Abstract
Because of the lack of information about effective analgesics in non-mammalian vertebrates, the potency of various non-opioid agents were tested in a model of analgesia by using Northern grass frogs (Rana pipiens). This alternative model has been used widely for investigating opioid analgesic action. Potential non-opioid analgesics tested included antipsychotic, benzodiazepine, barbiturate, antihistamine, non-steroidal anti-inflammatory (NSAID), and partial opioid agents. Northern grass frogs were acclimated to lab conditions in individual cages. Drugs were administered systemically through the dorsal lymph sac, and analgesic effects were estimated by using the acetic acid test (AAT). The AAT is done by placing logarithmic dilutions of acid dropwise on the dorsum of the animal’s thigh until a wiping response is obtained. At various doses, chlorpromazine and haloperidol (antipsychotics), chlordiazepoxide (a benzodiazepine), buprenorphine (a partial opioid agonist), and diphenhydramine (a histamine antagonist) produced moderate to strong analgesic effects. Indomethacin and ketorolac (NSAIDs), butorphanol (a partial opioid agonist), and pentobarbital (a barbiturate) produced weaker but noticeable analgesic effects. Our results are the first to document the effectiveness of a wide array of pharmacologically active agents in a novel amphibian model for analgesia. These findings provide needed data regarding the use of alternative, non-opioid agents for the treatment of pain in amphibians and other poikilothermic species.
Practicing veterinarians and animal care personnel at research institutions increasingly are called upon to provide analgesia for herpetological species after surgical or experimental procedures. There is a growing literature on the relative potency and dosage of various full opioid agonists used to produce analgesia (1–6) and on the characterization of opioid receptors (7–13) in amphibians. However, there is little information regarding the potency and suitability of non-opioid analgesics in earlier-evolved vertebrates. Previous studies in amphibians demonstrated the analgesic efficacy of alpha2 adrenergic agonists, such as clonidine, dexmedotomidine, and xylazine (14–16). In addition, a single NSAID agent, flunixin meglumine, and the partial opioid agonist butorphanol were effective analgesics after intracelomic injection in Rana pipiens (16). In a model of anesthesia using adult bullfrogs (Rana catesbeiana), it was noted that responses to strong forceps pinching of the hindlimb remained intact in animals anesthetized with thiopental (17). Further information on non-opioid analgesic effects in amphibian species is not available
In mammals, the antipsychotic agents chlorpromazine and haloperidol produce dose-dependent analgesia that is not blocked by opioid antagonists (18–20). Using common algesiometric tests in rodents, the benzodiazepines chlordiazepoxide (21, 22) and midazolam (23) show dose-dependent analgesia. With regard to barbiturates, it is a common belief that these agents may be hyperalgesic however, recent studies show that select agents produce attenuation of the response to noxious stimuli (24, 25). There is good evidence that diphenhydramine, an H1 histamine antagonist, produces analgesia in mammals after systemic (26, 27) and central (28) administration. NSAID agents, such as indomethacin and ketorolac, show analgesic effects after peripheral and central administrations (29, 30). Finally, the partial opioid agonists butorphanol and buprenorphine show mild and moderate analgesic effects in rats and mice (31).
In this study, we assayed the analgesic effects of potential non-opioid analgesics including antipsychotic, benzodiazepine, barbiturate, antihistamine, and non-steroidal anti-inflammatory (NSAID) agents by using the acetic acid test in Rana pipiens. The partial opioid agonists butorphanol and buprenorphine also were tested, and a high dose of the full opioid agonist morphine was included for comparison. The agents tested were chosen for wide availability in animal care facilities and for the lack of Schedule II controlled-substances licensing. Key drugs of the described classes of agents were tested for the effective analgesic dosage range, with an emphasis on a wide variety of agents rather than a complete characterization of individual classes or agents. Although obtaining lethality and toxicity data were not a goal of the study, animals were assessed for survivability after administration of the highest doses of the tested agents.
These studies further validate an alternative model for pain research that is based on the comparative neurology of pain and a phylogenetic perspective (32, 33). The present results are useful for the clinical care of amphibian species and in experimental protocols in which postoperative analgesics may be warranted.
Materials and Methods
Animals
Northern grass frogs (Rana pipiens-, snout-vent length, 2.5 to 3.0 in.; average body weight, 26 g) of both sexes were obtained from a commercial source (Sullivans, Nashville, Tenn.). Frogs were kept in groups of 48 in free-flowing tap water and were fed live crickets 3 to 4 times weekly. At least 2 days before the start of an experiment, animals were assigned randomly to individual cages (standard mouse cages with wood-screen tops) in the lab for acclimatization. The Institutional Animal Care and Use Committee at Oklahoma State University—College of Osteopathic Medicine (Tulsa, Okla.) approved the animal protocols used in this study.
Drug preparation and administration
Drugs were obtained from commercial sources as follows: buprenorphine hydrochloride, butorphanol tartrate, chlordiazepoxide hydrochloride, chlorpromazine hydrochloride, diphenhydramine hydrochloride, haloperidol base, indomethacin base, morphine sulfate, and pentobarbital sodium salt were all purchased from Sigma-Aldrich (St. Louis, Mo.); and ketorolac was kindly provided by Dr. Peggy Borgese (Roche Laboratories, Nutley, N J.). Drugs were mixed in sterile saline (0.9%) to yield nmol/g doses of the active base and administered in a volume of 10 μl/g body weight. All injections were made subcutaneously into the dorsal lymph sac by using a 1-cc tuberculin syringe (29-gauge needle). Each animal received only one drug or a saline control injection, and animals were euthanized by an overdose of tricaine methylsulfonate (MS-222, Sigma-Aldrich) at the end of the study.
Algesiometric measurement
The nociceptive, or pain, threshold in amphibians can be estimated by using the acetic acid test (34). Briefly, 11 serial dilutions of glacial acetic acid are made, with concentration “0” being the weakest and concentration “10” being glacial. Starting with the lowest concentration, a single drop is placed on the dorsum of the frog’s thigh, and the animal is observed for a wiping response with either hindlimb. If a response does not occur within 5 sec, the drop is rinsed thoroughly with distilled water, and testing continues on the opposite thigh with the next higher concentration of acid. The nociceptive threshold (NT) is recorded as the raw code number of the concentration that elicits a wiping response.
Data collection and analysis
Treatment and saline-control groups consisted of 4 to 6 animals each. For agonist effects, the NT was determined before the administration of the test agent (baseline NT) and at 1, 3, and 5 h after administration. Each individual animal’s NT was transformed into the maximum percentage effect (MPE) at each time point by using the following formula:
MPE data were plotted for dose groups as a time course after administration, and the maximum MPE values over that time course were pooled from individual animals with the same treatment for the construction of the peak effect graph. Computer software (Pharm/PCS, Microcomputer Specialists, Philadelphia, Pa.) was used to test differences between treatment groups and control animals by using Student’s t-test. The level of significance was set at P< 0.05.
Behavioral assessment
As previously discussed, studies of analgesic activity using behavioral models relying on a motor response must incorporate additional precautions to ensure that the observed analgesia is not confounded by underlying general sedation or motor dysfunction (35). Therefore, in animals showing significant analgesic effects, general behavior was observed to assess overt sedation. The following reflexes were assessed after the 5-h time course in these animals to determine the motor effects (if any) of tested agents: corneal reflex (light touch of corneal surface normally elicits blinking response), righting reflex (untreated animals respond to placement on back with rapid turnover), and the hindlimb withdrawal reflex (normal animals rapidly withdraw hindlimb after a sharp pinch to the webbing of the foot).
Results
None of the agents tested produced greater than an 80% analgesic effect at doses that were not lethal within 24 h (Tables 1 and 2). All of the agents administered at the doses listed in Table 1, which includes the pharmacological classes of these compounds, did not show any untoward behavioral effects such as overt sedation or motor dysfunction as tested by using the righting, hindlimb withdrawal, and corneal reflexes. Agents that produced significant analgesia compared to saline and greater than 40% analgesic effects in the acetic acid test were chlorpromazine (30 nmol/g; P= ), chlordiazepoxide (300 nmol/g; P= ), buprenorphine (30 nmol/g; P= ), diphenhydramine (200 nmol/g; P= ), and haloperidol (30 nmol/g; P = ). [Please provide exact P values as indicated.] Morphine administered at the high dose of 300 nmol/g (114 mg/kg) produced the maximum analgesic effect (100% MPE) in all animals.
Table 1.
Agent, class, and analgesic effects of drugs tested in the amphibian acetic acid test
| Agent | Class | Tested dosea | Equivalent mg/kg | Maximum percent effectb |
|---|---|---|---|---|
| Morphine | Opioid analgesic | 300 | 114 | 100 |
| Chlorpromazine | Antipsychotic | 100 | 32 | 62.9 |
| Chlordiazepoxide | Benzodiazepine | 300 | 90 | 51.3 |
| Buprenorphine | Partial opioid agonist | 30 | 14 | 48.6 |
| Chlorpromazine | Antipsychotic | 30 | 10 | 47.8 |
| Diphenhydramine | H1 antagonist | 200 | 51 | 43.2 |
| Haloperidol | Antipsychotic | 30 | 11 | 41.1 |
| Indomethacin | NSAID | 300 | 107 | 39.6 |
| Ketorolac | NSAID | 100 | 26 | 39.1 |
| Chlordiazepoxide | Benzodiazepine | 100 | 30 | 37.5 |
| Butorphanol | Partial opioid agonist | 100 | 33 | 36.9 |
| Indomethacin | NSAID | 1000 | 358 | 29.2 |
| Pentobarbital | Barbiturate | 30 | 8 | 27.7 |
| Butorphanol | Partial opioid agonist | 30 | 10 | 25.7 |
| Haloperidol | Antipsychotic | 10 | 4 | 25.7 |
| Pentobarbital | Barbiturate | 10 | 3 | 22.9 |
| Saline control | Vehicle | 10 | not done | 11.4 |
NSAID, non-steroidal anti-inflammatory drug
nmol/mg except for the saline control, which is μl/mg [please confirm Greek symbol].
Average maximum percent effect of analgesia observed at that dose (n = 4 to 8). [The Methods say “4 to 6” in a group—please correct/clarify as needed]
Table 2.
Lethality data for the tested agents after subcutaneous administration in Rana pipiens
| Agent | Class | Tested dose (nmol/g) | Lethalitya |
|---|---|---|---|
| Buprenorphine | Partial opioid agonist | 100 | 3/4 |
| Butorphanol | Partial opioid agonist | 300 | 4/4 |
| Diphenhydramine | HI antagonist | 250 | 4/4 |
| Chlorpromazine | Antipsychotic | 1000 | 5/6 |
| Haloperidol | Antipsychotic | Not doneb | Not determinedb |
| Chlordiazepoxide | Benzodiazepine | 1000 | 3/6 |
| Pentobarbital | Barbiturate | 100 | 3/6 |
| Ketorolac | NSAID | 1000 | 1/4 |
| Indomethacin | NSAID | Not doneb | Not determinedb |
NSAID, non-steroidal anti-inflammatory’ drug
Number of animals dead within 24 h/total number of animals tested at that dose.
Higher doses not tested, because of insolubility of drug into vehicle at the higher concentration.
Less robust but still significant analgesic effects were observed with indomethacin (300 nmol/g; P= ), ketorolac (100 nmol/g; P = ), chlordiazepoxide (100 nmol/g; P = ), butorphanol (100 nmol/g; P= ), indomethacin (1000 nmol/g; P= ), pentobarbital (30 nmol/g; P= ), butorphanol (30 nmol/g; P= ), haloperidol (10 nmol/g; P=), and pentobarbital (10 nmol/g; P= ). [Please provide exact P values as indicated] Administration of saline to control animals produced a slight elevation of the nociceptive thresholds, resulting in an 11.4% MPE (Table 1).
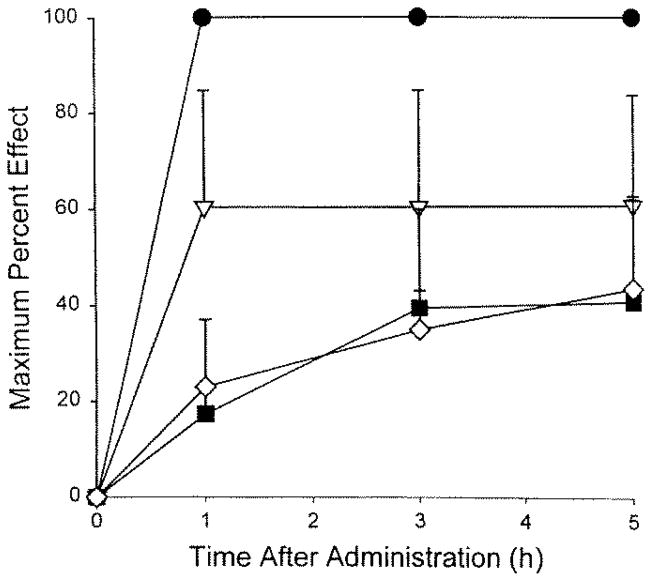
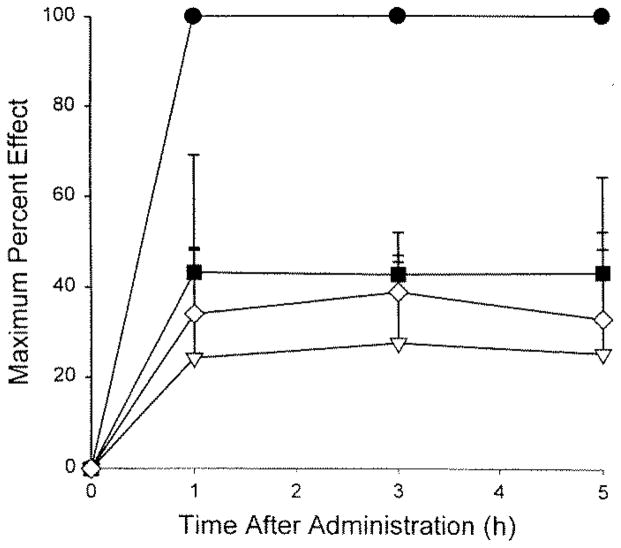
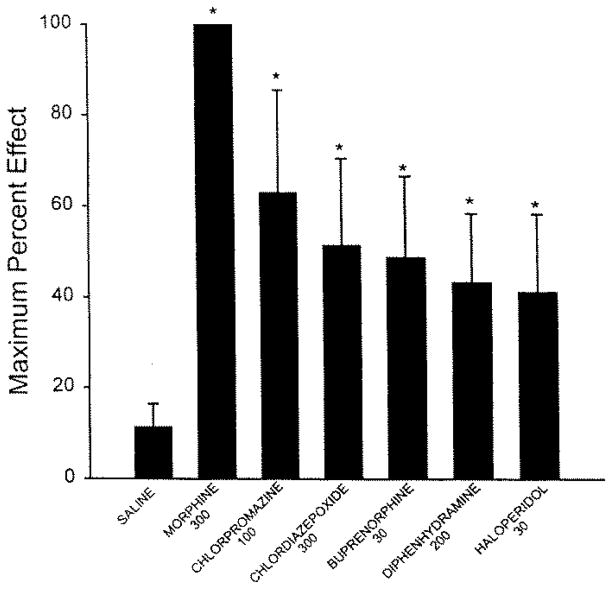
The time course of analgesic effects of the more potent agents over the 5-h post-treatment assessment was unremarkable with respect to levels of analgesia obtained at the first post-treatment test time (1 h) and the last (5 h; Figs. 1 and 2). Significant analgesic effects were evident by 1 h after administration and remained elevated throughout the 5-h time course. Peak analgesic effects for the highest doses of the more potent agents showed a rank order of morphine > chlorpromazine > chlordiazepoxide > buprenorphine > diphenhydramine > haloperidol (Fig. 3).
Figure 1.
Time course of the analgesic effects of morphine, chlorpromazine, chlordiazepoxide, and buprenorphine after systemic administration in amphibians (n = 4 to 6 per group).○, morphine (300 nmol/g); ▽, chlorpromazine (100 nmol/g);■, chlordiazepoxide (300 nmol/g); ⋇, buprenorphine (30 nmol/g). [“Key” deleted from illustration] Saline-injected control group did not give a significant effect and is omitted for clarity.
Figure 2.
Time course of the analgesic effects of morphine, chlorpromazine, diphenhydramine, and buprenorphine following systemic administration in amphibians (n = 4 to 6 animals per group). ●, morphine (300 nmol/g); ▽, chlorpromazine (30 nmol/g); ■, diphenhydramine (200 nmol/g); ⋄, haloperidol (30 nmol/g). [“Key” deleted from illustration] Saline-injected control group did not give a significant effect and is omitted for clarity.
Figure 3.
Mean peak analgesic effect [Is rewording accurate?] of morphine, chlorpromazine, chlordiazepoxide, and buprenorphine, diphenhydramine, and buprenorphine after systemic administration in amphibians (n = 4 to 6 per group). Saline-injected control group is included for comparison. *, P< 0.05 (Student’s t test) versus saline control group. [Please add “nmol/g” to doses given on s-axis of figure. Please “define” bars—1 standard deviation?]
Although the safety index of tested agents was not determined, doses that produced lethality 24 h after administration of test agents are noted in Table 2. At these doses, animals could not be tested because of loss of righting, hindlimb withdrawal, and/or corneal reflexes.
Discussion
The systemic administration of various doses of non-opioid analgesic agents produced mild to moderate analgesic effects in amphibians. The present results were garnered in the context of an experimental model of analgesic efficacy by using a behavioral test in amphibians. The acetic acid test in the frog Rana pipiens has been shown to activate small-diameter nociceptive afferents, and the wiping response used as an endpoint is specific to the applied noxious stimulus (32). All the doses reported as analgesic in this study were without untoward adverse effects, and animals responded normally to testing of the righting, hindlimb withdrawal, and corneal reflexes. Although the study was not designed to document the toxicity and lethality of these agents in amphibians, the results shown in Table 2 suggest that, for many agents, there is a narrow range of therapeutic doses.
The antipsychotic agents chlorpromazine and haloperidol produced moderate analgesic effects that, were without untoward side effects at the doses used in the acetic acid test. The older phenothiazine agent, chlorpromazine, has mixed antagonist activities at dopamine, cholinergic, and adrenergic receptors, whereas the newer agent, haloperidol, is more selective for dopamine receptors. The fact that both agents induced an elevation of the nociceptive threshold suggests that dopaminergic systems are involved in nociceptive modulation in amphibians. The benzodiazepine chlordiazepoxide and the barbiturate pentobarbital produced mild to moderate analgesic effects. These agents work by interaction at ā-aminobutyric acid (GABA) receptors, enhancing the efficacy of the inhibitory GABA neurotransmitter at its receptor. Agents enhancing GABA activity are shown to be effective analgesics in mammals (36). In contrast to the present results, a recent study using anesthetic doses of thiopental administered to bullfrogs reported that a strong forceps pinch to the hindlimb elicited a withdrawal response, even though these animals were unable to complete a righting reflex (17). It is likely that differences in the intensity of the noxious stimuli used in the present and former study (weak, cutaneous stimulus versus a strong, deeply activating forceps pinch) could account for this apparent discrepancy.
The histamine (H1) antagonist diphenhydramine produced moderate analgesia, albeit at the fairly high dose of 200 nmol/g. Diphenhydramine is classified as a histamine antagonist, although at high doses this agent also is known to block muscarinic cholinergic receptors. After systemic administration in amphibians, diphenhydramine produced greater than 40% analgesia. The NSAID agents indomethacin and ketorolac produced mild analgesic effects after systemic administration in amphibians. Interestingly, in common rodent measures of analgesia (such as the hot-plate and tail-flick tests), analgesia after systemic NSAIDs is not seen (37). The mammalian studies cited in the Introduction in which NSAID analgesic effects were reported used either an inflammatory pain stimulus, such as the injection of formalin into the rodent hindpaw, or visceral noxious chemical stimulus. This difference suggests that the amphibian model may be an alternative screening model for NSAID analgesia, as the acetic acid test uses an acute and self-limiting weak noxious stimulus. The analgesic effect seen here after NSAID administration confirms the results of an earlier study that used a similar model of nociceptive measurement in Rana pipiens after flunixin meglumine administration (16). Results after the administration of the partial opioid agonists buprenorphine and butorphanol also confirm the findings of an earlier report of butorphanol analgesia that used the same model (16). In this study, the authors also report that xylazine hydrochloride, an alpha,, adrenergic agonist, provided good analgesia that lasted longer than the effects from butorphanol or flunixin (16).
Regarding comparing results from similar studies in mammalian species, the present findings in amphibians suggest that there is a commonality of nociceptive processing in these two classes of vertebrates. Although there is a dearth of knowledge on nociceptive pathways and endogenous analgesic systems in non-mammalian vertebrates, amphibian nervous tissue is replete with endogenous opioids (38, 39, 9), substance P (40–42), serotonin (43–45), calcitonin-gene related peptide (46, 47), and other substances shown to modulate nociceptive processing (4). With regard to receptor sites mediating the analgesic effects of putative analgesic agents, there is much less information. Specific receptors for opioids (11–13), GABA (48, 49), and benzodiazepines (50) have been characterized in amphibian nervous system tissues. Clearly there is a need for further neuropharmacological studies in non-mammalian vertebrates.
Our results suggest that careful clinical use of non-opioid analgesics and partial opioid agonists may be warranted in amphibians and other herpetological species. Previous work demonstrated that clinically used opioids, such as morphine, meperidine, and fentanyl, are potent and safe analgesics in amphibians (5, 51). However, full opioid agonists are controlled substances (Schedule II) that require special licensing and reporting requirements as well as tight security of drug holdings. The non-opioid agents tested in this study may be more accessible than opioids when mild to moderate analgesia is needed. In this regard, the partial opioid agonists can be used judiciously, as butorphanol is a nonscheduled agent, and buprenorphine is a Schedule V controlled substance.
Importantly, our results provide guidance for the clinician who needs to provide analgesic treatment in amphibians and possibly other earlier-evolved vertebrate species. The actual doses used in the present amphibian model may not be appropriate in other amphibian or herpetological species. In addition, the present data on the use of non-opioid analgesics in amphibians come from an experimental pain model. The acetic acid test used here is an experimental model to determine analgesic effects in amphibians and is based on a weak noxious stimulus that is applied cutaneously. Pain arising from surgical procedures or visceral structures or that is neuropathic in origin may not respond to the same analgesic agents or dosages shown in the present study.
Specific recommendations for analgesic agents and doses in amphibians should be extrapolated cautiously from the present results. Comparison of the lethality data, although limited, suggests that the safety index for these agents is quite narrow. Finally, regarding alternatives to pharmacological interventions for producing analgesia in amphibians, a recent study examined the effect of hypothermia on nociceptive thresholds in Rana pipiens (52). Poikilothermic animals such as amphibians may be ideally suited to use such non-pharmacological means of analgesic treatment.
Acknowledgments
Research support was generously provided by the American College of Laboratory Animal Medicine (ACLAM) Foundation Research Grant. We thank Dr. Marty Morin for his encouragement and Dr. Sally Wixson and the list members of COMPMED for suggestions on the selection of test agents. Don Maclver was supported by a summer research fellowship from the Auxiliary to the Oklahoma Osteopathic Association.
References
- 1.Stevens CW, Pezalla PD. A spinal site mediates opiate analgesia in frogs. Life Sci. 1983;53:2097–2103. doi: 10.1016/0024-3205(83)90333-8. [DOI] [PubMed] [Google Scholar]
- 2.Stevens CW, Pezalla PD. Naloxone blocks the analgesic action of levorphanol but not of dextrorphan in the leopard frog. Brain Res. 1984;301:171–174. doi: 10.1016/0006-8993(84)90418-9. [DOI] [PubMed] [Google Scholar]
- 3.Pezalla PD, Stevens CW. Behavioral effects of morphine, levorphanol, dextrorphan, and naloxone in the frog Rana pipiens. Pharm Biochem Behav. 1984;21:213–217. doi: 10.1016/0091-3057(84)90217-x. [DOI] [PubMed] [Google Scholar]
- 4.Stevens CW. Opioid antinociception in amphibians. Brain Res Bull. 1988;21:959–962. doi: 10.1016/0361-9230(88)90034-2. [DOI] [PubMed] [Google Scholar]
- 5.Stevens CW, Klopp AJ, Facello JA. Analgesic potency of mu and kappa opioids after systemic administration in amphibians. J Pharmacol Exp Ther. 1994;269:1086–1093. [PubMed] [Google Scholar]
- 6.Stevens CW, Rothe-Skinner K. Supraspinal administration of opioids with selectivity for μ-, δ-, and κ-opioid receptors produces analgesia in amphibians. Eur J Pharmacol. 1997;331:15–21. doi: 10.1016/s0014-2999(97)01026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevens CW, Kirkendall K. Time course and magnitude of tolerance to the analgesic effects of systemic morphine in amphibians. Life Sci. 1993;52:PL111–116. doi: 10.1016/0024-3205(93)90097-m. [DOI] [PubMed] [Google Scholar]
- 8.Stevens CW, Pezalla PD, Yaksh TL. Spinal antinociceptive action of three representative opioid peptides in frogs. Brain Res. 1987;402:201–203. doi: 10.1016/0006-8993(87)91069-9. [DOI] [PubMed] [Google Scholar]
- 9.Stevens CW, Sangha S, Ogg BG. Analgesia produced by immobilization stress and an enkephalinase-inhibitor in amphibians. Pharmacol Biochem Behav. 1995;51:675–680. doi: 10.1016/0091-3057(94)00436-m. [DOI] [PubMed] [Google Scholar]
- 10.Stevens CW, Newman LC. Spinal administration of selective opioid antagonists in amphibians: evidence for an opioid unireceptor. Life Sci. 1999;64:PL125–PL130. doi: 10.1016/s0024-3205(99)00013-2. [DOI] [PubMed] [Google Scholar]
- 11.Newman LC, Wallace DR, Stevens CW. Characterization of 3H-diprenorphine binding in Rana pipiens-. observations of filter binding enhanced by naltrexone. J Pharmacol Toxicol Methods. 1999;41:43–48. doi: 10.1016/s1056-8719(99)00020-9. [DOI] [PubMed] [Google Scholar]
- 12.Newman LC, Wallace DR, Stevens CW. Selective opioid agonist and antagonist displacement of [3H]-naloxone binding in amphibian brain. Eur j Pharmacol. 2000;397:255–262. doi: 10.1016/s0014-2999(00)00265-x. [DOI] [PubMed] [Google Scholar]
- 13.Newman LC, Wallace DR, Stevens CW. Selective opioid receptor agonist and antagonist displacement of [3H]-naloxone binding in amphibian spinal cord. Brain Res. 2000;884:184–191. doi: 10.1016/s0006-8993(00)02967-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brenner GM, Klopp AJ, Deason LL, et al. Analgesic potency of alpha adrenergic agents after systemic administration in amphibians. j Pharmacol Exp Ther. 1994;270:540–545. [PubMed] [Google Scholar]
- 15.Stevens CW, Brenner GM. Spinal administration of adrenergic agents produces analgesia in amphibians. Eur J Pharmacol. 1996;316:205–210. doi: 10.1016/s0014-2999(96)00681-4. [DOI] [PubMed] [Google Scholar]
- 16.Terril-Robb L, Suckow M, Grigdesby C. Evaluation of the analgesic effects of butorphanol tartarate, xylazine hydrochloride, and flunixin meglumine in leopard frogs (Rana pipiens). Contemp Top Lab Anim Sci. 1996;35:54–56. [Google Scholar]
- 17.Downes H, Koop BR, Klopfenstein B, et al. Retention of nociceptor responses during deep barbituate anesthesia in frogs. Comp Biochem Physiol. 1999;124:203–210. doi: 10.1016/s0742-8413(99)00069-9. [DOI] [PubMed] [Google Scholar]
- 18.Otsuki T, Agatsuma Y, Jokura H, et al. Monosodium urate test: a new analgesic test by crystal-induced monoarthritis in rats. J Neurosci Methods. 1990;33:229–231. doi: 10.1016/0165-0270(90)90026-c. [DOI] [PubMed] [Google Scholar]
- 19.Sawynok J. GABAergic mechanisms of analgesia: an update. Pharmacol Biochem Behav. 1987;26:463–474. doi: 10.1016/0091-3057(87)90148-1. [DOI] [PubMed] [Google Scholar]
- 20.Bodnar RJ, Nicotera N. Neuroleptic and analgesic interactions upon pain and activity measures. Pharmacol Biochem Behav. 1982;16:411–416. doi: 10.1016/0091-3057(82)90444-0. [DOI] [PubMed] [Google Scholar]
- 21.Boulter N, Serrao JM, Gent J, et al. Spinally mediated and nociception following intrathecal chlordiazepoxide—further evidence for a benzodiazepine spinal analgesic effect. Eur J Anaesthesiol. 1991;8:407–411. [PubMed] [Google Scholar]
- 22.Kunchandy J, Kulkarni SK. Naloxone-sensitive and GABAA receptor mediated analgesic response of benzodiazepines in mice. Meth. Find. [This seems odd—is it correct?] Exp Clin Pharmacol. 1987;9:95–99. [PubMed] [Google Scholar]
- 23.Goodchild CS, Serrao jM. Intrathecal midazolam in the rat: evidence for spinally-mediated analgesia. Brit j Anaesth. 1987;59:1563–1570. doi: 10.1093/bja/59.12.1563. [DOI] [PubMed] [Google Scholar]
- 24.Kitahata LM, Saberski L. Are barbiturates hyperalgesic? Anesthesiol. 1992;77:1059–1061. doi: 10.1097/00000542-199212000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Carter RB. Differentiating analgesic and non-analgesic drug activities on rat hot plate: effect of behavioral endpoint. Pain. 1991;47:211–220. doi: 10.1016/0304-3959(91)90207-E. [DOI] [PubMed] [Google Scholar]
- 26.Ghelardini C, Galeotti N, Bartolini A. No development of tolerance to analgesia by repeated administration of HI antagonists. Life Sci. 1998;63:317–322. doi: 10.1016/s0024-3205(98)00483-4. [DOI] [PubMed] [Google Scholar]
- 27.Leza J, Lizasoain I, Lorenzo P. H1-and H2-histamine receptor blockers and opiate analgesia in mice. Meth. Find. Exp Clin Pharmacol. 1990;12:671–678. [PubMed] [Google Scholar]
- 28.Chung YH, Miyake H, Kamei C, et al. Analgesic effect of histamine induced by intracerebral injection into mice. Agents Actions. 1984;15:137–142. doi: 10.1007/BF01972339. [DOI] [PubMed] [Google Scholar]
- 29.Herrero JF, Parrado A, Cervero F. Central and peripheral actions of the NSAID ketoprofen on spinal cord nociceptive reflexes. Neuropharmacology. 1997;36:1425–1431. doi: 10.1016/s0028-3908(97)00120-2. [DOI] [PubMed] [Google Scholar]
- 30.Malmberg AB, Yaksh TL. Antinociceptive actions of spinal nonsteroidal anti-inflammatory agents on the formalin test in the rat. J Pharmacol Exp Ther. 1992;263:136–146. [PubMed] [Google Scholar]
- 31.Gades N, Danneman PJ, Wixson S, et al. The magnitude and duration of the analgesic effect of morphine, butorphanol, and buprenorphine in rats and mice. Contemp Top Lab Anim Sci. 2000;39:8–13. [PubMed] [Google Scholar]
- 32.Stevens CW. Alternatives to the use of mammals for pain research. Life Sci. 1992;50:901–912. doi: 10.1016/0024-3205(92)90167-n. [DOI] [PubMed] [Google Scholar]
- 33.Stevens CW. An amphibian model for pain research. Lab Anim. 1995;24:32–36. [Google Scholar]
- 34.Pezalla PD. Morphine-induced analgesia and explosive motor behavior in an amphibian. Brain Res. 1983;273:297–305. doi: 10.1016/0006-8993(83)90854-5. [DOI] [PubMed] [Google Scholar]
- 35.Stevens CW, Yaksh TL. Dynorphin A and related peptides administered intrathecally in the rat: a search for putative kappa opiate receptor activity. j Pharmacol Exp Ther. 1986;238:833–838. [PubMed] [Google Scholar]
- 36.Aran S, Hammond DL. Antagonism of baclofen-induced antinociception by intrathecal administration of phaclofen or 2-hydroxy-saclofen but not delta-aminovaleric acid in the rat. j Pharmacol Exp Ther. 1991;257:360–368. [PubMed] [Google Scholar]
- 37.Antognini JF. Intrathecal acetyl salicylic acid and indomethacin are not analgesic for a subramaximal stimulus. Anesth Analges. 1993;76:1079–1082. doi: 10.1213/00000539-199305000-00029. [DOI] [PubMed] [Google Scholar]
- 38.Cone RI, Goldstein A. A dynorphin-like opioid in the central nervous system of an amphibian. Proc Nat Acad Sci USA. 1982;79:3345–3349. doi: 10.1073/pnas.79.10.3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolters JG, Ten Donkelaar HJ, Verhofstad AAJ. Distribution of some peptides (substance P, [Leu]enkephalin, [Met]enkephalin) in the brain stem and spinal cord of a lizard, Varanus exantkematims. Neuroscience. 1986;18:917–946. doi: 10.1016/0306-4522(86)90109-0. [DOI] [PubMed] [Google Scholar]
- 40.Lorez HP, Kemali M. Substance P, met-enkephalin and somatostatin-like immunoreactivity distribution in the frog spinal cord. Neurosci Lett. 1981;26:119–124. doi: 10.1016/0304-3940(81)90336-0. [DOI] [PubMed] [Google Scholar]
- 41.Inagaki S, Senba E, Shiosaka S, et al. Regional distribution of substance P-like immunoreactivity in the frog brain and spinal cord: immunohistochemical analysis. J Comp Neurol. 1981;201:243–254. doi: 10.1002/cne.902010208. [DOI] [PubMed] [Google Scholar]
- 42.Adli DS, Rosenthal BM, Yuen GL, et al. Immunohistochemical localization of substance P, somatostatin, enkephalin, and serotonin in the spinal cord of the Northern leopard frog, Rana pipiens. J Comp Neurol. 1988;275:106–116. doi: 10.1002/cne.902750109. [DOI] [PubMed] [Google Scholar]
- 43.Mensah PL, Glanzman DL, Levy WB, et al. The effects of 5,6-dihydroxytryptamine in the amphibian spinal cord using silver staining techniques. Brain Res. 1974;78:255–261. doi: 10.1016/0006-8993(74)90550-2. [DOI] [PubMed] [Google Scholar]
- 44.Tan H, Miletic V. Electrophysiological properties of frog spinal dorsal horn neurons and their responses to serotonin: an intracellular study in the isolated hemisected spinal cord. Brain Res. 1990;528:344–348. doi: 10.1016/0006-8993(90)91680-f. [DOI] [PubMed] [Google Scholar]
- 45.Tan H, Miletic V. Bulbospinal serotoninergic pathways in the frog Rana pipiens. J Comp Neurol. 1990;292:291–302. doi: 10.1002/cne.902920211. [DOI] [PubMed] [Google Scholar]
- 46.Yui R. Immunohistochemical studies on peptide neurons in the hypothalamus of the bullfrog Rana calesbeiana. Gen Comp Endocrinol. 1982;49:195–209. doi: 10.1016/0016-6480(83)90136-3. [DOI] [PubMed] [Google Scholar]
- 47.Gibson SJ, Polak JM, Bloom SR, et al. Calcitonin generelated peptide immunoreactivity in the spinal cord of man and of eight other species. j Neurosci. 1984;4:3101–3111. doi: 10.1523/JNEUROSCI.04-12-03101.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ono H-, Akahane K, Fukuda H, et al. Supersensitivity of the GABA receptor in the frog spinal cord, as induced by kainic acid. Comp Biochem Physiol. 1983;76C:231–236. doi: 10.1016/0742-8413(83)90070-1. [DOI] [PubMed] [Google Scholar]
- 49.Nistri A, Berti C. GABA-induced depolarizing responses of the frog spinal cord can be either enhanced or antagonized by the benzodiazepine midazolam and the methyixanthine caffeine. Neurosci Lett. 1984;47:277–281. doi: 10.1016/0304-3940(84)90526-3. [DOI] [PubMed] [Google Scholar]
- 50.Tavolaro R, Canonaco M, Franzoni MF. A quantitative autoradiographic study of GABAA and benzodiazepine receptors in the brain of the frog Rana esculenta. Brain Behav Evol. 1993;42:171–177. doi: 10.1159/000114150. [DOI] [PubMed] [Google Scholar]
- 51.Stevens CW. Relative analgesic potency of mu, delta, and kappa opioids after spinal administration in amphibians. J Pharmacol Exp Ther. 1996;276:440–448. [PubMed] [Google Scholar]
- 52.Suckow M, Terril L, Grigdesby C, et al. Evaluation of hypothermia-induced analgesia and influence of opioid antagonists in leopard frogs (Rana fripiens). Pharmacol Biochem Behav. 1999;63:39–43. doi: 10.1016/s0091-3057(98)00237-8. [DOI] [PubMed] [Google Scholar]