Abstract
Purpose of review
This review covers topics relevant to olivocochlear-efferent anatomy and function for which there are new findings in papers from 2009 to early 2010.
Recent findings
Work within the review period has increased our understanding of medial efferent (MOC) mechanisms in outer hair cells, MOC reflex tuning, MOC effects on distortion product otoacoustic emissions, the time course of MOC effects, MOC effects in psychophysical tests and on understanding speech, MOC effects in attention and learning, and lateral efferent function in binaural hearing. In addition, there are new insights into efferent molecular mechanisms and their effect on cochlear development.
Summary
Techniques for measuring efferent effects using otoacoustic emissions are now well developed and have promise in clinical applications ranging from predicting which subjects are susceptible to acoustic trauma to characterizing relationships between efferent activation and learning disabilities. To realize this promise, studies are needed in which these techniques are applied with high standards.
Keywords: medial olivocochlear, hearing, auditory psychophysics, descending control, auditory development
INTRODUCTION
This review will cover papers that significantly enhance understanding about olivocochlear (OC) efferents. Most of the papers studied medial OC (MOC) efferents, the myelinated efferents that innervate outer hair cells (OHCs) in mature animals. However, there is work on lateral OC (LOC) efferents that innervate auditory-nerve fibers under inner hair cells (IHCs), and on acetylcholine receptors -- the receptors for the main efferent neurotransmitter. For more detailed background see previous reviews [1, 2].
MOC Mechanisms
One way MOC synapses may inhibit is by shunting OHC receptor currents [3]. Although measurements in isolated OHCs suggested that the OHC capacitance would severely limit this shunting [4], a new model that includes both piezoelectric and elastic OHC properties shows that the OHC capacitance is not as limiting as was believed [5**]. This model demonstrates that, in OHCs driving a load, the capacitance is strongly coupled to OHC motility and that MOC synaptic shunting can indeed reduce OHC responses leading to reduced cochlear amplification.
In the cochlear base, MOC activity reduces basilar-membrane responses to sound by reducing the gain of cochlear amplification [6]. Recordings from auditory-nerve fibers show that MOC effects are different in the apical and basal halves of the cochlea [7]. To understand the mechanical basis for apical MOC effects, responses to sound were measured in the organ of Corti from Hensen cells in the guinea-pig apex [8]. MOC stimulation slightly reduced AC responses and had a larger, but still small (3 dB), effect on DC responses. Much remains to be learned about apical cochlear mechanics and MOC effects.
MOC reflex properties
The signature events of the “MOC reflex” are MOC excitation by sound and the resulting cochlear changes. Because MOC fibers have narrow tuning curves and innervate the cochlea tonotopically, the MOC reflex has been thought to provide narrowly-tuned negative feedback to cochlear places excited by sound [9]. MOC reflex effects can be noninvasively assayed using otoacoustic emissions (OAEs), which are ear-canal sounds whose amplitude depends on cochlear amplification. MOC-induced reductions of OAEs are sometimes called “suppressions,” but because the reduction is due to MOC synaptic effects, not two-tone suppression, we refer to these reductions as MOC inhibition.
MOC-reflex tuning was recently measured using stimulus-frequency OAEs (SFOAEs) near 1 kHz, with MOC activity elicited by half-octave bands of noise varied over a 5 octave range [10*]. The MOC reflex had broad frequency tuning, and in some subjects noise bands centered 2.5 octaves above or below the probe tone produced significant MOC effects. For probes near 1 kHz, the most effective elicitor was 0.5–1 octave below the probe frequency (not at the probe frequency as expected). Similar tests using 0.5 and 4 kHz probes also showed broad tuning, but for 0.5 kHz the most effective elicitor was above the probe frequency (opposite the direction for 1 kHz probes), while for 4 kHz, the most effective elicitor was centered at the probe frequency, and, in addition, elicitors in a broad, low-frequency region produced MOC activation [11, Lilaonitkul and Guinan, unpublished data]. These human results are similar to the pattern of inhibition versus characteristic frequency for auditory-nerve fibers found in cats [12], taking into account that the range of hearing in cats is 1–1.5 octaves higher than in humans. In another human study, MOC effects were measured by a method that extracts nonlinear SFOAE components plus changes in the linear component [13*]. This study found that elicitor-noise frequencies below the 4 kHz probe were primarily responsible for producing MOC activity, consistent with the above results.
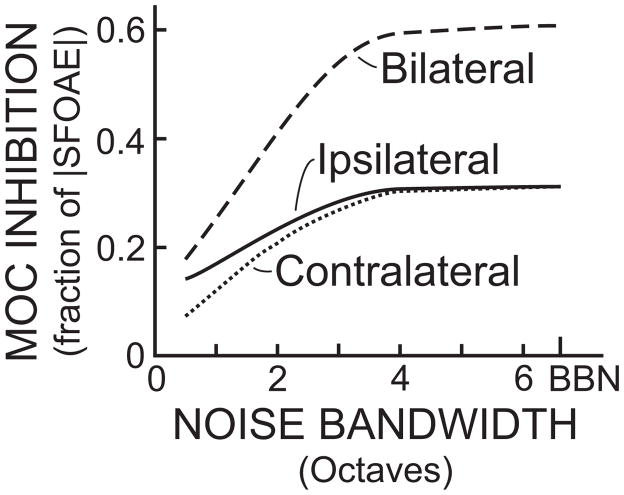
MOC reflex frequency selectivity was also measured as a function of noise bandwidth for constant-SPL elicitors presented ipsilateral, contralateral or bilateral relative to the measurement ear [14*]. Probe frequencies of 0.5, 1 and 4 kHz were used. As noise bandwidth was increased up to 4–6.7 octaves, MOC effects, measured by the change in SFOAEs, grew even though noise spectral density in the frequency region of the probe decreased (Fig. 1). These results indicate that sound excitations over almost the whole range of hearing summate to elicit the MOC effects seen at a single frequency. The experiments also revealed an interesting aspect of reflex laterality. For narrow-band noises, ipsilateral effects were twice as large as contralateral effects, whereas for broad-band noises, ipsilateral and contralateral elicitors had similar effects (Fig. 1). Perhaps strong MOC reflexes produce similar ipsilateral and contralateral effects on low-frequency responses so that binaural sound localization abilities are preserved.
Figure 1.
Medial olivocochlear (MOC) inhibition increases as the bandwidth of a constant-level elicitor increases, showing that sound excitations over almost the whole range of hearing are integrated in activating the efferents that affect one cochlear frequency region. For narrow-band elicitors (0.5 octaves), ipsilateral noise was approximately twice as effective as contralateral noise, but for wide-band elicitors (>6 octaves), ipsilateral and contralateral elicitors were equally effective. Patterned after the data Lilaonitkul and Guinan [14] for probe frequencies near 1 kHz, and 60 dB SPL elicitors centered on the probe frequency.
The potential for the MOC reflex to affect binaural sound localization is shown by the large latency change it can produce. Changes in OAE latencies were measured to determine the change in cochlear tuning produced by the MOC reflex (filter theory indicates that broader filters have shorter delays) [15*]. Significant reductions in OAE delays (5% for 0.5–2 kHz) were found, which indicates that MOC activity slightly widens cochlear tuning. The large change in cochlear response latency implied by the measured 0.5 ms change in OAE delay would have a profound effect on binaural localization if it were not balanced, e.g. by the MOC system producing similar changes in both ears.
MOC effects on DPOAEs
One common method for measuring MOC effects is by the change in distortion product OAEs (DPOAEs) at the frequency 2f1-f2 (where f2>f1). MOC activity typically reduces DPOAEs, but sometimes it enhances them. Two recent papers [16*, 17*] show how these different effects are produced by interference from the two kinds of OAE sources (distortion and reflection sources [18]). These studies used DPOAE measurements with fine frequency steps to separate the distortion and reflection source components, and found that: (1) MOC stimulation inhibits (i.e. reduces) both components and shifts their phase, with the reflection component affected more than the distortion component, (2) DPOAE dips are produced by phase cancellation of the two components, and MOC-induced phase changes move the cancellation frequencies upward, (3) reduction of cancellations, particularly at dips, produces MOC-induced DPOAE enhancements, and (4) consistent DPOAE reductions (~2 dB) are found if measurements are made at fine-structure peaks [16*, 17*].
Recent measurements show that larger MOC changes are found in the DPOAEs at f2-f1 than at 2f1-f2 [19*]. Unfortunately, f2-f1 DPOAEs are too small to measure in most human subjects. The relative sizes of f2-f1 and 2f1-f2 DPOAEs are controlled by the OHC-stereocilia operating point which can be varied using a low-frequency “bias” tone. The MOC effect on f2-f1 DPOAEs was found to interact with the bias-tone effect in a way that indicates that MOC action slightly changes the operating point of cochlear amplification [20*].
The time course of MOC effects
Two papers studied the effects of prolonged sound stimulation in producing MOC effects. In awake humans, 16 minutes of contralateral noise (interrupted twice for measurements) produced sustained inhibition of transient-evoked OAEs (TEOAEs) with perhaps a small increase in inhibition over the 16 minutes [21*]. In anesthetized guinea pigs, the MOC inhibition of ipsilateral cochlear responses (compound action potentials, DPOAEs, and round-window noise) began at the onset of the elicitor and showed an increase over 2–3 minutes that was partially sustained during the 15 minute contralateral noise [22*]. The slow increase in MOC inhibition was interpreted as not being due to the MOC slow effect [23, 24], which decreases after a few minutes of stimulation. The slow change might be due to an increase in MOC firing during the sustained noise [22*]. After termination of the noise, the human data showed a significant enhancement of TEOAE amplitude [21*]. After-stimulation enhancement was also seen in experiments demonstrating the slow effect [23, 24], but these enhancements may have an entirely different origin than the slow effect [25].
A problem in OAE tests for MOC effects is that the sound that evokes the OAE may also evoke MOC activity [26]. One way of avoiding this confound is by studying MOC effects on spontaneous OAEs (SOAEs). MOC effects elicited by contralateral sound on SOAEs showed onset and offset time constants in the few hundred ms range (similar to those seen in SFOAEs [27]) as well as slower changes and an SOAE enhancement after the sound burst. [28*]
MOC effects on Speech in Noise
It is hypothesized that MOC inhibition enhances the ability to discriminate signals in noise [29]. One way to gain understanding of this is by making a cochlear model that includes MOC effects (i.e. lower cochlear-amplifier gain) and by determining how MOC effects change perceptual abilities. Two recent studies did this using cochlear models as inputs to computerized speech recognition systems [30*, 31*]. Although the model systems differed significantly, with no MOC activation both showed that speech in silence was recognized well, but speech in background noise was not. The addition of MOC effects improved speech recognition in background noise in both models. Thus, a model that mimics the speech recognition errors produced by real people shows an efferent enhancement of performance [30*]. Interestingly, optimum performance was obtained when the amount of efferent activity was proportional to the noise level [31*]. Such models show how MOC inhibition may improve speech in noise detection in humans [32].
MOC activity may enhance speech perception even when there is no background noise. Following identification tests of speech that was partially time reversed, subjects with the best identification scores had larger MOC inhibition of CEOAEs than subjects with the worst scores [33*]. The difference was strongest in the right ear. MOC cochlear effects may have directly aided performance, or alternately, both MOC activation and speech performance may be correlated (e.g. from left-hemisphere mechanisms) without MOC activity actually aiding performance.
MOC effects in psychophysics
The psychophysical phenomena called the “temporal effect” or “overshoot” has been suggested to be due to MOC inhibition. Overshoot is the phenomena that a brief sound has a lower threshold when presented 100 ms or more after the start of a noise burst compared to its threshold near the start of the noise burst. It is hypothesized that (1) the noise burst elicits MOC activity that builds up slowly and eventually decreases cochlear-amplifier gain, and (2) the decrease in cochlear-amplifier gain reduces the response to the low-level noise more than it reduces the response to the brief, high-level tone. If this is true, MOC activity during overshoot should decrease OAEs, and two recent studies looked for such a change. One study [34*] did not find an OAE change that corresponded to overshoot, while another did [35**]. The different results appear attributable to the different methods used. Overall, the results indicate that overshoot is due, at least in part, to MOC activity.
The amount and frequency specificity of MOC activity must vary continually in response to the recent history of sound stimulation and subject attention. Thus, cochlear amplifier gain can be expected to vary during psychophysical tests. Recent papers provide evidence for this [36, 37, 38].
Attention, learning and plasticity
A recent study found a reduction in MOC reflex activity (compared to passive, no-task listening) when a subject performed a task requiring attention to a sound [39*]. Tasks have been reported to produce both increases and decreases in MOC activity, but clear rules for which occurs have not emerged. An attractive hypothesis is that MOC activity increases in tasks when the MOC activity confers a benefit, but it decreases in tasks when it confers no benefit. Data are needed that test this hypothesis.
Over the last few years evidence has built up for a role of efferents in learning and plasticity. [40]. New evidence for a role in learning comes from the finding that MOC effects on OAEs were significantly lower at some frequencies in children with auditory listening problems compared to normal children [41]. A role of the descending auditory system in neural plasticity associated with sound localization has been shown by experiments in ferrets [42*]. Normally ferrets can relearn to accurately localize after one ear is plugged, but this ability was greatly reduced after selective destruction of cortical cells that project to the inferior colliculus. Whether this plasticity involves MOC neurons is not known. It is noteworthy, however, that MOC neurons exhibit immunofluorescence that indicates they contain enzymes that are involved in producing neural plasticity [43]. Furthermore, in frogs, nitric oxide (which helps produce neural plasticity) may slowly enhance efferent synaptic effects on hair cells, which suggests that nitric oxide might be involved in producing the MOC slow effect [44].
MOC tests in various populations
MOC tests, done by eliciting MOC activity with contralateral noise and measuring changes with OAEs, have been used in a variety of circumstances. Two papers indicate that OAE amplitudes and/or MOC effects on OAEs decrease in older subjects [45, 46]. One paper indicates that smoking lowers TEOAEs and MOC effects on TEOAEs [47], while another found MOC effects on TEOAEs went down with age in smokers, but not in non-smokers [48]. MOC effects were reported to be generally lower in subjects with tinnitus than in normals [49, 50]. Finally, in children with type-I diabetes mellitus, MOC inhibition of TEOAEs was lower than normal, which may be an early manifestation of diabetic neuropathy [51]. These results should be interpreted cautiously because the methods were often weak [2], e.g. the OAEs had low signal-to-noise ratios (only 3 dB), or OAE differences were not normalized thereby biasing the results.
LOC function in binaural hearing
LOC efferents have been suggested to have a role in balancing the outputs of the right and left cochleae to achieve optimum binaural hearing [1, 52]. However, following manipulations that lowered the output of one cochlea, the output of the other cochlea remained constant, which argues against the hypothesis [53*].
Manipulations of cochlear neuroreceptors
MOC inhibition involves multiple steps that have been targets for manipulation and study. The efferent neurotransmitter, acetycholine (ACh), has receptors (AChRs) in the cochlea that are composed of α9 and α10 subunits [54]. Acetylcholine released by MOC terminals acts onα9α10AChRs that allow entry of Ca2+ ions into the OHC. These Ca2+ ions activate nearby calcium-activated potassium channels, called SK2 channels, that allow potassium to exit and hyperpolarize the OHC. A study using animals with a mutation inα9AChRs that increased AChR sensitivity found that this increased protection from acoustic trauma [55**]. This result provides a strong confirmation of the role of MOC synapses in trauma protection and the potential value of MOC reflex testing for predicting which subjects are susceptible to acoustic trauma. A study using SK2-null mice confirmed that electrically driven MOC effects are lost without functional SK2 channels, and also found down-regulation of OHC ryanodine receptors that normally increase the SK2 activation [56]. This study also found, in double-null mice lacking both α10AChR and SK2 genes, that there was a down regulation of α9nAChR expression [56]. These results indicate that there are interlocking developmental signals such that SK2 channels are necessary for long-term survival of olivocochlear fibers and synapses. Another study showed a range of developmental anomalies in α9AChR knockout animals [57].
Mice with the α9AChR gene deleted have no MOC cochlear inhibition [58] and provide a way of assessing MOC function. In signal-in-noise sound-localization tests, α9AChR-knockout mice had surprisingly normal behavior, and the authors suggested that central compensation via MOC collateral branches to the cochlear nucleus was responsible [59]. However, a recent study of α9AChR-knockout mice found no obvious changes in the central morphology of OC neurons [60*]. Perhaps, as later suggested [61], MOC activity normally aids sound localization in noise, but knockouts showed no deficit because long training allowed them to develop alternate listening strategies. Whether such compensation involves MOC collaterals to the cochlear nucleus is unknown, but recent studies support older anatomical work indicating that MOC collaterals excite certain classes of cochlear-nucleus neurons [62]. Furthermore, animals that were unilaterally deafened indicate that MOC collaterals provide a slow excitation to cochlear-nucleus neurons [63*].
Efferent feedback that releases ACh on hair cell organs is phylogenetically old. New data indicate that the ACh receptors in mammals (α9α10AChRs) evolved recently, presumably to control the phylogenetically-new, prestin-based cochlear amplification [64]. Two papers point out that AChRs are a possible pharmacotherapeutic target in conditions such as noise-induced hearing loss, tinnitus and auditory processing disorders [65, 66].
GABA
A variety of data indicate that gamma-aminobutyric acid (GABA) is a second neurotransmitter released at LOC and MOC synapses, at least in some species. In α9AChR-knockout animals, there was an unexpected increased expression of genes encoding GABA receptor subunits and the GABA synthetic enzyme [67]. These data suggest a developmental association in the cochlea between nicotinic cholinergic and GABAergic systems. In animals with a GABA receptor subunit (GABAb1) knocked out, MOC function assessed by DPOAE inhibition was normal, but these animals showed increased resistance to permanent (but not temporary) acoustic trauma [68*]. Immunostaining indicates that GABAb1 receptors are located in type II afferents that synapse on OHCs, which indicates a role for type II synapses in acoustic trauma and perhaps in cochlear amplification [68*].
MOC fibers and development
During mammalian development, MOC fibers briefly form synapses on IHCs (with α9α10ACh receptors) before detaching and continuing on to innervate OHCs [69]. The role of this transient MOC innervation of IHCs is not clear. α10ACh receptors on IHCs normally disappear with the disappearance of MOC innervation. Genetically-manipulated mice that have α10AChRs in adulthood do not show ACh currents, presumably because IHC SK2 channels are also down-regulated [70]. Experiments in α10AChR-knockout animals show that the development of basic IHC properties does not require the α10AChR subunit [71]. Another study showed that while hearing was severely impaired in mice lacking the Ca2+ channel subunit CaVbeta2, the transient MOC innervation of IHCs still occurs during development [72].
CONCLUSION
Recent work provides a new understanding of MOC reflex tuning during passive listening but more work is needed to provide an understanding of MOC tuning during active listening. The interpretation of OAE changes in terms of MOC effects is now well understood for all OAE types. The main challenge in applying OAE methods to clinically relevant questions is the need for good methodology, e.g. the signal-to-noise ratio required for measuring MOC effects is much higher than what is required for simply measuring OAEs [2]. Measuring MOC effects and relating them to learning and disabilities seems particularly ripe for exciting new work. Finally, continued use of animals with new molecular knock-outs can be expected to provide important insights into cochlear development and how efferents produce their effects.
Acknowledgments
I thank Dr. Jeffery Lichtenhan and Ms. Maria Berezina for comments on the manuscript. Supported by NIH NIDCD RO1 DC005977, RO1 DC000235, and P30 DC005209.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Guinan JJ., Jr . The Physiology of Olivocochlear Efferents. In: Dallos PJ, Popper AN, Fay RR, editors. The Cochlea. New York: Springer-Verlag; 1996. pp. 435–502. [Google Scholar]
- 2.Guinan JJ., Jr Olivocochlear efferents: anatomy, physiology, function, and the measurement of efferent effects in humans. Ear Hear. 2006:589–607. doi: 10.1097/01.aud.0000240507.83072.e7. [DOI] [PubMed] [Google Scholar]
- 3.Fex J. Efferent inhibition in the cochlea related to hair-cell dc activity: Study of postsynaptic activity of the crossed olivo-cochlear fibers in the cat. J Acoust Soc Am. 1967;41:666–75. doi: 10.1121/1.1910395. [DOI] [PubMed] [Google Scholar]
- 4.Santos-Sacchi J. On the frequency limit and phase of outer hair cell motility: Effects of the membrane filter. J Neurosci. 1992;12:1906–16. doi: 10.1523/JNEUROSCI.12-05-01906.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rabbitt RD, Clifford S, Breneman KD, et al. Power efficiency of outer hair cell somatic ** electromotility. PLoS computational biology. 2009;5:e1000444. doi: 10.1371/journal.pcbi.1000444. This detailed model shows how OHCs can deliver energy for cochlear amplification at frequencies far above the membrane cut-off frequency of an isolated OHC, and how shunting by efferent synapses acts to lower this energy output and produce efferent inhibition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper NP, Guinan JJ., Jr Efferent-Mediated Control of Basilar Membrane Motion. J Physiol. 2006;576:49–54. doi: 10.1113/jphysiol.2006.114991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guinan JJ, Jr, Lin T, Cheng H. Medial-olivocochlear-efferent inhibition of the first peak of auditory-nerve responses: Evidence for a new motion within the cochlea. J Acoust Soc Am. 2005;118:2421–33. doi: 10.1121/1.2017899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper N, Guinan JJ., Jr Efferent inhibition of the apical cochlea’s mechanical responses to sound. Asso Res Otolaryngol Abstr. 2010;34:41. (#120) [Google Scholar]
- 9.Winslow RL, Sachs MB. Effect of electrical stimulation of the crossed olivocochlear bundle on auditory nerve response to tones in noise. J Neurophysiol. 1987;57:1002–21. doi: 10.1152/jn.1987.57.4.1002. [DOI] [PubMed] [Google Scholar]
- 10.Lilaonitkul W, Guinan JJ., Jr Reflex control of the human inner ear: a half-octave offset in * medial efferent feedback that is consistent with an efferent role in the control of masking. J Neurophysiol. 2009;101:1394–406. doi: 10.1152/jn.90925.2008. This study shows the tuning of MOC reflex effects for ipsilateral, contralateral, and bilateral, 60 dB SPL, ½octave, noise and tone elicitors, as well as the first tuning curves for MOC effects in humans. The findings show that, for 1 kHz probes, the most effective elicitors are at frequencies below the probe frequency. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lilaonitkul W, Guinan JJ., Jr Medial olivocochlear (MOC) efferent acoustic reflexes in humans are widely tuned and have their biggest effects at frequencies above that of the eliciting sound. Asso Res Otolaryngol Abstr. 2008;31:284. (#835) [Google Scholar]
- 12.Warren EH, III, Liberman MC. Effects of contralateral sound on auditory-nerve responses. II. Dependence on stimulus variables. Hearing Res. 1989;37:105–22. doi: 10.1016/0378-5955(89)90033-6. [DOI] [PubMed] [Google Scholar]
- 13.Walsh KP, Pasanen EG, McFadden D. Properties of a nonlinear version of the stimulus* frequency otoacoustic emission. J Acoust Soc Am. 2010;127:955–69. doi: 10.1121/1.3279832. This paper used a variant of SFOAE methodology in humans and showed that sounds below a 4 kHz probe frequency were most effective in eliciting MOC inhibition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lilaonitkul W, Guinan JJ., Jr Human Medial Olivocochlear Reflex: Effects as Functions of * Contralateral, Ipsilateral, and Bilateral Elicitor Bandwidths. J Assoc Res Otolaryngol. 2009;10:459–70. doi: 10.1007/s10162-009-0163-1. This paper measured MOC effects with 0.5, 1 and 4 kHz probes as functions of the noise bandwidth of ipsilateral, contralateral and bilateral elicitors. MOC effects increased up to bandwidths of 6. octaves (i.e. 0.1–10 kHz) which indicate that the MOC reflex integrates excitation from most of the cochlea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Francis NA, Guinan JJ., Jr Medial olivocochlear efferent stimulation by contralateral noise * induces similar changes in stimulus-frequency and click-evoked otoacoustic measurements of cochlear filter-related delay. Hear Res. 2010 doi: 10.1016/j.heares.2010.04.009. in Press. This paper measured MOC-induced delay reductions in SFOAEs and TEOAEs in humans and interpreted them in terms of the cochlear filter-widening that would have produced such delay changes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdala C, Mishra SK, Williams TL. Considering distortion product otoacoustic emission * fine structure in measurements of the medial olivocochlear reflex. J Acoust Soc Am. 2009;125:1584–94. doi: 10.1121/1.3068442. DPOAEs measured at fine frequency steps in humans were decomposed into distortion and reflection components. Contralateral-noise-elicited MOC inhibition was larger on the reflection component than on the distortion component. Interference of these components explains MOC-induced enhancement of DPOAEs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deeter R, Abel R, Calandruccio L, Dhar S. Contralateral acoustic stimulation alters the * magnitude and phase of distortion product otoacoustic emissions. J Acoust Soc Am. 2009;126:2413–24. doi: 10.1121/1.3224716. This shows that DPOAE dips in humans are caused by interference of distortion and reflection components and that MOC inhibition and phase shifts are greater on the reflection component. These effects produce the upward shift in the frequency of dips and MOC- induced enhancement of DPOAEs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shera CA, Guinan JJ., Jr Evoked otoacoustic emissions arise by two fundamentally different mechanisms: A taxonomy for mammalian OAEs. J Acoust Soc Am. 1999;105:782–98. doi: 10.1121/1.426948. [DOI] [PubMed] [Google Scholar]
- 19.Wittekindt A, Gaese BH, Kossl M. Influence of contralateral acoustic stimulation on the * quadratic distortion product f2-f1 in humans. Hear Res. 2009;247:27–33. doi: 10.1016/j.heares.2008.09.011. This study shows there are stronger MOC effects on f2-f1 distortion products than on 2f1-f2 distortion products. [DOI] [PubMed] [Google Scholar]
- 20.Abel C, Wittekindt A, Kossl M. Contralateral acoustic stimulation modulates low-frequency * biasing of DPOAE: efferent influence on cochlear amplifier operating state? J Neurophysiol. 2009;101:2362–71. doi: 10.1152/jn.00026.2009. This presents evidence from gerbils that the OHC-stereocilia operating point is slightly changed by MOC inhibition. [DOI] [PubMed] [Google Scholar]
- 21.van Zyl A, Swanepoel D, Hall JW., 3rd Effect of prolonged contralateral acoustic * stimulation on transient evoked otoacoustic emissions. Hear Res. 2009;254:77–81. doi: 10.1016/j.heares.2009.04.013. This paper showed sustained MOC effects on TEOAEs over the 15 minutes of a contralateral elicitor noise in humans. [DOI] [PubMed] [Google Scholar]
- 22.Larsen E, Liberman MC. Slow build-up of cochlear suppression during sustained * contralateral noise: Central modulation of olivocochlear efferents? Hear Res. 2009;256:1–10. doi: 10.1016/j.heares.2009.02.002. This paper found, in guinea pigs, that MOC inhibition increased during the first 2–3 minutes of contralateral noise and presented evidence that this was due to a slow increase in the firing of MOC fibers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sridhar TS, Liberman MC, Brown MC, Sewell WF. A novel cholinergic “slow effect” of olivocochlear stimulation on cochlear potentials in the guinea pig. J Neurosci. 1995;15:3667–78. doi: 10.1523/JNEUROSCI.15-05-03667.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooper NP, Guinan JJ., Jr Separate mechanical processes underlie fast and slow effects of medial olivocochlear efferent activity. J Physiol. 2003;548:307–12. doi: 10.1113/jphysiol.2003.039081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maison SF, Vetter DE, Liberman MC. A novel effect of cochlear efferents: in vivo response enhancement does not require alpha9 cholinergic receptors. J Neurophysiol. 2007;97:3269–78. doi: 10.1152/jn.00067.2007. [DOI] [PubMed] [Google Scholar]
- 26.Guinan JJ, Backus BC, Lilaonitkul W, Aharonson V. Medial olivocochlear efferent reflex in humans: otoacoustic emission (OAE) measurement issues and the advantages of stimulus frequency OAEs. J Assoc Res Otolaryngol. 2003;4:521–40. doi: 10.1007/s10162-002-3037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Backus BC, Guinan JJ., Jr Time course of the human medial olivocochlear reflex. J Acoust Soc Am. 2006;119:2889–904. doi: 10.1121/1.2169918. [DOI] [PubMed] [Google Scholar]
- 28.Zhao W, Dhar S. The effect of contralateral acoustic stimulation on spontaneous otoacoustic * emissions. J Assoc Res Otolaryngol. 2010;11:53–67. doi: 10.1007/s10162-009-0189-4. This study used a time-frequency analysis to show that the time course of MOC effects on SOAEs in humans includes a gradual adaptation and an overshoot in both SOAE magnitude and frequency. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawase T, Delgutte B, Liberman MC. Anti-masking effects of the olivocochlear reflex, II: Enhancement of auditory-nerve response to masked tones. J Neurophysiol. 1993;70:2533–49. doi: 10.1152/jn.1993.70.6.2533. [DOI] [PubMed] [Google Scholar]
- 30.Messing DP, Delhorne L, Bruckert E, et al. A non-linear efferent-inspired model of the * auditory system; matching human confusions in stationary noise. Speech Communication. 2009;51:668–83. This study used an auditory model that produces realistic speech identification errors and showed that the inclusion of a phenomenological MOC model enhances speech identification in the presence of noise. [Google Scholar]
- 31.Brown GJ, Ferry RT, Meddis R. A computer model of auditory efferent suppression: * implications for the recognition of speech in noise. J Acoust Soc Am. 2010;127:943–54. doi: 10.1121/1.3273893. Using a cochlear model as input to a speech recognition system, this study shows that optimal performance is obtained when efferent inhibition proportional to the background noise is included. [DOI] [PubMed] [Google Scholar]
- 32.Kumar UA, Vanaja CS. Functioning of olivocochlear bundle and speech perception in noise. Ear Hear. 2004 Apr;25:142–6. doi: 10.1097/01.aud.0000120363.56591.e6. [DOI] [PubMed] [Google Scholar]
- 33.Grataloup C, Hoen M, Veuillet E, et al. Speech restoration: an interactive process. J Speech * Lang Hear Res. 2009;52:827–38. doi: 10.1044/1092-4388(2008/06-0235). This study found that subjects with a high ability to correctly perceive time-reversed speech had a high contralateral-sound-evoked MOC inhibition of TEOAEs in the right ear, which suggests a role for MOC activity in understanding speech even when there is no background noise. [DOI] [PubMed] [Google Scholar]
- 34.Keefe DH, Schairer KS, Ellison JC, et al. Use of stimulus-frequency otoacoustic emissions * to investigate efferent and cochlear contributions to temporal overshoot. J Acoust Soc Am. 2009;125:1595–604. doi: 10.1121/1.3068443. This study looked for an OAE correlate of behavioral overshoot in humans using an unusual method that measured SFOAE “thresholds.” No difference in threshold was found for early versus late tone bursts, but the interpretation of this is unclear because MOC activity may have inhibited both the SFOAE residual and the noise-interval residual used as the reference for the SFOAE threshold. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walsh KP, Pasanen EG, McFadden D. Overshoot measured physiologically and * psychophysically in the same human ears. Hear Res. 2010 doi: 10.1016/j.heares.2010.04.007. in Press. This study measured behavioral overshoot at 4 kHz, and in the same subjects, an OAE-based metric that showed an overshoot-like change in all but one subject. It is the first study to show a physiological measure of MOC effects that may account, at least partly, for overshoot. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jennings SG, Strickland EA, Heinz MG. Precursor effects on behavioral estimates of frequency selectivity and gain in forward masking. J Acoust Soc Am. 2009;125:2172–81. doi: 10.1121/1.3081383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wojtczak M, Oxenham AJ. On- and off-frequency forward masking by Schroeder-phase complexes. J Assoc Res Otolaryngol. 2009;10:595–607. doi: 10.1007/s10162-009-0180-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou X, Henin S, Thompson SE, et al. Sensitization to masked tones following notched-noise correlates with estimates of cochlear function using distortion product otoacoustic emissions. J Acoust Soc Am. 2010;127:970–6. doi: 10.1121/1.3277156. [DOI] [PubMed] [Google Scholar]
- 39.Harkrider AW, Bowers CD. Evidence for a cortically mediated release from inhibition in * the human cochlea. J Am Acad Audiol. 2009;20:208–15. doi: 10.3766/jaaa.20.3.7. This study showed that MOC inhibition declined (compared to passive listening) when subjects paid attention to either the ipsilateral TEOAE-producing clicks or the contralateral MOC-eliciting noise. [DOI] [PubMed] [Google Scholar]
- 40.de Boer J, Thornton AR. Neural correlates of perceptual learning in the auditory brainstem: efferent activity predicts and reflects improvement at a speech-in-noise discrimination task. J Neurosci. 2008;28:4929–37. doi: 10.1523/JNEUROSCI.0902-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yalcinkaya F, Yilmaz ST, Muluk NB. Transient evoked otoacoustic emissions and contralateral suppressions in children with auditory listening problems. Auris Nasus Larynx. 2010;37:47–54. doi: 10.1016/j.anl.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 42.Bajo VM, Nodal FR, Moore DR, King AJ. The descending corticocollicular pathway * mediates learning-induced auditory plasticity. Nat Neurosci. 2010;13:253–60. doi: 10.1038/nn.2466. In ferrets, relearning of sound localization after plugging one ear was lost after eliminating corticocollicular neurons, although sound-localization accuracy was unaffected. This shows involvement of the descending auditory system in sound-localization plasticity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reuss S, Disque-Kaiser U, Antoniou-Lipfert P, et al. Neurochemistry of olivocochlear neurons in the hamster. Anat Rec (Hoboken) 2009;292:461–71. doi: 10.1002/ar.20881. [DOI] [PubMed] [Google Scholar]
- 44.Lv P, Rodriguez-Contreras A, Jeong Kim H, et al. Release and Elementary Mechanisms of Nitric Oxide in Hair Cells. J Neurophysiol. 2010 Mar 17; doi: 10.1152/jn.00017.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keppler H, Dhooge I, Corthals P, et al. The effects of aging on evoked otoacoustic emissions and efferent suppression of transient evoked otoacoustic emissions. Clin Neurophysiol. 2010;121:359–65. doi: 10.1016/j.clinph.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 46.Oliveira JR, Fernandes JC, Costa Filho OA. Age impact on the efferent system activities in cochlear mechanical properties in normal hearing individuals. Brazilian journal of otorhinolaryngology. 2009;75:340–4. doi: 10.1016/S1808-8694(15)30648-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paschoal CP, Azevedo MF. Cigarette smoking as a risk factor for auditory problems. Brazilian journal of otorhinolaryngology. 2009;75:893–902. doi: 10.1016/S1808-8694(15)30556-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vinay Effect of smoking on transient evoked otoacoustic emissions and contralateral suppression. Auris Nasus Larynx. 2010;37:299–302. doi: 10.1016/j.anl.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 49.Fernandes L, Santos TM. Tinnitus and normal hearing: a study on the transient otoacoustic emissions suppression. Brazilian journal of otorhinolaryngology. 2009;75:414–9. doi: 10.1016/S1808-8694(15)30660-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paglialonga A, Del Bo L, Ravazzani P, Tognola G. Quantitative analysis of cochlear active mechanisms in tinnitus subjects with normal hearing sensitivity: multiparametric recording of evoked otoacoustic emissions and contralateral suppression. Auris Nasus Larynx. 2010;37:291–8. doi: 10.1016/j.anl.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 51.Ugur AK, Kemaloglu YK, Ugur MB, et al. Otoacoustic emissions and effects of contralateral white noise stimulation on transient evoked otoacoustic emissions in diabetic children. International journal of pediatric otorhinolaryngology. 2009;73:555–9. doi: 10.1016/j.ijporl.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 52.Darrow KN, Maison SF, Liberman MC. Cochlear efferent feedback balances interaural sensitivity. Nat Neurosci. 2006;9:1474–6. doi: 10.1038/nn1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Larsen E, Liberman MC. Contralateral cochlear effects of ipsilateral damage: no evidence * for interaural coupling. Hear Res. 2010;260:70–80. doi: 10.1016/j.heares.2009.11.011. Manipulations that produced threshold shifts in one ear of guinea pigs did not change either threshold or supra-threshold measures of cochlear function in the opposite ear. This finding indicates that lateral efferents do not act to balance outputs from the two ears. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elgoyhen AB, Vetter DE, Katz E, et al. alpha10: a determinant of nicotinic cholinergic receptor function in mammalian vestibular and cochlear mechanosensory hair cells. Proc Natl Acad Sci U S A. 2001;98:3501–6. doi: 10.1073/pnas.051622798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taranda J, Maison SF, Ballestero JA, et al. A point mutation in the hair cell nicotinic ** cholinergic receptor prolongs cochlear inhibition and enhances noise protection. PLoS biology. 2009;7:e18. doi: 10.1371/journal.pbio.1000018. This study used genetically modified mice in which MOC effects were increased and found that these mice had lower permanent hearing loss following exposure to intense noise. This result provides strong evidence that MOC efferents help to prevent damage due to traumatic sounds. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murthy V, Maison SF, Taranda J, et al. SK2 channels are required for function and long-term survival of efferent synapses on mammalian outer hair cells. Mol Cell Neurosci. 2009;40:39–49. doi: 10.1016/j.mcn.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murthy V, Taranda J, Elgoyhen AB, Vetter DE. Activity of nAChRs containing alpha9 subunits modulates synapse stabilization via bidirectional signaling programs. Developmental neurobiology. 2009;69:931–49. doi: 10.1002/dneu.20753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vetter DE, Liberman MC, Mann J, et al. Role of alpha 9 nicotinic ACh receptor subunits in the development and function of cochlear efferent innervation. Neuron. 1999;23:93–103. doi: 10.1016/s0896-6273(00)80756-4. [DOI] [PubMed] [Google Scholar]
- 59.May BJ, Prosen CA, Weiss D, Vetter D. Behavioral investigation of some possible effects of the central olivocochlear pathways in transgenic mice. Hear Res. 2002;171:142–57. doi: 10.1016/s0378-5955(02)00495-1. [DOI] [PubMed] [Google Scholar]
- 60.Brown MC, Vetter DE. Olivocochlear neuron central anatomy is normal in alpha 9 * knockout mice. J Assoc Res Otolaryngol. 2009;10:64–75. doi: 10.1007/s10162-008-0144-9. This study examined the central anatomy of olivocochlear efferents and found no difference between α9AChR-knockout and normal mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.May BJ, Budelis J, Niparko JK. Behavioral studies of the olivocochlear efferent system: learning to listen in noise. Arch Otolaryngol Head Neck Surg. 2004;130:660–4. doi: 10.1001/archotol.130.5.660. [DOI] [PubMed] [Google Scholar]
- 62.Mulders WH, Paolini AG, Needham K, Robertson D. Synaptic responses in cochlear nucleus neurons evoked by activation of the olivocochlear system. Hear Res. 2009;256:85–92. doi: 10.1016/j.heares.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 63.Bledsoe SC, Jr, Koehler S, Tucci DL, et al. Ventral cochlear nucleus responses to * contralateral sound are mediated by commissural and olivocochlear pathways. J Neurophysiol. 2009;102:886–900. doi: 10.1152/jn.91003.2008. Using unilaterally deafened mice and selective central damage produced by a neurotoxin, this study provides evidence that the collaterals of MOC axons produce slow excitatory effects in the cochlear nucleus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Elgoyhen AB, Franchini LF. Prestin and the cholinergic receptor of hair cells: Positively-selected proteins in mammals. Hear Res. 2010 doi: 10.1016/j.heares.2009.12.028. in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Elgoyhen AB, Katz E, Fuchs PA. The nicotinic receptor of cochlear hair cells: a possible pharmacotherapeutic target? Biochemical pharmacology. 2009;78:712–9. doi: 10.1016/j.bcp.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McIntosh JM, Absalom N, Chebib M, et al. Alpha9 nicotinic acetylcholine receptors and the treatment of pain. Biochemical pharmacology. 2009;78:693–702. doi: 10.1016/j.bcp.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Turcan S, Slonim DK, Vetter DE. Lack of nAChR activity depresses cochlear maturation and up-regulates GABA system components: temporal profiling of gene expression in alpha9 null mice. PloS one. 2010;5:e9058. doi: 10.1371/journal.pone.0009058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maison SF, Casanova E, Holstein GR, et al. Loss of GABAB receptors in cochlear neurons: * threshold elevation suggests modulation of outer hair cell function by type II afferent fibers. J Assoc Res Otolaryngol. 2009;10:50–63. doi: 10.1007/s10162-008-0138-7. This study found that GABA(B1)-deficient mice had DPOAE thresholds that were ~10 dB elevated and MOC inhibition of DPOAEs that was normal. These animals showed increased resistance to permanent, but not temporary, threshold shifts, which indicates a role of GABA in the prevention of permanent threshold shifts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Simmons DD. Development of the inner ear efferent system across vertebrate species. J Neurobiol. 2002;53:228–50. doi: 10.1002/neu.10130. [DOI] [PubMed] [Google Scholar]
- 70.Taranda J, Ballestero JA, Hiel H, et al. Constitutive expression of the alpha10 nicotinic acetylcholine receptor subunit fails to maintain cholinergic responses in inner hair cells after the onset of hearing. J Assoc Res Otolaryngol. 2009;10:397–406. doi: 10.1007/s10162-009-0173-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gomez-Casati ME, Wedemeyer C, Taranda J, et al. Electrical properties and functional expression of ionic channels in cochlear inner hair cells of mice lacking the alpha10 nicotinic cholinergic receptor subunit. J Assoc Res Otolaryngol. 2009 Jun;10:221–32. doi: 10.1007/s10162-009-0164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Neef J, Gehrt A, Bulankina AV, et al. The Ca2+ channel subunit beta2 regulates Ca2+ channel abundance and function in inner hair cells and is required for hearing. J Neurosci. 2009;29:10730–40. doi: 10.1523/JNEUROSCI.1577-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]