Abstract
Although it is well recognized that the tumor microenvironment plays a key role in regulating tumor progression the mechanisms through which this occurs need to be defined. Current international research activities towards defining the role of the tumor microenvironment in cancer progression were the subject of the 1st Tianjin Forum on Tumor Microenvironment held at Nankai University in Tianjin, China, July 2 to 4, 2010. The importance of variety of processes, such as inflammation and angiogenesis, in the role of tumor progression were described for multiple tumor types including breast, prostate, and hepatic cancers as well as the process of bone metastasis. Identification of novel signaling pathways that impact both angiogenesis and bone remodeling were presented. Several themes emerged from this meeting including that (1) tumor cells modify the microenvironment to enhance their own survival and progression; (2) targeting host factors, in addition to targeting tumor cells, will have important therapeutic effects; and (3) host cells distribution within the tumor has both prognostic and therapeutic significance. Several priorities for future research were defined including use of a systems biology approach to define the role of host factors in tumor progression; defining the importance of targeting both arms of the bone remodeling process for therapy of bone metastasis and determining how different cell subsets contribute to microenvironment-mediated regulation of tumor progression.
Keywords: tumor microenvironment, cancer therapy, angiogenesis, bone metastasis, inflammation
Introduction
The composition of the tumor cells and host microenvironment, known as the tumor microenvironment, has been unequivocally demonstrated to play a key role in tumor progression. Developing a clear understanding of mechanisms through which the tumor microenvironment contribute to tumor progression is key to providing a framework towards developing effective anti-tumor therapies. Specifically, it will afford the opportunity to target not only tumor cells, but also the microenvironment itself. Progress in delineating mechanism through which the tumor microenvironment promotes tumor progression was the subject of the 1st Tianjin Forum on Tumor Microenvironment, an international conference held at Nankai University in Tianjin, China, July 2 to 4, 2010.
The meeting began with I. Witz (Tel Aviv University, Tel Aviv, Israel) providing an overview of the complexity of the tumor microenvironment including the many different cells and stromal components that contribute to tumor progression. Evidence for how some of these cells and factors modify tumor progression was provided in presentations discussed below.
Induction of neovascularization in tumor progression
It is well recognized that the vascular component of the microenvironment is a critical mediator of cancer progression (1). Several presentations addressed regulators of angiogenesis. R. Kerbel (University of Toronto, Toronto, Canada) discussed that maximum tolerated doses of chemotherapeutics can cause an increase of circulating endothelial precursor cells (EPC) to localize to tumors, which accelerates tumor recovery. He also demonstrated that low-dose metronomic chemotherapy (i.e. daily low doses of therapeutic agent) does not induce the host EPC spike (2). EPCs were also discussed by L. Li (Nankai University, Tianjin, China) who spoke about modulation of bone marrow derived hematopoietic stem cell (HSC) differentiation into EPC by a tumor necrosis factor (TNF) family member, vascular endothelial cell growth inhibitor (VEGI; TNFSF15). VEGI is produced by endothelial cells and induces apoptosis in proliferating endothelial cells. It had been previously identified that VEGI is downregulated in tumor vasculature allowing for angiogenesis (3). Dr. Li presented new findings that downregulation of VEGI in a tumor vasculature may be mediated by cytokines, most likely interferon–γ, produced by tumor-infiltrating T-cells. Dr. Li also showed that VEGI inhibits EPC adhesion on fibronectin, laminin, and vitronectin, as well as EPC migration and capillary formation in vitro. Additionally, VEGI induces apoptosis to E-selectin positive EPC through a mechanism partially dependent on death receptor-3. Another novel regulator of angiogenesis was discussed by Z. Wang (University of Pittsburgh, Pittsburgh, PA). Upregulated gene 19 (U19)/ELL-associated factor 2 (Eaf2) is a putative tumor suppressor that exhibits frequent loss of expression in high-grade prostate cancer (4). Dr. Wang developed a murine U19/Eaf2-knockout model that developed lung adenocarcinoma, B-cell lymphoma, hepatocellular carcinoma and prostate intraepithelial neoplasia. They found that the expression of an anti-angiogenic protein, thrombospondin-1 (TSP-1) is down-regulated in the prostate and liver of U19/EAF2 knockout mouse. They also determined that U19/EAF2 promotes the expression of TSP-1 via blocking p53 repression of the TSP-1 promoter. This suggests a mechanism through which loss of U19/EAF2, as observed in prostate cancer, promotes angiogenesis. The concept of vascular formation was extended by T. Tammela (University of Helsinki, Helsinki, Finland) to the process of lymphangiogenesis. He demonstrated that intralymphatic tumor cells were foci of tumor relapse. VEGFR- 3, a receptor tyrosine kinase, is restricted to lymphatic endothelium in adults and is activated by VEGF-C and VEGF-D. VEGF-C expression in human tumors is correlated with poor prognosis and increased lymph node metastases (5). Dr. Tammela demonstrated that blocking VEGF-C/VEGFR-3 results in decreased sprouting, vascular density, vessel branching and suppression of lymph node metastasis in preclinical tumor models. These results indicate that VEGFR-3 could serve as a target for anti-metastatic therapy. Another method of vascular modulation was discussed by Z. Qin (Institute of Biophysics, Chinese Academy of Sciences, Beijing, China) who showed that stromal fibroblasts secrete a variety of cytokines that regulate tumor angiogenesis. He demonstrated that the fibroblast inhibits tumor growth through IFNγ-mediated down-regulation of VEGF expression by these cells.
Altered vascularity as a mediator of tumor progression
In addition to formation of vessels to promote metastasis, Y. Luo (Tsinghua University, Beijing, China) discussed that in melanoma (B16/F10) and breast cancer (MDA-MB-231) models in mice increased metastatic ability was mediated through promoting pulmonary vascular destabilization. They determined that the combination of angiopoietin 2 (Angpt2), matrix metalloproteinase (MMP) 3, and MMP10 were up-regulated in tumor cells circulating through the lung which led to the increased permeability of pulmonary vasculature and extravasation of tumor cells.
Bone metastasis: Osteoblast and tumor interactions
The skeleton is a common metastatic site. Normal bone remodeling consists of a tightly controlled balance between the osteoclasts, that resorb bone, and the osteoblasts, that produce bone (6). Metastases interfere with the balance of bone remodeling resulting in either osteolytic (marked bone resorption) or osteoblastic (marked mineral production) lesions. Several presentations addressed the mechanisms through which tumors induce dysregulation of bone remodeling and how the bone microenvironment promotes metastatic tumor growth. E. Keller (University of Michigan, Ann Arbor, Michigan) discussed that as opposed to most solid tumors that produce osteolytic lesions, prostate cancer produces osteoblastic lesions (7). He presented data that demonstrated prostate cancer induces osteoblastic activity in the bone, in part, through downregulation of the Wnt inhibitor, dikkopf-1 (DKK-1). Wnts are molecules important in many aspects of morphogenesis and have been shown to be required for normal bone development. Dr. Keller’s group identified that prostate cancers express many Wnts; however, their activity is kept in check by the Wnt inhibitor DKK-1 (8). He presented novel work to account for the mechanism through which DKK-1 regulates PCa growth. Specifically, that DKK-1 downregulates the cell cycle inhibitor p21, which then allows for increased PCa in the bone. His lab team also demonstrated that DKK-1 is decreased in clinical prostate cancer bone metastases and overexpression of DKK-1 in a prostate cancer cell line converted it to a highly osteolytic cell line. These results indicate that loss of DKK-1 allows for Wnt function to be realized resulting in osteoblastic activity. These results contrast to those presented by D. Roodman (University of Pittsburgh, Pittsburgh, Pennsylvania) who discussed why multiple myeloma (MM) is so osteolytic. Originating from B-lymphocytes that have become dysregulated plasma cells, multiple myeloma consists of marked osteolytic lesions (9). Dr. Roodman discussed that even after tumor burden is resolved, the bone does not fully remodel resulting in persistent osteolytic lesions. He indicated that the marked osteolytic nature of MM is due to both activation of the osteoclast, as well as suppression of the osteoblast. He found that soluble factors produced by myeloma cells, including tumor necrosis factor-α and interleukin-7, suppressed osteoblast differentiation. They identified repression is mediated through upregulating the transcriptional repressor Gfi-1. Gfi-1, in turn, suppressed expression of two key factors needed for osteoblast differentiation, Runx2 and osterix. He demonstrated the clinical relevance of these findings by observing that Gfi-1 expression was upregulated in the marrow of MM patients. Taken together these data suggest an as of yet unreported mechanism, in which Gfi-1 is an important transcriptional suppressor of Runx2 in osteoblast precursors in MM. In another scenario of the importance of bone remodeling, X. Cao, (Johns Hopkins University, Baltimore, Maryland) presented data that clarified the role that transforming growth factor-β-1 (TGF-β1) plays on bone remodeling (10). TGF-β1 is released from bone during bone resorption in an inactive form that is activated by osteoclast-mediated lowering of pH and production of proteases. It has been previously demonstrated that inhibition of TGF-β1 leads to bone production and that overexpression of TGF-β1 results in bone loss. However, TGF-β1 does not alter osteoblast activity which is somewhat confounding in light of the changes in overall bone remodeling. To shed light on the role of TGF-β1 in bone remodeling Dr. Cao developed a mouse model consisting of a knockout of both TGF-β1 and recombination-activating gene-2 (Rag2). The loss of the Rag2 gene prevents the autoimmune phenomenon that occurs in mice with a knockout of TGF-β1 alone. Using these mice, Dr. Cao demonstrated that fluorescently-labeled bone marrow stromal cells (BMSC), which are the precursors of osteoblasts, migrate and bind to the bone trabeculae, where bone formation occurs, in the wildtype mice, but the BMSC did not localize to the bone trabeculae in the TGF-β1/Rag2 knockout mice. A series of additional studies confirmed this initial observation using mice in which a point mutation in TGF-β1 was created identical to that found in Camurati-Engelmann disease (CED) in which patients suffer from thickened bone (11). Additionally, he demonstrated that an inhibitor of TGF-β1 was able to restore the bone to normal in the CED model mice. These results underscore an important novel function of TGF-β1 in that it promotes bone production through directing BMSC to sites of bone resorption on trabeculae. This finding has important implications for bone metastasis, in which bone resorption is often increased and TGF-β1 levels are elevated in the bone microenvironment. Importantly, targeting TGF-β1 may help restore the overall bone remodeling cycle towards a proper balance in the case of bone metastasis when the cycle is dysregulated.
Bone metastasis: Osteoclast and tumor interactions
In addition to the osteoblast, the osteoclast plays a key role in the establishment and progression of bone metastasis. C. Wang (University of California Los Angeles, Los Angeles, California) reported that NF-κB is constitutively activated in breast cancer cells lines and in clinical breast cancer tissues (12). He further demonstrated that breast cancer induces osteoclastogenesis through NF-κB-induced production of granulocyte macrophage colony stimulating factor (GM-CSF) from the breast cancer cells. This induction of GM-CSF-mediated osteoclastogenesis was required for establishment of bone metastasis in a murine model. In a follow up to these previously published data, Dr. Wang presented new studies in which his groups sought to determine what induced constitutive NF-κB in breast cancer cells, Dr. Wang screened molecules that regulated NF-κB and identified that transducin-β-like protein 1 (TBL1), a component of the SMRT complex, was required for NF-κB activation in breast cancer cells. Knockdown of TBL1 inhibited NF-κB-dependent gene expression and diminished invasive growth in vitro and tumor metastasis in vivo. These results indicate that TBL1 alters the tumor microenvironment to promote bone metastasis through induction of osteoclastogenesis through induction of NFκB-induced activation of GM-CSF. Another key regulator of osteoclastogenesis was discussed by G. Xiao (University of Pittsburgh, Pittsburgh, Pennsylvania). He explored the role that activating transcription factor 4 (ATF4) had on osteoclast differentiation (13). To explore this, he overexpressed or knocked down ATF4 expression specifically in murine osteoclasts. Absence of ATF4 expression resulted in loss of diminished osteoclast differentiation in bone marrow monocyte cultures. Furthermore, transgenic overexpression of ATF4 targeted to osteoclasts induced severe marked osteoclastogenesis and osteopenia in mice. To extend these studies to cancer, his group examined for ATF4 in MM. Their novel observation was that ATF4 was upregulated by MM cell-derived factors such as TNFα in primary mouse bone marrow monocyte cultures. Taken together, these results indicate that ATF4 is a candidate target to decreased bone metastasis through inhibiting alteration of the tumor microenvironment that leads to osteoclastogenesis.
Immune cells and tumor progression
Inflammation has been postulated to contribute to tumor initiation and progression (14). J. Pollard (Albert Einstein College of Medicine, New York, New York) discussed the role of macrophages in tumor progression. Macrophages enhance malignancy by stimulating angiogenesis and tumor cell invasion and suppressing anti-tumor immunity (15). Dr. Pollard demonstrated that at metastatic sites, macrophages promote tumor cell extravasation, survival and subsequent growth. This concept has been further refined based on the identification of macrophage polarization (16). Specifically, macrophages can become activated by their cytokine milieu into one of two broad forms: M1, which are characterized, in part by high expression of IL-12 and IL-23, low expression of IL-10 and production of free radicals and M2, which are characterized by low expression of IL-12 and IL-23, high expression of IL-10 and high levels of mannose and galactose receptors. Dr. Pollard described that these subpopulations of macrophages induce different signaling cascades and thus open the door to identification of novel therapeutic targets. The theme of macrophages in tumor progression was extended by J. Zhang (University of Michigan, Ann Arbor, Michigan) who demonstrated, using a knockout mouse model of urokinase plasminogen activator (uPA), the novel finding that loss of host-produced uPA results in decreased tumor-associated macrophages (TAMs) resulting in decreased tumor growth. One mechanism as to why decreased TAM leads to decreased tumor growth was addressed by L. Zheng (Sun Yat-Sen University, Guangzhou, China). He explored the interaction between TAM and T cells and addressed the concept that T cell phenotype can influence cancer progression. Two T cell types that have received a lot of attention recently include Th17 cells and regulatory T cells (Treg). Th17 cells are a member of the CD4+ T effector cells (in addition to classical Th1 and Th2 cells) characterized by production of IL-17 family cytokines that induced chemotaxis of granulocytes and macrophages. Tregs are generally characterized as CD4+ T cells that express FoxP3 and function to limit the immune response through suppressing function of other T cell types. Dr. Zheng’s group demonstrated that activated monocytes in peritumoral stroma suppressed tumor-specific T cell immunity by expressing B7-H1 molecules, which represents a novel link between inflammation and immune tolerance in the tumor. In addition they also observed that proinflammatory Th17 cells accumulate in hepatocellular carcinoma (HCC) tissue, where they promoted angiogenesis and progression. Another link between lymphocytes and tumor progression was discussed by W. Fridman (University Pierre et Marie Curie, Paris, France). He demonstrated that lymphocytes are not randomly scattered within the tumor but appear organized in the center of the tumor, its invasive margin and in tertiary lymphoid follicles adjacent to the tumor nests. His lab analyzed large cohorts of colorectal and lung cancers and identified that a high infiltration of Th1 cells in both the center and the invasive margin of the primary tumor was a major prognostic factor for tumor recurrence.
Conclusions
The meeting touched on many aspects and novel pathways of the microenvironment that contribute to tumor progression (summarized in Fig. 1). Several consensus points were identified: (1) tumor cells modify the microenvironment to enhance their progression; (2) in addition to directly targeting tumor cells, targeting the microenvironment will have important therapeutic anti-tumor effects; and (3) host cells within the tumor microenvironment are heterogeneously distributed throughout the tumor, which has implications for both prognosis and therapy. It was also discussed that future areas of research that should receive priority include (1) a systems biology approach towards defining the functions f the host component of the tumor microenvironment; (2) defining the importance of simultaneously targeting the bone remodeling process itself, including both osteolytic and osteoblastic components of bone metastasis; and (3) determining how different subsets of immune cells (e.g., M1 versus M2 macrophages, or Treg versus Th17 lymphocytes) contribute to cancer progression.
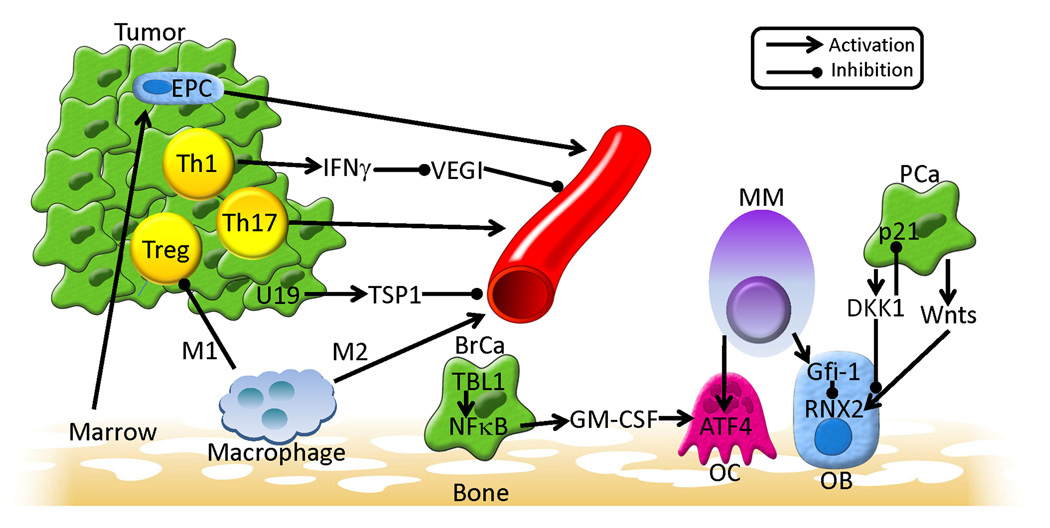
Figure 1. Summary of selected key points from The First Tianjin, China Forum on Tumor Microenvironment.
A variety of host and tumor interactions can promote angiogenesis. (1) Marrow-derived endothelial precursor cells (EPC) can localize to tumor where they can participate in vascular formation; (2) Intratumoral Th1 cells, which produce interferon-gamma (IFNγ) can inhibit vascular endothelial growth cell inhibitor (VEGI), which itself is an inhibitor of angiogenesis, thus resulting in an overall pro-angiogenic effect; (3) Both intratumoral Th17 cells and M2-differentiated macrophages promote angiogenesis through cytokine secretion; and (4) tumor-associated decrease of upregulated gene 19 (U19) expression results in decreased thrombospondin-1 (TSP) expression, which itself inhibits angiogenesis, resulting in an overall pro-angiogenic effect. Macrophages can also differentiate along an M1 pathway, resulting in inhibition of T regulatory (Treg) cells which will result in derepressing a variety of T cell responses that can promote tumor growth and angiogenesis. In addition to angiogenic effects, there were multiple interactions identified that result in impacting the bone microenvironment. In breast cancer (BrCa) it was show that transducin-β-like protein 1 (TBL1) was required for induction of NFκB activity, which resulted in production of granulocyte monocyte-colony stimulating factor (GM-CSF) and osteoclast (OC) production. Multiple myeloma (MM) was also shown to simulate OC differentiation through activation of activating transcription factor 4 (ATF4). In addition to its impact on OCs, MM also promotes overall bone loss through inhibition of osteoblasts (OB) by upregulating Gfi-1 expression, which in turn inhibits the RNX2 transcription factor that is required for osteoblastogenesis. In contrast, prostate cancer (PCa) was shown to promote osteoblast activity through downregulation of dikkopf-1 (DKK1), which allows for Wnts to promote osteoblastic activity. DKK1 also was shown to promote the cell cycle through activation inhibition of the cell cycle inhibitor p21, which overall promotes tumor growth.
Supplementary Material
Acknowledgments
The authors apologize to speakers whose work is not discussed due to space limitations (complete speakers list is presented as a supplemental file). This work was supported by National Institutes of Health Grant P01CA093900 to E.T. Keller and The Ministry of Science and Technology of China Grant 2009CB918900 to L.Y. Li.
References
- 1.Furuya M, Yonemitsu Y, Aoki I., III Angiogenesis: complexity of tumor vasculature and microenvironment. Curr Pharm Des. 2009;15:1854–1867. doi: 10.2174/138161209788453275. [DOI] [PubMed] [Google Scholar]
- 2.Kerbel RS. Issues regarding improving the impact of antiangiogenic drugs for the treatment of breast cancer. Breast. 2009;18 Suppl 3:S41–S47. doi: 10.1016/S0960-9776(09)70271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tian F, Liang PH, Li LY. Inhibition of endothelial progenitor cell differentiation by VEGI. Blood. 2009;113:5352–5360. doi: 10.1182/blood-2008-08-173773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao W, Zhang Q, Habermacher G, Yang X, Zhang AY, Cai X, et al. U19/Eaf2 knockout causes lung adenocarcinoma, B-cell lymphoma, hepatocellular carcinoma and prostatic intraepithelial neoplasia. Oncogene. 2008;27:1536–1544. doi: 10.1038/sj.onc.1210786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su JL, Yen CJ, Chen PS, Chuang SE, Hong CC, Kuo IH, et al. The role of the VEGF-C/VEGFR-3 axis in cancer progression. Br J Cancer. 2007;96:541–545. doi: 10.1038/sj.bjc.6603487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crofton PM. Bone and bone turnover. Endocr Dev. 2009;15:77–100. doi: 10.1159/000207611. [DOI] [PubMed] [Google Scholar]
- 7.Hall CL, Keller ET. The role of Wnts in bone metastases. Cancer Metastasis Rev. 2006;25:551–558. doi: 10.1007/s10555-006-9022-2. [DOI] [PubMed] [Google Scholar]
- 8.Hall CL, Bafico A, Dai J, Aaronson SA, Keller ET. Prostate cancer cells promote osteoblastic bone metastases through Wnts. Cancer Res. 2005;65:7554–7560. doi: 10.1158/0008-5472.CAN-05-1317. [DOI] [PubMed] [Google Scholar]
- 9.Roodman GD. Pathogenesis of myeloma bone disease. Leukemia. 2009;23:435–441. doi: 10.1038/leu.2008.336. [DOI] [PubMed] [Google Scholar]
- 10.Tang Y, Wu X, Lei W, Pang L, Wan C, Shi Z, et al. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med. 2009;15:757–765. doi: 10.1038/nm.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janssens K, Vanhoenacker F, Bonduelle M, Verbruggen L, Van Maldergem L, Ralston S, et al. Camurati-Engelmann disease: review of the clinical, radiological, and molecular data of 24 families and implications for diagnosis and treatment. J Med Genet. 2006;43:1–11. doi: 10.1136/jmg.2005.033522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park BK, Zhang H, Zeng Q, Dai J, Keller ET, Giordano T, et al. NF-kappaB in breast cancer cells promotes osteolytic bone metastasis by inducing osteoclastogenesis via GM-CSF. Nat Med. 2007;13:62–69. doi: 10.1038/nm1519. [DOI] [PubMed] [Google Scholar]
- 13.Cao H, Yu S, Yao Z, Galson DL, Jiang Y, Zhang X, et al. Activating transcription factor 4 regulates osteoclast differentiation in mice. J Clin Invest. 2010;120:2755–2766. doi: 10.1172/JCI42106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balkwill F, Mantovani A. Cancer and inflammation: implications for pharmacology and therapeutics. Clin Pharmacol Ther. 2010;87:401–406. doi: 10.1038/clpt.2009.312. [DOI] [PubMed] [Google Scholar]
- 15.Coffelt SB, Hughes R, Lewis CE. Tumor-associated macrophages: effectors of angiogenesis and tumor progression. Biochim Biophys Acta. 2009;1796:11–18. doi: 10.1016/j.bbcan.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.