [DOI] [PubMed] [Google Scholar]
- Wilson EM, Rotwein P. Selective control of skeletal muscle differentiation by Akt1. J Biol Chem. 2007;282:5106–5110. doi: 10.1074/jbc.C600315200. [DOI] [PubMed] [Google Scholar]
- Xu J, Liao K. Protein kinase B/AKT 1 plays a pivotal role in insulin-like growth factor-1 receptor signaling induced 3T3-L1 adipocyte differentiation. J Biol Chem. 2004;279:35914–35922. doi: 10.1074/jbc.M402297200. [DOI] [PubMed] [Google Scholar]
- Xu MQ, Xing QH, Zheng YL, Li S, Gao JJ, He G, Guo TW, Feng GY, Xu F, He L. Association of AKT1 gene polymorphisms with risk of schizophrenia and with response to antipsychotics in the Chinese population. J Clin Psychiatry. 2007;68:1358–1367. doi: 10.4088/jcp.v68n0906. [DOI] [PubMed] [Google Scholar]