Abstract
Migration of human dermal fibroblasts (HDFs) is critical for skin wound healing. The mechanism remains unclear. We report here that platelet-derived growth factor-BB (PDGF-BB) is the major promotility factor in human serum for HDF motility on type I collagen. PDGF-BB recapitulates the full promotility activity of human serum and anti-PDGF neutralizing antibodies completely block it. Although collagen matrix initiates HDF migration without growth factors, PDGF-BB–stimulated migration depends upon attachment of the cells to a collagen matrix. The PDGF-BB's role is to provide directionality and further enhancement for the collagen-initiated HDF motility. To study the collagen and PDGF-BB “dual signaling” in primary HDF, we establish “gene cassettes” plus lentiviral gene delivery approach, in which groups of genes are studied individually or in combination for their roles in HDF migration. Focal adhesion kinase, p21Rac,CDC42-activated kinase and Akt are grouped into an upstream kinase gene cassette, and the four major mitogen-activated protein kinases (extracellular signal-regulated kinase 1/2, p38, c-Jun NH2-terminal kinase, and extracellular signal-regulated kinase 5) are grouped into a downstream kinase gene cassette. The experiments demonstrate 1) the genes' individual roles and specificities, 2) their combined effects and sufficiency, and 3) the mechanisms of their intermolecular connections in HDF migration driven by collagen and PDGF-BB.
INTRODUCTION
Soluble growth factors and extracellular matrix (ECM) are the two key extracellular signals that directly influence the cell's decision to move or to stop (Lauffenburger and Horwitz, 1996). Directional cell migration toward a gradient of soluble growth factors is often referred as chemotaxis. Cell migration toward an ECM gradient in the absence of growth factors is also defined as haptotaxis (Carter, 1967). The transition from nonmotile to motile cells is often triggered by quantitative or qualitative alterations of ECMs and growth factors. In intact skin, for example, the epidermal and dermal cells are bathed in interstitial fluid, largely a filtrate of plasma. In an acute skin wound, however, the cells at the cut edge of the wound become in contact with serum for the first time. Both newly formed ECMs and newly generated serum factors are absent in the previously unwounded environment. These changes are likely responsible for the nonmotile to motile transition of the skin cells. Indeed, our recent study showed that human serum promotes human keratinocyte polarization and directional migration, whereas human plasma does not (Henry et al., 2003). This study suggests that new serum factors, which are absent in plasma, promote skin cell migration.
Human dermal fibroblasts (HDFs) play essential roles in cutaneous wound repair and remodeling. They proliferate to expand, migrate into the wound bed, synthesize new ECM, and express thick actin bundles as myofibroblasts (Singer and Clark, 1999). A number of growth factors/cytokines have been reported to affect, directly or indirectly, HDF motility. They include basic and acidic fibroblast growth factors, transforming growth factor-β 1 and β 2, and vascular endothelial growth factor and platelet-derived growth factor (PDGF) (reviewed by Singer and Clark, 1999; Imanishi et al., 2000). Among them, the function of PDGF-BB was best characterized (Deuel et al., 1991; Heldin and Westermark, 1999). Studies in ex vivo revealed that PDGF-BB is a mitogen and a motogen for HDF chemotaxis (Seppa et al., 1982). PDGF stimulates HDF production of matrix proteins, including fibronectin (Blatti et al., 1988), collagen (Lepisto et al., 1996), and hyaluronic acid (Heldin et al., 1989). PDGF also stimulates synthesis of collagenases in HDFs (Bauer et al., 1985). In vivo, direct application of PDGF-BB skin wounds induces increased formation of granulation tissue (Grotendorst et al., 1985; Sprugel et al., 1987) and increased wound-breaking strength, thereby increasing the rate of healing (reviewed by Pierce et al., 1994). Type I collagen, fibronectin, fibronogen, fibrin, and vitronectin are the reported ECMs that play important roles in various stages of wound healing. Among them, type I collagen is the most plentiful ECM in the dermis (Singer and Clark, 1999).
PDGF-BB elicits its biological responses via binding to its specific cell surface receptors, PDGFRs (in β β, β α, or α α dimers). PDGF binding activates the PDGFR' protein tyrosine kinase within seconds, leading to the receptor autophosphorylation and recruitments of a handful of downstream signaling molecules such as phospholypase Cγ, phosphatidylinisitol 3′-kinase, pp60src, SHC, and the Grb2-Sos complex, just to mention a few. These receptor-bound proteins in turn activate their immediate downstream targets, including protein kinase C, Akt, Rac, and Ras. Within minutes, the above-mentioned immediate early events are followed by peak activation of more downstream signaling molecules. Among them, the mitogen-activated protein kinases (MAPKs), including extracellular signal-regulated kinase (ERK) 1/2, c-Jun NH2-terminal kinase (JNK), p38 and ERK5, are best characterized. The activated MAPKs are believed to translocate to the nucleus and regulate expression of so-called early genes such as c-fos, c-myc, and c-jun (reviewed by Heldin and Westermark, 1999), which leads to waves of more gene expression and ultimately to a completion of a specific cellular response such as cell migration.
Integrins determine the interactions of cells with specific ECMs (Hynes, 1992). Integrins are bona fide signaling receptors that perform both so-called inside-out and outside-in signaling (Giancotti and Ruoslahti, 1999; Miranti and Brugge, 2002; Schwartz and Ginsberg, 2002). In the insideout signaling during cell adhesion, the cells rapidly change integrin function by altering the binding affinity of these integrin receptors for ligands. In the outside-in signaling during cell cycle progression, migration, and differentiation, the activated integrins engage in both parallel and vertical signaling events. In parallel signaling, integrins associate with and modulate the functions of other receptor tyrosine kinases (RTKs) at the cell surface. Integrins can even trigger activation of growth factor receptors independently of their ligand binding, suggesting that integrins and RTKs biochemically and functionally interact (reviewed by Eliceiri, 2001). In vertical signaling, integrins activate intracellular signaling molecules, such as focal adhesion kinase (FAK), pp60src, p130cas, and paxillin, and also orchestrate formation of the focal adhesions (Miranti and Brugge, 2002). Some of these molecules, such as FAK, can act as a liaison between the integrin and receptor tyrosine kinases (Sieg et al., 2000).
Despite previous studies, important questions remain unanswered. What is the specific role of a growth factor versus an ECM, when they are copresent, in the control of cell migration? Are their promotility effects simply additive or combined? How do intracellular signaling networks interpret the “dual signaling” of growth factor and ECM? In this study of HDF motility, we provide evidence that PDGF-BB is the main factor in human serum that stimulates HDF motility on type I collagen. We found, however, PDGF-BB does not have primary promotility effect, because it cannot initiate HDF motility without an ECM. Collagen matrix initiates HDF motility in the absence of any growth factors. The PDGF-BB's role is to provide directionality and enhancement to collagen-initiated HDF motility. Furthermore, to overcome two fundamental difficulties of studying the signaling mechanisms in primary human cells: 1) involvement of multiple parallel signaling pathways, and 2) demands for single and multiple gene delivery with high gene transduction efficiency, we established “Gene Cassette” approach to compare groups (“cassettes”) of related yet functionally independent genes for their roles in PDGF-BB and collagen signaling in the same experiments. Findings of this study may apply broadly to other promotility growth factors and ECMs.
MATERIALS AND METHODS
Primary HDFs were purchased from Cascade Biologics (Portland, OR) and cultured in DMEM supplemented with 10% fetal bovine serum (FBS), according to the manufacturer's instruction. HDFs at passages 4–5 were used throughout this study. Native rat-tail type I collagen (it works the same as the human's, in motility assays), human collagen IV, human fibronectin, and human vitronectin were from BD Biosciences (Bedford, MA). Human sera were either from Sigma-Aldrich (St. Louis, MO) or prepared from volunteer blood donors at University of Southern California. PDGF-BB, PDGF-AA, poly-l-lysine (P-1274), and colloidal gold (gold chloride, G4022) were purchased from Sigma-Aldrich. Epidermal growth factor (EGF) and transforming growth factor-á (TGFα) were from BD Biosciences. Vascular endothelial growth factor, basic and acidic fibroblast growth factor, insulin, insulin-like growth factor-1, nerve growth factor, and HGF were from Sigma-Aldrich. TGFβ 1, and interleukin (IL)-4 were from Leinco Technologies (St. Louis, MO). The wild-type (wt) and various mutant genes of human Pak1, mouse Akt1, mouse FAK, mouse p38α, human MEK1, human JNK and JNKK2, and human MEK5 were kindly provided by G. Bokoch, D. Schlaepfer, and J. Han (The Scripps Research Institute, La Jolla, CA), N. Hay (University of Illinois at Chicago, Chicago, IL), N. Ahn (University of Colorado, Boulder, CO), Denis Templeton (The Ohio Sate University, Columbus, OH), A. Lin (University of Chicago), and S. Gutkind (National Institutes of Health), respectively. Anti-PDGF-BB neutralizing antibody (P6101; Sigma-Aldrich), anti-Pak antibodies (sc-882, sc-1871, and sc-7117), anti-FAK antibody (sc-557), anti-p38 (α, β, γ, and δ) antibodies (sc-535, sc-6176, sc-6022, and sc-7585), anti-JNKK2 antibody (T-19), and anti-Mek5 antibody (sc-94) were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-Mek1 antibody (#9122) was from Cell Signaling Technology (Beverly, MA). Anti-ERK1/2 antibody (03-6600) was from Zymed Laboratories (South San Francisco, CA). Anti-phospho-ERK1/2 antibody (V803A) was from Promega (Madison, WI). Anti-phospho-p38 antibody (#9211), anti-Akt, anti-phospho-Akt antibodies (#9272 and #9271), anti-JNK and anti-phospho-JNK antibody kit (#9250) were from Cell Signaling Technology. All restriction enzymes, T4 DNA ligase, and calf intestine phosphatase were from New England BioLabs (Beverly, MA). SB202190, SB202474, U0126, and SP600125 were purchased from Calbiochem (San Diego, CA). Pertussis toxin (#180) was from the List Biological Laboratories (Campbell, CA). GM6001 and matrix metalloproteinase (MMP) Inhibitor III were from Calbiochem. Bovine TIMP-1 was purchased from Chemicon International (Temecula, CA). Thymidine-[methyl-3H] (#2406701) was from ICN Biomedicals (Costa Mesa, CA). Plasmid Midi kit (#12143) was from QIAGEN (Valencia CA). XL-10 Gold Ultra competent cells (XL-10 Gold) were from Stratagene (Kingsport, TN). All other reagents and supplies, unless indicated, were from VWR (Bristol, CT) or Sigma-Aldrich.
Colloidal Gold Cell Motility Assay and Computerassisted Data Analyses
Cell migration was examined by using the colloidal gold migration assay, as described by Albrecht-Buehler (1977) and modified for a computer-assisted analysis (Woodley et al., 1988). Briefly, coverslips (35 mm in diameter) were precoated with 1% freshly prepared bovine serum albumin (BSA) in phosphate-buffered saline (PBS), dried by air, and placed into 12-well tissue culture plates with one coverslip per well. Colloidal gold chloride solution (colloidal gold chloride suspension [6.85 mg/ml H2O:30% Na2CO3:H2O, 0.9 ml:3 ml:5.5 ml]) was heated in an 50-ml Erlenmeyer flask with constant swirling until boiling and then removed from the heat source. An equal volume of freshly prepared 0.1% formaldehyde solution was slowly added to the gold salt mixture with swirling. The mixture (turns to purple-brown) was immediately plated into the 12-well plates with the coverslips at 1 ml/well and left undisturbed for 2 h to let the colloidal gold particles settle on the coated BSA. After removal of the supernatant, the wells were gently rinsed once with 1 ml of Hanks' balanced salt solution (HBSS) without Ca2+ and then incubated with 1 ml of HBSS containing 5 mM Ca2+ and various concentrations of either native rat tail type I collagen or polylysine as the control matrix at 37°C for 2 h. Unattached collagens/polylysine were removed and the plates were rinsed once with HBSS without Ca2+. Uncoated areas of the colloidal gold were further blocked by incubating with 0.1% BSA to prevent possible additional peptide coating, such as by HDF-secreted ECMs. The latter procedure was meant to ensure HDF motility on the coated collagens. Serum-starved (DMEM with 0.2% FBS, overnight) HDFs were trypsinized, resuspended in the same media, and the cell numbers were counted. Three thousand cells/well were plated and allowed to migrate for different periods of time, up to 16 h. Cells were fixed in 0.1% formaldehyde in PBS for 10 min.
Migration in each well was visualized under a dark field microscope that is linked to a computer via a real-time charge-coupled device camera (KP-MIU; Hitachi Denshi, Woodberry, NY). Fifteen randomly selected and two to three single cell track-containing fields in each well were analyzed by the computer using NIH Image 1.6 program, which calculates migration index (MI). The MI represents the percentage of the field area consumed by cell migration tracks over the total field area viewed under the microscope. The images of migration tracks were photographed. For the experiments where kinase inhibitors, toxin and MMP inhibitors were used, HDFs were pretreated with SB202190 (10–25 μM), SB202474 (10–25 μM, negative control for SB202190), U0126 (10–30 μM), SP600125 (20–50 μM), pertussis toxin (1 ng/ml, 10 ng/ml, and 30 ng/ml), GM6001 (1 μg/ml, 5 μg/ml, and 25 μg/ml), MMP Inhibitor III (3 μM, 10 μM, and 30 μM) for 30 min before being subjected to trypsinization. Continued presence of the inhibitors at the same concentrations throughout migration assays was ensured.
Statistical Analysis
The methodology to determine whether the differences in MIs between experimental sets of migrating HDFs are significant has been published by us (Chen et al., 1993). In brief, statistical analyses of differences in MIs between triplicate sets of experimental conditions were performed using the Microsoft Excel. Confirmation of a difference in migration as statistically significant requires rejection of the null hypothesis of no difference between mean migration indices obtained from replicate sets at the p = 0.05 level with the Student's t test (Chen et al., 1993).
In Vitro Wound Healing (“Scratch”) Assay
Twelve-well plates were precoated with collagen (45 μg/ml) or control polylysine (30 μg/ml) in triplicates, followed by further BSA blocking. A sufficient number of serum-starved HDFs were plated, so that they became confluent in the wells right after attachment (∼1–2 h). Scratches were then made as described previously (O'Toole et al., 1997). Floating cells were removed by PBS washing. Media containing 0.2% FBS without or with indicated concentrations of PDGF-BB were added to the wells and incubated for additional 16 h. Mitomycin C (10 μg/ml) was always included in the media to prevent cell proliferation. Five representative images of the scratched areas under each condition were photographed. To estimate the relative migration of the cells, the unclosed cell-free areas from five prints under each condition were excised and weighed on a scale (Mettler AE50). We used “average gap” (AG, %) to quantify the data. The collagen alone or polylysine alone at 0 h was considered 100% AG. The wells that showed the most HDF migration had the least AG values.
Rhodamine-conjugated Phalloidin Staining and Microscopic Analyses
Cells were cultured on square glass coverslips precoated with indicated matrices (polylysine or collagen) in six-well tissue culture plates for 24 h. Cells then were serum-starved overnight and treated with or without PDGF-BB for various periods of time. The detailed protocols for fixation, permeabilization, and rhodamine-conjugated phalloidin staining of the cells were described previously by us (Chen et al., 2000). Images of stained cells were visualized under fluorescent microscope (Axioplan; Carl Zeiss, Thornwood, NY) and photographed by an attached digital camera (AxioCam MRm; Carl Zeiss).
Subcloning, Production of Lentiviral Stocks, and Infection
The lentivirus-derived vector pRRLsinhCMV was inserted with wild type (wt) or various mutants of Pak1, Akt, FAK, p38α, MEK1, JNKK2, and MEK5 genes at EcoRV and SalI, SalI and EcoRI, EcoRV, XbaI, and SalI, and BamHI and SacII, respectively. These constructs were used to cotransfect 293T cells together with two packaging vectors pCMVΔ R8.2 and pMDG, as described previously by us (Chen et al., 2003). Conditioned media were collected, filtered, and concentrated (if necessary) before being used for infecting HDFs. HDFs were plated 1 day before infection in six-well tissue culture plates. When the culture reaches 25% confluence, they were subjected to infection as described previously (Chen et al., 2003). Forty-eight hours after infection, HDFs were subjected to biochemical, cell migration, or cell proliferation experiments.
Cytotoxicity after infection, or chemical or toxin treatment was examined by a trypan blue exclusion assay and a cell proliferation assay, as described previously (Li et al., 2001). An agent was considered noncytotoxic, if >90% of the cells excluded the trypan blue dye. Our second criterion for the lack of cytotoxicity was that the cells were able to proliferate in culture at or above the 90% level of the unexposed cells after exposure to a given agent.
Measurement of Protein Expression Levels in Infected Cells
Expression of gene products was detected by immunoblotting the lysates of infected cells with antibodies against corresponding gene products. The methods used to detect activation of the protein kinases were antiphosphorylated form antibodies and in vitro kinase assays. Briefly, 48 h after infection, the cells were solubilized in lysis buffer (Li et al., 2001). The protein contents of the postnuclear lysates were equalized in Bio-Rad protein assay by a spectrophotometer at wavelength of 595 nm. To measure the protein levels of exogenously expressed gene products over their endogenous counterparts, 50 μg of total cellular proteins per sample was subjected to Western blot analysis with antispecific protein antibodies. To detect activation of the kinases in response to PDGF-BB, lysates of the cells were immunoblotted with antibodies specifically against the phosphorylated forms of the proteins, followed by horseradish peroxidase-conjugated secondary antibodies. Results were finally visualized by enhanced chemiluminescence reactions. Unsaturated exposures were used to compare the relative fold increases by scanning densitometry. The mean densitometry from three different exposures of the same experiment was calculated into relative fold increases of PDGF stimulation over unstimulated controls.
RESULTS
PDGF-BB Is the Major Promotility Factor in Human Serum for HDFs
We have recently shown that human serum, but not human plasma, promotes keratinocyte migration (Henry et al., 2003). This finding suggests that promotility activities are increased when plasma is converted to serum, such as during acute skin wounding. Although it is unclear what serum factor(s) actually participates in promoting HDF migration in vivo, we investigated whether a single serum factor could replace the entire HDF promotility activity in human serum in vitro. Previous studies suggest that, among the half a dozen reported HDF promotility growth factors, PDGF-BB is a strong candidate (Deuel et al., 1991; Imanishi et al., 2000; Rönnstrand and Heldin, 2001).
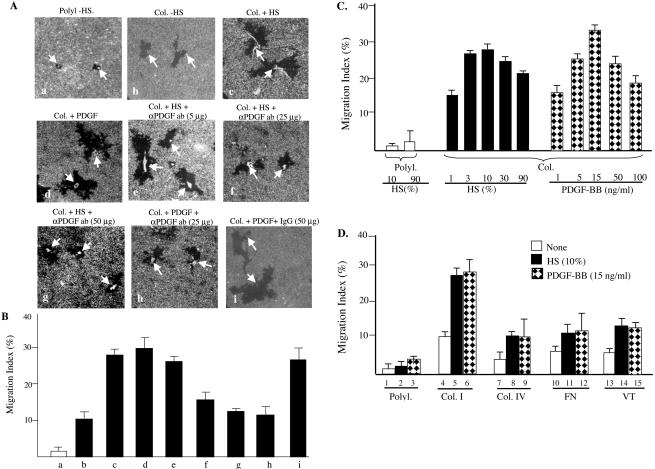
To test the above-mentioned hypothesis, serum-starved quiescent HDFs were subjected to colloidal gold migration assays in the absence or presence of human serum or human recombinant PDGF-BB, in which HDFs were either attached to a type I collagen matrix or a polylysine control matrix. As shown in Figure 1A, HDFs that were attached to a polylysine-coated surface failed to migrate (a, arrows point to attached cells). Little HDF migration was detected on polylysine even in the presence of either 10% human serum or PDGF-BB (15 ng/ml) (our unpublished data; Figure 1D). This finding suggests that cell attachment to physiological ECM is a prerequisite for migration. Consistent with this notion, HDFs that were attached to a collagen-coated surface were able to migrate, although modestly, even in the absence of any growth factors or serum (b). Addition of human serum to HDFs on collagen dramatically enhanced the migration, leaving behind large migration tracks (c). Interestingly, replacement of the human serum with PDGF-BB (15 ng/ml) generated a similar degree of enhancement (d). These data suggest that PDGF-BB may represent the major HDF promotility activity in human serum.
Figure 1.
PDGF-BB is the major promotility factor in human serum for HDFs. Primary neonatal HDFs, serum starved for 18 h, were seeded onto colloidal gold coverslips, which were coated with either poly-l-lysine (Polyl., 10 μg/ml) as a nonphysiological control matrix or type I collagen (Col, 45 μg/ml) for 2 h. Cells were incubated in media with or without indicated concentrations of either human serum (HS, 10% or as indicated) or human recombinant PDGF-BB (15 ng/ml or as indicated). To block PDGF-BB' action in humans serum, human serum-containing media were added with either anti-PDGF neutralizing antibodies (5, 25, or 50 μg/ml) or control IgG for 45 min before being used in migration assays. HDF migration caused colloidal gold-free areas (“tracks”), which were quantified by a computer-assisted analysis as MI (see MATERIALS AND METHODS). (A) The representative images of single-cell migration tracks under indicated conditions. (B) Computer-assisted quantification of 15 randomly selected microscopic fields per condition. (C) HDF migration in response to various doses of HS or PDGF-BB. (D) Comparison of HDF motility on four indicated ECMs without or with HS or PDGF-BB. Col. IV, collagen IV, FN, fibronectin, VT, vintronectin. The results represent those of three independent experiments.
To further investigate this observation, we used anti-PDGF-BB neutralizing antibodies to block the action of PDGF-BB in human serum. We found that this antibody inhibited human serum-induced migration in a dose-dependent manner (e–g). A complete inhibition of the PDGF-BB–stimulated HDF migration (over the collagen alone-induced migration) was detected by addition of 25–50 μg/ml the anti-PDGF antibody (f and g versus b). As the positive control, inhibition of PDGF-BB–induced migration by the same neutralizing antibodies was included (h). As the negative control, the addition of irrelevant goat IgG showed no inhibitory effect (i). Quantitation of the above-mentioned migrations was shown in Figure 1B. It can be seen that, in comparison with no migration on polylysine, collagen alone was able to produce MI ∼10 (b versus a). Human serum enhanced the MI up to 30 (c) and so did PDGF-BB (d). Addition of 25 μg/ml anti-PDGF neutralizing antibodies almost completely reduced the human serum-stimulated migration to the level of collagen alone-induced migration with MIs of ∼10–12 (f and g versus b). The same amount of antibodies also completely inhibited the PDGF-BB-enhanced MI (h). Control IgG had no effect (i).
Optimization of the amounts of serum and PDGF-BB is shown in Figure 1C. Note that 3–10% of human serum already showed the strongest induction of HDF migration on a collagen matrix. PDGF-BB at 15 ng/ml achieved the maximum promotility effect of human serum on HDFs.
We compared the effects of other growth factors, which have previously been reported to stimulate HDF migration, with the effect of human serum and PDGF-BB. These factors included EGF (Adelmann-Grill et al., 1990), FGF (Thomas, 1987), IL-4 (Postlethwaite and Seyer, 1991), IL-1 (Heckmann et al., 1993), PDGF-AA (Siegbahn et al., 1990), PDGF-AB (Kirchberg et al., 1995), and TGFβ 1 (Postlethwaite et al., 1987), although antimotility effect of TGFβ 1 was also reported (Ellis et al., 1992). None of these agents, either alone or in combination, was able to generate >30% of human serum's effect on HDF migration. TGFβ 1 showed a clear and potent inhibitory effect (our unpublished data). Together, our data suggests that PDGF-BB is the main promotility factor in human serum for HDFs.
It is known that type I collagen is an abundant ECM in dermis. To study whether type I collagen is also a potent promotility ECM for HDFs, we performed migration assays, in which HDFs were attached to polylysine (Polyl), collagen I (Col-I), collagen IV (Col-IV), fibronectin (FN), or vitronectin (VT), and incubated with or without human serum or PDGF-BB. The attachment and spreading of HDFs on all ECMs were significantly similar. On polylysine, complete HDFs' attachment took additional 1 h (totally 2 h) than HDFs on all other ECMs, and less cell spreading was observed (our unpublished data; Figure 3). As shown in Figure 1D, type I collagen showed the strongest promotility ECM for HDFs (4) and exhibited the strongest synergistic effect with human serum and PDGF-BB (3 and 4). Although other ECMs had significantly weaker promotility effect on HDFs, all were able to significantly promote HDF migration in the absence of growth factors (7, 10, and 13), in comparison with the effect of polylysine (1).
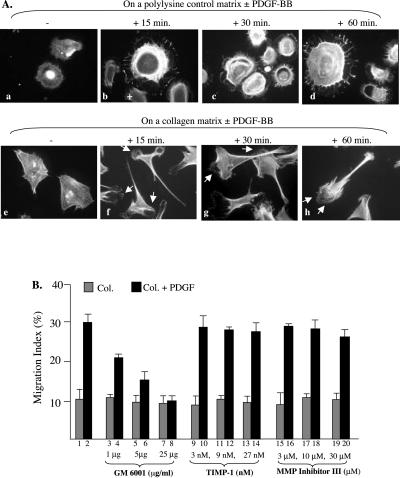
Figure 3.
PDGF-BB stimulates polarization of HDFs on collagen and MMP participation. (A) HDFs were seeded on polylysine or on collagen-coated coverslips. After attachment, cells were serum starved overnight and then untreated or treated with PDGF-BB (15 ng/ml) for the indicated time. Cells were fixed and stained with rhodamine-conjugated phalloidin (see MATERIALS AND METHODS). Morphological differences of the cells on either polylysine (a–d) or on collagen (f–i) in the absence (a and f) or presence (b, c, d and g, h, i) of PDGF-BB were analyzed under a fluorescent microscope and the representative images photographed. Approximately 80 randomly selected cells per condition were examined and quantified by taking the percentage of polarized HDFs. Less than 14% and >78% of HDFs were polarized in the absence or presence of PDGF-BB, respectively. (B) Serum-starved HDFs were subjected to colloidal gold migration assays in the absence or presence of indicated concentrations of MMP inhibitors, GM6001, TIMP-1 and MMP Inhibitor III. After 14 h of incubation, migration was analyzed as described previously. Results from one of two similar experiments are presented.
The “Active” and “Passive” Promotility Effects of Collagen Matrix and PDGF-BB
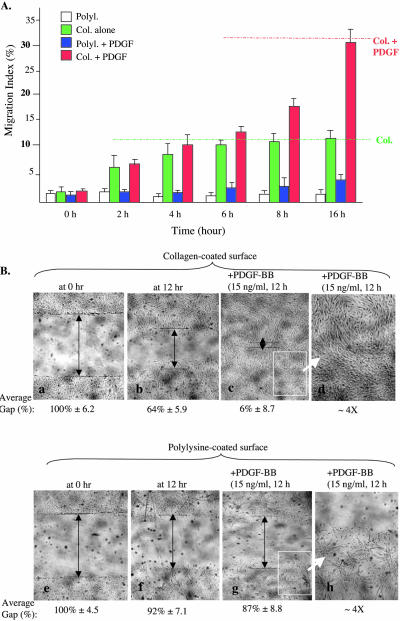
The above-mentioned results indicate that a physiological ECM, such as collagen, is able to initiate HDF migration, whereas growth factors such as PDGF-BB is unable to initiate migration in the absence of an ECM. We further verified these observations by carrying out kinetic analyses of HDF migration under the four well-defined conditions: 1) polylyisne control matrix alone (no serum or PDGF-BB); 2) plylysine control matrix plus PDGF-BB; 3) collagen matrix alone; and 4) collagen matrix plus PDGF-BB. We found that collagen's promotility effect acts at the early phase of HDF migration, and PDGF-BB's enhancement effect comes in at the later phase of the migration. As shown in Figure 2A, PDGF-BB could not induce HDF migration on polylysine (blue bars versus white bars), indicating again that PDGF-BB cannot initiate migration in the absence of a physiological ECM. In contrast, in the absence of PDGF-BB, collagen was able to initiate migration and remained a driving force for the initial 4 h (green bars versus red bars). After 4 h, collagen's promotility effect reached a plateau, and only when PDGF-BB was added, did the cells exhibit continued migration over the plateau level (red bars after 4 h). The effect of PDGF-BB then became the major driving force for the remaining period of cell migration (red bars versus green bars at 8 and 16 h). The delayed promotility effect of PDGF-BB suggests that PDGF-BB-stimulated HDF migration requires de novo protein synthesis. Consistent with this notion, addition of cycloheximinde, a protein synthesis inhibitor, blocked the late phase HDF motility driven by PDGF-BB (our unpublished data).
Figure 2.
Collagen initiates and PDGF further enhances HDF migration. Serum-starved HDFs were subjected to colloidal gold migration assay or in vitro wound healing assay. (A) Time course of HDF migration under four well-defined conditions: i) on polylysine (white bars), on collagen (green bars), on polylysine plus PDGF-BB (blue bars), and on collagen plus PDGF-BB (red bars). Migration was stopped at the indicated time points by cell fixation and subjected to computer-assisted analyses (for MIs). Representative results of three independent experiments are shown. (B) Serum-starved HDFs were plated in six-well tissue culture dishes precoated with either polylysine or collagen. After cell attachment (∼2 h), “wounds” (scratches) were made at the middle of the wells, and free cells were removed. Media were then changed to fresh ones with or without PDGF-BB and incubated for additional 14 h. Mitomycin C (to block proliferation) was present in all wells. Closure of wound was photographed and quantified as described in MATERIALS AND METHODS. d and h, amplified partial images of c and g, respectively, to closely visualize changes of the cells on polylysine or collagen after PDGF-BB treatment. This experiment was repeated four times and similar results were obtained.
The findings on the functional relationship of collagen and PDGF were further confirmed by an independent migration assay, the in vitro wound-healing (scratch) assay. It is shown in Figure 2B that HDFs migrated on collagen alone (b versus a), but not on polylysine (f versus e). The addition of PDGF-BB further enhanced HDF migration only on collagen (c), but not on polylysine (g). PDGF-BB stimulation caused dramatic HDF clustering on polylysine (h). This clustering effect was not detected with HDFs on collagen (d). Because of the presence of mitomycin C throughout these experiments, any of the “wound closure” was not due to cell proliferation.
PDGF-BB Stimulates Polarization Only When HDFs Are Attached to an ECM
We investigated why cell attachment to an ECM is a prerequisite for serum factors, such as PDGF-BB, to elicit its promotility effect. We analyzed the actin cytoskeletal structure of HDFs on polylysine or collagen in the absence or presence of PDGF-BB for indicated times. As shown in Figure 3A, in the absence of PDGF-BB, there is some morphological difference between HDFs on polylysine (a) and collagen (e). HDFs on collagen spread more than the cells on polylysine. Dramatic morphological changes occurred on both matrices when PDGF-BB was added. HDFs on polylysine formed lamellepodia all around them in a time-dependent manner and showed no sign of polarization (b–d). In contrast, PDGF stimulated lamellepodium formation at the cells' leading edge as rapidly as 15 min and in a time-dependent manner (f–h). Because protrusion and polarization are essential for directional cell migration, the above-mentioned study provides an explanation for the dependence of HDF motility on ECM.
In addition to the critical role of collagen itself, it is likely that modification of collagen matrix by HDF-secreted MMPs in response to PDGF-BB stimulation also contributed to the enhancement and directionality effects of PDGF-BB. To test this hypothesis, we pretreated HDFs with increasing concentrations of three MMP inhibitors: TIMP-1 (a peptide inhibitor for MMP9), GM6001 (N-[(2R)-2-(hydroxamidocarbonylmethyl)-4-methylpentanoyl]-l-tryptophan methylamide, or Galardin, a broad MMP inhibitor), and MMP Inhibitor III (hydroxamidocarbonylmethyl)-4-methylpentanoyl]-l-tryptophan, a broad MMP inhibitor). As shown in Figure 3B, although neither TIMP-1 (9–14) nor MMP Inhibitor III (15–20) showed any significant inhibition, GM6001 potently inhibited PDGHF-stimulated HDF migration in a dose-dependent manner (3–8). Although the identity of the specific MMP(s) involved remains to be studied, these results suggest that MMP(s) plays a critical role in HDF motility on collagen.
Gene Cassette Approach for Multiple Signaling Networks in HDF Migration
The conventional approach of studying signaling mechanisms often focused on the function of a single signaling molecule or a linear signaling pathway. Results of this approach were unclear and often conflicting, likely due to the reasons of 1) lack of adequate internal controls for the specificity; 2) cell type specificities (the same gene may act differently in different types of cells); and 3) participations of multiple parallel pathways at all levels. Therefore, to study the migratory signaling program in primary HDFs, we faced two fundamental problems: the complexity of the nonlinear and multiple genes-participating signaling networks and the technical limitations of using primary human cells. For example, establishment of stabled cell lines is not possible and transient drug selection after a lower efficiency transfection often causes “sickness” of the cells. We established Gene Cassette approach to combat these two problems. First, to examine multiple parallel pathways, we used groups or cassettes of genes that either belong to a gene family or are related because they act in parallel at the similar cellular levels. This would allow comparisons of multiple related genes in the same cells in response to the same stimuli. Second, to efficiently deliver the genes, individually or in combination, to primary HDFs and achieve sustained expression, we made use of a lentiviral vector system, pRRLsinhCMV, that offers >90% gene transduction efficiency to primary HDFs, as shown previously by us (Chen et al., 2003).
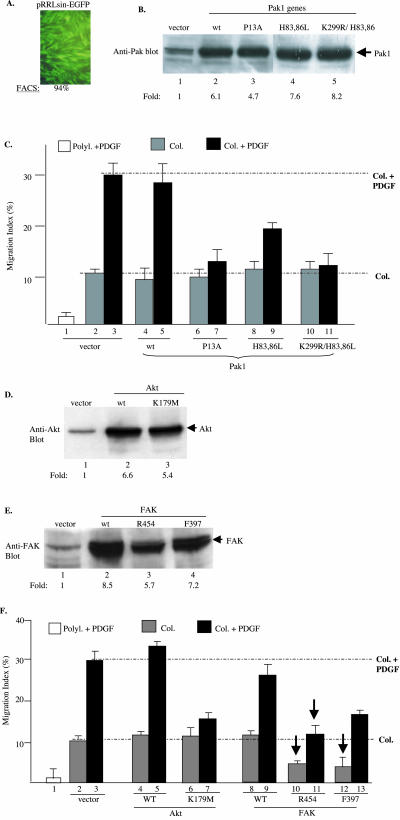
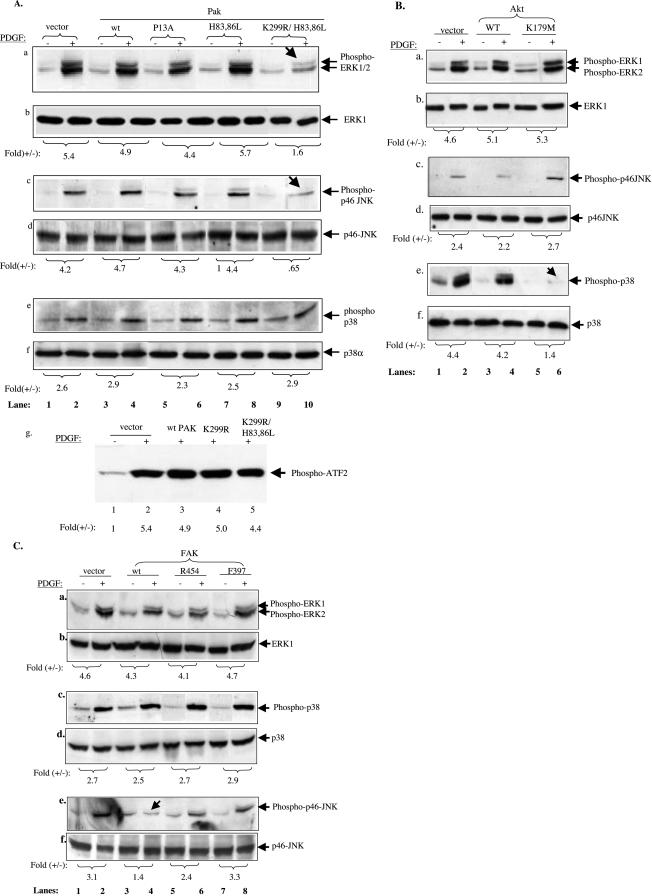
Three protein kinases, p21rac/cdc42-activated protein kinase (Pak), protein kinase B/Akt, and FAK, were grouped into one gene cassette (cassette I), based on 1) their ubiquitous expression and 2) their immediate downstream receptor signaling and membrane-binding property. Both wild types and mutants of these genes were cloned into pRRLsinhCMV, and virus production was carried out as described previously (Chen et al., 2003). Three mutants of Pak1 gene used were 1) Pak1-K299R/H83,86L (kinase-defective and defective in binding to Rac and Cdc42); 2) Pak1-H83, 86L (defective in binding to Rac and Cdc42; and 3) Pak1-P13A (defective in binding to SH3-containing adapter proteins). The two mutants of FAK gene were 1) R454 (kinase-defective) and F397 (tyrosine 397 phosphorylation mutant). The two mutant of Akt1 used was the kinase-defective Akt-KD (K179M) and constitutively active Akt-myr. First, we studied the individual roles of these genes by infecting HDFs with each gene separately. However, to have these 10 constructs serve as specificity controls for each other, they all were used to infect the same HDF culture at the same time. As shown in Figure 4A, pRRLsinhCMV infection achieved 94% gene transduction efficiency by using an inserted GFP gene. Before infecting HDFs with these genes for motility assays, the titers of the viruses were further adjusted such that similar expression levels of these Pak genes in HDFs were ensured.
Figure 4.
Specificities of cassette I genes in collagen- and PDGF-BB–driven HDF motility HDFs were infected with lentiviruses carrying wild-type (wt) or mutants of Pak, Akt, and FAK genes or a control EGFP gene. Infection was stopped after 6 h and cells were incubated in growth media for additional 48 h. After serum starvation, cells were subjected to Western blot analyses for protein expression and colloidal gold migration assays. (A) Fluorescence-activated cell sorting analysis of EGFP-positive cells over total cells (%). (B, D, and E) Immunoblots of equal amounts of cell lysates (50 μg of total proteins) with indicated antibodies. Intensity of the bands was quantified by scanning densitometry and estimated as fold increases in reference with their endogenous expressions (lanes 1). (C and F) MIs of the cells on polylysine or collagen with or without PDGF-BB (15 ng/ml) are presented. (C) Effects of wt or mutants of Pak1. (F) Effects of wt or mutant Akt and FAK on collagen- or collagen plus PDGF-stimulated HDF migration. A representative experiment of four independent experiments is shown.
For the Pak genes, the lentiviral vector-mediated expression was five- to eightfold higher than the endogenous Pak (Figure 4B). These cells were subjected to migration assays, as described previously. Due to space limitations, only the computer-assisted analyses of the migration assays are shown here. As shown in Figure 4C, HDF migration on polylysine was used as the baseline control (1). HDFs infected with empty vector migrated on collagen with MIs between 10 and 12 in the absence of PDGF-BB (2), and with MIs ∼30 in the presence of PDGF-BB (3). Overexpression of the wild-type Pak showed little effect on either the collagen-driven or PDGF-BB–enhanced migration (4 and 5). Interestingly, none of the three Pak mutants showed any significant inhibition on collagen-initiated migration (6, 8, and 10). In contrast, Pak-P13A and Pak-K299R/H83,86L triple mutants dramatically inhibited the PDGF-BB–enhanced HDF migration (bars 7 and 11). The Pak-H83,86L mutant showed less, but significant, inhibition (9), as well. These results suggest that Pak's SH3-binding, kinase activity and/or its membrane translocation are critical for mediating PDGF-BB signaling. Because neither Pak-H83,86L nor Pak-K299R/H83,86L was able to bind to upstream Rho family GTPases, the observed inhibitory effects could not be due to nonspecific “titration” of the upstream activators such as GTP-bound Rac.
Similarly, five- to ninefold expressions of Akt and FAK genes over their endogenous counterparts are shown in Figure 4, D and E. As shown in Figure 4F, the wild-type Akt had little effect on either collagen-driven or collagen plus-PDGF-BB–driven migration (4 and 5). The Akt-K179M mutant showed no significant inhibition of the collagen-initiated migration (6). However, again, Akt-K179M almost completely blocked PDGF-stimulated HDF migration (7). Therefore, similar to Pak, Akt is only involved in PDGF-BB signaling.
In contrast, FAK plays a critical role in both collagen-initiated and PDGF-BB–enhanced HDF migration. The FAK-R454 and FAK-F397 mutants reduced collagen-initiated migration by >60% (10 and 12 versus 2, pointed by arrows). Interestingly, it also blocked PDGF-BB–enhanced migration (11 and 13 versus 3). The wild-type FAK showed a slightly enhancing effect on HDF migration (8 and 9). These results suggest that FAK participates in both collagen and PDGF-BB signaling.
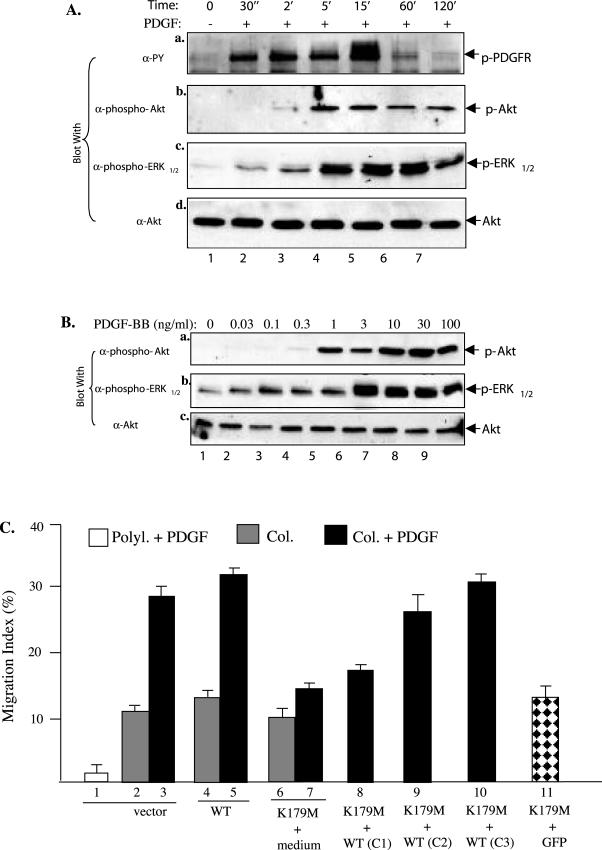
To ensure that these are the primary and specific effects of the genes, we used Akt as an example for further detailed analyses, because its activation by PDGF-BB was less studied. As shown in Figure 5A, Akt is rapidly activated (at 2 min) in HDFs after PDGF stimulation (b) and it occurs after the PDGFR activation (a) and at the similar time of ERK1/2 activation (c). Furthermore, PDGF-BB activates Akt in a dose-dependent manner with as low as 0.3 ng/ml PDGF-BB (B, a). These data indicate that activation of Akt is an early event of PDGFR signaling. We then tested whether coinfection of increasing amounts of wt Akt with a fixed amount of Akt-K1789M would override the dominant negative effect of Ake-K179M. As clearly shown in Figure 5C, the coinfected wt Akt was able to completely reverse the inhibitory effect of Akt-K179M (8, 9, and 10 versus 7). Coinfection with a GFP gene, however, showed no reversing effect on Akt-K179M (11). Together, these data demonstrate that Akt transmits the primary signals from the activated PDGFR.
Figure 5.
Akt is a primary target of PDGF-BB signaling. (A) Kinetics of PDGF-BB–stimulated activation of PDGFR, Akt and ERK1/2 in HDFs. HDFs were serum starved and treated without or with PDGF-BB (15 ng/ml) for the indicated times. Equalized cell lysates (50 μg of total proteins) were immunoblotted with the indicated antibodies. The anti-Akt blot was included as a loading control. (B) Comparison of the sensitivities of Akt and ERK1/2 activations in response to increasing concentrations of PDGF-BB. HDFs were treated with the indicated concentrations of PDGF-BB for 5 min and analyzed for activation of Akt and ERK1/2 by blotting with the indicated antibodies, as described above. Akt protein levels were used as a control for equal loadings. (C) Reversal of Akt-K179M' inhibition by overexpressing wt Akt. HDFs were infected with vector (1–3), wt Akt (4 and 5), Akt-K179M or Akt-K179M mixed with increasing amounts of wt Akt. The total volume of infection was 2 ml, in which 1 ml of virus was mixed with 1 ml of medium for all single infections. For coinfections, 1 ml of Akt-K179M virus was mixed with 1 ml of 25% (C1), 50% (C2), and 75% (C3) of wt Akt viruses. The cells were then subjected to colloidal gold migration assays. This experiments was repeated twice and similar results were obtained.
Specificity of Four Major MAPK Cascades in Collagen and PDGF-BB Signaling in HDF Migration
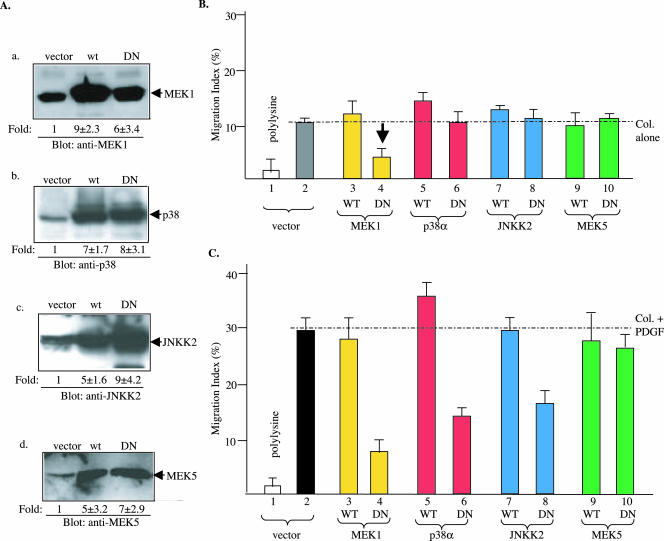
To investigate the downstream signaling by cassette I genes, we chose the four major MAPK cascades, ERK1/2, p38, JNK and ERK5 (reviewed by Chen et al., 2001; Chang and Karin, 2001), and grouped them into a second gene cassette (cassette II). First, study of cassette II genes altogether in HDFs would determine the specificities of these MAPK cascades in a given cellular response, i.e., motility. HDFs were infected with lentiviruses that carried either wild types or dominant negative mutants of MEK1 (K97M), p38α (AF), JNKK2 (KM), and MEK5 (AA). As shown in Figure 6A, pRRLsinhCMV vector expressed the protein products of the genes 5- to 10-fold higher than their endogenous counterparts (a–d). The HDFs, infected with each of the genes were subjected to migration assays under the four previously defined conditions. As shown in Figure 6B, as expected, vector-infected control HDFs did not migrate on polylysine (1), but migrated significantly on collagen in the absence of PDGF-BB or serum (2). Overexpression of the wild-type genes of MEK1, p38α, JNKK2, and MEK5 showed either no effect or a moderate enhancing effect on collagen-initiated HDF migration (3, 5, 7, and 9). Neither did the three of the four dominant negative mutants, p38α-AF, JNKK2-KM, and MEK5-AA (6, 8, and 10). Interestingly, the only MEK1-K97M mutant showed a strong inhibition of collagen-initiated HDF migration (4 versus 2, pointed by an arrow). We conclude that, among the four MAPK cascades, only the ERK1/2 cascade is specifically involved in collagen-driven motility of HDFs.
Figure 6.
Strong specificities of cassette II MAPKs between collagen- and PDGF-BB–driven HDF migration HDFs were infected with lentivirus carrying either a control EGFP gene (vector) or the wild-type or mutant genes of four MAPK pathways as indicated. (A) Equalized lysates (50 μg of total proteins) of the infected cells were analyzed for expression of the corresponding MAPK pathway genes 48 h after infection by Western blots using indicated antibodies. The fold increases in intensity over the endogenous expression are estimated by densitometry scanning. (B) MIs of HDF migration on collagen in the absence of PDGF-BB showing the effects of MAPK genes. (C) MIs of HDF migration on collagen plus PDGF-BB showing the effects of MAPK genes. Open bar, polylysine control; all other bars, Col alone or Col plus PDGF-BB. A representative experiment of three independent experiments is presented.
In contrast with the results on collagen-driven HDF motility, we found that three of the four MAPK cascades play critical roles in PDGF-BB–enhanced HDF migration. As shown in Figure 6C, MEK1-K97M, p38α-AF, and JNKK2-KM inhibited, with variable degrees, PDGF-BB–stimulated HDF migration (4, 6, and 8). The only exception was MEK5-AA that showed insignificant inhibitory effect (10). MEK5-AA indeed blocked the ERK5 pathway in infected HDFs, because the TNF-α–induced expression of MMP-9 gene, a downstream target for the MEK5-ERK5 cascade (Mehta et al., 2003), could be inhibited in HDFs (our unpublished data). Therefore, the p38 and JNK cascades are specifically involved in PDGF-BB signaling, whereas the ERK1/2 cascade takes part in both collagen signaling and PDGF-BB signaling. ERK5, however, is not required for HDF migration.
These findings were further supported by an independent study using chemical inhibitors for selected MAPKs. SB202190, SP600125, and U0126 were used to inhibit p38, JNK, and MEK1, respectively. As shown in Table 1, we found that, at concentrations (10–15 μM) where HDF viability was not compromised, SB202190 and SP600125 selectively blocked PDGF-BB signaling. In contrast, U0126 inhibited both collagen and PDGF-BB signaling (Table 1).
Table 1.
Effects of MAPK inhibitors on HDF migration (MIs)
| Inhibitors/MIs | Polylysine | Collagen | Collagen + PDGF |
|---|---|---|---|
| DMSO | 2 ± 0.7 | 9.3 ± 2.1 | 32 ± 2.7 |
| U0126 | 1.4 ± 1.7 | 3.0 ± 1.4 | 7.8 ± 2.3 |
| SB202190 | 2.4 ± 0.5 | 10.1 ± 2.2 | 12 ± 14 |
| SP600125 | 1.8 ± 0.2 | 8.4 ± 0.6 | 17 ± 1.4 |
DMSO, dimethyl sulfoxide.
The concentrations of U0126, SB202190, and SP600125 used for those experiments were 10, 25, and 20 μM, respectively. The data are the average of three independent experiments. Inhibition is emphasized by bold.
Cassette I Upstream Kinases Uniquely Connect with the Cassette II MAPKs in HDFs
After establishing the individual roles of the four MAPK cascades, we studied how the cassette I members (Pak, Akt, and FAK) are connected to the MAPK cascades in cassette II in HDFs. To do so, the HDFs, which were infected with the wt and mutants of PAK, Akt, or FAK, were tested for activation of the MAPKs in response to PDGF-BB, by using antibodies specifically against phosphorylated forms of the MAPKs and by in vitro kinase assays. ERK5 was exempted from the study due to lack of reliable detection reagents and its lesser role in HDF motility. We reasoned, for example, if Pak acts upstream of any of the downstream MAPKs, PAK mutants would block PDGF-BB–stimulated activation of that MAPK pathway. Results of Pak genes on the three MAPKs are shown in Figure 7A. Although wild-type Pak, Pak-P13A, and Pak-H83,86L showed no significant effects on the activation of ERK1/2 (a, lanes 1–8), the Pak-K299/H83,86L triple mutant clearly reduced ERK1/2 activation from 5.4- to 1.6-fold (lane 10 versus lane 2) (pointed by an arrow). These data suggest that although the SH3-binding and RhoGTPase-binding domains of Pak are not essential for activating ERK1/2, the kinase activity plus its binding to RhoGTPases are critical for mediating PDGF-BB–stimulated ERK1/2 activation.
Figure 7.
Cassette I kinases connect to distinct cassette II downstream MAPK cascades. Serum-starved HDFs expressing wt or mutants of Pak1, Akt, or FAK were either untreated (-) or treated (+) for 10 min with PDGF-BB (15 ng/ml). Equalized cell extracts (50 μg of total proteins) were subjected to analyses for activation of ERK1/2, p38, and JNK by using corresponding anti-phospho-MAPK antibodies. Duplicate blots were probed with anti-ERK1/2, anti-p38, and anti-JNK antibodies to show the loaded protein levels. (A) Effects of wt and mutants of Pak1 on PDGF-stimulated activation of ERK1/2 (a and b), JNK (c and d), and p38 (e and f). Each of the top panels (a, c, and e) was blotted with anti-phospho-MAPK antibodies, and the bottom panels (b, d, and f) with corresponding anti-MAPK protein antibodies. (g) The same set of cell extracts was immunoprecipitated with an anti-p38 antibody, and the immunocomplexes were tested for p38 kinase activity toward purified ATF-2 by using an in vitro p38 kinase assay kit (#9820; Cell Signaling Technology). (B and C) HDFs, expressing the wt or mutants of Akt or FAK were subjected to similar experiments as in A with indicted antibodies. Fold (+/-) referred to PDGF-stimulated over unstimulated activation of the indicated MAPKs. The data were quantified by densitometry scanning of the phosphorylated bands against their corresponding protein control bands. Calculations were based on references to each of their corresponding MAPK protein bands. Four independent experiments were carried out for Pak-, Akt-, and FAK-infected HDFs. In statistical evaluations comparing before and after PDGF stimulation, all Pak, Akt, or FAK mutants gave p < 0.05–0.01 by paired t tests.
Similar results were obtained with PDGF-BB–stimulated activation of JNK. The Pak-K299R/H83,86L mutant significantly reduced PDGF-stimulated p46-JNK activation (c, lane 10 versus lanes 2 and 4). The wild type, P13A, and H83,86L showed neither stimulatory nor inhibitory effect (c, lanes 4, 6, and 8). Interestingly, neither the wild type nor any of the mutants of Pak showed any effect on PDGF-stimulated activation of p38 (e, lanes 6, 8, and 10 versus lanes 2 and 4). This suggests that Pak does not act upstream of p38 in PDGF-BB signaling in HDFs. This finding is inconsistent with the previous reports that Pak is an upstream activator of p38 in several other cell types in response to PDGF-BB (Bagrodia et al., 1995; Zhang et al., 1995; Wery-Zennaro et al., 2000; Dechert et al., 2001; Lee et al., 2001b). Therefore, we further verified this observation by carrying out an in vitro kinase assay of p38. Anti-p38 immunoprecipitates were assessed for their kinase activity toward purified transcription factor ATF-2. As clearly shown in g, none of the Pak gene infections inhibited PDGF-stimulated phosphorylation of ATF2 by immunoprecipitated p38 (lanes 4 and 5 versus lanes 2 and 3).
Akt, however, clearly connects to a distinct MAPK. As shown in Figure 7B, neither wild-type nor kinase-defective (K179M) Akt significantly affected PDGF-BB–stimulated activation of ERK1/2 (a and b) or JNK (c and d). However, Akt-K179M almost completely inhibited the activation of p38 (e, lane 6 versus lane 2) (pointed by an arrow). In contrast, wt Akt showed neither stimulatory nor inhibitory effects (lanes 3 and 4).
Surprisingly, FAK did not seem to play a clear part in PDGF-stimulated activation of all three MAPKs. As shown in Figure 7C, although it clearly had little effect on ERK1/2 and p38 activation (a–d), it negatively regulated PDGF-BB–stimulated activation of JNK. Overexpression of wt FAK significantly reduced PDGF-BB–stimulated JNK activation (e, lane 4 versus lane 2) (pointed by an arrow). This inhibitory effect by wt FAK was partially abolished by the R454 or F397 mutations in FAK (lanes 6 and 8).
Taken together, the above-mentioned results indicated that 1) PDGF-BB stimulation activates ERK1/2, p38, and JNK in HDFs migrating on collagen; 2) Pak mediates, at least partially, PDGF-BB–induced activation of ERK1/2 and JNK, and it does not require its SH3-binding and RhoGTPase-binding domains; 3) Akt mediates PDGF-BB–stimulated activation of p38, but not ERK1/2 or JNK; and 4) FAK negatively regulates JNK activation in HDFs in response to PDGF-BB. These results are summarized in Table 2.
Table 2.
Relationships of cassette I and cassette II genes in HDFs

, positive regulator

, negative regulator
, no relations
Multiple Gene Coinfection and Combined Effects of Cassette I and Cassette II Genes in HDF Motility
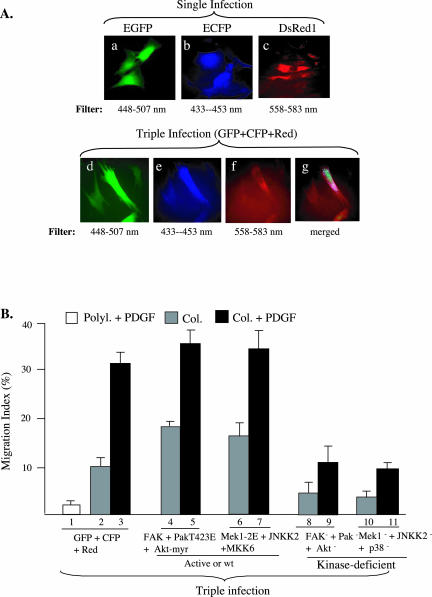
The previous studies with individual gene infections indicate that all cassette I kinases and three of the four cassette II MAPKs are required for HDF migration on collagen in response to PDGF-BB. An important question was whether simultaneously introduction of these gene cassettes into the same HDFs would be sufficient to “reconstitute” the migratory signals or to replace PDGF-BB in HDF migration. First, we tested whether the lentiviral system was capable of introducing multiple genes into the same HDFs at the same time. We individually cloned enhanced green fluorescent protein (EGFP) (green), enhanced cyan fluorescent protein (ECFP) (blue), and DsRed1 (red) genes into pRRLsinhCMV and infected HDFs with either a single gene or all three genes. It is shown in Figure 8A that individually infected HDFs showed expression of EGFP (a), ECFP (b), and DsRed1 (c). The same triple-infected cells could be viewed for expression of EGFP (d), CGFP (e), and DsRed1 (f) or merged (g). These data indicate that the lentiviral system was able to introduce multiple genes into the same HDFs at the same time. Taking advantage of this observation, we studied the combined effects of cassette I and cassette II genes. We chose to use either constitutively active forms of the kinases (PakT423E, Akt-myr, Mek1-2E, and MKK6), if available, or the kinase-deficient mutants, as described previously. There are currently no constitutively activated mutants for FAK or the JNK pathway. As shown in Figure 8B, coinfection of the three fluorescent genes had little effects on collagen or collagen plus PDGF-BB–stimulated HDF migration (1–3). Interestingly, triple infections of HDFs with the wt or activated cassette I kinases significantly, but not as fully as PDGF-BB, augmented collagen-driven HDF migration (4 versus 2). The PDGF-BB–stimulated migration was also slightly increased (5 versus 3). Similar results were obtained from HDFs triple infected with the wt or activated cassette II kinases (6 and 7). These data suggest that both cassette I and cassette II kinases are able to partially replace the PDGF-BB' promotility effect. In contrast, triple infections of the three kinase-deficient mutants from cassette I or II synergistically blocked both collagen and PDGF-BB–stimulated HDF migration (8 and 9 and 10 and 11).
Figure 8.
Combined effects of cassette I and cassette II genes after multiple lentiviral coinfections. (A) HDFs were either infected with a single or coinfected with three lentiviruses carrying an EGFP, an ECFP, or a DsRed gene. Individual expression of the three genes shows the expected colors of fluorescence (a–c). Simultaneous expression of three genes in the same cells shows the feasibility of introducing multiple genes into cells by the lentiviral system. (B) HDFs were triple infected with either the three fluorescent genes (1–3), wt FAK plus PAK-T423E plus Akt-myr (4 and 5), or Mek1–2E plus wt JNKK2 plus MKK6 (6 and 7), or kinase-dead FAK, Pak, and Akt (8 and 9), or kinase-dead Mek1, JNKK2, and p38 (10 and 11). Cells were then subjected to colloidal gold migration assays in the absence or presence of PDGF-BB (15 ng/ml), as described.
DISCUSSION
Despite previous studies on individual functions and signaling cross talk of ECMs and growth factors in control of cell motility (reviewed by Eliceiri, 2001), it remained unclear how ECMs and growth factors, which often simultaneously coexist in vivo, work collaboratively to control optimal cell motility. To gain new insights into these questions, we have systematically investigated the individual and combined roles of collagen and PDGF-BB in the regulation of HDF migration. We report for the first time that PDGF-BB is the main HDF promotility factor in human serum. However, PDGF-BB has no migration-initiating effect in the absence of ECM. Collagen provides the initiation signals for HDF migration, and PDGF-BB stimulates polarization and provides enhancement and directionality for collagen-driven HDF migration. Therefore, it is collagen that “jump-starts” HDF migration. Once the initiation signals from collagen are ensured, PDGF-BB will “fuel” and optimize the collagendriven migration. For the biological relevance, throughout our studies, we chose to use primary human cells, HDFs. To better appreciate the complexity of dual signaling by colla-gen and PDGF-BB and to overcome technical limitations of using primary HDFs for these studies, we established gene cassettes with lentiviral gene delivery approach. This approach allowed us to efficiently deliver multiple genes, individually or in combination, into HDFs in a single experiment, and to study effect of each individual gene, in comparison with other cassette genes, and the combined effect of all the cassette genes.
Human serum is the main source for growth factors during skin wound healing. Our study provides evidence that a single growth factor, PDGF-BB, can replace the entire effect of human serum for driving HDF migration on collagen. Blockade of PDGF-BB action in human serum by anti-PDGF–neutralizing antibodies diminishes the promotility effect of human serum on HDFs. In comparison, other growth factors and cytokines, previously reported to promote HDF migration, showed no >30% of the promotility effects of either PDGF-BB or human serum. Although these data clearly demonstrate that PDGF-BB is the primary promotility factor in human serum for HDF migration on collagen, these in vitro data do not exclude the possibility that in vivo other growth factors may also be directly or indirectly involved in various stages of wound healing. Clark and his colleagues showed that multiple growth factors/cytokines may be directly or indirectly involved in HDF motility control. PDGF-BB promoted a decrease in α 1 and an increase in α 5 at the cell surface, whereas IL-1, tumor necrosis factor-α, and interferon-γ caused increases in α 1 and α 5 (Gailit et al., 1996). In addition, PDGF-BB–induced expression of the integrins also depends upon the ECMs, to which HDFs are attached (Xu et al., 1996). Alterations of integrin expression profiles can indirectly influence HDF motility by switching to different ECMs.
We found that ECM initiates HDF motility in the absence of soluble growth factors. However, it is possible that collagen matrix first induces secretion of growth factors, which in turn act on the same cells in an autocrine manner. To rule out this possibility, conditioned media were prepared from HDF cultures on a collagen matrix for up to 4 d and tested for their motility-enhancing effects by adding to HDFs on a collagen matrix. None of the conditioned media show any enhancement of HDF motility on collagen. Therefore, collagen does not jump-start HDF motility by inducing a growth factor autocrine loop. However, we found that different forms of collagen have distinct effects on HDF motility. Only immobilized (substratum-bound) collagens are able to initiate HDF migration. Soluble (in suspension) collagens do not drive HDF migration. Instead, addition of soluble collagens to HDFs already on an immobilized collagen matrix or leaving the extra and unattached collagens unwashed would attenuate HDF migration by >50%. In this case, it is possible that the suspended collagen molecules compete for the same pool of integrins and diminish the adhesion-forming integrins bound to the immobilized collagen.
It is now clear that multiple parallel intracellular pathways are often simultaneously used for executing a specific cellular response, such as cell motility (Valius and Kazlauskas, 1993; Fambrough et al., 1999; Dey et al., 2000; Gilman et al., 2002 [Alliance for Cellular Signaling]). To gain new insights into the complexity of these signaling networks, i.e., the specificity, necessity and sufficiency, the conventional approach to focus on a single signaling molecule or pathway has proven to be ineffective and less desirable. We propose gene cassettes with lentiviral gene delivery approach to study the above-mentioned three fundamental issues of signal transduction. First, this approach makes it technically easy to compare the individual functions of family or related signaling genes in a single experiment under identical experimental settings (i.e., cell type, stimuli, and a defined cellular response). Because each of the family genes tested serves as an internal control for rest of the genes being tested in the same experiment, this approach is ideal for testing the specificity of an individual gene. Second, application of the lentivirus' multiple infection ability with an extremely high gene transduction efficiency in primary human cells enables one to carry out gene “add-on” experiments to study signal sufficiency, thereby leading ultimately to a comprehensive understanding of a given signaling networks. For example, expression of an increasing number of constitutively activated genes, which are unknown to play an important role, can shed light on the question of “how much is needed for executing the full biological response.” Third, this approach offers a unique opportunity for piecing together connections and hierarchical orders among signaling molecules. By expressing single or multiple either constitutively activated or dominant negative mutants of one gene cassette, and then monitoring the activation or inhibition of another gene cassette, one would be able to elucidate at least the frame of the signaling networks.
In the current study, the Gene Cassette approach has generated several significant findings. First, collagen and PDGF-BB use distinct and overlapping signaling pathways to control HDF migration. Among the seven protein kinases tested, only FAK and ERK1/2 pathways are involved in the initiation of HDF migration by collagen. Other kinases, however, are required specifically for PDGF-BB signaling, which mainly causes polarization, enhances motility, and provides directionality. It would be difficult to make these observations if focus is on a single signaling molecule/pathway. Second, our data show that no single kinase tested is sufficient to replace the effect of collagen or PDGF-BB. Furthermore, even overexpression of the wt or constitutively activated genes of the entire cassette could not replace the cell surface signals. These results indicate either that a larger number of parallel signaling pathways are required to accommodate the “size” of the promotility signals or that these parallel pathways are strictly coordinated in terms of who goes first and how much signaling strength each pathway outputs, or both. Third, we also tested the individual roles of cassette I and cassette II genes in PDGF-stimulated DNA synthesis in the same cells. We found that Akt in cassette I and ERK1/2 and JNK in cassette II are also required for DNA synthesis (our unpublished data). It suggests that these three kinases are capable of receiving more than one kind of signals and bifurcating the signals to distinct downstream pathways. For example, Akt processes both migratory and proliferative signals from PDGF receptor. However, it relays only the migratory, but not the proliferative, signal to downstream effector, p38, because p38 is only required for HDF migration but not DNA synthesis. Therefore, Akt must transmit the proliferative signal to some other effector(s) in the same cells. Similarly, although ERK1/2 and JNK pathways are used by both migration and DNA synthesis signals, these kinases seem to be able to interpret the difference from different upstream activators. For example, ERK1/2 are able to distinguish activating signals coming through Pak or coming via Ras and Raf. The former is for motility and the latter for mitogenesis. It is of great interest to further investigate how these kinases dissect the different signals. A schematic representation of the migration signal transduction in HDFs is shown in Figure 9. Currently, we are expanding the Gene Cassette approach by introducing single or multiple small interfering RNAs against the signaling molecules into HDFs by lentiviral vectors. This added approach provides an excellent opportunity to study the specificities of gene isoforms.
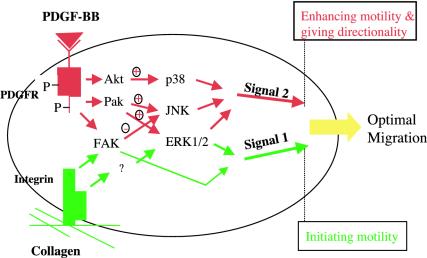
Figure 9.
A schematic dual signaling model of collagen and PDGF-BB in the control of HDF migration. Collagen matrix initiates HDF migration (signal 1, green). PDGF-BB enhances and provides directionality for the collagen-driven migration (signal 2, red). We propose that collagen and PDGF-BB together determine the optimal HDF motility. Collagen matrix and PDGF-BB use specific and common signaling pathways. Arrows do not implicate direct activations.
Finally, this study has pointed out new directions. First, pertussis toxin at 10 ng/ml completely abolished PDGF-BB-, but not collagen-stimulated HDF migration (our unpublished data), suggesting that heterotrimeric G proteins are involved in PDGF-BB signaling, as well. Although the exact mechanism of this cross talk remains to be further studied, a recent report indicated that Akt mediates phosphorylation of G protein-coupled receptor, EGD-1 during endothelial cell motility (Lee et al., 2001a). It is highly likely that Akt communicates with multiple effectors, such as p38 and EGD-1, even just for cell motility purpose. Second, we showed that GM6001 (Galardin), a general small molecule inhibitor for MMPs, potently blocked PDGF-BB–stimulated HDF migration. Whereas, two other MMP inhibitors, small molecule inhibitor MMP Inhibitor III and peptide inhibitor TIMP-1 had little effect even at high concentrations. In particular, the chemical structures of GM6001 and MMP Inhibitor III are strikingly similar, yet their effects are all or none. The function of the MMP(s) involved is to modulate the collagen matrix, such as exposing potential cryptic RGD sites (Xu et al., 2001, Hangai et al., 2002; Giannelli and Antonaci, 2002). It is one of the many “end products” that directly execute cell motility signals. However, induction of the MMP(s) by PDGF-BB could be mediated by one of the kinase cascades we have studied here.
Acknowledgments
We thank the following scientists for reagents: J. Han (p38), A. Lin (JNKK2), N. Ahn (MEK1), D. Schlaepfer (FAK), N. Hay (Akt), and S. Gutkind (MEK5). This study was supported by National Institutes of Health grant GM/AR67100-01 (to W.L.) and AR46538 (to D.T.W.).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–05–0352. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-05-0352.
References
- Albrecht-Buehler, G. (1977). The phagokinetic tracks of 3T3 cells. Cell 11, 395-404. [DOI] [PubMed] [Google Scholar]
- Adelmann-Grill, B.C., Wach, F., Cully, Z., Hein, R., and Krieg, T. (1990). Chemotactic migration of normal dermal fibroblasts towards epidermal growth factor and its modulation by platelet-derived growth factor and transforming growth factor-beta. Eur. J. Cell Biol. 51, 322-336. [PubMed] [Google Scholar]
- Bagrodia, S., Derijard, B., Davis, R.J., and Cerione, R.A. (1995). Cdc42 and PAK-mediated signaling leads to Jun kinase and p38 mitogen-activated protein kinase activation. J. Biol. Chem. 270, 27995-27998. [DOI] [PubMed] [Google Scholar]
- Bauer, E.A., Cooper, T.W., Huang, J.S., Altman, J., and Deuel, T.F. (1985). Stimulation of in vitro human skin collagenase expression by platelet-derived growth factor. Proc. Natl. Acad. Sci. USA 82, 4132-4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatti, S.P., Foster, D.N., Ranganathan, G., Moses, H.L., and Getz, M.J. (1988). Induction of fibronectin gene transcription and mRNA is a primary response to growth-factor stimulation of AKR-2B cells. Proc. Natl. Acad. Sci. USA 85, 1119-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, L., and Karin, M. (2001). Mammalian MAP kinase signalling cascades. Nature 410, 37-40. [DOI] [PubMed] [Google Scholar]
- Carter, S.B. (1967). Haptotaxis and the mechanism of cell motility. Nature 2, 256-261. [DOI] [PubMed] [Google Scholar]
- Chen, J.D., Kim, J.P., Zhang, K., Sarret, Y., Wynn, K.C., Kramer, R.H., and Woodley, D.T. (1993). Epidermal growth factor (EGF) promotes human keratinocyte locomotion on collagen by increasing the alpha 2 integrin subunit. Exp. Cell Res. 209, 216-223. [DOI] [PubMed] [Google Scholar]
- Chen, M., She, H., Kim, A., Woodley, D.T., and Li, W. (2000). Nck-beta adapter regulates actin polymerization in NIH 3T3 fibroblasts in response to platelet-derived growth factor-BB. Mol. Cell. Biol. 20, 7867-7880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, M., Li, W., Fan, J., Kasahara, N., and Woodley, D. (2003). An efficient gene transduction system for studying gene functions in primary human. Exp. Dermatol. 28, 193-199. [DOI] [PubMed] [Google Scholar]
- Chen, Z., Gibson, T.B., Robinson, F., Silvestro, L., Pearson, G., Xu, B., Wright, A., Vanderbilt, C., and Cobb, M.H. (2001). MAP kinases. Chem. Rev. 101, 2449-2476. [DOI] [PubMed] [Google Scholar]
- Dechert, M.A., Holder, J.M., and Gerthoffer, W.T. (2001). p21-activated kinase 1 participates in tracheal smooth muscle cell migration by signaling to p38 MAPK. Am. J. Physiol. 281, C123-C132. [DOI] [PubMed] [Google Scholar]
- Deuel, T.F., Kawahara, R.S., Mustoe, T.A., and Pierce, A.F. (1991). Growth factors and wound healing: platelet-derived growth factor as a model cytokine. Annu. Rev. Med. 42, 567-584. [DOI] [PubMed] [Google Scholar]
- Dey, A., She, H., Kim, L., Woodley, D., and Li, W. (2000). Gamma interferon induces expression of MAD1 gene in macrophage, which inhibits colonystimulating factor-1 (CSF-1)-dependent mitogenesis. Mol. Biol. Cell 72, 232-241. [PubMed] [Google Scholar]
- Eliceiri, B.P. (2001). Integrin and growth factor receptor crosstalk. Circ. Res. 89, 1104-1110. [DOI] [PubMed] [Google Scholar]
- Ellis, I., Grey, A.M., Schor, A.M., and Schor, S.L. (1992). Antagonistic effects of TGF-beta 1 and MSF on fibroblast migration and hyaluronic acid synthesis. Possible implications for dermal wound healing. J. Cell Sci. 102, 447-456. [DOI] [PubMed] [Google Scholar]
- Fambrough, D., McClure, K., Kazlauskas, A., and Lander, E.S. (1999). Diverse signaling pathways activated by growth factor receptors induce broadly overlapping, rather than independent, sets of genes. Cell 97, 727-741. [DOI] [PubMed] [Google Scholar]
- Gailit, J., Xu, J., Bueller, H., and Clark, R.A. (1996). Platelet-derived growth factor and inflammatory cytokines have differential effects on the expression of integrins alpha 1 beta 1 and alpha 5 beta 1 by human dermal fibroblasts in vitro. J. Cell Physiol. 69, 281-289. [DOI] [PubMed] [Google Scholar]
- Giancotti, F.G., and Ruoslahti, E. (1999). Integrin signaling. Science 285, 1028-1032. [DOI] [PubMed] [Google Scholar]
- Giannelli, G., and Antonaci, S. (2002). Gelatinases and their inhibitors in tumor metastasis: from biological research to medical applications. Histol. Histopathol. 17, 339-345. [DOI] [PubMed] [Google Scholar]
- Gilman, A.G., et al. (2002). Overview of the Alliance for Cellular Signaling. Nature 420, 703-706. [DOI] [PubMed] [Google Scholar]
- Grotendorst, G.R., Martin, G.R., Pencev, D., Sodek, J., and Harvey, A.K. (1985). Stimulation of granulation tissue formation by platelet-derived growth factor in normal and diabetic rats. J. Clin. Investig. 76, 2323-2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangai, M., Kitaya, N., Xu, J., Chan, C.K., Kim, J.J., Werb, Z., Ryan, S.J., and Brooks, P.C. (2002). Matrix metalloproteinase-9-dependent exposure of a cryptic migratory control site in collagen is required before retinal angiogenesis. Am. J. Pathol. 161, 1429-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckmann, M., Adelmann-Grill, B.C., Hein, R., and Krieg, T. (1993). Biphasic effects of interleukin-1 alpha on dermal fibroblasts: enhancement of chemotactic responsiveness at low concentrations and of mRNA expression for collagenase at high concentrations. J. Investig. Dermatol. 100, 780-784. [DOI] [PubMed] [Google Scholar]
- Heldin, P., Laurent, T.C., and Heldin, C.H. (1989). Effect of growth factors on hyaluronan synthesis in cultured human fibroblasts. Biochem. J. 258, 919-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin, C.H., and Westermark, B. (1999). Mechanism of action and in vivo role of platelet-derived growth factor. Physiol. Rev. 79, 1283-1316. [DOI] [PubMed] [Google Scholar]
- Henry, G., Li, W., Garner, W., and Woodley, D.T. (2003). Migration of human keratinocytes in plasma and serum and wound re-epithelialisation. Lancet 361, 574-576. [DOI] [PubMed] [Google Scholar]
- Hynes, R.O. (1992). Integrins: versatility, modulation, and signaling in cell adhesion. Cell 69, 11-25. [DOI] [PubMed] [Google Scholar]
- Imanishi, J., Kamiyama, K., Iguchi, I., Kita, M., Sotozono, C., and Kinoshita, S. (2000). Growth factors: importance in wound healing and maintenance of transparency of the cornea. Prog. Retin. Eye Res. 19, 113-129. [DOI] [PubMed] [Google Scholar]
- Kirchberg, K., Lange, T.S., Klein, E.C., Jungtaubl, H., Heinen, G., Meyer-Ingold, W., and Scharffetter-Kochanek, K. (1995). Induction of beta 1 integrin synthesis by recombinant platelet-derived growth factor (PDGF-AB) correlates with an enhanced migratory response of human dermal fibroblasts to various extracellular matrix proteins. Exp. Cell Res. 220, 29-35. [DOI] [PubMed] [Google Scholar]
- Lauffenburger, D.A., and Horwitz, A.F. (1996). Cell migration: a physically integrated molecular process. Cell 84, 359-369. [DOI] [PubMed] [Google Scholar]
- Lee, M.J., et al. (2001a). Akt-mediated phosphorylation of the G protein-coupled receptor EDG-1 is required for endothelial cell chemotaxis. Mol. Cell 8, 693-704. [DOI] [PubMed] [Google Scholar]
- Lee, S.H., Eom, M., Lee, S.J., Kim, S., Park, H.J., and Park, D. (2001b). BetaPix-enhanced p38 activation by Cdc42/Rac/PAK/MKK3/6-mediated pathway. Implication in the regulation of membrane ruffling. J. Biol. Chem. 276, 25066-25072. [DOI] [PubMed] [Google Scholar]
- Lepisto, J., Peltonen, J., Vaha-Kreula, M., Soderstrom, K., Niinikoski, J., and Laato, M. (1996). Selective modulation of collagen gene expression by different isoforms of platelet-derived growth factor in experimental wound healing. Cell Tissue Res. 286, 449-455. [DOI] [PubMed] [Google Scholar]
- Li, W., Nadelman, C., Gratch, N.S., Li, W., Chen, M., Kasahara, N., and Woodley, D.T. (2001). An important role for protein kinase C-delta in human keratinocyte migration on dermal collagen. Exp. Cell Res. 273, 219-228. [DOI] [PubMed] [Google Scholar]
- Mehta, P.B., Jenkins, B.L., McCarthy, L., Thilak, L., Robson, C.N., Neal, D.E., and Leung, H.Y. (2003). MEK5 overexpression is associated with metastatic prostate cancer, and stimulates proliferation, MMP-9 expression and invasion. Oncogene 6, 1381-1389. [DOI] [PubMed] [Google Scholar]
- Miranti, C.K., and Brugge, J.S. (2002). Sensing the environment: a historical perspective on integrin signal transduction. Nat. Cell Biol. 4, E83-E90. [DOI] [PubMed] [Google Scholar]
- O'Toole, E.A., Marinkovich, M.P., Peavey, C.L., Amieva, M.R., Furthmayr, H., Mustoe, T.A., and Woodley, D.T. (1997). Hypoxia increases human keratinocyte motility on connective tissue. J. Clin. Investig. 100, 2881-2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce, G.F., Tarpley, J.E., Allman, R.M., Goode, P.S., Serdar, C.M., Morris, B., Mustoe, T.A., and Vande Berg, J. (1994). Tissue repair processes in healing chronic pressure ulcers treated with recombinant platelet-derived growth factor BB. Am. J. Pathol. 145, 1399-1410. [PMC free article] [PubMed] [Google Scholar]
- Postlethwaite, A.E., Keski-Oja, J., Moses, H.L., and Kang, A.H. (1987). Stimulation of the chemotactic migration of human fibroblasts by transforming growth factor beta. J. Exp. Med. 165, 251-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwaite, A.E., and Seyer, J.M. (1991). Fibroblast chemotaxis induction by human recombinant interleukin-4. Identification by synthetic peptide analysis of two chemotactic domains residing in amino acid sequences 70-88 and 89-122. J. Clin. Investig. 87, 2147-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönnstrand, L., and Heldin, C-H. (2001). Mechanisms of platelet-derived growth factor-induced chemotaxis. Int. J. Cancer 91, 7575-7562. [DOI] [PubMed] [Google Scholar]
- Schwartz, M.A., and Ginsberg, M.H. (2002). Networks and crosstalk: integrin signalling spreads. Nat. Cell Biol. E65-E78. [DOI] [PubMed]
- Seppa, H., Grotendorst, G., Seppa, S., Schiffmann, E., and Martin, G.R. (1982). Platelet-derived growth factor in chemotactic for fibroblasts. J. Cell Biol. 92, 584-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieg, D.J., Hauck, C.R., Ilic, D., Klingbeil, C.K., Schaefer, E., Damsky, C.H., and Schlaepfer, D.D. (2000). FAK integrates growth-factor and integrin signals to promote cell migration. Nat. Cell Biol. 2, 249-256. [DOI] [PubMed] [Google Scholar]
- Siegbahn, A., Hammacher, A., Westermark, B., and Heldin, C.H. (1990). Differential effects of the various isoforms of platelet-derived growth factor on chemotaxis of fibroblasts, monocytes, and granulocytes. J. Clin. Investig. 85, 916-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer, A.J., and Clark, R.A. (1999). Cutaneous wound healing. N. Engl. J. Med. 341, 738-746. [DOI] [PubMed] [Google Scholar]
- Sprugel, K.H., McPherson, J.M., Clowes, A.W., and Ross, R. (1987). Effects of growth factors in vivo. I. Cell ingrowth into porous subcutaneous chambers. Am. J. Pathol. 129, 601-613. [PMC free article] [PubMed] [Google Scholar]
- Thomas, K.A. (1987). Fibroblast growth factors. FASEB J. 1, 434-440. [DOI] [PubMed] [Google Scholar]
- Wery-Zennaro, S., Zugaza, J.L., Letourneur, M., Bertoglio, J., and Pierre, J. (2000). IL-4 regulation of IL-6 production involves Rac/Cdc42- and p38 MAPK-dependent pathways in keratinocytes. Oncogene 19, 1596-1604. [DOI] [PubMed] [Google Scholar]
- Valius, M., and Kazlauskas, A. (1993). Phospholipase C-gamma 1 and phosphatidylinositol 3 kinase are the downstream mediators of the PDGF receptor's mitogenic signal. Cell 73, 321-334. [DOI] [PubMed] [Google Scholar]
- Woodley, D.T., Bachmann, P.M., and O'Keefe, E.J. (1988). Laminin inhibits human keratinocyte migration. J. Cell Physiol. 136, 140-146. [DOI] [PubMed] [Google Scholar]
- Xu, J., Rodriguez, D., Petitclerc, E., Kim, J.J., Hangai, M., Moon, Y.S., Davis, G.E., Brooks, P.C., and Yuen, S.M. (2001). Proteolytic exposure of a cryptic site within collagen type IV is required for angiogenesis and tumor growth in vivo. J. Cell Biol. 2001 154, 1069-1079. Erratum in J. Cell Biol. 155, 859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J., Zutter, M.M, and Santoro, S.A, and Clark, R.A. (1996). PDGF induction of alpha 2 integrin gene expression is mediated by protein kinase C-zeta. J. Cell Biol. 134, 1301-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S., Han, J., Sells, M.A., Chernoff, J., Knaus, U.G., Ulevitch, R.J., and Bokoch, G.M. (1995). Rho family GTPases regulate p38 mitogen-activated protein kinase through the downstream mediator Pak1. J. Biol. Chem. 270, 23934-23936. [DOI] [PubMed] [Google Scholar]