Summary
NsrR is a nitric oxide (NO)-sensitive transcription repressor that controls NO metabolism in a wide range of bacteria. In Bacillus subtilis, NsrR represses transcription of the nitrite reductase (nasDEF) genes that are under positive control of the ResD-ResE two-component signal transduction system. Derepression is achieved by reaction of NO with NsrR. Unlike some NsrR orthologues that were shown to contain a NO-sensitive [2Fe-2S] cluster, B. subtilis NsrR, when purified anaerobically either from aerobic or anaerobic Escherichia coli and B. subtilis cultures, contains a [4Fe-4S] cluster. [4Fe-4S]-NsrR binds to around the −35 element of the nasD promoter with much higher affinity than apo-NsrR and binding of [4Fe-4S]-NsrR, but not apo-protein, is sensitive to NO. RNA polymerase and phosphorylated ResD make a ternary complex at the nasD promoter and NsrR dissociates the preformed ternary complex. In addition to the −35 region, NsrR binds to two distinct sites of the upstream regulatory region where ResD also binds. These interactions, unlike the high-affinity site binding, do not depend on the NsrR [4Fe-4S] cluster and binding is not sensitive to NO, suggesting a role for apo-NsrR in transcriptional regulation.
Keywords: Bacillus subtilis, NsrR, ResD-ResE, nitric oxide, Fe-S cluster
Introduction
In order to adapt to oxygen limitation, Bacillus subtilis undergoes a global change in its transcriptional profile (Ye et al., 2000). This includes the upregulation of genes specifying products that function in anaerobic respiration. The ResDE two-component signal transduction system composed of the ResE sensor kinase and the cognate ResD response regulator plays an important role in regulating anaerobic respiration as well as aerobic respiration (Nakano et al., 1996, Sun et al., 1996). ResE, upon sensing an unidentified signal, undergoes autophosphorylation and promotes phosphorylation of ResD. Phosphorylated ResD (ResD∼P) activates transcription of genes involved in the respiratory pathway that transfers electrons to oxygen (under aerobic conditions) or to nitrate (under anaerobic conditions). Among ResDE-controlled genes, nasDEF and hmp, which encode nitrite reductase and flavohemoglobin respectively, are the most highly induced during nitrate respiration (Ye et al., 2000) or by nitric oxide (NO) (Moore et al., 2004, Nakano, 2002). Later studies showed that strong induction of these genes is caused by inactivation of the NO-sensitive NsrR transcriptional regulator (Nakano et al., 2006, Yukl et al., 2008).
NsrR belongs to a recently discovered family of transcription regulators that directly sense NO [reviewed in (Spiro, 2007)]. Investigators using a comparative genomic approach proposed that NsrR is a master regulator in NO metabolism both in gram-positive and gram-negative bacteria (Rodionov et al., 2005). This was further confirmed by studies in various bacteria including Escherichia coli (Bodenmiller & Spiro, 2006, Filenko et al., 2007, Rankin et al., 2008), Neisseria gonorrhoeae (Isabella et al., 2008, Isabella et al., 2009, Overton et al., 2006), Neisseria meningitidis (Heurlier et al., 2008, Rock et al., 2007), Salmonella enterica serovar typhimurium (Bang et al., 2006), and Moraxella catarrhalis (Wang et al., 2008) as well as in B. subtilis (Nakano et al., 2006). Survival of an nsrR mutant of S. enterica serovar Typhimurium in IFN-γ-stimulated macrophages was reduced compared to the wild-type strain due to enhanced sensitivity to oxidative stress (Gilberthorpe et al., 2007), suggesting the importance of NsrR in bacterial pathogenesis. Transcription of NsrR-controlled genes is upregulated by either an nsrR null mutation or with NO, hence NsrR functions as a transcription repressor, the activity of which is sensitive to NO. The B. subtilis nsrR gene was originally identified by a transcription factor-transformation array analysis as the site of mutation that resulted in aerobic derepression of hmp (Nakano et al., 2006). NsrR represses ResD-controlled nasDEF and hmp transcription and repression is relieved by an exogenous NO donor (such as spermine NONOate) and endogenous NO that is produced by nitrate respiration.
Although the importance of NO in mammalian physiology and pathophysiology is well known [reviewed in (Gross & Wolin, 1995)], it has begun to emerge that NO also plays a pivotal role in bacterial physiology including bacterial infection and persistence in host organisms (Fang, 2004). As NO is an intermediate metabolite in denitrification and is freely diffusible across membranes, not only denitrifiers but also bacteria that cohabit with denitrifiers in various environments constantly encounter NO. Furthermore, it has been shown that even non-denitrifying bacteria endogenously produce NO during nitrate respiration (Ji & Hollocher, 1988). For example, Salmonella nitrate reductase (NarGHJI) (Gilberthorpe & Poole, 2008) and E. coli nitrite reductases (NrfA and NirB; Corker & Poole, 2003, Weiss, 2006) were shown to generate NO.
Our previous study showed that anaerobically purified B. subtilis NsrR (BsNsrR) bears a [4Fe-4S] cluster that, upon exposure to NO, forms dinitrosyl iron complexes (Yukl et al., 2008). Aerobically purified NsrR from N. gonorrhoeae (NgNsrR) (Isabella et al., 2009) and Streptomyces coelicolor (ScNsrR) (Tucker et al., 2008) were shown to contain a NO-sensitive [2Fe-2S] cluster, causing some uncertainty as to the form of Fe-S cluster that is physiologically relevant. Here, we report that anaerobically purified BsNsrR (from either E. coli or B. subtilis cultures) contains a [4Fe-4S] cluster. We also demonstrate that NsrR binds around the −35 element of the nasD promoter and to a further upstream region that overlaps with the ResD-binding sites. The binding of NsrR to the −35 region is greatly enhanced by the presence of the [4Fe-4S] cluster, whereas the binding to the upstream region does not require the Fe-S cluster.
Results
Anaerobically purified NsrR either from aerobic or anaerobic E. coli cultures contains a [4Fe-4S] cluster
In order to eliminate the possibility that the [4Fe-4S] cluster detected with N-terminal and C-terminal His6-tagged BsNsrR (Yukl et al., 2008) is the result of an erroneous Fe-S cluster formation, we constructed a C-terminal Strep-tag BsNsrR and purified the protein from E. coli under anaerobic conditions. The Strep-tag BsNsrR, like the C-terminal His6-tagged BsNsrR (Yukl et al., 2008), is functional when produced in B. subtilis (Fig. S2). Absorbance spectra in the visible region for Strep-tag NsrR showed that anaerobically purified protein has a [4Fe-4S] cluster like the His6-tagged proteins (Fig. S1). The presence of a [4Fe-4S] cluster was further confirmed by resonance Raman (RR) spectroscopy (Fig. S1) and electron paramagnetic resonance (EPR) spectroscopy (data not shown). We also noted that even when E. coli cells were grown and handled aerobically prior to lysis, the [4Fe-4S]form of NsrR was observed if purified anaerobically, suggesting that native BsNsrR harbors a [4Fe-4S] cluster. These data ruled out any concern of interference from the His6-tag on the Fe-S cluster, and subsequent experiments were carried out with the C-terminal His6-NsrR (NsrR-His6)protein.
The [4Fe-4S] cluster is required for high-affinity DNA-binding of NsrR
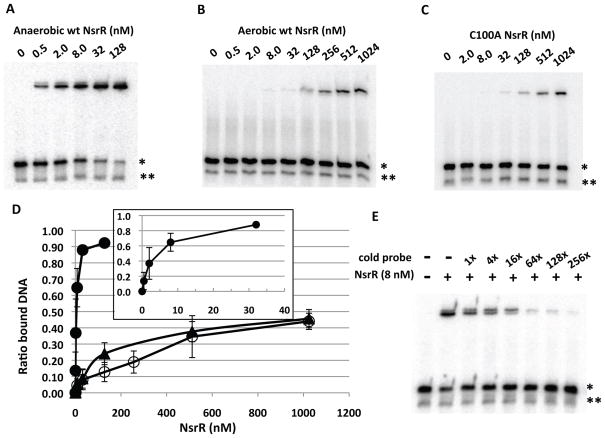
We examined whether the [4Fe-4S] cluster is essential for binding of NsrR to nasD by electrophoretic mobility shift assays (EMSA). As a probe, we used a 30-base pair double-stranded nasD DNA carrying the putative NsrR-binding site previously identified (Nakano et al., 2006) (−44 to −19 in Fig. 1). The double-stranded DNA was generated by annealing complementary oligonucleotides (Experimental procedures). Anaerobically purified [4Fe-4S]-NsrR (average [4Fe-4S]-cluster incorporation was 28%) bound to the double-stranded DNA (marked with *) and not to the single-stranded DNA (marked with **) (Fig. 2A). At 0.5 nM, [4Fe-4S]-NsrR began to bind nasD with apparent half-saturation that occurs between 2 and 8 nM depending on the NsrR preparation. Corrected for the amount of apo-protein present, the Kd of [4Fe-4S]-NsrR can be estimated to be below 2 nM. Two shifted bands with slightly different electrophoretic mobilities were observed and intensities of the faster migrating band increased as NsrR concentration increased. We assume that one of the shifted bands is likely the complex between target DNA and a heterodimer formed by [4Fe-4S]-NsrR and apo-NsrR for the following reasons. First, our previous result showed that NsrR forms a dimer regardless of the Fe-S cluster (Yukl et al., 2008). Second, both shifted bands are sensitive to spermine NONOate (see Fig. 4A), thus NsrR bound to the probe contains a Fe-S cluster, which is further supported by the finding that DNA-bound apo-NsrR migrates slower than the two shifted bands (data not shown). Excess amounts of cold nasD DNA competed for NsrR binding to the radioactive probe (Fig. 2E).
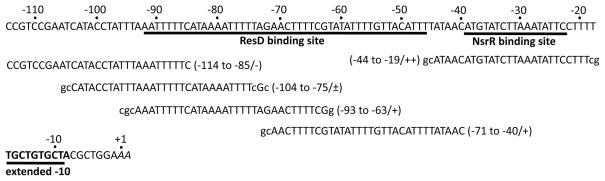
Figure 1.
Nucleotide sequence of the nasD regulatory region. The italicized adenine residues indicate the previously identified transcription start sites (Nakano et al., 1998). The sequence of the coding strand from −114 to +2 (relative to one of the transcription start sites) is shown. Underlined are the extended −10 element, the previously identified ResD-binding (Geng et al., 2004, Nakano et al., 2000) and NsrR-binding (Nakano et al., 2006) sites. The sequence of oligonucleotides used to generate EMSA probes are also listed. The numbers in the parentheses are relative to the transcription start site. The oligonucleotide marked with ++ indicates the site where [4Fe-4S]-NsrR preferentially binds with high affinity. Apo-NsrR binds to the regions marked with +, weakly binds to the site marked with ±, does not bind to the site marked with −.
Figure 2.
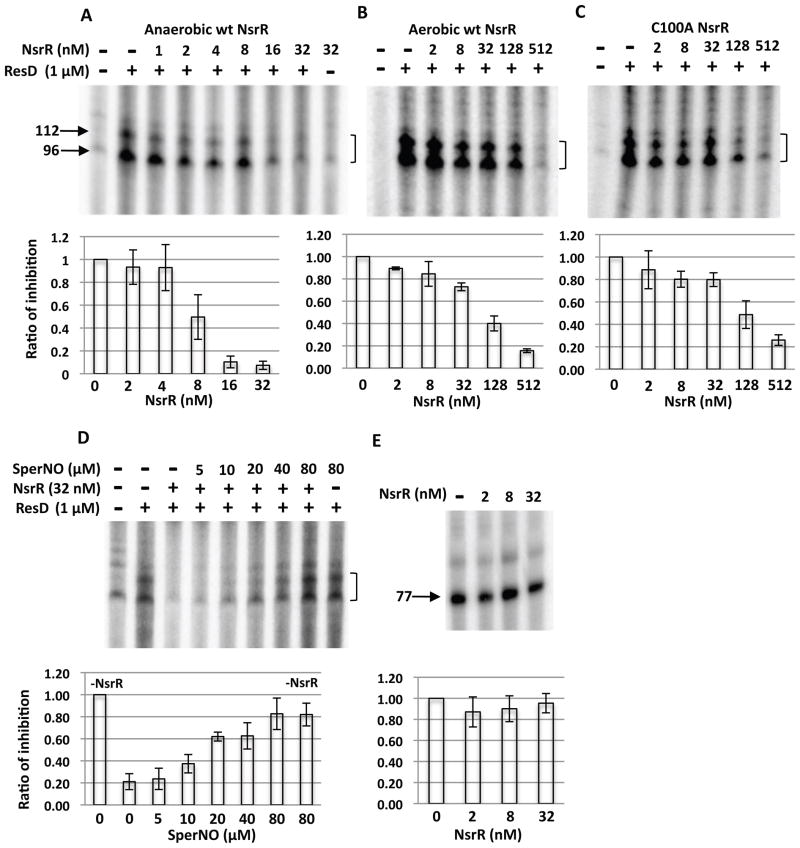
Binding assay of NsrR to the nasD promoter. The sequence of the nasD probe (−44 to −19) that contains the proposed NsrR-binding site is shown in Figure 1. The radiolabelled probe (0.1 nM) was incubated with increasing concentrations of wild-type NsrR-His6 purified under anaerobic conditions (A), aerobic conditions (B), or with the NsrR (C100A) mutant purified under anaerobic conditions (C). When anaerobically purified NsrR was used, the binding reaction as well as gel electrophoresis was carried out under anaerobic conditions as described in Experimental procedures. A single asterisk shows the double-stranded DNA and a double asterisk shows the single-stranded DNA, unannealed radiolabelled DNA oligonucleotide.
D. ImageJ was used to quantify the ratio of shifted band to total double-stranded probe bands from multiple EMSA experiments (n=6 for A and n=3 for B and C) and the average values are shown with standard deviations. Symbols: closed circles, anaerobically purified NsrR; open circles, aerobically purified NsrR; closed triangles, anaerobically purified NsrR(C100A) mutant protein. The inset shows a binding curve with anaerobic NsrR at concentrations less than 32 nM.
E. Competition assay. Anaerobically purified wild-type NsrR and radiolabelled nasD probe DNA were used with or without excess cold nasD DNA of the corresponding sequence.
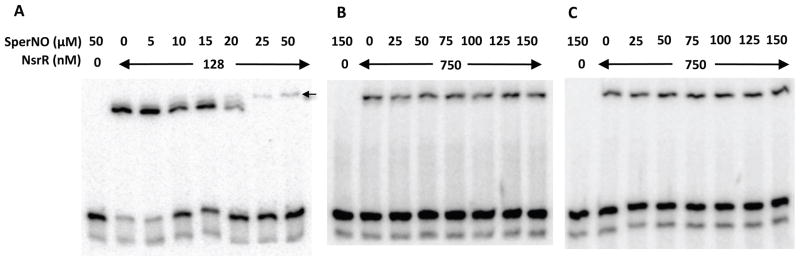
Figure 4.
Effect of spermine NONOate on DNA-binding activity of NsrR. The nasD DNA (−44 to −19) was incubated with indicated concentrations of either wild-type NsrR-His6 purified under anaerobic conditions (A), aerobic conditions (B), or C100A mutant NsrR (C). Increasing concentrations of SperNO were added to the reaction as indicated. EMSA reactions and electrophoresis were carried out in an anaerobic chamber as described in Experimental procedures.
The aerobically purified NsrR (apo-NsrR) contains essentially no Fe-S cluster as judged by the measurement of Fe (less than 0.75% incorporation of the [4Fe-4S] cluster). The binding of apo-NsrR to nasD was much weaker compared to [4Fe-4S]-NsrR and the Kd was too high to be assigned at least within the range of concentrations used (Fig. 2B). These results demonstrated that the [4Fe-4S] cluster is essential for high-affinity binding of NsrR to the target promoter.
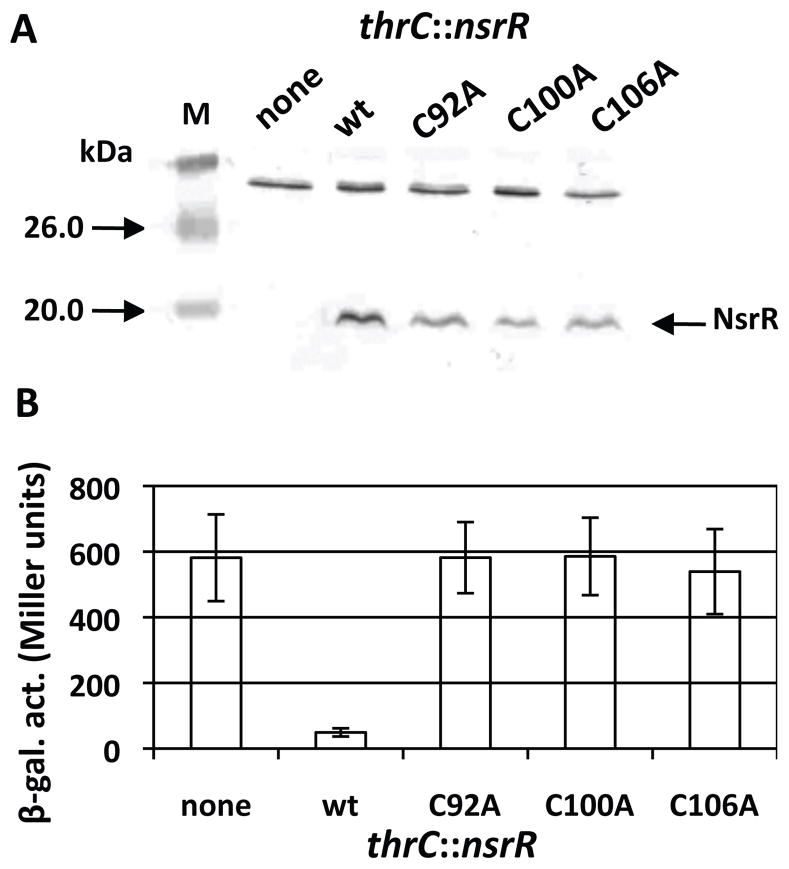
NsrR has three cysteines that likely serve to coordinate the [4Fe-4S] cluster. To assess the importance of cysteines for NsrR function, mutant NsrR proteins with Cys to Ala substitutions were produced. Western blot analysis showed that all mutant proteins were produced in B. subtilis (Fig. 3A). nasD expression was severely repressed in cells producing the wild-type NsrR, whereas the expression was derepressed in cells producing the mutant proteins to a level similar to that in the nsrR null mutant (Fig. 3B). This result demonstrated that the three cysteine residues are important for NsrR repressor activity.
Figure 3.
Effect of cysteine mutations on in vivo NsrR activity.
A. Western blot analysis of the wild-type and mutant NsrR in B. subtilis. Cell lysate was prepared from a culture of an nsrR null mutant (none) as well as from the mutant carrying ectopically expressed wild-type nsrR (wt) or mutant alleles of nsrR (C92A, C100A, or C106A) at the thrC locus. The same amount of total protein from each lysate was resolved on an SDS-polyacrylamide gel and NsrR was detected by anti-NsrR antibody as described in Experimental procedures.
B. Strains carrying nasD-lacZ were grown in 2xYT supplemented with 0.5% glucose, 0.5% pyruvate, and appropriate antibiotics. Samples were collected at 1-h intervals to measure β-galactosidase activity and the activity at T1 (1 h after the end of exponential growth) is shown. The activities were measured at least three times in cultures of two independent clones and the average values of the data are presented along with standard deviations.
The C100A mutant protein was purified from E. coli to determine whether the loss of repressor activity was caused by a weaker binding affinity for the nasD promoter due to the lack of the [4Fe-4S] cluster. No measurable iron was detected in the mutant protein when purified anaerobically by the same protocol as used for wild-type NsrR purification, suggesting that the cysteine is important for ligation of the [4Fe-4S] cluster. NsrR(C100A) protein showed a weak DNA-binding activity equivalent to the aerobically purified apo-NsrR (compare Figs. 2B and 2C), confirming the requirement of the Fe-S cluster in efficient DNA binding to repress transcription.
Spermine NONOate abrogates binding of NsrR to nasD
To address the effect of NO on binding of NsrR to nasD, we used a NO donor spermine NONOate (hereafter referred to as SperNO). One mole of SperNO generates two moles of NO with a half-life of 39 min at 37°C and 230 min at 20–25°C (at pH 7.4, Cayman Chemical). Under our EMSA conditions, 1 μM or less NO is likely released from 10 μM SperNO solution. SperNO, at a concentration of 5 to 10 μM, inhibited the binding of [4Fe-4S]-NsrR (Fig. 4A) as seen by an increase of the free probe. At higher concentrations of SperNO, a small amount of a nasD-NsrR complex with a slower electrophoretic mobility appeared (marked with an arrow in Fig. 4A). This NO-induced complex migrated to the same position in a native gel as a complex formed with apo-NsrR (data not shown), suggesting that this complex was formed with nasD and apo-NsrR (see Fig. 7C as well). In contrast to [4Fe-4S]-NsrR, binding of apo-NsrR and NsrR(C100A) to nasD was not affected by SperNO (Figs. 4B and 4C). Taken together, these results clearly showed that the [4Fe-4S] cluster is essential for NO-responsive NsrR activity.
Figure 7.
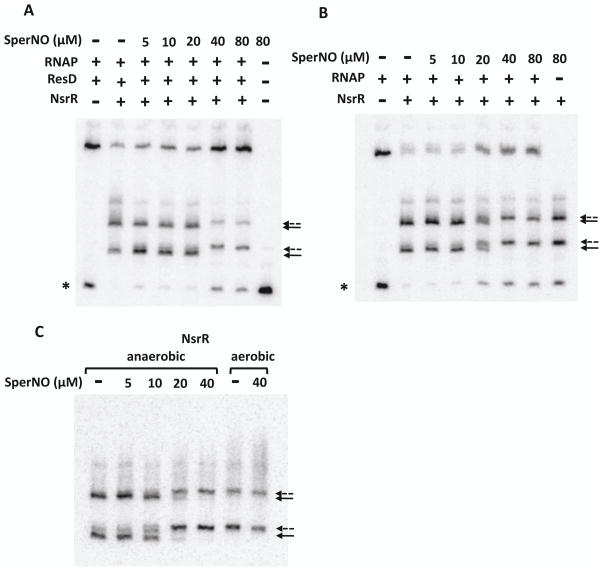
Effect of spermine NONOate on DNA-binding activity of NsrR. The nasD (−114 to −4) probe was incubated with RNAP (15 nM), ResD∼P (0.5 μM), and anaerobically purified NsrR-His6 (128 nM) (A), or with RNAP and NsrR-His6 (B) in the absence or presence of increasing concentrations of SperNO. Full arrows and broken arrows show the nasD-NsrR complex formed in the absence and presence of SperNO, respectively. A single asterisk shows the free probe.
C. The nasD probe was incubated with either anaerobically or aerobically purified NsrR-His6 in the absence or presence of SperNO. The free probe ran off the gel. All EMSA reactions and electrophoresis were carried out in an anaerobic chamber.
NsrR represses ResD-controlled nasD transcription in vitro and NO partially alleviates repression
To assess whether the EMSA results described above explain the negative effect of NsrR on nasD transcription as well as the antagonistic role of NO in NsrR-dependent repression, we performed in vitro transcription assays of nasD. Compared to apo-NsrR (Fig. 5B) and NsrR(C100A) (Fig. 5C), [4Fe-4S]-NsrR was able to repress the transcription at much lower concentrations (Fig. 5A). Quantitative analysis using ImageJ showed that minimal concentrations required for repression were slightly variable in the case of anaerobically purified NsrR, which is likely caused by variations in Fe-S cluster incorporation from prep to prep (Fig. 5A). SperNO at 20 μM largely relieved NsrR-dependent nasD repression (Fig. 5D), but had no significant effect on nasD transcription in the absence of NsrR, confirming that NO stimulates nasD transcription by alleviating NsrR-dependent repression. Transcription of rpsD encoding ribosomal protein S4 was not repressed by NsrR (Fig. 5E), which is consistent with in vivo result (Nakano et al., 2006).
Figure 5.
In vitro transcription of nasD. The nasD template (−170 to +96) was incubated without or with σARNAP, ResD∼P (1 μM) and increasing concentrations of wild-type NsrR-His6 purified under anaerobic conditions (A), aerobic conditions (B) or C100A mutant NsrR (C). An arrow with numbers shows the size of transcript in nucleotides. A bracket shows two nasD transcripts (96 and 112 base) that are generated by transcription in vitro as previously described (Geng et al., 2004) and the 96-base transcript corresponding to RNA transcribed in vivo.
D. Effect of spermine NONOate on repressor activity of NsrR. SperNO at the indicated concentration was added in the reaction with anaerobically purified NsrR.
E. In vitro transcription of rpsD using anaerobically purified NsrR. The 77 base transcript is marked.
The intensity of the corresponding bands was quantified with ImageJ and is shown as the ratio of transcript level in the presence of NsrR to that in the absence of NsrR (A, B, C and E), or as the ratio of transcript level in the presence of NsrR (and SperNO) to that in the absence of NsrR/SperNO (D). The average values (n=6 for A and n=3 for the rest) are shown with standard deviation.
NsrR inhibits the interaction of RNA polymerase-ResD∼P with the nasD promoter and NO relieves the inhibition
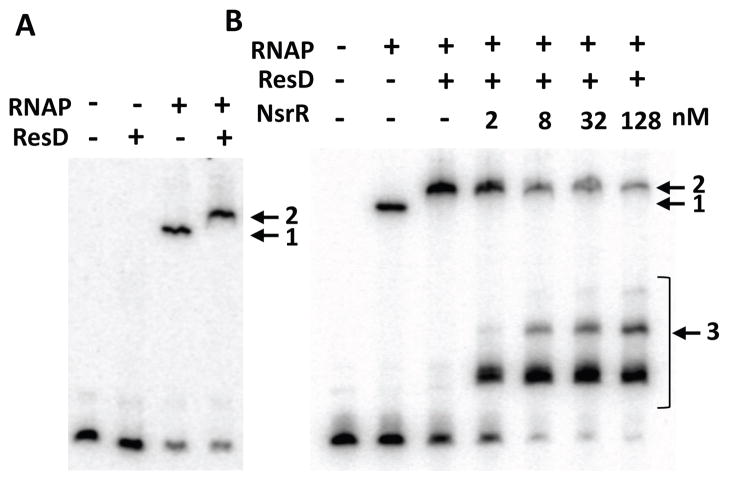
The EMSA results described above argue that the binding of NsrR to the −35 region plays a pivotal role in NsrR-dependent repression of nasD. As the ResD-ResE signal transduction proteins are essential for activation of nasD (Nakano et al., 1998), we examined how NsrR interferes with ResD-dependent transcription of nasD. To this end, anaerobic EMSA experiments were carried out with a longer nasD (−114 to −4) probe that also includes the previously identified ResD-binding region (Geng et al., 2004, Nakano et al., 2000) (Fig. 1). ResD∼P alone at 0.5 μM did not bind to the nasD promoter, whereas RNA polymerase (RNAP) as low as 15 nM interacted with the probe (shown by the arrow with #1 in Fig. 6A). When RNAP was present, 0.5 μM ResD∼P supershifted the nasD-RNAP complex (the arrow with #2). These data suggested that the role of ResD∼P in nasD transcription is not simply to recruit RNAP to the promoter.
Figure 6.
Analysis of RNAP, ResD∼P, and NsrR binding to the nasD promoter. The nasD probe encompassing −114 and −4 of the promoter region was used. All EMSA reactions and electrophoresis were carried out in an anaerobic chamber.
A. The probe (0.2 nM) was incubated with 0.5 μM ResD phosphorylated with 0.5 μM ResE, 15 nM RNAP, or both.
B. The probe was incubated with 15 nM RNAP in the absence or presence of 0.5 μM ResD∼P and increasing concentrations of NsrR-His6 purified under anaerobic conditions.
To determine whether [4Fe-4S]-NsrR disrupts the preformed nasD-RNAP-ResD∼P transcription initiation complex, we allowed the nasD-RNAP-ResD∼P ternary complex to form, followed by addition of NsrR. NsrR at 2 nM started to bind the probe and increasing concentrations of NsrR displaced the preformed ternary complex, which was accompanied by formation of multiple nasD-NsrR complexes (the arrow with #3 in Fig. 6B). These results indicated that [4Fe-4S]-NsrR efficiently disrupts the nasD-RNAP-ResD∼P initiation complex by binding to the nasD promoter and that the second and third NsrR-binding sites exist in the region between −114 and −4 (Fig. 6B). The nasD-RNAP complex was also disrupted in the presence of 8 nM NsrR, and a small amount of a supershifted band appeared at higher concentrations of NsrR (the arrow with #2 in Fig. S3A). This band could represent nasD-RNAP-NsrR complex resulted from binding of apo-NsrR to the upstream nasD as described later. Although ResD∼P does not efficiently bind to nasD as shown in Figure 6A, it weakly binds at 1 μM (the arrow with #4 in Fig. S3B). In the presence of 2 nM NsrR, a supershifted band appeared, which was likely formed by binding of ResD∼P and [4Fe-4S]-NsrR to nasD.
As observed in EMSA using a shorter probe (Fig. 4A), the presence of SperNO at concentrations of 20 to 40 μM and higher altered the electrophoretic mobility of the nasD-NsrR complexes (shown with solid arrows in Fig. 7), generating new complexes (broken arrows) with slower mobilities. At these SperNO concentrations, we also observed a simultaneous increase in the amount of nasD-RNAP-ResD∼P ternary complex and free probe (Fig. 7A). Similarly, SperNO restored nasD-RNAP binary complex formation (Fig. 7B). SperNO showed no significant effect on the binding of either RNAP alone or RNAP-ResD∼P to nasD (data not shown), demonstrating that SperNO is directed only at [4Fe-4S]-NsrR.
What is the nature of the nasD-NsrR complex generated by SperNO? Figure 7C shows that the complexes formed by anaerobically purified NsrR in the presence of SperNO migrated at the same position in a native gel as those formed by aerobically purified apo-NsrR. In this experiment we used 128 nM NsrR, the concentration of which was shown to be sufficient for apo-NsrR to bind to nasD (Fig. 2B). As shown in Figure 2B, 8 nM apo-NsrR is unable to bind the nasD probe carrying only the primary NsrR-binding site. If the shifted band with the slowest mobility that was generated by SperNO is indeed the nasD-apo-NsrR complex, then the band should not be generated when 8 nM NsrR is used. The result showed that it is indeed the case (data not shown). Therefore, the SperNO-induced nasD-NsrR complexes in Figures 4A and 7 are likely formed with apo-NsrR either present in anaerobically purified NsrR protein or generated by SpeNO, although we could not completely eliminate the possibility that the complex contains DNIC-NsrR.
Figure 8.
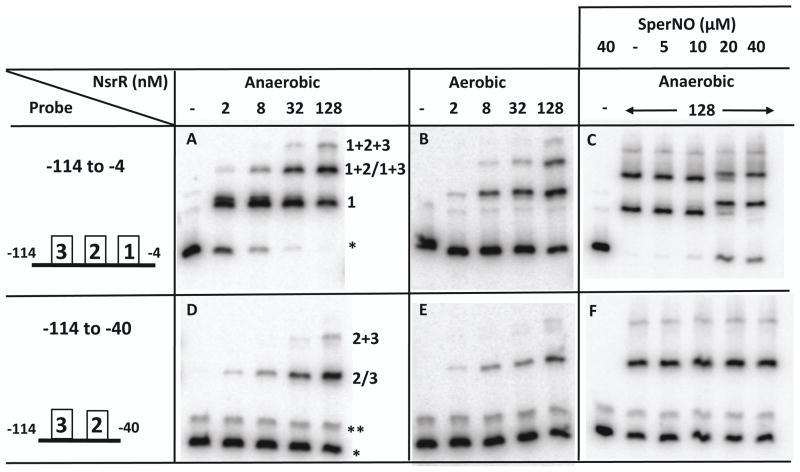
Binding of NsrR to the nasD promoter with or without the primary NsrR-binding site. Two nasD DNA fragments were used for probes, one of which (−114 to −4) contains the primary NsrR-binding site, and the other (−114 to −40) lacks the binding site. Increasing concentrations of anaerobically or aerobically purified NsrR-His6 and SperNO were used as indicated. A single asterisk shows a free probe and a double asterisk shows an unidentified DNA fragment generated in small amounts by PCR (note that this DNA does not bind to NsrR). Schematic views of the probe are shown in the leftmost panels and include the primary NsrR-binding site (marked with boxed 1) and secondary binding sites (marked with boxed 2 and 3). The three shifted bands in panel A correspond to the NsrR complexes interacting with the binding site 1 (shown as 1), binding site 1 and 2 or 1 and 3 (1+2/1+3), and the binding site 1, 2, and 3 (1+2+3) of the nasD probe. The two shifted bands in panel D correspond to the NsrR complexes interacting with the binding site 2 or 3 (2/3), and the binding site 2 and 3 (2+3).
Apo-NsrR binds to the ResD-binding region of nasD
The results described in Figures 6 and 7 suggested that NsrR interacts with nasD by binding to the second and third sites in addition to the primary site around the −35 region. To understand the role of the secondary binding sites in regulation of nasD transcription, we determined the location of the secondary NsrR-binding sites and whether binding affinities of these cis elements are different between [4Fe-4S]-NsrR and apo-NsrR. To localize the secondary sites, we generated a probe (−114 to −40) that lacks the primary NsrR-binding site. Three important results were obtained by comparing EMSA results of NsrR between the full-length (−114 to −4) probe and the deleted (−114 to −40) probe. First, at higher concentrations of NsrR, three nasD-NsrR complexes were formed with the full-length probe, while only two complexes were detected with the deleted probe (Fig. 8). Based on this result, we concluded that the second and third NsrR-binding sites locate in the region between −114 and −40. Second, when the full-length probe was used, the nasD-NsrR complex with the fastest mobility (marked as #1 in Fig. 8A) was formed with [4Fe-4S]-NsrR at much lower concentrations than with apo-NsrR (compare Figs. 8A and 8B), whereas [4Fe-4S]-NsrR and apo-NsrR bound to the deleted probe with a similar affinity to form the first complex (marked #2/3, and compare Figs. 8D and 8E). Furthermore, apo-NsrR bound similarly to these three sites (compare Fig. 8B and 8E). These results argued that the NsrR-binding site around the −35 element is the only site that [4Fe-4S]-NsrR binds with a higher affinity than apo-NsrR does. Third, the nasD promoter lacking the primary NsrR-binding site did not generate NO-specific complexes with slow elecrophoretic mobilities (compare Figs. 8C and 8F), which is in good agreement with the interaction of apo-NsrR with the upstream binding sites.
To further define the location of the upstream NsrR-binding sites, we generated four overlapping short double-stranded oligonucleotides that cover the region between −114 to −40 of nasD (Fig. 1). To determine with which probe apo-NsrR interacts, we performed EMSA experiment (Fig. S4). The result showed that the two binding sites reside in −93 to −63 and −71 to −40 of nasD. The probe from −104 to −75 exhibited a very weak activity, suggesting that the region between −93 and −75 serves as a core-binding site and DNA from −74 to −63 is required for stabilizing the nasD-NsrR complex. EMSA using [4Fe-4S]-NsrR showed an almost identical result (data not shown). Interestingly the region from −93 to −40 where apo-NsrR binds was identified as the ResD-binding site in our previous studies (Geng et al., 2004, Nakano et al., 2000).
A high concentration of NsrR is detected in B. subtilis cells
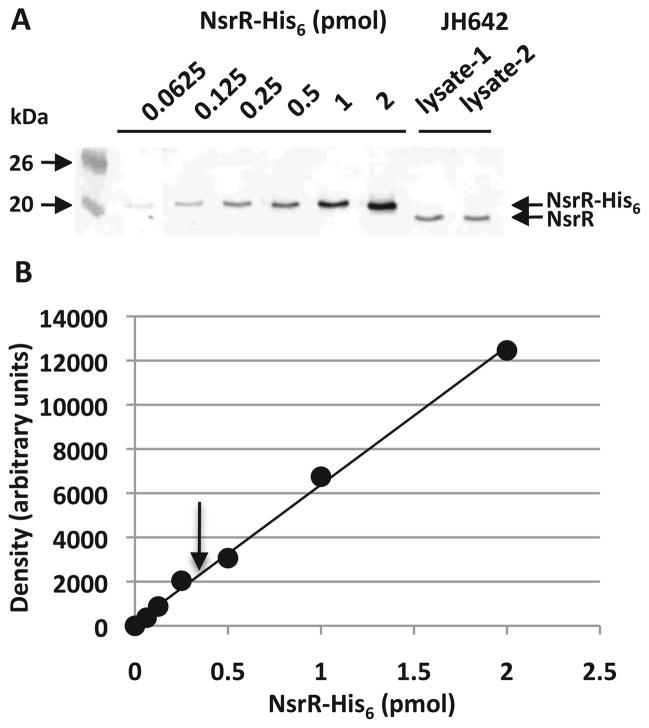
The results described above showed that apo-NsrR binds to the nasD regulatory region that overlaps with ResD-binding site. The question remains whether the interaction between apo-NsrR and nasD is physiologically relevant, particularly because the affinity of apo-NsrR to these sites is weaker than that of [4Fe-4S]-NsrR to the primary binding element. To evaluate whether there is a good possibility that the apo-NsrR-DNA interaction occurs in vivo, we measured NsrR concentration in B. subtilis cells using quantitative western blotting as previously described (Cai & Inouye, 2002) with some modification (Experimental procedures) (Fig. 9). As a standard, we used various concentrations of the purified NsrR-His6 protein. The cell lysate prepared from B. subtilis JH642 was resolved on an SDS-PAGE gel together with the standard NsrR protein. Polyclonal anti-His6-NsrR antiserum (Nakano et al., 2006) was used to determine concentrations of NsrR in the western blot. We repeated the experiments using three independently prepared cell lysate and three different purification batches of NsrR proteins as standards. The aqueous volume of a B. subtilis cell was estimated as 1 x 10−14 liter by previous study (McCabe & Gollnick, 2004). By calculating with this value of cell volume, we found that in vivo NsrR concentration is 1.34 ± 0.21 μM (n=7), which corresponds to 8,066 ± 1,264 monomers in the cell. The result, together with the EMSA result in Figure 8, suggests that the cellular NsrR concentration is likely sufficient to allow NsrR to interact with low-affinity binding sites of nasD.
Figure 9.
Determination of in vivo NsrR concentration in B. subtilis cells using quantitative western blot analysis.
A. Western blot of increasing amounts of purified NsrR-His6 together with B. subtilis cell lysate pepared from independent cultures. Molecular weight markers are marked.
B. The standard curve was plotted by quantifying band densitities of NsrR-His6 shown in panel A. An arrow indicates the intensity of NsrR detected in the cell lysate shown in panel A.
Discussion
The NO-sensitive transcription regulator NsrR plays an important role in the control of the NO stress response in a wide range of bacteria [reviewed in (Tucker et al., 2010)]. We presented data here to confirm our earlier study (Yukl et al., 2008) that B. subtilis NsrR proteins, when purified from either aerobic or anaerobic E. coli cultures, carry a [4Fe-4S] cluster. In addition, while revising this manuscript, we successfully purified the C-terminal His6-NsrR from B. subtilis (see supporting information). NsrR purified from the native host carries a [4Fe-4S] cluster and the Fe-S incorporation (average 65%) was much higher than the heterologously expressed protein (average 28%). The NsrR protein behaves similarly to the protein purified from E. coli with respect to DNA-binding activity and NO sensitivity (Fig. S5). From these results we conclude that Bs-NsrR contains a [4Fe-4S] cluster in B. subtilis. The [4Fe-4S] cluster is maintained in aerobic cultures presumably because the concentration of intracellular oxygen remains sufficiently low. In addition, glutathione was postulated to be involved in stabilizing or reactivating oxidized Fe-S clusters (Song et al., 2006) and a similar role might be played by low-molecular-weight thiols such as cysteine and bacillithiol (Newton et al., 2009) in B. subtilis that lacks glutathione. In either case, the higher in vivo sensitivity of NsrR to NO than to oxygen is apparent from the previous in vivo result that ResD-independent hmp expression is repressed by NsrR in B. subtilis cells cultured aerobically and the repression is relieved by SperNO (Nakano et al., 2006).
Although NsrR-dependent repression of nasD is relieved by SperNO, NO-dependent upregulation of nasD transcription still requires ResD. We have previously shown that certain amino acid residues of the carboxy-terminal domain of the α subunit (αCTD) are required for ResD-dependent transcription of fnr (Geng et al., 2007) and nasD (H.G. and M.M.N. unpublished results). Transcriptional activators that requireαCTD are known to bind upstream of the −35 element and recruit RNAP via interaction with αCTD (Busby & Ebright, 1999). This recruitment model of gene activation could not explain how ResD∼P plays a role in activation of nasD, as RNAP alone binds to nasD and stimulates binding of ResD∼P to nasD (Fig. 6A). We noticed that the nasD promoter contains a 5′-TtTG-3′ sequence one base pair upstream of the −10 hexamer (5′-TGTGCT-3′) (the downstream 5′-TGCTACGCT-3′ sequence, which overlaps with the putative −10 sequence, could also serve as an extended −10 promoter). “Extended −10 promoters” carrying the 5′-TG-3′ (and often 5′-TRTG-3′) motif have been found in E. coli (Mitchell et al., 2003) and are more common in B. subtilis (Helmann, 1995, Voskuil & Chambliss, 1998). E. coli extended −10 promoters generally show weak matches to the consensus −35 element, whereas B. subtilis extended −10 promoters have highly conserved −35 sequences. Given that the nasD promoter contains a non-canonical −10 hexamer (TGTGCT) and lacks −35 hexamer, one could envisage that the interaction between ResD∼P and αCTD assists in productive binding of RNAP to nasD.
This study showed that the preformed nasD-RNAP-ResD∼P complex is dissociated by NsrR (Fig. 6B), partly, if not solely, by competing with RNAP for interaction with the high-affinity NsrR-binding site (−39 and −24) (Fig. S3). The ternary complex formation was more strongly inhibited by the higher concentrations of NsrR that promotes secondary site binding (Fig. 6B). The secondary sites were localized in two distinct regions, within −93 to −63 and −71 to −40, which overlap the previously identified ResD-binding region (around −91 to −46). Neither apo-NsrR nor [4Fe-4S]-NsrR bound the nasD(−114 to −85) fragment, indicating that apo-NsrR interacts with the secondary sites in a sequence-specific manner. Interestingly, anaerobically purified NsrR and aerobically purified NsrR bind with a similar affinity to these secondary sites, which is in sharp contrast to the primary NsrR-binding site where the [4Fe-4S] cluster is required for efficient binding.
There is a precedent for both holo- and apo-forms of Fe-S transcription regulator playing roles in gene regulation. E. coli IscR, a [2Fe-2S] cluster-carrying transcription regulator (Schwartz et al., 2001), and NsrR are similar in primary structure. IscR controls transcription of the iscRSUA and sufABCDSE operons encoding proteins that function in assembly of Fe-S clusters. Under oxidative stress and iron limiting conditions, apo-IscR activates sufA transcription (Yeo et al., 2006) and [2Fe-2S]-IscR-dependent repression of iscR is concomitantly relieved (Giel et al., 2006) in order to meet the cell’s need for Fe-S reassembly. The two forms of IscR recognize sequences specific to promoters of each class (Giel et al., 2006, Nesbit et al., 2009). A partial inverted repeat sequence ATRTATYTtAAATATAT was previously proposed as a putative B. subtilis NsrR-binding site (Nakano et al., 2006). Consistent with this notion, [4Fe-4S]-NsrR preferentially binds a 30-mer oligonucleotide that includes the putative binding site (Fig. 1 and Fig. 2). The EMSA results in Figure S4 (and see Fig. 1 for sequence) indicated that apo-NsrR-binding elements reside within two separate 30 nucleotide regions. We could not find high sequence similarity between the sites recognized by apo-NsrR and the [4Fe-4S]-NsrR-binding element. The two regions recognized by apo-NsrR show some similarity (Fig. 1), but further study is required to deduce the consensus binding sequence for apo-NsrR.
The observation that anaerobically and aerobically purified NsrR bound to the upstream sites with a similar affinity raises two alternative possibilities. One possibility is that apo- and [4Fe-4S]-NsrR indeed have a similar affinity to these sites. The other possibility is that apo-NsrR binds with a higher affinity than [4Fe-4S]-NsrR. A larger population of apo-NsrR present in anaerobically purified NsrR made it difficult to measure the binding affinity of [4Fe-4S]-NsrR in anaerobically purified protein sample. Although we could not eliminate the former possibility, we assume the latter possibility is more likely. The EMSA result with the probe (−114 to −40) lacking the high-affinity binding site indicated that the nasD complex formed by anaerobically purified NsrR did not promote the supershift usually observed in the presence SperNO (Fig. 8F and compare with Fig. 8C). The result suggests that NsrR molecules bound to the probe were almost exclusively apo-protein, in keeping with the preferential recognition of the two upstream binding sites by apo-NsrR. In the case of IscR, a recent study showed that both apo- and [2Fe-2S]-IscR bind to sufA-class promoters in a sequence-specific manner (Nesbit et al., 2009).
To our knowledge, this is the first report suggesting that apo- and [Fe-S]-NsrR interact with two distinct classes of DNA targets that have different recognition sequences. Furthermore, instead of separate sets of genes, the two classes of NsrR-target sequences reside in the single nasD gene promoter region. It remains to be determined what function these two NsrR-target sites perform in the regulation of nasD expression. Assuming that apo-NsrR concentration increases relative to [4Fe-4S]-NsrR under conditions that disrupt the Fe-S cluster formation, one could envisage apo-NsrR occupying the ResD-binding region. Simultaneously, RNAP might successfully compete for the −35 region to which apo-NsrR weakly binds. The outcome is that, instead of a nasD-RNAP-ResD complex, a nasD-RNAP-NsrR complex might be formed to initiate nasD transcription. This is a very speculative view but is worth examining in future. The results of this study also suggest that apo-NsrR might function independently of holo-NsrR in regulation of yet-unidentified genes.
Experimental procedures
Bacterial strains and culture conditions
The strains and plasmids used in this study are listed in Table S1. E. coli DH5α was used for cloning of the recombinant plasmids. E. coli BL21 (DE3)/pLysS was used to produce NsrR proteins and ER2566 (New England Biolabs) was used for production of ResD and ResE. All B. subtilis strains are isogenic derivatives of JH642. ORB6559 was constructed by transforming pCm::Sp (Steinmetz & Richter, 1994) into ORB6179 (Nakano et al., 2006), which replaces the chloramphenicol-resistance marker in nsrR with spectinomycin resistance. Antibiotic concentrations used in growth media were ampicillin (50 μg ml−1), chloramphenicol (5 μg ml−1), spectinomycin (75 μg ml−1), and erythromycin/lincomycin (1 μg ml−1and 25 μg ml−1, respectively).
Alanine substitutions of cysteine residues in NsrR
In order to substitute each cysteine in NsrR (amino acid residues 92, 100, and 106) and ectopically express the mutant nsrR genes in strain ORB6559 (nsrR:spc), the following plasmids were constructed using a thrC integration vector pDG795 (Guérout-Fleury et al., 1996). Plasmids pHG64 and pHG66, pUC18 derivatives carrying nsrR(C92A) and nsrR(C106A) respectively, were digested with EcoRI and BamHI and the nsrR genes were inserted into pDG795 digested with the same enzymes to generate pMMN666 and pMMN667, respectively. pDG795 carrying nsrR(C100A) was generated by two-step PCR as follows. Two PCR products amplified from pHG56 using oligonucleotide pairs − oHG89 (5′-AAGAATCTCGCTGTTATTTCCCCGGTT-3′)/oHG94 (5′-GAGGATCCGCTTTTGAC CTT-3′: BamHI site is italicized) and oHG90 (5′-AACCGGGGAAATAACAGCGAGA TTCTT-3′)/oHG93 (5′-CGGAATTCGCACTTGCTTTC-3′: EcoRI site is italicized). The PCR products were annealed and used as template in the second PCR with oHG93 and oHG94 as primers. The resultant PCR product was digested with EcoRI and BamHI and inserted into EcoRI/BamHI-cleaved pDG795, resulting in pMMN669. A pDG795 derivative carrying nsrR(wt), pMMN668, was constructed by subcloning the nsrR gene from pHG56 into pDG795 in a similar way described above. nsrR in each plasmid was verified by sequencing analysis. These four pDG795 derivatives were used to transform ORB6559 (nsrR::spc) with selection for erythromycin- and spectinomycin-resistance and Thr-colonies generated by a double-crossover recombination were chosen as ORB6629 [nsrR::spc thrC::nsrR(C92A)], ORB6630 [nsrR::spc thrC::nsrR(C106A)], ORB6631 [nsrR::spc thrC::nsrR (wt)], and ORB6632 [nsrR::spc thrC::nsrR(C100A)]. Strains ORB6629 to 6632 were transduced by SPβ phage carrying nasD-lacZ (Nakano et al., 1998) to generate ORB6640 to 6643, respectively.
β-Galactosidase activity measurement
Two independent isolates of each B. subtilis strain were cultured at 37°C under anaerobic conditions in 2x yeast extract-tryptone (YT) media (Nakano et al., 1988) with 0.5% glucose and 0.5% pyruvate supplemented with antibiotics, if necessary. Cells were harvested at 1-h intervals and β-galactosidase activity was measured as described (Miller, 1972). The activity at T1 (1 h after the end of the exponential growth) was shown.
Western blot analysis
Cells were cultured at 37°C in 2xYT supplemented with 0.5% glucose, 0.5% pyruvate, and appropriate antibiotics. When the optical density at 600 nm (OD600) of the cultures reached approximately 0.4, cells were harvested and cell lysate was prepared by passing through a French press. Twentyμg of total protein was applied to sodium dodecylsulfate-polyacrylamide (15%) (SDS-PAGE) gel and western blot analysis was carried out using anti-His6-NsrR antibody as described (Nakano et al., 2006).
Determination of cellular concentration of NsrR by quantitative western blot analysis
The intracellular concentration of NsrR was determined in JH642 cells grown anaerobically in 2xYT supplemented with 0.5% glucose and 0.5% pyruvate. Cells were harvested at OD600=0.4 to 0.5, and cell lysate was prepared from the 45-ml cultures using the protoplast lysis method as previously described (Baruah et al., 2004). Around 15 μg of total protein in 15 μl was applied to a 15% SDS-PAGE. NsrR-His6 was purified as described below and 0.0625 to 2 pmoles protein in 15 μl was used as standard. Detection of NsrR was carried out by western blot using anti-His6-NsrR antibody as described (Nakano et al., 2006). Colony-forming units were determined from the cultures by serial dilution and plating on LB agar.
Anaerobic and aerobic purification of NsrR-His6
NsrR-His6 protein was overproduced in E. coli BL21(DE3)/pLysS carrying pMMN740 as previously described (Yukl et al., 2008). For anaerobic protein purification, cell pellet was transferred to an anaerobic chamber (Coy Laboratory Products) containing 5% H2 and 95% N2, where the centrifuge tube was kept with its lid open until chamber oxygen level was below 5 ppm. Cell suspension was transferred into a French press cell and all French press cell components were assembled in the anaerobic chamber. Cell lysate obtained by passage through the French press was collected into a centrifuge tube while being purged with N2. Aerobic purification was performed outside the anaerobic chamber in the same way as anaerobic purification.
The NsrR(C100A) mutant protein was purified from BL21(DE3)/pLysS carrying pMMN805 under anaerobic conditions following the same procedure as for wild-type NsrR. To construct pMMN805, nsrR(C100A) was amplified by PCR using oMN05-296 (5′-GGCGCGGGCATATGAAGTTAACCAATTATAC-3′: NdeI site is italicized) and oMN05-304 (5′-CGCTCTCGAGTTCCTTCATTTTTAAAAGC-3′: XhoI site is italicized) with pMMN732 as template. The PCR product digested with NdeI and XhoI was inserted into pET23a(+) digested with the same enzymes to generate pMMN805. The nsrR coding sequence was confirmed by sequencing.
Purification of ResD, ResE, RNAP, and σA
ResD and ResE were overproduced in E. coli ER2566 (New England Biolabs) and purified as previously described (Geng et al., 2004) except that ResD protein was dialyzed against 25 mM Tris-HCl buffer (pH 8.0) containing 50 mM NaCl, 5 mM MgCl2, 5% glycerol, and 5 mM β-mercaptoethanol before storage at −80°C. RNAP and σA were purified as previously described (Nakano et al., 2006).
Measurement of protein concentration and iron content
Protein concentrations were determined by Bio-Rad protein assay using bovine serum albumin as a standard. As seen with N-terminal His6-NsrR (Yukl et al., 2008), total amino acid analysis of the C-terminal His6-NsrR (AAA Service Laboratory Inc., Damascus, Oregon) showed that the Bio-Rad protein assay overestimates NsrR concentrations by ~26%. [4Fe-4S]-NsrR concentrations were calculated accordingly. The iron content of NsrR at two different protein concentrations (1 and 4 μM for anaerobically purified protein and 10 and 20 μM for C100A mutant and aerobically purified protein) was determined with a ferene assay as described (Yukl et al., 2008) and QuantiChrom iron assay kit (BioAssay Systems). The minimum amount detected was around 0.45 μM. Both methods gave similar results.
Electrophoretic mobility shift assay (EMSA) of NsrR, ResD∼P and RNAP
EMSA was carried out under anaerobic and aerobic conditions as previously described except that the reaction buffer was slightly modified [50 mM Tris-HCl, pH 7.5, 100 mM KCl, 0.5 mM DTT, 5 mM MgCl2, 5 μg ml−1 BSA, 10 μg ml−1 poly(dI-dC), 10% glycerol] and native gels were run in TGE buffer (50 mM Tris, 0.38 M glycine, 2 mM EDTA; pH not adjusted). For anaerobic experiments, EMSA was performed in the anaerobic chamber as carried out with other Fe-S proteins (Edwards et al., 2010, Reents et al., 2006). The solutions and electrophoresis buffer were degassed by purging with N2 and were transferred to the anaerobic chamber one day before the experiment. Polyacrylamide gels assembled with the electrophoresis apparatus were placed in the anaerobic chamber and allowed to stand for at least 2 to 3 h before the gel was pre-run at 150 V for 1h. For EMSA using only NsrR, a shorter nasD probe (−45 to −20) was generated by annealing complementary 30-mer oligonucleotides oMN09-489 (5′-GCATAACATGTATCTTAAATATTCCTTTCG-3′; coding strand) and oMN09-491 (5′-CGAAAGGAATATTTAAGATACATGTTATGC-3′; noncoding strand). The annealed oligonucleotides cover the putative NsrR-binding site of nasD (Nakano et al., 2006). oMN09-489 (2 μM) was radiolabelled using 50 μCi [γ-32P]-ATP (800 Ci/mmol) and T4 polynucleotide kinase at 37°C for 30 min. The reaction was stopped by heating at 65°C for 15min. The labelled oligonucleotide was purified using nucleotide removal kit (Qiagen) and was mixed with 2 μM of unlabelled oMN09-491 in New England Biolabs restriction enzyme buffer 2. To anneal the oligonucleotides, the mixture was heated at 90°C for 5 min in a heat block filled with water and the heat block was removed from the apparatus to slowly cool to the room temperature. Four probes (−114 to −85, −104 to −75, −93 to −63, and −71 to −40) used in the experiment of Figure S4 were generated similarly by annealing a labelled oligonucleotide shown in Figure 1 and the complementary unlabelled noncoding strand. Approximately 0.1 nM of the probe was used in 20 μl of the reaction buffer without or with NsrR.
For EMSA using ResD∼P, RNAP, and NsrR, a longer nasD probe (−114 to −4) containing ResD-binding sites (Geng et al., 2004, Nakano et al., 2000) and the NsrR-binding site was generated as follows. Oligonucleotide oSK42 (5′-CCGTCCGAATCATACCTATT-3′) was radiolabelled using T4 polynucleotide kinase and [γ-32P]-ATP. The probe DNA was generated by PCR from pMMN406 (Nakano et al., 2000) using labelled oSK42 and unlabelled oSK43 (5′-AGCGTAGCACAGCAAAAAGG-3′). The amplified PCR product was purified by PCR purification kit (Qiagen). A nasD probe (−114 to −40) that lacks the primary NsrR-binding site was similarly generated using oligonucleotides oSK42 and oSK79 (5′-GTTATAAAATGTAACAAAATATACG-3′).
To determine the effect of NsrR on a preformed nasD-RNAP complex, the nasD probe (around 0.2 nM) and RNAP (15 nM) were mixed in 20 μl of the EMSA reaction buffer. To determine the effect on the nasD-ResD∼P, or nasD-RNAP-ResD∼P complex, ResD (0.5 μM) was phosphorylated with ResE (0.5 μM) in 20 μl of the EMSA reaction buffer, to which nasD probe and RNAP (for the ternary complex formation) were added. The reaction mixture containing the preformed binary or ternary complex was incubated for 10 min at room temperature. Increasing concentrations of NsrR were added to the reaction, which was further incubated for 15 min. To examine the effect of NO, SperNO (dissolved in 10 mM NaOH) was added to the reaction 10 min after the addition of NsrR, and incubated at room temperature for additional 20 min. As a control 10 mM NaOH at the same volume as the SperNO solution was included in the reaction. After complexes were resolved by polyacrylamide gel electrophoresis, the gel was dried and radioactive bands were analysed with a Typhoon Trio+ variable imager (GE Healthcare). The intensity of bands was quantified using ImageJ (NIH).
In vitro transcription assay
The effect of NsrR on ResD-activated nasD transcription was determined using in vitro transcription assays as previously described (Nakano et al., 2006) with minor modifications. In short, a nasD template (−170 to +96 with respect to the transcription start site) was generated by PCR using oSK-34 (5′-TTTAATCGGGGAAGCCTTAGA-3′) and oHG-1 (5′-TATCTCTTCAATGGCCCTTA-3′) and purified by low-melting agarose gel and PCR purification kit (Qiagen). As a control, an rpsD promoter template was generated by PCR using oSN03-86 and oSN03-87 (Nakano et al., 2006). All reactions were performed in the Coy anaerobic chamber except otherwise stated. ResD (1 μM) and ResE (1 μM) were mixed in the transcription buffer (Nakano et al., 2006) containing RNasin (Promega) and incubated at room temperature for 10 min. Then 5 nM of the nasD template, NsrR at different concentrations, RNAP (15 nM), and σA (7.5 nM) were added and incubated at room temperature for 10 min, followed by the addition of SperNO, where indicated. Transcription was initiated by adding [α-32P]-UTP and NTPs. The reaction mixture was further incubated for 10 min. After the reaction was stopped, samples were taken out from the chamber, precipitated with ethanol, and separated on a pre-run 6% polyacrylamide-urea gel at 500 V for approximately 40 min. The gel was dried and analysed with a Typhoon Trio+ variable imager and the intensity of bands was quantified using ImageJ.
Supplementary Material
Acknowledgments
We thank Peter Zuber for valuable comments on the manuscript and Cole Zuber for technical assistance. This work was supported in part by a grant GM74785 (P.M-L.) from the National Institute of Health, MCB0110513 (M.M.N.) from the National Science Foundation, and Vertex pharmaceutical scholarship (T.H.).
References
- Bang IS, Liu L, Vazquez-Torres A, Crouch ML, Stamler JS, Fang FC. Maintenance of nitric oxide and redox homeostasis by the Salmonella flavohemoglobin hmp. J Biol Chem. 2006;281:28039–28047. doi: 10.1074/jbc.M605174200. [DOI] [PubMed] [Google Scholar]
- Baruah A, Lindsey B, Zhu Y, Nakano MM. Mutational analysis of the signal-sensing domain of ResE histidine kinase from Bacillus subtilis. J Bacteriol. 2004;186:1694–1704. doi: 10.1128/JB.186.6.1694-1704.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodenmiller DM, Spiro S. The yjeB (nsrR) gene of Escherichia coli encodes a nitric oxide-sensitive transcriptional regulator. J Bacteriol. 2006;188:874–881. doi: 10.1128/JB.188.3.874-881.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busby S, Ebright RH. Transcription activation by catabolite activator protein (CAP) J Mol Biol. 1999;293:199–213. doi: 10.1006/jmbi.1999.3161. [DOI] [PubMed] [Google Scholar]
- Cai SJ, Inouye M. EnvZ-OmpR interaction and osmoregulation in Escherichia coli. J Biol Chem. 2002;277:24155–24161. doi: 10.1074/jbc.M110715200. [DOI] [PubMed] [Google Scholar]
- Corker H, Poole RK. Nitric oxide formation by Escherichia coli. Dependence on nitrite reductase, the NO-sensing regulator Fnr, and flavohemoglobin Hmp. J Biol Chem. 2003;278:31584–31592. doi: 10.1074/jbc.M303282200. [DOI] [PubMed] [Google Scholar]
- Edwards J, Cole LJ, Green JB, Thomson MJ, Wood AJ, Whittingham JL, Moir JW. Binding to DNA protects Neisseria meningitidis fumarate and nitrate reductase regulator (FNR) from oxygen. J Biol Chem. 2010;285:1105–1112. doi: 10.1074/jbc.M109.057810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang FC. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Microbiol. 2004;2:820–832. doi: 10.1038/nrmicro1004. [DOI] [PubMed] [Google Scholar]
- Filenko N, Spiro S, Browning DF, Squire D, Overton TW, Cole J, Constantinidou C. The NsrR regulon of Escherichia coli K-12 includes genes encoding the hybrid cluster protein and the periplasmic, respiratory nitrite reductase. J Bacteriol. 2007;189:4410–4417. doi: 10.1128/JB.00080-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng H, Nakano S, Nakano MM. Transcriptional activation by Bacillus subtilis ResD: Tandem binding to target elements and phosphorylation-dependent and -independent transcriptional activation. J Bacteriol. 2004;186:2028–2037. doi: 10.1128/JB.186.7.2028-2037.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng H, Zhu Y, Mullen K, Zuber CS, Nakano MM. Characterization of ResDE-dependent fnr transcription in Bacillus subtilis. J Bacteriol. 2007;189:1745–1755. doi: 10.1128/JB.01502-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giel JL, Rodionov D, Liu M, Blattner FR, Kiley PJ. IscR-dependent gene expression links iron-sulphur cluster assembly to the control of O2-regulated genes in Escherichia coli. Mol Microbiol. 2006;60:1058–1075. doi: 10.1111/j.1365-2958.2006.05160.x. [DOI] [PubMed] [Google Scholar]
- Gilberthorpe NJ, Lee ME, Stevanin TM, Read RC, Poole RK. NsrR: a key regulator circumventing Salmonella enterica serovar Typhimurium oxidative and nitrosative stress in vitro and in IFN-gamma-stimulated J774.2 macrophages. Microbiology. 2007;153:1756–1771. doi: 10.1099/mic.0.2006/003731-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilberthorpe NJ, Poole RK. Nitric oxide homeostasis in Salmonella typhimurium: roles of respiratory nitrate reductase and flavohemoglobin. J Biol Chem. 2008;283:11146–11154. doi: 10.1074/jbc.M708019200. [DOI] [PubMed] [Google Scholar]
- Gross SS, Wolin MS. Nitric oxide: pathophysiological mechanisms. Annu Rev Physiol. 1995;57:737–769. doi: 10.1146/annurev.ph.57.030195.003513. [DOI] [PubMed] [Google Scholar]
- Guérout-Fleury AM, Frandsen N, Stragier P. Plasmids for ectopic integration in Bacillus subtilis. Gene. 1996;180:57–61. doi: 10.1016/s0378-1119(96)00404-0. [DOI] [PubMed] [Google Scholar]
- Helmann JD. Compilation and analysis of Bacillus subtilis sigma A-dependent promoter sequences: evidence for extended contact between RNA polymerase and upstream promoter DNA. Nucleic Acids Res. 1995;23:2351–2360. doi: 10.1093/nar/23.13.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heurlier K, Thomson MJ, Aziz N, Moir JW. The nitric oxide (NO)-sensing repressor NsrR of Neisseria meningitidis has a compact regulon of genes involved in NO synthesis and detoxification. J Bacteriol. 2008;190:2488–2495. doi: 10.1128/JB.01869-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isabella V, Wright LF, Barth K, Spence JM, Grogan S, Genco CA, Clark VL. cis- and trans-acting elements involved in regulation of norB (norZ), the gene encoding nitric oxide reductase in Neisseria gonorrhoeae. Microbiology. 2008;154:226–239. doi: 10.1099/mic.0.2007/010470-0. [DOI] [PubMed] [Google Scholar]
- Isabella VM, Lapek JD, Jr, Kennedy EM, Clark VL. Functional analysis of NsrR, a nitric oxide-sensing Rrf2 repressor in Neisseria gonorrhoeae. Mol Microbiol. 2009;71:227–239. doi: 10.1111/j.1365-2958.2008.06522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji XB, Hollocher TC. Reduction of nitrite to nitric oxide by enteric bacteria. Biochem Biophys Res Commun. 1988;157:106–108. doi: 10.1016/s0006-291x(88)80018-4. [DOI] [PubMed] [Google Scholar]
- McCabe BC, Gollnick P. Cellular levels of trp RNA-binding attenuation protein in Bacillus subtilis. J Bacteriol. 2004;186:5157–5159. doi: 10.1128/JB.186.15.5157-5159.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JH. Experiments in molecular genetics. Cold Spring Harbor Laboratory; Cold Spring Harbor, N.Y: 1972. [Google Scholar]
- Mitchell JE, Zheng D, Busby SJ, Minchin SD. Identification and analysis of 'extended -10' promoters in Escherichia coli. Nucleic Acids Res. 2003;31:4689–4695. doi: 10.1093/nar/gkg694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CM, Nakano MM, Wang T, Ye RW, Helmann JD. Response of Bacillus subtilis to nitric oxide and the nitrosating agent sodium nitroprusside. J Bacteriol. 2004;186:4655–4604. doi: 10.1128/JB.186.14.4655-4664.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano MM. Induction of ResDE-dependent gene expression in Bacillus subtilis in response to nitric oxide and nitrosative stress. J Bacteriol. 2002;184:1783–1787. doi: 10.1128/JB.184.6.1783-1787.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano MM, Geng H, Nakano S, Kobayashi K. The nitric oxide-responsive regulator NsrR controls ResDE-dependent gene expression. J Bacteriol. 2006;188:5878–5887. doi: 10.1128/JB.00486-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano MM, Hoffmann T, Zhu Y, Jahn D. Nitrogen and oxygen regulation of Bacillus subtilis nasDEF encoding NADH-dependent nitrite reductase by TnrA and ResDE. J Bacteriol. 1998;180:5344–5350. doi: 10.1128/jb.180.20.5344-5350.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano MM, Marahiel MA, Zuber P. Identification of a genetic locus required for biosynthesis of the lipopeptide antibiotic surfactin in Bacillus subtilis. J Bacteriol. 1988;170:5662–5668. doi: 10.1128/jb.170.12.5662-5668.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano MM, Zhu Y, LaCelle M, Zhang X, Hulett FM. Interaction of ResD with regulatory regions of anaerobically induced genes in Bacillus subtilis. Mol Microbiol. 2000;37:1198–1207. doi: 10.1046/j.1365-2958.2000.02075.x. [DOI] [PubMed] [Google Scholar]
- Nakano MM, Zuber P, Glaser P, Danchin A, Hulett FM. Two-component regulatory proteins ResD-ResE are required for transcriptional activation of fnr upon oxygen limitation in Bacillus subtilis. J Bacteriol. 1996;178:3796–3802. doi: 10.1128/jb.178.13.3796-3802.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesbit AD, Giel JL, Rose JC, Kiley PJ. Sequence-specific binding to a subset of IscR-regulated promoters does not require IscR Fe-S cluster ligation. J Mol Biol. 2009;387:28–41. doi: 10.1016/j.jmb.2009.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton GL, Rawat M, La Clair JJ, Jothivasan VK, Budiarto T, Hamilton CJ, Claiborne A, Helmann JD, Fahey RC. Bacillithiol is an antioxidant thiol produced in Bacilli. Nat Chem Biol. 2009;5:625–627. doi: 10.1038/nchembio.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton TW, Whitehead R, Li Y, Snyder LA, Saunders NJ, Smith H, Cole JA. Coordinated regulation of the Neisseria gonorrhoeae truncated denitrification pathway by the nitric oxide-sensitive repressor, NsrR, and nitrite-insensitive NarQ-NarP. J Biol Chem. 2006;281:33115–33126. doi: 10.1074/jbc.M607056200. [DOI] [PubMed] [Google Scholar]
- Rankin LD, Bodenmiller DM, Partridge JD, Nishino SF, Spain JC, Spiro S. Escherichia coli NsrR regulates a pathway for the oxidation of 3-nitrotyramine to 4-hydroxy-3-nitrophenylacetate. J Bacteriol. 2008;190:6170–6177. doi: 10.1128/JB.00508-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reents H, Gruner I, Harmening U, Bottger LH, Layer G, Heathcote P, Trautwein AX, Jahn D, Hartig E. Bacillus subtilis Fnr senses oxygen via a [4Fe-4S] cluster coordinated by three cysteine residues without change in the oligomeric state. Mol Microbiol. 2006;60:1432–1445. doi: 10.1111/j.1365-2958.2006.05198.x. [DOI] [PubMed] [Google Scholar]
- Rock JD, Thomson MJ, Read RC, Moir JW. Regulation of Denitrification Genes in Neisseria meningitidis by Nitric Oxide and the Repressor NsrR. J Bacteriol. 2007;189:1138–1144. doi: 10.1128/JB.01368-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodionov DA, I, Dubchak L, Arkin AP, Alm EJ, Gelfand MS. Dissimilatory metabolism of nitrogen oxides in bacteria: comparative reconstruction of transcriptional networks. PLoS Comput Biol. 2005;1:e55. doi: 10.1371/journal.pcbi.0010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CJ, Giel JL, Patschkowski T, Luther C, Ruzicka FJ, Beinert H, Kiley PJ. IscR, an Fe-S cluster-containing transcription factor, represses expression of Escherichia coli genes encoding Fe-S cluster assembly proteins. Proc Natl Acad Sci USA. 2001;98:14895–14900. doi: 10.1073/pnas.251550898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JY, Cha J, Lee J, Roe JH. Glutathione reductase and a mitochondrial thioredoxin play overlapping roles in maintaining iron-sulfur enzymes in fission yeast. Eukaryot Cell. 2006;5:1857–1865. doi: 10.1128/EC.00244-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro S. Regulators of bacterial responses to nitric oxide. FEMS Microbiol Rev. 2007;31:193–211. doi: 10.1111/j.1574-6976.2006.00061.x. [DOI] [PubMed] [Google Scholar]
- Steinmetz M, Richter R. Plasmids designated to alter the antibiotic resistance expressed by insertion mutations in Bacillus subtilis, through in vivo recombination. Gene. 1994;142:79–83. doi: 10.1016/0378-1119(94)90358-1. [DOI] [PubMed] [Google Scholar]
- Sun G, Sharkova E, Chesnut R, Birkey S, Duggan MF, Sorokin A, Pujic P, Ehrlich SD, Hulett FM. Regulators of aerobic and anaerobic respiration in Bacillus subtilis. J Bacteriol. 1996;178:1374–1385. doi: 10.1128/jb.178.5.1374-1385.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker NP, Hicks MG, Clarke TA, Crack JC, Chandra G, Le Brun NE, Dixon R, Hutchings MI. The transcriptional repressor protein NsrR senses nitric oxide directly via a [2Fe-2S] cluster. PLoS ONE. 2008;3:e3623. doi: 10.1371/journal.pone.0003623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker NP, Le Brun NE, Dixon R, Hutchings MI. There's NO stopping NsrR, a global regulator of the bacterial NO stress response. Trends Microbiol. 2010;18:149–156. doi: 10.1016/j.tim.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Voskuil MI, Chambliss GH. The −16 region of Bacillus subtilis and other gram-positive bacterial promoters. Nucleic Acids Res. 1998;26:3584–3590. doi: 10.1093/nar/26.15.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Richardson AR, Martens-Habbena W, Stahl DA, Fang FC, Hansen EJ. Identification of a repressor of a truncated denitrification pathway in Moraxella catarrhalis. J Bacteriol. 2008;190:7762–7772. doi: 10.1128/JB.01032-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B. Evidence for mutagenesis by nitric oxide during nitrate metabolism in Escherichia coli. J Bacteriol. 2006;188:829–833. doi: 10.1128/JB.188.3.829-833.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye RW, Tao W, Bedzyk L, Young T, Chen M, Li L. Global gene expression profiles of Bacillus subtilis grown under anaerobic conditions. J Bacteriol. 2000;182:4458–4465. doi: 10.1128/jb.182.16.4458-4465.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo WS, Lee JH, Lee KC, Roe JH. IscR acts as an activator in response to oxidative stress for the suf operon encoding Fe-S assembly proteins. Mol Microbiol. 2006;61:206–218. doi: 10.1111/j.1365-2958.2006.05220.x. [DOI] [PubMed] [Google Scholar]
- Yukl ET, Elbaz MA, Nakano MM, Moënne-Loccoz P. Transcription factor NsrR from Bacillus subtilis senses nitric oxide with a 4Fe-4S cluster. Biochemistry. 2008;47:13084–13092. doi: 10.1021/bi801342x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.