Abstract
Background
We have shown that glucagon-like peptide-1 (GLP-1 [7–36] amide), stimulates myocardial glucose uptake in dilated cardiomyopathy (DCM) independent of an insulinotropic effect. The cellular mechanisms of GLP-1 induced myocardial glucose uptake are unknown.
Methods and Results
Myocardial substrates and glucoregulatory hormones were measured in conscious, chronically instrumented dogs at Control (n=6), DCM (n=9), and DCM following treatment with a 48-hour infusion of GLP-1 (7–36) amide (n=9) or vehicle (Veh, n=6). GLP-1 receptors and cellular pathways implicated in myocardial glucose uptake (MGU) were measured in sarcolemmal membranes harvested from the four groups. GLP-1 stimulated MGU (DCM: 20±7; DCM+GLP-1: 61±12 nmol/min/g, p=0.001) independent of increased plasma insulin levels. The GLP-1 receptors were up-regulated in the sarcolemmal membranes (Con: 98±2; DCM: 256±58 DU, p=0.046) and were expressed in their activated (65 kDa) form in DCM. The GLP-1 induced increases in MGU did not involve adenylyl cyclase or Akt activation, but was associated with marked increases in p38α MAP kinase activity (DCM + Veh: 97±22 DCM+GLP-1: 170±36 pmol ATP/mg/min, p=0.051), induction of nitric oxide synthase 2 (NOS2) (DCM +Veh: 151±13; DCM+GLP-1: 306±12 DU, p=0.001), and GLUT-1 translocation (DCM+Veh: 21±3%; DCM+GLP-1: 39±3% membrane bound, p=0.005). The effects of GLP-1 on MGU were blocked by pretreatment with the p38α MAP kinase inhibitor or non-specific NOS inhibitor, nitro-L-arginine.
Conclusions
GLP-1 stimulates myocardial glucose uptake through a non Akt-1 dependent mechanism by activating cellular pathways that have been identified in mediating chronic hibernation and the late phase of ischemic pre-conditioning.
Keywords: glucagon-like peptide-1, myocardial glucose uptake, MAP kinase, nitric oxide synthase, dilated cardiomyopathy
Introduction
Glucagon-like peptide-1 (GLP-1 [7–36] amide) is a member of the pro-glucagon family of naturally occurring incretins released by L-cells in the intestine in response to nutrient ingestion (1–3). The cellular signaling mechanisms have been studied extensively in β–cells of the pancreas that serve as the principle physiological target, stimulating insulin release (4, 5). However, recent investigation has focused on other extra-pancreatic targets of GLP-1 action including direct effects on skeletal and cardiac muscle to stimulate glucose uptake, independent of its insulinotropic effects (6–9). Several laboratories have demonstrated GLP-1 transcripts in hearts of rodents (10) and humans (11) and recently GLP-1 receptors were localized to cardiomyocytes and coronary endothelial cells in mice hearts (12). Our laboratory has demonstrated that dilated cardiomyopathy (DCM) in conscious dogs is associated with the development of insulin resistance (13) and that the impaired insulin actions are most evident on myocardial glucose uptake and coronary blood flow, as opposed to whole body glucose disposition. We have observed the GLP-1 (7–36) amide stimulates myocardial glucose uptake in conscious dogs with DCM (14) and in isolated normal and post-ischemic rat hearts (15). Despite the emergence of evidence to support direct cardiac effects (16), the mechanism of myocardial cellular effects of GLP-1 to stimulate myocardial glucose uptake and induce cardio-protection in large animal models of non-ischemic cardiomyopathy remains unknown.
Accordingly, the purpose of the present study was to determine the presence of GLP-1 receptors in the canine myocardium and whether the receptors are down-regulated in heart failure. A second goal was to determine the mechanisms whereby GLP-1 increases myocardial glucose uptake and specifically whether these mechanisms involved increased Akt phosphorylation and GLUT-4 translocation as is true for classical myocardial insulin signaling. A third goal was to determine whether myocardial glucose uptake induced by GLP-1could be attenuated by inhibition of identified steps in the signaling cascade.
Methods
Instrumentation
Forty-two mongrel dogs of either sex weighing 15–20 kg were instrumented as described previously from our laboratory (13, 14). The dogs were allowed to recover from the surgical procedure for 2 weeks, during which time they were trained to lie quietly on the experimental table in a conscious, unrestrained state. Hemodynamic measurements were made with the dogs fully awake, lying quietly on their right side.
Hemodynamic measurements
Baseline experiments consisted of left ventricular (LV) and systemic hemodynamic recordings to determine LV contractility (LV dP/dt), stroke volume (SV), cardiac output (CO), and coronary blood flow (CBF). Six dogs did not undergo pacing and served as normal controls. DCM was induced by rapid right ventricular (RV) pacing (240 min−1) for 28 days in 36 dogs, as described previously (13,14). At that time, LV and systemic hemodynamics were measured with pacing suspended for 30 minutes. At this point (DCM), 9 dogs were euthanized to determine the effects of DCM on specified cellular signaling components in myocardial tissue. In the remaining 15 dogs, pacing rates were reduced to 180 min−1 for 48 hours during which time they were randomized to receive a continuous infusion of synthetic GLP-1 (7–36) amide (2.5 pmol/kg/min) through the right atrial catheter (DCM+GLP-1, n=9) or vehicle (saline 2.8 ml+0.2 ml plasma/day; DCM+veh, n=6). After the additional 48 hours, the infusions were continued while the pacemakers were deactivated for 4 hours prior to the final hemodynamic and metabolic determinations. GLP-1 (7–36) amide was prepared as described previously (14).
In order to determine whether the effects of GLP-1 on myocardial glucose uptake were p38α MAP kinase-dependent, we studied an additional 6 dogs, chronically instrumented as described above, prior to and following 28 days of rapid pacing. Myocardial glucose uptake was measured at baseline and following a 6-hour infusion of GLP-1 before and after the administration of the p38α MAP kinase inhibitor, SB 203580 (Calbiochem: 1 mg/kg IV) over 60 minutes.
In order to determine whether the effects of GLP-1 on myocardial glucose uptake were nitric oxide (NO) dependent, we studied an additional 6 dogs, chronically instrumented as described above, prior to and following 28 days of rapid pacing. Myocardial NO uptake was measured at baseline and following a 6-hour infusion of GLP-1 before and after the administration of the non-specific NO inhibitor, nitro-L-arginine (30 mg/kg IV) over 30 minutes. The CBF response to IV acetylcholine injection (5μg/kg) was measured before and after N-LA administration to establish NO inhibition.
Metabolic Determinations
All dogs were fed a standard diet with fixed carbohydrate and fat content. Body weights were monitored weekly. Metabolic parameters were measured at 8 am, following an overnight fast. Arterial and coronary sinus blood samples were obtained in all dogs at control and 28 days following initiation of rapid pacing. Transmyocardial substrate balance was calculated as the difference between arterial and coronary sinus content. Basal myocardial substrate uptake was calculated as the product of myocardial substrate balance and CBF, normalized for LV mass and expressed as nmol/min/g LV myocardium. Plasma glucose, NEFA and NO levels were measured as described previously (13, 14, 20). Myocardial insulin sensitivity was assessed in the a) baseline state (n=18), b) following the development of DCM (day 28, n=9), and c) after a 48-hour infusion of GLP-1 (day 30, n=9) or vehicle (day 30, n=6) using the hyperinsulinemic-euglycemic clamp technique as described previously (12–14).
Cellular Mechanisms
Following the completion of the final clamp studies, GLP-1 or Veh was continued for an additional 120 minutes following which time the animals were anesthetized and intubated. LV samples were taken from the heart and sarcolemmal membrane preparations developed as described previously (13, 15). GLP-1 receptors were measured in crude sarcolemmal membrane preparations using a rabbit poly-clonal antibody (Alpha Diag. International, San Antonio, TX), as described previously (13, 15). Goat anti-rabbit IgG-horseradish peroxidase conjugate, anti-Akt-1, and anti-phospho-Akt-1 (Ser-473) were purchased from BD Transduction Laboratories (San Diego, CA). Phospho-Akt-1 specificity was confirmed using phosphorylated and non-phosphorylated NIH/3T3 cell extracts purchased from Cell Signaling Technology, Inc. (Beverly, MA).
For the measurement of GLUT translocation, purified light (sarcolemmal) and heavy (intracellular) membrane vesicles and cytosolic fractions were isolated from LV myocardium using a sucrose gradient (15, 21). Membranes were probed with rabbit anti-GLUT-4 and anti-GLUT-1 polyclonal antibody (1:1000) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) overnight at 4°C. Densitometric analysis of the bands was carried out using a Personal Densitometer SI and ImageQuaNT™ Software (Molecular Dynamics, Sunnyvale, CA) and expressed as the % sarcolemmal membrane bound to total GLUT in LV homogenates (15).
The AMP-activated protein kinase (AMPK) activity in the LV myocardium was determined as described previously (15). Activity was expressed as incorporated ATP (picomoles)per milligram of protein per minute. Total p38α MAP kinase (Santa Cruz Biotechnology, Santa Cruz, CA) was assayed by Western blot and p38 MAP kinase activity was measured using an approach that measures the transfer of phosphate from ATP to tarfets of p38 ( ). NOS isoforms were measured using antibodies purchased from Santa Cruz Biotechnology, Santa Cruz, CA.
Adenylyl cyclase activity was assayed as described previously from our laboratory using this canine model (22) Cardiac membranes (25–50,g of protein) were added to a solution containing 1 mM ATP (2–3 × 106 counts/min of [a-32P]ATP), 20 mM creatine phosphate, creatine phosphokinase (1 U), 1 mM cAMP (2,000–3,000 counts/min of 3[H]cAMP as an internal standard), 25 mM Tris, 5 mM MgCl2, 1 mM EDTA, and the test substance to measure adenylyl cyclase activity [GTP (0.1 mM), isoproterenol (0.1 mM), Gpp(NH)p (0.1 mM), NaF (10 mM), and forskolin (0.1 mM)]. 10 Ml of stopping solution (20 mM ATP, 10 mM cAMP, 2% sodium dodecyl sulfate [SDS]) were added to each tube to terminate the reaction, and the tubes were heated on a dry bath at 100°C for 3min. Cyclic AMP was separated, as previously described (22).
Mitochondrial Isolates
Crude mitochondrial isolates vere prepared from canine myocardium using a trypsin digestion procedure as described previously (23). The purity of the mitochondrial isolates was established by demonstrating the absence of Na+/K+ ATPase activity in mitochondrial preparations. Membrane samples (5μg/lane) for uncoupling protein-3 [UCP-3, Alpha Diagnostics, San Antonio, TX] and 2.5μg/lane for succinate dehydrogenase [SDHA, Abcam, Cambridge, MA] and mitochondrial cytochrome oxidase-1 [MCO1, Abcam, Cambridge, MA]) and manganese superoxide dismutase (MnSOD, Abcam, Cambridge, MA) were subjected to electrophoretic separation by SDS-PAGE and quantified as described previously (23). Reactive oxygen species were determined as a product of lipid peroxidation and measured as malondialdehyde using a commercially available assay from Cayman Chemicals (Ann Arbor, MI).
Statistical analysis
All measurements are expressed as means ± SEM. The hemodynamic (Table 1) and metabolic (Table 2) responses to the Veh or GLP-1 infusion were analyzed by repeated measures ANOVA. Absolute p valves for the interaction of treatment effect with evolution of DCM were reported. The cellular signaling data in the four groups in Figures 1–6 were analyzed by one way ANOVA after a Levene’s test confirmed homogeneity of variances. Alternatively, a Kruskal-Wallis test was used under circumstances where variances were inhomogeneous. Differences in protein abundance and activity were compared between Control and DCM or between DCM+Veh and DCM+GLP-1 using an unpaired Student t-test.
Table 1.
The Effects of GLP-1 Infusion on Hemodynamics in Dogs with Dilated Cardiomyopathy (DCM)
| DCM + Vehicle | DCM +GLP-1 | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | DCM28 days | DCM30 days | Baseline | DCM28 days | DCM30 days | p Value for Interaction | |
| LV Pressure(mmHg) | 121±6 | 103±2 | 104±3 | 126±6 | 105±3 | 114±4 | 0.20 |
| LV End Diastolic Pressure(mmHg) | 8±1 | 32±4 | 30±4 | 9±2 | 32±3 | 20±2* | 0.001 |
| LV dP/dt(mmHg) | 2887±198 | 1542±106 | 1602±124 | 2980±241 | 1378±211 | 2150±210* | 0.010 |
| Heart Rate(min−1) | 80±6 | 121±9 | 108±9 | 84±4 | 115±8 | 89±5* | 0.001 |
| Mean Arterial Pressure(mmHg) | 92±8 | 86±4 | 85±3 | 96±4 | 86±5 | 83±4 | 0.44 |
| Cardiac Output(l/min) | 2.3±0.1 | 1.5±0.1 | 1.7±0.2 | 2.4±0.2 | 1.6±0.1 | 2.1±0.2* | 0.007 |
| Coronary Blood Flow(ml/min) | 21±3 | 30±2 | 30±4 | 22±4 | 32±3 | 35±4 | 0.50 |
| LV Ejection Fraction (%) | 49±4 | 22±3 | 22±4 | 51±4 | 20±4 | 39±3* | 0.002 |
| MVO2 mlO2/min | 215±13 | 318±38 | 320±43 | 200±9 | 307±26 | 324±38 | 0.93 |
significant difference in response to DCM+GLP-1 compared to DCM+vehicle
Table 2.
Metabolic Parameters
| DCM+Vehicle (n=6) | DCM+GLP-1 (n=9) | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | DCM28 days | DCM30 days | Baseline | DCM28 days | DCM30 days | p Value for Interaction | |
| Plasma Glucose mmol/l | 5.6±0.2 | 5.5±0.2 | 5.4±0.2 | 5.3±0.3 | 5.4±0.4 | 5.2±0.2 | 0.74 |
| Plasma NEFAμmo/l | 598±54 | 623±67 | 676±104 | 643±91 | 553±76 | 648±45 | 0.89 |
| Plasma Insulin pmol/l | 38±8 | 114±20 | 80±9 | 56±8 | 99±16 | 58±9* | 0.007 |
| Plasma Glucagon pg/ml | 23±5 | 39±6 | 41±4 | 29±2 | 47±6 | 31±3* | 0.03 |
| Arterial NO μM | 15.9±1.1 | 11.8±1.0 | 12.2±0.9 | 16.7±1.1 | 10.8±1.0 | 17.3±1.0* | 0.04 |
| Plasma NE nmol/l | 0.47±0.04 | 1.21±0.1 | 1.07±0.12 | 0.39±0.09 | 1.17±0.1 | 0.81±0.09* | 0.03 |
| Glucose Clamp | |||||||
| Basal(μmol/min/g) | 1.1±0.4 | 1.8±0.5 | 2.1±0.6 | 0.9±0.4 | 1.9±0.7 | 5.8±1.1* | 0.002 |
| Hyperinsulinema(μmol/min/g) | 15±3 | 7±3 | 7±3 | 14±3 | 6±3 | 21±4* | 0.0001 |
significant difference in response DCM+GLP-1 compared to DCM+vehicle
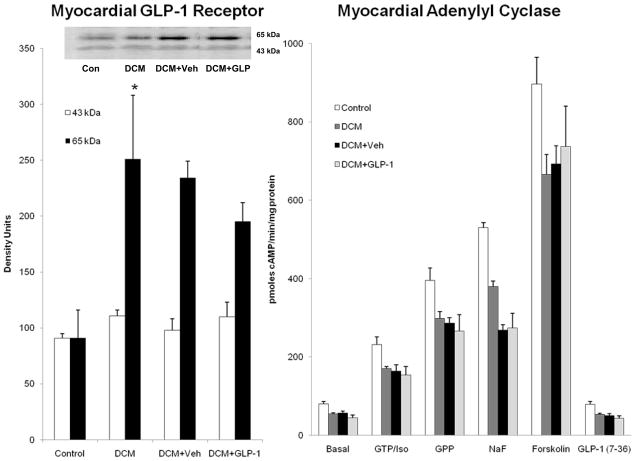
Figure 1.
The sarcolemmal expression of GLP-1 receptor isoforms (Figure 1A) and basal and agonist stimulated myocardial adenylyl cyclase activity (Figure 1B) in Control dogs (n=6), DCM alone (n=6), DCM+Veh (n=6) and DCM+GLP-1 (n=6).
* p=0.046 Control compared to DCM
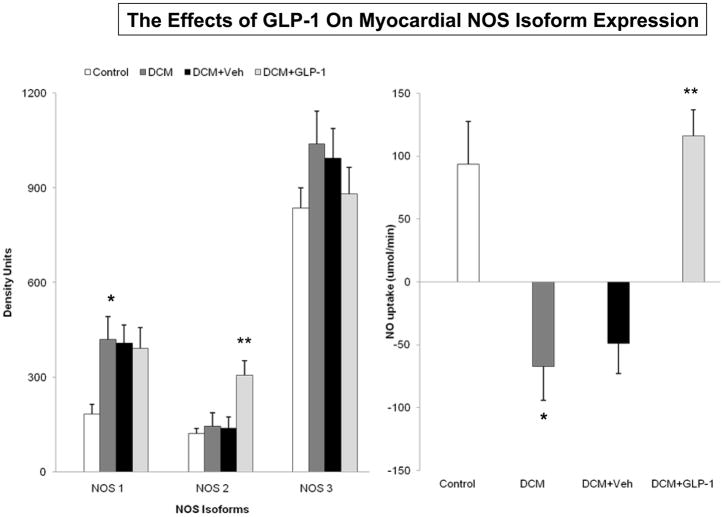
Figure 6.
The effects of GLP-1 treatment on myocardial NOS isoform expression (6A) and on myocardial NO uptake (6B) in Control dogs (n=6), DCM alone (n=9), DCM+Veh (n=6) and DCM+GLP-1 (n=9). One way ANOVA NOS1 p=0.0111, NOS2 p=0.0002; NOS3 p=0.4371. One way ANOVA NO uptake p=0.0001.
*p=0.0042 Control compared to DCM. ** **p=0.0010 DCM+ Veh compared to DCM+GLP-1.
Results
The Effects of GLP-1 on LV and Systemic Hemodynamics
Table 1 illustrates the hemodynamic perturbations associated with the development of DCM in 15 dogs and the effects of infusion with vehicle or GLP-1 on hemodynamic recovery. At 28 days, the development of DCM was characterized by significant decreases in LV systolic pressure, LV dP/dt, CO and LVEF, and significant increases in LV end diastolic pressure, heart rate, CBF and MVO2 in both groups. GLP-1 infusion was associated with improvements in LV end diastolic pressures (p<0.001); LV dP/dt (p<0.010), CO (p<0.007) and LV ejection fraction (p<0.002) compared to DCM+Veh.
Table 2 illustrates the effects of the development of GLP-1 treatment on plasma substrates and glucoregulatory hormones. Fasting plasma glucose and NEFA levels were normal in DCM and remained unchanged following treatment with Veh or GLP-1. Both plasma insulin and glucagon levels increased during the development of DCM in both groups. However, plasma insulin levels (p=0.005) and plasma glucagon levels (p=0.0254) were reduced following GLP-1 treatment compared to the response in DCM+ Veh infusion. The salutary responses to GLP-1 treatment were associated with increases in plasma arterial NO levels (p=0.046) and decreases in plasma norepiephrine levels (p<0.016) compared to DCM+Veh infusion.
Table 2 illustrates the results of the hyperinsulinemic-euglycemic clamps in the two treatment groups. GLP-1infusion increased basal myocardial glucose uptake by three-fold (DCM+vehicle: 22±6; DCM+GLP-1: 61±12 nmol/min/g, p<0.001) in dogs with DCM. The GLP-1 induced increase in basal myocardial glucose uptake occurred despite lower basal insulin levels (Table 2). Insulin-stimulated myocardial glucose uptake during hyperinsulinemia was impaired significantly during the development of DCM in both treatment groups. However, GLP-1 infusion markedly augments insulin-stimulated myocardial glucose uptake (DCM+vehicle: 74±3; DCM+GLP-1: 266±43 nmol/min/g, p<0.001) compared to DC+Veh.
GLP-1 Receptors and Adenylyl Cyclase (AC) Activity in Canine Myocardium
Figure 1A reveals the expression of GLP-1 receptors in canine myocardium in all groups. The receptor was present in two isoforms. There was no diffdrence in the 43kDa isoform of the receptor among the groups. However, there was up-regulation of the 65kDa isoform in dogs with DCM compared to control (p=0.046). There was no difference in GLP-1 receptor expression between Veh and GLP-1 treated groups.
Figure 1B illustrates the effects of GLP-1 treatment on the adenylyl cyclase (AC) activity in sarcolemmal membrane preparations harvested from 6 Control dogs, 6 dogs with DCM, 6 dogs with DCM+vehicle and 6 dogs with DCM+GLP-1. DCM was associated with decreased basal and agonist stimulated AC activity compared to Control, consistent with heterologous desensitization. There was no significant impact of GLP-1 treatment on basal or stimulated AC activity. In separate experiments, sarcolemmal membranes incubated with increasing concentrations of GLP-1 (0.06–12 pmol/μg protein) had no significant effect to stimulate cyclic AMP generation (122 to 150 pmol/min/μg protein) in contrast to other conventional agonists such as dobutamine that increased cyclic AMP production fivefold (98 to 546 pmol/min//μg protein, p<0.001). Thus, in contrast to what has been observed in pancreatic β-cells, GLP-1 had no measurable effects on AC activity in myocardium either in vivo or in vitro.
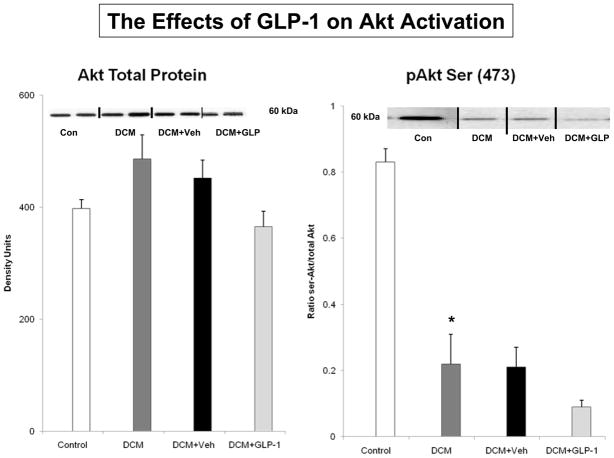
The Effects of GLP-1 on Akt Activation and Membrane Expression of GLUT Transporters
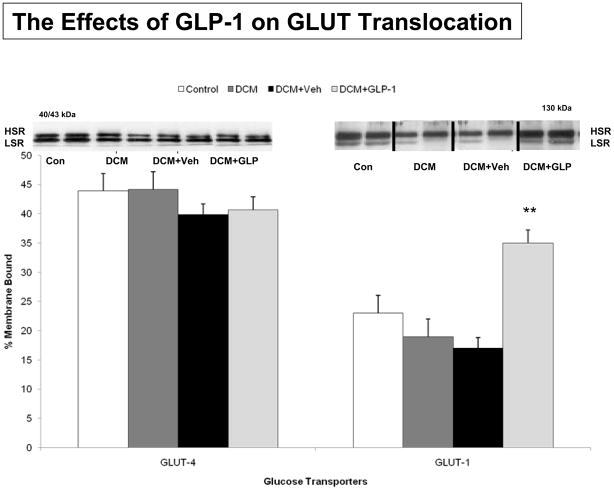
To determine whether GLP-1 increased myocardial glucose uptake through the PI3-kinase/Akt activation, we examined the phosphorylation of Akt in the four groups (Figure 2). There was no difference in Akt abundance, but there was significant reduction in Akt phosphorylation at serine 473 among groups (p<0.001). Specifically, there was a significant reduction in Akt phosphorylation between Control and DCM (p<0.003), but no difference between Veh and GLP-1 treated dogs (p=0.1042). Figure 3 illustrates the effects of GLP-1 treatment on GLUT expression in purified sarcolemmal membranes as a percent of total GLUT expression. There was no difference in GLUT-4 expression in the various cellular compartments or in the percent membrane bound among the four groups (Figure 3A, p=0.5643). However, there was a significant increase in GLUT-1 expression in purified sarcolemmal membranes following GLP-1 treatment (Figure 3B, p<0.001). Specifically, while there was no difference in GLUT-1 expression between Control and DCM (p=0.4809), there was a significant increase in GLUT-1 membrane expression in GLP-1 treated dogs compared to Veh (p<0.01) Figure 3 Supplement illustrates the changes in the expression of GLUT 1 transporters in LV homogenates as well as cytosolic, intracellular and sarcolemmal compartments. GLP-1 treatment was associated with small increases in GLUT-1 expression in LV homogenates and intracellular vesicles, but not in cytolsolic fractions.
Figure 2.
Akt-1 protein expression and activation as measured by serine 473 phosphorylation in Control dogs (n=6), DCM alone (n=9), DCM+Veh (n=6) and DCM+GLP-1 (n=9). One way ANOVA, p=0.001.
* p=0.003 Control compared to DCM
Figure 3.
GLUT–4 and GLUT–1 translocation in response to GLP-1 treatment in Control dogs (n=6), DCM alone (n=9), DCM+Veh (n=6) and DCM+GLP-1 (n=9). Transporter translocation is expressed as the % membrane bound/total transporter concentration in membrane and cytosolic fractions. One way ANOVA p=0.0013 for GLUT-1.
**p=0.0013 DCM+ Veh compared to DCM+GLP-1.
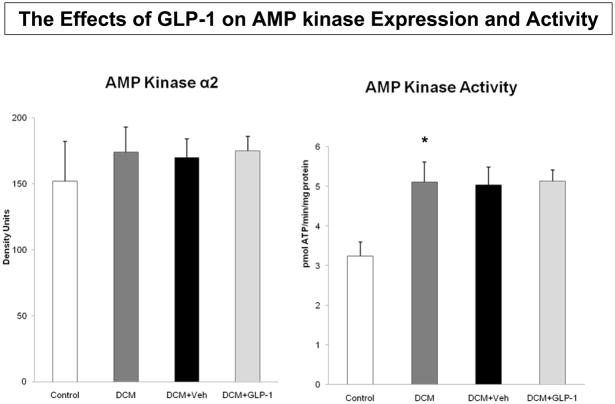
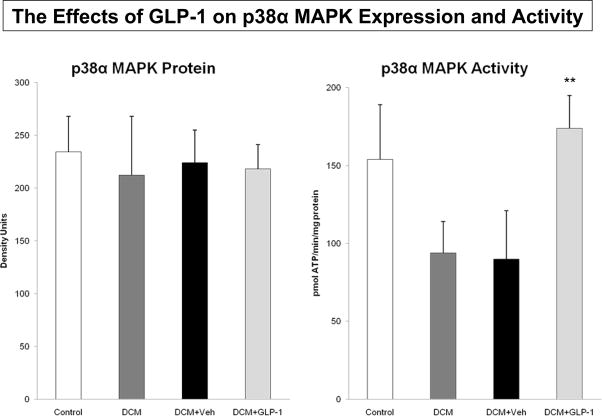
To examine the role of non-Akt dependent mechanisms, we measured AMP kinase abundance and activity in the respective groups (Figure 4). There was no difference in protein expression (Figure 4A), but there was an increase in AMP kinase activity among the four groups (Figure 4B, p=0.059). Specifically, there was a significant increase in AMP kinase activity between Control and DCM dogs (p<0.02), but not between GLP-1 and Veh treated dogs. Similarly, there was no difference in protein expression of the p38α isoform of MAP kinase (Figure 5A). However, there were differences in p38α MAP kinase activity among the groups (Figure 5B). Specifically, GLP-1 treatment was associated with increased p38αMAP kinase activity (p<0.04) compared to the response in Veh treated dogs.
Figure 4.
The effects of GLP-1 treatment on AMP kinase protein expression (4A) and activity (4B) in Control dogs (n=6), DCM alone (n=9), DCM+Veh (n=6) and DCM+GLP-1 (n=9). One way ANOVA for AMP kinase activity p=0.0590.
*p=0.0181 Control compared to DCM.
Figure 5.
The effects of GLP-1 treatment on p38α MAP kinase expression (5A) and activity (5B) in Control dogs (n=6), DCM alone (n=9), DCM+Veh (n=6) and DCM+GLP-1 (n=9). One way ANOVA p=0.2869 for p38 MAP kinase activity.
**p=0.0431 DCM+ Veh compared to DCM+GLP-1.
Figure 6 shows the impact of GLP-1 infusion in DCM on the expression of nitric oxide synthase isoforms. There was no difference in NOS3 isoform expression, but there was a doubling of NOS2 expression in the dogs with DCM during GLP-1 infusion compared to Veh infusion ((p<0.001). Notably, DCM was associated with increased NOS1 expression compared to Control (p<0.004), which was maintained but not enhanced by GLP-1 (Figure 6A). The altered NOS isoform expression was associated with an increase in arterial NO concentrations (p<0.05, Table 2) and myocardial NO uptake in dogs with DCM+GLP-1 compared to DCM+vehicle (p<0.001, Figure 6B).
The Effects of p38 MAP kinase and NO Inhibition on GLP-1 induced Myocardial Glucose Uptake
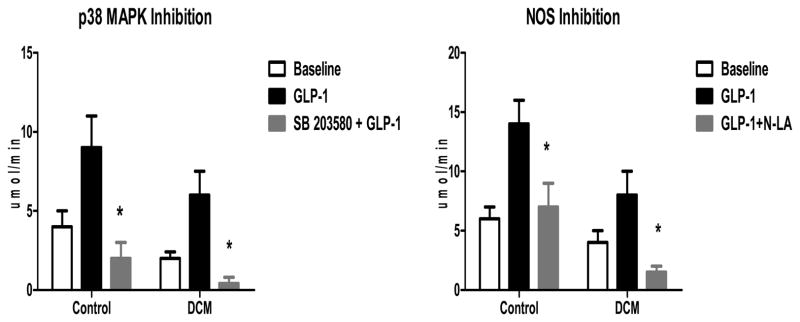
In order to determine whether the increases in p38 MAP kinase activity and NOS2 expression were linked to the GLP-1 induced increases in myocardial glucose uptake, we conducted acute 6 hour infusions of GLP-1 in the presence and absence of acute pharmacological blockade of these pathways. Figure 7 illustrates the effects of inhibition of p38 MAP kinase (Figure 7A, n=6) and non-specific NOS inhibition with nitro-L-arginine (Figure 7B, n=6) on GLP-1 induced myocardial glucose uptake following an acute 6 hour infusion. Inhibition of p38 MAP kinase attenuated the GLP-1 induced increase in myocardial glucose uptake in both Control dogs (p<0.01) and dogs with DCM (p<0.02). Pretreatment with N-LA markedly attenuated the GLP-1 induced increase in myocardial glucose uptake in both Control (p<0.04) and DCM dogs (p<0.02). The effects were unrelated to differences in insulin levels or significant reductions in CBF, but were associated with a decrease in glucose extraction.
Figure 7.
The effects of p38 MAP kinase inhibition with SB 203580 (7A) and NOS inhibition with nitro L-arginine (N-LA; 7B) on GLP-1 mediated myocardial glucose uptake in Control (n=6) and DCM (n=6).
*p<0.02 GLP-1 compared to Blockade +GLP-1
The Effects of GLP-1 Infusion on Mitochondrial Function in DCM
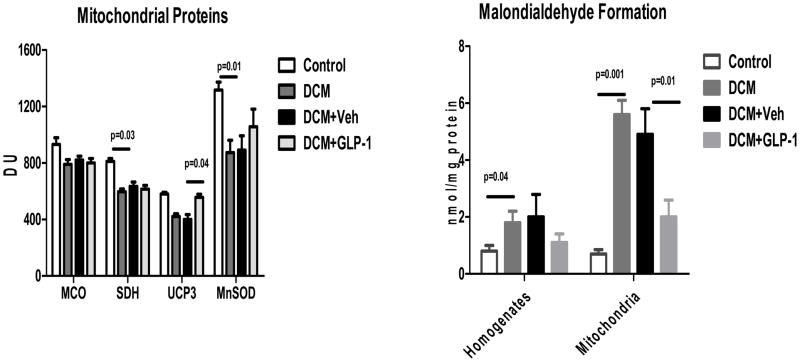
Figure 8 demonstrates the effects of a 48 hour infusion of GLP-1 on mitochondrial proteins and mitochondrial lipid peroxidation. DCM was associated with reductions in MCO-1, SDH, and UCP-3 expression (p<0.002). There was also a significant reduction in MnSOD expression. Neither MCO-1 nor SDH were altered by GLP-1 or Veh infusion. However, GLP-1 increased significantly the expression of UCP-3 (p<0.02) and tended to increase MnSOD in DCM compared to Veh (p=0.0876) Figure 8A). The development of DCM was associated with increased lipid peroxidation in whole cell homogenates (p<0.02) and isolated mitochondria (p<0.001) and the levels were reduced following a 48 hour infusion of GLP-1 but not Veh (p<0.01, Figure 8B).
Figure 8.
The effects of GLP-1 treatment on mitochondrial protein expression (8A) and lipid peroxidation (8B) in Control dogs (n=5), DCM alone (n=5), DCM+Veh (n=4) and DCM+GLP-1 (n=5). mCO = mitochrondrial cytochrome oxidase; SDH = succinate dehydrogenase;UCP-3 = uncoupling protein-3, Mn SOD= manganese superoxide dismutase.
One way ANOVA MCO p=0.001; SDH p=0.001; UCP-3 p=0.001; MnSOD p=0.00
Discussion
In the present study, we demonstrate that GLP-1 increases both basal and insulin-stimulated glucose uptake in the myocardium of conscious dogs with DCM. We demonstrated that the increase in myocardial glucose uptake was independent of Akt phosphorylation. Neither the improvement in myocardial function nor the increase in myocardial glucose uptake was associated with increased adenylyl cyclase activity. During DCM, AMP kinase activity was significantly increased in all groups, but the activity was sustained but not augmented by GLP-1 infusion. However, GLP-1 increased GLUT-1 expression in LV sarcolemmal membranes independent of any increase in plasma insulin levels, and was associated with increases in p38α MAP kinase activity, increased expression of NOS2 and increased myocardial uptake of nitric oxide. GLP-1 induced increases in myocardial glucose uptake in the failing heart were attenuated by blockade of either p38 MAP kinase or NOS. Taken together, these data suggest that GLP-1 has direct effects on myocardial glucose uptake mediated through a pathway distinct from classical insulin-mediated glucose uptake.
Prior studies have demonstrated the presence of the GLP-1 receptor in the myocardium (10-12), but ours is the first study to demonstrate an increase in GLP-1 receptor in DCM. The 65kDa isoform is considered to represent the glycosylated and active form (17) of the G-protein coupled receptor and was increased in DCM and remained increased in DCM+GLP-1 treated groups. The presence of the receptor supports the notion that GLP-1 may subtend physiological functions over and above those associated with its role in pancreatic insulin release.
We observed that GLP-1 infusion did not increase myocardial AC activity in Control or DCM dogs in contrast to what has been reported in beta cells (3–5). Our findings are in keeping with recent observations in extra-pancreatic tissues where the actions of GLP-1 are unassociated with cAMP generation (8, 9). The difference in pancreatic and extra-pancreatic responses to GLP-1 may reflect differences in the expression of specific isoforms of AC or receptor coupling. Notably, AC type VIII predominates in the β-cell (24,25) whereas type V/VI is the predominant isoform in the heart (26). Notably, there may be important species differences in protein sequences in the third intracellular loop of the GLP-1 receptor that govern coupling to specific Gs or Gi proteins (27,28).
The significant improvement in LV function following 48 hour infusion of GLP-1 raises the possibility that these salutary improvements could be mediated through β-adrenergic mechanisms. There are several lines of evidence that argue against this possibility. First, the failure to increase cAMP argues against a conventional β1-adrenergic mechanism. Secondly, GLP-1 infusion was associated with decreases in plasma NE indicating that GLP-1 does not act by increasing SNS activation (Table 2). Thirdly, we have shown previously that acute GLP-1 infusion at doses of 1–20 pmol/kg/min does not increase heart rate or LV dp/dt in conscious dogs under Control or following the development of DCM, in contrast to the response to dobutamine (14, see Supplemental Materials). Finally, we have also shown previously that there is a temporal dissociation between the GLP-1 mediated increases in myocardial glucose uptake that occur within 3–6 hours of GLP-1 infusion and GLP-1 associated increases in contractility which occur only after 12–24 hours of GLP-1 infusion (see Supplemental Materials). This temporal dissociation indicates a distinct process from that associated with β-adrenergic stimulation. Taken together, these data suggest that the hemodynamic improvements are not adrenergically mediated.
Alternative signaling mechanisms include the fact that GLP-1 has been shown to couple through its receptor on pancreatic β-cells via a Giα2, p38 MAP kinase-dependent mechanism (19). p38 MAP kinase activataon is a potent stimulus to NOS2 activity and has been implicated as a mechanism involved in both chronic myocardial hibernation and the late phase of ischemic pre-conditioning (29). Our laboratory has shown previously that GLP-1 enhanced myocardial recovery following both brief periods of coronary artery occlusion and reperfusion in both conscious dogs (30) and isolated rat hearts (15) as well as in dogs with DCM induced by rapid pacing (14). We have also demonstrated that rapid pacing induces a state of chronic myocardial stunning in conscious dogs (31). Our data suggest that this alternative pathway is activated in response to GLP-1 infusion in DCM.
Prior studies have demonstrated that GLP-1 inhibits gut motility in an NO-dependent fashion (32) and relaxes pulmonary (33) but not coronary vessels (34) in an NO-dependent fashion. NO has been established to play a critical role in contraction-dependent glucose uptake by stimulation of both GLUT-1 (35) and GLUT-4 translocation (36) as well as vascular effects in isolated mouse heart preparations (12). In our study, increased NOS2 expression and increased myocardial NO production was observed in association with increased p38 MAP kinase activity and sarcolemmal GLUT-1 expression following GLP-1 infusion. p38 MAP kinase activation has also been associated with GLUT-1 translocation (37,38). These events were in turn associated with increased myocardial glucose uptake, independent of increased plasma insulin levels or Akt-1 activation. We showed that both p38 MAP kinase inhibition and non-specific NOS inhibition independently blocked the GLP-1 induced increase in myocardial glucose uptake, implicating NO as a critical step in GLP-1 mediated glucose uptake in myocardium. However, we did not measure GLUT-1 expression following pharmacological blockade and therefore cannot conclude definitively that inhibition of p38 MAP kinase and NOS2 limits GLUT-1 expression as the mechanism of altering myocardial glucose uptake.
GLP-1 treatment in DCM was also associated with changes in mitochondrial protein expression and reduced oxidative stress as assessed by lipid peroxidation. Specifically, UCP-3 activity has been shown to reduce ROS production (39, 40) in mitochondria by contributing to decreased transmembrane potential (Δϕ). Increased oxidative stress has been associated with opening of the mitochondrial permeability transition leading to mitochondrial swelling and ultimately rupture and necrosis (39, 40). We have shown previously that DCM is associated with myocardial mitochondrial vacuolization and swelling consistent with MPT opening (23). The GLP-1 associated increase in UCP-3 is consistent with the observed reduction in oxidative stress. We did observe that GLP-1 treatment was associated with a trend toward increased expression of MnSOD suggesting that GLP-1 may affect other mitochondrial defense mechanisms against oxidative stress besides UCP-3. Further studies will be needed to understand the complete effects of GLP-1 on mitochondrial activity.
In conclusion, GLP-1 stimulates myocardial glucose uptake in conscious dogs with pacing-induced DCM through a p38α MAP kinase-mediated and NO-dependent mechanism. The effects are neither adenylyl cyclase nor Akt dependent in the heart of normal dogs or dogs with DCM. The availability of an alternative pathway to stimulate glucose uptake in the presence of insulin resistance holds therapeutic promise.
Clinical Perspective.
Glucagon-like peptide -1 is a naturally occurring incretin that has proven to be an effective treatment for type 2 diabetes. Our laboratory has shown that GLP-1 treatment improves LV and systemic hemodynamics in both conscious dogs with dilated cardiomyopathy and in humans with Class III/IV heart failure. While the mechanism whereby GLP-1 stimulates insulin release from the beta cells of the pancreas are now well established, the cellular pathways whereby GLP-1 acts in the myocardium are not fully understood. To that end, we studied a 48 hour continuous infusion (2.5 pmol/kg/min) of GLP-1(7–36) amide in conscious dogs with pacing induced cardiomyopathy and examined the cellular basis for its effects. GLP-1 receptors were present in the myocardium but did not couple through adenylyl cyclase or Akt-1 suggesting that the mechanism were distinct from those reported in the pancreas or in the hearts of rats and mice. We determined that GLP-1 activated the p38 MAP kinase pathway and induced NOS2 expression and increased sarcolemmal GLUT-1 expression, consistent with activation of pathways implicated in the late phase of ischemic preconditioning. Moreover, GLP-1 treatment was associated with increased complex IV and UCP-3 expression in mitochondria and reduced mitochondrial lipid peroxidation. The effects of GLP-1 on myocardial glucose uptake were blocked by inhibitors of p38 MAP kinase or NOS2. Thus, in conscious dogs with dilated cardiomyopathy, GLP-1 acts through a novel non-cAMP dependent, non-Akt dependent pathway that has been implicated in cardioprotection.
Supplementary Material
Acknowledgments
Sources of Funding:
This work has been supported in part by US Public Health Service Grants AG-023125
Footnotes
Disclosures:
None.
References
- 1.Drucker DJ. Development of glucagon-like peptide-1-based pharmaceuticals as therapeutic agents for the treatment of diabetes. Curr Pharm Des. 2001;7:1399. doi: 10.2174/1381612013397401. [DOI] [PubMed] [Google Scholar]
- 2.Perfetti R, Merkel P. Glucagon-like peptide-1: a major regulator of pancreatic beta-cell function. Eur J Endocrinol. 2000;143:717–25. doi: 10.1530/eje.0.1430717. [DOI] [PubMed] [Google Scholar]
- 3.Doyle ME, Egan JM. Glucagon-like peptide-1. Recent Prog Horm Res. 2001;56:377–99. doi: 10.1210/rp.56.1.377. [DOI] [PubMed] [Google Scholar]
- 4.Holst JJ, Gromada J. Role of incretin hormones in the regulation of insulin secretion in diabetic and nondiabetic humans. Am J Physiol Endocrinol Metab. 2004;287:E199–206. doi: 10.1152/ajpendo.00545.2003. [DOI] [PubMed] [Google Scholar]
- 5.MacDonald PE, El-Kholy W, Riedel MJ, Salapatek AM, Light PE, Wheeler MB. The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes. 2002;51:S434–42. doi: 10.2337/diabetes.51.2007.s434. [DOI] [PubMed] [Google Scholar]
- 6.Vila Petroff MG, Egan JM, Wang X, Sollott SJ. Glucagon-like peptide-1 increases cAMP but fails to augment contraction in adult rat cardiac myocytes. Circ Res. 2001;89:445–52. doi: 10.1161/hh1701.095716. [DOI] [PubMed] [Google Scholar]
- 7.Egan JM, Montrose-Rafizadeh C, Wang Y, Bernier M, Roth J. Glucagon-like peptide-1 (7–36) amide (GLP-1) enhances insulin-stimulated glucose metabolism in 3T3-L1 adipocytes: one of several potential extra-pancreatic sites of GLP-1 action. J Endocrinology. 1994;135:2070–5. doi: 10.1210/endo.135.5.7956929. [DOI] [PubMed] [Google Scholar]
- 8.Luque MA, Gonzalez N, Marquez L, Acitores A, Redondo A, Morales M, Valverde I, Villanueva-Penacarrillo ML. Glucagon-like peptide-1 (GLP-1) and glucose metabolism in human myocytes. J Endocrinol. 2002;173:465–73. doi: 10.1677/joe.0.1730465. [DOI] [PubMed] [Google Scholar]
- 9.Acitores A, Gonzalez N, Sancho V, Valverde I, Villanueva-Penacarrillo ML. Cell signaling of glucagon-like peptide-1 action in rat skeletal muscle. J Endocrinol. 2004;180:389–98. doi: 10.1677/joe.0.1800389. [DOI] [PubMed] [Google Scholar]
- 10.Bullock BP, Heller RS, Habener JF. Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide-1 receptor. Endocrinology. 1996;137:2968–78. doi: 10.1210/endo.137.7.8770921. [DOI] [PubMed] [Google Scholar]
- 11.Wei Y, Mojsov S. Distribution of GLP-1 and PACAP receptors in human tissue. Acta Physiol Scand. 1996;157:355–7. doi: 10.1046/j.1365-201X.1996.42256000.x. [DOI] [PubMed] [Google Scholar]
- 12.Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and Vasodilatory Actions of Glucagon-Like Peptide 1 Receptor Are Mediated Through Both Glucagon-Like Peptide 1 Receptor–Dependent and –Independent Pathways. Circulation. 2008;117:2340–50. doi: 10.1161/CIRCULATIONAHA.107.739938. [DOI] [PubMed] [Google Scholar]
- 13.Nikolaidis LA, Stuzu A, Stolarrki C, Alahi D, Shen YT, Shannon RP. The development of myocardial insulin resistance in conscious dogs with advanced dilated c!rdiomyopathy. Cardiovasc Res. 2004;61:297–306. doi: 10.1016/j.cardiores.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 14.Nikolaidis LA, Elahi D, Hentosz T, Doverspike A, Huerbin R, Zourelias L, Stolarski C, Shen YT, Shannon RP. Recombinant GLP-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation. 2004;110:955–961. doi: 10.1161/01.CIR.0000139339.85840.DD. [DOI] [PubMed] [Google Scholar]
- 15.Zhao T, Parikh P, Bhashyam S, Bolukoglu H, Poornima IG, Shen YT, Shannon RP. The Direct Effects of Glucagon-Like Peptide-1 (GLP-1) on Myocardial Contractility and Glucose Uptake in Normal and Post-Ischemic Isolated Rat Hearts. J Pharmacol Exp Ther. 2006;317:1106–13. doi: 10.1124/jpet.106.100982. [DOI] [PubMed] [Google Scholar]
- 16.Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes. 2005;54:146–151. doi: 10.2337/diabetes.54.1.146. [DOI] [PubMed] [Google Scholar]
- 17.Goke R, Just R, Lankat-Buttgereit B, Goke B. Glycosylation of the GLP-1 receptor is a prerequisite for regular receptor function. Peptides. 1994;15:675–81. doi: 10.1016/0196-9781(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 18.Widmann C, Dolci W, Thorens B. Internalization and homologous desensitization of the GLP-1 receptor depend on phosphorylation of the receptor carboxyl tail at the same three sites. Mol Endocrinol. 1997;11:1094–102. doi: 10.1210/mend.11.8.9959. [DOI] [PubMed] [Google Scholar]
- 19.Montrose-Rafizadeh C, Avdonin P, Garant MJ, Rodgers BD, Kole S, Yang H, Levine MA, Schwindinger W, Bernier M. Pancreatic glucagon-like peptide-1 receptor couples to multiple G proteins and activates mitogen-activated protein kinase pathways in Chinese hamster ovary cells. Endocrinology. 1999;140:1132–40. doi: 10.1210/endo.140.3.6550. [DOI] [PubMed] [Google Scholar]
- 20.Miles AM, Wink DA, Cook JC, Grisham MB. Determination of nitric oxide using fluorescence spectroscopy. Methods in Enzymology. 1996;268:105–20. doi: 10.1016/s0076-6879(96)68013-6. [DOI] [PubMed] [Google Scholar]
- 21.Mead F, Williams AJ. Block of the ryanodine receptor channel by neomycin is relieved at high holding potentials. Biophys J. 2002;82:1953–63. doi: 10.1016/S0006-3495(02)75544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiuchi K, Shannon RP, Komamura K, Cohen DJ, Bianchi C, Homcy CJ, Vatner SF, Vatner DE. Myocardial β-adrenergicreceptor function during the development of pacing induced heart failure. J Clin Invest. 1993;91:907–14. doi: 10.1172/JCI116312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhashyam S, Parikh P, Bolukoglu H, Shannon AH, Porter JH, Shen YT, Shannon RP. Aging is associated with myocardial insulin resistance and mitochondrial dysfunction. Am J Physiol Heart Circ Physiol. 2007;293:H3063–71. doi: 10.1152/ajpheart.00163.2007. [DOI] [PubMed] [Google Scholar]
- 24.Delmeire D, Flamez D, Hinke SA, Cali JJ, Pipeleers D, Schuit F. Type VIII adenylyl cyclase in rat beta cells: coincidence signal detector/generator for glucose and GLP-1. Diabetologia. 2003;46:1383–93. doi: 10.1007/s00125-003-1203-8. [DOI] [PubMed] [Google Scholar]
- 25.Leech CA, Castonguay MA, Habener JF. Expression of adenylyl cyclase subtypes in pancreatic beta cells. Biochem Biophys Res Commun. 1999;254:703–6. doi: 10.1006/bbrc.1998.9906. [DOI] [PubMed] [Google Scholar]
- 26.Ishikawa Y, Homcy CJ. The adenylyl cyclases as integrators of transmembrane signal transduction. Circ Res. 1997;80:297–304. doi: 10.1161/01.res.80.3.297. [DOI] [PubMed] [Google Scholar]
- 27.Bavec A, Hällbrink M, Langel U, Zorko M. Different role of intracellular loops of glucagon-like peptide-1 receptor in G-protein coupling. Regul Pept. 2003;111:137–44. doi: 10.1016/s0167-0115(02)00282-3. [DOI] [PubMed] [Google Scholar]
- 28.Heller RS, Kieffer TJ, Habener JF. Point mutations in the first and third intracellular loops of the glucagon-like peptide-1 receptor alter intracellular signaling. Biochem Biophys Res Commun. 1996;223:624–32. doi: 10.1006/bbrc.1996.0945. [DOI] [PubMed] [Google Scholar]
- 29.McFalls EO, Hou M, Bache RJ, Best A, Marx D, Sikora J, Ward HB. Activation of p38 MAPK and increased glucose transport in chronic hibernating swine myocardium. Am J Physiol Heart Circ Physiol. 2004;287:H1328–34. doi: 10.1152/ajpheart.01188.2003. [DOI] [PubMed] [Google Scholar]
- 30.Nikolaidis LA, Doverspike A, Hentosz T, Zourelias L, Shen YT, Elahi D, Shannon RP. Glucagon-like peptide-1 limits myocardial stunning following brief coronary occlusion and reperfusion in conscious canines. J Pharmacol Exp Ther. 2005;312:303–8. doi: 10.1124/jpet.104.073890. [DOI] [PubMed] [Google Scholar]
- 31.Nikolaidis LA, Hentosz T, Doverspike A, Huerbin R, Stolarski C, Shen YT, Shannon RP. Mechanisms whereby rapid RV pacing causes LV dysfunction: perfusion-contraction matching and NO. Am J Physiol. 2001;281:H2270–81. doi: 10.1152/ajpheart.2001.281.6.H2270. [DOI] [PubMed] [Google Scholar]
- 32.Tolessa T, Gutniak M, Holst JJ, Efendic S, Hellstrom PM. Inhibitory effect of glucagon like peptide-1 on small bowel motility. Fasting but not fed motility inhibited via nitric oxide independently of insulin and somatostain. J Clin Invest. 1998;102:764–74. doi: 10.1172/JCI942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Golpon HA, Puechner A, Welte T, Wichert PV, Feddersen CO. Vasorelaxant effect of glucagons like peptide (7–36) amide and amylin on the pulmonary circulation of the rat. Regul Pept. 2001;102:81–6. doi: 10.1016/s0167-0115(01)00300-7. [DOI] [PubMed] [Google Scholar]
- 34.Nystrom T, Gutniak M, Zhang Q, Holst JJ, Ahren B, Sjoholm A. Effects of glucagons like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol. 2004;287:E1209–15. doi: 10.1152/ajpendo.00237.2004. [DOI] [PubMed] [Google Scholar]
- 35.Van Dyke DA, Walters L, Frieswyk D, Kohmeyer D, Louters LL. Acute effects of troglitizone and nitric oxide on glucose uptake in L929 fibroblasts cells. Life Sci. 2003;72:2321–7. doi: 10.1016/s0024-3205(03)00119-x. [DOI] [PubMed] [Google Scholar]
- 36.Etgen GJ, Fryburg DA, Gibbs EM. Nitric oxide stimulates skeletal muscle glucose transport through a calcium/contraction and pjosphatidylinositol-3-kinase independent pathway. Diabetes. 1997;46:1915–9. doi: 10.2337/diab.46.11.1915. [DOI] [PubMed] [Google Scholar]
- 37.Denton RM, Tavare JM. Does mitogen activated protein kinase have a role in insulin action? The case for and against. Eur J Biochem. 1995;227:597–611. doi: 10.1111/j.1432-1033.1995.tb20179.x. [DOI] [PubMed] [Google Scholar]
- 38.Taha C, Tsakiridis T, McCall A, Klip A. Glucose transport expression in L6 muscle cells: regulation through insulin and stress activated pathways. Am J Physiol. 1997;273:E68–E76. doi: 10.1152/ajpendo.1997.273.1.E68. [DOI] [PubMed] [Google Scholar]
- 39.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murphy E, Steenbergen C. Preconditioning: the mitochondrial connection. Annu Rev Physiol. 2007;69:51–67. doi: 10.1146/annurev.physiol.69.031905.163645. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.